Abstract
Although commercial lithium-ion batteries have been widely used in portable electronics and electric vehicles, they are still plagued by the uncontrollable dendritic lithium under extreme conditions. Herein, an efficient strategy is developed to produce a MXene-configured graphite via an electrostatic interaction between MXene and silane coupling agent-modified graphite. Typically, MXene layers are adhered onto the basal plane of graphite, with good preservation of the uncovered lateral edges of graphite, effectively strengthening the adsorption energy of Li+ and reducing the lithium nucleation energy barrier. Moreover, the MXene interface possesses good lattice compatibility with Li (110) plane, greatly promoting the homogeneous growth of Li along the preferable plane under extreme conditions. Even at −20 °C, Ah-level pouch cell with MXene-configured graphite electrode delivers a high capacity retention of 93% after 1200 cycles (273 Wh kg−1) at 1 C, exceeding lithium-ion batteries with bare graphite electrode (43% capacity retention, 191 Wh kg−1).
Similar content being viewed by others
Introduction
Lithium-ion batteries (LIBs) have attracted worldwide attention as a power source for electric vehicles, owing to their high specific energy, long cycle life and cost-effectiveness1,2,3,4,5. In general, LIBs are built from graphite electrode and transition metal oxide/phosphide positive electrodes in organic electrolytes, exhibiting high energy densities of 140–280 Wh kg−1 at ambient conditions6,7. However, under extreme conditions such as sub-zero temperatures and high rates, LIBs commonly have poor electrochemical performance owing to high electrochemical polarizations at the electrolyte/electrode interface and inhomogeneous reaction currents associated with inevitable formation of lithium dendrites at edges of graphite8,9,10,11. The emerged dendrites would further block the insertion of Li+ into the interlayers of graphite, severely aggravating the polarizations and uncontrollable growth of lithium dendrites, which largely hamper the practical applications of LIBs in some scenarios12,13.
To address the issues of large electrochemical polarizations and uncontrollable lithium dendrites, some strategies involving in promoting Li+ migration in solid electrolyte interphase (SEI) layers and enhancing Li+ diffusion coefficient in negative electrodes have been explored as follows: (1) exploiting advanced electrolytes such as localized high-concentration electrolytes to facilitate the formation of an anion-derived interface with high ionic conductivities, improving the reversibility of Li plating11,14,15; (2) building artificial interface layers (such as inorganic layers) to reduce the Li+ de-solvation energy barrier at the interface between electrolyte and graphite, accelerating the interfacial reaction kinetics16,17,18,19,20; (3) homogenizing Li+ flux by adding hard carbons or amorphous carbons with high specific surface areas into the graphite electrodes, inhibiting metallic Li plating21,22,23. Although these strategies enable to alleviate electrochemical polarizations and lithium dendrites to some extent, it remains a big challenge to eliminate the dendritic lithium in graphite electrodes under extreme conditions such as at low temperatures (<0 °C) and high rates (>4 C, the goal of United States advanced battery consortium)13,24,25.
Here, MXene-configured graphite was produced via an electrostatic interaction between MXene and aminopropyltrimethoxysilane (APS)-modified graphite, where the MXene layers were mainly adhered onto the basal plane of graphite, with good preservation of the uncovered lateral edges of graphite, overcoming the diffusion limitation of two-dimensional (2D) materials in composites and electrodes. Unlike bare graphite, the MXene-configured graphite possesses an enhanced adsorption energy of Li+ and efficiently reduces nucleation energy barrier of Li. Under extreme conditions, the outmost MXene not only facilitates the growth of dense packing body-centered cubic (bcc) Li along preferable (110) plane on MXene owing to their good lattice compatibility, but also efficiently homogenizes the reaction currents. Remarkably, even at a low temperature of −20 °C, Ah-level pouch cell with MXene-configured graphite electrode delivers a high capacity retention of 93% after 1200 cycles and a high specific energy of 273 Wh kg−1 (based on the mass of the whole pouch cell), exceeding that with bare graphite electrode (43% capacity retention, 191 Wh kg−1, based on the mass of the whole pouch cell).
