Abstract
Mitotic DNA synthesis (MiDAS) serves to complete the replication of genomic loci that are not fully replicated in S phase in response to replication stress. Previous studies suggest that MiDAS might proceed via break-induced DNA replication, a sub-pathway of homologous recombination repair activated at broken or collapsed replication forks. We set out to define whether DNA double strand break end-resection factors play a role in MiDAS. Here, we show that several core end-resection factors, including MRE11, CtIP and BRCA1 are essential for MiDAS. In addition, while loss of WRN or DNA2 impairs MiDAS, there is no requirement for other known end-resection factors such as EXO1 and BLM. Moreover, both the exonuclease and the helicase activities of WRN contribute to MiDAS. Because oncogene-induced replication stress is common in cancers, targeting of WRN or other factors required for MiDAS could facilitate the development of targeted cancer therapies.
Similar content being viewed by others
Introduction
DNA replication stress (RS) is a collective term for the slowing or stalling of replication fork progression in S phase1. One of the consequences of RS is that cells can enter mitosis with regions of under-replicated genomic DNA (UR-DNA)2. These regions contains one or more of the following features: repetitive sequences that are prone to forming DNA secondary structures, a lack of active DNA replication origins, and genes that are naturally late replicating3,4. The best characterized of these regions are common fragile sites (CFSs)5,6,7,8,9,10 and telomeres11,12. When cells are challenged with RS caused by oncogene activation or aphidicolin (APH; a B family DNA polymerase inhibitor), mitotic DNA synthesis (MiDAS) can occur to help cells to replicate the UR-DNAs at CFS13,14,15,16,17 and telomeres (reviewed in ref. 18), as well as other uncharacterized regions19,20. Although most frequently observed in aneuploid human cancer cells12,13,14,21,22,23, MiDAS has been shown to occur in multiple eukaryotic cell types, including yeast24, chicken cells25, mouse cells21, and normal human cells14,26, particularly when the cells have been exposed to RS. Moreover, it has been demonstrated that MiDAS is a common feature of cancer cell lines, irrespective of whether their telomeres are maintained by telomerase or by the alternative lengthening of telomeres (ALT) mechanism12.
Given the significant association of MiDAS with RS and genome instability in cancer cells, it is important to understand the mechanism underlying this unusual form of DNA replication. To date, it is clear that the occurrence of MiDAS involves several pathways and a diverse set of proteins (reviewed in ref. 27). Briefly, using CFSs as an example, the FANCD2-FANCI protein complex forms foci at CFS loci in the late G2 phase and mitosis when cells are challenged with RS in S phase16. These FANCD2-FANCI foci co-localize with SLX4 and can serve as a surrogate marker of the location of CFSs in G2/M phase cells14. Upon mitotic entry, the phosphorylation of EME1 promotes the association of MUS81-EME1 with SLX4 at under-replicated CFS loci13,14,28,29, which then promotes MiDAS in prophase and early prometaphase14. It has been demonstrated that MiDAS requires SLX4, MUS81-EME1, the non-catalytic subunit of DNA polymerase δ, POLD3 (Pol32 in yeast), RAD5213,14,30, and the PIF131 and RTEL121 helicases. Furthermore, it was reported recently that MiDAS requires the action of at least two different DNA polymerases; Pol δ and Pol ζ, the latter of which is not required for bulk S-phase replication32. Why there is a requirement for the use of two polymerases in MiDAS remains to be determined, but it could reflect the sequential use of two polymerases during the same process or the fact that DNA synthesis during MiDAS can reflect an ongoing involvement of at least two separate processes. For example, at least a proportion of MiDAS events require RAD52, although these events can apparently proceed without the central recombinase in homologous recombination (HR), RAD5113,33. Nevertheless, RAD51 is essential for a DNA damage response (DDR)-dependent form of MiDAS at non-CFS loci34. It has also been demonstrated that MiDAS can occur at folate-sensitive fragile site loci, including at the FRAXA locus harboring an abnormally expanded CGG trinucleotide repeat sequence. In this case, however, the DNA synthesis is fully dependent on RAD5126.
It is well documented that the DNA synthesis associated with MiDAS can often occur on only one of the two sister-chromatids (∼50% of MiDAS at CFSs and ∼70% of MiDAS at FRAXA with CGG expansion), while the remaining MiDAS is found on both chromatids or in a complex pattern13,26. These data indicate that at least some of the MiDAS events apparently arise via a conservative form of DNA synthesis that is distinct from conventional semi-conservative DNA replication occurring in S-phase. One proposal is that this conservative synthesis proceeds via break-induced replication (BIR), a recombination-based form of DNA repair that has been studied most extensively in yeast35,36. In yeast, BIR requires Rad51, Pol32 and the Pif1 helicase, and is an alternative pathway for repairing one-ended double-strand breaks (DSBs) generated by, for example, the cleavage of a replication fork by the SLX4-MUS81-EME1 complex. These breaks cannot be repaired using conventional DSB repair37 without the inadvertent generation of genomic rearrangements. BIR is initiated following DNA end-resection that generates a ssDNA overhang, which can then act as a primer to anneal to or invade into a region of homology and promote DNA synthesis. In human cells, BIR requires the function of POLD3, RAD51, RAD52, and the PIF1 helicase31,38, which is akin to the requirements for BIR in yeast. It is well-established that end-resection of the 5’ terminated DNA strand is required for all HR processes, including single-strand annealing (SSA), synthesis-dependent strand annealing (SDSA), the canonical double Holliday junction repair (DSBR), long-tract gene conversion (LTGC) and BIR37,39. In yeast, DNA end-resection comprises two steps: short-range resection catalyzed by Mre11-Rad50-Xrs2 and Sae2 (CtIP in humans), and long-range resection promoted by either Sgs1-Dna2 or Exo139.
Considering that resection pathways are highly conserved between yeast and humans, we hypothesized that DSB end-resection factors would likely also be required to promote MiDAS. Our results indicate that many of the canonical DSB end-resection factors, including MRE11, CtIP, BRCA1, and DNA2 contribute significantly to MiDAS. Intriguingly, while the BLM helicase (Sgs1 homolog) is implicated as the primary factor cooperating with DNA2 for long range resection in most cases of HR in human cells, we find that it is the WRN helicase, and not BLM, that is essential for MiDAS. These data reveal a specific role for WRN in the maintenance of genome stability in human cells undergoing RS, which may have implications for the pathogenesis of the premature aging condition, Werner’s syndrome, caused by mutations in WRN.
Results
MRE11 is essential for MiDAS
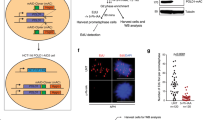
Amongst the factors that promote DSB end-resection, MRE11 is the catalytic component of the MRE11-RAD50-NBS1 (MRN) complex, which is one of the first proteins to be recruited to DSBs, and is required both for initiation of resection and for promoting the activation of ATM (reviewed in ref. 37). MRN acts as both a 3’ to 5’ exonuclease and an endonuclease. Because the processing of stalled replication forks by endonucleases associated with SLX4 leads to the formation of one-ended DSBs40,41, it seemed plausible that MRE11 might act to initiate MiDAS through DSB end-resection to facilitate the recruitment of the DNA synthesis machinery needed for MiDAS. Since MRE11 is essential for viability in human cells and plays roles at different stages of the cell cycle, it can be confusing to interpret results obtained by long-term siRNA deletion of MRN. For this reason, we established an HCT116-MRE11-AID ‘degron’ cell line following a previously published genome editing methodology42. This cell line shows stable expression of OsTIR1 (an auxin-binding receptor) and has both alleles of MRE11 fused in frame with a mAID-Clover (mAC) sequence (Supplementary Fig. 1a). These two features allow for degradation of MRE11 within only 30 minutes following treatment of the cells with auxin (IAA) (Supplementary Fig. 1a–d). We could also show that auxin-mediated degradation of MRE11 is comparable with that of siRNAs targeting MRE11 (Supplementary Fig. 1e–f). Subsequently, we used these cells to assess the role of MRN in MiDAS. For this, we quantified EdU incorporation in early mitosis in the HCT116-MRE11-AID degron cell line following an established protocol43. We observed that the frequency of MiDAS decreased significantly following short-term depletion of MRE11 (Fig. 1a–d). An equivalent observation was made when the HCT116-MRE11-AID degron cells were treated with Mirin, an inhibitor of MRE11’s exonuclease activity44, which was only added as cells entered mitosis (Fig. 1e–g). As another specificity control, we used siRNAs to deplete MRE11 in two different cell lines, HeLa and U2OS, with equivalent results (Supplementary Fig. 2). Because MiDAS is proposed to be a rescue strategy for cells to survive following RS, we assessed whether MRE11 degradation or inhibition could lead to reduced cell survival by performing colony formation assays with mitotic cells harvested after MRE11 degradation (HCT116-MRE11-AID cells; Supplementary Fig. 3a–d), or Mirin treatment (U2OS cells; Supplementary Fig. 3e–g). Our results indicate that cell survival is compromised following RS when MRE11 function is inhibited specifically in mitosis.
a Experimental workflow for analyzing MiDAS in HCT116 MRE11-AID cells following APH treatment. To enrich mitotic cells, cells were treated with RO3306 for 6 hours, and then released into M phase with medium containing EdU, and with (or without) IAA for 30 minutes, which can induce rapid degradation of MRE11. Mitotic cells were then harvested by mitotic shake-off before being fixed and analyzed for MiDAS. b Western blot analysis of the expression of MRE11 following a one-hour incubation with IAA. GAPDH was used as loading control. Each experiment was repeated independently with similar results at least three times. Representative immunofluorescence images (c) and quantification (d) of prometaphase cells (HCT116 MRE11-AID) showing MiDAS. e Experimental workflow for analyzing MiDAS in HCT116 MRE11-AID cells following APH treatment in S-phase and inhibition of MRE11 function by mirin in early mitosis. Representative immunofluorescence images (f) and quantification (g) of prometaphase cells (HCT116 MRE11-AID) with MiDAS. h Experimental workflow for analyzing MiDAS in U2OS cells following APH treatment in S-phase and inhibition of MRE11 function by MRE11 inhibitors PFM01 or PGM39 in early mitosis. Representative immunofluorescence images (i) and quantification ( j) of prometaphase cells (U2O) with MiDAS. In all cases, ongoing MiDAS was labeled using EdU (red), and DNA was stained with DAPI (blue). Scale bars, 10 μm. In each quantification, data are presented as mean ± s.e.m. of 3 independent experiments. n = 150: 50 cells were analyzed in each condition in each replicate. In total, 150 cells were analyzed for each condition. Statistical p values were calculated using two-tailed non-parametric Mann–Whitney tests and indicated in quantification graphs. Source data are provided as a Source Data file.
