Abstract
The NiOx/perovskite interface in p-i-n inverted perovskite solar cells (PSCs) suffers from trap-assisted nonradiative recombination, chemical reactions and weak adhesion. The self-assembled molecules are usually designed to address the above issues. However, absonant bilateral bond strength with NiOx and perovskite hinders the realization of efficient and stable PSCs. Herein, a bilateral bond strength equilibrium strategy is proposed to stabilize the buried interface in inverted PSCs through functional group and spatial conformation engineering. 1-(benzothiaxole-2-ylthio)succnic acid (BTSA) is adsorbed on the surface of NiOx through the S atom, π-ring, and N atom in the benzothiazole, making benzothiazole ring parallel to the NiOx surface, which is beneficial for passivating bilateral defects and improving hole transport. This strategy leads to effective interfacial defect passivation, interfacial chemical reaction suppression and ameliorated electrical properties of NiOx films, enabling 1.53 eV PSCs and large-area module (764 cm2) with a PCE of 26.98% (certified 26.65%) and 21.98%, respectively.
Similar content being viewed by others
Introduction
Inverted perovskite solar cells (PSCs) have achieved significant advancements in long-term stability and power conversion efficiency (PCE)1,2,3,4,5,6,7,8. The hole transport layer (HTL) plays a critical role in realizing high-performance inverted PSCs9. Currently, almost all high-efficiency inverted PSCs are fabricated based on the following hole transport materials (HTMs), including poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine] (PTAA)3, self-assembled monolayers (SAMs)10,11, and nickel oxide (NiOx)12,13. However, each HTM has its limitations. PTAA suffers from poor reproducibility and wettability, which hinders its large-scale application14,15. Pure SAMs, though efficient, have weak adhesive force Indium Tin Oxide (ITO), impeding the realization of long-term stability16,17. NiOx inorganic HTM has the advantages of high hole mobility, low cost, high light transmittance, good stability and easy large-scale preparation, which has attracted wide attention. Although inverted PSCs based on NiOx have achieved a PCE exceeding 26%4,5,18, its long-term operational stability is still relatively poor. It has been revealed that chemical reaction would happen at the interface between NiOx and perovskite, where organic ammonium cation would deprotonate to generate organic amine and high valency nickel would oxidize iodide into I213,19,20. The illumination and heating conditions would accelerate the above interfacial chemical reactions. The defects at NiOx/perovskite interface, such as undercoordinated Pb2+, undercoordinated Ni3+ or Ni2+, halide vacancy, oxygen vacancy defects, etc., would also deteriorate the intrinsic stability of inverted PSCs. In addition, the energy level mismatch between NiOx and the perovskite layer leads to serious open-circuit voltage (VOC) loss13. Higher Ni3+ and Ni2+ content will be beneficial to improve the conductivity and stability of NiOx films, respectively, while Ni4+ is generally detrimental due to its strong oxidizing ability, which can oxidize I⁻ into I213,21,22. Therefore, further optimizing the Ni3+/Ni2+ ratio can achieve an optimal balance between improved stability and enhanced conductivity.
It was reported that SAMs have difficulty achieving uniform distribution on the surface of transparent electrodes (e.g., ITO or Fluorine-doped Tin Oxide (FTO)), which reduces the reproducibility of device performance23. The research shows that compared with ITO or FTO, NiOx can form stronger chemical bond with SAMs, which is beneficial to deposit uniform SAMs at NiOx surface, thereby enhancing device photovoltaic performance and reproducibility10,24,25. This suggests that adopting self-assembled organic molecules to modify NiOx is expected to simultaneously overcome the instability induced by SAMs or NiOx. In view of the above considerations, various SAMs have been employed to modify NiOx and construct dual HTLs24,25,26,27. For example, Chen et al.26 used 4-chlorobenzene sulfonic acid (CBSA) to modify NiOx/perovskite interface. Chlorine atoms on the benzene ring can provide growth sites for perovskites and release interfacial stress, while the sulfonic acid group (-SO3H) at the other end of the benzene ring can passivate the Ni3+ defect. CBSA modification can suppress interfacial chemical reactions, finally enhancing device stability and PCE. Besides, You et al.24 employed [4-(3,6-dimethyl-9H-carbazol-9-yl)butyl]phosphonic acid (Me-4PACz) SAMs to modify NiOx/perovskite interface. Yan et al.25 first used Me-4PACz SAMs to modify NiOx and then deposited Lewis base molecule 1,3-bis(diphenylphosphino)propane (DPPP), which passivated undercoordinated Pb2+ defects. As a result, the PCE of the modulated device was slightly increased after 3500 h of maximum power point (MPP) tracking. Zhu et al.27 adopted -OCH3 group functionalized SAM (4-(3,11-Dimethoxy-7H-dibenzo[c,g]carbazol-7-yl)butyl)phosphonic Acid (MeO-4PADBC) to stabilize NiOx/perovskite interface. The -PO3H2 at one end of MeO-4PADBC can anchor to the surface of NiOx and passivate the uncoordinated Ni3+ and Ni2+ defects, while the -OCH3 at the other end of MeO-4PADBC can passivate the uncoordinated Pb2+ defects through coordination action, improving the stability of the interface and device.
Although constructing SAM/NiOx dual HTLs exhibited immense potential, currently reported SAMs still face several challenges. First, reported SAMs can not simultaneously suppress deprotonation of organic cations and oxidation of I−, passivate undercoordinated Pb2+ defects, passivate undercoordinated Ni3+ and Ni2+ defects, and modulate interfacial energy band alignment. Second, reported SAMs usually have strong interaction with NiOx but do not have or have very weak interaction with perovskites because there is a lack of active functional groups at the end of the SAM molecule. This would not only make SAMs fail to passivate the defects at the bottom surface of perovskite films but also result in incongruous bond strength of SAMs with perovskite and NiOx. It has been reported that absonant bilateral bond strength is not conducive to defect passivation and interface stability28. Finally, most reported SAMs exhibit strong hydrophobicity and thus poor surface wettability, which is not favorable for following perovskite film deposition. Consequently, there is an urgent need to develop multifunctional self-assembled molecules by elaborate molecular design to simultaneously overcome the above challenges.
In this study, we proposed bilateral bond strength equilibrium strategy to stabilize buried interface in inverted PSCs. 2-((carboxymethyl)thio)succinic acid (CSA), thiodisuccinic acid (TSA), and 1-(benzothiaxole-2-ylthio)succnic acid (BTSA) were used to modify the interface between NiOx and perovskite. All three molecules can passivate interface defects via interaction with both NiOx and perovskite layers. However, compared with CSA and TSA, BTSA possessed more harmonious bond strength with NiOx and perovskite, resulting in its best defect passivation effect and the most robust buried interface. Except for bilateral defect passivation, BTSA can also inhibit the interfacial chemical reaction between NiOx and perovskite, enhance the conductivity of NiOx by regulating Ni3+/Ni2+ ratio, as well as optimize interfacial energy band alignment. As a result, the BTSA modulated inverted regular bandgap 1.53 eV device delivered a champion PCE of 26.98% (certified 26.65%), the record PCE value reported for the devices using vacuum flash evaporation. Our interface modification strategy is also effective for 1.66 eV wide-bandgap PSCs, demonstrating an attractive PCE of 24.50%. After continuous operation for 2000 h, the BTSA-based device maintained 93.4% of the initial PCE value, while the control devices maintained only about 71.9%. This work provides insights into strengthening buried interface stability by simultaneously passivating defects, suppressing interfacial chemical reactions, reducing interfacial energy level mismatch, and improving the electrical property of NiOx via meticulous self-assembled molecular design.