Results
The MXene-configured graphite was fabricated via an electrostatic interaction between negatively charged MXene and amino group-modified graphite. Initially, flake graphite was grafted via aminosilane coupling agent APS (Supplementary Figs. 1 and 2), introducing abundant positively charged amino (-NH2) groups on the surface26,27. In comparison, MXene is negatively charged with a zeta potential of −29.7 mV (Supplementary Fig. 1) owing to large presence of -OH, -O and -F terminations on the surface28. Consequently, once MXene Ti3C2Tx was added into the suspension of positively charged APS-modified graphite with a zeta potential of +6.5 mV, the MXene layers would be spontaneously adsorbed onto the graphite surface through their electrostatic interaction, leaving a transparent aqueous dispersion (Supplementary Fig. 3) and affording a sandwiched MXene-graphite-MXene heterostructure (referred as MXene-configured graphite), with an optimal MXene content of ~1 wt.%, as demonstrated in Supplementary Figs. 4–8. The energy dispersive X-ray spectroscopy (EDS) elemental mapping and atomic force microscopy (AFM) images (Supplementary Figs. 9–16) clearly disclose that many MXene nanosheets with lateral sizes of 0.5–4 μm and thickness of ~2 nm are attached onto the basal plane of graphite rather than the edge plane. The EDS line scan analysis along the cross section of MXene-configured graphite (Supplementary Fig. 17) shows that C species is mainly located in the middle of the heterostructure, while Ti species is presented on both sides, clearly demonstrating a sandwiched MXene-graphite-MXene structure (Supplementary Fig. 18). Moreover, unlike conventional chemical modified or coated graphite29, it is found that MXene layers are mainly adhered onto the basal plane of graphite with good preservation of the uncovered lateral edges of graphite. Such basal plane attachment of MXene to graphite should be attributed to their relatively smaller lateral sizes (0.5–4 μm) than that of bare graphite (~300 μm) as well as strong face-to-face interactions between MXene and the basal plane of graphite, affording an efficient interface reconfiguration from carbon to MXene and the well-maintained lattice parameters of graphite, as confirmed by synchrotron radiation XRD patterns (Fig. 1 and Supplementary Figs. 19–22). Density functional theory (DFT) calculations reveal that such interface reconfiguration from carbon to MXene would largely increase the adsorption energy of Li to −3.41 eV, which is ~3 times that of bare graphite (−1.03 eV) (Supplementary Fig. 23). Experimental results demonstrate that the nucleation overpotential (|ηn | ) is only 12 mV for the MXene interface, about a quarter of bare graphite (42 mV). Since that the Li nucleation energy barrier (ΔGn*) is directly proportional to overpotential based on classical nucleation theory (ΔGn*∝z | ηn | , where z is the charge of a cation), the MXene interface would undoubtedly reduce the Li nucleation energy barrier, efficiently promoting the homogeneous nucleation of Li on the MXene-configured graphite electrode.
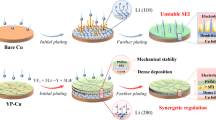
Schematic diagram of MXene-configured graphite electrode in LIBs under extreme conditions, where the interface reconfiguration from carbon to MXene delivers a high lattice matching between Li and MXene interface, and a low Li nucleation energy barrier, facilitating homogeneous lithium plating on MXene interface. Under an extreme condition at −20 °C for 1200 cycles, the capacity retention of MXene-configured graphite with MXene interface is 2.2 times that of bare graphite. Under an extreme condition at 10 C for 1200 cycles, the capacity retention of MXene-configured graphite with MXene interface is 2.1 times that of bare graphite.
Li plating mechanism at MXene interface for MXene-configured graphite
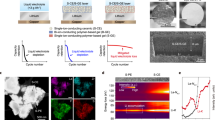
To gain insight into lithium plating behaviors on MXene (Ti3C2Tx) interface, cryogenic transmission electron microscopy (cryo-TEM) was conducted after plating lithium at various levels from 0.3 to 0.7 mAh cm−2 (Fig. 2a–e and Supplementary Fig. 24). As shown in Fig. 2b, at a plating level of 0.3 mAh cm−2, some isolated domains with diameters of 10–20 nm are observed on the layers. Upon increasing the plating level to 1.0 mAh cm−2, extensive metallic lithium is homogeneously grown on the layers without visible dendritic lithium (Fig. 2c). Remarkably, the plated lithium is exclusively bcc Li with (110) plane, exhibiting a lattice spacing of 2.48 Å (Fig. 2d, e). This is in contrast to the dendritic lithium (Supplementary Fig. 25) randomly grown along the (110), (200) or (211) multiple planes on carbon films30. The preferential growth of lithium along the (110) plane on MXene could be further demonstrated via in situ X-ray diffraction (XRD) measurement. As increasing the lithium plating level to 10 mAh cm−2, the intensity of the (110) plane is progressively enhanced, which is absent in the cases of graphite and Cu-Li electrodes (Supplementary Fig. 26). Moreover, the growth of Li along (110) plane is achieved on both Ti3C2Tx with -F and -OH terminations (Fig. 2c and Supplementary Figs. 27 and 28), demonstrating a functional group-independent Li deposition behavior.
a Schematic diagram of lithium nucleation, horizontal accumulation and planar growth on MXene Ti3C2Tx interface. Cryo-TEM images at lithium plating levels of 0.3 (b) and 1.0 (c) mAh cm−2 with a current density of 0.5 mA cm−2, in which the red, blue and yellow regions represent crystalline Li metal, LiF and Li2O nanocrystals, respectively. Insets: fast Fourier transform patterns acquired from the plated Li metal region. d Magnified image of boxed regions in blue from (c), showing well crystal structure. e Atomic-resolution TEM image of the boxed regions from (d), disclosing an interplanar spacing of 2.48 Å for the distance between (110) facets. f Lattice mismatch between (110), (200), (211) and (310) planes of lithium and the hexagonal crystal plane of MXene Ti3C2Tx interface. Insets: the lattice matching diagrams between the different crystal planes of Li and MXene Ti3C2Tx interface. g Adsorption energies of Li atoms grown along horizontal or vertical orientation at substrates of lithium, MXene Ti3C2Tx, graphene and copper.
