Abstract
As intrinsic differences in humoral immune response to SARS-CoV-2 between children and adults remain unclear, we improved characterisation by defining the kinetics, specificity and function of antibodies to SARS-CoV-2 in children (n = 146, aged 9.4 ± 4.8 years with n = 257 samples) compared to adults (n = 85, aged 39.5 ± 15.2 years with n = 122 samples). We used plasma samples from an infection and vaccination-naive cohort study with RT-PCR confirmed ancestral B.1* SARS-CoV-2 virus infection with asymptomatic or mild disease, collected in Hong Kong between March to December 2020, from acute (0–14 days post infection) to convalescent (15–206 days) timepoints. Children had significantly lower primary antibody responses against SARS-CoV-2 proteins overall, leading to a less isotype switched response. While children had lower OC43 Spike and SARS-CoV-2 S2 IgG and avidity than adults, they exhibited higher avidities for SARS-CoV-2 whole Spike and Nucleocapsid, and higher levels of Spike FcγR-binding antibodies. Adults’ SARS-CoV-2 antibody responses could be derived from high avidity pre-existing cross-reactive common cold coronavirus B cell responses, whilst children appear to generate a de novo SARS-CoV-2- specific Spike and Nucleocapsid IgG with robust Fc receptor (FcR) binding ability and high avidity at a higher proportion than adults, thus their responses are more targeted and functional for SARS-CoV-2.
Similar content being viewed by others
Introduction
Children infected with SARS-CoV-2 experience a different spectrum of disease from adults. The immunological underpinnings of this remain unclear. The majority of COVID cases in children of ancestral Wu-1 infection in the pre-variant pre-vaccine pandemic era, were mild or asymptomatic1,2, with lower infection fatality ratios3, and rare cases of severe disease characterised by acute respiratory distress4, and even rarer multisystem inflammatory syndrome (MIS-C)5. Reduced infection fatality ratios in children were also observed in other non-endemic coronaviruses, SARS-CoV and MERS-CoV, however both also had low infection rates in children compared to adults6,7. Lower COVID morbidity and mortality in children may be due to intrinsic differences between adults and children in terms of immune cell composition, receptor distribution and baseline inflammation, and due to different immune histories affecting pre-existing immunity8.
Here we aim to explore the differences in humoral immune response between children and adults to better understand the qualitative and quantitative immunological differences following mild and asymptomatic infection with first SARS-CoV-2 infection. The adaptive T cell compartment characterisation showed that children exhibit lower CD4+ and CD8+ structural-specific T cell responses than adults9,10, with a response mostly directed towards ORF1ab10. Children also have lower pro-inflammatory type cytokine profiles, countered by higher Th2 and growth factor cytokine responses (IL-4, IL-5, IL-25, IL-33)11.
In the humoral compartment, children also exhibit lower IgG directed towards Spike, Membrane, ORF3 and ORF7 proteins than adults12, with lower levels of memory B cells13, seropositivity rates and neutralising antibodies9,14,15. This suggests that the magnitude of the adaptive immune response is not specifically protective of symptomatic or severe SARS-CoV-2 infection. Qualitative differences based on prior immune responses may play a role in early kinetics of the response, and may impact the sustained children’s response up to 500 days post infection, compared to waning adults response16. Fc receptor (FcR) functions targeting multiple SARS-CoV-2 antigens have been shown to be enriched in moderate and severe adult cases compared to fatal17,18 and add to the protective function of monoclonal antibody therapy in animal models19,20. FcγRIIa-binding for Antibody Dependent Cellular Phagocytosis (ADCP) has also been shown to correlate with phagocytic function against SARS-CoV-2 and other virus proteins21,22 which is also linked to protection from mortality in humans and animals23,24.
Different levels in cross-reactivity of response between children and adults may also shape differences in response. Children may first become seropositive for common cold coronavirus (CCCoVs) infection before age 3.5 years25,26 and during a lifetime, people become infected with CCCoVs every 2 to 7 years27, whilst reinfection with SARS-CoV-2 due to antigenic variation is shorter at around 6 months28. SARS-CoV-2 uninfected children have lower levels of CCCoV antibodies29, higher SARS-CoV-2 S2 reactive antibodies30, and higher SARS-CoV-2 Spike IgM than uninfected adults31. However pre-existing cross-reactive antibodies generated by infection with related β-CoVs OC43 and HKU1 do not impact outcomes of SARS-CoV-2 infection32,33.
In this work, we assess the quantitative and qualitative changes in B.1* SARS-CoV-2 antibody responses early in the pandemic, in a unique cohort of mild and asymptomatically infected children compared to adults during primary SARS-CoV-2 infection over time and in the absence of vaccination. We show that children can generate a functional serological response against SARS-CoV-2 with a unique dynamic compared to adults, driven by lower magnitude of class switched IgG antibodies at convalescent timepoints, but higher avidity and FcR-binding antibodies. A remarkable driver of differences between adults and children is the OC43 S-specific antibody responses, which correlates with SARS-CoV-2 responses in adults but not children, suggesting a cross-reactive recall response in adults.
Results
Cohort description
Blood samples were obtained from opportunistic sampling of volunteers with RT-PCR confirmed SARS-CoV-2 primary infection, who were previously infection and vaccination-naïve. Participants were recruited between March 2020 and December 2020 and prior to the introduction of COVID-19 vaccines. Children (0.25–17 years) and adults (18-71 years) were recruited from hospitals based on RT-PCR positivity, lack of admission to ICU and by WHO severity score < 2 (mild)34. Due to the zero-COVID policy in place in Hong Kong until late 2022, less than 1% of the population was infected in the first two years of the pandemic and even mild or asymptomatic individuals were hospitalised for isolation purposes. Adult samples were selected based on severity ( <2) and timepoints to match available samples from children, despite a higher proportion of mild cases (85.9% for adults than children 56.8%, p < 0.0001), than asymptomatic (Supplementary Fig. 1C). There was also more repeated sampling in children than adults (Table 1, p = 0.0018). No critical or fatal COVID-19 cases were included and those taking medications other than steroids or antivirals were excluded. Due to limited volume and sample availability, a subset of 160 children’s samples were included for non-Spike and antibody avidity assessment (with the same age range 0.25–17 years and average±SD 9.3 ± 4.7 years old). For multivariate analysis, 75 samples from 52 children and 111 samples from 80 adults who had data collected from most immune measures tested were included (Table 1).
Acute antibody responses are similar between children and adults
The early acute (\(\le\)14 days) SARS-CoV-2 infection serological response was assessed in infected children, adults, and uninfected controls (Fig. 1). Infected children and adults had significantly higher acute S-IgG compared to uninfected controls, but there was no significant difference between infected age groups (mean 0.12 for children and 0.39 O.D. for adults, Fig. 1A). Similarly, there was no difference by age in infected groups in the magnitude of IgG responses against Nucleocapsid (N) (Fig. 1B) or replication dependent open reading frame 8 (ORF8) (Fig. 1C) with acute N-IgG at 0.063 for children and 0.14 O.D. for adults (Fig. 1B) and ORF8-IgG 0.32 for children and 0.58 O.D. for adults (Fig. 1C). At acute infection, most infected children and adults had S-IgG responses below the cut-off of detection, with only 37.3% of children and 47.5% of adults seropositive for S-IgG responses above the pre-pandemic responder cut-off (Fig. 1A), which was also similar for N (Fig. 1B) and ORF8 (Fig. 1C) IgG seropositivity.