Results
Design of self-assembled molecules
To effectively passivate interface defects, -COOH and -S- functional groups were introduced into modification molecules29,30,31. It was inferred that these functional groups should be able to passivate undercoordinated Pb2+, undercoordinated Ni3+/Ni2+, halide vacancy, and oxygen vacancy defects. In addition, multiple -COOH groups were incorporated to chemically bridge NiOx and perovskite layers by bilateral polydentate chelating interaction, thereby heightening the stability of the buried interface32. The bilateral bond strength of modifiers with NiOx and perovskite can be regulated by the number of -COOH and the introduction of benzothiazole, aiming to achieve harmonious bilateral bond strength and thus robust buried interface for realizing long-term stability and efficient hole extraction and transport. It was speculated that -COOH can ionize protons to suppress the deprotonation of organic cations (e.g., FA+ and MA+)33. To minimize the effect of interface modification on wettability, molecular conjugation was reduced as far as possible through connecting -COOH and -S- groups via multiple single bonds instead of double bonds and aromatic rings. Based on the above considerations, CSA, TSA, and BTSA were designed and screened to manipulate the interface between NiOx and perovskite.
Investigation on the interaction of modifiers with NiOx and perovskite
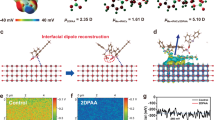
We first analyzed three molecules and their interactions with the NiOx and perovskite from a theoretical simulation perspective. Figure 1a shows the chemical structures and electrostatic potential (ESP) of three molecules. Obviously, the negative charge is mainly distributed on -C=O group at -COOH, and N/S atom in the benzothiazole. We calculated the charge density differences for the adsorption of three molecules on the NiOx surface (Fig. 1b). All modifiers can firmly bond with Ni through anchoring of -COOH and -S- functional groups12,16,34,35,36. BTSA consists of a benzothiazole, two -COOH groups and one -S- group. DFT calculations prove that the BTSA have the lowest adsorption energy and is most stable when the benzothiazole ring lies parallel to the NiOx surface. The results show that CSA and TSA bond to the Ni atom on the NiOx surface through the -S- and -COOH groups. For CSA and TSA, only the -COOH groups on both sides can anchor onto the NiOx surface. Attracted by the exposed O atom on the NiOx surface, some -COOH groups will inevitably ionize H+, which decreases the p-type conductivity. In contrast, BTSA was proven to strongly bond to the Ni atom of the NiOx surface through the S atom, π-ring, and N atom in the benzothiazole, which makes benzothiazole ring parallel to the NiOx surface. This parallel adsorption configuration of BTSA keeps the -COOH group away from the NiOx surface but are inclined to combine with uncoordinated Pb2+ at the bottom surface of perovskite films, and thus prevents -COOH from ionizing H+ to bond with O atoms on the NiOx surface, which increases the p-type conductivity. The charge transfer value for the three molecules on NiOx surface is 0.73 e, 0.95 e, and 1.8 e for CSA, TSA, and BTSA, respectively (Fig. 1c). This charge transfer should be feasible to increase the proportion of Ni3+ in the NiOx layer, thereby enhancing the p-type conductivity of NiOx37,38,39. Studies have shown that exposed Ni atoms on the surface of NiOx will act as electron donors, enhancing the n-type conductivity, while the exposed O atoms act as electron acceptors, contributing to p-type conductivity. Therefore, to enhance the p-type conductivity of NiOx based HTLs, it is crucial to passivate the exposed Ni atoms while avoiding the passivation of exposed O atoms, achieving precise control over its conductivity.
a Structure and electrostatic potential of CSA, TSA, and BTSA. b Adsorption structures and electrostatic potential of CSA, TSA, and BTSA on the NiO surface (cyan indicates a decrease in charge density, yellow indicates an increase in charge density). c One-dimensional planar-averaged charge density difference along the z-direction (The peak indicated by the arrow corresponds to the net charge transfer from the NiO surface to the molecule). d Binding energy of CSA, TSA, and BTSA with FAPbI3. e Binding energy of CSA, TSA, and BTSA with NiO. f Bar chart of the binding energy of CSA, TSA, and BTSA with NiOx.
We then investigated the chemical interaction from the differences in binding energies of three molecules with NiOx and perovskite (Fig. 1d, e). As exhibited in Fig. 1f, the DFT calculation results show that CSA, TSA, and BTSA with NiOx showed a Eb value of −2.52 eV, −2.23 eV and −1.22 eV, respectively, while they exhibited a Eb value of −1.47 eV, −1.40 eV, and −1.10 eV with FAPbI3 perovskite, respectively. The bilateral binding energy differences ΔEb (ΔEb = Eb(perovskite) − Eb(NiOx)) for CSA, TSA, and BTSA molecules are 1.05 eV, 0.83 eV, and 0.12 eV, respectively. As we mentioned above, the disharmonious bilateral bond strength is not beneficial for defect passivation and interface stability28. The minimum binding energy difference indicates that BTSA should be the most effective in defect passivation and strengthening buried interface stability.
Investigation of electrical property of NiOx films
It is well known that the content of Ni2+, Ni3+, and Ni4+ in NiOx has a significant impact on its conductivity. Ni3+ will enhance the p-type conductivity of NiOx, Ni2+ is beneficial for the stability of perovskite, while Ni4+ is usually harmful for its strong oxidizing property. To quantify the content of Ni in different valence states, we performed X-ray photoelectron spectroscopy (XPS) measurements to evaluate the interaction between the NiOx and three molecules. Through S 2p XPS peaks analysis, we first confirmed the presence of the three molecules on the surface of the NiOx film (Supplementary Fig. 1)35,40. The Ni 2p XPS peaks of the pristine NiOx films revealed a shift in binding energy after three molecules modulation, which indicates the chemical interaction of three molecules with NiOx (Supplementary Fig. 2)25. The chemical composition of the near-surface region was analyzed using XPS for the control and three molecules modulated NiOx samples, where fitting of the Ni 2p spectral envelope revealed at least three distinct species (Ni4+, Ni3+, and Ni2+). According to the specific details of the chemical states of Ni after fitting the Ni 2p spectra (Fig. 2a-d), we compiled the peak area of Ni4+, Ni3+, and Ni2+ into a bar chart (Fig. 2e)21. The fitting results indicate that the proportion of Ni4+ in NiOx was reduced from 36.4% of control film to 30.5%, 29.0% and 20.0% for CSA, TSA and BTSA, respectively, while the proportion of Ni2+ was increased from 30.1% of control film to 36.4% of CSA, 36.8% of TSA and 33.8% of BTSA. Specifically, as for the BTSA-modulated NiOx samples, the proportion of Ni3+ was increased from 33.5% to 46.2%. This indicates that the modification of BTSA can effectively diminish the content of Ni4+ and increase the content of Ni2+ and Ni3+. In general, the presence of Ni3+ facilitates p-type hole transport in NiOx HTL. We actually observed a significant improvement in hole mobility (Fig. 2f) and conductivity (Fig. 2g) in the three modulated NiOx films, especially in the BTSA modulated films, whereas the enhancements were less pronounced in NiOx films modulated with CSA or TSA.
