Abstract
Supported small and dense high-entropy-alloy nanoparticles (HEA-NPs) are promising functional materials for many applications. However, their synthesis remains a grand challenge because the extreme heating typically required to raise the entropic contribution to the formation of a solid solution unavoidably causes the sintering of HEA-NPs. Herein, we present a one-step continuous-flow spray pyrolysis strategy to synthesize multicomponent (from quinary to denary) HEA-NPs with an average size of <2 nanometers and metal loadings of ~30 wt% uniformly dispersed on various carbon substrates, including graphene and carbon black. In addition, this flow-type synthesis is featured with notable advantages in facile gram scalability and reproducible production when compared with the existing methods. We identify a hydrocarbothermal synthesis mechanism that in situ generates H2 via the reaction between carbon and water in the aerosol droplets to realize the complete metal reduction and alloying at a reduced temperature. The relatively mild reaction condition, combined with the short heating duration and the strong metal-support interaction, enables the simultaneous achievements of small size and high loading in HEA-NPs. The quinary FeCoNiCuPt HEA-NPs are demonstrated as highly efficient electrocatalysts toward the oxygen reduction and hydrogen evolution reactions.
Similar content being viewed by others
Introduction
High-entropy alloy nanoparticles (HEA-NPs) consisting of five or more metal elements in a solid solution at the nanoscale have received increasing attention as promising functional materials in diverse applications, particularly in catalysis1,2,3,4,5,6. To achieve acceptable catalytic performance, it is necessary to immobilize HEA-NPs on appropriate supports, which not only serve to facilitate the dispersion and stabilization of metals but also could influence their reactivity via the metal-support interaction7,8,9,10. Among the various supports, nanocarbons stand out for their high surface area, chemical inertness, good electrical conductivity, and low cost, making nanocarbon-supported HEA-NPs attractive catalysts for various electrochemical energy conversion reactions11,12,13,14,15. To realize the full potential of HEA-NPs, it is desirable to reduce their size into a small range (for example, <2 nm) for achieving a high proportion of surface atoms, large mass-specific activity, and reduced cost (especially when precious metals are involved)16,17,18,19,20,21. Additionally, increasing the metal loading in carbon-supported HEA-NPs is beneficial for constructing high-power electrochemical energy conversion devices as it can effectively decrease the catalyst layer thickness and thus lower the mass transport resistance22,23.
However, it remains a daunting task to synthesize carbon-supported HEA-NPs with high metal loading while maintaining a small size (<2 nm). The substantial immiscibility of multiple mixing metal elements with vastly different physicochemical properties leads to highly positive mixing enthalpy (∆Hmix)1,24,25. Therefore, to drive the formation of single-phase solid solution within high entropy alloys (HEAs), the adoption of high synthesis temperature is critical so that the entropic term (T∆Smix, T is temperature and ∆Smix is mixing entropy) can dominate over ∆Hmix to afford a negative mixing Gibbs free energy (∆Gmix) according to the formula ∆Gmix = ∆Hmix − T∆Smix. Consequently, various thermochemical approaches (for example, Joule heating, microwave irradiation, fast-moving bed pyrolysis, and laser ablation)26,27,28,29,30,31,32,33,34, featured with rapid quenching to preserve the high-entropy state, have been developed to synthesize carbon-supported HEA-NPs. However, high temperature would unavoidably accelerate the atomic diffusion and particle coarsening, which is particularly severe at high metal loadings when particles are in closer proximity35. As a result, the synthesized HEA-NPs are generally larger than 2 nm, and a tradeoff between their size and loading is commonly encountered within the existing strategies, as summarized in Table 1.
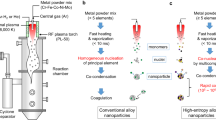
On the other hand, the currently available synthetic methods are typically based on batch-type processes, which suffer from the difficulties in ensuring the uniformity of different batches and scaling up production that is limited by the size of the reactor36,37. In comparison, continuous-flow synthesis is desirable for its notable processing merits such as high reproducibility (as a result of the stable reaction condition) and easy scalability, and it has been applied to prepare various chemicals and materials38,39,40,41,42. For example, IrPdPtRhRu HEA-NPs with a small size (1.32 nm) were synthesized at room temperature by a liquid-phase flow process with an organic reducing agent and then loaded onto carbon supports by wet impregnation36. Nevertheless, this method is limited by the low metal loading (7 wt%), weak metal-support interaction, and poor generality (only suitable for noble metals that are readily reduced). Up to now, the development of a continuous-flow strategy for the controlled synthesis of carbon-supported small and dense HEA-NPs with a wide range of elemental combinations remains highly desirable yet challenging.