Children (Spike (S) n = 126, Nucleocapsid (N) ORF8, and OC43-S n = 78) in red and adults (n = 40) in black (A–C, G) IgG and (DEFH) (children n = 22, adults n = 40) IgM responses to (A, D) S, (B, E) N, (C, F) ORF8 and OC43 S (G, H) at acute infection timepoints (day 0–14 post infection) compared to children without PCR confirmed SARS-CoV-2 infection (n = 12, red open circles) and pre-pandemic adult controls (n = 48, also used to define a responder cut off as mean +2 SD, shown by dotted lines). Percentage donors with responses above the cut off are shown in grey boxes under groups. Individual data points are shown, with lines and error bars showing mean and standard deviations. Lower bars are not shown if at 0 or below. Differences between groups assessed using two-sided Kruskal Wallis test with Dunn’s Multiple comparisons, differences between children and adult responder status assessed with Fisher’s Exact test. All differences with p < 0.05 shown. Source data are provided as a Source Data file.
Acute serum IgM responses, known as an early antibody immune response, to S, N and ORF8 proteins were equivalent in children and adults (Fig. 1D–F). Furthermore, uninfected children’s IgM levels were equivalent to those in the infected children group indicating either a high cross-reactive baseline, or an immature and non-specific response. The uninfected children’s IgM responses were significantly higher than uninfected adults (S-IgM 5.5-fold, p < 0.0001, N-IgM 4.5-fold, p < 0.0001, ORF8-IgM 3.4-fold, p = 0.0002, Fig. 1D–F), as previously seen by others for S-IgM31. SARS-CoV-2 infected participants response to CCCoV OC43 Spike showed no significant differences between infected and uninfected children (though from only a small number (n = 12) of uninfected children), but was significantly higher in infected than uninfected adults IgG (p = 0.0033) and IgM (p = 0.0325) (Fig. 1G, H).
Temporal patterns result in significantly lower SARS-CoV-2 antibodies in children than adults at convalescence
While neutralising antibodies at convalescent ( >14 days) timepoints were equivalent between children and adults (Fig. 2A), there was a significantly lower IgG magnitude response in children than adults for both SARS-CoV-2 Spike (p < 0.0001), Fig. 2B), and OC43 Spike (p = 0.0001, Fig. 2C). The difference in SARS-CoV-2 response was driven by significant differences (p < 0.0001) in S2 domain IgG (Fig. 2D), while Receptor Binding Domain (RBD) and S1-N-Terminal Domain (NTD) IgG were equivalent at acute and convalescent timepoints (Supplementary Fig 2A, B, D, E). ORF8-IgG convalescent responses were also lower in children (p = 0.0267), whilst there were no significant differences between children and adults for Nucleocapsid (Fig. 2E, F). IgG responses also increased with age as a continuous variable (S-IgG Spearman coefficient r = 0.336, p < 0.0001, Supplementary Fig 3). The higher response against SARS-CoV-2 Spike in adults was also observed in a longitudinal analysis, for which we fitted a Generalised Additive Mixed Model (GAMM) accounting for repeated samples from the same individuals. The S-IgG response was on average 66.9% decreased in children compared to adults (p < 0.0001), and 26.9% against Nucleocapsid (p = 0.046, Fig. 2G, H).
Children samples in red (PRNT50 n = 58 Spike (S) n = 111 OC43 S/ Nucleocapsid (N)/ ORF8 (n = 82), S2 (n = 81) and adults in black (PRNT50 n = 40, Spike n = 82, OC43 S/S2/N/ORF8 n = 80), neutralising antibody titres (A), IgG responses (B–F) at convalescent timepoints (from day 15 post symptom onset or hospital admission). Dotted lines represent a responder cut off based on the mean+2 SD response of 48 pre-pandemic controls, and % donors with responses above the cut off shown in grey boxes below. Individual data points are shown, with lines and error bars showing mean and standard deviations. Lower bars are not shown if at 0 or below. Differences between groups were assessed using a two-sided Mann-Whitney test with exact p values up to 4 significant figures shown. Antibody levels directed against SARS-CoV-2 S up to 180 days post symptom onset or hospital admission (G) or against SARS-CoV-2 N up to 138 days (H). For S-IgG,122 samples from 85 adults are depicted in black and 231 samples from 137 children are shown in red (G) while, for N-IgG, points represent 113 samples from 84 adults in black and 158 samples from 106 children in red (H). Ratio between IgG and IgM in infected children, adults, uninfected children and uninfected adults against S, N, ORF8 and OC43 S (I–L) with dotted likes at 1 for equal ratio. Differences between groups assessed with two-sided Kruskal-Wallis test with Dunn’s multiple comparisons correction and exact p values up to 4 significant figures shown. M Longitudinal plot of IgG-to-IgM ratios targeting S tracked until day 104. Data include 103 samples from 77 adults and 55 samples from 42 children. (N) Longitudinal plot of IgG-to-IgM ratios targeting nucleocapsid up to day 138. Points represent n = 104 samples from 79 adults in grey and n = 74 samples from 52 children in red. G, H, M, N Fitted values are derived from GAMM accounting for participants’ ID as a random effect and, as a fixed effect, age group, symptoms and sex (G), age group and symptoms (M) or only age group (H, N). 95% confidence intervals are depicted by the shaded areas. The p-value corresponds to the significance of age group as a parametric coefficient in the model and the average decrease of response in children compared to adults is indicated. Source data are provided as a Source Data file.
When defined in terms of the IgG/IgM ratio, indicative of a class switched and maturing antibody response, adults had significantly more S-specific SARS-CoV-2 and OC43 class-switched antibodies (IgG>IgM) than children at convalescent timepoints (p = 0.0074 for SARS-CoV-2, p < 0.0001 for OC43, Fig. 2I, J) and over time. Our GAM models confirmed lower IgG/IgM ratios in children, with ratios on average 58.1% and 74.6% lower than in adults for SARS-CoV-2 and OC43 Spikes respectively (Fig. 2M, N, Supplementary Fig 4A). While IgG/IgM ratios for SARS-CoV-2 N and ORF8 proteins were not significantly lower in children at convalescent timepoints (Fig. 2K, L), they were significantly lower over time compared to adults, as shown by our GAM models accounting for participants’ ID as random effects and age group as fixed effect. IgG/IgM ratios for children were indeed respectively 60.7% (N) and 61.9% (ORF8) of the adults’ ratios (Fig. 2L, Supplementary Fig 4B). SARS-CoV-2 S-IgM were equivalent between children and adults at convalescent timepoints and over time (Supplementary Fig 5A, G), thus higher ratios in adults are accounted for by increased IgG responses. OC43 S-IgM remained significantly higher in children (p = 0.0002, Supplementary Fig 5D) at convalescent timepoints and over time (on average +56.8% in children, p < 0.0001, Supplementary Fig. 5J). Children’s total serum IgM was not different compared to adults (Supplementary Fig 5E, K), and children also had higher RSV-F IgM at steady state, potentially suggesting the presence of non-specific IgM responses in children (Supplementary Fig 5F, L).