To further gain insight into the Li plating mechanism at the MXene interface, the lattice compatibility between electrodeposited lithium along diverse planes and MXene-configured graphite was calculated via Bramfitt two-dimensional mismatch formula31:
Where \({\delta }_{{{\mathrm{Li}}}({{hkl}})}^{{{\mathrm{2D}}}}\) represents the lattice mismatch between the exposed plane of 2D materials and the (hkl) plane of Li metal, \(d{\left[{uvw}\right]}_{{{\mathrm{2D}}}}^{i}\) refers to the interatomic spacing along the low-index direction [uvw]2D of 2D materials. \(d{\left[{uvw}\right]}_{{{\mathrm{Li}}}}^{i}\) stands for the interatomic spacing along low-index direction in Li(hkl) plane and θ is the angle between the low-index direction [uvw]2D and [uvw]Li. The computational results show that the lattice mismatch between the (110) plane of Li and hexagonal crystal plane of MXene Ti3C2Tx is only 7.5%, which is much lower than those of other Li planes: 36.5% for the (200) plane, 32.4% for the (211) plane and 70.7% for the (310) plane (Fig. 2f and Supplementary Table 1), suggesting that Li atoms are prone to densely grow along the close-packed (110) planes at MXene interface, in good agreement with above cryo-TEM and XRD analyses. In contrast, the lattice mismatch between (110) plane of Li and hexagonal crystal plane of graphite is as high as 39.6% (Supplementary Fig. 29 and Supplementary Table 2), which is ~5 times that of the MXene interface. It is clear that our interface reconfiguration from carbon to MXene is efficient to manipulate the lithium plating behaviors in the graphite electrodes. To further elucidate the packing way of Li atoms along horizontal or vertical orientation at MXene interface during lithium plating, DFT calculations were conducted. In Fig. 2g, along horizontal accumulation, the adsorption energy is −3.05 eV for MXene Ti3C2Tx, which is much higher than those for bare Li (−1.65 eV), graphene (−1.46 eV) and copper (−2.33 eV). Conversely, for vertical accumulation, the adsorption energy is −0.33 eV for MXene Ti3C2Tx, which is much lower than those of bare Li (−1.01 eV), graphene (−1.54 eV) and copper (−1.01 eV). Driven by the adsorption energy difference, lithium has a pronounced tendency to grow horizontally at MXene interface, possibly solving the notorious issue of vertical growth of dendritic Li on bare lithium and many other substrates (Supplementary Fig. 30).
The electrical and electrochemical properties of MXene-configured graphite electrode
To obtain LIBs with good low-temperature and high-rate performance, graphite electrode usually features optimal active mass loadings of ≤8 mg cm−2, since that high mass loading would have a large influence on the electron transfer and ion diffusion ability, leading to large electrochemical polarizations9,18,32,33. Differently, our MXene-configured graphite electrode with a high mass loading of ~19.3 mg cm−2 not only could be facilely fabricated via a traditional slurry method owing to the high viscosity (3451 mPa s−1, Supplementary Fig. 31)34, but also exhibited a high electrical conductivity of 1084 S cm−1 (Supplementary Fig. 32), which is about 2.5 times that of bare graphite electrode (350–400 S cm−1). Such high conductivity should be originated from three-dimensional conducting network built from highly electrical MXene interface (4545 S cm−1) on graphite (Supplementary Figs. 32 and 33). Moreover, owing to the interaction between the functional groups of MXene and binders, our electrode has a high peel strength of 11.9 N m−1 (Supplementary Fig. 34), ensuring the integrity of the thick electrode. Based on galvanostatic intermittent titration technique (GITT) measurement (Supplementary Fig. 35), MXene-configured graphite has an average Li+ diffusion coefficient of 8.73 × 10−11 cm2 s−1 with a mass loading of 3 mg cm−2, which is much higher than that of MXene-graphite mixture (3.8 × 10−11 cm2 s−1) and graphite (1.2 × 10−11 cm2 s−1). Such difference is further enhanced as increasing the mass loading in the electrodes. As increasing the mass loading to 12 mg cm−2, the average Li+ diffusion coefficient in MXene-configured graphite is 2.87 × 10−11 cm2 s−1 in the electrode, which is about one order magnitude of MXene-graphite mixture (1.7 × 10−12 cm2 s−1) and graphite (3.0 × 10−12 cm2 s−1), possibly attributed to the generation of LiF in the SEI layers (Supplementary Fig. 36)35,36,37. Such accelerated Li+ diffusion could be further demonstrated by the distribution of relaxation times (DRT) measurement (Supplementary Fig. 35), in which the MXene-configured graphite electrode has a much lower Li+ diffusion impedance (Rdiff-Gr) of 971 Ω s−1 at −20 °C than MXene-graphite mixture (6962 Ω s−1). Correspondingly, the Li+ desolvation activation energy of MXene-configured graphite electrode (49.0 kJ mol−1, Fig. 3a) is lower than that of graphite (58.5 kJ mol−1). Thus, at a typical low temperature of −20 °C, a half-cell with MXene-configured graphite electrode shows a much lower electrochemical polarization (Fig. 3b and Supplementary Figs. 37 and 38, 138 mV for the potential gap between the lithiation/delithiation peaks) than that with bare graphite electrode (223 mV). Such low electrochemical polarization could be further confirmed by the cyclic voltammetry curves (Supplementary Fig. 39) and electrochemical impedance spectroscopy (EIS) spectra (Supplementary Fig. 40). At −20 °C, the MXene-configured graphite electrode delivers a high specific capacity of 298 mAh g−1, which accounts for 81% of the capacity (365 mAh g−1) at 25 °C (Supplementary Figs. 6 and 37).
a The activation energy of Li+ desolvation in MXene-configured graphite electrode, showing a low activation energy. b The dQ/dV curves of MXene-configured graphite electrode at −20 °C in half cell, exhibiting a low electrochemical polarization. c DCM curve of MXene-configured graphite electrode at −20 °C, in which the low slope indicates a low surface area of plated lithium on the MXene interface. Cycling performance (d) and corresponding CC/CV charging stages (e) of Ah-level pouch cell with MXene-configured graphite electrode at the temperatures from 40 to −20 °C, disclosing a good low-temperature capability. f Photographs associated with SEM images of fully lithiated MXene-configured graphite electrode after 100 cycles at 1 C@ − 20 °C, exhibiting a dendrite-free morphology.
