Abstract
Nitrogen use efficiency (NUE), a critical determinant of crop productivity and agricultural sustainability, varies significantly between indica and japonica subspecies. Here, we identify three coding-region SNPs in OsNLP4 underlying this divergence. These SNPs enhance the binding affinity of OsNLP4indica to nitrate response elements (NREs), amplifying transcriptional activation of nitrogen metabolism and iron homeostasis genes. Introgression of the OsNLP4indica allele into elite japonica cultivar XS134 increases both grain yield and NUE by 12–25% across multi-location field trials under varying nitrogen regimes. Heterologous expression in Arabidopsis increases shoot biomass by 23%, demonstrating possible conservation of function in dicots. Mechanistically, the indica allele’s stronger NRE-binding capacity synergistically modulates downstream pathways. Furthermore, combining OsNLP4indica with balanced nitrogen-iron fertilization boosts NUE by 30–32%. Our findings resolve a critical genetic basis of indica-japonica NUE divergence, provide a validated strategy for improving yield and NUE of commercial japonica varieties, and highlight OsNLP4indica as a cross-species genetic resource for sustainable agriculture.
Similar content being viewed by others
Introduction
Rice stands as a linchpin of global food security, nourishing over half of the world’s population1. However, its productivity hinges heavily on the intensive application of fertilizers, especially nitrogen (N) fertilizer2. Despite this, the nitrogen use efficiency (NUE) of rice, defined by the grain yield per unit of available N in the soil3, remains disconcertingly low, hovering below 40%. This not only imposes a significant financial burden on farmers but also exacts a toll on the environment through detrimental ecological impacts4,5,6. Therefore, the imperative of developing novel rice varieties endowed with enhanced NUE has emerged as a pressing priority to ensure both global food security and the sustainability of agricultural practices.
The differences between the indica and japonica subspecies of Asian cultivated rice underscore the complexity of NUE7,8,9,10. Japonica, favored for its superior quality and steadfast performance in temperate zones, grapples with a chronic challenge of low NUE11. Addressing this challenge necessitates a nuanced understanding of the underlying mechanisms driving the disparity in NUE between indica and japonica rice during their evolutionary trajectories.
The difference in NUE between indica and japonica was reflected in N absorption. Phylogenetic analyses have unraveled conserved single-nucleotide polymorphisms (SNPs) within the nitrate (NO3-) transporter gene OsNRT1.1B between the two subspecies, which was likely selected during rice domestication. Interestingly, the improved NO3- absorption and utilization capacity conferred by the indica allele of OsNRT1.1B underscores its pivotal role in bolstering grain yield and NUE in japonica varieties12. Moreover, the interplay of OsNRT1.1B with rhizosphere microbial communities further accentuates its multifaceted impact on NUE enhancement in rice13. Artificial selection has driven allelic differentiation, resulting in distinct structural variations of OsNR2 proteins between indica and japonica OsNR2 alleles. Specifically, OsNR2indica demonstrates increased enzyme activity compared to its japonica counterpart. Furthermore, OsNR2indica facilitates NO3- uptake through a feed-forward interaction with OsNRT1.1B. These characteristics endow OsNR2indica with the ability to enhance effective tiller number, grain yield, and NUE in japonica rice varieties14.
Beyond genetic disparities in N sensing, transport, and assimilation, variations in the expression levels of N-responsive genes contribute substantially to the NUE gap between indica and japonica rice. REGULATOR OF N-RESPONSIVE RSA ON CHROMOSOME 10 (RNR10) inhibits the degradation of rice NUE quantitative trait locus-encoded DULL NITROGEN RESPONSE1 (qDNR1) protein through ubiquitination, reducing auxin synthesis and eventually leading to decreased responsiveness of japonica rice roots to external N and decreased NUE15,16. Moreover, differences in the expression levels of ABC1-1 REPRESSOR 1 (OsARE1), OsMYB61, AMINO ACID PERMEASE 3 (OsAPP3), and OsAPP5 between indica and japonica subspecies also directly or indirectly contributed to the differences in NUE17,18,19,20, further underscoring the intricate regulatory landscape governing NUE divergence between indica and japonica subspecies.
Central to the orchestration of primary N metabolism in rice are the NIN-LIKE PROTEINs (NLPs), with OsNLP4 emerging as a key regulator of N metabolism, iron (Fe) metabolism, tillering and panicle development21,22,23. Structural prediction reveals three conserved domains in OsNLP4: an N-terminal GAF domain functioning as a chemo sensor for small signal molecules, a central RWP-RK domain mediating specific DNA binding to nitrate response elements (NREs) in target promoters, and a C-terminal PB1 domain enabling protein-protein interaction24.
In this work, we unveil distinct SNPs within the OsNLP4 GAF domain, delineating a stark contrast between indica and japonica subspecies. These SNPs endow indica OsNLP4 with augmented binding affinities to NREs, thereby amplifying the transcriptional regulation of genes pivotal for N metabolism and yield traits. Leveraging this insight, we demonstrate the efficacy of introgressing the indica OsNLP4 allele into elite japonica cultivars, yielding elite japonica cultivars with improved NUE and grain yield, even under low N conditions. This study reveals a valuable germplasm resource for enhancing NUE in commercial japonica varieties through conventional breeding or base editing, thereby surmounting the longstanding challenge of low NUE in japonica rice.
Results
OsNLP4 is divergent between indica and japonica rice
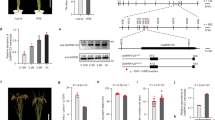
To explore whether OsNLP4 plays a role in the domestication of indica and japonica, we analyzed the OsNLP4 genome sequences of 3,000 rice varieties25 and identified two distinct haplotypes defined by the four SNPs in the OsNLP4 coding region between indica and japonica populations (Fig. 1a). The SNP1 (C303 to A303), SNP3 (A569 to G569) and SNP4 (G763 to A763) resulted in missense mutations, with arginine (Arg), serine (Ser) and threonine (Thr) in Hap-indica corresponding to serine (Ser), asparagine (Asn) and alanine (Ala) in Hap-japonica, respectively, whereas SNP2 was a synonymous nucleotide substitution (C531 to T531) without an amino acid change (Fig. 1a).
a Conserved single-nucleotide polymorphism (SNP) differentiation between the indica and japonica OsNLP4 proteins. Locations of functional domains are indicated by black bars. Single nucleotide diversities, characterizing the distribution ratio of SNP1 (b), SNP3 (c) and SNP4 (d) from two OsNLP4 haplotypes in 4726 diverse rice accessions (indica: 2759, japonica: 1512, aus: 269, aromatic: 96, intermediate: 90, O. rufipogon: 28, O. glaberrima: 11, O. barthii: 10). e NUE of rice varieties carrying different OsNLP4 haplotypes (Hap-indica, Hap-japonica). All data are from plants grown under normal paddy-field fertilization conditions at Wenjiang in 2023 (Hap-indica, n = 143; Hap-japonica, n = 69). f The chlorate sensitivity of the parental plants Xiushui134 (XS134) and 9311, SSSL95, and XS134indica-NLP4 (NIL) was tested. Scale bar = 2.0 cm. 15N uptake assays in XS134 and XS134indica-NLP4 (NIL-1, NIL-2) plants labeled with 15N-nitrate (g) and 15N-ammonium (h). i Total N content of XS134, NIL-1, and NIL-2. Seedlings were grown in modified Kimura B solution (pH 5.8) supplemented with 2 mM KNO3 for 18 days. j Growth phenotype of representative XS134, NIL-1, and NIL-2 at the maturing stage in the field trial at Hefei in 2022. Scale bar = 8.0 cm. Tiller number per plant (k), actual yield per plot (l), and NUE (m) of XS134, NIL-1, and NIL-2 in the field trial. The values are the means ± SDs (n = 8 replicates, 6 seedlings per replicate for g–i, n = 16 plants for k, n = 16 panicles for l, and n = 4 replicates, 80 plants per replicate for m). The different letters above the bars denote significant differences (P < 0.05) according to a two-side Duncan’s multiple range test for g–i and k-m, and a two-sided Student’s t-test for e. Source data are provided as a Source Data file.