a–d XPS spectra of Ni 2p core levels for the control, CSA, TSA, and BTSA-modulated NiOx films. e Bar chart of the relative XPS peak area values of Ni4+, Ni3+, and Ni2+ extracted from the XPS spectra of Ni 2p core levels for the control, CSA, TSA, and BTSA-modulated NiOx films. The hole mobility (f) and the conductivity (g) of the control, CSA, TSA, and BTSA-modulated NiOx films. h Photographs of NiOx films before and after treatment with spin-coating FAI in DMF solution (Film 1), BTSA/FAI in DMF solution (Film 2), and bare DMF solution (Film 3). i UV-visible absorption spectra of the films of fresh and aged (UV illumination for 24 h) ITO/NiOx/FAI and ITO/NiOx/BTSA/FAI.
Suppression of interfacial chemical reaction
We have demonstrated that BTSA can effectively reduce the content of Ni4+, which should contribute to inhibiting the interfacial reaction between Ni4+ and I-. To confirm this point, we conducted a direct aging experiment by spin-coating an FAI in DMF solution (Film 1), a BTSA/FAI in DMF solution (Film 2), and bare DMF solution (Film 3) onto NiOx films (Fig. 2h). These treated NiOx films were exposed to UV-ozone for 5 min. Usually, NiOx films exhibited black color due to a high proportion of high-valence Ni. It is seen that acting as the reference samples, Film 3 showed no color change, indicating that NiOx exhibits no obvious decomposition, ruling out the influence of the DMF solvent. However, Film 1 exhibited discoloration from black to gray, indicating a reaction between FAI and NiOx13,37. In contrast, Film 2 retained its original black color, confirming that BTSA effectively prevented the reaction between FAI and NiOx. Subsequently, we quantitatively measured the absorption spectra of ITO/NiOx/FAI and ITO/NiOx/BTSA/FAI films before and after 24 h of UV light exposure. Normally, the synthesized black NiOx nanoparticles with outstanding hole transporting performance usually exhibit significant light absorption characteristics in the visible light range. In contrast, the generated I2 by interface reaction has a clear characteristic absorption peak at ~400 nm. As presented in Fig. 2i, in the UV-Vis absorption spectrum range of 400–900 nm, we confirm the higher intensity indicates a superior hole transport performance of NiOx with high Ni≤3+ content. The pristine NiOx/FAI film exhibited a severe decline in absorption intensity in the wavelength range of 400–900 nm and a slight increase in the region of 300–400 nm. The color change and absorbance changes of NiOx/FAI samples indicate a significant redox reaction, revealing the decreased content of Ni≤3+ and increased I2. In contrast, the BTSA-modulated film showed minimal change, further confirming that BTSA could inhibit the chemical reaction at NiOx/FAI or perovskite interface.
Characterizations of perovskite film quality
Considering the significant effect of substrates on the morphology and crystallization of the deposited perovskite films, we explored the morphology of perovskite films deposited on the NiOx substrates without or with CSA, TSA, and BTSA modification by performing atomic force microscope (AFM) and scanning electron microscope (SEM) measurements. The Root Mean Square value was 2.5 nm for the pristine NiOx film, while it was reduced to 1.8 nm after modulation with BTSA, indicating that BTSA can improve the uniformity of NiOx surface (Supplementary Fig. 3). From SEM images of the bottom surface of perovskite films, we can observe evident large pinholes in the control perovskite film whereas the pinholes disappeared in modulated perovskite films (Fig. 3a). From SEM images and the statistical graphs of grain size distribution of the top surface of perovskite films, CSA and TSA modulated perovskite films exhibited much smaller pinholes than control perovskite film (Supplementary Fig. 4). In comparison, no pinholes were found in BTSA modulated perovskite film. Moreover, the grain size was enlarged after interface modification. This indicates that all SAMs can improve perovskite crystallization but BTSA is the most effective. From the XRD patterns, we can observe the improved crystallinity of perovskite films modulated with these three molecules, and the full-width half-maximum (FWHM) of the characteristic peak (100) of perovskite modulated by BTSA is the lowest, indicating that BTSA has the best effect on perovskite crystallization (Supplementary Fig. 5).
a SEM images of the bottom surface of the perovskite films deposited on different HTLs. Steady-state PL (b) and TRPL (c) spectra of the 1.53 eV Cs0.05MA0.05FA0.9PbI3 based perovskite films deposited on the nonconductive glass substrates without or with CSA, TSA, and BTSA modulation, which was measured from glass side. d TAS of the control and BTSA-modulated devices. e TPV curves of the control, CSA, TSA, and BTSA-treated devices. f VOC-light intensity dependence of the control and BTSA-based devices. g Dark J-V measurements of the control, CSA, TSA and BTSA-based inverted devices.
We then investigated the photoluminescence (PL) emission intensity and carrier lifetimes of perovskite films using steady-state PL and time-resolved photoluminescence (TRPL). As shown in Fig. 3b, the PL intensity was gradually increased from control, CSA, TSA and BTSA-modulated perovskite films, suggesting that these SAMs can passivate the defects at the bottom surface of perovskite films but BTSA is optimal. As exhibited in Fig. 3c, the average carrier lifetimes of the control, CSA, TSA, and BTSA-modulated perovskite films were 247 ns, 458 ns, 778 ns, and 1559 ns, respectively. Obviously, BTSA-modulated perovskite film had the longest carrier lifetime, implying that BTSA is the most efficient in passivating interface defects due to harmonious bilateral bond strength. In addition, the BTSA-modulated perovskite film had fewer defect densities, which could be supported by the space-charge-limited current of the hole-only device with the calculating formula of Nt = 2ε0εrVTFL∕qL241,42. From Supplementary Fig. 6, we can see that the trap-filled limited voltage (VTFL) of control, CSA, TSA, and BTSA-modulated perovskite films was decreased from 0.22 V to 0.13 V, 0.12 V and 0.10 V, indicating that BTSA can effectively passivate the defects at the NiOx/perovskite buried interface. Subsequently, we used thermal admittance spectroscopy (TAS) to investigate the impact of BTSA modification on perovskite defects (Fig. 3d). Compared to the control, the BTSA-modulated perovskite film exhibited lower shallow-level and deep-level defect densities, demonstrating excellent passivation ability of BTSA43,44.