Therefore, while acute IgG and IgM responses are not different between children and adults, temporal patterns IgG magnitude and class switching resulted in significantly lower SARS-CoV-2 Spike IgG in children than adults by convalescence. This was not dependent on symptomatic illness, as there were no differences seen between symptomatic and asymptomatic children or adults, either longitudinally, or at convalescent timepoints (Supplementary Fig 6).
High SARS-CoV-2 and not OC43 IgG avidity suggests de novo antibody responses in children
The longitudinal avidity, representative of affinity maturation, while accounting for increased strength of binding by multivalent responses35 of S-IgG demonstrated higher avidity which increased over time in children compared to adults (Fig. 3A, p = 0.0098), and S-IgG avidity at convalescence was significantly higher in children than adults (p < 0.0001,Fig. 3B). The proportion of the avidity response of high avidity ( >50%) was 56.0% in children compared to 22.8% adults. This was driven by higher RBD-IgG avidity in children than adults (p = 0.0440, Fig. 3C), while S1-NTD and S2 domains had equivalent or lower avidity in children than adults (Supplementary Fig 2F, G). Like S, the avidity of N-IgG is consistently higher in children than adults over time (23.8% higher avidity in children, p < 0.0001, Fig. 3D) and significantly higher at convalescence (children: 78.0% versus adults: 31.6%, p < 0.0001, Fig. 3E). The avidity of the OC43 S-IgG response showed the opposite pattern, with significantly higher avidity in adults (100%) than in children (79.6%, p < 0.0001) at convalescent timepoints and over time (on average 37.6% lower in children) (Figure. 3F, G). OC43-S IgG avidity was comparable between infected and uninfected adults (Fig. 3G), thus there is no further affinity maturation of the OC43-S B cell response during SARS-CoV-2 infection.
IgG avidity of response based on response following 8 M Urea treatment for removal of low avidity IgG against SARS-CoV-2 whole Spike, and RBD domains (A–C), and Nucleocapsid (D, E) and OC43 Spike (F, G). Data is representative of individual values in children (red) and adults (black). Data shown over time up to 138 days post infection with predictions from a generalised additive mixed model accounting for participants’ IDs as a random effect and age group as a fixed effect (A, D, F), and as convalescent avidities after day 14 post infection with mean and SD in error bars (BCEG). Lower bars are not shown if at 0 or below. For convalescent timepoints (BCEG), the sample sizes were n = 50 children’s samples and n = 57 adults’ samples (B), n = 73 children and 77 adults in (C), n = 51 children and n = 57 adults (E), or n = 51 infected children, 56 infected adults, 10 non-infected children, and 9 non-infected adults in (G). A, D, F Data for longitudinal graphs originated from 62 samples from 45 children and 91 samples from 68 adults in (A), 70 samples from 48 children and 93 samples from 69 adults in (D), and 69 samples from 47 children and 95 samples from 69 adults in (F). Dotted lines in graphs show 50% avidity with high avidity responses above the line. Percentage donors with high avidity responses are shown in grey boxes (B, C, E, G). Statistical differences in avidity were assessed with two-sided Mann-Whitney test (B, C, E) to compare 2 groups, and two-sided Kruskal-Wallis with Dunn’s multiple comparisons to include comparisons with uninfected children (-red open circles) and uninfected adults (black open circles) with significant p-values shown (G). P values in (A, D, F) correspond to the significance of age group as a parametric coefficient in the model and the average difference in percentage is indicated when significant. 95% confidence intervals are depicted by the shaded areas (ADF). Source data are provided as a Source Data file.
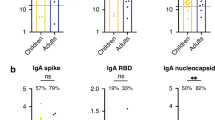
The IgA responses are not impacted by age for SARS-CoV-2 S, N and ORF8 IgA (Supplementary Fig 7A–C), but are significantly higher in children for OC43 S-IgA (p = 0.0245, Supplementary Fig 7D). The class switched response from IgM to IgA at convalescent timepoints was also significantly higher in adults than children against SARS-CoV-2 N (p = 0.0043, Supplementary Fig 7F).
Antibody FcR binding is higher in children compared to adults
The functional quality of antibodies to mediate effector functions was assessed by Fc gamma receptor (FcγR) binding. FcγRIIIa-binding correlates (r = 0.5114, p = 0.0005) with NK cell degranulation in adults (Supplementary Fig. 8) and is a proxy and correlate for NK cell mediated ADCC killing36. This correlation is consistent with other pathogens like influenza virus and HIV in adults and children37,38. Although used as in vitro assays, experimental ADCC is also a correlate of protection against severe and fatal SARS-CoV-2 infections in humans17,18.
Adults and children had the same pattern of S-FcγRIIa longitudinal response for ADCP with an increase over time and maintained until at least 4 months, as shown by GAM model accounting for individual participants (Fig. 4A, p = 0.15). At convalescent timepoints, responses were comparable, with significantly lower responses in uninfected children and adults (p < 0.0001 for both, Fig. 4B). As a proportion of the total IgG response (measured as a ratio FcγRIIa-binding to total S-IgG), children had significantly higher proportion S-IgG with FcγRIIa-binding capacity (p = 0.0004, Fig. 4C), indicating a functional enrichment of ADCP responses.
Spike specific antibodies that bind to FcγRIIa (A–C) and FcγRIIIa (D–F) in infected children (red) and adults (black) (A, D) over time shown as individual values with predictions from a generalised additive mixed model and 95% CI and at (BCEF) convalescent ( > 14 days post infection) timepoints (n = 53 children and 71 adults in (B), 34 children and 71 adults in (C), and 111 (E) or 110 (F) children and 82 adults in (E, F)). 12 (B, E) or 10 (E, F) non-infected children and 10 non-infected adults were tested in (B, C, E, F). Responses shown with bars for mean ± SD and as (B, E) raw FcR binding responses and (C, F) ratio of FcR response to total IgG. Lower bars are not shown if at 0 or below. Groups were compared with two-sided Kruskal-Wallis with Dunn’s multiple comparisons. A, D Longitudinal responses are plotted up to 138 days and represent 75 samples from 52 children and 105 samples from 79 adults (A) or up to 180 days with 231 samples from 137 children and 122 samples from 85 adults (D). The GAMMs account for participants’ IDs as random effect and age group as fixed effects (A, D). The p-values correspond to the significance of age group as a parametric coefficient in the model and the average increase of response in children compared to adults is indicated. 95% confidence intervals are depicted by the shaded areas (A, D). Source data are provided as a Source Data file.
Meanwhile, S-FcγRIIIa-binding response for ADCC was significantly higher in children than adults over time (Fig. 4D), ( + 73.3% higher on average in children than adults, p < 0.0001, GAMM accounting for repeated measures). At convalescent timepoints, children thus had increased S-FcγRIIIa responses compared to adults (p < 0.0001). Similarly, the S-FcγRIIIa to IgG ratio was significantly higher in infected children than adults (p < 0.0001, Fig. 4F).