The SNP1, SNP3 and SNP4 of the OsNLP4indica haplotypes represented 92.0%, 95.3% and 95.4% of the 2749 indica accessions, respectively, which represented only 0.6%, 0.9% and 0.9% of the 1512 japonica accessions, suggesting the divergence of OsNLP4 between indica and japonica subspecies (Fig. 1b–d). In addition, the SNP1 type of the OsNLP4indica haplotype accounted for only 7.1%, 0% and 0% of the total nucleotide type of O. rufipogon, O. glaberrima, and O. barthii, respectively, indicating that it might be derived from the rare variation in O. rufipogon. In contrast, the SNP3 and SNP4 nucleotide types of the OsNLP4indica haplotype were widely distributed in the ancestor species, accounting for 89.3%, 100% and 100%, respectively (Fig. 1b–d). These findings suggest that OsNLP4indica was subjected to artificial selection during the domestication of indica varieties and has not been introgressed into japonica varieties.
OsNLP4 indica substantially enhances NUE and grain yield in japonica rice
To verify whether the natural variations in OsNLP4 between indica and japonica contribute to the divergence in NUE, we analyzed 143 indica varieties and 69 japonica varieties carrying two OsNLP4 haplotypes, respectively, and we found that the NUE of indica varieties containing OsNLP4indica was significantly higher than that of japonica varieties (Fig. 1e).
To introgress OsNLP4indica to japonica rice, we crossed indica rice 9311, an elite parent of hybrid rice as the donor parent, with XS134, an elite japonica cultivar widely cultivated in regions along Yangtze River, as the recurrent parent to construct a set of single segment substitution lines (SSSLs), each containing a single chromosome segment from 9311 substituted into the XS134 genetic background26. the near-isogenic line the near isogenic line XS134indica-NLP4 (NIL). Given the phylogenetic divergence of OsNLP4 between indica and japonica, coupled with its well-documented regulatory role in NUE21,23, we specifically selected SSSL-95 harboring the 9311-derived OsNLP4indica allele for functional characterization. Chlorate sensitivity assays revealed significantly enhanced susceptibility in SSSL-95 compared to the recurrent parent XS134, prompting its selection for development of the near isogenic line XS134indica-NLP4 (NIL) (Fig. 1f). Fine mapping was performed for a cross between SSSL-95 (donor parent) and XS134 (recurrent parent), and the region containing the complete OsNLP4 locus was narrowed down to an ~25-kb region flanked by the markers VG5 and VG6 (Supplementary Fig. 1). Sequence comparison of OsNLP4 coding region revealed the expected 4 SNPs between NIL and XS134 (Supplementary Data 1), indicating a successful introgression of OsNLP4indica allele into XS134.
Compared with XS134, XS134indica-NLP4-1 and −2 (NIL-1, NIL-2), two parallel lines from independent events, showed greater 15NO3- and 15NH4+ accumulation, eventually leading to a significant increase in total N content (Fig. 1g–i). In the field trial in Hefei, Anhui Province, China (E117°, N31°), NILs exhibited more tillers and more grains per panicle than those of the recipient control. Consequently, the yield significantly increased by an average of 25% (Fig. 1j–m). These results clearly show that OsNLP4indica confers improved NUE and yield to japonica plants.
When grown in nutrient solution containing different NO3- concentrations (0.02 mM, 0.2 mM, and 2 mM), NILs showed obvious growth advantages, and the seedling biomass increased significantly by an average of 9.9%, 20.4% and 23.8%, respectively, compared with that of XS134 under different N conditions (Fig. 2a, b). We then performed long-term pot experiments under low (LN, 1 mM KNO3) and high (HN, 5 mM KNO3) N supplies. NILs exhibited greater grain yields than XS134, even under LN conditions (Fig. 2c, d).
a Phenotypes of the XS134 and NILs (NIL-1, NIL-2) plants grown in hydroponic media supplemented with different nitrate concentrations for 18 days. Scale bar = 2.0 cm. b Biomass of the plants under different nitrate conditions, as shown in (a). c Growth phenotype at the maturing stage of representative XS134, NIL-1, and NIL-2 plants grown in vermiculite pots and provided with different nitrate concentrations. Images of four-month-old plants were taken. LN: 1 mM KNO3, HN: 5 mM KNO3. Scale bar = 8.0 cm. d Images of total grains per plant for XS134, NIL-1, and NIL-2 under different nitrate conditions, as shown in c. Scale bar = 2.0 cm. e Growth phenotype of representative XS134, NIL-1, and NIL-2 at maturing stage in the field under different N conditions (Changxing, Zhejiang province, China). LN: 90 kg urea/ha, NN: 180 kg urea/ha, HN: 360 kg urea/ha. Scale bar = 8.0 cm. f Images of total grains per plant for XS134, NIL-1, and NIL-2 under different N conditions as shown in (e). Scale bar = 1.0 cm.Tiller number per plant (g), actual yield per plot (h), and NUE (i) of XS134, NIL-1, and NIL-2 in the field under different N conditions as shown in (e). Grain yield per plot (j), and NUE (k) of XS134, NIL-1, and NIL-2 in the large-scale field under different N conditions (Lingshui, Hainan province, China). Each plot is 66 m2. LN: 75 kg urea/ha, HN: 150 kg urea/ha (local routine N application levels). The values are the means ± SDs (n = 16 plants for b and g, n = 4 replicates, 80 plants per replicate for h and i, n = 4 replicates, 1700 plants per replicate for j and k. The different letters above the bars denote significant differences (P < 0.05) according to a two-sided Duncan’s multiple range test. Source data are provided as a Source Data file.
To further confirm that OsNLP4indica improves NUE, we conducted field trials at Changxing, Zhejiang Province, China (E119°, N31°) under LN, NN and HN conditions. The NILs exhibited substantial increases in the grain number per panicle and tiller number per plant (Fig. 2e–g), resulting in an average increase of actual yield per plot by 21.1% under LN, 25.8% under NN and 20.1% under HN compared with the recipient control XS134 (Fig. 2h). The NUE was also improved by 21.1%, 25.8% and 20.1%, respectively (Fig. 2i).
Furthermore, we conducted a large-scale field trial in Lingshui, Hainan Province, China (E109°, N18°), and found that the yield of NILs was also significantly improved compared with those of XS134, increasing by 12.5% under LN and 15.7% under HN (local routine N application level) (Fig. 2j). The NUE achieved obvious improvements similar to the yield (Fig. 2k). Similar increases in yield and NUE under different N conditions were reproduced in multiple independent large-scale field experiments (Supplementary Fig. 2). Taken together, our results clearly demonstrate that OsNLP4indica introgression significantly enhances grain yield and NUE in japonica.
