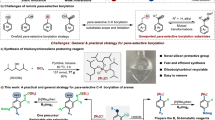
Abstract
Arenes bearing silyl and boryl, or germyl and boryl groups, represent a significant class of bifunctional molecules that have garnered considerable attention in organic synthesis due to their unique reactivity and versatility in undergoing diverse chemical transformations. Despite their importance, the synthesis of these mixed-metalloid compounds, particularly those with ortho-substituted aromatic frameworks, often involves complex, multi-step procedures. Herein, we present a highly efficient, single-step method for synthesizing ortho-boronated arylhydrosilanes and arylgermanes via catalytic C−H borylation reaction. The use of Si- and Ge-containing directing groups has been demonstrated to effectively suppress undesirable metathesis reactions, a side effect typically associated with their metallic nature. Our approach employs an iridium catalyst in combination with a ligand and an acetate base, which facilitates the formation of benzo-fused four-membered metallacycles within the catalytic cycle. The method’s broad applicability and practicality are further underscored by its ability to generate a wide array of valuable intermediates that can be easily transformed into various functionalized molecules. Both experimental and computational studies provide profound insights into the reaction dynamics and the factors influencing the formation of these strained metallacycles.
Similar content being viewed by others
Introduction
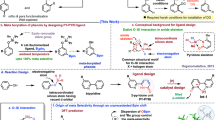
Catalytic C−H activation has emerged as a powerful strategy for modifying carbon centers in organic molecules, offering atom-efficient and streamlined synthetic pathways1,2,3,4,5,6,7,8,9,10. In complex molecules where multiple C–H bonds often reside in similar environments, the incorporation of a directing group has proven to be the most effective method for achieving selective transformation (Fig. 1a)11,12,13,14,15,16. These directing groups, whether inherently present or artificially introduced, serve to precisely position the metal catalyst at the desired C–H bond. Such process is thermodynamically favored due to the formation of stable five-, six-, and seven-membered metallacyclic intermediates, which ensure regioselectivity through conformational rigidity. Non-metallic elements, particularly those from the chalcogen (O, S) and pnictogen (N, P) groups, are widely utilized as coordinating atoms in directing groups. In contrast, the integration of directing groups containing metalloid elements has seen limited progress, primarily due to their propensity to induce undesirable metathesis reactions in the presence of transition metals, a consequence of their metallic character17. Although some success has been achieved with silicon atoms, current methods are only confined to specific substrate classes that form five-membered metallacyclic intermediates, significantly limiting their broader applicability18,19,20. Consequently, there is a pressing need to develop more versatile C−H activation strategies that can accommodate a wider range of metalloid elements while moving beyond the conventional constraints of rigid metallacycle formation.
Arenes ortho-bisfunctionalized with silyl and boryl, or germyl and boryl groups, have been extensively utilized in photoelectronics, semiconductors, catalysis, and organic synthesis due to the unique properties of these distinct metalloids21,22,23,24,25,26,27. However, current synthetic methods for these compounds often require intricate, multi-step processes, particularly when forming two C−metalloid bonds individually28,29,30,31. Seminal studies by Milstein32,33, Tilley, and Bergman34 have demonstrated that iridium complexes derived from triphenylsilane can produce benzo-fused four-membered silametallacycles through oxidative addition of ortho C−H bonds (Fig. 1b). Building on these pioneering works, we are inspired to explore three key questions: whether such strained metallacycles can be generated in a catalytic fashion, whether they can further couple with boron species, and whether the metalloid elements can be extended to germanium atoms. Notably, Gaunt and coworkers have reported palladium-catalyzed β-selective C−H activation of aliphatic amines, predominantly achieved via the formation of four-membered metallacycles (Fig. 1c)35,36. In contrast, catalytic C−H borylation leading to the formation of benzo-fused metallacycles introduces even greater ring strain and a metalloid motif that is more susceptible to uncharted side reactions37,38,39,40,41.
Here, we introduce an ortho-selective C−H borylation directed by silicon and germanium atoms (Fig. 1d). Our system, which utilizes arylhydrosilanes or arylgermanes as substrates, combines an iridium catalyst with a bipyridine-type ligand, an acetate base, and HBpin to efficiently facilitate the formation of a benzo-fused four-membered metallacycle within the catalytic cycle. Importantly, this methodology effectively overcomes the common issue of byproduct formations from C−metalloid cleavage26,42,43,44,45 and metalloid−H bond cleavage46,47,48,49,50. This approach enables the one-step preparation of a diverse array of ortho-boronated arylhydrosilanes and arylgermanes, offering superior efficiency in terms of atom, step and redox economy. The ability to selectively functionalize these aryl compounds with boron atoms while maintaining the integrity of the metalloid atoms represents a significant advancement in the field of C−H activation.