Next, we investigated whether the endonuclease and/or the exonuclease activity of MRE11 is required for MiDAS. For this, we exposed U2OS cells to the PFM01 or PFM39 inhibitors of MRE11, which are specific for the endonuclease and exonuclease activity respectively. These inhibitors were applied to the cells only at the end of G2 phase and in early mitosis (for a total 90 minutes) to avoid perturbation of S-phase. Interestingly, we observed a significant decrease in the frequency of MiDAS with both MRE11 inhibitors (Fig. 1h–j). Given that MRE11 works in the complex with NBS1 and RAD50, and functions at a very early stage in the DSB end-resection process, we investigated whether depletion of MRE11 influences the recruitment of other factors required for HR to the chromatin following RS. We observed that depletion of MRE11 in U2OS cells led to a reduction in the chromatin bound fraction of NBS1, RAD50, RPA, CtIP, BRCA1, DNA2, WRN, BLM, RAD52, RAD51 and POLD3 to different degrees in early mitotic cells. Significantly, however, there was no effect on the association of EXO1 (Fig. 2). In addition, we could verify this finding by treating these cells with a MRE11 inhibitor (PFM39) at G2/M phase boundary (in total 2 hours) (Supplementary Fig. 4). Taken together, these data demonstrate that both the endonuclease and exonuclease activities of MRE11 are required for efficient MiDAS to be conducted in response to RS, and that the function of MRE11 is required to promote the chromatin association of several end-resection and MiDAS factors. These data are consistent with a requirement for the nuclease activities of MRE11 in promoting MiDAS via DSB end-resection.
a Experimental workflow of cell synchronization for analyzing the chromatin-bound proteins at different stages of the cell cycle following siRNA to deplete MRE11 and APH treatment in S-phase. b Western blot analysis of soluble or chromatin-bound fraction of BRCA1, WRN, RAD50, BLM, DNA2, CtIP, phosphorylated CtIP, NBS1, POLD3, RAD52, RAD51, and RPA32 at the indicated cell cycle phases. A black arrow indicates the band of BLM detected with BLM antibody, while the star indicates a non-specific band. GAPDH or Histone H3 was used as loading control for soluble and chromatin bound proteins respectively. This experiment was repeated independently with similar results for three times. Source data are provided as a Source Data file.
Our data indicate that DSB end-resection factors play a role in MiDAS. However, it was not clear whether MRE11 might be involved in catalyzing not only end-resection, but also the initial cleavage of the stalled replication forks prior to MiDAS. Previously, it was always assumed that this fork processing required SLX4-associated nucleases29,41. To address this, we quantified the level of DSBs on metaphase chromosomes in U2OS cells following inhibition of MRE11 in early mitosis. The DSBs at CFSs and other late-replicating regions were detected by the co-localization of foci for γH2AX and FANCD2 on metaphase chromosomes, as has been shown previously13,14. For this analysis, cells were treated with Mirin during early mitosis following a 16-hour treatment of APH (Supplementary Fig. 5a). We observed that there was no difference in the frequency of co-localization of γH2AX and FANCD2 following exposure of cells to Mirin in mitosis (Supplementary Fig. 5b, c). In contrast, there was a significant reduction in the frequency of co-localization of γH2AX and FANCD2 following depletion of MUS81 (Supplementary Fig. 5d–f), which is consistent with a role of the SLX4/MUS81-EME1 complex in the generation of one-ended DSBs at replication forks14.
CtIP and BRCA1 are required for MiDAS
Next, we investigated whether CtIP and/or BRCA1 are required for MiDAS, since both are known to stimulate the activity of MRN complex for cells to commit and promote homology-directed repair mechanisms instead of classical NHEJ (c-NHEJ) to repair DSBs37. It is known that CtIP associates with MRN complex in response to the formation of DSBs, where it promotes MRE11 endonuclease activity to initiate DNA end-resection and the generation of a short stretch of 3’-ssDNA45,46,47. Together with its partners PALB2 and BARD1, BRCA1 promotes ubiquitination of histones in the vicinity of DSBs48,49,50. BRCA1 also cooperates with MRN complex and forms a complex with CtIP, which can promote end-resection in the early steps of at least some sub-pathways of HR, such as the synthesis-dependent strand annealing (SDSA) pathway51,52,53.
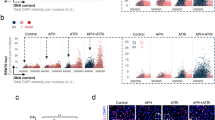
We treated U2OS cells with specific siRNAs to deplete either CtIP or BRCA1 and then harvested cells during prometaphase for MiDAS analysis following APH treatment for 18 hours (Fig. 3a). We observed that, following CtIP depletion, there was a significant reduction in the frequency of MiDAS, suggesting that CtIP might co-operate with MRE11 to facilitate MiDAS (Fig. 3b–d). To investigate whether there is a requirement for BRCA1 in MiDAS, we analyzed cells deficient in either BRCA1 or BARD1, since BARD1 is an obligate BRCA1 binding partner. Depletion of BRCA1 reduced the frequency of MiDAS (Fig. 3e–g), as did the degradation of BARD1 in early mitosis in an HCT116-BARD1-AID degron cell line54 (Supplementary Fig. 6a–d). Furthermore, we confirmed the role of BRCA1/BARD1 in MiDAS by treating U2OS cells with BRCA1-(BRCT)2 protein interaction inhibitor BRCA1-IN-255 (Supplementary Fig. 6e–g).
a Experimental workflow for analyzing MiDAS in U2OS cells following APH treatment in S-phase and depletion of CtIP or BRCA1 by siRNAs. b Western blot analysis of the expression of CtIP at the end of G2 phase. Tubulin was used as loading control. This experiment was repeated independently with similar results at least three times. Representative immunofluorescence images (c) and quantification (d) of prometaphase cells showing MiDAS. e Western blot analysis of the expression of BRCA1 at the end of G2 phase. Tubulin was used as loading control. This experiment was repeated independently with similar results at least three times. Representative immunofluorescence images (f) and quantification (g) of prometaphase cells (U2OS). In all cases, ongoing MiDAS was labeled using EdU (red), and DNA was stained with DAPI (blue). Scale bars, 10 μm. In each quantification, data are presented as mean ± s.e.m. of at least 3 independent experiments. n = 150: 50 cells were analyzed in each condition in each replicate. In total, 150 cells were analyzed for each condition. Statistical p values were calculated with two-tailed non-parametric Mann–Whitney tests and indicated in quantification graphs. Source data are provided as a Source Data file.
Next, we analyzed the chromatin recruitment of DSB end resection factors in both asynchronous and mitotic cells following siRNA-mediated knockdown of BRCA1 or CtIP (Supplementary Fig. 7). We observed that factors associated with MiDAS, such as RAD52 and POLD3, are significantly depleted from the chromatin fraction specifically in mitotic cells in both cases. Furthermore, the chromatin recruitment of other DSB end resection factors, including BLM, RAD51, WRN, DNA2, RPA32, and, unexpectedly, MRE11, are all reduced to different degrees upon BRCA1 knockdown. This was also the case for most of these factors (with the exceptions being BLM and RAD51) in the case of CtIP knockdown. These data suggested that BLM and RAD51 are very unlikely to function in the same pathway as that of CtIP in MiDAS. It was also observed that BRCA1 knockdown impairs CtIP chromatin loading, while CtIP knockdown reduces BRCA1 chromatin loading in mitotic cells. Taken together, these analyses suggest that CtIP, BRCA1 and MRE11 act together to promote MiDAS.
DNA2 and WRN, but not EXO1 or BLM, promote MiDAS
In the classical HR pathway, once short DNA overhangs (of a few hundred nucleotides) are generated by MRN/CtIP, they are subjected to further resection by either EXO1 or DNA2 acting together with the BLM or WRN helicases45, which leads to longer overhangs of up to several kilobases56,57. EXO1 is a 5’ to 3’ exonuclease that can degrade 5’-terminating DNA strands within dsDNA58. In human cells, it has been observed that EXO1 activity can be stimulated by MRN and BLM59. DNA2 is a bifunctional enzyme containing a RecB-like nuclease domain and a superfamily I helicase domain60,61,62,63. In contrast to MRE11, DNA2 only degrades ssDNA64. Studies in yeast indicated that the helicase domain of Dna2 cannot unwind dsDNA unless its nuclease activity is inactivated65,66. Therefore, it is generally thought that DNA2 requires a separate helicase as a partner when resecting the 5'-end of a dsDNA end. In yeast, this partner is Sgs1, while in human cells DNA2 can be partnered by either of the two Sgs1 homologs, BLM or WRN56,57,67,68,69.