OsNLP4 indica enhances yield and NUE in transgenic plants
To further confirm that natural variations in OsNLP4 contribute to the divergence in NUE between indica and japonica, we generated transgenic plants in the DJ background either expressing self-promoter-driven OsNLP4 derived from Dongjin (DJ, japonica) (gDJ-1, gDJ-2) or 9311 (g9311-1, g9311-2). Field trials in Hefei, Anhui Province, China (E117°, N31°) showed that OsNLP4indica transgenic plants exhibited improved performance, with an average increase in tiller number, yield and NUE of 22.9%, 24.8% and 21.1%, respectively, compared with those of DJ, whereas OsNLP4japonica transgenic plants increased by only 8.2%, 9.8% and 9.8%, respectively (Fig. 3a–d).
a Growth phenotype of representative DJ, OsNLP4 japonica transgenic plants (gDJ-1, gDJ-2), and OsNLP4indica transgenic plants (g9311-1, g9311-2) at the maturing stage in a field trial at Hefei in 2023. Scale bar = 8.0 cm. Tiller number per plant (b), actual yield per plot (c), and NUE (d) of DJ, gDJ-1, gDJ-2, g9311-1, and g9311-2 in the field trial. e Phenotypes of DJ, gDJ-1, gDJ-2, g9311-1, and g9311-2 grown in hydroponic medium with different nitrate concentrations for 18 days. Scale bar = 2.0 cm. f Biomass of the plants under different nitrate conditions, as shown in (e). g Growth phenotype of representative DJ, OsNLP4japonica transgenic plants (gDJ-1), and OsNLP4indica transgenic plants (g9311-2) at maturing stage in the field under different N conditions (Changxing, Zhejiang Province, China). LN: 90 kg urea/ha, NN: 180 kg urea/ha, HN: 360 kg urea/ha. Scale bar = 8.0 cm. Actual yield per plot (h), and NUE (i) of DJ, gDJ-1, and g9311-2 in the field trial. j Phenotypes of 2-week-old wild-type Arabidopsis (Col-0), OsNLP4japonica transgenic plants (OsNLP4DJ-1, OsNLP4DJ-2), and OsNLP4indica transgenic plants (OsNLP49311-1, OsNLP49311-2) grown in soil. k Shoot fresh weight of the plants under the conditions shown in (j). l Hierarchical clustering analysis was conducted on expression profiles of 20 N metabolism-associated genes in Col-0, OsNLP4japonica transgenic plants (OsNLP4DJ-1), and OsNLP4indica transgenic plants (OsNLP49311-1). Transcriptome profiling was performed using 16-day-old Arabidopsis seedlings grown on MS medium. The heatmap displays log2-transformed expression ratios of N metabolism marker genes. Data points meeting statistical significance thresholds (P < 0.05) were selected for visualization using a graduated color scale. FPKM (fragments per kilobase of transcript per million mapped fragments) values represent normalized transcript abundance. Values represent the means ± SDs (n = 16 plants for b, f and k, n = 4 replicates, 80 plants per replicate for c, d, h, and i, and n = 3 replicates for l). The different letters above the bars denote significant differences (P < 0.05) according to a two-sided Duncan’s multiple range test. Source data are provided as a Source Data file.
Under hydroponic culture, the OsNLP4indica transgenic plants exhibited significant growth advantages under all NO3- concentrations over OsNLP4japonica transgenic plants except for biomass under 0.02 mM NO3− (Fig. 3e, f). In the field trial in Changxing, Zhejiang Province, China (E119°, N31°), similar to the NILs, the yield of OsNLP4indica transgenic plants increased by 17.3%, 30.1%, and 25.5%, respectively, under LN, NN, and HN conditions, compared with DJ wild-type control plants, whereas the OsNLP4japonica transgenic lines only increased by 2.7%, 6.7% and 10.2%, respectively (Fig. 3g, h). The NUE showed similar changes to yield between OsNLP4indica and OsNLP4japonica transgenic plants under different N conditions (Fig. 3i).
Phylogenetic analyses suggest that NLP family members may be functionally conserved in different species (Supplementary Fig. 3), which led us to assume that the functions of OsNLP4indica in the monocot rice may also be conserved in dicot plants. To verify our hypothesis, we generated Arabidopsis transgenic plants expressing either self-promoter-driven OsNLP4indica or OsNLP4japonica. The OsNLP4indica plants exhibited a significant 23% increase in shoot biomass compared with the OsNLP4japonica transgenic plants (Fig. 3j, k). This phenotypic divergence correlated with differential induction of N metabolism-related marker genes, which showed substantially stronger upregulation in OsNLP4indica transgenic lines compared to OsNLP4japonica plants (Fig. 3l, Supplementary Fig. 4). Interestingly, these results recapitulate the functional divergence between the two rice orthologs within a heterologous dicot system. Collectively, our findings highlight OsNLP4indica cross-species potential as a strategic genetic target for enhancing NUE in both monocot and dicot crops.
Three SNPs enhance the binding affinity of OsNLP4indica for NREs
To investigate the genetic basis of natural OsNLP4 variations contributing to yield enhancement and improved NUE, we first compared the promoter sequences of OsNLP4 between indica and japonica populations and revealed substantial sequence polymorphisms, including SNPs, insertion-deletions (INDELs), and short tandem repeats (STRs) (Supplementary Table 1). Interestingly, transcript level analysis demonstrated comparable OsNLP4 expression between NILs and XS134, as well as among transgenic plants, ruling out OsNLP4 expression levels as the determinant for NUE differences between subspecies (Supplementary Fig. 5). Moreover, sequence alignment of OsNLP4 coding regions among 9311, XS134, and DJ accessions identified no amino acid substitutions beyond the conserved SNPs (Supplementary Data 1). OsNRT1.1B and OsNR2, as known NUE differentiation factors12,14, were also confirmed to maintain identical coding sequences between XS134 and NILs (Supplementary Data 2 and 3). These cumulative findings conclusively attribute the observed NUE enhancement in NILs to three coding SNPs within the OsNLP4 locus.
Intriguingly, transcriptome profiling identified significant upregulation of key N metabolism genes containing NRE cis-elements in NIL plants, including OsNRT1.1B and OsNR2, along with Fe metabolism marker genes (Supplementary Fig. 6). Considering that the SNPs reside in the GAF domain, which affects DNA binding ability upon nitrate sensing27, we examined NRE-binding activity by performing an electrophoretic mobility shift assay (EMSA) and found that OsNLP4indica exhibited stronger NRE-binding ability than OsNLP4japonica in vitro (Fig. 4a). Subsequent dual-luciferase (LUC) reporter assays also confirmed this finding in vivo. Greater LUC activity was detected when the effector construct 35S-OsNLP4indica was cotransfected with the reporter construct NRE:LUC than when the effector 35S-OsNLP4japonica was cotransfected (Fig. 4b). To quantitatively assess the functional consequences of OsNLP4 allelic variation on NRE interaction dynamics, we employed microscale thermophoresis (MST). Recombinant MBP-NLP4 fusion proteins (japonica and indica variants) expressed in Escherichia coli exhibited distinct binding profiles across NRE concentration gradients. Quantitative MST analysis showed that the dissociation constant (Kd) of OsNLP4indica (1.099 µM) was approximately one-third that of OsNLP4japonica (3.135 µM) (Fig. 4c), confirming superior NRE-binding capacity of the indica allele.
