Abstract
Soybeans fix atmospheric N2 through symbiosis with rhizobia. The relationship between rhizobia and soybeans, particularly those with high nitrous oxide (N2O)-reducing (N2OR) activities, can be leveraged to reduce N2O emissions from agricultural soils. However, inoculating soybeans with these rhizobia under field conditions often fails because of the competition from indigenous rhizobia that possess low or no N2OR activity. In this work, we utilize natural incompatibility systems between soybean and rhizobia to address this challenge. Specifically, Rj2 and GmNNL1 inhibit certain rhizobial infections in response to NopP, an effector protein. By combining a soybean line with a hybrid accumulation of the Rj2 and GmNNL1 genes and bradyrhizobia lacking the nopP gene, we develop a soybean-bradyrhizobial symbiosis system in which strains with high N2OR activity predominantly infect. Our optimize symbiotic system substantially reduces N2O emissions in field and laboratory tests, presenting a promising approach for sustainable agricultural practices.
Similar content being viewed by others
Introduction
While the expansion of food production is essential to support the growing human population, agricultural lands are major sources of anthropogenic nitrous oxide (N2O), a greenhouse gas (GHG) with a global warming potential ~300 times greater than that of carbon dioxide (CO2)1. Meanwhile, chemical nitrogen fertilizers, synthesized from fixed atmospheric N2 via the Haber-Bosch process using fossil fuels, serve as the primary source of nitrogen for current intensive farming systems1,2,3. Then, nitrogen sources, including fertilizers and biological residues, are converted to inorganic nitrogen by soil microorganisms through the nitrogen cycle4,5,6. Soil nitrogen, existing as ammonia and nitrate inorganic compounds, transitions between these states through nitrification and denitrification. Among these processes, denitrification involves multiple reduction reactions (NO3− → NO2− → NO → N2O → N2)4,7.
While many soil bacteria lack specific genes that encode reductase for complete denitrification, thereby acting as sources and sinks for N2O7,8,9, soil rhizobial bacteria can perform biological N2 fixation through symbiosis with leguminous plants. For example, Bradyrhizobium, a genus comprising N2-fixing bacteria, has symbiotic interactions with legumes, such as soybeans, by infecting the plants, forming nodules in plant roots, and converting N2 into ammonium in the nodules10,11,12,13. This type of biological N2 fixation provides N2 to host plants without the economic effects and GHG emissions associated with chemical N2 fertilizer production14,15. However, the nitrogen released from aging and decaying nodules is also a source of N2O in the soil16,17,18,19,20. Globally, N2O emissions from soybean plant residues were estimated to be 19,685 kt CO2 eq in 2020 (FAO, 2024 https://www.fao.org/faostat/en/#home).
Recent phylogenomic analyses of Bradyrhizobium species have revealed diverse lifestyles and complex evolutionary histories related to nitrogen fixation21,22, photosynthesis23,24, degradation of aromatic compounds25, and symbiotic interactions with a wide range of leguminous plants26. Non-symbiotic members of Bradyrhizobium are frequently found in forest ecosystems25 and agricultural environments21,22. In contrast, Bradyrhizobium strains that nodulate soybean plants are largely restricted to three major species—B. japonicum, B. diazoefficiens, and B. elkanii—which are recognized as indigenous soybean bradyrhizobia27,28,29,30 and are also commonly used as commercial inoculants31,32.
B. diazoefficiens strains possess a complete denitrification pathway from nitrate (NO3−) to dinitrogen (N2) (NO3− → NO2− → NO → N2O → N2), catalyzed by four key enzymes: periplasmic NO3− reductase (Nap), copper (Cu)-containing NO2− reductase (NirK), c-type NO reductase (cNor), and N2O reductase (N2OR)5,7. In contrast, B. japonicum strains exhibit partial denitrification, reducing nitrate (NO3−) to nitrous oxide (N2O) (NO3− → NO2− → NO → N2O), due to the absence of the nos genes encoding the N2OR system29,33,34. B. elkanii apparently lacks all denitrification steps35,36.
Indigenous strains of B. diazoefficiens, including well-studied experimental strains such as USDA110 and USDA122, have been identified in paddy fields in Japan29, but are rarely detected in field soils of North America30,37. Consequently, N2O-reducing soybean bradyrhizobia are phylogenetically restricted to B. diazoefficiens, which constitutes a minor group among indigenous soybean bradyrhizobia. Notably, B. diazoefficiens strains with active N2OR have been experimentally inoculated onto soybean seeds in field trials conducted in Japan, France, and South America, leading to successful reductions in N2O emissions from soils16,38,39,40,41,42.
Genomic analysis of several B. diazoefficiens USDA110 mutants with enhanced N2OR activity identified mutations in the nasS gene40,43. Subsequent biochemical and genetic studies revealed that the two-component regulatory system NasST represses nos gene expression through an antitermination mechanism acting upstream of the nos gene cluster in B. diazoefficiens USDA1105,44,45. These findings suggest that genetically modified B. diazoefficiens strains with inactivated antitermination systems could serve as agricultural inoculants to enhance the reduction. During a survey for wild-type soybean bradyrhizobia with high N2OR activity, B. ottawaense strains were isolated from sorghum roots in Fukushima, Japan. These strains naturally nodulate soybean and exhibit higher N2OR activity than wild-type B. diazoefficiens USDA11022. Under anaerobic conditions, the expression levels of nosZ (encoding N2OR) in B. ottawaense strains SG09 and OO99T were more than 100-fold higher than those in B. diazoefficiens USDA110, which may explain their elevated N2OR activity20. However, the mechanisms underlying this enhanced N2OR activity may involve: (i) regulation of nos expression via signal transduction pathways responsive to external stimuli such as O2, NO3−, redox state, and copper5,7, and (ii) regulation of electron allocation among different denitrification reductases (e.g., Nap/Nar vs. Nos)46,47,48.
As the fourth most important crop worldwide, soybeans provide essential vegetable protein and oil for both humans and animals14,49,50. In 2022, the global area under soybean cultivation reached 133,791,632 hectares, accounting for ~8% of the global total of cropland (1,617,392,600 hectares) (Our World in Data, 2022; https://ourworldindata.org/). Thus, introducing soybean symbiosis with wild-type strains of B. ottawaense exhibiting high N2OR activities can substantially reduce N2O emissions from soybean agriculture. However, competition from indigenous soil rhizobia hinders the infection of promising wild-type B. ottawaense following soybean inoculation. For example, the occupancy rate of B. diazoefficines mutants with higher N2OR activities in soybean nodules remains unstable, ranging between 20% and 60%41. Consequently, the rhizobial competition problem has been a major barrier to the successful introduction of promising rhizobia into agricultural fields10,51,52,53,54. Thus, establishing a soybean symbiotic system that predominantly supports the infection by B. ottawaense with higher N2OR activities is essential for addressing this issue and maximizing the N2O-reducing potential of B. ottawaense.
While various factors influence the nodule occupancy rate of rhizobia, the primary determinant is the genotype of the host plant53,54,55,56. Specifically, soybean incompatibility-a symbiotic inhibition response triggered by effector proteins secreted by rhizobia via the Type III secretion system-is defined by the interaction between specific soybean genes and corresponding rhizobial effector proteins involved56,57,58. Currently, the genes involved in incompatibility include Rj2, Rfg1, Rj4, and GmNNL159,60,61,62,63,64. Rj2 and GmNNL1 are Toll/interleukin -1 receptor-nucleotide-binding site-leucine-rich repeat (TIR-NBS-LRR) resistance R genes63,64,65. Rj2 induces incompatibility by recognizing NopPUSDA122, an effector protein derived from B. diazoefficiens USDA12260. In contrast, both NopPUSDA6 from B. japonicum USDA6 and NopPUSDA110 from B. diazoefficiens USDA110 are recognized by GmNNL164. Most nodulating Bradyrhizobium spp. harbor nopP58. In addition, Sugawara et al.60 analyzed the nopP sequences from a collection of bradyrhizobial isolates from soil samples across 32 locations in Japan and found that NopPUSDA122, NopPUSDA110, and NopPUSDA6 (ST2) were widespread in Japan. We hypothesized that soybean lines carrying both Rj2 and GmNNL1 may suppress the infection of indigenous rhizobia harboring nopPUSDA6, nopPUSDA110, or nopPUSDA122.
In this work, we develop a soybean line carrying both Rj2 and GmNNL1 through genetic crossing. Additionally, we generate NopP-deficient B. ottawaense with high N2OR activity via induced spontaneous mutations60. Taken together, in this study, we show that these NopP-deficient strains bypass symbiotic incompatibility with Rj2 and GmNNL1, enabling successful infection of the Rj2/GmNNL1 soybean line. The incompatibility-bypassing strains achieve high nodule occupancy rates in the Rj2/GmNNL1 soybean line when co-inoculated with B. japonicum USDA6, B. diazoefficiens USDA110, and B. diazoefficiens USDA122, demonstrating substantial N2O-reducing capacity. We then examine whether these incompatibility-bypassing B. ottawaense strains can outcompete indigenous rhizobia in soybean field soil, thereby reducing N2O emissions from the soybean rhizosphere.