To further investigate the origination of the enhanced electrochemical performance of MXene-configured graphite electrode at low temperatures, in situ dynamic capacitance measurement (DCM) was conducted. As displayed in Supplementary Fig. 41, in the case of the MXene-configured graphite electrode at −20 °C, the onset time of Li plating is 19,549 s, which is much later than that for bare graphite electrode (14,449 s), demonstrating that the MXene interface enables to efficiently postpone initial time of lithium plating. Moreover, the MXene-configured graphite electrode exhibits a low slope of 0.8 × 10−6 for the capacitance Cs variation vs. Li plating time, merely one fifth of graphite electrode (4.2 × 10−6, Fig. 3c). According to the literature on DCM tests38, the slop is positively related to electrochemical active surface area of plated lithium on the electrodes. This can be further demonstrated by SEM images of MXene-configured graphite after discharging from 100% to 200% state of charge (SoC), in which all the electrode surfaces are smooth (Supplementary Fig. 42). In contrast, numerous lithium dendrites are observed on bare graphite electrode, similar to the reported graphite electrodes under extreme conditions14,17. Remarkably, the excessive plating lithium on MXene-configured graphite (200% SoC) delivers a high I(110)/I(200) value of 3.8 in the XRD patterns, nearly three times that on bare graphite electrode (1.1, Supplementary Fig. 43), in good agreement with above dominated (110) observations from cryo-TEM images and DFT calculations. Based on the reported lithium electrodes, the densest packed (110) plane of Li has the lowest migration energy barrier of 0.11 J m−2, possessing good reversibility39,40,41,42. After 100 cycles of lithium plating/stripping, the surface of the MXene-configured graphite electrode is still smooth without any silver-white dead lithium, which is largely presented on the bare graphite (Supplementary Fig. 44).
The electrochemical performance of pouch cells with MXene-configured graphite electrode under extreme conditions
Based on the aforementioned MXene-configured graphite electrode with a high mass loading of ~19.3 mg cm−2, Ah-level pouch cells with tunable negative-to-positive electrode capacity ratios (N/P ratios) from 0.98 to 1.08 were assembled with LiNi0.8Co0.1Mn0.1O2 (NCM811) positive electrode in a layer-by-layer configuration and tested using a constant current (CC)-constant voltage (CV) charging protocol. As shown in Supplementary Fig. 45, as the N/P ratio is 1.08, the initial Coulombic efficiency (ICE) is 92.3% for MXene-configured graphite, comparable to those of bare graphite (91.8%). Unexpected, as decreasing the N/P value from 1.08 to 0.98, the ICE difference between MXene-configured graphite and graphite is increased from 0.5% to 1.7%, which should be relative to the lithium plating behaviors at low N/P levels. As demonstrated in Supplementary Fig. 46, there are obvious lithium dendrites on bare graphite surface (N/P = 0.98), whereas the dendrites are absent on MXene-configured graphite (N/P = 0.98). Thus, to balance the high ICE and high specific energy, in our pouch cell, N/P ratio was set as 1.02.
To evaluate the temperature tolerance of MXene-configured graphite, pouch cells were measured at different temperatures from 40 to −20 °C at 1 C (1 C = 200 mA g−1). As depicted in Fig. 3d, the capacity of the pouch cell slightly decreases from 1.06 to 0.98 Ah (Fig. 3d), whereas the cell with bare graphite dramatically decreases to 0.47 Ah. Moreover, at −20 °C, the pouch cell with MXene-configured graphite exhibits a low capacity contribution of 14% at the CV state (Fig. 3e), much lower than that of the cell with bare graphite electrode (86%). According to the literatures on charging process18,43, the long potentiostatic stage at the CV state correlates with the lithium plating levels. Consequently, the level of plated lithium in pouch cell with MXene-configured graphite electrode is much lower than that with bare graphite, in good agreement with above DCM results. Furthermore, the plated lithium has a smooth surface, in stark contrast to the dendritic or mossy lithium on bare graphite electrode (Fig. 3f). The dendrite-free lithium plating should also be originated from the good lattice compatibility between the Li (110) plane and the hexagonal crystal structure of MXene, as validated by XRD analysis (Supplementary Fig. 43).
It is well-known that stress change is an important factor to assess commercial LIBs44,45. In Fig. 4a, in situ pressure monitoring device was assembled to evaluate the stress fluctuation in the pouch cell with MXene-configured graphite electrode. At −20 °C, a small stress fluctuation with an average pressure increment of 0.12 kPa is observed (Fig. 4b), which is 5% of that in the cell with bare graphite electrode (2.37 kPa), clearly demonstrating that our pouch cell has a negligible volume change during cycling under low temperatures. Such low stress fluctuation is beneficial for the long-term cycling performance under extreme conditions, including low temperatures and high rates. When cycled at 1 C@ − 20 °C, the specific energy is up to 273 Wh kg−1 (based on the mass of the whole pouch cell, Supplementary Table 3), which is 84% of that cycled at 25 °C (324 Wh kg−1, based on the mass of the whole pouch cell). Remarkably, after 1200 cycles at the low temperature, the capacity retention is up to 93% (Fig. 4c). By contrast, the pouch cell with bare graphite electrode shows relatively low specific energy (191 Wh kg−1, based on the mass of the whole pouch cell) and capacity retention (43%) under the same testing conditions, similar to the state-of-art LIBs under the similar conditions (144–176 Wh kg−1, Fig. 4d)10,46,47. Moreover, at a rate of 10 C, our pouch cell still exhibits a high capacity retention of 94% after 1200 cycles and a high specific energy of 287 Wh kg−1 (based on the mass of the whole pouch cell, Supplementary Fig. 47), exceeding the pouch cells with MXene-graphite mixture (Supplementary Figs. 48 and 49), bare graphite electrode and reported LIBs (<10 C, 180–265 Wh kg−1)9,18,21,48,49. This strategy of interface reconfiguration to MXenes can be further extended to other active materials such as silicon/silicon oxides and lithium metal to significantly improve the electrochemical performance of LIBs (Supplementary Figs. 50–55), underscoring the potential of MXenes utilized in next-generation commercial LIBs with good electrochemical performance under wide operating conditions.