In this work, we report a generalized continuous-flow method to synthesize carbon-supported HEA-NPs with a high metal loading of up to 36.7 wt% and an average size of down to 1.62 nm (in the case of the quinary FeCoNiCuPt), representing improved values compared to those in the literature (Table 1, Supplementary Fig. 1 and Supplementary Table 1). This synthetic method involves a facile, one-step spray pyrolysis process where aerosol droplets containing metal salts, carbon precursor, and H2O solvent are directly passed through the heating furnace with Ar as the carrier gas (that is, without externally provided reducing agents). Detailed investigations identify a “hydrocarbothermal reduction” mechanism that in situ generates H2 locally via the reaction between carbon and H2O (C + 2H2O → CO2 + 2H2) in the aerosol droplets to achieve the complete metal reduction and alloying at the temperature of 850 °C. This relatively mild reaction condition, compared to previous thermochemical approaches, combined with the transient heating of spray pyrolysis and the strong anchoring effect of the oxygenated carbon substrate, enables the simultaneous achievements of small size and dense distribution of HEA-NPs. The generality of our method is showcased by increasing the number of disparate metal elements up to ten (Ru, Rh, Pd, Ir, Pt, Fe, Co, Ni, Cu and Zn) that have a diverse range of physiochemical properties (Supplementary Table 2) as well as by utilizing graphene or carbon black as the supporting substrates, all offering HEA-NPs with small size (<2 nm) and high metal loadings (~ 30 wt%). The simplicity of the method, together with the inherent strength of the flow-type reactor, endows it with facile gram scalability and high reproducibility. As proof of utility, the graphene-supported quinary FeCoNiCuPt HEA-NPs are employed as bi-functional electrocatalysts for the oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER). The noble metal-normalized mass activity of this catalyst reaches 1.94 ± 0.01 A mgPt−1 at 0.90 V versus the reversible hydrogen electrode (RHE) for the ORR and 32.71 ± 1.74 A mgPt−1 at −0.05 V versus RHE for the HER.
Results
Synthesis and characterization of materials
The reactor and preparation procedure for synthesizing the carbon-supported HEA-NPs, using graphene-supported quinary FeCoNiCuPt (denoted as HEA-NPs/G) as the representative example, is schematically illustrated in Fig. 1, and the detailed description is provided in the Experimental Section. Briefly, an aqueous dispersion containing graphene oxide (GO) and metal salts MClxHy (M = Fe, Co, Ni, Cu, or Pt) was first prepared. With good colloidal stability (Supplementary Fig. 2), the dispersion was nebulized by an ultrasonic atomizer to generate aerosol droplets that were then passed through a tube furnace preheated at 850 °C with Ar carrier gas at a flow rate of 2 L min−1, after which the powdery materials exiting the reactor were collected by a filter collector. During the spray pyrolysis, the fast heating caused the thermal decomposition and reduction of the metal salts by H2 that was in situ generated (discussed later), while the fast quenching was critical to preserve the high entropy state of HEA-NPs (Supplementary Fig. 3)43. This thermal shock-type pyrolysis was also important for achieving a small size and uniform distribution of HEA-NPs. The residence time of the aerosol droplets in the heating zone was estimated to be ~ 3 s and the heating/cooling rate was ~ 1000 °C s−1 (Supplementary Fig. 4). Besides, the spray pyrolysis thermally reduced GO for it to be electrically conductive (Supplementary Fig. 5). In addition, it transformed the sheet-like morphology of graphene substrate into crumpled particle driven by the rapid solvent evaporation44,45, endowing the sample with more opened structure and a high surface area of 350 m2 g−1 (Supplementary Fig. 6).
Scanning electron microscopy (SEM) images revealed that HEA-NPs/G consists of crumpled graphene particles with a size ranging from 200 nm to 500 nm, and no metal particles were identified at the surface of graphene due to their small size (Fig. 2a and Supplementary Fig. 7). From the annular dark-field scanning transmission electron microscopy (ADF-STEM) images (Fig. 2b, c), numerous HEA-NPs are densely and uniformly decorated on the graphene substrate. High-resolution ADF-STEM image showed that the nanoparticles in HEA-NPs/G are of good crystallinity and have an average diameter of 1.62 ± 0.83 nm (Fig. 2d and Supplementary Fig. 8). The inductively coupled plasma mass spectrometry (ICP-MS) determined that the total metal content was 36.7 wt% and the atomic ratio of Pt, Fe, Co Ni and Cu was 1.00: 1.09: 1.23: 1.17: 1.08 (Supplementary Table 3). The energy-dispersive spectroscopy (EDS) revealed that the atomic percentages of the five metal elements in the individual nanoparticles of different sizes fall within the range of 5–35 at% (Supplementary Table 4), meeting the compositional requirement of HEAs. Fourier-transform infrared spectroscopy (FT-IR) suggested that there was a chemical interaction between the HEA-NPs and the oxygen functional groups from the graphene substrate (Supplementary Fig. 9). Such a strong metal-support interaction is important in achieving the small size and uniform distribution of the HEA-NPs by suppressing interparticle sintering46, as further evidenced by the observation that lowering the amounts of oxygen functional groups resulted in larger particle size and less uniform distribution (Supplementary Fig. 10).