Based on this background, we analyzed whether any of the long-range DNA end-resection factors are required for MiDAS. Using validated siRNAs, we observed that there was a dramatic decrease in the frequency of MiDAS following DNA2 depletion (Fig. 4a–d). The effect of DNA2 on the efficiency of MiDAS was confirmed by disrupting DNA2 function using a short-term exposure of mitotic cells to a small molecule inhibitor, C5, that targets the nuclease activity of DNA270 (Fig. 4e–g). For analysis of EXO1, we analyzed a U2OS cell line in which the EXO1 gene had been inactivated using CRISPR-Cas971. We observed that MiDAS was not significantly affected in the EXO1 knockout cells following treatment with APH (Fig. 4h–k). These data indicate that the DNA2 branch of the long-range resection process is necessary for MiDAS and cannot be substituted by the alternative EXO1-dependent branch.
a Experimental workflow for analyzing MiDAS in U2OS cells following APH treatment in S-phase and depletion of DNA2 by siRNAs. b Western blot analysis of the expression of DNA2 at the end of G2 phase. Tubulin was used as loading control. This experiment was repeated independently with similar results at least three times. Representative immunofluorescence images (c) and quantification (d) of prometaphase cells (U2OS). e Experimental workflow for analyzing MiDAS in U2OS cells following APH treatment in S-phase and inhibition of DNA2 activity in early mitosis using the inhibitor C5. Representative immunofluorescence images (f) and quantification (g) of prometaphase cells with MiDAS. h Experimental workflow for analyzing MiDAS in U2OS cells with or without EXO1 expression following APH treatment in S-phase. i Western blot analysis of the expression of EXO1 in EXO1 (+/+) and EXO1 (−/−) U2OS cells. Tubulin was used as loading control. This experiment was repeated independently with similar results at least three times. Representative immunofluorescence images (j) and quantification (k) of prometaphase cells (U2OS). In all cases, ongoing MiDAS was labeled using EdU (red), and DNA was stained with DAPI (blue). Scale bars, 10 μm. In each quantification, data are presented as mean ± s.e.m. of at least 3 independent experiments. n = 150: 50 cells were analyzed in each condition in each replicate. In total, 150 cells were analyzed for each condition. Statistical p values were calculated with two-tailed non-parametric Mann–Whitney tests and indicated in quantification graphs. Source data are provided as a Source Data file.
Given the requirement for DNA2 in MiDAS, we investigated whether the BLM and/or WRN helicases partner DNA2 in this process. First, we analyzed a pair of isogenic U2OS cell lines, one of which has the BLM gene inactivated by gene targeting72 (Supplementary Fig. 8a–b). We observed that loss of BLM had no significant effect on the frequency of MiDAS (Supplementary Fig. 8b–d). We confirmed that BLM plays no role in MiDAS by depleting the protein from parental U2OS cells using siRNAs (Supplementary Fig. 8e–h). Hence, we then analyzed WRN, which was depleted using siRNAs. In this case, we observed a significant decrease in MiDAS. Depletion of WRN combined with loss of BLM had no additive effects on the frequency of MiDAS (Supplementary Fig. 8c–d). To validate these findings further, we took the advantage of a recently established WRN degron cell line HCT116-WRN-BdTAG in which WRN can be degraded rapidly and specifically by incubation of the cells with AGB-173, and performed MiDAS analysis in combination of depletion of BLM with siRNA (Fig. 5a). Our results demonstrated that, indeed, when these cells were challenged with APH and BLM depletion, MiDAS was not affected. However, when WRN was degraded at G2/M phase boundary, MiDAS was reduced significantly, whether or not BLM was present (Fig. 5b–d). Furthermore, we confirm WRN’s role in MiDAS by performing MiDAS analysis in combination with treating U2OS cells with a validated inhibitor of WRN helicase activity, HRO76174, which was applied only at G2/M phase boundary (for a total 90 minutes). Our results indicate that HRO761 reduced MiDAS significantly (Fig. 5e–g). Taken together, these data demonstrate that DNA2 and WRN, but not BLM, are required for MiDAS.
a Experimental workflow for analyzing MiDAS in HCT116 WRN-BdTAG cells coupled with siRNA treatment to deplete BLM. b Western blot analysis of BLM and WRN following siRNA transfection in HCT116 WRN-BdTAG cells where WRN could be degraded upon 30 minutes treatment with AGB1. A black arrow indicates the band of BLM detected with BLM antibody, while the star indicates a non-specific band. GAPDH was used as loading control. This experiment was repeated independently with similar results at least three times. Representative immunofluorescence images (c) and quantification (d) of prometaphase cells with MiDAS. e Experimental workflow for analyzing MiDAS in U2OS cells following APH treatment in S-phase inhibition of WRN helicase activity by HR0761 at late G2 and early M phase (1.5 hours in total). Representative immunofluorescence images (f) and quantification (g) of prometaphase cells showing MiDAS. In all cases, ongoing MiDAS was labeled using EdU (red), and DNA was stained with DAPI (blue). Scale bars, 10 μm. In each quantification, data are presented as mean ± s.e.m. of 3 independent experiments. n = 150: 50 cells were analyzed in each condition in each replicate. In total, 150 cells were analyzed for each condition. Statistical p values were calculated with two-tailed non-parametric Mann–Whitney tests and indicated in quantification graphs. Source data are provided as a Source Data file.
WRN has been shown to promote the survival of cancer cells with microsatellite instability (MSI)75,76,77,78,79,80. Therefore, we addressed whether WRN’s contribution to MiDAS might be selective for MSI cancer cells. For this, we quantified MiDAS in two WRN degron cell lines (SW620-WRN-BdTAG and HCT116-WRN-BdTAG). In both cases, WRN can be degraded rapidly by incubation of the cells with AGB-173. For the analysis of MiDAS in the microsatellite stable SW620-WRN-BdTAG cell line, we carried out conventional MiDAS analysis (Fig. 6a) and observed that, upon WRN degradation in early mitosis, there was an approximately a 40% decrease in the frequency of MiDAS (Fig. 6b–d). These data indicate that cells do not require microsatellite instability to show impaired MiDAS in the absence of WRN. For the analysis of the HCT116-WRN-BdTAG cell line that has microsatellite instability (MSI), in order to enrich mitotic cells, we adopted a 16-hour thymidine treatment to synchronize cells at the G1/S phase boundary (Fig. 6e and Supplementary Fig. 9a–c) before releasing the cells into S-phase in the presence of APH. Subsequently, WRN was degraded in late G2 and early M phase by AGB-1 treatment before MiDAS was quantified (Fig. 6e and Supplementary Fig. 9d–f). A similar level of reduction in MiDAS was seen in these MSI cells as in the microsatellite stable cells (Fig. 6e–h).
a Experimental workflow for analyzing MiDAS in SW620 WRN-BdTAG cells following APH treatment in S-phase and degradation of WRN with AGB1 treatment for one hour at late G2 phase early M phase. b Western blot analysis of the expression of WRN at the end of G2 phase. Tubulin was used as loading control. This experiment was repeated independently with similar results at least three times. Representative immunofluorescence images (c) and quantification (d) of prometaphase cells (U2OS). e Experimental workflow for analyzing MiDAS in HCT116 WRN-BdTAG cells following APH treatment in S-phase and degradation of WRN with AGB1 treatment for one hour at late G2 phase early M phase. This batch of cells were also subject to transfection with plasmids expressing wild type (WT) or mutant WRN with inactivation of exonuclease function (WRN E84A), or helicase function (WRN K577M), or both (WRN E84A/K577M). Representative immunofluorescence images (f) and quantification (h) of prometaphase cells with MiDAS. g Western blot analysis of the expression of endogenous WRN and Myc-tag of exogenous expressed WRN-myc. Tubulin was used as loading control. This experiment was repeated independently with similar results at least three times. In all cases, ongoing MiDAS was labeled using EdU (red), and DNA was stained with DAPI (blue). Scale bars, 10 μm. In each quantification, data are presented as mean ± s.e.m. of at least 3 independent experiments. n = 150: 50 cells were analyzed in each condition in each replicate. In total, 150 cells were analyzed for each condition. Statistical p values were calculated with two-tailed non-parametric Mann–Whitney tests and indicated in quantification graphs. Source data are provided as a Source Data file.
Next, we analyzed whether the exonuclease domain and/or the helicase domain of WRN is required for efficient MiDAS. For this, we ectopically expressed wild type WRN or previously validated mutant versions of WRN75 in these cells (Fig. 6e). We observed that the expression of wild-type (WT) WRN could rescue MiDAS upon WRN degradation in early M phase. In contrast, neither the E84A exonuclease dead mutant, nor the K577M helicase dead mutant (or indeed the E84A/K577M double mutant) could rescue MiDAS upon WRN degradation in early M phase (Fig. 6f–h). Similar results were also shown in U2OS cells when their endogenous WRN was depleted using siRNA targeting the 5’ untranslated region (5’ UTR) of WRN, and transfected with plasmids expressing either WT or mutant forms of WRN (Supplementary Fig. 10). Furthermore, we performed colony formation assays using SW620 and HCT116 WRN-BdTAG cell lines to evaluate the impact of MRE11 inhibition or WRN inhibition on cell survival, particularly when the inhibition is applied only at the MiDAS pathway (2 hour at G2/M phase boundary) (Supplementary Fig. 11). The results from this set of experiments demonstrated that, first, APH treatment alone could cause reduced cell survival, which is consistent with the notion that increased RS is detrimental to cell survival; second, SW620 or HCT116 responded to treatment with MRE inhibitor or WRN inhibitor very differently, which is predictable considering each cell line has a very different spectrum of genetic changes, which is also consistent with published data that MSI+ve cells are vulnerable to WRN inhibition74; third, the treatment of combination of APH and MRE11 inhibition, or APH and WRN inhibition reduced the cell survival on both cell lines, which is consistent with our results above. Together, these data indicate that both the helicase and the exonuclease activities of WRN are required for its function in the MiDAS pathway, which is not affected by the MSI status of the cells.