a Diagram of the MXene-configured graphite || NCM811 pouch cell and the structure scheme of an in situ pressure monitoring device. The stress fluctuations (b) and long-term cycling performance (c) of the pouch cell with MXene-configured graphite electrode at −20 °C, showing a negligible stress fluctuation and good cycling stability, respectively. After 1200 cycles at −20 °C, the capacity retention of MXene-configured graphite with MXene interface is 2.2 times that of bare graphite. d Comparison of the specific energy of pouch cells with MXene-configured graphite and bare graphite electrodes as well as the reported pouch cells, showing the highest specific energy retention under wide operating temperatures from 25 to −20 °C.
In conclusion, an efficient strategy of interface reconfiguration from carbon to MXene in graphite electrode is explored to eliminate lithium dendrites for commercial LIBs under extreme conditions. The outmost MXene interface could not only promote the electrical conductivity and effectively reduce the nucleation barrier of Li in the electrode, but possess a good lattice compatibility with plated lithium, facilitating the horizontal growth of dense packing bcc Li along (110) plane under extreme conditions. Thus, an Ah-level pouch cell with MXene-configured graphite electrode delivers a long durability (93% capacity retention) after 1200 cycles, a high specific energy of 273 Wh kg−1 (based on the mass of the whole pouch cell) at 1 C@ − 20 °C, and good rate capability (10 C), exceeding that with bare graphite electrodes. The interface reconfiguration strategy to MXenes provides a valuable opportunity to develop dendrite-free electrode materials for next-generation LIBs.
Methods
Synthesis of MXenes
MXene Ti3C2Tx was synthesized by selectively etching the A-element layers from MAX phases50. Typically, 1 g Ti3AlC2 powder (JiNan SCMXene Tech. Co., Ltd, 99%) was immersed into a mixture with 40 mL of 12 M HCl (Aladdin, 36%) and 3.2 g LiF (Aladdin, 99%), and heated at 35 °C for 24 h under magnetic stirring. The suspension was repeatedly washed with deionized water and then ultrasound for several hours, producing Ti3C2Tx suspension after centrifugation treatment. The Ti3C2Tx suspension was further free-dried to obtain Ti3C2Tx powder. The terminations on MXene were modulated by dispersing the Ti3C2Tx powder into 1 M NaOH solution (Aladdin, 33%) and stirring for 3 h.
Synthesis of MXene-configured graphite
The MXene-configured graphite was fabricated via an electrostatic interaction between negatively charged MXene and amino group-modified graphite. APS-modified graphite particles were firstly synthesized by dispersing 1 g of graphite particles into 100 mL of APS (Aladdin, 97%) aqueous solution (0.15 mg mL−1), subsequently stirring at 60 °C for 12 h and finally washing with deionized water for several cycles. Then, the MXene-configured graphite was produced by dropwise adding 50 mL of MXene aqueous solution (0.2 mg mL−1) to 100 mL of the APS-modified graphite aqueous solution (10 mg mL−1), stirring for 2 h and subsequent centrifugation treatment. In practice, the morphologies of graphite (particle distribution, lateral dimensions and thickness), the uniformity of APS coating and the dimensions/thickness/concentrations of MXene should be taken into consideration to achieve selective attachment of MXene onto the basal plane of graphite.
Preparation of MXene-configured graphite electrode
The MXene-configured graphite electrode was prepared by a classic slurry method. Specifically, 98 wt.% MXene-configured graphite, 1 wt.% carboxymethyl cellulose (CMC) binder (Dodochem, 99.9%), and 1 wt.% Super P (Dodochem, 99%) were successively dispersed in deionized water to form a uniform slurry. The slurry was then coated on both sides of copper foil (6 μm) and dried under vacuum at 80 °C for 15 h, producing MXene-configured graphite electrode. After calendaring, the MXene-configured graphite electrode was cut into rectangular pieces to assemble pouch cells.
Preparation of NCM positive electrode
The NCM positive electrode was prepared by a classic slurry method. Specifically, 98 wt.% NCM811 (Shenzhen Kejing Star Technology Company, 99%), 0.8 wt.% Super P (Dodochem, 99%), and 1.2 wt.% polyvinylidene fluoride (PVDF, Mv~1200000, Dongguan Kelude Experimental Equipment Technology Co., Ltd, 99.9%) binder (Dodochem, 99.9%) were successively dispersed in N-methyl-2-pyrrolidone solution to form a uniform slurry (Dodochem, 99.9%). The slurry was then coated on both sides of the 10 μm-thick aluminum foil and dried in a vacuum oven at 80 °C. After calendaring the coated electrode, the NCM electrode was cut into rectangular pieces for the assembly of pouch cells. The mass loading of NCM811 on the positive electrode was 39.2 mg cm−2.