Multiparametric analysis confirms distinct coordinated immune responses in children and adults
Correlation analyses show distinct overall serological responses based on the parameters measured above. Adults have a larger cluster of positively correlated serological responses linking several IgG, FcγRIIa- and FcγRIIIa-binding responses against SARS-CoV-2 S, RBD, S1, N, OC43 and PRNT50 than children (Fig. 5A, B). Children’s SARS-CoV-2 S, N, ORF8 IgG and S FcR-binding responses are more strongly positively correlated together (r = 0.46 for S–N, 0.57 for FcγRIIa—S, and 0.54 for FcγRIIa—FcγRIIIa in adults against 0.72, 0.78 and 0.90 for children respectively). Children’s responses also show a strong positive correlation with increasing time post infection (0.60 N-IgG, 0.43 for FcγRIIIa and 0.50 for FcγRIIa, Fig. 5B). OC43-S-IgG is significantly positively correlated with SARS-CoV-2 proteins in adults (r = 0.36 for S-IgG, p < 0.05, 0.61 for N-IgG in adults, p < 0.0001, and 0.46 for ORF8-IgG, p < 0.0001, Fig. 5A) and with neutralisation (r = 0.53). On the contrary, no significant correlations for OC43 S-IgG were evident in children, except for age and avidity for OC43 (Fig. 5B). Univariate correlations similarly highlighted more positive correlations of antibody responses with increasing time for children, and negative correlations for adults (Supplementary Fig 9), whilst emphasising the strong positive correlations and link between IgG features (avidity and FcR) in adults.
A subset of samples with multiple measurements was selected for the multivariate analyses. It consists of 111 samples from 80 adults and 75 samples from 52 children. A, B Spearman correlations between all antibody measurements were calculated and depicted as a network for samples from adults (A) or children (B) participants. Each node corresponds to a variable, and edges between them represent positive correlations in red and negative correlations in blue. The thickness of the edge represents the correlation coefficient, with fine lines indicating a coefficient below 0.4. Only significant correlations after Bonferroni correction for multiple testing are shown. Nodes were grouped together when strongly correlating for children. Features are colour coded, with grey for donor information, blue for IgG, pink for IgM, green for IgA, orange for IgG avidity, purple for Fc-receptors and red for neutralisation (PRNT). C, D A principal component analysis with all numerical variables except age was performed and points for dimensions 3 and 4 are shown in (C) coloured by age group with children in red and adults in grey. 95% concentration ellipses are overlaid to indicate clustering patterns. A two-sided Analysis of Similarities test (ANOSIM) was used to assess if the two age groups were statistically different: p-value and R statistic are indicated. R ranges from -1 to 1, if around 0 the groups are similar, the closest to 1 the most the groups are separated. The contribution of each variable to each dimension of the PCA are shown in (D) with the size of the dots while the colour of the dots represents the loadings on each principal component. Only significant contributions are represented, where a contribution is considered significant if it exceeds 3.7%, corresponding to the expected value under a uniform distribution. E, F Supervised multiple factor analysis illustrating the distribution of samples colour-coded by sex, symptoms or age group (E). ANOSIM p-values and R statistic are indicated. The loadings of each variable are shown in (F) on the x-axis, with bars colour-coded to indicate their contribution to the principal component, non-significant contributions are in grey. Contributions are considered significant if they exceed 3%, corresponding to the expected value under a uniform distribution. Source data are provided as a Source Data file.
Unsupervised principal component analysis highlighted that, when taking into account all the antibody features measured in our study and the time post onset of disease, children’s and adults’ samples clustered separately on principal components 3 and 4 as confirmed by analysis of similarities (ANOSIM) for which p = 0.001 and R = 0.32 (the closest to 1 the most distinct are the groups, Fig. 5C and Supplementary Fig 10A). This clustering was not based on symptomatic or asymptomatic infection (Supplementary Fig. 10B), or on the sex of participants (Supplementary Fig 10C). The variables driving this separation on Dimension 3 were mostly avidity for S-OC43 IgG (16.2% contribution, -0.6 loading), S-FcγRIIIa (13.1% contribution, 0.59 loading), and RSV-IgM (11.9% contribution, 0.56 loading) (Fig. 5D). Positive loadings are associated with children while negative are associated with adults.
Finally, to consider both the quantitative and qualitative variables (age group, symptomology and sex), a multiple factor analysis (MFA) was performed (Fig. 5E). The MFA confirmed a distinct separation of samples along Dimension 1 based on age group (R = 0.56, p = 0.001). Specifically, samples from children clustered in the negative space of Dimension 1, while those from adults were positioned in the positive space. In contrast, separation based on symptoms or sex was less pronounced, despite a significant difference in symptoms between adults and children (Table 1); as both groups were mild/asymptomatic this did not drive major antibody differences. The main contributors to Dimension 1 after age groups, are IgG avidity for OC43 that contributes for 16.4% with +0.77 loading, avidity for N-IgG with 6.5% and -0.49 loading, and RSV-IgM with 5.3% contribution and -0.49 loading (Fig. 5F). Separation along Dimension 2 is mainly driven by Time (24.5%, −0.12 loading) and Fc-receptors responses, with S-FcγRIIa contributing for 16.1% and S-FcγRIIIa for 9.9%. Our multiparametric analyses thus confirms our previous univariate findings for distinct serological signatures between adults and children based on specificity, avidity and Fc effector functions.
Discussion
Overall, we found that children have unique SARS-CoV-2 antibody dynamics compared to adults, driven by lower magnitude of class switched IgG antibodies at convalescent timepoints, but higher avidity and FcR-binding antibodies. The ability of children to generate functional SARS-CoV-2 specific serological responses was evident in this study. This is promising for children experiencing their first exposures of current and future SARS-CoV-2 variants, and in the event of potential future coronavirus pandemics, where children may similarly generate de novo immune responses with higher avidity and FcR functions than adults.
We showed the observed differences between children and adults are driven by age alone, with little differences by multiparametric analysis shown by symptom severity (asymptomatic vs mild/ moderate) or sex (male or female). These differences are likely driven by children generating a SARS-CoV-2 specific de novo response, highlighted by correlation analysis of increasing IgG and FcR binding responses with time. Differences between adults and children are driven by OC43 S-specific antibody responses, and OC43 S-IgG strongly correlates with SARS-CoV-2 responses in adults and not children, suggesting a cross-reactive recall response in adults but not children. This may be due to greater cumulative CCCoV infections with increasing age29. While antibodies to other CCCoV HKU1, 229E and NL63 were not tested in our study, OC43 was used as a closely related common beta-coronavirus, and accounts for the highest antibody levels out of the 4 endemic CCCoV39. Both children and adults have high pre-existing OC43-S specific antibodies, but with lower OC43-S IgG in children, also seen by others40, and with significantly lower OC43-S specific IgG/IgM ratio and IgG avidity in children, with more ‘immature’ type of high OC43-S IgM response. Children are known to have greater innate-like B cells expressing natural IgM than adults41,42. This suggests that adults have a class-switched cross-reactive memory antibody response to OC43, while children’s is lower avidity and more innate-like from fewer exposures and lower OC43 immune memory. In adults, pre-existing antibodies towards CCCoVs have been previously shown to not be protective and may indeed hinder an effective SARS-CoV-2 specific response32,33. This could be due to competition between the high cross-reactive memory B cell responses to conserved epitopes in the S2 domain in adults, with novel S1/RBD specific responses derived from naïve B cells43. We found adults had higher S2 and S2 avidity responses compared to children, driving their higher total response, while RBD-directed response and neutralisation was equivalent in children, supporting the greater de novo response in children. Epitope mapping between adults and children’s antibody responses by techniques such as Electron Microscope Poly Epitope Mapping (EMPEM) may reveal specific early targeting for epitope dominance in further detail44. While our results confirm the lower antibody magnitude in children than adults found previously14,15, other studies contrastingly found higher IgG in children than adults, which may be explained by using different inclusion criteria measuring only seropositive children, or comparing later timepoints16,40.