a SEM image of HEA-NPs/G. b Low-magnification STEM image of HEA-NPs/G. c Enlarged STEM image of HEA-NPs/G. d ADF-STEM image of HEA-NPs/G. The inset shows the statistical histogram of particle size and average size of HEA-NPs. The size is given as the mean ± standard deviation. e XRD pattern of HEA-NPs/G, indicating a single-phase FCC crystalline structure. f Atomic-resolution ADF-STEM image of an individual HEA-NP with a lattice spacing of 2.25 Å that corresponds to the (111) plane. g EDS elemental maps of HEA-NPs/G composed of five elements (Fe, Co, Ni, Cu, Pt) at low (top), medium (middle), and high (bottom) magnifications. h Size tunability by varying the flow rate of the Ar carrier gas during spray pyrolysis. i Stable reaction condition and high reproducibility of the synthetic method, demonstrated by the similar size and metal loading between samples collected at different times (1 h and 5 h). The data were shown as the mean ± standard deviation, where the mean values were derived from three independent measurements (with data points shown as circles). j A photograph of 1.25562 g of HEA-NPs/G in an analytical balance collected from a one-time continuous reaction.
Powdery X-ray diffraction (XRD) pattern displayed one set of diffraction patterns in HEA-NPs/G and the peaks at ~ 42° and ~ 49° were characteristic of the (111) and (200) planes, respectively, of a face-centered cubic (FCC) crystalline structure (Fig. 2e)47. The positions of the above two peaks are between the corresponding peaks of the Pt standard and non-precious transition metal standards, suggesting the successful formation of a single-phase solid-solution structure in HEA-NPs/G. The additional peak at about 25° originated from the (002) plane of the graphene substrate. The atomic-resolution ADF-STEM image of a 1.9 nm-sized HEA-NP shows the 2.25 Å lattice spacing that corresponds to the (111) facet of an FCC structure, and the slight lattice distortion could be ascribed to the different atomic size of the elements in the HEA-NP (Fig. 2f). The EDS elemental maps at low magnification revealed the full and homogeneous distribution of the constituent elements across the entire graphene substrate (Fig. 2g, top), highlighting the dense and close packing of HEA-NPs. Elemental maps at medium magnification (Fig. 2g, middle) depicted the correlated spatial distribution of the nanoparticles and the elements, while that at high magnification (Fig. 2g, bottom and Supplementary Figs. 11 and 12) revealed the homogeneous mixing of the elements within a single HEA-NP, suggesting no apparent elemental segregation or phase separation.
The size of HEA-NPs can be tuned by adjusting the carrier gas flow rate or the pyrolysis temperature. Specifically, the average particle size can be tuned between ~ 1.6 nm to ~ 8.4 nm by varying the carrier gas flow rate (Fig. 2h and Supplementary Fig. 13). The increase in the pyrolysis temperature caused the enlargement of the particle size (Supplementary Fig. 14). The attempt to synthesize smaller HEA-NPs by decreasing the pyrolysis temperature was unsuccessful due to the occurrence of phase separation or incomplete alloying (Supplementary Fig. 15). Benefited from the stable reaction condition of the flow-type reactor, our method has high reproducibility, as demonstrated by the almost same average particle size (1.59 nm versus 1.61 nm) and total metal loading (36.7 wt% versus 36.4 wt%) between samples collected at different reacting time (1 h and 5 h) (Fig. 2i, Supplementary Fig. 16 and Supplementary Table 5), and it can be readily scaled up to gram-scale level by simply extending the reacting time to ~12 h (Fig. 2j).
Hydrocarbothermal reduction mechanism
With ultrafast heating/cooling capability and short reaction duration to prevent phase separation, spray pyrolysis has been previously used to synthesize HEA-NPs43,48,49, and therefore, we focus our discussion on the reduction mechanism in our synthesis strategy that enables the achievements of small size and dense distribution in HEA-NPs/G. In the previous synthesis (Fig. 3a)43, the aerosol droplets containing only metal salts (i.e., carbon-free) were firstly dried (i.e., H2O-free) before being pyrolyzed, and the metal salts were reduced to metal by externally supplied H2 at a significantly higher pyrolysis temperature of >1100 °C. The lack of carbon substrate and the significantly higher pyrolysis temperature caused severe particle sintering, resulting in HEA-NPs with a much larger size (~60 nm) and a wide size distribution. In our method (Fig. 3b), the synthesis was completed within a single step via directly pyrolyzing the aerosol droplets containing metal salts + H2O + carbon at a significantly lower temperature (850 °C) with Ar carrier gas (with no externally provided reducing agents). We found that the introduction of both carbon and H2O was critical for achieving small HEA-NPs and enabling the complete reduction of metal salts by the in situ generated H2 via the hydrocarbothermal reduction process (C + 2H2O → CO2 + 2H2; xH2 + MOx → xH2O + M), which were evidenced by the following control experiments.