Results
Generation of incompatibility-bypassing bradyrhizobia with high N2O-reducing activity
To establish Bradyrhizobium strains capable of overcoming incompatibility mediated by NopP effectors, it is necessary to delete the effector gene or disable its function to establish bradyrhizbial strains that can overcome incompatibility with NopP effectors. When Rj2 soybeans are inoculated with bradyrhizobia strains carrying nopPUSDA122, large mature nodules are rarely formed60. When rhizobia carrying the NopPUSDA122 effector encountered Rj2 soybean, the interaction triggered a spontaneous mutation in the nopPUSDA122 gene via the transposition of an endogenous transposon, resulting in the emergence of strains capable of overcoming Rj2 incompatibility. Where nodules do form, they are often infected by spontaneous nopP-deficient mutants. Notably, none of the eleven strains of B. ottawaense previously isolated for their high N2O-reducing activity encode nopPUSDA12220.
To produce incompatibility-bypassing rhizobia, we surveyed the B. ottawaense strains carrying nopPUSDA122 strains in Japanese soybean fields. As a result, we obtained two target strains of B. ottawaense carrying nopPUSDA122 (FY2 and GMA461) from Numata (Gunma Prefecture) and one strain identified as a natural nopP mutant (OSA024) from Yao (Osaka Prefecture).
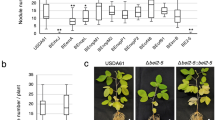
The 16S-23S internal transcribed spacer (ITS) sequence of these three strains (OSA024, FY2, and GMA461) showed over 99% identity to the high N2O-reducing strain SG0920, indicating close phylogenetic relationships (Fig. 1). Additionally, these strains exhibited high N2O-reducing activity in free-living cells, comparable to B. ottawaense SG09 and substantially higher than B. diazoefficiens USDA110 (Fig. 1). The O2 respiration activities of B. ottawaense strains—including the newly isolated strains OSA024, GMA461, and FY2—were comparable to that of B. diazoefficiens USDA110T (Supplementary Fig. 1, Supplementary Method 1). Despite similar respiration levels, the B. ottawaense strains exhibited higher N2OR activity than B. diazoefficiens USDA110T (Fig. 1), likely due to differences in electron flow through the respiratory chain.
The ITS phylogenetic tree was constructed by aligning ITS sequences using ClustalW and applying the neighbor-joining method. The N2O-reducing activities were determined via gas chromatography20. *Strains of B. ottawaense isolated in this study. Boxplots indicate medians (centerlines), interquartile ranges (box edges), individual data points (dots), and mean values (red crosses). Each strain was analyzed with three biological replicates (n = 3). Different letters indicate statistically significant differences (p < 0.05; Multiple comparisons with correction were performed using one-way analysis of variance (ANOVA) followed by Tukey’s HSD test). Source data are provided as a Source data file.
Next, we attempted to isolate mutants with spontaneously disrupted nopPUSDA122 by inoculating the Rj2 soybean cultivar Hardee with the FY2 or GMA461 strain carrying nopPUSDA122. Based on visual assessment of nodule shape and color, any spherical structure exhibiting red coloration exceeding 1 mm in diameter was defined and counted as a mature nodule. When FY2 was inoculated onto 30 Rj2 soybean plants at a concentration of 109 bacteria per seed, most plants did not form nodules. However, in four plants, a single large nodule exceeding 5 mm in diameter was observed. Among the four rhizobial isolates obtained from these nodules, three harbored insertion sequences (ISs) within nopPUSDA122. One of these isolates was designated as the representative strain, FY2-m1. Similarly, GMA461-inoculated Rj2 soybean plants (n = 30) formed four large nodules. Two of the four isolates obtained from these nodules exhibited IS insertions in nopPUSDA122, and one isolate was designated the representative strain, GMA461-m4 (Fig. 2a, b). ISs are mobile DNA elements that can transpose within the genome. In Bradyrhizobium, various types of ISs are known to be scattered throughout the genome33. Sequence analysis of nopP in FY2-m1 and GMA461-m4 showed that ISRj266 was inserted in both strains, but the positions of IS insertion differed (Fig. 2b, c). In the natural nopP mutant OSA024, ISBj1166 was inserted in nopPUSDA6 (Fig. 2b, c).
a A large nodule (white arrowhead) formed in the Rj2 soybean cultivar “Hardee” following inoculation with B. ottawaense GMA461. b Positions of endogenous transposon insertions in nopP of FY2-m1, GMA461-m4, and OSA024. Insertions of ISRj2 were detected in nopP of FY2-m1 and GMA461-m4, and ISBj11 in nopP of OSA024. White arrowheads indicate the positions of the four amino acid residues that define the NopP type; corresponding residues and positions are labeled above each arrowhead. c PCR amplification of the nopP region using nopP-specific primers. Bands with increased molecular weight, indicating transposon insertion, are marked with red arrowheads. The experiment was repeated two times independently with similar results. Source data are provided as a Source data file.
Generation of soybean lines accumulating Rj2 and GmNNL1 incompatibility genes
We selected the parent plants for accumulating Rj2 and GmNNL1 through the crossing and initially chose the well-known Japanese cultivar “Bonminori,” carrying the Rj2 genotype67. Additionally, 192 accessions from a soybean mini-core collection with available whole-genome resequencing data68 were analyzed for the Rj2 and GmNNL1 genotypes. We extracted genetic variations in Rj2 and GmNNL1 by detecting single-nucleotide polymorphisms (SNPs), small insertions and deletions (InDels), and structural variations (SVs) across the 193 accessions. Since the Rj2/rj2 genotype is determined by an SNP (C/T) in the second exon, which causes a single amino acid substitution (R490 to I490)61, we identified 10 accessions with the Rj2 genotype (Supplementary Data 1). Conversely, the null Gmnnl1 genotype results from a 179-bp SINE-like transposon insertion in the second exon69. Only 4 accessions possessed a functional GmNNL1 genotype (Supplementary Data 1). The individual occurrence percentages of Rj2 and NNL1 functional genotypes within the mini-core collection were 5.18% and 2.07%, respectively. Lastly, no accession had a functional genotype of Rj2 and GmNNL1.
Then, we selected the cultivars “Bonminori” and GmWC108 (Karasu-mame), which had similar flowering times, as parent plants for crossing to accumulate Rj2 and GmNNL1. After crossing, we selected 1 line that was homozygous for Rj2/GmNNL1 and another line that was homozygous for rj2/Gmnnl1 from the F2 seed population. From the progenies, we obtained the F3 seeds. The Rj2 and rj2 and the GmNNL1 and Gmnnl1 genotypes of the seeds could be distinguished based on the PCR-amplified fragments of varying sizes that covered an SV near Rj2 and GmNNL1 and were generated using the cotyledon DNA from F2 seeds (Fig. 3a).
a Electrophoresis images of molecular markers for Rj2 and GmNNL1 in soybean lines. Number of mature nodules on the roots of Rj2/GmNNL1-accumulated soybean inoculated with FY2 or FY2-m1 (b), and GMA461 or GMA461-m4 (c). Photographs of each nodulated root were shown below each graph. Boxplots indicate medians (centerlines), interquartile ranges (box edges), individual data points (dots), and mean values (red crosses). Each inoculation combination was performed with n = 4 biological replicates. Asterisks indicate statistically significant differences (Two-sided Wilcoxon rank-sum test, p < 0.05). Source data are provided as a Source data file.
Evaluation of the symbiosis ability of incompatibility-bypassing N2O-reducing rhizobia
Then, the Rj2/GmNNL1 line was inoculated with FY2 and GMA461, which carried nopPUSDA122, and with FY2-m1 and GMA461-m4, which exhibited the loss of nopPUSDA122 function (Fig. 3b, c). Premature nodule-like structures were observed upon inoculation with FY2 or GMA461. In contrast, inoculation with FY2-m1 and GMA461-m4 resulted in the development of multiple mature nodules. These findings indicate that the loss of nopPUSDA122 function due to endogenous transposon insertion enabled FY2-m1 and GMA461-m4 to overcome incompatibility mediated by Rj2 and GmNNL1.
Nodule occupancy, N2O flux, and symbiotic phenotypes in infection competition between incompatibility-bypassing and other rhizobia
We simulated the infection competition between indigenous rhizobia and inoculated rhizobia that bypassed Rj2/GmNNL1 incompatibility by conducting a simultaneous inoculation experiment using four strains: USDA6, USDA110, USDA122, and an incompatibility-bypassing strain. First, we compared infection competition among the four strains by assessing nodule occupancy rates. For each individual nodule formed on the root, the symbiotic rhizobial strain was identified based on the differences in the length of restriction enzyme-treated nopP fragments (PCR-RFLP, Supplementary Fig. 2). The nodule occupancy rate was calculated as the number of nodules colonized by the target strain divided by the total number of nodules analyzed. Then, the nodule occupancy rates of the strains in mature nodules of Rj2/GmNNL1 and rj2/Gmnnl1 soybeans following simultaneous inoculation with strain FY2-m1 and USDA6/USDA110/USDA122 were compared (Fig. 4a). In the Rj2/GmNNL1 soybean, the FY2-m1 strain occupied 95.3% of the mature nodules, while in the rj2/Gmnnl1 soybean, its occupancy was 56.1%. Similarly, the simultaneous inoculation of GMA461-m4 with USDA6/USDA110/USDA122 resulted in a nodule occupancy rate of 92.1% in the Rj2/GmNNL1 line and 54.7% in the rj2/Gmnnl1 line (Fig. 4b). For OSA024, the simultaneous inoculation with USDA6/USDA110/USDA122 led to 97.4% nodule occupancy in the Rj2/GmNNL1 line and 27.9% in the rj2/Gmnnl1 line (Fig. 4c). These results indicate that the USDA6/USDA110/USDA122 strains were unable to evade Rj2/GmNNL1 incompatibility, resulting in the predominant infection by incompatibility-bypassing strains in the Rj2/GmNNL1 soybean.