The response in children is also characterised by higher avidity S and N IgG responses than adults, maintained at 6 months with minimal waning, particularly for N, suggesting a longevity of N antibodies in children. Indeed, N-IgG has been shown to be a correlate of protection in children from subsequent Omicron infection, while S-IgG response was not45. Higher S-IgG avidity in children, driven in part by RBD-IgG avidity, suggests higher quality de novo response, also shown by other work finding high RBD-specific avidity (by lower dissociation rates) in children46.
The functionality of the S antibodies was also different by age and effector functions. FcγRIIIa-binding, which indicates ADCC function, is a correlate of protection against severe disease and death17,18 and here was significantly higher in children than adults, warranting further investigation of NK cellular response in COVID. FcγRIIIa-binding is also a major driver of difference between children and adults’ overall response by multiparametric analysis, highlighting the importance of this response in children. Others have additionally seen higher NK cell activation by NKp30 and CD107a in children compared to adults with SARS-CoV-2 infection47, which could be driven by differences in Fc core fucosylation of children compared to adults48. Others have found no difference between children and adults for either ADCP or NK cell activation when assessing responses to S1 subunit49, suggesting these differences are driven by S2. Further research into IgG subclasses with different FcR affinities50, and Fc glycosylation patterns in children is warranted.
Overall, the kinetics and quality of antibody responses are inherently different with age during SARS-CoV-2 infection. Whilst there was equivalent acute IgG in children and adults against SARS-CoV-2 proteins, children had increased avidity and FcγRIIIa function at convalescence, which may indicate the formation of B cell germinal centres which could be harnessed by vaccination. High total SARS-CoV-2 specific S-IgG but lower functional quality in adults may be driven by immune competition from pre-existing OC43 antibodies which are not protective32. Mechanistic studies are needed for B cell function in children and the impact of prior immunity for epitope specificity.
This study confirms that children generate high quality but low IgG magnitude immune responses to SARS-CoV-2 mild and asymptomatic infections. Further study is needed to understand the protective thresholds and mechanisms in children. Ultimately, the ability for children to generate higher quality antibody responses than adults is promising in the event of future endemic SARS-CoV-2 infections or coronavirus pandemics in this age group.
Methods
Patients and samples collection
Heparinised blood samples were taken from RT-PCR confirmed SARS-CoV-2 infected 146 children (9.4 ± 4.8, 0.25–17 years old) and 85 adults (39.5 ± 15.2, 18–71 years old) with asymptomatic and mild/ moderate COVID-19 (Table 1, Supplementary Fig 1). From infected children, we used a total of 257 COVID-19 children plasma samples from acute (n = 128, 0–14 days) and convalescent (n = 129, 15–206 days) timepoints, among which 193 were longitudinal samples from 82 participants with 2–4 sampling timepoints. From infected adults we used a total of 122 COVID-19 plasma samples from acute (n = 40, 2–14 days) and convalescent (n = 82, 15–180 days) timepoints. There were 72 longitudinal samples from 35 participants with 2 to 3 sampling timepoints. Data is presented throughout as responses from distinct samples. Sample day was defined as day post-symptom onset or RT-PCR confirmation for asymptomatic cases through contact tracing or quarantine. The COVID-19 patient study was approved by the institutional review board of the respective hospitals, viz. Kowloon West Cluster (KW/EX-20-039 (144-27)), Kowloon Central / Kowloon East cluster (KC/KE-20-0154/ER2) and HKU/HA Hong Kong West Cluster (UW 20-273, UW20-169), Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee (CREC 2020.229). All adult participants and parents for the children provided written informed consent and assent. Sex of participants was self-reported and considered in the study.
The negative control plasma samples used in this study were from Hong Kong blood donors (confirmed to be negative for COVID-19), from a total of 60 plasma samples including negative paediatric samples (n = 12, collected in 2020) and negative adult samples (n = 48, collected in 2017). The collection of negative control blood donors was approved by the Institutional Review Board of The Hong Kong University and the Hong Kong Island West Cluster of Hospitals (approval number: UW16-254 and UW 20-273). Written informed consent was obtained from the participants or their legal representants. Sex of participants was self-reported and considered in the study. Plasma samples were collected from heparinised blood. All samples from COVID-19-positive participants or negative controls were heat-inactivated prior to experimental use at 56 °C for 30 min. Heat-inactivated and non-heat inactivated samples were initially tested in the laboratory and by others51,52.
Antibody binding by Enzyme-linked immunosorbent assay (ELISA) for antigen specificity, avidity and FcR binding
Flat-bottom 96-well plates (Nunc MaxiSorp, Thermofisher Scientific) were coated with SARS-CoV-2 or OC43 proteins (SARS-CoV-2 and OC43 Spike proteins (expressed in Baculovirus-Insect cells, Sinobiological), RBD, S1-NTD and S2 domain proteins (expressed in HEK293 mammalian cells, Sinobiological), Nucleocapsid (expressed in Baculovirus-Insect cells, from Gaya Amarasinghe, University of Washington, St Louis, USA), ORF8 (expressed in tobacco BY-2 cells, from Masashi Mori, Ishikawa Prefectural University, Japan)53, RSV-F (expressed in mammalian cells, MyBiosource) diluted in PBS and incubated overnight at 4 °C. For IgG, IgM and IgA detection, plates were coated with S1 + S2, RBD, S1-NTD, S2, N and RSV-F proteins at 250 ng/mL, ORF8 at 300 ng/mL. For FcR binding ELISAs, plates were coated with protein at 500 ng/mL. Following protein incubation, plates were blocked with PBS with 0.1% FBS for 1 h then washed with PBS with 0.1% Tween-20 and dried. Heat inactivated (56 °C for 30 mins) plasma diluted at 1:100 in PBS with 0.1% FBS, 0.05% Tween-20 was added to wells and incubated at RT for 2 h. For FcR binding ELISAs, incubations were at 37 °C for 1 h. Plates were washed, dried and incubated for a further 2 h with secondary HRP-conjugated detection antibody (IgG-HRP (1:5000, G18 145; BD), IgM-HRP (1:1000, HP6083, Invitrogen) or IgA-HRP (1:1000, BFG-267, Invitrogen)) in PBS with 0.1% FBS, 0.05% Tween-20. For FcR ELISAs, plates were incubated with biotinylated dimeric recombinant soluble (rs)FcγRIIIa-V158 at 100 ng/mL or rsFcγRIIa-H131 at 200 ng/mL (from Mark Hogarth and Bruce Wines, Burnet Institute, Australia) for 1 h at 37 °C then with streptavidin-HRP (1:10,000, BD). Plates were washed again and HRP was revealed by stabilised hydrogen peroxide and tetramethylbenzidine (R&D systems) for 20 mins, stopped with 2 N H2SO4 and O.D. analysed with an absorbance microplate reader at 450 nm wavelength (Tecan Life Sciences). For avidity ELISAs, matched plasma plates were incubated for 5 mins with 8 M urea (Invitrogen) 3 times before incubation with secondary HRP-conjugated antibody. Avidity measures were calculated by dividing the response following urea treatment by the total protein specific response and transformed to a percentage. Avidity measures were calculated only for detectable IgG or IgM responses and were maximised at 110%.