Schematic illustration of (a) previous method and (b) current method for synthesizing HEA-NPs using the continuous-flow spray pyrolysis technique. In the previous method, the aerosol droplets containing only metal salts are dried before being spray pyrolyzed at 1100 °C with H2 as the reducing agent. The resulting HEA-NPs are substrate-free and have a large diameter with a wide size distribution. In the current method, the aerosol droplets containing both carbon precursor and metal salts are directly spray pyrolyzed at a lower temperature of 850 °C with Ar as the carrier gas. The obtained HEA-NPs are supported on carbon with a small size, dense and uniform distribution, and strong metal-support interaction. c Schematic illustration of the different reaction pathways of the spray pyrolysis synthesis with varied components contained in the aerosols. STEM image and EDS elemental maps of the control sample prepared by spray pyrolysis without H2O (d) or carbon precursor (e), showing the co-presence of metal phase (highlighted by the green contour) and oxide phase (highlighted by the orange contour). f Radar plot summarizing the EDS-determined atomic percentages of constituent elements within the metallic region shown in (d) and (e). g XRD patterns of the samples prepared by hydrocarbothermal synthesis and the two control samples. Standard patterns are included for reference, including Fe3O4 (PDF#72-2303) in green, CuO (PDF#78-0428) in orange, CoO (PDF#70-2855) in purple and NiO (PDF#89-7130) in yellow. h GC analysis of the exhaust gas collected from the hydrocarbothermal synthesis and the control experiment without H2O. i Ellingham diagram that shows the oxidation potential of different elements. Tsynthesis is the spray pyrolysis temperature (850 °C).
In the first control experiment, the aerosols used for spray pyrolysis contained only metal salts and carbon precursor (H2O solvents were removed by a silica dryer placed at the upstream) (Fig. 3c), while all other synthetic conditions were kept the same to those of HEA-NPs/G. In this way, the generation of H2 via the above-proposed hydrocarbothermal process was disenabled and the metal salts were reduced to metal mainly by carbon via carbothermal reduction (xC + MOx → xCO + M). TEM and EDS elemental maps of the as-obtained product depicted the inhomogeneous distribution of constituent elements and the co-presence of metallic particle and metal oxide impurities (Fig. 3d). From the radar plot that summarized the EDS-determined atomic percentages of constituent elements within a single metallic particle (Fig. 3f), the particle possessed higher atomic ratios of Pt, Fe and Cu than Co and Ni, in contrast to the near-equal atomic ratio of the particle in HEA-NPs/G. In the second control experiment, the aerosols contained only metal salts and H2O (without the addition of carbon precursor) (Fig. 3c). In this case, neither H2 nor carbon reductants were available and therefore metal salts could be reduced only by thermal decomposition (2HxMCly → (y-x)Cl2 + 2 M + 2xHCl). The as-synthesized sample experienced apparent phase separation and incomplete metal reduction (Fig. 3e, f), which is within expectation considering that the formation of HEAs via the salt self-decomposition would require an extremely high temperature above the decomposition temperature of the involved metal salts or even the melting point of the constituent metals29. In addition, without the carbon support, this control sample suffered from severe particle aggregation (Supplementary Fig. 17).
XRD patterns confirmed the co-presence of metal phase and metal oxide phase in the two control samples, in contrast to the single-phase structure in HEA-NPs/G (Fig. 3g). Additionally, as indicated by the position of the XRD peak associated with alloy (~42°) and the intensity of the peaks assigned to metal oxides, the degree of alloying and reduction follows the order of hydrocarbothermal synthesis > first control > second control, which reflected the differed reducing power of the corresponding reduction mechanisms involved in these processes. These results highlighted the critical role of H2O/carbon and thus the in situ generated H2 in our synthesis for achieving complete reduction and alloying. This was corroborated by the successful formation of HEA-NPs through the spray pyrolysis synthesis without H2O but with H2 supplied externally (Supplementary Fig. 18), and by the identification of H2 via gas chromatography (GC) in the exhaust gas collected from the hydrocarbothermal synthesis (while no H2 was detected if H2O was removed) (Fig. 3h).