Previous studies have indicated that WRN is required for telomere maintenance in cells utilizing the ALT mechanism, which appears to rely on a BIR-like process81,82,83. Given that the U2OS and SW620 cells analyzed here depend on ALT, we considered whether telomeric MiDAS might be particularly affected by loss of WRN. To address this, we analyzed MiDAS in telomerase-positive HeLa cells in which WRN was depleted. However, we observed a similar reduction in MiDAS following WRN depletion in this cell line (Supplementary Fig. 12). Nevertheless, we analyzed whether WRN might still promote MiDAS more prominently at telomeres. For this, we performed MiDAS analysis in combination of telomeric-PNA FISH for assessing its co-localization with telomeres, or immunofluorescence with FANCD2 antibody for assessing its co-localization with CFSs in U2OS cells. Our results indicate that depletion of WRN has a very similar effect on the frequency of MiDAS at telomeres (Fig. 7a–g) and at CFS loci (Fig. 7h–j). Hence, WRN is required for protection of cancer cells against RS genome-wide regardless of their telomerase status.
a Experimental workflow for analyzing MiDAS in metaphase spreads of U2OS cells coupled with telomere detection with FISH using a telomere PNA probe, and IF analysis of co-localization of MiDAS and FANCD2. b Western blot analysis of the expression of WRN at the end of G2 phase following siRNA and APH treatment. Tubulin was used as loading control. This experiment was repeated independently with similar results at least three times. c Representative immunofluorescence images, and quantification (d–g) of MiDAS (red) and telomeres (green) in metaphase chromosomes. n = 90: 30 cells were analyzed in each condition in each replicate (90 cells were analyzed for each condition in total) in metaphase chromosome analysis. h Representative immunofluorescence images, and quantification (i) of MiDAS (red) and FANCD2 (green) in prometaphase cells. DNA was stained with DAPI (blue). Scale bars, 10 μm. In each quantification, data are presented as mean ± s.e.m. of at least 3 independent experiments. n = 120: 40 cells were analyzed in each condition in each replicate (120 cells were analyzed for each condition in total) in prometaphase cell analysis. Statistical p values were calculated with two-tailed non-parametric Mann–Whitney tests in d, e, i, and unpaired two-sided t-tests in f and g. P values are indicated in the quantification graphs. Source data are provided as a Source Data file.
Loss of end-resection factors leads to genome instability
Earlier studies indicated that when the MiDAS pathway is suppressed, cells display elevated mitotic abnormalities, as well as micronuclei (MN) and 53BP1 bodies in newly born daughter cells11,12,17. To evaluate the consequences of a decrease in the efficiency of MiDAS caused by the impairment in DSB end resection, we examined MN and 53BP1 body formation in pairs of G1 daughter cells in the new cell cycle. We observed that, upon depletion of BRCA1, DNA2, MRE11, or WRN, there was a significant increase in the frequency of MN in G1 phase cells in the following cell cycle, while the frequency of 53BP1 bodies was unchanged or decreased (Fig. 8a–e). To verify these data, inhibitors of MRE11 (PMF39) or DNA2 (C5) were applied to cells only at the G2/M phase boundary to inhibit enzyme function in early mitosis (Fig. 8f). We observed that the frequency of 53BP1 bodies and MN were both increased in comparison to the controls (Fig. 8g–i), indicating increased genome instability. The lack of an effect of CtIP depletion on markers of genome instability might reflect the incomplete removal of the protein using siRNA treatment in comparison with the use of small molecule inhibitors.
a Experimental workflow for analyzing 53BP1 bodies or MN in the next G1 phase cells following APH treatment in S-phase and depletion of BRCA1, DNA2, CtIP, MRE11, or WRN by siRNAs in U2OS cells. b Western blot analysis of the expression of BRCA1, DNA2, CtIP, MRE11, or WRN at the end of G2 phase. Tubulin was used as loading control in all cases. This experiment was repeated independently with similar results at least three times. Representative immunofluorescence images (c) and quantification of 53BP1 bodies (d) or MN (e). DNA was stained with DAPI (blue). 53BP1 was detected with antibody and is shown red. MN was shown by DAPI and indicated by white arrows. Scale bars, 10 μm. f Experimental workflow for analyzing 53BP1 bodies or MN in the next G1 phase cells following APH treatment in S-phase and inhibition of the function of MRE11 or DNA2 by PMF39 or C5 respectively. Representative immunofluorescence images (g) and quantification of 53BP1 bodies (h) or MN (i). DNA was stained with DAPI (blue). 53BP1 was detected with antibody and is shown red. MN was shown by DAPI and indicated by white arrows. Scale bars, 10 μm. In each quantification, data are presented as mean ± s.e.m. of at least 3 independent experiments. n = 150: 50 pair of new G1 cells were analyzed in each condition in each replicate. In total, 150 pair of new G1 cells were analyzed for each condition. Statistical p values were calculated with two-tailed non-parametric Mann–Whitney tests and indicated in quantification graphs. Source data are provided as a Source Data file.
Discussion
In this study, we adopted a candidate approach to examine the involvement of a panel of seven DSB HR repair end-resection factors in MiDAS. Consistent with a model proposed previously that 50% or more of the MiDAS events might be conducted via a BIR-like pathway (reviewed in ref. 27), several key end-resection factors essential for the HR-based repair facilitate MiDAS; namely, MRE11, CtIP, BRCA1, and DNA2. Nevertheless, not all resection factors that play a role in canonical HR in the S/G2-phases are required for MiDAS. Most strikingly, it is generally accepted that HR in interphase proceeds via either an EXO1-dependent or a BLM/DNA2-dependent pathway56,69. However, neither EXO1 nor BLM plays any discernible role in MiDAS. Instead, it is the Werner helicase, WRN, that plays a crucial role in MiDAS. To our knowledge, this is a newly defined function for WRN in DNA repair/recombination that cannot be substituted by BLM.
These findings are potentially relevant to the etiology of Werner’s syndrome (see below) and support the notion that MiDAS is an atypical DNA repair pathway. To verify our findings collectively, we performed RPA foci analysis following MRE, DNA2 or WRN inhibition in early M phase in U2OS cells in parallel. Our data showed that, indeed, following APH treatment in S phase, when cells were only treated with inhibitors to MRE11, DNA2, or WRN for only one hour at G2/M phase boundary, the RPA foci were significantly reduced in all the treated conditions compared with the untreated control cells (Fig. 9a–c). Furthermore, taking advantage of an antibody that can detect WRN in fixed cells, we performed IF with this antibody and a FANCD2 antibody to evaluate the recruitment of WRN to the loci undergoing RS. Our results indicated that, consistent with the previous finding that WRN is recruited to chromatin in all the phases of the cell cycle74, its co-localization with FANCD2 is present in G2 phase and M phase, but was significantly increased when cells are treated with APH in G2 phase or early M phase (Fig. 9d–f).
a Experimental workflow for analyzing RPA foci following APH treatment in prometaphase cells coupled with short inhibition to MRE11 (with PFM39), DNA2 (with C5) or WRN (with HRO761) at G2/M boundary. Representative immunofluorescence images (b) and quantification (c) of RPA foci (red). DNA was stained with DAPI (blue). Scale bars, 10 μm. n = 102: over 30 cells were analyzed in each condition in each replicate. In total, 102 cells were analyzed for each condition. d Experimental workflow for analyzing WRN’s localization at loci with replication stress marked by FANCD2 in G2 and early M phase with or without the treatment of APH. Representative immunofluorescence images (e) and quantification of foci (f) that have WRN (red) and FANCD2 (green) co-localization in the nucleus. DNA was stained with DAPI (blue). Scale bars, 10 μm. In each quantification, data are presented as mean ± s.e.m. of at least 3 independent experiments. n = 105: over 30 cells were analyzed in each condition in each replicate. In total, 105 cells were analyzed for each condition. Statistical p values were calculated with two-tailed non-parametric Mann–Whitney tests and indicated in quantification graphs. Source data are provided as a Source Data file.
It is worth noting that our data on the involvement of BRCA1 in MiDAS could be confounded by the fact that BRCA1 promotes MUS81 recruitment at collapsed forks during BIR, which is correlated with reduced EdU foci in mitosis84. In our study, for analyzing the involvement of BRCA1 in MiDAS, in addition to using siRNA for depletion BRCA1, we employed a degron cell line to rapidly degrade its interaction partner BARD1 at the boundary of G2/M phase, or an inhibitor to inhibit BRCA1 and proteins with BRCT domain interaction (Supplementary Fig. 6). Our results indicate that BRCA1 needs to interact with BARD1 to promote MiDAS, which may not be the case for its action in recruiting MUS81 at a BIR site. Further analysis is warranted to clarify BRCA1’s role in this pathway.
Despite the clear association between DSB end-resection factors and MiDAS, questions remain about how exactly end-resection can occur in MiDAS. For example, does MiDAS need ATM activation? Based on the fact that mitotic kinases such as CDK1 and PLK1 trigger inhibitory phosphorylation on several DDR proteins, such as XRCC1, 53BP1, RAD51, BRCA2 and RPA in late G2/M, it is most likely that the conventional DNA DSB repair pathways are normally suppressed during mitosis85,86,87,88,89,90,91. This suggests that processing of the stalled forks might sometimes occur in the G2 phase, although the requirement for TRAIP, which unloads remaining replisomes from the chromatin at the entry into mitosis might suggest otherwise. In the future, it would be also interesting to investigate mechanistically how WRN could contribute to MiDAS, considering that both its helicase and exonuclease activities are required for MiDAS. Does it cooperate with DNA2 in promoting MiDAS (as most models of resection would suggest) or does it act alone or via an interaction with another protein?