Plaque reduction neutralisation tests (PRNT)
PRNT was performed as previously described54 in a biosafety level 3 facility using the virus isolate BetaCoV/Hong Kong/VM20001061/2020. Serial dilutions of serum samples were incubated with 30–40 plaque-forming units of virus for 1 h at 37 °C. The virus–serum mixtures were added onto Vero E6 cell monolayers and incubated 1 h at 37 °C in 5% CO2 incubator. Plates were overlaid with 1% agarose in cell culture medium and incubated for 3 days when the plates were fixed and stained. Antibody titres were defined as the highest serum dilution that resulted in >50% (PRNT50) reduction in the number of plaques.
Assessment of total IgM
Total plasma IgM was measured using the Human IgM ELISA Kit (Invitrogen) according to manufacturer’s instructions. Briefly, plasma samples were diluted 1:10,000 with 1x Assay Buffer (PBS with 1% Tween20, 10% BSA) and added to pre-washed microwell 96-well plate provided and precoated with anti-human IgM, along with 50 µL diluted HRP-conjugated antibody. Plates were covered with adhesive film and incubated at room temperature with shaking for 1 hour. Plates were washed 4x with PBS with 1% Tween20) then incubated with 100 µL tetramethyl-benzidine substrate solution for 30 min in the dark. 1 M Phosphoric acid stop solution was added to stop further colour change and analysed with an absorbance microplate reader at 450 nm wavelength.
ADCC functional assay
NK-92 cells modified to stably express FcγRIIIa (Fox Chase Cancer Centre) were passaged in Alpha-MEM supplemented with 2 mM L-Glutamax (Thermofisher Scientific), 0.2 mM Myo-inositol (Sigma), 10% heat inactivated horse serum (Invitrogen), 10% heat inactivated HyClone characterised FBS (Cytiva), 2.5 µM Folic Acid (Sigma), 1 mM NA Pyruvate (Thermofisher Scientific), 100 µg/mL penicillin/streptomycin (Thermofisher Scientific), 0.0001 M β-mercaptoethanol (Sigma) and 100 IU/mL human recombinant IL-2 (Roche). SARS-CoV-2 Spike protein (S1 + S2, Sinobiological) at 4 µg/mL was used to coat U bottom 96-well plates (Nunc Maxisorp, Thermofisher) overnight at 4 °C. Plates were washed with PBS and incubated with heat inactivated plasma at 1:20 dilution for 2 h at 37 °C. Plates were again washed and incubated with 100,000 NK-92 cells in 100 µL per well for 4 h at 37 °C, 5% CO2. Wells were washed with PBS and cells stained for viability (Zombie-NIR, 1:100, Biolegend) for 30 mins 4 °C, then anti-human CD56-PE (clone 5.1H11, dilution 1:200, Biolegend), anti-CD107a-APC (clone H4A4, dilution 1:50, Biolegend) for 30 mins 4 °C, fixed (Cytofix/ cytoperm) and acquired by flow cytometry (AttuneNxT Invitrogen) and analysed by FlowJo v10 (gating strategy shown in Supplementary Fig 8B). For positive control, CD16-LEAF (1:100, Biolegend), and negative control, HIV gp120 protein (Sinobiological), were used to coat plates in the place of Spike protein.
Statistical analysis
Univariate analysis comprised of two-sided Kruskal-Wallis test with Dunn’s multiple comparisons to measure statistical differences between infected children and adults and uninfected children and adults. Where responses were only compared between children and adults, two-sided Mann-Whitney test was used. Data was assumed to be non-normally distributed for statistical analysis. Graphpad Prism 9 was used for these analyses and graphing. For other analyses, RStudio Version 2024.04.2 + 764 with R version 4.4.1 (2024-06-14)55 was used. Data handling and visualisation were performed using the following packages: readxl56, dplyr57, ggplot2(version 3.5.1)58, itsadug59, xlsx60, writexl61, tidyverse62, scales63, Matrix64 and ggnewscale65.
Longitudinal analyses to assess changes with age or time were conducted using generalised additive mixed models (GAMMs) to account for repeated measurements and possible cofounding variables. Exact models used are specified in the legends of the figures. These analyses were performed with the function gam from the mgcv package (version 1.9-1)66 using REML estimation. We imputed participants’ ID as a random effect and used a smooth term for time that varies by the level of age group and used age group and other possible cofounding variables as fixed effects. The formula used in most figures is: antibodymeasure ~ s(Time, by = Age group) + s(participantsID, bs = “re”) + Age group. Sex and symptomology were added when specified in the figure legend. We selected the best model using parsimony principle and picking the model with smallest AIC if the analysis of deviance table showed a significant improvement in the model compared to the simplest model. We also used lmtest67 to compare models. P-values of the parametric coefficient for age group are indicated on the graphs. When significant, we estimated the average increase of response in children using the intercept of the model as the average response for adults the parametric coefficient of the children: \({{{\rm{percen}}}}{{{{\rm{t}}}}}_{{{{\rm{increase}}}}}=\frac{{{{\rm{parametric}}}}\; {{{\rm{coefficient}}}}\; {{{\rm{for}}}}\; {{{\rm{children}}}}}{{{{\rm{average}}}}\; {{{\rm{response}}}}\; {{{\rm{for}}}}\; {{{\rm{adults}}}}}\) or \({10}^{{{{\rm{parametric}}}}\; {{{\rm{coefficient}}}}\; {{{\rm{for}}}}\; {{{\rm{children}}}}-1}\) for logarithmic scales.
Spearman’s correlations were calculated to assess IgG levels evolution with age and linear regressions from geom_smooth from ggplot2 were added to the graphs. Spearman’s correlations between antibody measurements were computed and plotted using rquery.cormat function (from https://www.sthda.com/upload/rquery_cormat.r that requires corrplot package68). The correlation networks were also based on Spearman correlations and were plotted using qgraph69. A Bonferroni correction was applied to correct for multiple testing and only the significant correlations are depicted on the figure. Fine lines correspond to a correlation coefficient under 0.4.
To investigate differences between antibody responses in adults and in children considering several all the antibody features measured, we performed principal component analysis (PCA) as unsupervised method and multiple factor analysis (MFA) as a supervised analysis handling both quantitative and qualitative variables. For both multivariate analyses, the packages FactoMineR (version 2.11)70 and factoextra71 were used. To handle missing values in the PCA, we also used missMDA package (version 1.19)72. Individuals are plotted in Figs. 5C and 5E, where each point represents a sample. A 95% concentration ellipse, assuming normality, is overlaid to illustrate clustering patterns. Separation between groups was assessed using an Analysis of Similarities (ANOSIM) test from the vegan package73.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Data from ELISA responses with background subtracted are indicated in the main text and Supplementary Figs. Datasets generated and/or analysed during the current study are provided with the paper or are appended as supplementary data. Source data are provided with this paper.