Results
Condition optimization
To establish a platform for this chemical process, we initially opted for the reaction between triphenylsilane (1a) and HBpin (Table 1). The reaction was employed [Ir(cod)OMe]2 as the catalyst along with a tridentate ligand L1 at a temperature of 125 °C in THF. However, merely trace amounts of the desired C–H borylation product 2a were observed via GC-MS analysis (entry 1). To enhance the overall yield of the reaction, we explored the influence of various additives. The incorporation of LiOAc into the reaction system could slightly improve the reactivity (entry 2). Additionally, we examined alternative salts such as NaOAc (19%) and KOAc (26%), which exhibited superior effectiveness in facilitating the formation of 2a (entries 3–4). Conversely, the utilization of K2CO3 resulted in a significant decrease in yield (entry 5). These discoveries underline the significance of both the K+ cation and OAc- anion in influencing the overall outcome of the reaction. Moreover, we investigated the impact of different ligands on the reaction. The inclusion of bipyridine-type ligands L2–L4 proved to be crucial in promoting the reaction (entries 6–8), with the highest achievable yield of 2a (75%) obtained in the presence of L4 (entry 8). Under the optimized reaction conditions, we observed the absence of diborylated products, with Ph-Bpin emerging as the primary byproduct, presumably resulting from C-Si bond cleavage. The structure of product 2a was further confirmed by X-ray crystallography. In contrast, employing 1,10-phenanthroline (L5) as the ligand led to a significantly lower yield (entry 9). Other solvents such as dioxane, toluene, and DMF resulted in much lower reactivity (entries 10–12). We also explored the potential of alternative boron reagents, such as B2pin2, which yielded inferior results (entries 13). Interestingly, alternative iridium sources such as [Ir(cod)Cl]2 and [Ir(cod)acac]2 resulted in a substantial decrease in yield (entries 14–15), while other transition metals like [Rh(cod)Cl]2 completely failed to promote the reaction (entry 16). Reducing the loading of [Ir(cod)OMe]2 to 1.0 mol% still exhibited an acceptable reactivity (entry 17). Finally, a control experiment confirmed that the reaction did not proceed in the absence of the metal catalyst (entry 18).
Substrate scope of silanes
Having the optimized reaction conditions in hand, we proceeded to explore the suitability of various arylhydrosilanes for ortho C–H borylation (Fig. 2). A wide range of triarylsilanes bearing substituents such as methyl (1b), isopropyl (1c), tert-butyl (1d), phenyl (1e), methoxy (1f), and methylthio (1g) were subjected to the system producing products 2b–2g with modest to good yields. Notably, the reaction of tri(naphthalen-2-yl)silane (1h), which possesses two potential sites for C–H borylation, demonstrated remarkable selectivity towards borylation at the less hindered C3 position. The structure of product 2h was also confirmed by X-ray crystallography. However, when identical reaction conditions were applied to dialkylarylhydrosilanes, the reaction efficiency decreased markedly, necessitating further optimization. The inclusion of NaOAc in the conditions with a mixture of B2pin2 and HBpin was found to significantly enhance the reaction efficiency. For example, silane 1i, bearing two isopropyl substituents, was successfully converted to product 2i in a 54% yield. In contrast, without NaOAc, the reaction became very chaotic. Substrate 1j, containing a para-methyl group, displayed favorable reactivity and selectivity at the less sterically hindered position. Conversely, substrate 1k, with a meta-methyl group, exhibited significantly reduced reactivity. Furthermore, other dialkylarylhydrosilanes with various substituents at the para position, including iPr (1m), tBu (1n), Ph (1o), OMe (1p), TMS (1q), F (1r), Cl (1s), CF3 (1t), and OCF3 (1u), were successfully utilized under these conditions. Additionally, the reaction of diisopropyl(naphthalen-2-yl)silane (1v) showed exclusive C3 selectivity. Under these optimized conditions, when diarylalkylhydrosilane 1w, containing two aryl and one methyl substituent, was tested, product 2h was only 17% yield, and a byproduct 2a, resulting from σ-bond metathesis, was observed. Interestingly, substrates with isopropyl (1x) and cyclopentyl (1y) groups exhibited compatible reactivity, indicating that the introduction of bulky substituents on the silicon atom can be advantageous in suppressing σ-bond metathesis. Finally, the reaction of silane 1z, containing two aryl and one benzyl substituent, displayed extraordinary selectivity for C–H bond activation within the benzyl motif, resulting in the formation of the dual C–H borylation product 2z with a yield of 68%. This intriguing outcome suggests a preferential formation of five-membered silametallacycles during the C–H activation process.