It should be noted that inactivation of MRE11, CtIP, BRCA1, DNA2, or WRN in early mitosis consistently only suppresses MiDAS maximally by about 75%. Although this observation might reflect incomplete depletion of the protein in some cases, it is possible also that a proportion of the MiDAS events do not proceed via a pathway in which there is a requirement for DNA end-resection. Indeed, this could be partially explained by the fact that approximately 50–75% of MiDAS events are conservative, while the others are either semi-conservative or a ‘complex type’13. Future analysis of the pattern of MiDAS foci on metaphase chromosomes following the depletion or inactivation of the relevant end-resection factors would be required to address this issue. Based on all data obtained from this study, we propose a model for the involvement of DSB end resection factors in MiDAS (Fig. 10).
In G2 phase, at loci where converging replication forks have not yet merged or are stalled due to replication stress in the human genome, the FANCD2 and SLX4 proteins are recruited. When cells enter the prophase of mitosis, the TRAIP ubiquitin ligase unloads the replisome, and DNA structure-specific endonucleases (SSEs) are recruited to the SLX4 scaffold. The stalled fork is then cleaved by one of the SLX4-associated SSEs to create a one-ended double strand break (DSB). Together with BRCA1, CtIP stimulates MRE11’s endonuclease activity and then exonuclease activity leading to a 5’-3’ end resection and creation of 3’ single-stranded DNA (ssDNA) overhang. The overhang continues to be extended, possibly with the cooperation of DNA2 and WRN. In the case of WRN, although it has been proposed to co-operate with DNA2 in long-range DSB resection in in vitro assays67, our results indicated that both its 3’-5’ exonuclease and 3’-5’ helicase activity are required for MiDAS. Subsequently, the action of WRN and DNA2 establishes a ssDNA overhang that is sufficiently long and bound by RPA. RAD52 then promotes the annealing of complementary single-stranded DNA (ssDNA) by interacting with RPA, and promoting the removal of RPA from ssDNA102. The RAD52-coated DNA promotes ssDNA annealing to the complementary parent strand, making it possible for POLD3-dependent MiDAS to occur in prophase and prometaphase.
Our results and conclusions also have implications for the etiology of Werner’s syndrome, a rare autosomal recessive disease with early onset signs of aging, and an increased incidence of cancer and diabetes caused by loss of function of WRN92,93. In comparison to the BLM helicase, whose primary cellular roles are suppressing sister chromatid exchanges and chromosomal aberrations including ultra fine DNA bridges (reviewed in ref. 94), WRN is crucial for maintaining replication fork stability and for DNA repair pathways that serve to promote telomere maintenance (reviewed in ref. 95). Here, we discovered an additional role for WRN in the MiDAS pathway for suppression of RS-induced challenges to genome maintenance that cannot be performed by BLM (Fig. 5 and Supplementary Fig. 8). This finding is in agreement with the role of the WRN-DNA2 complex in promoting long-range DNA end resection96,97. Moreover, our data indicate that the exonuclease and the helicase activities of WRN are both contributing to MiDAS (Fig. 6 and Supplementary Fig. 10). This begs the question of how WRN’s exonuclease function could promote MiDAS, given that the prevailing model of end-resection posits that only the WRN helicase function is required alongside DNA2. Previous studies indicated that the WRN exonuclease activity has a DnaQ-like proofreading activity that is stimulated by Ku70/8098, and is essential to promote an increase in the fidelity of Y-family translesion (TLS) polymerases in human cells99. In future studies, it would be most interesting to investigate whether Ku proteins or the other TLS family members play a role in MiDAS, particularly given the knowledge that REV1 and Pol ζ contribute to MiDAS32. Overall, given that both the exonuclease and helicase functions are inactivated in Werner patient cell lines100, it is plausible that defective MiDAS plays a role in the etiology of this disorder.
The clinical phenotypes associated with loss of either WRN or BLM are markedly different, which indicates at least one unique role for each of these RecQ helicases. We propose that a function for WRN via MiDAS in suppressing genome instability in difficult-to-replicate loci such as CFSs and telomeres might represent at least one of the unique roles of WRN. Given that WRN is implicated in supporting the ALT mechanism of telomere maintenance and MiDAS, we suggest it is plausible that a major role for WRN is to support BIR or a BIR-like process such as microhomology-mediated BIR. Indeed, our colony formation assays (Supplementary Fig. 11) showed that a WRN inhibitor strongly perturbs the survival of cells with MSI74. Nevertheless, cancer cells with no MSI are also vulnerable to WRN inhibition when they are challenged with RS.
Loss of function mutations in WRN are the cause of the premature aging disorder Werner’s syndrome (WS). Development of this disorder appears to require the loss of both the helicase and the exonuclease activities of WRN. Hence, any function assigned to WRN that might plausibly be responsible for suppressing WS needs to take account of the requirement for combined loss of both enzymatic activities. We have shown that WRN’s role in MiDAS requires both activities, indicating that the loss of MiDAS could potentially be the key function underlying the widespread premature aging features characteristic of WS. Further work will be required to test this hypothesis.
Methods
Cell lines and cell culture
The osteosarcoma cell line (U2OS), cervical carcinoma cell line (HeLa) and colorectal carcinoma cell line (HCT116) were obtained from the American Type Culture Collection (ATCC). The isogenic pair of U2OS BLM (+/+) and BLM knock out (BLM−/−) cell lines were kindly provided by Dr. E. L. Denchi (National Cancer Institute, NIH, Bethesda, USA)72. The isogenic pair of U2OS (EXO1 + /+) and EXO1 knock out (EXO1−/−) cells were kindly provided by Prof. P. Cejka (Università della Svizzera italiana, Bellinzona, Switzerland)71. The HCT116-BARD1-AID cell line was kindly provided by Prof. J. Ross Chapman (University of Oxford, UK)54. The HCT116-WRN-Bd-TAG and SW620-WRN-Bd-TAG cell lines were kindly provided by Prof. J.Rouse (University of Dundee, UK)73. All cell lines were authenticated by the corresponding vendors or establishers. In addition, metaphase chromosome analysis was performed to confirm that these cell lines had the expected ploidy and chromosome number.
All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM/Glutamax, ThermoFisher Scientific) supplemented with 10% FBS (ThermoFisher Scientific) and 1% pen/strep (ThermoFisher Scientific), at 37 °C in a humidified incubator with 5% CO2. Cells were regularly tested for mycoplasma (MycoAlert, Lonza) and shown to be negative.
To establish the HCT116:MRE11-mAID cells, a parental cell line expressing OsTIR1 (with puromycin resistance)101 was transfected with a CRISPR plasmid targeting the C-terminus coding region of the MRE11 gene (5’-CTGAGAAACATGCAAGATAC−3’) and two donor plasmids encoding mAID-EGFP and Neomycin or Hygromycin resistance respectively, following a published protocol42. After selecting clones in the presence of Neomycin (700 µg/mL) and hygromycin (100 µg/mL), the bi-allelic insertion was confirmed by genomic PCR. Subsequently the expression of the MRE11-mAID-EGFP protein was confirmed by Western blot analysis. The HCT116-MRE11-mAID cells were cultured in DMEM medium with addition of puromycin (0.8 μg/ml) and Neomycin (600 μg/ml) routinely for maintaining the constructs. The selected cell line could be confirmed to be from the parental HCT116 cell line judging by the consistency of morphology, phenotype, and chromosome numbers among the clones.
Cell treatment and mitotic DNA synthesis (MiDAS) analysis
To induce replication stress, cells were treated with either 0.3 or 0.4 μM APH, depending on the cell line, for the duration specified in the flow chart of each experiment. For MiDAS analysis, to enrich mitotic cells, asynchronous cells were arrested in the G2 phase using CDK1 specific inhibitor RO3306 (APExBIO; 7 µM) as shown in the flow chart of each experiment. Cells were then washed with pre-warmed (37 °C) PBS (ThermoFisher Scientific) three times within 5 minutes and subsequently released into pre-warmed (37 °C) culture medium containing EdU (ThermoFisher Scientific; 40 µM) for 30 minutes. Cells were then harvested by mitotic shake-off for immunofluorescence (IF) analysis following a protocol described previously43.
Other cell treatment
To silence the expression of genes of interests, cells were transfected with 80 nM siRNAs. The transfection was performed using Lipofectamine RNAimax transfection reagent (ThermoFisher Scientific), following the manufacture’s protocol. SiRNAs used in this study are listed and referenced in Supplementary Table 1.
To inhibit the function of proteins of interests, the following reagents are used: 3-Indoleacetic acid (IAA) (Santa Cruz Biotechnology, Cat. sc-254494; 500 µM), BromoTag® AGB1 (Biotechne, Cat. 7686; 500 nM), BromoTag® cis-AGB1 (Biotechne, Cat. 7687; 500 nM), Mirin (Sigma-Aldrich, Cat. M9948; 50 μM), C5 (MedChemExpress, Cat. HY-128729; 100 μM), PFM01 (MedChemExpress, Cat. HY-116770; 100 μM), PFM39 (MedChemExpress, Cat. HY- 120951; 100 μM), BRCA1-IN-2 (MedChemExpress, Cat. HY-100862; 100 μM), HRO761 (MedChemExpress, Cat. HY-148699; 10 μM). The duration and timing of the treatment of these reagents is described in corresponding figures.