The Ellingham diagram is often used as a guideline for the thermochemical synthesis of HEAs as it illustrates the oxidation potential of the constituent elements and the relative ease of reducing an oxide as a function of temperature50. As illustrated in Fig. 3i, at the synthesis temperature (850 °C), H2 has a lower oxidation potential than the constituent elements (Pt, Fe, Co, Ni, and Cu) and therefore it is thermodynamically feasible for H2 to reduce these elements. It is noted that carbon is predicted by the Ellingham diagram to be a stronger reducing agent than H2 at the synthesis temperature, but obvious formation of metal oxides occurred, as demonstrated in the first control experiment discussed above. We ascribed this observation to kinetic considerations, which are especially important in transient synthesis. Specifically, it is considered that H2 has a faster reduction kinetics than carbon since it has a higher atom mobility and its reaction with metal salts involves gas-solid reaction, as opposed to the solid-solid reaction in the case of carbon. The reducing capability (from both thermodynamic and kinetic aspects) of carbon could be improved by increasing the pyrolysis temperature. Indeed, the formation of HEA-NPs was realized when the carbothermal synthesis temperature was raised to be higher than 950 °C, but at the cost of severe particle growth (Supplementary Fig. 19).
Overall, in the continuous-flow spray pyrolysis with the simultaneous presence of metal salts, H2O, and carbon in the aerosols, we identified a hydrocarbothermal reduction mechanism for achieving the complete metal reduction and formation of HEA-NPs.
Generality of the synthetic strategy
The hydrocarbothermal continuous-flow synthesis can be readily generalized to more complex multicomponent HEA-NPs, including the senary (FeCoNiCuPtPd), septenary (FeCoNiCuZnPtPd), octonary (FeCoNiCuZnPtPdRh), novenary (FeCoNiCuZnPtPdRhIr), and denary (FeCoNiCuZnPtPdRhIrRu) HEA-NPs/G, by simply adding additional types of metal salts in the nebulizing precursor solutions. These alloying elements have different physiochemical properties such as preferred crystal structures (FCC, hexagonal close-packed, or body-centered cubic), atomic radius (124.1 to 137.6 pm), reduction potentials (−0.76 to 1.41 V versus the standard hydrogen electrode), and melting temperatures (420 to 2446 °C) (Supplementary Table 2), which would conventionally prevent single-phase uniform mixing. The EDS elemental maps at high and low magnifications showed the even distribution of constituent elements throughout the graphene sheet as well as on the single particle (Fig. 4a, b and Supplementary Figs. 20-23), highlighting the dense packing of the nanoparticles and their compositional uniformity. XRD patterns confirmed the single-phase structure of nanoparticles in these samples (Fig. 4c). ADF-STEM images revealed the uniform and dense distribution of nanoparticles on the graphene substrate (Supplementary Figs. 24–28). From the statistical analyses of size distribution from ADF-STEM images and ICP-MS results (Fig. 4d and Supplementary Table 3), these samples are all featured with small size of <2 nm and high metal loadings of >30 wt% (senary: 1.39 ± 0.66 nm, 32.9 wt%; septenary: 1.54 ± 0.90 nm, 32.9 wt%; octonary: 1.53 ± 0.84 nm, 37.7 wt%; novenary: 1.54 ± 0.96 nm, 35.8 wt%; denary: 1.46 ± 0.74 nm, 36.3 wt%).
EDS elemental maps of senary HEA-NPs/G (a) and denary HEA-NPs/G (b) at low (top) and high (bottom) magnifications. c XRD pattern of senary to denary HEA-NPs/G. d Particle size distribution and average size in different HEA-NPs/G samples. The size is given as the mean ± standard deviation. e, Scheme of the HEA-NPs supported on a carbon black supraparticle. f Low-magnification STEM image of HEA-NPs/CB. g Enlarged STEM image of HEA-NPs/CB. h ADF-STEM image of HEA-NPs/CB. i Atomic-resolution ADF-STEM image of an individual HEA-NP in HEA-NPs/CB. EDS elemental maps of quinary HEA-NPs/CB at low (j) and high (k) magnifications.