Reaction conditions: [Ir(cod)OMe]2 (2.5 mol%), L4 (5.0 mol%), 1 (0.50 mmol), HBpin (1.25 mmol), KOAc (1.25 mmol) in 1.5 mL of THF at 125 °C for 6 h under argon; For entries 8–25: [Ir(cod)OMe]2 (5.0 mol%), L4 (10.0 mol%), HBpin (0.15 mmol), B2pin2 (0.75 mmol), NaOAc (1.25 mmol) at 150 °C. aIsolated yield.
Substrate scope of germanes
Based on the above findings, we extended our investigation to explore the application of hydrogermanes in C–H borylation (Fig. 3). Initially, when triphenylgermane (3a) was substituted with 1a under the standard conditions, a significant presence of byproducts arising from σ-C–Ge bond metathesis was observed. To address this challenge, we performed a comprehensive screening and identified an optimized reaction protocol employing a stoichiometric quantity of the tridentate ligand L1. This ligand effectively inhibited the metathesis pathway, enabling the formation of the desired product 4a with a 72% yield (entry 1). Diminishing the catalyst load significantly reduced the yield of the target product (entry 2). Nonetheless, decreasing the ligand amount to 30 mol% still facilitated the formation of 4a with an acceptable yield (56%). Notably, substituting ligand L1 with L4 resulted in only trace amounts of the desired product (entry 4). Additionally, KOAc was found to be indispensable for the reaction’s progression (entry 5). Leveraging these insights, we investigated the feasibility of C–H borylation using a variety of arylhydrogermanes. Triarylhydrogermanes with different substituents on the benzene ring, such as methyl (3b), isopropyl (3c), methoxy (3d), methylthio (3e), fluoro (3f), and trimethylsilyl (3g), were tested in the reaction system, yielding products 4b–4g in moderate yields. Notably, the reaction involving tri-3-methylphenylgermane (3h) and tri-2-naphthylgermane (3i), which have two potential sites for borylation, showed a significant preference for the less sterically hindered position. Furthermore, hydrogermanes 3j and 3k, substituted with two phenyl groups and one alkyl group, were effectively transformed into products 4j and 4k with high efficiency. However, under these updated conditions, the reaction of a hydrogermane, containing one phenyl and two isopropyl substituents, completely failed. Instead, we only observed the byproduct resulting from σ-bond metathesis (not shown in the Figure).
Product derivatization
The adept synthesis of mixed-metalloid compounds has paved the way for more in-depth investigations into their reactivity (Fig. 4). Our initial studies focused on the reactivity of ortho-boronated arylhydrosilanes (Fig. 4a). For instance, compound 2a can efficiently undergo a Pd-catalyzed Suzuki–Miyaura coupling reaction with iodoarenes 5, leading to the rapid formation of biaryl product 6 in a yield of 54%. Subsequently, in the presence of Wilkinson’s catalyst, compound 6 can be further transformed through dehydrogenative cyclization, yielding silole 7 with an impressive 88% yield51. Moreover, the reaction of compound 2a with ClCH2I and nBuLi results in the in situ generation of the CH2BrLi reagent, which ultimately leads to the formation of boronate 8, now featuring an additional methylene unit52. Notably, the methylation of the Si–H bond in 2a, achieved via treatment with CH2I2 and Et2Zn, delivers product 9 with full conversion. This compound can then be effectively oxidized using NaBO3·4H2O, providing phenol 10 in nearly quantitative yield. Further diversification of compound 9 is achieved by subjecting it to NIS in the presence of CuI, resulting in the iodination product 11 with a yield of 73%. Additionally, we developed a Rh-catalyzed cyclization protocol wherein compound 9 reacts with alkyne 12, enabling the synthesis of benzosilole 13 with a 63% yield53. We also explored the reactivity of ortho-boronated arylgermanes, using reagent 3a as the starting material (Fig. 4b). Through a sequential C–H borylation and methylation of the Ge atom, compound 14 can be synthesized in a one-pot process with a yield of 64%. Utilizing the above rhodium-catalyzed cyclization conditions, we successfully obtained benzogermole 15 with a good yield from compound 14 and alkyne 12.