To obtain prometaphase cells, asynchronous cells were arrested in the G2 phase using CDK1 specific inhibitor RO3306 (APExBIO; 7 µM), as described previously14. Cells were then washed with pre-warmed (37 °C) PBS (ThermoFisher Scientific) and subsequently released into pre-warmed (37 °C) culture medium for 30 minutes, which allowed cells to progress from G2 into prophase. Mitotic cells were collected by mitotic shake-off. For IF analysis, cells were subsequently seeded on poly-L-lysine-coated slides (Sigma Aldrich).
To obtain metaphase chromosomes, asynchronous cells were arrested in the G2 phase using the CDK1 specific inhibitor RO3306 (APExBIO; 7 µM), as described previously14. Cells were then washed with pre-warmed (37 °C) PBS and subsequently released into pre-warmed (37 °C) culture medium with addition of colcemid (100 ng/ml; ThermoFisher Scientific) for 1 hour, which allowed cells progress from G2 phase into metaphase. Mitotic cells were harvested by mitotic shake-off, swollen in pre-warmed (37 °C) hypotonic solution (10 mM Tris-HCl, pH 7,5, 10 mM NaCl, 5 mM MgCl2) for 20 minutes at 37 °C, and then fixed with methanol:acetic acid 3:1. The spreads were prepared by dropping swollen and fixed metaphase cells onto glass slides and then fixed in 4% PFA fixation buffer (4% PFA, 250 mM HEPES, 1x PBS, 0,4% Triton X-100) for 20 minutes at 4 °C.
To obtain anaphase cells, prometaphase cells were collected as described above and reseeded onto pre-warmed (37 °C), poly-L-lysine-coated slides (Sigma Aldrich) for an additional 20-30 minutes to allow their progression into anaphase.
To obtain G1 phase cells for analysis of micronuclei and 53BP1 foci after the treatment of APH in the previous S phase, prometaphase cells collected from mitotic-shake off were re-seeded onto pre-warmed slides coated with Poly-L-Lysine. Subsequently, the cells were incubated for an additional 3 hours to facilitate progression into the next G1 phase.
To synchronize cells at G1/S phase boundary, cells were treated with thymidine (Sigma Aldrich Cat. T1895; 2 mM) for 16 hours.
Plasmids and transfection
Plasmids expressing wild type or mutant WRN (pLX209-neo-active WRN, pLX209-neo-WRN E84A, pLX209-neo-WRN K577M, pLX209-neo-WRN E84A K577M) were obtained from Addgene75. Once the cells reached 70% confluency, the culture medium was replaced with Opti-MEM Reduced Serum Medium (ThermoFisher, Cat. 31985070). A transfection mixture was prepared by combining plasmid DNA (2 μg per well of a 6-well plate) with FuGENE HD Transfection Reagent (6 μL per well; Promega, Cat. E2312,) and DMEM following manufacturer’s instructions. The mixture was incubated at room temperature (RT) for 15 minutes, then added dropwise to the cells.
Colony formation assay
Following specific treatment, cells were harvested and re-seeded into 6-well plate (150–300 cells per well) or into 10 cm dish (300 cells per dish). The cells were then allowed to grow for 12 days to form colonies. The colonies were rinsed once with PBS and then fixed and stained with 0.1% crystal violet containing 70% ethanol. The colonies were counted manually.
IF analysis on fixed cells
The cells were treated as described in the Fig. legends before they were fixed on coverslips with PTMEF fixation buffer (4% paraformaldehyde, 200 mM PIPES, pH 6.8, 200 mM MgCl2, 10 mM EGTA, pH 8.0, 0,2% Triton X-100) for 10 minutes at RT. Cells were washed with PBS and blocked with 3% BSA in 1× PBST (PBS supplemented with 0,5% Triton X-100) overnight at 4 °C. The samples were then incubated overnight with primary antibody (diluted in 3% BSA in 1× PBST) at 4 °C and washed three times with 3% BSA in 1× PBST, and subsequently incubated with secondary antibodies (diluted in 3% BSA in 1× PBST) at RT for 1 hour. Following three additional washes with 3% BSA in 1× PBST, samples were mounted with DAPI-containing Vectashield (Vector Laboratories). Between each wash, the samples were incubated with corresponding buffers for 20 minutes and protected from light. For RPA foci detection in mitotic cells, samples were pre-extracted by an incubation with ice-cold 0.5% Triton X-100 in 1x PBS for 5 minutes at RT, followed by a fixation in freshly prepared PTMEF buffer for 10 minutes at RT, and an additional incubation with 0.2% NP-40 in 1x PBS for 10 minutes at RT. Samples were then incubated with a RPA antibody and corresponding secondary antibody, and washed with 3% BSA in 1× PBST after each incubation. The IF images were captured using an Olympus BX63 microscope (Olympus) and analyzed using CellSens (Olympus) and Fiji-ImageJ software. Antibodies and their dilutions used in this study are listed and referenced in Supplementary Table 2.
Telomere Fluorescence in situ Hybridization (FISH) and EdU detection
After metaphase chromosomes are fixed on the slides, a FITC-labeled PNA telomere probe (Panagene; Cat. F1009-5) was used for telomeric FISH according to manufacturer’s protocol with the following modifications: 1x blocking reagent (11096176001, Roche) was added into the hybridization buffer, probe incubation was performed at 37 °C for at least 2 hours, and the slides containing fixed chromosomes were incubated in Washing Solution I (1x PBS with 0.1% Tween 20; pre-warmed at 57 °C) for two times of 15 minutes. At the end of the telomere FISH procedure, cells were re-fixed with 4% formaldehyde in 1x PBS for 4 minutes at RT, and EdU detection was carried out using Click-iT® Plus EdU Alexa Fluor® 594 Imaging Kit (C10339 Thermo Fisher Scientific). Slides containing fixed chromosomes were incubated with the reaction mix for 1 hour at RT in the dark and were then washed in 1x PBS containing 3% BSA in and 0.5% Triton-X100 for three times (20 minutes each time). Slides were then mounted using Vectashield mounting medium with DAPI (Vector Labs) for microscopic analysis. Images were captured using an Olympus BX63 microscope and analyzed using CellSens (Olympus).
Western blot analysis
Cells were harvested by trypsinization and centrifugation at 300 g for 5 minutes. Cell pellets were lysed with cell extraction buffer (ThermoFisher Scientific) supplemented with PMSF (1 mM; Sigma Aldrich), PhosSTOP (Merck) and Complete protease inhibitor cocktail (Merck) on ice for 30 minutes. The lysates were then sonicated using a Soniprep 150 Plus sonicator (MSE). A Pierce BCA Protein Assay Kit (ThermoFisher Scientific) was used to quantify the protein concentration following the manufacturer’s protocol. Then, 20–40 µg protein per sample were mixed with NUPAGE SDS sample buffer (ThermoFisher Scientific) and incubated at 90 °C for 5 minutes before being separated on a NuPAGE 4–12% bis-tris gel (ThermoFisher Scientific) and transferred to a PVDF membrane (Amersham Pharmacia) at RT. The membrane was blocked with 5% skimmed-milk powder (Sigma Aldrich) in PBST buffer for 1 hour at RT before further incubation at 4 °C with primary antibody (diluted in 5% milk) overnight. The membrane was then washed three times with PBST (with 20 minutes incubation between each time), incubated with a secondary antibody diluted in 5% milk for 1 hour at RT and then washed an additional three times in PBST (with 20 minutes incubation between each time). The membrane was then imaged using Luminata Forte HRP substrate (Merck Millipore) and an Imager 600 system (Amersham). Antibodies and their dilutions used in this study are listed and referenced in Supplementary Table 2.
Chromatin binding assay
Cells were harvested by trypsinization or mitotic shake-off and centrifugation at 400 g for 5 minutes. To separate chromatin bound proteins, cell pellets were lysed in pre-chilled (4 °C) 1x RIPA buffer for 1 min. The samples were centrifuged at 10,000 g for 20 minutes at 4 °C, the supernatant was then saved as a soluble fraction, and the pellet was further dissolved in cell extraction buffer additionally supplemented with Benzonase (Sigma) on ice for at least 30 minutes. The chromatin fraction was then sonicated using a Bioruptor® Pico; Diagenode, B01060010, before analyzing all samples by western blot analysis as described above.
FACS analysis
Following various treatment, cells were harvested and then fixed with ice-cold 70% ethanol. After 24 hours incubation at −20 °C, fixed cells were collected by centrifugation at 500 g at 4 °C. The fixed cells were then washed with ice cold PBS/0,3% BSA 3 times, and then stained with 0.5–1 ml of staining solution consist of 1x PBS, 80 µg/ml propidium iodide (Invitrogen) and 100 µg/ml RNase A (Sigma Aldrich) at 37 °C for 30 minutes. Fluorescence-activated cell sorting (FACS) analysis for each sample was carried out on a FACSCelesta flow cytometer (BD Biosciences). 50,000 cells were analyzed for each condition. The data was extracted using BD FACSDiva software, and displayed with FlowJO software.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The raw data of graphs/statistics and western blots generated in this study are provided as Source Data file with this paper. All original images generated in this study have been deposited at Figshare (https://doi.org/10.6084/m9.figshare.29328551). Source data are provided with this paper.
References
Zeman, M. K. & Cimprich, K. A. Causes and consequences of replication stress. Nat. Cell Biol. 16, 2–9 (2014).
Bertolin, A. P., Hoffmann, J. S. & Gottifredi, V. Under-replicated DNA: the byproduct of large genomes? Cancers (Basel) 12, https://doi.org/10.3390/cancers12102764 (2020).
Hua, B. L. & Orr-Weaver, T. L. DNA replication control during drosophila development: insights into the onset of S phase, replication initiation, and fork progression. Genetics 207, 29–47 (2017).