Besides the graphene substrate, our method can be used to synthesize HEA-NPs supported on carbon black (HEA-NPs/CB). HEA-NPs/CB were synthesized following the same protocol of HEA-NPs/G except that GO in the nebulizing solution was replaced by oxidized carbon black (CB). The oxidizing treatment of CB is necessary to make it dispersible in H2O solvent so as to enable the nebulizing process. From the SEM and low-magnification ADF-STEM images of the quinary FeCoNiCuPt HEA-NPs/CB (Fig. 4e, f, and Supplementary Fig. 29), the CB particles self-assembled into spherical super-structured particles (supraparticles) with a size ranging from several hundred nanometers to a few microns. The self-assembly process was driven by fast evaporation during spray pyrolysis51. ADF-STEM images at higher magnifications revealed the formation of homogeneous and high-density small crystalline HEA-NPs on the CB substrates (Fig. 4g, h). The average particle diameter was 1.42 ± 0.73 nm (Fig. 4d, last panel), and the total metal loading was 27.9 wt% by ICP-MS. The atomic-scale ADF-STEM image revealed the FCC crystalline structure with 2.28 Å lattice spacing of the (111) plane (Fig. 4i). EDS elemental mappings of an individual supraparticle and HEA-NPs both depicted the homogeneous distribution of the five metal elements, confirming the high metal loading density and the absence of obvious phase segregation. Thus, our method is general to synthesize a variety of small and dense HEA-NPs supported on different carbon substrates.
Demonstration of HEA-NPs/G as multifunctional electrocatalysts
To explore HEA-NPs/G as multifunctional electrocatalysts, we evaluated the catalytic performances of the quinary FeCoNiCuPt HEA-NPs/G toward the ORR and HER. The ORR test was conducted in 0.1 M HClO4 with a rotating disk electrode (RDE) in a typical three-electrode configuration. The activity of FeCoNiCuPt HEA-NPs/G was optimized by varying the temperature and flow rate of carrier gas during spray pyrolysis synthesis (Supplementary Fig. 30). The optimal sample has an average size of 3.3 nm, and its composition was summarized in Supplementary Table 6. For comparison purpose, binary PtCo-NPs/G and quaternary FeCoNiCu-NPs/G (prepared by the same protocol of FeCoNiCuPt HEA-NPs/G except that the metal precursors were varied), along with commercial Pt/C catalyst, were tested under identical conditions. Figure 5a shows the ORR linear sweep voltammetry (LSV) curves (after iR compensation) of the optimized FeCoNiCuPt HEA-NPs/G, PtCo-NPs/G, FeCoNiCu-NPs/G and commercial Pt/C. The corresponding curves before iR compensation were provided in Supplementary Fig. 31. HEA-NPs/G exhibited a more positive half-wave potential (E1/2) of 0.94 V versus RHE than PtCo-NPs/G of 0.92 V and Pt/C of 0.90 V. FeCoNiCu-NPs/G presented negligible activity, suggesting that Pt served as the main active site. The hydrogen underpotential deposition (HUD) region of the cyclic voltammetry (CV) curves recorded in N2-saturated electrolyte was used to determine the electrochemically active surface area (ECSA) of the catalysts (Supplementary Fig. 32). HEA-NPs/G possessed a higher ECSA of 83.0 m2 gPt−1 than Pt/C of 76.5 m2 gPt−1. The ECSA values for the catalysts determined by the CO stripping method are slightly larger than those by the HUD method (Supplementary Fig. 33). The mass activity and specific activity of the catalysts at 0.90 V versus RHE were obtained by normalizing the kinetic currents (calculated from the Koutecký-Levich equation) with the mass loading and HUD-determined ECSA of Pt, respectively. The mass activity and specific activity of HEA-NPs/G (1.94 ± 0.01 A mgPt−1, 2.34 ± 0.11 mA cmPt−2) are significantly higher than PtCo-NPs/G (0.90 ± 0.04 A mgPt−1, 1.29 ± 0.06 mA cmPt−2) and Pt/C (0.22 ± 0.02 A mgPt−1, 0.30 ± 0.07 mA cmPt−2) (Fig. 5b). The mass activity and specific activity of Pt/C obtained here are comparable to those reported previously, confirming the validity of the measurements52. Notably, the mass activity of HEA-NPs/G is competitive to previously reported HEAs-based ORR catalysts (Fig. 5c and Supplementary Table 7). The mass activity and specific activity of the catalysts at 0.95 V were provided in Supplementary Fig. 34, showing similar activity trend among HEA-NPs/G, PtCo-NPs/G and Pt/C. The catalytic stability of HEA-NPs/G was evaluated by accelerated durability test between 0.6 and 1.0 V versus RHE at a scan rate of 500 mV s−1. The CV and ORR polarization curves of HEA-NPs/G after 10000, 20000 and 30000 cycles almost overlapped with the initial ones, suggesting its excellent durability (Fig. 5d). The slight increase in the current associated with the hydrogen adsorption/desorption as well as Pt oxidation/reduction observed in the CV curves with continued cycling could be ascribed to the removal of surface oxide or leaching of non-precious metals that led to enhanced exposure of Pt on the surface. As shown in Fig. 5e, there are small variations in the mass activity along the cycling test, and its value dropped by only 5.5% after 30000 cycles, and the post-cycling specific activity values were maintained at a similar level to the initial value. The structural stability of HEA-NPs/G after 30000 cycles was verified by ADF-STEM and EDS elemental maps (Supplementary Figs. 35 and 36), demonstrating that the high-entropy state and size distribution of the HEA-NPs were well maintained.