Preparation of MXene-Li electrode
First, in an Ar-filled glovebox with H2O and O2 levels of <0.1 ppm, 13.5 g of lithium metal (China Energy Lithium Co., Ltd., 99%) was placed in a stainless-steel tank and heated at 200 °C until completely melting. Then, 1.5 g of MXene was slowly added into molten lithium and continuously stirred for 2 h at 80 r min−1, producing MXene-Li composite. After cooling down, the resultant MXene-Li was repeatedly rolled with multiple times via electric vertical double-roller machine (GRS-DG300L) to produce MXene-Li electrode with a thickness of 50 μm.
Coin cell fabrication and electrochemical tests
In an Ar-filled glove box (O2 < 0.1 ppm, H2O < 0.1 ppm), all coin cells were assembled as CR2032 type coin cells by using MXene-configured graphite (single-side coated on Cu foils) or MXene-Li electrode (thickness: 50 μm; diameter: 16 mm), commercial Li foils (thickness: 500 μm, diameter: 16 mm), polypropylene (PP, porosity: 40%, average pore size: 60 nm, Dongguan Kelude Experimental Equipment Technology Co., Ltd) separators and 80 μL of 1 M LiPF6 in ethylene carbonate (EC)/dimethyl carbonate (DMC) (1:1 [v/v]) with 10 wt.% fluoroethylene carbonate (FEC) addition. The Li | | MXene-configured graphite coin cells were operated in a voltage window of 0.01–1.5 V using a Landt CT2001A battery cycler (Landt Instrument).
Pouch cell fabrication and electrochemical tests
All pouch cells were fabricated using a stacking process in a dry room (dew point: <−40 °C, constant temperature: 20 °C). The mass loadings of positive and negative electrodes were 39.2 and 19.3 mg cm−2, respectively. The positive and negative electrodes were cut using a semi-automatic electrode sheet die-cutting machine (GRS-MQ280) with dimensions of 39 mm × 24 mm and 41 mm × 26 mm, respectively. All the electrodes were stacked with PP separators by semi-automatic stacking machine (GRS-BDP300-D). After welding the positive and negative electrodes to the Al and Ni tabs by ultrasonic welder (GRS-4008), respectively, the stacked electrodes were packed into a laminate bag, followed by top and side sealed to fabricate dry cells. The dry cells were further treated in vacuum at 80 °C for 24 h to remove moisture and then placed into an Ar-filled glovebox for vacuum sealing (GRS-DJY200) after electrolyte injection and gas release. The electrolyte is a mixture of 1 M LiPF6 in EC/DMC (1:1 [v/v]) with 10 wt.% FEC addition (Dodochem). The sealed pouch cells were finally put into a constant-temperature chamber at 40 °C for thermostatic activation before tests. The electrochemical performance of pouch cells was characterized in the voltage range from 2.5 to 4.2 V using a Landt CT2001A battery cycler. Particularly, MXene-graphite || NCM811 pouch cells were tested using a practical constant current (CC)-constant voltage (CV) charging protocol with the external pressure of ~300 MPa. The electrolyte loading used in this work was 2.0 g Ah−1 and the N/P ratio of MXene-configured graphite || NCM was ~1.02. The electrochemical energy storage tests were conducted in a climatic chamber at controlled temperatures (−40 to 60 °C).
Characterizations
The morphology and microstructure were characterized by field emission scanning electron microscopy (ZEISS, GeminiSEM 300) and transmission electron microscopy (TEM, FEI, Tecnai G2 F30) with X-ray Energy Dispersive Spectrometer (EDS, Oxford). The zeta potentials and particle size distribution were measured by Zetasizer Nano (ZS). The content of MXene in MXene-configured graphite was evaluated by thermogravimetric analysis (TGA) measurement. The viscosity of MXene-configured graphite and graphite was quantified via a viscosimeter (NDJ-5S8S). Cryogenic transmission electron microscopy (cryo-TEM) with a Gatan 698 cryo-transfer holder was executed based on a TEM sample prepared by positioning MXene layers on TEM grids and subsequently plating Li. In situ X-ray diffraction (XRD) measurements were conducted on a D/MAX-2500 X-ray diffractometer at 40 kV and 30 mA with a scan rate of 5° min−1 from 3° to 80°. Fourier Transform Infrared Spectrometer (FTIR) spectra were recorded on a Thermo Scientific Nicolet 6700 spectrometer. Electrochemical impedance spectroscopy (EIS) tests were conducted with a CHI 760E electrochemical workstation (Chenhua, Shanghai) over a frequency range from 1 MHz to 10 mHz and an amplitude of 5 mV. Dynamic capacitance measurement (DCM) test was performed using a Solartron 1470E electrochemical workstation conducted by overlying AC current on a DC current with a given C-rates, where the amplitude of AC currents was lower than 5.0% of the DC current. Pressure test of pouch cells was carried out on a customized in situ pressure monitoring device equipped with a pressure sensor (DS2-50N-XD).