Mechanistic investigation
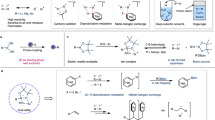
A series of deuterium labeling experiments were initially conducted in the absence of HBpin to elucidate the potential reaction intermediates (Fig. 5). When the reaction utilizing Ph3Si–D (1a–d) was performed without KOAc, 76% deuterium incorporation was observed at the ortho position of 1a, while only 5% D was detected at the silicon atom (Fig. 5a). The observed D incorporation suggests that the oxidative addition of Si–H bond to iridium center is irreversible and that the reaction may proceed through a four-membered silacyclic metal intermediate. The ortho C–D incorporation increased upon the addition of KOAc, indicating that KOAc accelerates the ortho C–H metalation. Furthermore, subsequent addition of D2O to this system resulted in significant deuterium labeling at the Si atom (43% D), suggesting the formation of a potassium silylide within the system, which can undergo hydrolysis with water. Importantly, no deuterium incorporation was observed in 1a with D2O in the absence of the catalyst (Fig. 5b). This discovery further confirms the essential presence of the iridium catalyst for the formation of four-membered silacyclic-iridium intermediate. A series of deuterium labeling experiments were then conducted under standard conditions using HBpin. During the work-up stage, deuterium was introduced into the reaction mixture of 1a and HBpin, resulting in 68% Si–D incorporation in the final product 2a (Fig. 5c). This observation confirms that the final product 2a is derived from a potassium silyl precursor. To further elucidate the mechanistic details of the Ir-catalyzed C–H borylation process, two kinetic isotope effect (KIE) experiments were performed. In the first experiment, parallel reactions were carried out using Ph3SiH (1a) and Ph3Si–D (1a–d1) with HBpin, yielding a KIE value of 1.29 (Fig. 5d). In the second experiment, parallel reactions were conducted using Ph3SiH (1a) and (C6D5)3Si-H (1a–d15) with HBpin, revealing a KIE value of 1.52 (Fig. 5e). These findings suggest that the cleavage of the Si–H bond and the ortho C–H bond are not the rate-determining steps in the reaction54.
DFT calculations
Based on the above results, density functional theory (DFT) calculations were conducted to elucidate the reaction pathway of triphenylsilane 1a in the absence of HBpin, aiming to gain a deeper understanding the formation of strained silametallacycles (Fig. 6). The catalytic cycle initiates with the dissociation of the dimeric iridium catalyst into the monomeric iridium intermediate INT1A. Following this, the Si−H bond of 1a undergoes oxidative addition to the iridium center of INT1A through the transition state TS2A, with an activation energy of 13.6 kcal mol−1 relative to the zero-energy point. In the absence of KOAc, the formed intermediate INT2A undergoes a reversible intramolecular oxidative addition of ortho C–H bond, forming a four-membered strained silametallacycles, designated as INT3A. In the presence of KOAc, intermediate INT2A activates the ortho C–H bond via a base-assisted internal electrophilic-type substitution (BIES) mechanism55,56. This process proceeds through the transition state TS3A-OAc, characterized by an activation energy of 18.1 kcal mol−1. Remarkably, this pathway is substantially more energetically favorable compared to the oxidative addition route through TS3A, which exhibits a significantly higher activation energy of 33.7 kcal mol−1. The reduced activation energy associated with the BIES mechanism underscores the efficiency of KOAc in facilitating the C–H activation, thereby promoting the formation of the strained silametallacyclic intermediate. Intermediate INT4A further undergoes reductive elimination via TS5A-Si with an activation energy of 25.3 kcal mol−1, transferring the hydrogen atom from silicon to the ortho-position of silicon center. This finding is consistent with experimental observations of deuterium incorporation.
We further investigated the competition reaction between silane 1a and HBpin with iridium catalyst (Fig. 7). The calculated energy barrier for the oxidative addition of the H–B bond to iridium is 18.8 kcal mol−1 relative to the energy of Ir-cat, significantly higher than that of the transition state TS2A for the oxidative addition of the H–Si bond (18.8 kcal mol−1 for TS2A-B vs 13.6 kcal mol−1 for TS2A). This energy difference suggests that silane 1a preferentially reacts with the iridium catalyst to form the strained silametallacyclic intermediate INT4A. This mechanistic pathway deviates notably from the previously directed aromatic C−H borylation triggered by Ir-Bpin species (see Supporting Information Figs. S1–6 for details)57,58,59.