a Electrophoretic mobility shift assay (EMSA) to detect OsNLP4japonica and OsNLP4 indica binding to the NRE. EMSAs were performed using a biotin-labeled NRE cis-fragment from the OsNIA1 gene as a probe and an unlabeled fragment as a competitor. Data are representative of three independent experiments, with similar results. b Dual-luciferase assays. The diagram depicts the effectors and reporter. The effector genes OsNLP4indica and OsNLP4japonica were under the control of the CaMV 35S promoter. The fragment containing the NRE cis-element from the promoter of OsNIA1 was fused to the LUC gene as a reporter. Firefly LUC and REN luciferase activities were detected by transient dual-luciferase reporter assays, and the LUC:REN ratio was calculated. c NRE binding to OsNLP4japonica and OsNLP4indica, as measured by microscale thermophoresis (MST). Kd: dissociation constant. d EMSA to detect OsNLP4japonica and OsNLP4indica binding to the OsD3 promoter. e Dual-luciferase reporting transient assays showed that OsNLP4indica had a greater ability to inhibit OsD3 expression than OsNLP4japonica. Data are representative of three independent experiments, with similar results. f The expression levels of OsD3 in the basal tissues of XS134 and NILs (NIL-1, NIL-2) plants cultured in soil for 30 days. g EMSA to detect OsNLP4japonica and OsNLP4indica binding to the OsRFL promoter. Data are representative of three independent experiments, with similar results. h Dual-luciferase reporting transient assays showed that OsNLP4indica had a greater ability to induce OsRFL expression than OsNLP4japonica. i The expression levels of the OsRFL in the XS134, NIL-1, and NIL-2 plants at the branch meristem formation stage (young panicles, 0.5 cm). The values are the means ± SDs (n = 3 replicates for b, c, e, f, h, and i). The different letters above the bars denote significant differences (P < 0.05) according to a two-sided Duncan’s multiple range test. Source data are provided as a Source Data file.
Similarly, we demonstrated the enhanced binding to the NRE in OsD3 and OsRFL promoter (Fig. 4d, e, g, h), consistent with the decreased transcript levels of OsD3, the increased OsD53 protein abundance, and the increased transcript levels of OsRFL, as well as decreased expression of OsCKX9 and OsFC1 downstream of OsD3 and increased tillers and panicle sizes in the introgression lines (Fig. 4f, i, Supplementary Figs. 7, 8). To dissect the functional contributions of individual SNPs, we performed site-directed mutagenesis to sequentially introduce indica alleles (A303, G569, A763) into the OsNLP4japonica background (C303, A569, G763) and conducted LUC experiments. Unexpectedly, none of the single-allele replacements restored the transcriptional activation potency of native OsNLP4indica (Supplementary Fig. 9). This result suggests that the functional enhancement of OsNLP4indica requires synergistic cooperation among all three SNPs.
Collectively, our results firmly establishes that the three SNPs at indica OsNLP4 synergistically confers superior NRE-binding capacity, thereby potentiating transcriptional activation of target genes and ultimately driving agronomic improvements in both yield and NUE.
NILs exhibit further enhanced yield and NUE under balanced N-Fe conditions
We previously discovered that a balanced N-Fe supply strongly induces OsNLP4 nuclear accumulation by reducing the levels of H2O2, promoting tillering and ultimately improving rice yield and NUE23. To evaluate the effect of N-Fe balance on the grain yield and NUE of NILs, we grew rice plants in pots filled with vermiculite under four N-Fe conditions. XS134 showed the worst growth under N-sufficient and Fe-deficient (HN-LFe) conditions (Supplementary Fig. 10), but produced more tillers under balanced and sufficient N and Fe (HN-HFe) than those under other conditions, which were 2.6, 1.6 and 3.9 times greater than those under LN-LFe, LN-HFe, and HN-LFe conditions, respectively, thus having the highest yield (Fig. 5a, b), indicating the tremendous impact of N-Fe balance on the growth of rice. Compared with XS134, NILs were more responsive to different N-Fe concentration variations. Under HN-HFe conditions, the NILs exhibited the greatest increase, with a 38.0% increase in tillers, a 39.0% increase in grain yield, and a 39.1% increase in NUE compared with those of XS134 (Fig. 5a–c). In contrast, under HN-LFe conditions, these plants produced the lowest tiller numbers, yields and NUE, with 87.1%, 95.8%, and 95.9% reductions, respectively, relative to those under HN-HFe conditions (Fig. 5a–c), consistent with our previous findings23.
Tiller number per plant (a), grain yield per plant (b) and NUE (c) of XS134 and NILs (NIL-1, NIL-2) plants grown in vermiculite pots under different N-Fe conditions. LFe: 50 μM Fe(II)-EDTA, HFe: 200 μM Fe(II)-EDTA, LN: 1 mM KNO3, HN: 5 mM KNO3. The values are the means ± SDs (n = 16 plants for a–c). Different letters above the bars denote significant differences (P < 0.05) according to a two-sided Duncan’s multiple range test. Source data are provided as a Source Data file.
Based on our previously confirmed efficacy of foliar spraying of N-Fe compound fertilizer in increasing yield and NUE23, we performed a field trial in Lingshui, Hainan Province, China (E109°, N18°), in which N-Fe fertilizers were sprayed on rice plants at the early stage of tillering. Compared with H2O-sprayed control treatment, the application of N-Fe compound fertilizer significantly increased the number of tillers, grain yield and NUE of XS134 by an average of 14.1%, 10.8% and 10.8%, respectively (Table 1). Compared with XS134 treated with H2O, the NIL-2 plants were more responsive to the N-Fe fertilizer and exhibited substantial increases in tiller number, yield, and NUE by 27.2%, 30.8% and 30.8%, respectively (Table 1).
Moreover, to adapt to conventional fertilization methods used for various crops without increase of labor costs, we integrated our N-Fe fertilizer with commercial compound fertilizer formula. In the field in Hefei, Anhui Province, China (E117°, N31°), with integrated fertilizer (N + Fe), the tiller number, yield and NUE of NIL-2 significantly increased by 28.9%, 32.4%, and 32.4%, respectively, whereas those treated with conventional fertilizer (N) increased by 16.2%, 20.5%, and 20.5%, respectively, compared with those of XS134 treated with conventional fertilizer (Table 1). Together, these results reaffirm the great efficacy of OsNLP4indica combined with balanced N-Fe fertilization in promoting the yield and NUE of japonica cultivars.
Discussion
NLPs are central regulators of primary N metabolism in plants28,29,30,31,32. OsNLP4, a key regulator of N response in rice, coordinately regulates the genes related to N metabolism, Fe metabolism, tillering and panicle development, thereby significantly improving rice yield and NUE21,22,23. However, OsNLPs have not been explored for their roles in the difference in NUE between indica and japonica rice. In this study, we presented concrete evidence that the natural variation at indica OsNLP4 enhanced the affinity of OsNLP4indica for NREs in the promoters of the target genes, thereby promoting downstream events such as N utilization and tillering and panicle development, and ultimately leading to a significant boost in yield and NUE, which is strongly supported by the introgression of OsNLP4indica into elite japonica varieties that effectively enhances yield and NUE. Importantly, combining with balanced N-Fe fertilizer, the introgressed japonica varieties achieved even higher productivity than with convention N fertilizer.