Nodule occupancy rates of USDA6, USDA110, USDA122, and incompatibility-bypassing rhizobial strains in Rj2/GmNNL1 and rj2/Gmnnl1 soybean lines inoculated with FY2-m1 (a; For each inoculation combination, 192 and 189 nodules were collected and analyzed from 6 plants, respectively), GMA461-m4 (b; For each inoculation combination, 190 and 192 nodules were collected and analyzed from 6 plants, respectively), and OSA024 (c; For each inoculation combination, 191 and 190 nodules were collected and analyzed from 5 plants, respectively). N2O emissions measured at 1, 2, and 3 weeks after decapitation (WAD) of soybean inoculated with FY2-m1 (d), GMA461-m4 (e), and OSA024 (f). The data are shown as mean ± s.e. Each inoculation combination was performed with n = 4 biological replicates. Asterisks indicate significant differences between Rj2/GmNNL1 and rj2/Gmnnl1 soybean lines on the same WAD (Two-sided Student’s t-test, p < 0.05). Source data are provided as a Source data file.
N2O flux generated from nodulated soybean roots was quantitatively analyzed in inoculated plots, where nodule occupancy was assessed. N2O flux was measured 1, 2, and 3 weeks after the senescence and decay induction of nodulated roots. There was no significant difference in N2O flux between samples inoculated with FY2-m1 and GMA461-m4 at 1 and 2 weeks after decapitation (WAD); however, a considerable decrease in N2O flux was detected at 3 WAD. In the case of inoculating with OSA024, there was a substantial decrease in N2O flux at 2 and 3 WAD. Across all three incompatibility-bypassing strains, a substantial decrease in N2O flux was recorded from the Rj2/GmNNL1 soybean compared with the rj2/Gmnnl1 soybean (Fig. 4d–f).
Additionally, a linear regression analysis was performed to compare the nodule occupancy rates of the incompatibility-bypassing N2O-reducing rhizobacteria in rj2/Gmnnl1 and Rj2/GmNNL1 lines at both 2 and 3 WAD. The nodule occupancy of the N2O-reducing rhizobacteria was negatively correlated with N2O flux (Supplementary Fig. 3). These findings indicate that FY2-m1, GMA461-m4, and OSA024, isolated as Rj2/GmNNL1 incompatibility-bypassing rhizobia, demonstrated remarkably high nodule occupancy in Rj2/GmNNL1 soybeans under competitive inoculation with other rhizobial strains, thereby achieving high N2O reduction capacity.
We conducted a comparative analysis of soybean growth and symbiotic phenotypes under Rj2 and GmNNL1 expression to address the concern that artificial accumulation of Rj2 and GmNNL1 in soybean lines could trigger defense responses against nopPUSDA6, nopPUSDA110, and nopPUSDA122 type rhizobia, potentially negatively affecting soybean growth and symbiotic phenotypes. We inoculated soybean lines Rj2/GmNNL1 and rj2/Gmnnl1 with B. japonicum USDA6, B. diazoefficiens USDA110 and USDA122, and an incompatibility-bypassing N2O-reducing bradyrhizobia.
Then, we assessed symbiotic phenotypes and soybean growth 5 weeks after inoculation. There was no significant difference in the number of nodules per plant (Supplementary Fig. 4a). However, nodule dry weight per plant and weight per nodule were substantially greater in the Rj2/GmNNL1 line than in the rj2/Gmnnl1 line (Supplementary Fig. 4b, c). N2-fixing activity was considerably higher in the Rj2/GmNNL1 and FY2-m1 combination (Supplementary Fig. 4d). Furthermore, shoot dry weight (SDW) and root dry weight (RDW) were substantially greater in Rj2/GmNNL1 than in rj2/Gmnnl1 (Supplementary Fig. 4e, f).
Comparative analysis of nodule occupancy and N2O flux between incompatibility-bypassing rhizobia and indigenous soil rhizobia
We tested the competition between incompatibility-bypassing rhizobacteria and indigenous rhizobia in the Rj2/GmNNL1 soybean, analyzing their effectiveness in nodule occupancy and N2O release using pot culture with field soil at the National Agriculture and Food Research Organization (NARO). Nodule occupancy by incompatibility-bypassing rhizobia was evaluated using nosZ-specific primers. In the mock-inoculated samples, nosZ amplicons corresponding to B. ottawaense (499 bp) and B. diazoefficiens USDA110 (832 bp) were absent in the majority of nodules, suggesting colonization by indigenous rhizobia lacking the nosZ gene. Notably, although detected at low frequency, PCR bands corresponding to the USDA110-type nosZ were observed, indicating that a subset of indigenous rhizobia capable of circumventing the weak incompatibility mediated by the GmNNL1 gene successfully infected the nodules. FY2-m1 and GMA461-m4 exhibited nodule occupancy rate of over 80% occupancy, whereas OSA024 showed less than 60% occupancy (Fig. 5a). GMA461-m4 had the lowest level of N2O release from the rhizosphere, including nodulated roots, following above-ground excision (Fig. 5b). Cumulative N2O emissions of FY2-m1, GMA461-m4 and OSA024 demonstrated a substantial reduction in N2O release to that of the mock inoculation (Fig. 5c).
a Nodule occupancy. For one experimental plot, 192 nodules were collected and analyzed from 6 plants. b Trends in N2O emissions following plant decapitation. The data are shown as mean ± s.e. Each inoculation combination was performed with n = 5 biological replicates for mock, FY2-m1, and GMA461-m4, and n = 3 biological replicates for OSA024. c Cumulative N2O emissions over 1–24 days after plant decapitation. Boxplots indicate medians (centerlines), interquartile ranges (box edges), individual data points (dots), and mean values (crosses). Each inoculation combination was performed with n = 5 biological replicates for mock, FY2-m1, and GMA461-m4, and n = 3 biological replicates for OSA024. Asterisks indicate significant differences between mock and incompatibility-bypassing bradyrhizobia strains on the same day (Multiple comparisons with correction were performed using one-way analysis of variance (ANOVA) followed by Dunnett’s test, *p < 0.05; **p < 0.01). Source data are provided as a Source data file.
The ability to reduce N2O by the high N2O-reducing bradyrhizobia strains, FY2-m1 and GMA461-m4, in soybean lines with and without the incompatibility genes was compared to examine the effects of the incompatibility genes. Besides the Rj2/GmNNL1 soybean line, the soybean variety “Akuden Shirazu”, lacking incompatibility genes (rj2/Gmnnl1), was included and examined as a negative control. FY2-m1 inoculation resulted in 69.8% and 25.0% nodule occupancy in the Rj2/GmNNL1 and rj2/Gmnnl1 soybeans, respectively (Supplementary Fig. 5a). N2O flux from the respective rhizosphere soils was considerably lower in the Rj2/GmNNL1 soybeans than in the rj2/Gmnnl1 soybeans (Supplementary Fig. 5b). GMA461-m4 inoculation yielded 77.6% and 12.9% nodule occupancy in the Rj2/GmNNL1 rhizosphere soil than in the rj2/Gmnnl1 rhizosphere soil (Supplementary Fig. 5a, b). The effect of soybean lines on N2O flux was most pronounced 8–18 days after decapitation when N2O flux values were highest (Supplementary Table 1, Supplementary Fig. 5b). In contrast, no significant effect was observed for rhizobial strain alone; however, a significant interaction between soybean line and rhizobial strain was detected on days 22 and 25. Furthermore, the cumulative N2O emission analysis indicated that the choice of soybean line could be a critical factor influencing total N2O release (Supplementary Table 1). The comparative analysis of cumulative N2O release over the measurement period showed that N2O release in FY2-m1/Rj2/GmNNL1 was 40% of that in FY2-m1/rj2/Gmnnl1, while N2O release in GMA461-m4/Rj2/GmNNL1 was only 16% of that in GMA461-m4/rj2/Gmnnl1 (Supplementary Fig. 5c). These results confirm a substantial reduction in N2O release associated with increased nodule occupancy in the two strains.
Nodule occupancy and N2O flux in soybean fields
The competition between B. ottawaense strains with high N2OR activity and indigenous rhizobia in the Rj2/GmNNL1 soybean and their effectiveness in nodule occupancy and N2O reduction in a soybean field were analyzed in the Kashimadai soybean field at Tohoku University. The nodule occupancy rates of the treatments were compared (Fig. 6a). The absence of the 499-bp nosZ amplicon specific to B. ottawaense in the uninoculated control indicates that indigenous B. ottawaense was not present in the field (Fig. 6a). GMA461-m4, a nopP mutant of B. ottawaense, showed a nodule occupancy of over 60%. In contrast, the parent strain GMA461, which carried nopPUSDA122, could not occupy nodules. Additionally, inoculation with SG09, which carried nopPUSDA6, showed a lower nodule occupancy of ~10%.
a Nodule occupancy by B. ottawaense-type rhizobia (A total of 186 nodules from 6 plants per plot). Boxplots indicate medians (centerlines), interquartile ranges (boxes), individual data points (dots), and mean values (crosses; n = 6 biological replicates). b N2O emissions from nodulated field-grown soybean immediately after shoot excision. Boxplots indicate medians (centerlines), interquartile ranges (boxes), individual data points (dots), and mean values (crosses; n = 4 biological replicates). Asterisks indicate statistically significant differences (p < 0.05). Multiple comparisons with correction were performed using one-way analysis of variance (ANOVA), followed by a two-sided Steel test in (a) and Dunnett’s test in (b), with mock inoculation used as the control. Source data are provided as a Source data file.