Based on the above results, we examined the pathway leading to the final products by DFT calculations (Fig. 8). A molecule of HBpin coordinates to the Ir center in INT4A, undergoing oxidative addition via transition state TS6A, with an energy barrier of 1.5 kcal mol−1, to form the Ir-H intermediate INT6A. Subsequently, reductive elimination and H2 release generate the penta-coordinated intermediate INT7A, with an activation energy of 2.3 kcal mol−1. A competitive pathway involving direct reductive elimination from INT6A to form the C–B bond was also considered but ruled out due to its prohibitively high energy barrier. The reductive elimination of INT7A proceeds through transition state TS8A, which has an activation energy of 20.3 kcal mol−1, leading to the formation of the desired C–B bond. In TS8A, the forming C–B bond length is 1.68 Å, indicating a well-defined transition state for this critical step. The generated intermediate INT8A then undergoes a transmetalation process with excess KOAc in the system, forming a silicate-potassium salt, pro-K. The structural information of transition state TS9A reveals the forming K–Si bond length to be 3.19 Å and the breaking Ir–Si bond length to be 3.45 Å. The activation energy barrier for this step is calculated to be 34.0 kcal mol−1 relative to INT6A, making it the highest energy barrier in the calculated free energy profile and thus the rate-determining step. During the work-up with D2O, the formed pro-K can further undergo hydrolysis, resulting in the formation of products 2a–d, as shown in the experimental results in Scheme 4a. Concurrently, the Ir intermediate INT10A, which is also generated, participates in an oxidative addition with another silane 1a, followed by a BIES-type C−H bond activation. This sequence of events culminates in the generation of the strained silametallacyclic intermediate INT4A, thereby completing the catalytic cycle efficiently.
Discussion
In conclusion, we have successfully developed a highly efficient and practical method for the synthesis of mixed-metalloid products through C−H borylation, enabled by iridium catalysts. This breakthrough addresses several long-standing challenges in the field of C−H activation, particularly the catalytic formation of strained benzo-fused metallacycles and the use of metalloid directing atom. The dual functionality of the formed mixed-metalloid compounds is a significant advantage, as it enables the synthesis of a wide range of valuable molecules with high versatility. The findings represent a significant advancement in organic synthesis, opening new avenues for the creation of complex and functionalized organic molecules with improved efficiency and selectivity.
Methods
General procedure for C−H borylation of hydrosilanes
For hydrosilanes 1a–1h
In a glove box, a 25 mL dried Schlenk tube equipped with a stirring bar was charged with silane (0.5 mmol), [Ir(cod)OMe]2 (8.3 mg; 0.0125 mmol, 2.5 mol%), dtbpy (6.7 mg; 0.025 mmol, 5 mol%), AcOK (123 mg; 1.25 mmol, 2.5 equiv.) and HBpin (160 mg; 1.25 mmol, 2.5 equiv.). The Schlenk tube was then removed from the glove box into air, and THF (1.5 mL, super dry) was added under a nitrogen flow. The mixture was heated to 125 °C and stirred for 6 h. The solvent was removed under reduced pressure, and the residue was purified by silica gel column chromatography to afford the products 2b–2h.
For hydrosilanes 1i–1z
In a glove box, a 25 mL dried Schlenk tube equipped with a stirring bar was charged with silane (0.5 mmol), [Ir(cod)OMe]2 (16.6 mg; 0.025 mmol, 5 mol%), dtbpy (13.4 mg; 0.05 mmol, 10 mol%), AcONa (102.5 mg; 1.25 mmol, 2.5 equiv.), HBpin (19.2 mg; 0.15 mmol, 30 mol%) and B2pin2 (190.6 mg; 0.75 mmol, 1.5 equiv.). The Schlenk tube was then removed from the glove box into air, and THF (1.5 mL, super dry) was added under a nitrogen flow. The mixture was heated to 150 °C and stirred for 6 h. The solvent was removed under reduced pressure, and the residue was purified by silica gel column chromatography to afford the products 2i–2z.
General procedure for C−H borylation of hydrogermanes
For hydrogermanes 3a–3k
In a glove box, a 25 mL Schlenk tube equipped with a stirring bar was charged with [Ir(cod)OMe]2 (6.64 mg; 0.01 mmol; 5 mol%), 2,2′:6′,2″-terpyridine (139.8 mg; 0.6 mmol; 3 equiv.), and AcOK (49 mg; 0.5 mmol; 2.5 equiv.). Then, the Schlenk tube was removed from the glove box into air. Germane (0.2 mmol), HBpin (64 mg; 0.5 mmol; 2.5 equiv.) and THF (3 mL) were added to the Schlenk tube under a nitrogen flow. The reaction mixture was then heated to 115 °C and stirred for 24 h. The solvent was removed under reduced pressure, and the residue was purified by silica gel column chromatography to afford the products 4b–4k.