Glover, T. W., Wilson, T. E. & Arlt, M. F. Fragile sites in cancer: more than meets the eye. Nat. Rev. Cancer 17, 489–501 (2017).
Burrow, A. A., Williams, L. E., Pierce, L. C. & Wang, Y. H. Over half of breakpoints in gene pairs involved in cancer-specific recurrent translocations are mapped to human chromosomal fragile sites. BMC Genom. 10, 59 (2009).
de Braekeleer, M. Fragile sites and chromosomal structural rearrangements in human leukemia and cancer. Anticancer Res. 7, 417–422 (1987).
De Braekeleer, M., Smith, B. & Lin, C. C. Fragile sites and structural rearrangements in cancer. Hum. Genet 69, 112–116 (1985).
Le Beau, M. M. Chromosomal fragile sites and cancer-specific rearrangements. Blood 67, 849–858 (1986).
Yunis, J. J. Fragile sites, mutagens and genomic rearrangements in cancer. Basic Life Sci. 43, 11–21 (1988).
Yunis, J. J. & Soreng, A. L. Constitutive fragile sites and cancer. Science 226, 1199–1204 (1984).
Sfeir, A. et al. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell 138, 90–103 (2009).
Ozer, O., Bhowmick, R., Liu, Y. & Hickson, I. D. Human cancer cells utilize mitotic DNA synthesis to resist replication stress at telomeres regardless of their telomere maintenance mechanism. Oncotarget 9, 15836–15846 (2018).
Bhowmick, R., Minocherhomji, S. & Hickson, I. D. RAD52 facilitates mitotic DNA synthesis following replication stress. Mol. Cell 64, 1117–1126 (2016).
Minocherhomji, S. et al. Replication stress activates DNA repair synthesis in mitosis. Nature 528, 286–290 (2015).
Naim, V. & Rosselli, F. The FANC pathway and BLM collaborate during mitosis to prevent micro-nucleation and chromosome abnormalities. Nat. Cell Biol. 11, 761–768 (2009).
Chan, K. L., Palmai-Pallag, T., Ying, S. & Hickson, I. D. Replication stress induces sister-chromatid bridging at fragile site loci in mitosis. Nat. Cell Biol. 11, 753–760 (2009).
Howlett, N. G., Taniguchi, T., Durkin, S. G., D’Andrea, A. D. & Glover, T. W. The Fanconi anemia pathway is required for the DNA replication stress response and for the regulation of common fragile site stability. Hum. Mol. Genet 14, 693–701 (2005).
Barnes, R. P., Thosar, S. A. & Opresko, P. L. Telomere fragility and MiDAS: managing the gaps at the end of the road. Genes (Basel) 14, https://doi.org/10.3390/genes14020348 (2023).
Ji, F. et al. Genome-wide high-resolution mapping of mitotic DNA synthesis sites and common fragile sites by direct sequencing. Cell Res. 30, 1009–1023 (2020).
Macheret, M. et al. High-resolution mapping of mitotic DNA synthesis regions and common fragile sites in the human genome through direct sequencing. Cell Res. 30, 997–1008 (2020).
Wu, W. et al. RTEL1 suppresses G-quadruplex-associated R-loops at difficult-to-replicate loci in the human genome. Nat. Struct. Mol. Biol. 27, 424–437 (2020).
Min, J., Wright, W. E. & Shay, J. W. Alternative lengthening of telomeres mediated by mitotic DNA synthesis engages break-induced replication processes. Mol. Cell. Biol. 37, https://doi.org/10.1128/MCB.00226-17 (2017).
Dilley, R. L. et al. Break-induced telomere synthesis underlies alternative telomere maintenance. Nature 539, 54–58 (2016).
Ivanova, T. et al. Budding yeast complete DNA synthesis after chromosome segregation begins. Nat. Commun. 11, 2267 (2020).
Pedersen, R. T., Kruse, T., Nilsson, J., Oestergaard, V. H. & Lisby, M. TopBP1 is required at mitosis to reduce transmission of DNA damage to G1 daughter cells. J. Cell Biol. 210, 565–582 (2015).
Garribba, L. et al. Folate stress induces SLX1- and RAD51-dependent mitotic DNA synthesis at the fragile X locus in human cells. Proc. Natl. Acad. Sci. USA, https://doi.org/10.1073/pnas.1921219117 (2020).
Bhowmick, R., Hickson, I. D. & Liu, Y. Completing genome replication outside of S phase. Mol. cell 83, 3596–3607 (2023).
Ying, S. et al. MUS81 promotes common fragile site expression. Nat. Cell Biol. 15, 1001–1007 (2013).
Wyatt, H. D., Sarbajna, S., Matos, J. & West, S. C. Coordinated actions of SLX1-SLX4 and MUS81-EME1 for Holliday junction resolution in human cells. Mol. Cell 52, 234–247 (2013).
Bursomanno, S. et al. Proteome-wide analysis of SUMO2 targets in response to pathological DNA replication stress in human cells. DNA Repair 25, 84–96 (2015).
Li, S. et al. PIF1 helicase promotes break-induced replication in mammalian cells. EMBO J. 40, e104509 (2021).
Wu, W. et al. Mitotic DNA synthesis in response to replication stress requires the sequential action of DNA polymerases zeta and delta in human cells. Nat. Commun. 14, 706 (2023).
Mocanu, C. et al. DNA replication is highly resilient and persistent under the challenge of mild replication stress. Cell Rep. 39, 110701 (2022).
Wassing, I. E. et al. The RAD51 recombinase protects mitotic chromatin in human cells. Nat. Commun. 12, 5380 (2021).
Saini, N. et al. Migrating bubble during break-induced replication drives conservative DNA synthesis. Nature 502, 389–392 (2013).
Donnianni, R. A. & Symington, L. S. Break-induced replication occurs by conservative DNA synthesis. Proc. Natl. Acad. Sci. USA 110, 13475–13480 (2013).
Scully, R., Panday, A., Elango, R. & Willis, N. A. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat. Rev. Mol. Cell Biol. 20, 698–714 (2019).
Hu, Q. et al. Break-induced replication plays a prominent role in long-range repeat-mediated deletion. EMBO J. 38, e101751 (2019).
Cejka, P. DNA end resection: nucleases team up with the right partners to initiate homologous recombination. J. Biol. Chem. 290, 22931–22938 (2015).
Knipscheer, P. et al. The Fanconi anemia pathway promotes replication-dependent DNA interstrand cross-link repair. Science 326, 1698–1701 (2009).
Klein Douwel, D. et al. XPF-ERCC1 acts in unhooking DNA interstrand crosslinks in cooperation with FANCD2 and FANCP/SLX4. Mol. Cell 54, 460–471 (2014).
Yesbolatova, A., Natsume, T., Hayashi, K. -i & Kanemaki, M. T. Generation of conditional auxin-inducible degron (AID) cells and tight control of degron-fused proteins using the degradation inhibitor auxinole. Methods 164-165, 73–80 (2019).
Garribba, L. et al. Inducing and detecting mitotic DNA synthesis at difficult-to-replicate loci. Methods Enzymol. 601, 45–58 (2018).
Dupre, A. et al. A forward chemical genetic screen reveals an inhibitor of the Mre11-Rad50-Nbs1 complex. Nat. Chem. Biol. 4, 119–125 (2008).
Anand, R., Ranjha, L., Cannavo, E. & Cejka, P. Phosphorylated CtIP functions as a co-factor of the MRE11-RAD50-NBS1 endonuclease in DNA end resection. Mol. cell 64, 940–950 (2016).
Cannavo, E. & Cejka, P. Sae2 promotes dsDNA endonuclease activity within Mre11-Rad50-Xrs2 to resect DNA breaks. Nature 514, 122–125 (2014).
Sartori, A. A. et al. Human CtIP promotes DNA end resection. Nature 450, 509–514 (2007).
Dai, L. et al. Structural insight into BRCA1-BARD1 complex recruitment to damaged chromatin. Mol. Cell 81, 2765–2777.e2766 (2021).
Sy, S. M., Huen, M. S. & Chen, J. PALB2 is an integral component of the BRCA complex required for homologous recombination repair. Proc. Natl. Acad. Sci. USA 106, 7155–7160 (2009).
Wang, B. et al. Abraxas and RAP80 form a BRCA1 protein complex required for the DNA damage response. Science 316, 1194–1198 (2007).
Ceppi, I. et al. Mechanism of BRCA1-BARD1 function in DNA end resection and DNA protection. Nature 634, 492–500 (2024).
Cruz-García, A., López-Saavedra, A. & Huertas, P. BRCA1 accelerates CtIP-mediated DNA-end resection. Cell Rep. 9, 451–459 (2014).
Li, Y. et al. USP13 regulates the RAP80-BRCA1 complex dependent DNA damage response. Nat. Commun. 8, 15752 (2017).
Nakamura, K. et al. H4K20me0 recognition by BRCA1-BARD1 directs homologous recombination to sister chromatids. Nat. Cell Biol. 21, 311–318 (2019).
Na, Z., Pan, S., Uttamchandani, M. & Yao, S. Q. Protein-protein interaction inhibitors of BRCA1 discovered using small molecule microarrays. Methods Mol. Biol. 1518, 139–156 (2017).
Zhu, Z., Chung, W. H., Shim, E. Y., Lee, S. E. & Ira, G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell 134, 981–994 (2008).
Mimitou, E. P. & Symington, L. S. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature 455, 770–774 (2008).
Tran, P. T., Erdeniz, N., Dudley, S. & Liskay, R. M. Characterization of nuclease-dependent functions of Exo1p in Saccharomyces cerevisiae. DNA Repair (Amst.) 1, 895–912 (2002).