In the evolution and domestication of rice, OsNLP4 has differentiated into two distinct haplotypes: Hap-indica and Hap-japonica with three SNPs, which play a crucial role in the divergence of NUE between indica and japonica (Fig. 1a–e). The japonica varieties are widely cultivated in N-rich areas, while the indica varieties are mainly distributed in N-poor regions6. SNPs in Hap-indica are broadly distributed in indica, but rarely occur in japonica (Fig. 1b–d), suggesting that OsNLP4indica may contribute to the geographical adaptation of rice varieties to N-poor soils. In Hap-indica, compared with the high occurrence frequency of SNP3 and SNP4 in wild rice, SNP1 is only derived from rare variation in O. rufipogon (Fig. 1b–d). During rice domestication, perhaps this rare variant was retained under the selective pressure of low N in the N-deficient regions, due to the high NUE conferred by the OsNLP4indica haplotype (Fig. 1e).
The absence of OsNLP4indica haplotype in japonica varieties (Fig. 1a–d) suggests that this haplotype has not been utilized in the breeding of the existing elite japonica varieties, thus offering tremendous potential for yield and NUE improvements of japonica. We demonstrated this by introgressing OsNLP4indica of 9311 into XS13433, which significantly increased yield by at least 12.5% under different N fertilizer conditions at different locations in rigorous large-scale field trials (Figs. 1j–m, 2, Supplementary Figs. 1, 2, Supplementary Data 1). XS134 is an elite commercial japonica variety widely cultivated in regions along Yangtze River, and the improved XS134indica-NLP4 can be directly applied in agriculture, providing immediate economic and ecological benefits to this area, in stark contrast to previous studies reporting large yield increases in noncommercial varieties12,14.
The natural variations in OsNLP4 caused much stronger affinity of OsNLP4indica for binding to NRE than that of OsNLP4japonica, enhancing the function of OsNLP4indica, rather than differences in expression levels (Fig. 4, Supplementary Figs. 5, 6, Supplementary Table 1). One likely reason is that the three conserved SNPs of OsNLP4 are all clustered in the GAF domain, affecting the perception of the N signal and thus changing the binding affinity of the RWP-RK domain to NRE27,34,35. This is also supported by OsNLP4indica enabled rice to achieve higher yield and NUE, as well as Arabidopsis to produce higher biomass than OsNLP4japonica, which suggests the prospect of OsNLP4indica in monocot and dicot crops (Fig. 3, Supplementary Figs. 3, 4). The enhanced N utilization capacity, increased rice tiller number and panicle size are crucial factors for the improved NUE of NILs, which was attributed to the strong transcriptional regulation of OsNLP4indica on target genes such as OsNRT1.1B, OsNR2, OsD3 and OsRFL, which contain NREs in their promoters (Figs. 1f–m, 2e-k, 4, Supplementary Figs. 6–8)21,22,23. The activation of multiple downstream events highlights the significant advantages of enhancing transcription factor functionality36. This enhancement is precisely what drives the substantial increase in yield and nitrogen use efficiency (NUE) when the OsNLP4indica gene is introduced into commercial japonica rice varieties.
Furthermore, based on previous findings23,37,38, we demonstrated a greater productivity of NILs with balanced N-Fe fertilizers, providing a promising strategy for enhancing yield and NUE of commercial japonica varieties. NIL-2 was more responsive to N-Fe fertilizers than XS134, and exhibited more tillers, higher yield and NUE under balanced and sufficient N-Fe conditions (Fig. 5a–c, Supplementary Fig. 10). This was confirmed by field trials, where foliar spraying balanced N-Fe fertilizer at almost negligible expense for an additional 20% yield return for NIL-2 compared to XS134 (Table 1). To improve conventional fertilization of rice, we integrated N-Fe fertilizer with commercial compound fertilizer and applied it in a traditional way, resulting in significant increases in yield and NUE similar to those of foliar spray (Table 1), reaffirming the tremendous potential of OsNLP4indica combined with balanced N-Fe fertilizer.
Overall, we revealed that natural variations in the coding region of OsNLP4 augment its binding affinity for NRE, enhancing OsNLP4 functions in downstream events, including N metabolism, tillering and panicle development, and ultimately boosting yield and NUE, which contribute to the NUE disparity between indica and japonica subspecies. The introgression of the OsNLP4 indica allele can effectively enhance the yield and NUE of commercial japonica varieties, especially when combined with balanced N-Fe fertilizer, which is anticipated to play a significant role in resolving the dilemma of NUE depression in japonica. Most importantly, our findings hold profound implications for enhancing NUE and yield across diverse crop species via gene editing and transgenic approach.
Methods
Plant materials
The rice (Oryza sativa L.) varieties Xiushui 134 (XS134), 9311 and Dongjin (DJ) were used in this study. The SSSLs constructed with 9311 as the donor and XS134 as the recipient were obtained from Dr. Chengcai Chu (South China Agricultural University). XS134indica-NLP4 was obtained by crossing SSSL-95 (donor parent) and XS134 (recurrent parent), from which positional cloning using 275 BC1F2 and 650 BC2F2 progeny plants. Self-promoter-driven OsNLP4indica and OsNLP4japonica constructs were constructed by inserting the 3000 bp promoter fragment of OsNLP4 connected to the genomic sequence spanning OsNLP4 from DJ and 9311, respectively, into pCB308R via the GATEWAY cloning system39. The binary vectors were subsequently transformed into Agrobacterium tumefaciens (EHA105) for rice transformation by Wuhan Biorun Bioscience Co., Ltd. (Wuhan, China) (https://plant.biorun.com/).
The Arabidopsis thaliana ecotype Columbia (Col-0) was used in this study. Self-promoter-driven OsNLP4indica and OsNLP4japonica constructs were constructed by inserting the 3000 bp promoter fragment of OsNLP4 connected to the genomic sequence spanning OsNLP4 from DJ and 9311, respectively, into pCB308R via the GATEWAY cloning system. Transgenic Arabidopsis plants were subsequently generated via the Agrobacterium-mediated floral-dip method.
Plant growth conditions
For the hydroponic culture, seeds of XS134, NIL-1, NIL-2, DJ, gDJ-1, gDJ-2, g9311-1, and g9311-2 were washed with distilled water and incubated at 37 °C for 3 days. Germinated seeds were transferred to modified Kimura B solution (pH 5.8) supplemented with different levels of N (0.02 mM of LN, 0.02 mM of NN, 0.2 mM of KNO3, 2 mM of HN, or KNO3) and 100 μM of Fe(II)-EDTA and then grown for 18 days. The nutrient solutions were replaced every 2 days. The climate chamber was maintained under a 16-h light (30 °C)/8-h dark (28 °C) cycle.