Data availability
Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2378961 (2a) and 2378962 (2h). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source Data are provided with this manuscript. Supplementary information and chemical compound information are available in the online version of the paper. Reprints and permission information is available online at http://www.nature.com/reprints/. Correspondence and requests for materials should be addressed to Z.S. & M.W. Source data are provided with this paper.
References
Bergman, R. G. C–H activation. Nature 446, 391–393 (2007).
Wencel-Delord, J., Dröge, T., Liu, F. & Glorius, F. Towards mild metal-catalyzed C–H bond activation. Chem. Soc. Rev. 40, 4740–4761 (2011).
Dong, Z., Ren, Z., Thompson, S. J., Xu, Y. & Dong, G. Transition-metal-catalyzed C–H alkylation using alkenes. Chem. Rev. 117, 9333–9403 (2017).
Yang, Y., Lan, J. & You, J. Oxidative C–H/C–H coupling reactions between two (Hetero)arenes. Chem. Rev. 117, 8787–8863 (2017).
Wang, C.-S., Dixneuf, P. H. & Soulé, J.-F. Photoredox catalysis for building C–C bonds from C(sp2)–H bonds. Chem. Rev. 118, 7532–7585 (2018).
Davies, H. M. L. & Liao, K. Dirhodium tetracarboxylates as catalysts for selective intermolecular C–H functionalization. Nat. Rev. Chem. 3, 347–360 (2019).
Gandeepan, P. et al. 3d transition metals for C–H activation. Chem. Rev. 119, 2192–2452 (2019).
Guillemard, L., Kaplaneris, N., Ackermann, L. & Johansson, M. J. Late-stage C–H functionalization offers new opportunities in drug discovery. Nat. Rev. Chem. 5, 522–545 (2021).
Zhao, B., Prabagar, B. & Shi, Z. Modern strategies for C–H functionalization of heteroarenes with alternative coupling partners. Chem 7, 2585–2634 (2021).
Dalton, T., Faber, T. & Glorius, F. C–H activation: toward sustainability and applications. ACS Cent. Sci. 7, 245–261 (2021).
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C−H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010).
Colby, D. A., Bergman, R. G. & Ellman, J. A. Rhodium-catalyzed C−C bond formation via heteroatom-directed C−H bond activation. Chem. Rev. 110, 624–655 (2010).
Zhang, F. & Spring, D. R. Arene C–H functionalisation using a removable/modifiable or a traceless directing group strategy. Chem. Soc. Rev. 43, 6906–6919 (2014).
Liu, B. et al. Transition-metal-catalyzed, coordination-assisted functionalization of nonactivated C(sp3)–H bonds. Chem. Rev. 121, 14957–15074 (2021).
Sambiagio, C. et al. Comprehensive overview of directing groups applied in metal-catalysed C–H functionalisation chemistry. Chem. Soc. Rev. 47, 6603–6743 (2018).
Li, Z. & Shi, Z. Late-stage diversification of phosphines by C–H activation: a robust strategy for ligand design and preparation. Acc. Chem. Res. 57, 1057–1072 (2024).
Castillo, I. & Tilley, T. D. Synthesis of Sm−SiH3 complexes via σ-bond metathesis of the Si−C bond of phenylsilane. Organometallics 19, 4733–4739 (2000).
Boebel, T. A. & Hartwig, J. F. Silyl-directed, iridium-catalyzed ortho-borylation of arenes. a one-pot ortho-borylation of phenols, arylamines, and alkylarenes. J. Am. Chem. Soc. 130, 7534–7535 (2008).
Cho, S. H. & Hartwig, J. F. Iridium-catalyzed borylation of secondary benzylic C–H bonds directed by a hydrosilane. J. Am. Chem. Soc. 135, 8157–8160 (2013).
Su, B. & Hartwig, J. F. Iridium-catalyzed, silyl-directed, peri-borylation of C−H bonds in fused polycyclic arenes and heteroarenes. Angew. Chem., Int. Ed. 57, 10163–10167 (2018).
Showell, G. A. & Mills, J. S. Chemistry challenges in lead optimization: silicon isosteres in drug discovery. Drug Discov. Today 8, 551–556 (2003).