DFT calculations
The absorption energies were calculated using DFT as implemented in VASP using the Perdew-Burke-Ernzerh of exchange-correlation functional51,52. For geometry optimization, the Brillouin-zone integration was performed using a 5 × 5 × 1 k-points mesh generated using the Monkhorst-Pack method53,54. The vacuum region was at least 20 Å to avoid interactions between neighboring images. A kinetic energy cutoff of 500 eV was used for all the calculations. Geometry optimizations were performed using the conjugated gradient method, the threshold was set at 10−5 eV/atom in energy, while the force was converged to 10−2 eV Å−1. The absorption energy (Ead) is calculated according to the following formula:
Where the ELi+substrate is the total energy of substrate and absorbed Li atoms, ELi-substrate is the total energy of substrate and absorbed Li atoms that are one less Li atom than Li+substrate system, ELi is the energy of Li atom in bulk state. The calculation parameters and configurations in Supplementary Data 1–5.
Data availability
All data are available in the main text or the Supplementary Information, which can also be available from the corresponding authors upon request. Source data are provided with this paper.
References
Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, 652–657 (2008).
Yuan, K. et al. High-safety anode materials for advanced lithium-ion batteries. Energy Environ. Mater. 7, e12759 (2024).
Chu, S., Cui, Y. & Liu, N. The path towards sustainable energy. Nat. Mater. 16, 16–22 (2017).
Winter, M., Barnett, B. & Xu, K. Before Li ion batteries. Chem. Rev. 118, 11433–11456 (2018).
Cai, W. et al. A review on energy chemistry of fast-charging anodes. Chem. Soc. Rev. 49, 3806–3833 (2020).
Nitta, N., Wu, F. X., Lee, J. T. & Yushin, G. Li-ion battery materials: present and future. Mater. Today 18, 252–264 (2015).
Dechent, P. et al. ENPOLITE: comparing lithium-ion cells across energy, power, lifetime, and temperature. ACS Energy Lett. 6, 2351–2355 (2021).
Li, S. Q. et al. Fast charging anode materials for lithium-ion batteries: current status and perspectives. Adv. Funct. Mater. 32, 2200796 (2022).
Wang, C. Y. et al. Fast charging of energy-dense lithium-ion batteries. Nature 611, 485–490 (2022).
Zhang, N. et al. Critical review on low-temperature Li-ion/metal batteries. Adv. Mater. 34, 2107899 (2022).
Liu, Y., Shi, H. & Wu, Z. S. Recent status, key strategies and challenging perspectives of fast-charging graphite anodes for lithium-ion batteries. Energy Environ. Sci. 16, 4834–4871 (2023).
Choi, J. H. et al. Multi-interface strategy for electrode tailoring toward fast-charging lithium-ion batteries. Adv. Funct. Mater. 34, 2400414 (2024).
Choi, J. H. et al. Fast charging of lithium-ion batteries: a review of materials aspects. Adv. Energy Mater. 11, 2101126 (2021).
Yue, X. et al. Reversible Li plating on graphite anodes through electrolyte engineering for fast-charging batteries. Angew. Chem. Int. Ed. 62, e202302285 (2023).
Jiang, L. L. et al. Inhibiting solvent co-intercalation in a graphite anode by a localized high-concentration electrolyte in fast-charging batteries. Angew. Chem. Int. Ed. 60, 3402–3406 (2021).
Sun, C. et al. 50 C fast-charge Li-ion batteries using a graphite anode. Adv. Mater. 34, 2206020 (2022).
Xu, X., Yue, X., Chen, Y. & Liang, Z. Li plating regulation on fast-charging graphite anodes by a triglyme-LiNO3 synergistic electrolyte additive. Angew. Chem. Int. Ed. 62, e202306963 (2023).
Tu, S. et al. Fast-charging capability of graphite-based lithium-ion batteries enabled by Li3P-based crystalline solid-electrolyte interphase. Nat. Energy 8, 1365–1374 (2023).
Wang, C. et al. Li3PO4-enriched SEI on graphite anode boosts Li+ de-solvation enabling fast-charging and low-temperature lithium-ion batteries. Angew. Chem. Int. Ed. 63, e202402301 (2024).
Chen, K. H. et al. Enabling 6 C fast charging of Li-ion batteries with graphite/hard carbon hybrid anodes. Adv. Energy Mater. 11, 2003336 (2021).
Lu, G., Nai, J., Luan, D., Tao, X. & Lou, X. W. Surface engineering toward stable lithium metal anodes. Sci. Adv. 9, eadf1550 (2023).
Zhou, J. et al. Eliminating graphite exfoliation with an artificial solid electrolyte interphase for stable lithium-ion batteries. Small 18, 2107460 (2022).
Luo, S. et al. Regeneration of spent graphite via graphite-like turbostratic carbon coating for advanced Li ion battery anode. Energy Storage Mater. 73, 103833 (2024).
Tu, S. et al. Integrated dual-phase ion transport design within electrode for fast-charging lithium-ion batteries. Adv. Funct. Mater. 34, 2402077 (2024).
Liu, Y. Y., Zhu, Y. Y. & Cui, Y. Challenges and opportunities towards fast-charging battery materials. Nat. Energy 4, 540–550 (2019).
Yang, S., Feng, X., Ivanovici, S. & Müllen, K. Fabrication of graphene-encapsulated oxide nanoparticles: towards high-performance anode materials for lithium storage. Angew. Chem. Int. Ed. 49, 8408–8411 (2010).
Jiang, D. et al. Improved thermal conductivity of epoxy resins using silane coupling agent-modified expanded graphite. J. Appl. Polym. Sci. 140, e53417 (2023).
Sun, L. et al. High-zeta-potential accelerates interface charge transfer in lithium anodes via MXene-graphdiyne heterojunction layers. Chem. Eng. J. 469, 144014 (2023).
Li, K. et al. 3D MXene architectures for efficient energy storage and conversion. Adv. Funct. Mater. 30, 2000842 (2020).