For long-term treatment in vermiculite, seeds of XS134, NIL-1, and NIL-2 were germinated and transferred to pots filled with vermiculite (pot dimensions, 15 × 15 × 15 cm3). One plant was grown per pot, and each treatment was applied to 30 pots per genotype. Plants were fed modified Kimura B solution (pH 5.8) with different levels of N (LN, 1 mM KNO3; HN, 5 mM KNO3) and 100 μM Fe(II)-EDTA or with different N-Fe solutions (LN, 1 mM KNO3; HN, 5 mM KNO3; LFe, 50 μM Fe(II)-EDTA; HFe, 200 μM Fe(II)-EDTA) until maturity. Each treatment was irrigated with 10 L of nutrient solution each time. Throughout the growth period, the plants were irrigated 12 times at intervals of 12 days. The plants were maintained under a 12-h light (30 °C)/12-h dark (28 °C) cycle in a greenhouse. NUE was defined as the grain yield per unit of available N (KNO3) in vermiculite3.
For the soil culture, seeds of XS134, NIL-1, NIL-2, DJ, gDJ-1, gDJ-2, g9311-1, and g9311-2 were washed with distilled water and incubated at 37 °C for 3 days. Germinated seeds were transferred to pots filled with soil (the pot dimensions were 15 × 15 × 15 cm3). Each treatment contained 30 pots for each genotype. A single plant was grown per pot. The provided growth conditions included a 12-h light (30 °C)/12-h dark (28 °C) cycle in a greenhouse. To evaluate the phenotype of the soil-grown transgenic Arabidopsis plants, the plants were germinated and grown in soil at 22 °C under a 16-h light/8-h dark photoperiod.
Field trials
Field tests were carried out in paddy fields under natural growth conditions from 2022–2024 at three experimental locations: Hefei (Anhui Province, China), Lingshui (Hainan Province, China), and Changxing (Zhejiang Province, China).
The field trials at Hefei were performed two times—in 2022 and 2024 (from May to October). Urea was used as the N source at 180 kg N/ha. The plants were transplanted in 8 rows with 10 plants in each plot (3.6 m2), and four replicates were used for each treatment.
The field trial at Lingshui was performed from December 2023 to April 2024, and urea was used as the N source, with 75 kg N/ha for low N and 150 kg N/ha for high N (local routine N application level). For field trials with different N levels, the plants were transplanted to 170 rows × 10 plants per plot (66 m2), and four replicates were used for each treatment. For the field trial with only a normal N level (150 kg N/ha), the plants were transplanted in 2 rows × 10 plants per plot (0.8 m2), and four replicates were used for each treatment.
The field trial at Changxing was conducted in 2023 (May to October). Urea was used as the N source, with 90 kg N/ha for normal N, 180 kg N/ha for normal N, and 360 kg N/ha for high N. The planting density was 8 rows × 10 plants for each plot (3.4 m2), with four replicates.
The field foliar spray experiment at Lingshui (December 2022 to April 2023) comprised two treatments: i) control (foliar application of H2O) and ii) foliar application of Fe (1.5 mM Fe(II)-EDTA) in a cocktail solution containing 0.6% urea. At the early tillering stage, plants received foliar spray only once. All foliar solutions were applied at 1600 liters/hectare. The foliar treatments were applied in the late sunny afternoon40. All treatments received the same basic management. The plants were transplanted in 10 rows × 10 plants per plot (4 m2), and four replicates were used for each treatment.
The field experiment at Hefei (May to October 2023) in which modified tillering fertilizer was applied included two treatments: i) urea and ii) N-Fe compound fertilizer (supplemented with Fe(II)-EDTA in urea to adjust the molar ratio to 67:1.5). All treatments involved traditional fertilization at the early tillering stage, and the plants received the same basic management practices. The plants were transplanted in 8 rows with 10 plants in each plot (3.6 m2), and four replicates were used for each treatment.
RNA-sequencing analysis
For rice experiments, approximately 100 seedlings per strain (XS134 and NILs) were hydroponically cultured in a climate-controlled growth chamber under the specified conditions described above. Following germination, seedlings were maintained in modified Kimura B solution supplemented with 2 mM KNO3. Whole plant tissues from 14-day-old seedlings were harvested for transcriptome profiling. In parallel Arabidopsis experiments, seeds were surface-sterilized and germinated on MS medium under controlled environmental conditions (22 °C with 16-h light/8-dark cycles). Similarly, 16-day-old whole Arabidopsis seedlings were collected for RNA extraction. Each experimental group consisted of 30 pooled seedlings per biological replicate, with triplicate independent replicates performed to ensure reproducibility. RNA library preparation and subsequent sequencing procedures were carried out according to established protocols23.
qRT‒PCR analyses
Total RNA was isolated from whole seedlings (16-day-old), basal tissue (1-month-old), or young panicles (0.5 cm, in the branch primordium stage) of plants grown in soil using TRIzol reagent (TransGen, no. ET111). Two micrograms of total RNA were used to synthesize cDNA using the Easyscript One-step gDNA Removal and cDNA Synthesis SuperMix reagent kit (TransGen, no. AE311-02). The qRT‒PCR step one Plus real-time PCR system was used for the TaKaRa SYBR premix Ex Taq II reagent kit (no. Q111‒02). Subsequent qRT‒PCR procedures were performed according to the manufacturer’s instructions, and each qRT‒PCR assay was repeated at least three times with three independent RNA preparations. Rice Actin1 was used as an internal reference. The primers used are shown in Supplementary Data 4.
EMSAs
The coding regions of OsNLP4indica and OsNLP4japonica were cloned and inserted into the pET30a vector, which contains an N-terminal MBP tag, His tag, and a tobacco etch virus (TEV) protease site between the MBP tag and target protein. MBP-OsNLP4indica and MBP-OsNLP4japonica fusion proteins were expressed in the Escherichia coli Rosetta strain (DE3). We purified the recombinant proteins and the MBP protein with a Hisprep IMAC column (GE Healthcare, no. 17092102). Cis-fragments of target gene promoters were synthesized, and biotin was used to label the DNA ends. Unlabeled fragments of biotin with the same sequence were used as competitors. EMSAs were performed according to the LightShift TM EMSA Optimization and Control Kit (Thermo Fisher Scientific, no. 20148). MBP-OsNLP4indica- and MBP-OsNLP4 japonica-related reactions were loaded onto a 2% agarose gel in 0.5% TBE buffer for electrophoresis. The results were detected using a CCD camera system (GE Healthcare, Image Quant LAS 4000).
Protoplast transfection and dual-luciferase reporter assay
Stems from 2–3-week-old rice plants were excised and incubated in freshly prepared enzyme solution (1.5% cellulose R-10, 0.75% macerozyme R-10, 0.6 M mannitol, 10 mM MES, 1 mM CaCl2, 0.1% bovine serum albumin, pH 5.7) and soaked for 3–4 h in the dark with gentle shaking (50 rpm). After adding an equal volume of W5 buffer (154 mM sodium chloride, 125 mM CaC12, 5 mM KCl, 2 mM MES, pH 5.7), the harvesting cells were resuspended in MMG buffer (0.6 M mannitol, 15 mM MgCl2, 4 mM MES, pH 5.7). For protoplast transient expression experiments, plasmids were transfected into protoplasts using 40% PEG buffer (0.6 M mannitol, 15 mM MgCl2, 4 mM MES, pH 5.7)6. One thousand bp upstream of the target gene initiation codon and the NREs (cis-fragments) were cloned and inserted into the vector pGreen II0800-LUC to generate the reporter genes for dual-luciferase detection. The full-length CDSs of OsNLP4indica and OsNLP4japonica were inserted into Pan580 to generate the effectors. Firefly luciferase (LUC) activity and Renilla luciferase (REN) activity were determined using a dual luciferase reporting system (Vazyme, no. DL101-01) 12–18 h after transfection. The LUC and REN activity ratios were determined at least three times.