Study on surface potential and energy levels of NiOx films
The interface energy level mismatch between NiOx and perovskite is also an important cause of severe recombination losses. Kelvin Probe Force Microscope (KPFM) was used to gain deep insights into the surface potentials of the NiOx films and the changes in the energy level structure inside the PSC device (Supplementary Fig. 7). From the statistical histogram of KPFM, we can obtain that the average surface potential for control, TSA, CSA, and BTSA modulated NiOx films was −1.10 V to −1.01 V, −1.06 V, and −0.76 V, respectively. This indicates that the modification of BTSA can maximize the surface potential of perovskite films, with an average increase of 0.34 V45. At the same time, BTSA has the narrowest FWHM value, which is 12.28 mV, much lower than the 16.05 mV, 14.21 mV, and 12.30 mV of NiOx films modulated by control, TSA, and CSA. The above results indicate that BTSA modulation could induce the most significantly increased surface potential and more uniform surface potential, which is favorable for an increase in the work function of NiOx HTL and homogenized charge transfer in the interface. From the ultraviolet photoelectron spectroscopy (UPS) of secondary electron cut-off and valence bands (Supplementary Fig. 8), the Fermi level (EF) values of NiOx, NiOx/CSA, NiOx/TSA, and NiOx/BTSA films were calculated to be −4.89 eV, −4.91 eV, −4.99 eV, and −5.04 eV, respectively. Then, the highest occupied molecular orbital (HOMO) levels were calculated to be −5.50 eV, −5.60 eV, −5.63 eV, and −5.68 eV, respectively. We also measured the corresponding energy level of Cs0.05MA0.05FA0.9PbI3 we used. The calculated valence band of perovskite at −5.93 eV. The energy level diagram (Supplementary Fig. 9) indicates that the energy difference between the NiOx and perovskite valance bands for hole extraction is the lowest when modifying NiOx with BTSA, leading to small energy loss at the interface for the solar cell devices46,47.
Characterization of interfacial carrier dynamics
The transient photocurrent (TPC) measurement was carried out to evaluate interfacial charge transfer. TPC in Supplementary Fig. 10 shows that the current decay times of control, CSA, TSA, and BTSA-modulated devices were 2.87 μs, 1.71 μs, 1.32 μs, and 1.00 μs, respectively. Compared to the control device, the CSA, TSA, and BTSA-modulated devices exhibited improved hole extraction and transfer at the buried interface, which could be mainly ascribed to reduced interface defects and increased hole mobility of NiOx films. The Mott-Schottky test was conducted to delve into carrier separation and transport at the perovskite/HTL interface (Supplementary Fig. 11)41. The built-in potential (Vbi) of the control, CSA, TSA, and BTSA-based devices was gradually increased, indicating promoted carrier separation and transport following interface modification, which is closely correlated with reduced interface defects, ameliorative band alignment and reinforced buried interface48.
The carrier recombination within devices was studied by Transient photovoltage (TPV). As shown in Fig. 3e, the voltage decay times of the control, CSA, TSA and BTSA-modulated devices were 34.4 μs, 170 μs, 221 μs, and 270 μs, respectively. This shows that BTSA-based device exhibited the least serious non-radiative recombination. Enhanced charge transport and reduced nonradiative recombination were further confirmed by electrochemical impedance spectroscopy (Supplementary Fig. 12) and open-circuit voltage (VOC) versus light intensity measurements (Fig. 3f). Compared with the control device (1.85), the BTSA-modulated device (1.28) had a much lower ideality factor, suggesting that BTSA modification resulted in remarkably reduced nonradiative recombination due to mitigated interface defects and improved electrical properties of NiOx. After CSA, TSA and BTSA modulation, the dark current density was diminished by 2 orders of magnitude due to the inhibition of non-radiative recombination at the interface (Fig. 3g)49. In a word, CSA, TSA and BTSA can suppress the nonradiative recombination at the buried interface but BTSA is the most effective due to its harmonious bilateral bond strength.
Photovoltaic performance of PSCs
We fabricated the inverted devices with the architecture of FTO/NiOx/CSA, TSA, or BTSA/perovskite/C60/BCP/Ag to evaluate the effect of interface modification on photovoltaic performance (Fig. 4a). Specifically, the perovskite layer was crystallized by employing a vacuum flash evaporation process in ambient conditions. The composition for fabricating perovskite films and devices was 1.53 eV Cs0.05MA0.05FA0.9PbI3 or 1.66 eV Cs0.05MA0.15FA0.8Pb(I0.76Br0.24)3. The CSA, TSA, and BTSA-modulated PSCs exhibited an average PCE value of 24.83%, 25.67% and 26.89%, respectively, which is much higher than 23.53% for the control devices (Fig. 4b). It is worth noting that the BTSA-modulated devices exhibited the most noticeable enhancement in VOC and fill factor (FF), leading to the highest PCE. The amplitude of the photovoltaic performance improvement follows the sequence of BTSA > TSA > CSA, which is consistent with the previous theoretical calculations of the binding energy differences. Figure 4c presents the champion J-V curves in reverse scan (RS) and forward scan (FS) for the control and modulated PSCs. The control, CSA, TSA and BTSA-modulated PSCs exhibited the best PCE values of 24.40% (a short-circuit current density (JSC) of 25.90 mA cm−2, a VOC of 1.148 V, and an FF of 82.05%), 25.47% (a JSC of 26.20 mA cm−2, a VOC of 1.174 V, and an FF of 82.78%), 25.93% (a JSC of 26.24 mA cm−2, a VOC of 1.177 V, and an FF of 83.94%), 26.98% (a JSC of 26.37 mA cm−2, a VOC of 1.191 V, and an FF of 85.87%), respectively. Our obtained 26.98% is the record PCE ever reported for PSCs using the vacuum flash evaporation method (Fig. 4d). The BTSA-modulated PSCs also achieved an attractive certified 300 s steady-state average PCE of 26.65% (Supplementary Fig. 13 and Supplementary Table 1). The integrated current density from external quantum efficiency (EQE) was 25.43, 25.49, 25.59, and 25.90 mA cm–2, which matches with the JSCs from J–V curves (Supplementary Fig. 14). Our BTSA modulation strategy was also successfully extended to 1.66 eV wide-bandgap (WBG) PSCs. As illustrated in Supplementary Fig. 15 and Supplementary Table 2, the control 1.66 eV WBG PSCs only exhibited a PCE of 21.38% (a JSC of 21.46 mA cm−2, a VOC of 1.242 V, and an FF of 80.20%) in RS mode and 20.35% (a JSC of 21.53 mA cm−2, a VOC of 1.238 V, and an FF of 76.34%) in FS mode. In contrast, the BTSA-modulated WBG PSCs exhibited an improved champion PCE of 24.50% (a JSC of 22.03 mA cm−2, a VOC of 1.314 V, and an FF of 84.64%) in RS mode and 24.44% (a JSC of 22.15 mA cm−2, a VOC of 1.313 V, and an FF of 84.03%) in FS mode. These results demonstrated the universality of our developed buried interface passivation method. Furthermore, the PCE of 24.50% is also the highest value reported for 1.66 eV WBG PSCs. The EQE curves of the device with a 1.66 wide-bandgap perovskite based on control and BTSA was 21.12 mA cm−2 and 21.43 mA cm−2, respectively, which matches with the JSC values from J-V curves (Supplementary Fig. 16). Benefiting from the excellent wettability of BTSA on NiOx surface, we then fabricated a large-area module by vacuum flash method to verify the effectiveness and universality of BTSA passivation, where 46 subcells were connected in series (Fig. 4e and Supplementary Fig. 17). The champion BTSA-modulated PSC module with an aperture area (including dead area) of 764 cm2 exhibited a remarkable PCE from of 21.98% (a ISC of 407.63 mA, a VOC of 51.05 V, and an FF of 80.7%).
a Schematic diagram of the CSA, TSA, and BTSA modulated NiOx-based inverted PSCs prepared by vacuum flash evaporation technique. b Statistical distribution diagram of photovoltaic parameters of the control, CSA, TSA, and BTSA-modulated devices (Error bars represent the range of the data, minimum to maximum values; the height of the rectangular bar represents the mean value of the dataset; individual data points are shown (n = 7 per group)). c J−V curves of the best-performing control, CSA, TSA, and BTSA-modulated 1.53 eV-perovskite-based devices. d Comparison of the historical PCEs of the PSCs prepared using vacuum flash technique. e I–V curves of the champion modules with the treatment of BTSA, the inset shows the photograph of a perovskite solar modules based on 30 cm × 30 cm (aperture area of 764 cm2). f MPPT stability of the encapsulated control and BTSA-modulated devices under continuous 1-sun-equivalent white-light LED illumination in ambient air.