Nimonkar, A. V. et al. BLM-DNA2-RPA-MRN and EXO1-BLM-RPA-MRN constitute two DNA end resection machineries for human DNA break repair. Genes Dev. 25, 350–362 (2011).
Kim, D. H. et al. Enzymatic properties of the Caenorhabditis elegans Dna2 endonuclease/helicase and a species-specific interaction between RPA and Dna2. Nucleic Acids Res. 33, 1372–1383 (2005).
Bae, S. H. & Seo, Y. S. Characterization of the enzymatic properties of the yeast Dna2 helicase/endonuclease suggests a new model for Okazaki fragment processing. J. Biol. Chem. 275, 38022–38031 (2000).
Kim, J. H. et al. Isolation of human Dna2 endonuclease and characterization of its enzymatic properties. Nucleic Acids Res. 34, 1854–1864 (2006).
Budd, M. E., Choe, W. C. & Campbell, J. L. The nuclease activity of the yeast Dna2 protein, which is related to the RecB-like nucleases, is essential in vivo. J. Biol. Chem. 275, 16518–16529 (2000).
Kao, H. I., Campbell, J. L. & Bambara, R. A. Dna2p helicase/nuclease is a tracking protein, like FEN1, for flap cleavage during Okazaki fragment maturation. J. Biol. Chem. 279, 50840–50849 (2004).
Pinto, C., Kasaciunaite, K., Seidel, R. & Cejka, P. Human DNA2 possesses a cryptic DNA unwinding activity that functionally integrates with BLM or WRN helicases. Elife 5, ARTN e18574 (2016).
Levikova, M., Klaue, D., Seidel, R. & Cejka, P. Nuclease activity of Saccharomyces cerevisiae Dna2 inhibits its potent DNA helicase activity. Proc. Natl. Acad. Sci. USA 110, E1992–E2001 (2013).
Sturzenegger, A. et al. DNA2 cooperates with the WRN and BLM RecQ helicases to mediate long-range DNA end resection in human cells. J. Biol. Chem. 289, 27314–27326 (2014).
Niu, H. Y. et al. Mechanism of the ATP-dependent DNA end-resection machinery from Saccharomyces cerevisiae. Nature 467, 108–U143 (2010).
Gravel, S., Chapman, J. R., Magill, C. & Jackson, S. P. DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Gene Dev. 22, 2767–2772 (2008).
Liu, W. et al. A selective small molecule DNA2 inhibitor for sensitization of human cancer cells to chemotherapy. EBioMedicine 6, 73–86 (2016).
Ceppi, I. et al. PLK1 regulates CtIP and DNA2 interplay in long-range DNA end resection. Genes Dev. 37, 119–135 (2023).
Loe, T. K. et al. Telomere length heterogeneity in ALT cells is maintained by PML-dependent localization of the BTR complex to telomeres. Genes Dev. 34, 650–662 (2020).
Tejwani, V. et al. PROTAC-mediated conditional degradation of the WRN helicase as a potential strategy for selective killing of cancer cells with microsatellite instability. Sci. Rep. 14, 20824 (2024).
Ferretti, S. et al. Discovery of WRN inhibitor HRO761 with synthetic lethality in MSI cancers. Nature 629, 443–449 (2024).
Chan, E. M. et al. WRN helicase is a synthetic lethal target in microsatellite unstable cancers. Nature 568, 551–556 (2019).
Kategaya, L., Perumal, S. K., Hager, J. H. & Belmont, L. D. Werner syndrome helicase is required for the survival of cancer cells with microsatellite instability. iScience 13, 488–497 (2019).
Lieb, S. et al. Werner syndrome helicase is a selective vulnerability of microsatellite instability-high tumor cells. Elife 8, https://doi.org/10.7554/eLife.43333 (2019).
Behan, F. M. et al. Prioritization of cancer therapeutic targets using CRISPR-Cas9 screens. Nature 568, 511–516 (2019).
McDonald, E. R. et al. Project DRIVE: a compendium of cancer dependencies and synthetic lethal relationships uncovered by large-scale, deep RNAi screening. Cell 170, 577–592 e510 (2017).
Barretina, J. et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–607 (2012).
Johnson, F. B. et al. The Saccharomyces cerevisiae WRN homolog Sgs1p participates in telomere maintenance in cells lacking telomerase. EMBO J. 20, 905–913 (2001).
Edwards, D. N., Machwe, A., Chen, L., Bohr, V. A. & Orren, D. K. The DNA structure and sequence preferences of WRN underlie its function in telomeric recombination events. Nat. Commun. 6, 8331 (2015).
Gocha, A. R. S., Acharya, S. & Groden, J. WRN Loss Induces Switching of Telomerase-Independent Mechanisms of Telomere Elongation. Plos One 9, https://doi.org/10.1371/journal.pone.0093991 (2014).
Xu, Y. et al. 53BP1 and BRCA1 control pathway choice for stalled replication restart. Elife 6, https://doi.org/10.7554/eLife.30523 (2017).
Ayoub, N. et al. The carboxyl terminus of Brca2 links the disassembly of Rad51 complexes to mitotic entry. Curr. Biol. 19, 1075–1085 (2009).
Giunta, S., Belotserkovskaya, R. & Jackson, S. P. DNA damage signaling in response to double-strand breaks during mitosis. J. Cell Biol. 190, 197–207 (2010).
van Vugt, M. A. et al. A mitotic phosphorylation feedback network connects Cdk1, Plk1, 53BP1, and Chk2 to inactivate the G(2)/M DNA damage checkpoint. PLoS Biol. 8, e1000287 (2010).
Peterson, S. E. et al. Cdk1 uncouples CtIP-dependent resection and Rad51 filament formation during M-phase double-strand break repair. J. Cell Biol. 194, 705–720 (2011).
Orthwein, A. et al. Mitosis inhibits DNA double-strand break repair to guard against telomere fusions. Science 344, 189–193 (2014).
Terasawa, M., Shinohara, A. & Shinohara, M. Canonical non-homologous end joining in mitosis induces genome instability and is suppressed by M-phase-specific phosphorylation of XRCC4. PLoS Genet 10, e1004563 (2014).
Benada, J., Burdová, K., Lidak, T., von Morgen, P. & Macurek, L. Polo-like kinase 1 inhibits DNA damage response during mitosis. Cell Cycle 14, 219–231 (2015).
Yu, C. E. et al. Positional cloning of the Werner’s syndrome gene. Science 272, 258–262 (1996).
Oshima, J., Sidorova, J. M. & Monnat, R. J. Werner syndrome: clinical features, pathogenesis and potential therapeutic interventions. Ageing Res. Rev. 33, 105–114 (2017).
Chu, W. K. & Hickson, I. D. RecQ helicases: multifunctional genome caretakers. Nat. Rev. Cancer 9, 644–654 (2009).
Orren, D. K. & Machwe, A. Response to replication stress and maintenance of genome stability by WRN, the Werner syndrome protein. Int. J. Mol. Sci. 25, https://doi.org/10.3390/ijms25158300 (2024).
Thangavel, S. et al. DNA2 drives processing and restart of reversed replication forks in human cells. J. Cell Biol. 208, 545–562 (2015).
Palermo, V. et al. CDK1 phosphorylates WRN at collapsed replication forks. Nat. Commun. 7, https://doi.org/10.1038/ncomms12880 (2016).
Perry, J. J. P. et al. WRN exonuclease structure and molecular mechanism imply an editing role in DNA end processing. Nat. Struct. Mol. Biol. 13, 414–422 (2006).
Yoon, J. H., Sellamuthu, K., Prakash, L. & Prakash, S. WRN exonuclease imparts high fidelity on translesion synthesis by Y family DNA polymerases. Gene Dev. 38, 213–232 (2024).
Moser, M. J. et al. WRN helicase expression in Werner syndrome cell lines. Nucleic Acids Res. 28, 648–654 (2000).
Natsume, T., Kiyomitsu, T., Saga, Y. & Kanemaki, M. T. Rapid protein depletion in human cells by auxin-inducible degron tagging with short homology donors. Cell Rep. 15, 210–218 (2016).
Liang, C. C. et al. Mechanism of single-stranded DNA annealing by RAD52-RPA complex. Nature 629, 697–703 (2024).
Acknowledgements
We thank members of Ying Liu group and Ian Hickson for helpful discussions, and Malgorzata Clausen for expert technical assistance. This work was supported by grants from the National Natural Science Foundation of China (32470812; W.W.), Dagmar Marshalls Fond (Project grant; P.H.R.), the Danish National Research Foundation (DNRF115; Y.L.), European Union (H2020/Marie Skłodowska-Curie Actions; Antihelix 859853; Y.L.), Danish Independent Research Fund (1030-00180B; Y.L.), NEYE Foundation (122479; Y.L.), and the JSPS KAKENHI (20H05396 and 21H04719; M.K.).
Author information
Authors and Affiliations
Contributions
S.A.B., K.L., W.W., P.H.R., L.R., R.B, M.M.G.D., and M.T.K. performed experiments. S.A.B., K.L., W.W., P.H.R., R.B., M.T.K., and Y.L. analyzed data. S.A.B., M.T.K., and Y.L. designed experiments. S.A.B. and Y.L. interpreted results. S.A.B., M.T.K., and Y.L. wrote the manuscript, and all authors edited it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Patrick Sung, who co-reviewed with Hardeep Kaur, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Barwacz, S.A., Lundgaard, K., Wu, W. et al. DNA double-strand break end resection factors and WRN facilitate mitotic DNA synthesis in human cells. Nat Commun 16, 7901 (2025). https://doi.org/10.1038/s41467-025-63292-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-63292-7