Franz, A. K. & Wilson, S. O. Organosilicon molecules with medicinal applications. J. Med. Chem. 56, 388–405 (2013).
Langkopf, E. & Schinzer, D. Uses of silicon-containing compounds in the synthesis of natural products. Chem. Rev. 95, 1375–1408 (1995).
Fleming, I., Barbero, A. & Walter, D. Stereochemical control in organic synthesis using silicon-containing compounds. Chem. Rev. 97, 2063–2192 (1997).
Nakao, Y. & Hiyama, T. Silicon-based cross-coupling reaction: an environmentally benign version. Chem. Soc. Rev. 40, 4893–4901 (2011).
Fricke, C., Deckers, K. & Schoenebeck, F. Orthogonal stability and reactivity of aryl germanes enables rapid and selective (multi)halogenations. Angew. Chem. Int. Ed. 59, 18717–18722 (2020).
Rogova, T., Ahrweiler, E., Schoetz, M. D. & Schoenebeck, F. Recent developments with organogermanes: their preparation and application in synthesis and catalysis. Angew. Chem. Int. Ed. 63, e202314709 (2023).
Tobisu, M., Onoe, M., Kita, Y. & Chatani, N. Rhodium-catalyzed coupling of 2-silylphenylboronic acids with alkynes leading to benzosiloles: catalytic cleavage of the carbon−silicon bond in trialkylsilyl groups. J. Am. Chem. Soc. 131, 7506–7507 (2009).
Ihara, H., Koyanagi, M. & Suginome, M. Anthranilamide: a simple, removable ortho-directing modifier for arylboronic acids serving also as a protecting group in cross-coupling reactions. Org. Lett. 13, 2662–2665 (2011).
Tobisu, M., Baba, K. & Chatani, N. Rhodium-catalyzed synthesis of germoles via the activation of carbon–germanium bonds. Org. Lett. 13, 3282–3284 (2011).
Ihara, H. & Suginome, M. Easily attachable and detachable ortho-directing agent for arylboronic acids in ruthenium-catalyzed aromatic C−H silylation. J. Am. Chem. Soc. 131, 7502–7503 (2009).
Aizenberg, M. & Milstein, D. Competitive generation of C–H and C–Si bonds by reductive elimination: formation of silametallacycles by metalation of silyl ligands. Angew. Chem., Int. Ed. 33, 317–319 (1994).
Aizenberg, M. & Milstein, D. Facial (methyl)(hydrido)(silyl) complexes of iridium: synthesis, X-ray structures, and reductive elimination reactions. Facile formation of silametalacycles by metalation of silyl ligands. J. Am. Chem. Soc. 117, 6456–6464 (1995).
Klei, S. R., Tilley, T. D. & Bergman, R. G. Reactions of Cp*(PMe3)Ir(Me)OTf with silanes: role of base-free silylene complexes in rearrangements of the resulting silicon-based ligands. Organometallics 21, 3376–3387 (2002).
McNally, A., Haffemayer, B., Collins, B. S. L. & Gaunt, M. J. Palladium-catalysed C–H activation of aliphatic amines to give strained nitrogen heterocycles. Nature 510, 129–133 (2014).
Willcox, D., Chappell, B. G. N., Hogg, K. F., Calleja, J., Smalley, A. P. & Gaunt, M. J. et al. A general catalytic β-C–H carbonylation of aliphatic amines to β-lactams. Science 354, 851–857 (2016).
Mkhalid, I. A. I., Barnard, J. H., Marder, T. B., Murphy, J. M. & Hartwig, J. F. C−H activation for the construction of C−B bonds. Chem. Rev. 110, 890–931 (2010).
Hartwig, J. F. Regioselectivity of the borylation of alkanes and arenes. Chem. Soc. Rev. 40, 1992–2002 (2011).
Ros, A., Fernández, R. & Lassaletta, J. M. Functional group directed C–H borylation. Chem. Soc. Rev. 43, 3229–3243 (2014).
Xu, L. et al. Recent advances in catalytic C−H borylation reactions. Tetrahedron 73, 7123 (2017).
Wright, J. S., Scott, P. J. H. & Steel, P. G. Iridium-catalyzed C−H borylation of heteroarenes: balancing steric and electronic regiocontrol. Angew. Chem., Int. Ed. 60, 2796–2821 (2021).
Fricke, C. & Schoenebeck, F. Organogermanes as orthogonal coupling partners in synthesis and catalysis. Acc. Chem. Res. 53, 2715–2725 (2020).