At the maturation stage, 15 weeks after seeding, N2O flux from the rhizosphere of each treatment was measured immediately following above-ground excision (Fig. 6b). The N2O fluxes of the SG09 and GMA461 inoculation were not significantly different from that of the uninoculated control, whereas the GMA461-m4 inoculation substantially reduced N2O flux compared to SG09 and GMA461. These results indicate that high nodule occupancy of GMA461-m4 contributes to a reduction in N2O flux even under field conditions (Fig. 6).
Discussion
The discovery of rhizobia capable of reducing N2O has paved the way for developing technologies aimed at reducing field-derived N2O emissions through rhizobial symbiosis16,34,38,41. Since soybean is the world’s leading legume crop and forms symbiotic associations with bradyrhizobia species49, soybean symbiotic systems have been the primary focus for developing N2O reduction technologies. Field inoculation with B. diazoefficiens USDA110 with N2OR activity and its mutant with higher N2OR activity has successfully reduced N2O emissions from soybean fields16,38,41. However, the nodule occupancy of these inoculants was unstable, remaining at 20% to 60% in farm-scale experiments41. The instability in nodule occupancy likely limits the inoculants’ ability to maximize their N2O-reducing potential.
In many cases, the introduction of beneficial rhizobium strains into the field is limited by the infection competition from indigenous rhizobia, a phenomenon known as the rhizobial competition problem52,53,54. The nodule occupancy of inoculated rhizobia is generally ~5%–40%70,71, highlighting the need to improve the nodule occupancy rates of inoculated rhizobia. Competition with indigenous rhizobia is influenced by the soil’s physical and chemical properties and by biotic factors, such as rhizobial chemotactic response to rhizospheric substances secreted by host plants and rhizobial adhesion to plant roots53. In addition, the competition between rhizobial strains for carbon resources provided by the host plant plays an essential role in infection competition72. Establishing a rhizobial symbiotic system that allows beneficial rhizobia to dominate infection is crucial for maximizing the impact of promising rhizobia on host plants14,53. However, the factors determining rhizobial competitive ability remain largely unknown. Although Cunningham et al.73 explored the possibility of chemical control to selectively express nodulation (nod) genes in distinct soybean bradyrhizobia lineages, this approach was unlikely to be adopted due to the cost of chemicals. Consequently, there are no reports on artificially controlling the competitive ability of rhizobial inoculants in practice.
The soybean symbiotic incompatibility induced by rhizobial NopP, as used in this study, represents a rare interaction mechanism because it involves well-characterized genes on both the rhizobial and host plant sides60,61,63,64. This unique mechanism suggests a potential for the functional use of this to control rhizobial infection competition67. Since the NopP function in rhizobia can be disrupted using Rj2 incompatibility as a selection pressure60, we developed two rhizobial strains, FY2-m1 and GMA461-m4, that bypassed inhibition of infection by incompatibility genes, Rj2 and GmNNL1. This study represents an innovative approach to artificially enhance the host selectivity of promising rhizobia by modifying the host plant and the rhizobia.
The inhibition of infection by specific effector-harboring rhizobia by host incompatibility genes is thought to have evolved as a strategy to eliminate rhizobia that are not beneficial to the host plant74. However, it remains unclear why most nodulating Bradyrhizobium spp. retain NopPs that induce such incompatibility58. Since Bradyrhizobium spp. can infect multiple host plants, a NopP protein that induces incompatibility in one host may not trigger incompatibility in another56,75. Additionally, NopP promotes rhizobial infection in certain host plants76, indicating that retaining NopP may enhance the probability of rhizobial survival by enabling movement across symbioses with multiple host plants.
Host incompatibility genes generally share homology with R genes, which control infection suppression in response to pathogen attack56,63,64,65. For example, incompatibility induced by B. diazoefficiens USDA122 via Rj2 functions as an effector-triggered immunity with expression of downstream defense response genes77. In addition, Rj2 incompatibility is systemically expressed and triggers foliar resistance to plant pathogens77. Defense response-related genes are also expressed following rhizobial inoculation in response to incompatibility induced by GmNNL164. These findings indicate that incompatibility can be interpreted as a defense response against rhizobia harboring specific effectors. Plants carrying R genes often experience a growth penalty associated with defense responses78,79,80,81. Trade-offs between plant growth and immunity have been widely reported across model plants and crop species, underscoring the need to balance productivity with resistance in crop breeding78,81. In our study, two incompatibility genes were accumulated; thus, it was necessary to verify whether the trade-offs between defense response and plant growth from R genes were evident. We evaluated the effect of incompatibility gene accumulation by comparing the Rj2/GmNNL1 and rj2/Gmnnl1 lines from the same breeding backgrounds. The Rj2/GmNNL1 lines, where incompatibility genes are functionally expressed through co-inoculation with USDA6, USDA110, and USDA122, and incompatibility genes were not expressed in the rj2/Gmnnl1 lines. The similarity in nodule numbers indicates that the accumulation of incompatibility genes does not affect nodule number control mechanisms. However, nodule dry weight per plant, nodule dry weight per nodule, SDW, and RDW in Rj2/GmNNL1 were substantially higher than in rj2/Gmnnl1. We observed no negative effects on symbiotic phenotypes or plant growth due to incompatibility gene accumulation within our system. Therefore, these phenotypes appear more influenced by the combination of host soybean and rhizobial strains than by the expression of incompatibility genes82.
According to the geographical distribution of rhizobia in Japanese soil, the major rhizobial flora in the Honshu-Kyushu area can be classified into USDA6 (B. japonicum) without nos genes and USDA110 types (B. diazoefficiens) with nos genes28,29. Although bacterial classification based on ITS and NopP sequences does not always align, NopP sequence analysis using the same bacterial library29 indicates the widespread presence of NopPUSDA110, NopPUSDA122, and NopPUSDA6 types of bradyrhizobia in Japan60. This finding suggests that GmNNL1 and Rj2 can effectively inhibit infection competition by indigenous rhizobia. Here, this hypothesis was tested through competitive inoculation experiments with strains USDA6, USDA110, USDA122, and the incompatibility-bypassing N2O-reducing rhizobacteria FY2-m1, GMA461-m4, and OSA024. Each incompatibility-bypassing strain had a significantly higher nodule occupancy than USDA6, USDA110, and USDA122. However, in experiments using field soil, the occupancy rate of OSA024 was lower than that of FY2-m1 and GMA461-m4, and the N2O release from the OSA024-inoculated soybean rhizospheres trended higher. These data indicate that OSA024 was less effective than the other strains in the field soil used.
In our field experiment, the nopP-deficient GMA461-m4 showed higher nodule occupancy than GMA461 with nopPUSDA122 and SG09 with nopPUSDA6. The lack of nodulation with GMA461 may be due to the strong incompatibility of Rj2 against the effector NopPUSDA12260. In contrast, GmNNL1 incompatibility against rhizobia with nopPUSDA6 or nopPUSDA110 blocks infection via infection threads but still allows infection through crack entry64. Thus, the nodule occupancy of SG09 in Rj2/GmNNL1 soybeans is likely higher than that of GMA461. Nodule occupancy of GMA461-m4, at over 60%, may substantially mitigate N2O emissions from the rhizosphere of Rj2/GmNNL1 soybeans. Therefore, this strain is thought to both outcompete indigenous rhizobia in Rj2/GmNNL1 symbiosis and exhibit N2O reduction potential under field soil conditions. Collectively, our findings demonstrate that optimizing the combination of incompatibility genes and effectors-deficient N2O-reducing rhizobia can lead to soybean cultivation systems that effectively reduce N2O emissions.
Globally, soil types vary substantially. In this study, based on the characteristics of Japanese soils, Rj2 and GmNNL1 were used as symbiotic incompatibility genes to prevent infection by the predominant indigenous rhizobia. The suppression of indigenous rhizobia by Rj2/GmNNL1 soybean may be applicable to soils where nopP-carrying rhizobia are prevalent. Since the dominant indigenous rhizobia may differ depending on the soils’ physical, chemical, and biological properties, applying this method broadly will require selecting incompatibility genes specific to the dominant rhizobia in a soil type53,83. NopP is widely conserved among nodulating Bradyrhizobium strains58. Furthermore, genes encoding NopP variants-such as NopPUSDA110, NopPUSDA122, and other types-are commonly found in B. japonicum and B. diazoefiiciens strains isolated from China (prefix CCBAU), Brazil (prefix SEMIA), and the United States (prefix USDA), as well as from Japan60. These findings suggest that our strategy may be applicable beyond Japan. Integration of nopP-based rhizobial community profiling will contribute to the development of a more precise and effective symbiotic system.