a ORR polarization curves with a sweep rate of 10 mV s−1 at a rotating speed of 1600 r.p.m in the electrolyte of O2-saturated 0.1 M HClO4 (pH = 1.04 ± 0.017), with 100% iR compensation. The R values and uncorrected polarization curves were provided in Supplementary Fig 31. The catalyst loading for all catalysts is 122.3 μg cm−2. b Mass activity and specific activity of FeCoNiCuPt-NPs/G, PtCo-NPs/G and commercial Pt/C. c Comparison in mass activity between FeCoNiCuPt-NPs/G and previously reported HEAs-based ORR catalysts in Supplementary Table 7. The mass activity was calculated based on Pt. d ORR polarization curves of FeCoNiCuPt-NPs/G before and after various numbers of CV cycling. The inset shows the corresponding CV curves before and after CV cycling. e The mass activity and specific activity evolution of FeCoNiCuPt-NPs/G before and after CV cycling. f HER polarization curves with a sweep rate of 10 mV s−1 in the electrolyte of 0.5 M H2SO4 (pH = 0.33 ± 0.0082), with 90% iR compensation. The R values and uncorrected polarization curves were provided in Supplementary Fig 37. The catalyst loading is 122.3 μg cm−2 for Pt/C and 15.3 μg cm−2 for other catalysts. g Comparison in mass activity between FeCoNiCuPt-NPs/G and previously reported HEAs-based HER catalysts in Supplementary Table 8. The mass activity was calculated based on all noble metals. h Stability assessment of the PEMWE assembled with FeCoNiCuPt-NPs/G as the cathode catalyst and commercial IrOx/Ir as the anode catalyst by the galvanostatic test at 1.0 A cm−2 for 1000 h. The error bars in (b, c and g) represent the standard deviation of at least three independent measurements (with data points shown in circles) of the same catalyst.
The HER performance of HEA-NPs/G was evaluated in N2-saturated 0.5 M H2SO4 with a typical three-electrode configuration. Figure 5f displays the HER polarization curves (after iR compensation) of HEA-NPs/G, along with PtCo-NPs/G, FeCoNiCu-NPs/G and commercial Pt/C. The corresponding curves before iR compensation were provided in Supplementary Fig. 37. HEA-NPs/G shows the lowest overpotential (η) and highest current density in the entire potential region, and it requires only a small η of 15 mV to deliver the benchmark current density of 10 mA cm−2. The negligible activity of FeCoNiCu-NPs/G highlights that Pt is the active site. The mass activity value (at η = 50 mV) for HEA-NPs/G is 32.71 ± 1.74 A mgPt−1, which is about 44 times higher than Pt/C (0.75 ± 0.06 A mgPt−1) and is higher than all previously reported HEAs-based HER catalysts (Fig. 5g and Supplementary Table 8). Furthermore, we constructed a proton exchange membrane water electrolyzer (PEMWE) by using HEA-NPs/G as the cathode catalyst and commercial IrOx/Ir as the anode catalyst. The as-assembled PEMWE needs a cell voltage of 1.66 V to achieve a current density of 1.0 A cm−2 (Supplementary Fig. 38), making it competitive to with commercial Pt/C as the cathode catalyst. Galvanostatic test at 1.0 A cm−2 revealed that the PEMWE could operate stably for at least 1000 h with a small degradation rate of 54 µV h−1 (Fig. 5h).
Discussion
In summary, we reported a straightforward continuous-flow method to synthesize carbon-supported HEA-NPs with small size and high metal loadings via a one-step spray pyrolysis of aerosol droplets containing metal salts, carbon precursor, and H2O. A hydrocarbothermal reduction mechanism was identified that in situ generated H2 to achieve the complete metal reduction and alloying at a mild reaction condition, which, combined with the transient heating of spray pyrolysis and strong metal-support interaction, led to the dense and uniform distribution of small HEA-NPs. The generality of the synthesis was evidenced by the high compositional diversity with metal elements up to 10, and the applicability for different carbon supports such as graphene and carbon black. The size of HEA-NPs could be readily tuned by controlling the synthetic parameters like the carrier gas flow rate and pyrolysis temperature. As a proof-of-demonstration, the quinary HEA-NPs were utilized as bifunctional electrocatalysts towards the ORR and HER, exhibiting competitive performances compared to the state-of-the-art HEAs-based counterparts. The simplicity of the synthesis, together with the high reproducibility and facile scalability of the flow-type reactor, makes it highly promising for the mass production of HEA-NPs. We believe that this method will pave the road toward the widespread exploration of high-performance HEA-NPs in the catalysis and energy fields.