The uptake of 15N-nitrate and 15N-ammonium
15N-accumulation assays after 15N-nitrate labeling were performed with 15N-labeled KNO3 (99 atom% 15N, Sigma‒Aldrich, no. 335134) or 15N-labeled NH4Cl (98 atom% 15N, Sigma‒Aldrich, no. 299251). For the 15N-nitrate accumulation assay, rice seedlings were cultured in Kimura B solution for 10 days. Next, the seedlings were pretreated with Kimura B solution for 2 h and then transferred to modified Kimura B solution containing 2 mM 15N-KNO3 for 24 h. At the end of labeling, the roots were washed for 1 min in 0.1 mM CaSO4. Seedlings (whole plants) were then dried at 70 °C to a constant weight and ground. For 15N-ammonium accumulation assays, the treatments were conducted as described above, except that 2 mM 15N-KNO3 was replaced with 2 mM 15N-NH4Cl. The 15N content was analyzed using a continuous-flow isotope ratio mass spectrometer (DELTA V Advantage) coupled with an elemental analyzer (EA-HT, Thermo Fisher Scientific, Inc., Bremen, Germany). The 15N uptake rate is calculated as the total amount of 15N accumulated per unit dry weight of the sample per unit time.
Total N content analyses
Eighteen-day-old hydroponic plants grown in modified Kimura B solution (pH 5.8) supplemented with 2 mM KNO3 were used for all assays in this study. For the total N content assay, whole plants were dried at 70 °C to a constant weight, ground and then analyzed with an NC analyzer (Vario EL III model, Elementar, Hanau, Germany) according to the manufacturer’s instructions.
Western blotting assay
RIPA lysis buffer (strong) (Beyotime, China, cat # P0013B) was used to extract proteins from the tiller bases of rice plants (30 days) grown in soil. The seedlings were frozen in liquid nitrogen and ground into powder. The protein extract was separated by SDS‒PAGE and transferred to a nitrocellulose membrane. The antibodies used for western blotting were as follows: anti-OsD53 (rabbit pAb, antibodies prepared by Xuelu Wang Laboratory, Henan University, Kaifeng, China)41, 1:1000 for western blotting. An anti-ACTIN antibody (M20009, Mouse mAb, Abmart, Shanghai, China) at a 1:1000 dilution was used for western blotting. Goat anti-mouse lgG-HRP (M21001, Abmart, Shanghai, China) and goat anti-rabbit lgG-HRP (M21002, Abmart, Shanghai, China) were used at 1:5000 for western blotting. An Image Quant LAS 4000 (GE, USA), used as the CCD camera system, was used for band intensity quantification with the Super Signal West Femto Trial Kit (Thermo, Rockford, IL, USA, cat # 34095).
Microscale thermophoresis (MST) analysis
The MST assay was performed by the NanoTemper monolith NT.115 instrument. The purified MBP, MBP-OsNLP4indica and MBP-OsNLP4japonica were labeled with a RED-NHS Labeling Kit (NanoTemper Technologies) in assay buffer (20 mM HEPES buffer pH 7.5, 150 mM NaCl, and 0.005% Tween-20) according to the manufacturer’s instructions. 13-step serial dilutions of NRE were prepared in the same buffer to ensure consistent buffer conditions. The protein was mixed with an equal volume of substrate at different concentrations and incubated at room temperature for 30 min. Samples were loaded into MO-Z0025 capillaries (Nano-Temper Technologies). The experiments were carried out at a constant temperature of 25 °C, with 20% LED power and 40% MST power. Kd values were calculated using the MO Affinity Analysis v.2.2.4 software.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The RNA-seq data have been uploaded to NCBI Sequence Read Archive (SRA) database under BioProject accessions PRJNA1297387 and PRJNA1297901. SNPs for accessions from the 3 K Rice Genomes Project can be accessed via RiceVarMap v2.0 (http://ricevarmap.ncpgr.cn/vars_in_gene/). The sequence data used in this study can be found in the Rice Genome Annotation Project (https://rice.uga.edu/analyses_search_locus.shtml) and the Arabidopsis Information Resource (https://www.arabidopsis.org/): OsNLP4/LOC_Os09g37710 [https://rice.uga.edu/cgi-bin/sequence_display.cgi?orf=LOC_Os09g37710.2]; OsD3/LOC_Os06g06050 [https://rice.uga.edu/cgi-bin/sequence_display.cgi?orf=LOC_Os06g06050.1]; OsCKX9/LOC_Os05g31040 [https://rice.uga.edu/cgi-bin/sequence_display.cgi?orf=LOC_Os05g31040.1]; OsFC1/LOC_Os03g49880 [https://rice.uga.edu/cgi-bin/sequence_display.cgi?orf=LOC_Os03g49880.1]; OsRFL/LOC_Os04g51000 [https://rice.uga.edu/cgi-bin/sequence_display.cgi?orf=LOC_Os04g51000.1]; OsNRT1.1B/LOC_Os10g40600 [https://rice.uga.edu/cgi-bin/sequence_display.cgi?orf=LOC_Os10g40600.1]; OsNR2/LOC_Os02g53130 [https://rice.uga.edu/cgi-bin/sequence_display.cgi?orf=LOC_Os02g53130.1]; AtNRT1.1/At1g12110 [https://www.arabidopsis.org/locus?key=26949]; AtNRT2.1/At1g08090 [https://www.arabidopsis.org/locus?key=137698]; AtNIA1/At1g77760 [https://www.arabidopsis.org/locus?key=137464]; AtNIR1/At2g15620 [https://www.arabidopsis.org/locus?key=33557]; AtGS2/At5g35630 [https://www.arabidopsis.org/locus?key=133555]. Source data are provided with this paper.
Change history
16 October 2025
In the version of this article initially published online, the last sentence of the Author contributions was mistaken and has been revised to read “C.B.X. revised the manuscript and supervised the project” in the HTML and PDF versions of the article.
References
Zuo, J. R. & Li, J. Y. Molecular genetic dissection of quantitative trait loci regulating rice grain size. Annu. Rev. Genet. 48, 99–118 (2014).
Zhang, X. et al. Managing nitrogen for sustainable development. Nature 528, 51–59 (2015).
Hawkesford, M. J. Reducing the reliance on nitrogen fertilizer for wheat production. J. Cereal Sci. 59, 276–283 (2014).
Luo, L., Zhang, Y. & Xu, G. How does nitrogen shape plant architecture?. J. Exp. Bot. 71, 4415–4427 (2020).
Guo, J. H. et al. Significant acidification in major Chinese croplands. Science 327, 1008–1010 (2010).
Liu, Y. et al. Genomic basis of geographical adaptation to soil nitrogen in rice. Nature 590, 600–605 (2021).
Jin, J. et al. Genetic control of rice plant architecture under domestication. Nat. Genet. 40, 1365–1369 (2008).
Konishi, S. et al. An SNP caused loss of seed shattering during rice domestication. Science 312, 1392–1396 (2006).
Wu, B. et al. Suppressing a phosphohydrolase of cytokinin nucleotide enhances grain yield in rice. Nat. Genet. 55, 1381–1389 (2023).