Long-term stability of PSCs
Finally, we evaluated the stability of the control and modulated PSCs. We first tracked the laser beam-induced current (LBIC) of the unencapsulated devices during long-term storage in an ambient atmosphere (Supplementary Fig. 18)2. LBIC revealed the photocurrent mapping and generated the distribution of internal trap defects in the devices on a macroscopic scale. We observed that the photocurrent of all devices increased after 4 days. However, the BTSA-based device continued to maintain an increase in photocurrent after 43 days, while the other devices showed significant degradation. After 67 days, the photocurrent of the CSA and TSA-based devices decreased to the same level as the control group, while the BTSA-based device still maintained a higher photocurrent. This indicates that the BTSA-based device significantly improved photocurrent and exhibits excellent stability when stored in air environment. We attribute the enhanced stability of the device to BTSA modulation, which effectively suppressed interfacial redox reactions, enhanced the quality of both the NiOx and perovskite films, passivated defects at the buried interface and heightened interface stability.
The long-term stability of devices with and without BTSA modulation was then systematically investigated. Unencapsulated devices were aged in ambient air (30 ± 5% relative humidity, 25 ± 5 °C) under dark conditions. After 1200 h, the BTSA-modulated device could retain 90.2% of its initial efficiency, whereas the control devices maintained only about 72.1% (Supplementary Fig. 19). Then, We documented Cyclic J−V sweep stability of the unencapsulated control and BTSA-modulated devices in ambient air, the BTSA-modulated device maintained a PCE of 26.85% without attenuation after 2200 cycles of J−V sweep, while the PCE of standard device attenuated from 24.17% to 23.21% (Supplementary Fig. 20). Additionally, the thermal stability of the unencapsulated devices was investigated by aging at 65 °C in nitrogen atmosphere for 630 h (Supplementary Fig. 21). It is revealed that the BTSA-based device could maintain 96.4% of the initial PCE value, while the control devices only maintained their initial PCE value of about 59.9%. This indicates that the modification of BTSA is also beneficial for improving the thermal stability of the PSC devices, which is mainly due to the ability of BTSA to suppress the negative chemical reaction process between NiOx and perovskite as well as reduced interfacial defects. We also conducted MPP tracking for the encapsulated control and BTSA-based devices under continuous 100 mW cm−2 white LED light irradiation at room temperature of 25 ± 5 °C (Fig. 4f). After continuous operation for 2000 h, the BTSA-based device maintained 93.4% of the initial PCE value, while the control devices maintained only about 71.9%.
In conclusion, we developed bilateral bond strength equilibrium strategy to stabilize buried interface in inverted PSCs. CSA, TSA, and BTSA were adopted to modulate the NiOx/perovskite interface. By bilateral polydentate chelating, these self-assembled molecules can passivate interface defects, inhibit interfacial chemical reactions and improve the electrical properties of NiOx. Due to its bilateral bond strength equilibrium of BTSA, the best defect passivation effect and the most robust buried interface were realized. The BTSA modification resulted in highly efficient inverted 1.53 eV PSCs with a champion PCE of 26.98% (certified 26.65%), which is the record PCE value reported for the devices using vacuum flash evaporation. After continuous MPP tracking for 2000 h, the BTSA-modulated device retained 93.4% of its initial PCE value. Remarkably, we also demonstrate champion efficiency of 21.98% on the large-area PSC module with aperture areas 764 cm2. This work provides an effective approach for enhancing buried interface stability by rational engineering of self-assembled molecules, which paves the way for the commercialization of perovskite photovoltaic technology.
Methods
Materials
Formamidine iodide (FAI, 99.5%), methylammonium iodide (MAI, 99.5%), methylamine hydrochloride (MACl, 99.5%) and methylammonium bromide (MABr, 99.5%) were purchased from Greatcell Solar Materials. Ltd. Lead iodide (PbI2, 99.999%), cesium iodide (CsI, 99.99%) and lead bromide (PbBr2, 99.999%) were purchased from Chengdu Alpha Metal Materials Co., Ltd. N,N-dimethylformamide (DMF, ≥99.5%), dimethyl sulfoxide (DMSO, 99.9%) and isopropanol (IPA, ≥99.7%) were purchased from Sigma-Aldrich. Bathocuproine (BCP, 99.5%) and C60 (99.9%) were purchased from Advanced Election Technology. 2-((Carboxymethyl)thio succinic acid (CSA, 95%), Thiodisuccinic Acid (TSA, 95.0%) and 1-(benzothiaxole-2-ylthio)succnic acid (BTSA, 98.0%) were obtained from MACKLIN. ITO and FTO substrates were purchased from Beijing Huamin New Materials Technology Co., Ltd.
Nickel(II) nitrate hexahydrate (Ni(NO3)2·6H2O) was purchased from Sigma-Aldrich for synthesizing the NiOx nanoparticles. All chemicals were used without further purification. NiOx nanoparticles were synthesized as follows: Firstly, 3 g of Ni(NO3)2·6H2O was dissolved in 50 mL of deionized (DI) water. The solution was stirred at room temperature for 30 min. Subsequently, a 1.0 M NaOH solution was slowly added with continuous magnetic stirring until the pH reached approximately 10. After an additional hour of stirring, a green precipitate was obtained via centrifugation at 9000 rpm for 6 min. The precipitate was washed three times with DI water. The collected green solid was frozen for 3 h, then freeze-dried at 5 Pa for 15 h, and finally calcined at 270 °C for 2 h to produce NiOx nanoparticles. Prior to use, the NiOx was dispersed in DI water by sonication for 10 min at a concentration of 10 mg/mL.
Preparation of perovskite precursor solutions and perovskite film deposition
1.53 eV-Cs0.05MA0.05FA0.9PbI3
The stoichiometric Cs0.05MA0.05FA0.9PbI3 precursor solution (1.73 M) was prepared by dissolving 20.8 mg CsI, 247.7 mg FAI, 12.7 mg MAI, 10 mg MACl, and 824.8 mg PbI2 in mixed solvents of DMF/DMSO (4:1, by volume). For the 1.53 eV-Cs0.05MA0.05FA0.9PbI3 film crystallization, the perovskite films were deposited by spin-coating the perovskite precursor solution on glass/FTO/NiOx/(CSA, TSA, or BTSA) substrate at 1000 rpm for 10 s and 2500 rpm for 30 s. The wet perovskite film was quickly put into a sample chamber connected to vacuum-pumping instrumentation (Internal space is 12 cm × 12 cm × 2.1 cm). By opening the valve connecting the specimen chamber to the pump system, the perovskite film was immediately exposed to low pressure maintained at 10 Pa for 30 s, followed by full pressurization by admitting ambient air into the specimen chamber. Subsequently, the perovskite film was annealed at 110 °C for 15 min in the air (RH = 30 ± 10%) for full crystallization.