Agricultural land is a major anthropogenic source of N2O emissions1. Under current global warming conditions, biological N2 fixation through rhizobial symbiosis holds substantial promise as an alternative for agricultural production systems reliant on synthetic nitrogen fertilizers50,51,56,84. In addition to nitrogen fertilizer applied to fields, nitrogen sources released from soybean plant residues contribute to N2O emissions17,18,19,85. A rhizobial symbiotic system with N2O-reducing rhizobia can help reduce N2O emissions from soybean fields by lowering the need for artificial nitrogen fertilizer, a primary source of N2O emissions, and by reducing N2O derived from soybean residues through the rhizobia’s N2O-reducing capability.
Methods
Isolation of B. ottawaense strains and incompatibility-bypassing strains
To investigate B. ottawaense strains harboring nopPUSDA122, root nodules were collected from soybean (Glycine max) plants of three cultivars. Nodules were obtained from cultivar “Yukine” cultivated in a field in Numata City, Gunma Prefecture, Japan (36.647167°N, 139.015782°E) on September 12, 2021, and from cultivar “Koihime” cultivated in a different field in the same city (36.6434028°N, 139.0157167°E) on July 10, 2022. Additional nodules were collected from cultivar “Ezo-midori” grown in pots under greenhouse conditions using field soil from Kashiwamura-Machi, Yao City, Osaka Prefecture, Japan (34.6132722°N, 135.6239806°E) on May 19, 2022-previously identified as a source of B. ottawaense86. From 1 to 4 individual soybean plants per location and year, 82–90 nodules were collected. Each nodule was surface-sterilized with 0.5% sodium hypochlorite (NaOCl), individually placed into a 96-well microplate containing 150 µL of sterile water, and crushed using a sterile toothpick. Large debris was removed using a toothpick to obtain a bacteroid suspension. A 75 µL aliquot of the suspension was transferred to a new 96-well microplate, mixed with 25 µL of 50% glycerol solution, and stored at −80 °C for subsequent strain isolation. The remaining 75 µL of solution was centrifuged (2200 × g, room temperature, 10 min), and the supernatant discarded. The pellet was washed with 50 µL of 1% NaCl, followed by centrifugation under the same conditions. After adding 40 µL of sterile distilled water, the suspension was transferred to a new 96-well PCR plate. To each well, 50 µL of BL buffer (40 mM Tris, 1% Tween20, 0.5% Nonidet P-40, and 1 mM EDTA, pH 8.0) and 10 μL of proteinase K (1 mg mL−1) were added. Samples were incubated at 60 °C for 20 min and then at 95 °C for 5 min. After centrifugation (300 xg, room temperature, 1 min), the supernatant solution was used as the PCR template87.
Polymerase chain reaction (PCR) was performed using the prepared cell lysates as templates with PCR primer sets targeting the nosZ genes of B. ottawaense SG09 and B. diazoefficiense USDA110 (Supplementary Table 2), producing expected amplicons of 499 bp and 832 bp, respectively87. Samples yielding only the 499 bp amplicon were designated as B. ottawaense candidates. For these candidates, the 16S-23S ITS region was amplified using the primer pair BraITS-F/R (Supplementary Table 2)88. The resulting PCR products were directly sequenced using the Sanger method with the primer BraITS-F to determine ITS sequences (Supplementary Table 2). Furthermore, the nopP gene was amplified from the same lysates using the primer pair nopP_F1/R6 (Supplementary Table 2)60. The resulting PCR products were directly sequenced using the Sanger method with the primer nopP_F6 to determine the nopP sequences of the B. ottawaense candidates. Sanger sequencing of both ITS and nopP regions was outsourced to Azenta Life Sciences (South Plainfield, NJ, United States). Among the candidates, strains exhibiting ≥ 99% ITS sequence identity to B. ottawaense SG09 and possessing the nopPUSDA122 sequence were designated as nopPUSDA122-carrying B. ottawaense. These strains were recovered from the −80 °C freeze stock plates and purified on HM-salt agar medium89. Finally, strains FY2 and GMA461—both carrying nopPUSDA122—were isolated from root nodules of soybean cultivars “Yukine” (2021) and “Koihime” (2022) in Numata City, respectively. Additionally, B. ottawaense strain OSA024, which harbors an IS within the nopP gene, was identified through nopP sequence analysis of isolates obtained from field soil in Yao City.
Measurement of N2O-reducing activity in cultured rhizobia
The N2O-reducing activity of B. ottawaense strains was measured20. N2O-reducing activity was determined by culturing the bacteria under anaerobic conditions with 1% N2O supplied as the sole electron acceptor. N2O concentrations were measured using a gas chromatograph (GC2014; Shimadzu, Kyoto, Japan) equipped with a thermal conductivity detector and a Porapak Q column (GL Sciences, Tokyo, Japan). Bacterial strains were first aerobically cultured for over 6 h in a 75-mL test tube with an air-permeable plug containing 10 mL of HM liquid medium89 supplemented with 0.1% (w/v) arabinose and 0.025% (w/v) yeast extract, at 28 °C with shaking at 200 rpm. Then, an appropriate volume of bacterial culture was transferred to new tubes containing 10 mL of HM medium to reach an optical density (OD) at 660 nm (OD660) of 0.05, measured in a 25-mm diameter test tube (TEST25NP; AGC Techno Glass Co., Ltd., Shizuoka, Japan). After the initial culture, the test tube was sealed with a butyl-rubber cap, and the gas phase was replaced with a mixture of 4.98% N2O + 95.02% N2 gas for 12–14 h to induce N2O reduction metabolism. Afterward, the gas phase was replaced with 100% N2 gas, and 100% N2O was added to adjust to a final concentration of 1%. The test tube was incubated at 28 °C with shaking at 200 rpm, and 100 µL samples of the gas phase were withdrawn every 1–3 h for analysis by gas chromatography.
Selection of incompatibility-bypassing strains
Strains FY2 and GMA461 harboring nopPUSDA122 were inoculated into soybean (Glycine max (L.) Merr. cv. Hardee) carrying the Rj2 incompatibility gene. Soybean seeds were sterilized using 0.5% sodium hypochlorite, sown in Leonardo Jar pots (five seeds per pot) containing sterilized vermiculite, and inoculated with FY2 and GMA461 at 1 × 109 cells per seed60. Six pots were prepared for each inoculated strain. Soybeans were grown in a growth chamber at 25 °C with a 16-h light and 8-h dark cycle for 3 weeks. Large nodules exceeding 5 mm in diameter were collected and surface-sterilized with 0.5% NaOCl and sliced with a sterile razor blade, and the internal bacteroids were spread on HM agar medium to isolate the bradyrhizobia strains. Single-colony isolates were subsequently inoculated onto Hardee soybeans. After 3 weeks of cultivation, the bradyrhizobia strains were re-isolated from nodules on soybean roots with green leaves. Lastly, nopP PCR was performed on the bradyrhizobial isolates to determine the presence of ISs in nopP60.
Genotypic analysis of Rj2 and GmNNL1 genes in soybean germplasm
For whole-genome resequencing of the soybean variety “Bonminori”, total DNA was extracted from leaves using the DNeasy Plant Mini Kit (Qiagen). The DNA library was subjected to 150-bp paired-end sequencing on an Illumina NovaSeq instrument (Illumina Co., Ltd.) to achieve 20× genome coverage.
The reads from “Bonminori” and 192 accessions of the mini-core collection were mapped to the G. max Williams 82 genome assembly (v4.0) using BWA-MEM90, and the duplicates were removed using Picard MarkDuplicates (http://broadinstitute.github.io/picard/). Variants calling followed GATK best practices for germline SNP/Indel discovery91, using GATK version 4.0.11.0. Variants were initially called individually for each sample with GATK HaplotypeCaller, followed by joint genotyping with GenotypeGVCFs to consolidate variants92. Variants were first filtered using GATK with the parameters:“QD < 5.0 || FS > 50.0 || SOR > 3.0 || MQ < 50.0 || MQRankSum < −2.5 || ReadPosRankSum < −1.0 || ReadPosRankSum > 3.5” Further filtering was conducted using the bcftools view93 with parameters: -m2 -M2 -g hom --output-type z --exclude-uncalled -e “MAF < 0.05 || F_MISSING > 0.25.” All variants were annotated for potential impact using SnpEff version 4.394. The genotype of Rj2 was determined based on an SNP (C/T) at Gm16: 37281186 bp, which causes a single amino acid substitution (R490 to I490)61. Since the genotype of GmNNL1 is defined by an SV, SV analysis was performed using Manta69, with SV files subsequently merged using SURVIVOR95.
Breeding soybean with accumulated incompatibility genes
The soybean varieties “Bonminori”, harboring Rj2, and GmWMC108 Karasu-mame, harboring GmNNL1, were grown simultaneously during flowering and crossbred. To facilitate DNA marker analysis of the Rj2 genotypes, an indel marker was developed based on an SV near Rj2 (Supplementary Table 2). An indel marker for GmNNL1 genotype analysis was developed using genomic sequences around the position of the SINE-like transposon. For genotyping, genomic DNA was extracted from thin slices of the resulting seed cotyledon96. The Rj2 and GmNNL1 markers were amplified using PCR to select the F1 seeds heterozygous for Rj2 and GmNNL1 based on migration patterns in agarose gel electrophoresis. Then, the F1 seeds were planted and grown. After harvesting the F2 seeds, the lines homozygous for Rj2 and GmNNL1 were selected. Then, F3 seeds were harvested from the resulting F2 plants to establish Rj2/GmNNL1 soybean lines.