Wang, C. L. et al. A natural gene drive system confers reproductive isolation in rice. Cell 186, 3577–3592 (2023).
Koutroubas, S. D. & Ntanos, D. A. Genotypic differences for grain yield and nitrogen utilization in Indica and Japonica rice under Mediterranean conditions. Field Crops Res. 83, 251–260 (2003).
Hu, B. et al. Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat. Genet. 47, 834–838 (2015).
Zhang, J. Y. et al. NRT1.1B is associated with root microbiota composition and nitrogen use in field-grown rice. Nat. Biotechnol. 37, 676–684 (2019).
Gao, Z. et al. The indica nitrate reductase gene OsNR2 allele enhances rice yield potential and nitrogen use efficiency. Nat. Commun. 10, 5207 (2019).
Huang, Y. Z. et al. Improving rice nitrogen-use efficiency by modulating a novel monouniquitination machinery for optimal root plasticity response to nitrogen. Nat. Plants 9, 1902–1914 (2023).
Zhang, S. Y. et al. Natural allelic variation in a modulator of auxin homeostasis improves grain yield and nitrogen use efficiency in rice. Plant Cell 33, 566–580 (2021).
Gao, Y. H. et al. MYB61 is regulated by GRF4 and promotes nitrogen utilization and biomass production in rice. Nat. Commun. 11, 5219 (2020).
Wang, Q. et al. Genetic variations in ARE1 mediate grain yield by modulating nitrogen utilization in rice. Nat. Commun. 9, 735 (2018).
Wang, J. et al. The amino acid permease 5 (OsAAP5) regulates tiller number and grain yield in rice. Plant Physiol. 180, 1031–1045 (2019).
Lu, K. et al. Blocking amino acid transporter OsAAP3 improves grain yield by promoting outgrowth buds and increasing tiller number in rice. Plant Biotechnol. J. 16, 1710–1722 (2018).
Wu, J. et al. Rice NIN-LIKE PROTEIN 4 plays a pivotal role in nitrogen use efficiency. Plant Biotechnol. J. 19, 448–461 (2021).
Wu, J. et al. The OsNLP3/4-OsRFL module regulates nitrogen-promoted panicle architecture in rice. N. Phytol. 240, 2404–2418 (2023).
Song, Y. et al. Balanced nitrogen–iron sufficiency boosts grain yield and nitrogen use efficiency by promoting tillering. Mol. Plant 16, 1661–1677 (2023).
Chardin, C., Girin, T., Roudier, F., Meyer, C. & Krapp, A. The plant RWP-RK transcription factors: key regulators of nitrogen responses and of gametophyte development. J. Exp. Bot. 65, 5577–5587 (2014).
Alexandrov, N. et al. SNP-Seek database of SNPs derived from 3000 rice genomes. Nucleic Acids Res. 43, D1023–D1027 (2015).
Xi, Z. Y. et al. Development of a wide population of chromosome single-segment substitution lines in the genetic background of an elite cultivar of rice (Oryza sativaL.). Genome 49, 476–484 (2006).
Liu, K. H. et al. NIN-like protein 7 transcription factor is a plant nitrate sensor. Science 377, 1419–1425 (2022).
Alvarez, J. et al. Transient genome-wide interactions of the master transcription factor NLP7 initiate a rapid nitrogen-response cascade. Nat. Commun. 11, 1157 (2020).
Mu, X. & Luo, J. Evolutionary analyses of NIN-like proteins in plants and their roles in nitrate signaling. Cell. Mol. Life Sci. 76, 3753–3764 (2019).
Ge, M. et al. The NIN-like protein 5 (ZmNLP5) transcription factor is involved in modulating the nitrogen response in maize. Plant J. 102, 353–368 (2020).
Gao, Y. et al. Barley transcription factor HvNLP2 mediates nitrate signaling and affects nitrogen use efficiency. J. Exp. Bot. 73, 770–783 (2021).
Jiang, S. Y. et al. NIN-like protein transcription factors regulate leghemoglobin genes in legume nodules. Science 374, 625–628 (2021).
Bernardo, R. Molecular Markers and Selection for Complex Traits in Plants: Learning from the Last 20 Years. Crop Sci. 48, 1649–1664 (2008).
Wu, J. et al. GAF domain is essential for nitrate-dependent AtNLP7 function. BMC Plant Biol. 22, 366 (2022).
Konishi, M. & Yanagisawa, S. Arabidopsis NIN-like transcription factors have a central role in nitrate signalling. Nat. Commun. 4, 1617 (2013).
Century, K., Reuber, T. L. & Ratcliffe, O. J. Regulating the regulators: the future prospects for transcription-factor-based agricultural biotechnology products. Plant Physiol. 147, 20–29 (2008).
Pal, V., Singh, G. & Dhaliwal, S. S. Yield enhancement and biofortification of chickpea (Cicer arietinum L.) grain with iron and zinc through foliar application of ferrous sulfate and urea. J. Plant Nutr. 42, 1789–1802 (2019).
Nasar, J. et al. Nitrogen fertilization coupled with iron foliar application improves the photosynthetic characteristics, photosynthetic nitrogen use efficiency, and the related enzymes of maize crops under different planting patterns. Front. Plant Sci. 13, 988055 (2022).
Lei, Z. Y. et al. High-throughput binary vectors for plant gene function analysis. J. Integr. Plant Biol. 49, 556–567 (2007).
Prom, U. T. C. et al. Simultaneous biofortification of rice with zinc, iodine, iron and selenium through foliar treatment of a micronutrient cocktail in five countries. Front. Plant Sci. 11, 589835 (2020).
Fang, Z. et al. Strigolactones and brassinosteroids antagonistically regulate the stability of the D53-OsBZR1 complex to determine FC1 expression in rice tillering. Mol. Plant 13, 586–597 (2020).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (grant no. 32321001 to C.B.X. and 32100208 to J.W.), the Strategic Priority Research Program of the Chinese Academy of Science (grant no. XDA24010303 to C.B.X.), the start-up funding of Anhui Agricultural University (grant no. RC422403 to J.W.), the Anhui Provincial Natural Science Foundation (grant no. 2108085QC103 to J.W.), and the Fundamental Research Funds for the Central Universities (grant no. WK9100000023 to J.W.). We thank Prof. Xue-Lu Wang (Henan University, Kaifeng, China) for providing antibodies against OsD53, Prof. Kai Hua (Center for Excellence in Molecular Plant Sciences, Shanghai, China) for providing the gene-editing techniques, and Prof. Chun-Ming Wang (Nanjing Agricultural University, Nanjing, China) for providing the pAN580 plasmid.
Author information
Authors and Affiliations
Contributions
C.B.X. and J.W. designed the experiments. J.W., Y.S., G.Y.W., L.Q.S., and J.X.W. performed the experiments and data analyses. C.C.C. and H.K.W. contributed XS134 and SSSLs. S.G.L. and P.Q. contributed yield data of multiple indica and japonica varieties. C.Z.M and S.M.W. contributed to the field trials. J.W. wrote the manuscript. C.B.X. revised the manuscript and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wu, J., Song, Y., Wan, G. et al. Nature variations of OsNLP4 responsible for nitrogen use efficiency divergence in the two rice subspecies. Nat Commun 16, 7681 (2025). https://doi.org/10.1038/s41467-025-63109-7
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63109-7