Li, Y. et al. Atomic structure of sensitive battery materials and interfaces revealed by cryo-electron microscopy. Science 358, 506–510 (2017).
Bramfitt, B. L. The effect of carbide and nitride additions on the heterogeneous nucleation behavior of liquid iron. Metall. Trans. 1, 1987–1995 (1970).
Yang, Y. et al. Rechargeable LiNi0.65Co0.15Mn0.2O2 || graphite batteries operating at 60 °C. Angew. Chem. Int. Ed. 61, e202209619 (2022).
Li, Z. et al. Inhibiting gas generation to achieve ultralong-lifespan lithium-ion batteries at low temperatures. Matter 6, 2274–2292 (2023).
Hu et al. Effects of adhesion and cohesion on the electrochemical performance and durability of silicon composite electrodes. J. Power Sources 397, 223–230 (2018).
Liu, Y. et al. Self-assembled monolayers direct a LiF-rich interphase toward long-life lithium metal batteries. Science 375, 739–745 (2022).
Tan, J. et al. A growing appreciation for the role of LiF in the solid electrolyte interphase. Adv. Energy Mater. 11, 2100046 (2021).
Sung, J. H. et al. Dynamic cycling of ultrathin Li metal anode via electrode-electrolyte interphase comprising lithiophilic Ag and abundant LiF under carbonate-based electrolyte. Adv. Energy Mater. 9, 2500279 (2025).
Xu, L. et al. Operando quantified lithium plating determination enabled by dynamic capacitance measurement in working Li-ion batteries. Angew. Chem. Int. Ed. 61, e202210365 (2022).
Røe, I. T. et al. Crystal structure influences migration along Li and Mg surfaces. J. Phys. Chem. Lett. 11, 2891–2895 (2020).
Zhao, Q. et al. On the crystallography and reversibility of lithium electrodeposits at ultrahigh capacity. Nat. Commun. 12, 6034 (2021).
Zhang, Y. et al. Synergetic regulation of SEI mechanics and crystallographic orientation for stable lithium metal pouch cells. Nat. Commun. 15, 4454 (2024).
Chen, H. et al. Synthesis of monocrystalline lithium for high-critical-current-density solid-state batteries. Nat. Synth.4, 552–561 (2025).
Kim, N., Chae, S., Ma, J., Ko, M. & Cho, J. Fast-charging high-energy lithium-ion batteries via implantation of amorphous silicon nanolayer in edge-plane activated graphite anodes. Nat. Commun. 8, 812 (2017).
Tavassol, H., Jones, E. M. C., Sottos, N. R. & Gewirth, A. A. Electrochemical stiffness in lithium-ion batteries. Nat. Mater. 15, 1182–1187 (2016).
Barai, P., Higa, K. & Srinivasan, V. Impact of external pressure and electrolyte transport properties on lithium dendrite growth. J. Electrochem. Soc. 165, A2654–A2666 (2018).
Smart, M. C., Ratnakumar, B. V., Chin, K. B. & Whitcanack, L. D. Lithium-ion electrolytes containing ester cosolvents for improved low temperature performance. J. Electrochem. Soc. 157, A1361–A1374 (2010).
Hu, A. et al. Ion transport kinetics in low-temperature lithium metal batteries. Adv. Energy Mater. 12, 2202432 (2022).
Kazyak, E., Chen, K. H., Chen, Y., Cho, T. H. & Dasgupta, N. P. Enabling 4 C fast charging of lithium-ion batteries by coating graphite with a solid-state electrolyte. Adv. Energy Mater. 12, 2102618 (2022).
Lu, D. et al. Transformed solvation structure of noncoordinating flame-retardant assisted propylene carbonate enabling high voltage Li-ion batteries with high safety and long cyclability. Adv. Energy Mater. 13, 2300684 (2023).
Naguib, M. et al. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 23, 4248–4253 (2011).
Kresse, G. & Hafner, J. Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements. J. Phys. Condens. Matter 6, 8245–8257 (1994).
Aldegunde, M., Hepplestone, S. P., Sushko, P. V. & Kalna, K. Multi-scale simulations of metal-semiconductor nanoscale contacts. J. Phys. Conf. Ser. 647, 012030 (2015).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant numbers 52125207, 52225208, 22475009 and 52202204) and Beijing Natural Science Foundation (Grant number 2242039). We thank the support from the High Performance Computing Center of Beihang University. We thank the 1W1A-Diffuse X-ray Scattering Beamline of Beijing Synchrotron Radiation Facility (https://cstr.cn/31109.02.BSRF.1W1A) for providing technical support and assistance in synchrotron radiation XRD data collection.
Author information
Authors and Affiliations
Contributions
S.Y. supervised the project. H.C. designed and carried out the most experiments. G.L. performed the cryo-TEM measurements. Z.C. performed the SEM and XRD measurements. Q.Z. (Qi Zhu) carried out the DFT calculations. Y.Y. performed the FTIR measurements. Y.G. conducted the DCM measurements. H.C., S.Y., Z.D. and X.T. cowrote the paper. Y.S., Q.Z. (Qi Zhao) and B.L. provided some suggestions on the experiments and paper writing. All authors discussed the results and assisted during manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Zhong-Shuai Wu, Chandramohan George and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, H., Lu, G., Cao, Z. et al. MXene-configured graphite towards long-life lithium-ion batteries under extreme conditions. Nat Commun 16, 8493 (2025). https://doi.org/10.1038/s41467-025-63443-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63443-w