Method of rhizobial inoculation for soybean
A rhizobial inoculum was prepared by suspending rhizobial strains in Broughton and Dilworth (B&D) solution97 at 6.7 × 102 cells/mL. Leonard jar pots filled with sterile vermiculite were pre-inoculated with 150 mL of the rhizobial inoculum, and 2 chlorine-gas-treated soybean seeds were sown per pot (1.0 × 105 cells/Leonard jar). Cultivation was conducted in an artificial climatic chamber set to 25 °C with a 16-h light and 8-h dark photoperiod. On the fourth day after sowing, seedlings were thinned to leave 1 well-germinated plant per pot and then cultivated for 3–5 weeks. Pots were periodically supplied with a nitrogen-free B&D solution.
Analysis of root nodule occupancy using competitive inoculation tests in Leonard jar pot experiments
B. diazoefficiens strains USDA110 and USDA122 and B. japonicum strain USDA6 were selected as competitor strains. Incompatibility-bypassing strains and competitor strains were each suspended in B&D solution. One of the incompatibility-bypassing strains was mixed with all 3 competitor strains at equal ratios, and a rhizobial inoculum mixture with a total bacterial concentration of 6.7 × 102 cells/mL was prepared. In Leonard jar pots filled with sterile vermiculite, 150 mL of the rhizobial inoculum mixture was pre-inoculated, and 2 chlorine-gas-treated Rj2/GmNNL1 or rj2/Gmnnl1 soybeans were sown per pot following the standard soybean inoculation method. At 5 weeks after inoculation, the number of mature nodules on the roots was counted. Then, mature nodules were collected and surface-sterilized by immersion in 0.5% NaOCl for 3 min. After surface sterilization, cell lysate extracted from crushed root nodules was used to amplify the nopP region by PCR using the primers 09010_F and nopP_R (Supplementary Table 2). Next, PCR-amplified fragments were digested with the restriction enzymes AluI and PstI and analyzed by agarose gel electrophoresis. The strains occupying mature nodules were identified based on the patterns of the restriction enzyme-digested PCR-amplified fragments.
N2O flux was measured as previously reported20. In brief, the soybean root system was gently immersed in water to remove excess vermiculite after competitive inoculation. Then, the roots were transferred to a 100-mL glass vial containing 30 mL of soil obtained from the Kashimadai experimental field (38°27′36.0″N, 141°05′24.0″E) with permission from Tohoku University, Japan. Kashimadai soils had been sieved through a 2-mm mesh to remove large aggregates and stones. Additionally, 5 mL of sterile distilled water was added to each vial. The vials containing roots, soil, and water were incubated aerobically at 25 °C for 20 days to induce nodule degradation. The vials were covered with a soft cloth to maintain aeration during incubation. Each week during the incubation, vials were sealed with butyl-rubber caps and kept under atmospheric conditions for 240 to 360 min to determine N2O flux. N2O concentrations in the vial gas phase were measured using a gas chromatograph (GC2014; Shimadzu) equipped with a 63Ni electron-capture detector and tandem Porapak Q columns (GL Sciences; 80/100 mesh; 3.0 mm × 1.0 m and 3.0 mm × 2.0 m).
Analysis of root nodule occupancy and N2O release in field simulation cultivation with field soil
Andosol soil collected from the NARO field was air-dried in a greenhouse and sieved using a motorized sifter fitted with a 4-mm square mesh (Sasagawa Agricultural Machinery Co., Ltd.). Three liters of the sieved soil were packed into Wagner pots. Four seeds were sown in a pot and inoculated with 1 mL of incompatibility-bypassing rhizobial solution at 1 × 109 cells/mL per seed. Eight pots were prepared for each test plot. After germination, two seedlings were retained per pot. After 42 days of cultivation, soybeans were harvested from three pots, and the number of nodules formed on the roots was recorded. Nodule occupancy was determined using PCR to detect the B. ottawaense-type nosZ gene87 (Supplementary Table 2).
For the remaining five pots, the above-ground portion of the soybeans was excised, and 30-mL gas samples were collected at 0, 20, and 40 min after covering the pots with acrylic plates. Sampling was conducted every 2–3 days, and N2O concentrations in the gas samples were quantitatively analyzed using gas chromatography98 (GC2014, Shimadzu, Kyoto, Japan) using a headspace auto-sampler (AOC5000, Shimadzu). The gas chromatography system analyzes N2O concentrations within 10 min using a single 1 mL injection of the sample gas. N2 was used as the carrier gas, and N2O concentrations were determined using a Nickel-63 (63Ni) electron-capture detector at 340 °C, doped with 5% CH4 containing Ar gas.
Analysis of nodule occupancy and N2O flux through field cultivation
Rj2/GmNNL1 soybean seeds were sown in the Kashimadai experimental field (38.46°N, 141.09°E) on June 13, 2023. Peat moss-based inoculants containing B. ottawaense strains SG09, GMA461, or GMA461-m4 (1.0 × 1010 CFU/seed) were applied to the planting holes before sowing. On July 27, 2023, nodule occupancy by the inoculant strains in mature nodules was quantified via PCR targeting the B. ottawaense-type nosZ gene87 (Supplementary Table 2). On September 25–26, 2023, N2O fluxes from the rhizosphere of each treatment were measured using a mobile mid-infrared N2O sensor (MIRA Ultra; Aeris Technologies, Hayward, CA, USA). Changes in N2O concentration within the chamber were recorded at 1-s intervals for 20–30 min. Subsequently, the N2O flux was calculated using the slope (ΔC/Δt, in ppb h−1) of the linear regression line between 100 and 1000 s after chamber closure, based on the following equation:
where F is the N2O flux (µg N m−2 h−1); V is the chamber volume (0.0099–0.0123 m3), which varied depending on the depth to which the frame was inserted into the soil; A is the chamber area (0.08703 m2); ρ is the gas density (1.977 kg m−3 for N2O at 0 °C); and T is the mean air temperature inside the chamber, assumed to be 30 °C throughout the measurements.
Data analysis
Statistical analysis was conducted using JMP16.2.0. The statistical analysis methods applied are as follows: Fig. 1 and Supplementary Fig. 4—Tukey’s HSD test; Fig. 3—Wilcoxon rank-sum test; Fig. 4 and Supplementary Fig. 5C—Student’s t-test; Figs. 5 and 6B—Dunnett’s test using mock inoculation as control; Fig. 6A—Steel test using mock inoculation as control.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw sequence data of “Bonminori” were deposited in the DDBJ Sequence Read Archive under accession DRA610375. The sequence data of Glyma.16g212300 (Rj2) [https://www.ncbi.nlm.nih.gov/nuccore/GU967682] and Glyma.02G076900 (GmNNL1) [https://www.ncbi.nlm.nih.gov/nuccore/LC885356.2/] are available for download from NCBI. The nucleotide sequences of nopPUSDA122 (JX135464 [https://www.ncbi.nlm.nih.gov/nuccore/JX135464.1/]) in Bradyrhizobium diazoefficiens USDA122 and nopPUSDA6 (JX135432 [https://www.ncbi.nlm.nih.gov/nuccore/JX135432.1/]) in B. japonicus USDA6 can be downloaded from NCBI. Source data are provided with this paper.
References
Tian, H. et al. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 586, 248–256 (2020).
Galloway, J. N. et al. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320, 889–892 (2008).
Gong, C. et al. Global net climate effects of anthropogenic reactive nitrogen. Nature 632, 557–563 (2024).
Kuypers, M. M. M., Marchant, H. K. & Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol 16, 263–276 (2018).
Sánchez, C. & Minamisawa, K. Nitrogen cycling in soybean rhizosphere: sources and sinks of nitrous oxide (N2O). Front. Microbiol. 10, 1943 (2019).
Uchida, Y. & Akiyama, H. Mitigation of postharvest nitrous oxide emissions from soybean ecosystems: a review. Soil Sci. Plant Nutr. 59, 477–487 (2013).
Torres, M. J. et al. Nitrous oxide metabolism in nitrate-reducing bacteria: physiology and regulatory mechanisms. Adv. Micro. Physiol. 68, 353–432 (2016).
Hiis, E. G. et al. Unlocking bacterial potential to reduce farmland N2O emissions. Nature 630, 421–428 (2024).
Lycus, P. et al. Phenotypic and genotypic richness of denitrifiers revealed by a novel isolation strategy. ISME J. 11, 2219–2232 (2017).
Nakei, M. D., Venkataramana, P. B. & Ndakidemi, P. A. Soybean-nodulating rhizobia: ecology, characterization, diversity, and growth promoting functions. Front Sustain. Food Syst. 6, 824444 (2022).
Poole, P., Ramachandran, V. & Terpolilli, J. Rhizobia: from saprophytes to endosymbionts. Nat. Rev. Microbiol 16, 291–303 (2018).
Roy, S. et al. Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation. Plant Cell 32, 15–41 (2020).