1.66 eV-Cs0.05MA0.15FA0.8Pb(I0.76Br0.24)3
The stoichiometric Cs0.05MA0.15FA0.8Pb(I0.76Br0.24)3 precursor solution (1.4 M) was prepared by dissolving 18.2 mg of CsI, 192.6 mg of FAI, 536 mg of PbI2 (10% of excess), 131 mg of PbBr2, and 23.5 mg MABr in mixed solvents of DMF/DMSO (4:1, by volume). For the 1.66 eV- Cs0.05MA0.15FA0.8Pb(I0.76Br0.24)3 film crystallization, the perovskite films were deposited by spin-coating the perovskite precursor solution on glass/ITO/NiOx/(Me-4PACz or BTSA) substrate at 1000 rpm for 10 s and 2500 rpm for 30 s (The device with the treatment of Me-4PACz is used as Control). The wet perovskite film was quickly put into a sample chamber connected to vacuum-pumping instrumentation. The precursor perovskite film was transferred to the hot plate and annealed at 100 °C for 15 min in the N2 for full crystallization.
Device fabrication
First, ITO or FTO substrate was etched by laser etching technology. Ultrasonic cleaning of ITO or FTO glass after etching was carried out with glass cleaning agent, DI water, and ethanol in sequence, and each step took 5 min. The wettability of the ITO or FTO substrate was enhanced by UV-ozone modulation for 20 min. Then, the ink of NiOx nanoparticles (10 mg NiOx nanoparticles dispersed into 1 ml DI water) was spin-coated on ITO or FTO glass at 2000 rpm for 30 s and annealed at 150 °C for 10 min at room temperature. For modulated NiOx films, the CSA, TSA or BTSA solution (0.5 mg/ml dissolved in IPA) was spin-coated at 5000 rpm for 30 s on substrate and then annealed at 110 °C for 10 min. For the 1.53 eV-Cs0.05MA0.05FA0.9PbI3 film crystallization, the perovskite films were deposited by spin-coating the perovskite precursor solution on glass/ITO or FTO/NiOx/(CSA, TSA, or BTSA) substrate at 1000 rpm for 10 s and 2500 rpm for 30 s. The wet perovskite film was quickly put into a sample chamber (10 Pa for 30 s), subsequently, the perovskite film was annealed at 110 °C for 15 min in the air (RH = 30 ± 10%) for full crystallization. For the 1.66 eV- Cs0.05MA0.15FA0.8Pb(I0.76Br0.24)3 film crystallization, the perovskite films were deposited by spin-coating the perovskite precursor solution on glass/ITO or FTO/NiOx/(Me-4PACz or BTSA) substrate at 1000 rpm for 10 s and 2500 rpm for 30 s. The wet perovskite film was quickly put into a sample chamber (10 Pa for 30 s), subsequently, the perovskite film was annealed at 100 °C for 15 min in the N2 for full crystallization. Then PDI2 (0.4 mg/ml dissolved in IPA) or EDAI2 (WBG, 1 mg/ml dissolved in IPA) was spin-coated on the crystallized perovskite film at 5000 rpm for 30 s, and annealed at 100 °C for 5 min. Then, 30 nm C60 and 7 nm BCP were thermally evaporated on the perovskite films under a high vacuum 5 × 10−4 Pa, sequentially. Finally, the 100 nm Ag/Cu electrode is thermally evaporated at the top. The PSCs with Cu electrodes were used for the stability test. For sealing the devices for MPP stability testing, a UV-curable adhesive (Eversolar AB-341, Everlight Chemical) was applied over the active area of the PSCs, followed by the placement of a highly transparent glass panel. The device was then compressed and cured under ultraviolet light for 3 min.
Fabrication of large-area PSC modules
For the 30 × 30 cm2 solar module, the FTO cleaning process is the same as above, the red laser of 1064 nm was used to scribe the 30 × 30 cm2 FTO substrate (P1). NiOx layer (15 nm) was grown by DC sputtering on washed FTO substrates at room temperature with a chamber pressure of 0.37 Pa. The BTSA layer was blade-coated with a blade gap of 120 µm above the NiOx layer at a speed of 8 mm/s. For perovskite films, 1 M perovskite precursor solution was coated on HTL substrate by slot-die method with a gap between the slip and the coated substrate of 110 μm, a feed pump speed of 10 μl/s, and a substrate moving speed of 8 mm/s. The perovskite wet film was quickly transferred to the sample chamber connected to the vacuum pump. The vacuum pump was turned on, and the perovskite film was immediately exposed to a low pressure of 10 Pa for 300 s. Subsequently, the resulting perovskite films were annealed at 110 °C for 25 min in air. Then, C60 (20 nm) and BCP (7 nm) were sequentially thermally evaporated onto the perovskite films in a vacuum chamber (<5 × 10−4 Pa) and a green laser of 532 nm (P2) was used to etch the obtained perovskite/C60/BCP films. Finally, a 110 nm Ag electrode was thermally evaporated on the BCP film in a vacuum chamber (<3 × 10−4 Pa) and the Ag electrode was divided by the green laser of 532 nm with a line width of 350 μm (P3). The modules fabricated on 30 × 30 cm2 perovskite solar modules with an effective area of 764 cm2 contain 46 series connected cells with a GFF of 85%.
Device characterization
The J–V characteristics were obtained using a Keithley 2400 source meter in a controlled environment simulating AM 1.5 solar irradiance (100 mW cm−2), facilitated by a standard xenon lamp solar simulator (7ISO503A, SOFN INSTRUMENTS). These measurements were conducted either via a forward scan, ranging from −0.02 to 1.20 V, or a reverse scan, from 1.20 V down to −0.02 V. The J–V measurements for the solar cells were conducted in ambient air conditions. The external quantum efficiency (EQE) was determined using an EQE measurement system from ENLITECH, Taiwan. All assessments were performed at room temperature in an ambient atmosphere, and the solar cells were not encapsulated during these measurements. LBIC mapping measurements were tested by LSD4 of ENLITECH.
Certification of PSCs
The PSC devices were sent to the Tianjin Institute of Metrological Supervision and Testing, China, for official certification. Specifically, we fill the prepared solar cell samples for certification testing with nitrogen, then store them with a vacuum sealer and always place them in a dark sealed box. During the certification test, the certification agency recalibrates the effective active area of our solar cells to ensure accurate evaluation of data such as photocurrent density. The test environment and test methods are recorded in the certification data in Supplementary Fig. 13.