Code availability
The code used to perform analyses and make the figures is available at (https://github.com/valkenburg-lab/children_adults_SARS-CoV-2-antibody-responses).
References
Du, W. et al. Clinical characteristics of COVID-19 in children compared with adults in Shandong Province, China. Infection 48, 445–452 (2020).
Chung, E. et al. Comparison of symptoms and RNA levels in children and adults with SARS-CoV-2 infection in the community setting. JAMA Pediatrics 175, e212025–e212025 (2021).
Levin, A. T. et al. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur. J. Epidemiol. 35, 1123–1138 (2020).
Zsigmond, B. et al. Very low rates of severe COVID-19 in children hospitalised with confirmed SARS-CoV-2 infection in London, England″. J. Infect. 85, 90–122 (2022).
Feldstein, L. R. et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 383, 334–346 (2020).
Thabet, F. et al. Middle East respiratory syndrome coronavirus in children. Saudi Med J. 36, 484–486 (2015).
Iannarella, R. et al. Coronavirus infections in children: from SARS and MERS to COVID-19, a narrative review of epidemiological and clinical features. Acta Biomed. 91, e2020032 (2020).
Rotulo, G. A. & Palma, P. Understanding COVID-19 in children: immune determinants and post-infection conditions. Pediatr. Res. 94, 434–442 (2023).
Pierce, C. A. et al. Immune responses to SARS-CoV-2 infection in hospitalized pediatric and adult patients. Sci. Transl. Med. 12, eabd5487 (2020).
Cohen, C. A. et al. SARS-CoV-2 specific T cell responses are lower in children and increase with age and time after infection. Nat. Commun. 12, 4678 (2021).
Rajamanickam, A. et al. Elucidating systemic immune responses to acute and convalescent SARS-CoV-2 infection in children and elderly individuals. Immun., Inflamm. Dis. 12, e1167 (2024).
Hachim, A. et al. SARS-CoV-2 accessory proteins reveal distinct serological signatures in children. Nat. Commun. 13, 2951 (2022).
Manfroi, B. et al. Preschool-age children maintain a distinct memory CD4+ T cell and memory B cell response after SARS-CoV-2 infection. Sci. Transl. Med. 16, eadl1997 (2024).
Weisberg, S. P. et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 22, 25–31 (2021).
Toh, Z. Q. et al. Comparison of seroconversion in children and adults with mild COVID-19. JAMA Netw. Open 5, e221313–e221313 (2022).
Joshi, D. et al. Infants and young children generate more durable antibody responses to SARS-CoV-2 infection than adults. iScience, 26, 107967 (2023).
Kaplonek, P. et al. Early cross-coronavirus reactive signatures of humoral immunity against COVID-19. Sci. Immunol. 6, p. eabj2901 (2021).
Yu, Y. et al. Antibody-dependent cellular cytotoxicity response to SARS-CoV-2 in COVID-19 patients. Signal Transduct. Target. Ther. 6, 346 (2021).
Dangi, T. et al. Improved control of SARS-CoV-2 by treatment with a nucleocapsid-specific monoclonal antibody. The Journal of Clinical Investigation, 132, e162282 (2022).
Chen, Y. et al. Engineered ACE2-Fc counters murine lethal SARS-CoV-2 infection through direct neutralization and Fc-effector activities. Sci. Adv. 8, eabn4188 (2022).
Cohen, C. A. et al. Antibody Fc receptor binding and T cell responses to homologous and heterologous immunization with inactivated or mRNA vaccines against SARS-CoV-2. Nat. Commun. 15, 7358 (2024).
Ackerman, M. E. et al. Enhanced phagocytic activity of HIV-specific antibodies correlates with natural production of immunoglobulins with skewed affinity for FcγR2a and FcγR2b. J. Virol. 87, 5468–5476 (2013).
Zohar, T. et al. Compromised humoral functional evolution tracks with SARS-CoV-2 mortality. Cell 183, 1508–1519 (2020).
Yasui, F. et al. Phagocytic cells contribute to the antibody-mediated elimination of pulmonary-infected SARS coronavirus. Virology 454-455, 157–168 (2014).
Dijkman, R. et al. Human coronavirus NL63 and 229E seroconversion in children. J. Clin. Microbiol 46, 2368–2373 (2008).
Humbert, M. et al. Functional SARS-CoV-2 cross-reactive CD4+ T cells established in early childhood decline with age. Proc. Natl. Acad. Sci. 120, e2220320120 (2023).
Edridge, A. W. D. et al. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 26, 1691–1693 (2020).
Ma, X. et al. The symptoms and interval of omicron SARS-CoV-2 reinfection among healthcare workers in a hospital of Southern China: a cross-sectional study. BMC Infect. Dis. 24, 354 (2024).
Tanunliong, G. et al. Age-associated seroprevalence of coronavirus antibodies: population-based serosurveys in 2013 and 2020, British Columbia, Canada. Front Immunol. 13, 836449 (2022).
Ng, K. W. et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 370, 1339–1343 (2020).
Selva, K. J. et al. Systems serology detects functionally distinct coronavirus antibody features in children and elderly. Nat. Commun. 12, 2037 (2021).
Lin, C.-Y. et al. Pre-existing humoral immunity to human common cold coronaviruses negatively impacts the protective SARS-CoV-2 antibody response. Cell Host Microbe 30, 83–96.e4 (2022).
Anderson, E. M. et al. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell 184, 1858–1864.e10 (2021).
Rubio-Rivas, M. et al. WHO ordinal scale and inflammation risk categories in COVID-19. comparative study of the severity scales. J. Gen. Intern Med 37, 1980–1987 (2022).
Bauer, G. The potential significance of high avidity immunoglobulin G (IgG) for protective immunity towards SARS-CoV-2. Int J. Infect. Dis. 106, 61–64 (2021).
Hagemann, K. et al. Natural killer cell-mediated ADCC in SARS-CoV-2-infected individuals and vaccine recipients. Eur. J. Immunol. 52, 1297–1307 (2022).
McLean, M. R. et al. Dimeric Fcγ receptor enzyme-linked immunosorbent assay to study HIV-specific antibodies: A new look into breadth of Fcγ receptor antibodies induced by the RV144 vaccine trial. J. Immunol. 199, 816–826 (2017).
Jia, J. Z. et al. Influenza antibody breadth and effector functions are immune correlates from acquisition of pandemic infection of children. Nat. Commun. 15, 3210 (2024).
Dowell, A. C. et al. Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection. Nat. Immunol. 23, 40–49 (2022).
Renk, H. et al. Robust and durable serological response following pediatric SARS-CoV-2 infection. Nat. Commun. 13, 128 (2022).
Carsetti, R. et al. The immune system of children: the key to understanding SARS-CoV-2 susceptibility? The. Lancet Child Adolesc. Health 4, 414–416 (2020).