Wang, Q., Liu, J. & Zhu, H. Genetic and molecular mechanisms underlying symbiotic specificity in legume-rhizobium interactions. Front. Plant Sci. 9, 313 (2018).
Goyal, R. K., Mattoo, A. K. & Schmidt, M. A. Rhizobial-host interactions and symbiotic nitrogen fixation in legume crops toward agriculture sustainability. Front. Microbiol. 12, 669404 (2021).
Graham, P. H. & Vance, C. P. Legumes: importance and constraints to greater use. Plant Physiol. 131, 872–877 (2003).
Akiyama, H. et al. Mitigation of soil N2O emission by inoculation with a mixed culture of indigenous Bradyrhizobium diazoefficiens. Sci. Rep. 6, 32869 (2016).
Inaba, S. et al. Nitrous oxide emission and microbial community in the rhizosphere of nodulated soybeans during the late growth period. Microbes Environ. 24, 64–67 (2009).
Inaba, S. et al. N2O Emission from degraded soybean nodules depends on denitrification by Bradyrhizobium japonicum and other microbes in the rhizosphere. Microbes Environ. 27, 470–476 (2012).
Toyoda, S. et al. Dynamics of N2O production and reduction processes in a soybean field revealed by isotopocule analyses. Soil Biol. Biochem. 191, 109358 (2024).
Wasai-Hara, S. et al. Bradyrhizobium ottawaense efficiently reduces nitrous oxide through high nosZ gene expression. Sci. Rep. 13, 18862 (2023).
Tao, J., Wang, S., Liao, T. & Luo, H. Evolutionary origin and ecological implication of a unique nif island in free-living Bradyrhizobium lineages. ISME J. 15, 3195–3206 (2021).
Wasai-Hara, S. et al. Diversity of Bradyrhizobium in non-leguminous sorghum plants: B. ottawaense isolates unique in genes for N2O reductase and lack of the type VI secretion system. Microbes Environ. 35, 19102 (2020).
Avontuur, J. R. et al. Genome-informed Bradyrhizobium taxonomy: where to from here?. Syst. Appl. Microbiol. 42, 427–439 (2019).
Avontuur, J. R. et al. Complex evolutionary history of photosynthesis in Bradyrhizobium. Micro. Genom. 9, 001105 (2023).
VanInsberghe, D. et al. Non-symbiotic Bradyrhizobium ecotypes dominate North American forest soils. ISME J. 9, 2435–2441 (2015).
Terra, L. A., Klepa, M. S., Nogueira, M. A. & Hungria, M. Pangenome analysis indicates evolutionary origins and genetic diversity: emphasis on the role of nodulation in symbiotic Bradyrhizobium. Front. Plant Sci. 6, 1539151 (2025).
Delamuta, J. R. M. et al. Polyphasic evidence supporting the reclassification of Bradyrhizobium japonicum group Ia strains as Bradyrhizobium diazoefficiens sp. nov. Int. J. Syst. Evol. Microbiol. 63, 3342–3351 (2013).
Saeki, Y. et al. Mathematical ecology analysis of geographical distribution of soybean-nodulating bradyrhizobia in Japan. Microbes Environ. 28, 470–478 (2013).
Shiina, Y. et al. Relationship between soil type and N2O reductase genotype (nosZ) of indigenous soybean bradyrhizobia: nosZ-minus population and dominant in andosoils. Microbes Environ. 29, 420–426 (2014).
Shiro, S. et al. Genetic diversity and geographical distribution of indigenous soybean-nodulating bradyrhizobia in the United States. Appl. Environ. Microbiol. 79, 3610–3618 (2013).
Bender, F. R. et al. Genetic variation in symbiotic islands of natural variant strains of soybean Bradyrhizobium japonicum and Bradyrhizobium diazoefficiens differing in competitiveness and in the efficiency of nitrogen fixation. Microb. Genom. 8, 000795 (2022).
Maluk, M. et. al. Biological nitrogen fixation by soybean (Glycine max [L.] Merr.), a novel, high protein crop in Scotland, requires inoculation with non-native bradyrhizobia. Front. Agron. 5, 1196873 (2023).
Kaneko, T. et al. Complete genome sequence of the soybean symbiont Bradyrhizobium japonicum strain USDA6T. Genes 2, 763–787 (2011).
Sameshima-Saito, R. et al. Symbiotic Bradyrhizobium japonicum reduces N2O surrounding the soybean root system via nitrous oxide reductase. Appl. Environ. Microbiol. 72, 2526–2532 (2006).
Sameshima-Saito, R., Chiba, K. & Minamisawa, K. Correlation of denitrifying capability with the existence of nap, nir, nor and nos genes in diverse strains of soybean bradyrhizobia. Microbes Environ. 21, 174–184 (2006).
Reeve, W. et al. High-quality permanent draft genome sequence of the Bradyrhizobium elkanii type strain USDA 76T, isolated from Glycine max (L.) Merr. Stand Genom. Sci. 12, 26 (2017).
Keyser, H. H., Weber, D. F. & Uratsu, S. L. Rhizobium japonicum serogroup and hydrogenase phenotype distribution in 12 states. Appl. Environ. Microbiol. 47, 613–615 (1984).
Hénault, C. & Revellin, C. Inoculants of leguminous crops for mitigating soil emissions of the greenhouse gas nitrous oxide. Plant Soil 346, 289–296 (2011).
Hénault, C., Barbier, E., Hartmann, A. & Revellin, C. New insights into the use of rhizobia to mitigate soil N2O emissions. Agriculture 12, 271 (2022).
Itakura, M. et al. Generation of Bradyrhizobium japonicum mutants with increased N2O reductase activity by selection after introduction of a mutated dnaQ gene. Appl. Environ. Microbiol. 74, 7258–7264 (2008).
Itakura, M. et al. Mitigation of nitrous oxide emissions from soils by Bradyrhizobium japonicum inoculation. Nat. Clim. Change 3, 208–212 (2013).
Melissa, O. et al. Evaluation of nitrous oxide emission by soybean inoculated with Bradyrhizobium strains commonly used as inoculants in South America. Plant Soil 472, 311–328 (2022).
Sánchez, C. et al. The nitrate-sensing NasST system regulates nitrous oxide reductase and periplasmic nitrate reductase in Bradyrhizobium japonicum. Environ. Microbiol. 16, 3263–3274 (2014).
Sánchez, C., Mitsui, H. & Minamisawa, K. Regulation of nitrous oxide reductase genes by NasT-mediated transcription antitermination in Bradyrhizobium diazoefficiens. Environ. Microbiol. Rep. 9, 389–396 (2017).
Sánchez, C., Siqueira, A. F., Mitsui, H. & Minamisawa, K. Identification of genes regulated by the antitermination factor NasT during denitrification in Bradyrhizobium diazoefficiens. Microbes Environ. 34, 260–267 (2019).
Gao, Y. et al. Competition for electrons favours N2O reduction in denitrifying Bradyrhizobium isolates. Environ. Microbiol. 23, 2244–2259 (2021).
Gao, Y. et al. Denitrification by Bradyrhizobia under feast and famine and the role of the bc1 complex in securing electrons for N2O reduction. Appl. Environ. Microbiol. 89, e0174522 (2023).
Mania, D., Woliy, K., Degefu, T. & Frostegård, Å A common mechanism for efficient N2O reduction in diverse isolates of nodule-forming bradyrhizobia. Environ. Microbiol 22, 17–31 (2020).
Hartman, G. L., West, E. D. & Herman, T. K. Crops that feed the World 2. Soybean-worldwide production, use, and constraints caused by pathogens and pests. Food Secur. 3, 5–17 (2011).
Rotundo, J. L. et al. European soybean to benefit people and the environment. Sci. Rep. 14, 7612 (2024).
Bourion, V. et al. Co-inoculation of a pea core-collection with diverse rhizobial strains shows competitiveness for nodulation and efficiency of nitrogen fixation are distinct traits in the interaction. Front. Plant Sci. 8, 2249 (2018).
Mendoza-Suárez, M. A. et al. Optimizing Rhizobium-legume symbioses by simultaneous measurement of rhizobial competitiveness and N2 fixation in nodules. Proc. Natl. Acad. Sci. USA 117, 9822–9831 (2020).
Mendoza-Suárez, M., Andersen, S. U., Poole, P. S. & Sánchez-Canizares, C. Competition, nodule occupancy, and persistence of inoculant strains: key factors in the Rhizobium-legume symbioses. Front. Plant Sci. 12, 690567 (2021).
Triplett, E. W. & Sadowsky, M. J. Genetics of competition for nodulation of legumes. Annu. Rev. Microbiol. 46, 399–428 (1992).
Burghardt, L. T., Epstein, B., Hoge, M., Trujillo, D. I. & Tiffin, P. Host-associated rhizobial fitness: dependence on nitrogen, density, community complesity, and legume genotype. Appl. Environ. Microbiol. 88, 15 (2022).
Grundy, E. B., Gresshogg, P. M., Su, H. & Ferguson, B. J. Legumes regulate symbiosis with rhizobia via their innate immune system. Int. J. Mol. Sci. 24, 2800 (2023).
Staehelin, C. & Krishnan, H. B. Nodulation outer proteins: double-edged swords of symbiotic rhizobia. Biochem. J. 470, 263–274 (2015).