Dahiya, A., Fricke, C. & Schoenebeck, F. Gold-catalyzed chemoselective couplings of polyfluoroarenes with aryl germanes and downstream diversification. J. Am. Chem. Soc. 142, 7754–7759 (2020).
Xu, M.-Y. & Xiao, B. Germatranes and carbagermatranes: (hetero)aryl and alkyl coupling partners in Pd-catalyzed cross-coupling reactions. Chem. Commun. 57, 11764–11775 (2021).
Dahiya, A., Schoetz, M. D. & Schoenebeck, F. Orthogonal olefination with organogermanes. Angew. Chem. Int. Ed. 62, e202310380 (2023).
Boebel, T. A. & Hartwig, J. F. Iridium-catalyzed preparation of silylboranes by silane borylation and their use in the catalytic borylation of arenes. Organometallics 27, 6013–6019 (2008).
de la Vega-Hernández, K. et al. Radical germylzincation of α-heteroatom-substituted alkynes. J. Am. Chem. Soc. 140, 17632–17642 (2018).
Debrauwer, V. et al. Ligand-controlled regiodivergent palladium-catalyzed hydrogermylation of ynamides. J. Am. Chem. Soc. 142, 11153–11164 (2020).
Shishido, R. et al. General synthesis of trialkyl- and dialkylarylsilylboranes: versatile silicon nucleophiles in organic synthesis. J. Am. Chem. Soc. 142, 14125–14133 (2020).
Marciniec, B., Pietraszuk, C., Pawluć, P. & Maciejewski, H. Inorganometallics (transition metal−metalloid complexes) and catalysis. Chem. Rev. 122, 3996–4090 (2022).
Ureshino, T., Yoshida, T., Kuninobu, Y. & Takai, K. Rhodium-catalyzed synthesis of silafluorene derivatives via cleavage of silicon−hydrogen and carbon−hydrogen bonds. J. Am. Chem. Soc. 132, 14324–14326 (2010).
Okamoto, K. et al. External flash generation of carbenoids enables monodeuteration of dihalomethanes. Chem. Eur. J. 29, e202301738 (2023).
Onoe, M. et al. Rhodium-catalyzed carbon–silicon bond activation for synthesis of benzosilole derivatives. J. Am. Chem. Soc. 134, 19477–19488 (2012).
Simmons, E. M. & Hartwig, J. F. On the interpretation of deuterium kinetic isotope effects in C–H bond functionalizations by transition-metal complexes. Angew. Chem. Int. Ed. 51, 3066–3072 (2012).
Balcells, D., Clot, E. & Eisenstein, O. C–H bond activation in transition metal species from a computational perspective. Chem. Rev. 110, 749–823 (2010).
Davies, D. L., Macgregor, S. A. & McMullin, C. L. Computational studies of carboxylate-assisted C–H activation and functionalization at group 8–10 transition metal centers. Chem. Rev. 117, 8649–8709 (2017).
Tamura, H., Yamazaki, H., Sato, H. & Sakaki, S. Iridium-catalyzed borylation of benzene with diboron. Theoretical elucidation of catalytic cycle including unusual iridium(V) intermediate. J. Am. Chem. Soc. 125, 16114–16126 (2003).
Vanchura, B. A. et al. Electronic effects in iridium C–H borylations: insights from unencumbered substrates and variation of boryl ligand substituents. Chem. Commun. 46, 7724–7726 (2010).
Roosen, P. C. et al. Outer-sphere direction in iridium C–H borylation. J. Am. Chem. Soc. 134, 11350–11353 (2012).
Acknowledgements
We would like to thank financial support from National Key R&D Program of China (2022YFA1503200), the National Natural Science Foundation of China (Grants 92361201, 22025104 and 22171134), the Natural Science Foundation of Jiangsu Province (Grant BK20240059, BK20220033), the Fundamental Research Funds for the Central Universities (Grant 020514380326) for their financial support. We are also grateful to the High-Performance Computing Center of Nanjing University for performing the numerical calculations in this paper on its blade cluster system.
Author information
Authors and Affiliations
Contributions
Q.C. performed the experiments, mechanistic study, and analyzed the data. Y.Z. performed the crystallographic studies. Z.S. conceived the study and wrote the paper. M.W. performed the DFT calculation and conceived the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, Q., Wang, M., Zhao, Y. et al. Catalytic aromatic C−H borylation via strained Si and Ge metallacycles. Nat Commun 16, 8163 (2025). https://doi.org/10.1038/s41467-025-63335-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-63335-z