Characterization of device stability
The operational MPPT stability of the encapsulated devices was assessed under continuous one-sun conditions using a white LED lamp without a UV filter in ambient conditions. These tests were conducted using an MPP tracking system (YH-VMPP-16, Yanghua, Suzhou). Specifically, 16 solar cell samples were simultaneously placed in aging equipment. The environment around solar cells is maintained at around 25 °C through a refrigeration system. Aging equipment is tracked and tested by continuously scanning and analyzing the maximum power point of the device. White LED light sources have a light intensity energy of approximately 100 mW cm−2. The recording of Long-term stability of the unsealed devices was done using the Keithley 2400 digital source meter, and the data was obtained using the Zeal Young software K2400 Experimental Platform-Professional2450V3.5.
Characterization of morphology and crystal structure of perovskite films
SEM measurement was carried out by JEOL JSM7610F SEM. The scanning voltage is 3 kV. X-ray diffraction (XRD) patterns were measured on Rigaku SmartLab X-ray diffractometer using Cu Ka target radiation (λ = 1.5405 A) (measurement power of 2 kW, scan rate of 8° min−1). Field-emission SEM (JEOL 7610 F) was used to scan the surface morphology of the film and the cross-sectional view of the device at 3 kV.
Photoluminescence and ultraviolet-visible absorption measurements
The SSPL and TRPL were measured by a fluorescence spectrometer (FLS1000, Edinburgh Instruments), and the incident direction of the laser was from glass side. The ultraviolet-visible absorption spectra of the films were measured by Shimadzu UV-1900 spectrophotometer.
Atomic force microscope measurements
The Asylum Research MFP-3D was used to measure AFM morphology and KPFM in tapping mode.
X-ray photoelectron spectroscopy and ultraviolet photoelectron spectroscopy measurements
XPS and UPS spectra were collected through Thermo ESCALAB XI+. Fourier transform infrared (FTIR) spectroscopy was recorded using the FTIR-805 spectrometer of Tianjin Gangdong SCI.&TECH. CO,.LTD.
Mott–Schottky and electrochemical impedance spectroscopy measurements
The Mott-Schottky measurements were performed using the AMETEK VersSTAT 3F electrochemical workstation at a fixed frequency of 1k Hz. The applied bias voltage range is 0–1.2 V. EIS measurements are made in the dark, with frequencies ranging from 0.1 Hz to 1,000,000 Hz, using the same instruments as the Mott-Schottky measuring instruments.
Transient photocurrent and photovoltage measurements
TPC/TPV was tested by PD-RS of ENLITECH, Taiwan. The long-term stability was conducted in ambient conditions at a relative humidity (RH) of 35 ± 5% and a temperature of 25 ± 5 °C.
Density functional theory calculations
The DFT calculations were carried out utilizing the Perdew-Burke-Ernzerhof exchange-correlation functional. Projector augmented-wave pseudopotentials were adopted with a plane-wave cutoff energy of 400 eV. To account for van der Waals interactions, the DFT-D3 method was included. Modeling of the FAPbI3 surface involved the use of a slab comprising five atomic layers, separated by a vacuum gap of 30 Å. Alternatively, the slab model of the NiOx surface contained four atomic layers and a vacuum gap of 20 Å. The dipole correction for slab calculations was taken into account. The convergence criterion for the atomic forces was set to 0.02 eV/Å. The binding energy of the passivator with the FAPbI3 or NiOx surface was defined as Eb = E(slab@passivator) – E(slab) – E(passivator), where E(slab@passivator) represents the total energy of the system combining the slab and the passivator, E(slab) is the energy of the isolated slab, and E(passivator) is the energy of the standalone passivator. The electrostatic potentials (φ) of the passivators were calculated at the B3LYP/def2TZVP level with DFT-D3 using the Gaussian 09 package.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. Additional details that support the findings of this study will be made available by the corresponding author upon request. Source data are provided with this paper.
References
Zhang, Z. et al. Rationally designed universal passivator for high-performance single-junction and tandem perovskite solar cells. Nat. Commun. 16, 753 (2025).
Gao, W. et al. Synergistic modulation of orientation and steric hindrance induced by alkyl chain length in ammonium salt passivator toward high-performance inverted perovskite solar cells and modules. Adv. Mater. 37, 2413304 (2025).
Li, H. et al. 2D/3D heterojunction engineering at the buried interface towards high-performance inverted methylammonium-free perovskite solar cells. Nat. Energy 8, 946–955 (2023).
Liu, S. et al. Buried interface molecular hybrid for inverted perovskite solar cells. Nature 632, 536–542 (2024).
Yang, Y. et al. Amidination of ligands for chemical and field-effect passivation stabilizes perovskite solar cells. Science 386, 898–902 (2024).
Tao, M. et al. Molecule-triggered strain regulation and interfacial passivation for efficient inverted perovskite solar cells. Joule 8, 3142–3152 (2024).
Qu, G. et al. Self-assembled materials with an ordered hydrophilic bilayer for high performance inverted Perovskite solar cells. Nat. Commun. 16, 86 (2025).
Qu, S. et al. Revealing and inhibiting the facet-related ion migration for efficient and stable perovskite solar cells. Angew. Chem. Int. Ed. 64, e202415949 (2025).
Chen, J. & Park, N.-G. Inorganic hole transporting materials for stable and high efficiency perovskite solar cells. J. Phys. Chem. C. 122, 14039–14063 (2018).
Li, L. et al. Flexible all-perovskite tandem solar cells approaching 25% efficiency with molecule-bridged hole-selective contact. Nat. Energy 7, 708–717 (2022).
He, R. et al. Improving interface quality for 1-cm2 all-perovskite tandem solar cells. Nature 618, 80–86 (2023).
Li, M. et al. Stabilizing perovskite precursor by synergy of functional groups for NiOx-based inverted solar cells with 23.5 % efficiency. Angew. Chem. Int. Ed. 61, e202206914 (2022).
Boyd, C. C. et al. Overcoming redox reactions at perovskite-nickel oxide interfaces to boost voltages in perovskite solar cells. Joule 4, 1759–1775 (2020).
Li, C. et al. Pros and cons of hole-selective self-assembled monolayers in inverted PSCs and TSCs: extensive case studies and data analysis. Energy Environ. Sci. 17, 6157–6203 (2024).
Yi, Z. et al. Achieving a high open-circuit voltage of 1.339 V in 1.77 eV wide-bandgap perovskite solar cells via self-assembled monolayers. Energy Environ. Sci. 17, 202–209 (2024).
Li, M., Liu, M., Qi, F., Lin, F. R. & Jen, A. K. Y. Self-assembled monolayers for interfacial engineering in solution-processed thin-film electronic devices: design, fabrication, and applications. Chem. Rev. 124, 2138–2204 (2024).
Dai, Z. et al. Interfacial toughening with self-assembled monolayers enhances perovskite solar cell reliability. Science 372, 618–622 (2021).
Chen, H. et al. Improved charge extraction in inverted perovskite solar cells with dual-site-binding ligands. Science 384, 189–193 (2024).
Wu, T. et al. Elimination of light-induced degradation at the nickel oxide-perovskite heterojunction by aprotic sulfonium layers towards long-term operationally stable inverted perovskite solar cells. Energy Environ. Sci. 15, 4612–4624 (2022).