Teulet, A. et al. Phylogenetic distribution and evolutionary dynamics of nod and T3SS genes in the genus Bradyrhizobium. Microb. Genom. 6, mgen000407 (2020).
Ratu, S. T. N. et al. Multiple domains in the rhizobial type III effector Bel2-5 determine symbiotic efficiency with soybean. Front. Plant Sci. 12, 689064 (2021).
Sugawara, M. et al. Variation in bradyrhizobial NopP effector determines symbiotic incompatibility with Rj2-soybeans via effector-triggered immunity. Nat. Commun. 9, 3139 (2018).
Sugawara, M. et al. Symbiotic incompatibility between soybean and Bradyrhizobium arises from one amino acid determinant in soybean Rj2 protein. PLoS ONE 14, e0222469 (2019).
Tang, F., Yang, S., Liu, J. & Zhu, H. Rj4, a gene controlling nodulation specificity in soybeans, encodes a thaumatin-like protein but not the one previously reported. Plant Physiol. 170, 26–32 (2016).
Yang, S., Tang, F., Gao, M., Krishnan, H. B. & Zhu, H. R gene-controlled host specificity in the legume-rhizobia symbiosis. Proc. Natl. Acad. Sci. USA 107, 18735–18740 (2010).
Zhang, B. et al. Glycine max NNL1 restricts symbiotic compatibility with widely distributed bradyrhizobia via root hair infection. Nat. Plants 7, 73–86 (2021).
Gourion, B., Berrabah, F., Ratet, P. & Stacey, G. Rhizobium-legume symbioses: the crucial role of plant immunity. Trends Plant Sci. 20, 186–194 (2015).
Iida, T. et al. Symbiosis island shuffling with abundant insertion sequences in the genomes of extra-slow-growing strains of soybean bradyrhizobia. Appl. Environ. Microbiol. 81, 4143–4154 (2015).
Shiro, S. & Saeki, Y. Breeding of Rj gene-accumulated soybean genotypes and their availability for improving soybean productivity. in Soybean—Recent Advances in Research and Applications https://doi.org/10.5772/intechopen.102833 (2022).
Kajiya-Kanegae, H. et al. Whole genome sequence diversity and association analysis of 198 soybean accessions in mini-core collections. DNA Res. 28, dsaa032 (2021).
Chen, X. et al. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics 32, 1220–1222 (2016).
McDermott, R. T. & Graham, H. P. Bradyrhizobium japonicum inoculant mobility, nodule occupancy, and acetylene reduction in the soybean root system. Appl. Environ. Microbiol. 55, 2493–2498 (1989).
Hungria, M., Boddey, L. H., Santos, M. A. & Vargas, M. A. T. Nitrogen fixation capacity and nodule occupancy by Bradyrhizobium japonicum and B. elkanii strains. Biol. Fertil. Soils 27, 393–399 (1998).
Rahman, A. et al. Competitive interference among rhizobia reduces benefits to hosts. Curr. Biol. 33, 3804 (2023).
Cunningham, S., Kollmeyer, W. D. & Stacey, G. Chemical control of interstrain competition for soybean nodulation by Bradyrhizobium japonicum. Appl. Environ. Microbiol. 57, 1886–1892 (1991).
Jimenez-Guerrero, I., Medina, C., Vinardell, J. M., Ollero, F. J. & López-Baena, F. J. The rhizobial type 3 secretion system: the Dr. Jekyll and Mr. Hyde in the rhizobium-legume symbiosis. Int. J. Mol. Sci. 23, 11089 (2022).
López-Baena, F. J. et al. The absence of Nops secretion in Sinorhizobium fredii HH103 increases GmPR1 expression in Williams soybean. Mol. Plant Microbe Interact. 22, 1445–1454 (2009).
Skorpil, P. et al. NopP, a phosphorylated effector of Rhizobium sp. strain NGR234, is a major determinant of nodulation of the tropical legumes Flemingia congesta and Tephrosia vogelii. Mol. Microbiol. 57, 1304–1317 (2005).
Shine, M. B. et al. Glycerol-3-phosphate mediates rhizobia-induced systemic signaling in soybean. Nat. Commun. 10, 5303 (2019).
Gao, M., Hao, Z., Ning, Y. & He, Z. Revisiting growth-defense trade-offs and breeding strategies in crops. Plant Biotech. J. 22, 1198–1205 (2024).
He, Z., Webster, S. & He, S. Y. Growth-defense trade-offs in plants. Curr. Biol. 32, R634–R639 (2022).
Karasov, T. L., Chae, E., Herman, J. J. & Bergelson, J. Mechanisms to mitigate the trade-off between growth and defense. Plant Cell 29, 666–680 (2017).
Ning, Y., Liu, W. & Wang, G. L. Balancing immunity and yield in crop plants. Trends Plant Sci. 22, 1069–1079 (2017).
Yuan, K. et al. Characterization of rhizobia for the improvement of soybean cultivation at cold conditions in central Europe. Microbes Environ. 35, ME19124 (2020).
Li, Y. et al. Natural variation of GmRj2/Rfg1 determines symbiont differentiation in soybean. Curr. Biol. 33, 2478–2490 (2023).
Guilpart, N., Iizumi, T. & Makowski, D. Data-driven projections suggest large opportunities to improve Europe’s soybean self-sufficiency under climate change. Nat. Food 3, 255–265 (2022).
Moriuchi, M. et al. Fusarium fungi produce nitrous oxide (N2O) from nitrite (NO2-) in a model pot system simulating the soybean rhizosphere. Microbes Environ. 40, ME24092 (2025).
Minakata, C., Wasai-Hara, S., Fujioka, S., Sano, S. & Matsumura, A. Unique rhizobial communities dominated by Bradyrhizobium liaoningense and Bradyrhizobium ottawaense were found in vegetable soybean nodules in Osaka prefecture, Japan. Microbes Environ. 38, ME22081 (2023).
Hara, S. et al. Does rhizobial inoculation change the microbial community in field soils? a comparison with agricultural land-use changes. Microbes Environ. 39, ME24006 (2024).
Saeki, Y. et al. Diversity and geographical distribution of indigenous soybean-nodulating bradyrhizobia in Japan. Soil Sci. Plant Nutr. 52, 418–426 (2006).
Cole, M. A. & Elkan, G. H. Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrob. Agents Chemother. 4, 248–253 (1973).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Van der Auwera, G. A. et al. From FastQ data to high-confidence variant calls: the genome analysis toolkit best practices pipeline. Curr. Protoc. Bioinform. 43, 11.10.1–11.10.33 (2013).
Poplin, R. et al. Scaling accurate genetic variant discovery to tens of thousands of samples. Preprint at bioRxiv https://doi.org/10.1101/201178 (2017).
Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 27, 2987–2993 (2012).
Cingolani, P. et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff. Fly 6, 80–92 (2012).
Jeffares, D. C. et al. Transient structural variations have strong effects on quantitative traits and reproductive isolation in fission yeast. Nat. Commun. 8, 14061 (2017).
Kamiya, M. & Kiguchi, T. Rapid DNA extraction method from soybean seeds. Breed. Sci. 53, 277–279 (2003).
Broughton, W. J. & Dilworth, M. J. Control of leghaemoglobin synthesis in snake beans. Biochem. J. 125, 1075–1080 (1971).
Sudo, S. & Yamamoto, A. Three-component simultaneous analysis device and three-component simultaneous analysis method. Patent JP 6843395 (2021).
Acknowledgements
We thank Shusei Sato (Tohoku University) for critical reading of this manuscript and useful comments. We also thank Xuelu Wang (Henan University) for providing the sequence data of GmNNL1. This research was supported by a JPNP18016 project commissioned by the New Energy and Industrial Technology Development Organization (NEDO).
Author information
Authors and Affiliations
Contributions
K.M. and H.I.-A. oversaw the project and designed the experiments. H.N., M.I., K.T.W., F. L., K.K., A.S. (Atsuo Suzuki), S.O., L.V.D., M.S. (Masayuki Sugawara), K.T., M.S. (Matthew Shenton), S.M., A.S. (Arisa Shibata), K.S., Y.F., M.T., H.A., Y.S., K.M., and H.I.-A. performed experiments. M.I., K.K., A.S. (Atsuo Suzuki), S.O., and K.M. isolated B. ottawaense. H.N., M.I., K.K., M.S. (Masayuki Sugawara), K.T.W., Y.F., Y.S., and H.I.-A. analyzed nodule occupancy of incompatibility-bypassing bradyrhizobia. M.I., K.T.W., S.O., M.T., and H.A. analyzed N2O flux from the rhizosphere. F.L. and M.S. (Matthew Shenton) analyzed the genotypes of incompatibility genes in the NARO soybean core collection and selected the Rj2/GmNNL1-accumulating soybean lines. K.T. crossbred soybean cultivars possessing incompatibility genes. S.M., A.S. (Arisa Shibata), and K.S. sequenced the whole genome of N2O-reducing bradyrhizobia. H.I.-A., K.M., H.N., M.I., and S.O. analyzed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nishida, H., Itakura, M., Win, K.T. et al. Genetic design of soybean hosts and bradyrhizobial endosymbionts reduces N2O emissions from soybean rhizosphere. Nat Commun 16, 8023 (2025). https://doi.org/10.1038/s41467-025-63223-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-63223-6