Ciocca, M. et al. Evolution of human memory B cells from childhood to old age. Front. Immunol. 12, 690534 (2021).
Fraley, E. et al. Cross-reactive antibody immunity against SARS-CoV-2 in children and adults. Cell. Mol. Immunol. 18, 1826–1828 (2021).
Bangaru, S. et al. Structural mapping of antibody landscapes to human betacoronavirus spike proteins. Sci. Adv. 8, eabn2911 (2022).
Dowell, A. C. et al. Nucleocapsid-specific antibodies as a correlate of protection against SARS-CoV-2 reinfection in children. J. Infect. 87, 267–269 (2023).
Yang, H. S. et al. Association of age with SARS-CoV-2 antibody response. JAMA Netw. Open 4, e214302–e214302 (2021).
Jeong, S. D. et al. Increased type III interferons and NK cell functions in SARS-CoV-2-infected children. Signal Transduct. Target. Ther. 8, 54 (2023).
Cheng, H. D. et al. IgG Fc glycosylation as an axis of humoral immunity in childhood. J. Allergy Clin. Immunol. 145, 710–713.e9 (2020).
Gelderloos, A. T. et al. Primary SARS-CoV-2 infection in children and adults results in similar Fc-mediated antibody effector function patterns. Clin. Transl. Immunol. 13, e1521 (2024).
de Taeye, S. W. et al. FcγR Binding and ADCC Activity of Human IgG Allotypes. Front Immunol. 11, 740 (2020).
Burbelo, P. D. et al. Antibody profiling by Luciferase Immunoprecipitation Systems (LIPS). J. Vis. Exp. (JoVE), 32, 1549 (2009).
Amanat, F. et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med 26, 1033–1036 (2020).
Imamura, T. et al. Production of ORF8 protein from SARS-CoV-2 using an inducible virus-mediated expression system in suspension-cultured tobacco BY-2 cells. Plant Cell Rep. 40, 433–436 (2021).
Perera, R. A. et al. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), March 2020. Eurosurveillance 25, 2000421 (2020).
Team, R. C. R.: a language and environment for statistical computing. r foundation for statistical computing. https://www.R-project.org (2024).
Wickham, H. & Bryan, J. _readxl: Read Excel Files_. R package version 1.4.3. 2023; https://CRAN.R-project.org/package=readxl (2023).
Wickham, H. et al. _dplyr: A Grammar of Data Manipulation_. R package version 1.1.4; https://CRAN.R-project.org/package=dplyr (2023).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag (2016).
van Rij, J. et al. itsadug: Interpreting Time Series and Autocorrelated Data Using GAMMs. R package version 2.4.1 https://CRAN.R-project.org/package=itsadug (2022).
Dragulescu, A. A. C. xlsx: Read, Write, Format Excel 2007 and Excel 97/2000/XP/2003 Files. 2020 R package version 0.6.5; https://CRAN.R-project.org/package=xlsx (2020).
J, O. writexl: Export Data Frames to Excel ‘xlsx’ Format. R package version 1.5.0; https://CRAN.R-project.org/package=writexl (2024).
Wickham, H. et al. Welcome to the Tidyverse. J. Open Source Softw. 4, 1686 (2025).
Wickham, H, P. T. & Seidel, D. scales: Scale Functions for Visualization. R package version 1.3.0 https://CRAN.R-project.org/package=scales (2023).
Bates, D, M. M. & Jagan, M. Matrix: Sparse and Dense Matrix Classes and Methods 2024; R package version 1.7-0 https://CRAN.R-project.org/package=Matrix (2024).
E, C. ggnewscale: Multiple Fill and Colour Scales in ‘ggplot2’. 2024; R package version 0.5.0]. https://CRAN.R-project.org/package=ggnewscale (2024).
Wood, S. N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. 73, 3–36 (2011).
Zeileis, A. H. Torsten. diagnostic checking in regression relationships. R. N. 2, 7–10 (2002).
Wei, T. & Simko, V. R package ‘corrplot’: Visualization of a Correlation Matrix (Version 0.95). Available from: https://github.com/taiyun/corrplot (2024).
Epskamp, S. et al. ggraph: network visualizations of relationships in psychometric data. J. Stat. Softw. 48, 1–18 (2012).
Le, S., Josse, J. & Husson, F. FactoMineR: an R package for multivariate analysis. J. Stat. Softw. 25, 1–18 (2008).
Kassambara, A. & Mundt, F. factoextra: extract and visualize the results of multivariate data analyses. R. package version 1, 7 (2020).
Josse, J. & Husson, F. missMDA: a package for handling missing values in multivariate data analysis. J. Stat. Softw. 70, 1–31 (2016).
Oksanen J, S. G. et al. vegan: Community Ecology Package_. R package version 2.6-10. https://CRAN.R-project.org/package=vegan (2025).
Acknowledgements
The authors thank the patients and their families for their participation, and are grateful to the hospital staff, clinicians and nurses, particularly Karen Y.S. Yui, for sample coordination, and Fionn N.L.M. for sample processing. We thank Professor J.T.W. and Dr.K.L. for providing donor plasma controls. We thank Niamh Meagher and Peta Edler from the Statistics Consulting service of the Peter Doherty Institute for Infection and Immunity for their advice on the statistical analysis. This study was partly supported by the Health and Medical Research Fund Commissioned Research Fund (Reference No COVID190126 (MP) and 190115 (SAV)) from the Food and Health Bureau of the Government of Hong Kong Special Administrative Region, Theme based Research Grants Scheme (T11-705/21 N, LLMP), National Institutes of Allergy and Infectious Diseases, National Institutes of Health (USA) (contract HHSN272201400006C, MP and GA). Fellowship support from HKPF from the HKSAR RGC (CAC) and NHMRC EL2 fellowship (SAV).
Author information
Authors and Affiliations
Contributions
Conceptualisation: S.A.V., C.A.C., L.G., M.P. Specialised data analysis: L.G., K.W.K.L. Specialised reagent: M.M., G.A. Sample acquisition: M.Y.W.K., W.H.C., Y.S.Y., S.S.C., O.T.Y.T., D.S.C.H., S.M.S.C., L.L.M.P., M.P. Funding acquisition: S.A.V., L.L.M.P., M.P., G.A. Supervision: S.A.V., M.P. Writing—original draft: C.A.C., L.G., S.A.V. Writing—review & editing: C.A.C., L.G., S.A.V., M.P.
Corresponding author
Ethics declarations
Competing interests
LLM Poon, M Peiris and SA Valkenburg (patent pending: US 17/997,434, China: 2021800455096, WO2021219029A1) for the use of ORF8 as diagnostics of SARS-CoV-2 infection. M Mori produced ORF8 protein for serological experiments by patent process based on US Patents 8,507,220 and 8,586,826. Other authors have no declaration of interest.
Peer review
Peer review information
Nature Communications thanks Kizzmekia Corbett, and the other, anonymous, reviewer for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cohen, C.A., Grzelak, L., Chiu, S.S. et al. SARS-CoV-2 antibody responses in children exhibit higher FcR engagement and avidity than in adults. Nat Commun 16, 7879 (2025). https://doi.org/10.1038/s41467-025-63263-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63263-y