Qu, S. et al. Redox mediator-modulated self-assembled monolayer stabilizes a buried interface in efficient inverted perovskite solar cells. Energy Environ. Sci. 18, 3186–3195 (2025).
Du, M. et al. Surface redox engineering of vacuum-deposited NiOx for top-performance perovskite solar cells and modules. Joule 6, 1931–1943 (2022).
Liu, Y. et al. Synergistic redox modulation for high-performance nickel oxide-based inverted perovskite solar modules. Adv. Sci. 11, 2309111 (2024).
Al-Ashouri, A. et al. Monolithic perovskite/silicon tandem solar cell with > 29% efficiency by enhanced hole extraction. Science 370, 1300–1309 (2020).
Yu, S. et al. Homogenized NiOx nanoparticles for improved hole transport in inverted perovskite solar cells. Science 382, 1399–1404 (2023).
Li, C. et al. Rational design of Lewis base molecules for stable and efficient inverted perovskite solar cells. Science 379, 690–694 (2023).
Zhang, J. et al. Elimination of interfacial lattice mismatch and detrimental reaction by self-assembled layer dual-passivation for efficient and stable inverted perovskite solar cells. Adv. Energy Mater. 12, 2103674 (2022).
Li, Z. et al. Stabilized hole-selective layer for high-performance inverted p-i-n perovskite solar cells. Science 382, 284–289 (2023).
Li, Q. et al. Harmonizing the bilateral bond strength of the interfacial molecule in perovskite solar cells. Nat. Energy 9, 1506–1516 (2024).
Zhu, L. et al. Trap state passivation by rational ligand molecule engineering toward efficient and stable perovskite solar cells exceeding 23% efficiency. Adv. Energy Mater. 11, 2100529 (2021).
Liu, B. et al. Interfacial defect passivation and stress release via multi-active-site ligand anchoring enables efficient and stable methylammonium-free perovskite solar cells. AEL 6, 2526–2538 (2021).
Zhou, Q. et al. Revealing steric-hindrance-dependent buried interface defect passivation mechanism in efficient and stable perovskite solar cells with mitigated tensile stress. Angew. Chem. Int. Ed. 32, 2205507 (2022).
Liu, B. et al. Polydentate ligand reinforced chelating to stabilize buried interface toward high-performance perovskite solar cells. Angew. Chem. Int. Ed. 63, e202317185 (2024).
Li, M. et al. Stabilizing perovskite precursor by synergy of functional groups for NiOx-based inverted solar cells with 23.5% efficiency. Angew. Chem. Int. Ed. 134, e202206914 (2022).
Zhang, H. et al. Formamidinium lead iodide-based inverted perovskite solar cells with efficiency over 25% enabled by an amphiphilic molecular hole-transporter. Angew. Chem. Int. Ed. 63, e202401260 (2024).
Li, S. et al. Water-resistant perovskite nanodots enable robust two-photon lasing in aqueous environment. Nat. Commun. 11, 1192 (2020).
Liu, C. et al. Bimolecularly passivated interface enables efficient and stable inverted perovskite solar cells. Science 382, 810–815 (2023).
Wang, S. et al. Critical role of removing impurities in nickel oxide on high-efficiency and long-term stability of inverted perovskite solar cells. Angew. Chem. Int. Ed. 61, e202116534 (2022).
Kim, J. H. et al. High-performance and environmentally stable planar heterojunction perovskite solar cells based on a solution-processed copper-doped nickel oxide hole-transporting layer. Adv. Mater. 27, 695–701 (2015).
Zhang, H., Zhao, C., Yao, J. & Choy, W. C. H. Dopant-free NiO nanocrystals: a low-cost and stable hole transport material for commercializing perovskite optoelectronics. Angew. Chem. Int. Ed. 62, e202219307 (2023).
Zhang, H. et al. Formamidinium lead iodide-based inverted perovskite solar cells with efficiency over 25% enabled by an amphiphilic molecular hole-transporter. Angew. Chem. Int. Ed. 136, e202401260 (2024).
Chen, X. et al. Minimizing the buried interfacial energy loss using a fluorine-substituted small molecule for 25.92%-efficiency and stable inverted perovskite solar cells. Energy Environ. Sci. 17, 7342–7354 (2024).
Yang, Y. et al. Bilateral chemical linking at NiOx buried interface enables efficient and stable inverted perovskite solar cells and modules. Angew. Chem. Int. Ed. 63, e202409689 (2024).
Fei, C. et al. Lead-chelating hole-transport layers for efficient and stable perovskite minimodules. Science 380, 823–829 (2023).
Ni, Z. et al. Resolving spatial and energetic distributions of trap states in metal halide perovskite solar cells. Science 367, 1352–1358 (2020).
Lan, Z. et al. Cascade reaction in organic hole transport layer enables efficient perovskite solar cells. Angew. Chem. Int. Ed. 63, e202402840 (2024).
Sandberg, O. J. et al. On the question of the need for a built-in potential in perovskite solar cells. Adv. Mater. Interfaces 7, 2000041 (2020).
Li, X. et al. Mapping the energy level alignment at donor/acceptor interfaces in non-fullerene organic solar cells. Nat. Commun. 13, 2046 (2022).
Xiao, X. et al. Surface chemical conversion of residual PbI2 enables efficient and stable perovskite solar cells by 1,3-diphenyl-benzimidazolium iodide treatment. Adv. Funct. Mater. n/a, 2418792 (2025).
Gao, D. et al. Managing interfacial defects and carriers by synergistic modulation of functional groups and spatial conformation for high-performance perovskite photovoltaics based on vacuum flash method. Adv. Mater. 35, 2301028 (2023).
Acknowledgements
This work was supported by the Science and Technology Development Fund, Macau SAR (No. 0009/2022/AGJ, J.T.). The National Natural Science Foundation of China (52462031 (C.C.), U21A2076 (C.C.), 62274018 (J.C.)). The S&T Program of Hebei (24464401D, C.C.). The Natural Science Foundation of Hebei Province (E2024202086 (C.C.), E2024202300, (C.C.)). Hebei Province Higher Education Science and Technology Research Project (JZX2024030, C.C.). Shijiazhuang Basic Research Project at Hebei-based Universities (241790847A, C.C.). Science and Technology Cooperation Special Project of Shijiazhuang (SJZZXC24005, C.C.).
Author information
Authors and Affiliations
Contributions
J.D., J.Z., J.T., J.C., and C.C. conceived the ideas. J.D. fabricated devices and samples for various characterizations. J.D., C.C. and J.Z. conducted theoretical calculation analysis. Y.L., Y.D., and J.S. assisted in the preparation and testing of large-area PSC module devices. H.L. assisted with FTIR and XPS measurements. M.L. performed SEM measurements. Q.M. and Z.Z. helped to conduct the TRPL and PL measurements and analysis. J.D. and C.C. wrote the first draft of the manuscript. J.C. and C.C. wrote the final version of the manuscript. J.C. and C.C. supervised this project. All authors discussed the results and contributed to the revisions of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ding, J., Liao, Y., Liu, H. et al. Stabilizing buried interface by bilateral bond strength equilibrium strategy toward efficient perovskite photovoltaics. Nat Commun 16, 8407 (2025). https://doi.org/10.1038/s41467-025-63389-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-63389-z