Methods
Chemicals
Iron (III) chloride hexahydrate (FeCl3·6H2O, 99%), cobalt (II) chloride hexahydrate (CoCl2·6H2O, 99%), nickel (II) chloride hexahydrate (NiCl2·6H2O, 99%), copper (II) chloride dihydrate (CuCl2·2H2O, ≥99%), zinc (II) chloride (ZnCl2, 99%), ruthenium (III) chloride hydrate (RuCl3·xH2O, 99.8%), rhodium (III) chloride trihydrate (RhCl3·3H2O, 98%), iridium (III) chloride (IrCl3, 99.8%), chloroplatinic (IV) acid (H2PtCl6, 99%), palladium (II) chloride (PdCl2, 99%) were purchased from Sigma-Aldrich and were used without further purification. Perchloric acid (HClO4, 70%) and sulfuric acid (H2SO4, 95%) were purchased from Sinopharm Chemical Reagent Co. Ltd. GO was prepared by the oxidation of graphite flakes (50 mesh, 99.5%) following a modified Hummers’ method53. OCB was prepared following the same oxidation treatment of GO with commercial carbon black (Ketjen Black, ECP600JD) as the precursor.
Synthesis of HEA-NPs/G and HEA-NPs/CB
In a typical synthesis of HEA-NPs/G, GO powders (100 mg) were dispersed in deionized H2O (30 mL) by sonication for 15 min to form a uniform colloidal suspension. Another aqueous solution was prepared by dissolving mixed metal salts with an equal molar ratio into H2O (20 mL), and the total weights of all metals were kept at 25 mg. Then, this metal precursor solution was added dropwise into the GO suspension under stirring, followed by sonication for 15 min to obtain the precursor dispersion for the following spray pyrolysis synthesis. Next, the as-prepared aqueous precursor dispersion was nebulized by an ultrasonic atomizer (1.7 MHz, Yuwell Medical Equipment Inc, China) to generate aerosol droplets, which were carried by Ar gas at 2 L min−1 to pass through a horizontal tube furnace preheated at 850 °C. The powdery products exiting the reactor were collected by a filter collector of polyvinylidene fluoride (Delvstlab, pore size of 0.22 μm) or compressed nickel foam (Suzhou Jiashide, 99.99%, thickness of 1.0 cm). The flow rate of the Ar carrier gas and the temperature of the tube furnace could be varied to tune the size of the HEA-NPs. HEA-NPs/CB was prepared following the same protocol of HEA-NPs/G except that the carbon precursor was changed from GO to OCB.
Characterization
XRD measurements were carried out on a Bruker D8 advanced diffractometer (Bruker, USA) fitted with Cu Kα radiation (wavelength λ = 1.5406 Å) with a scan rate of 5° min−1. SEM images were obtained with a HITACHI Regulus 8100 cold field emission scanning electron microscope at an acceleration voltage of 15 kV. FT-IR data were collected on a Nicolet iS20 FT-IR spectrometer (Thermo Fisher Scientific Co) at room temperature. TEM images were taken using a FEI Themis Z electron microscope at an acceleration voltage of 100 kV. HRTEM, ADF-STEM, and the corresponding EDS were conducted on a Thermo Fisher Scientific FEI Titan G2 60-300 TEM instrument at a 200 kV accelerating voltage. The detection of exhaust gas collected from the spray pyrolysis synthesis was conducted by GC (Panna A91 Plus). ICP analysis was performed on an Agilent ICP-MS 7900 instrument.
Electrochemical measurements
A CHI 760E electrochemical station (Shanghai Chenhua, CH Instrument) and a rotating disk electrode setup (RDE, Pine Instrument, USA) were used to evaluate the electrocatalytic performances of the catalysts. The ORR measurements were conducted in a 0.1 M HClO4 electrolyte with a conventional three-electrode cell system, which consists of a catalyst-loaded working electrode, a Hg/Hg2SO4 (filled with saturated K2SO4) reference electrode, and a Pt wire counter electrode. The electrolyte was freshly prepared and promptly utilized (pH = 1.04 ± 0.017), which was prepared by adding 7.07 g of HClO4 (70%) into 200 mL of deionized water, followed by adjusting the volume to 500 mL in a volumetric flask. The measured potential against reference electrode was converted to RHE by \({E}_{{{\rm{RHE}}}}={E}_{{{\rm{Hg}}}/{{{\rm{Hg}}}}_{2}{{{\rm{SO}}}}_{4}}+0.723\) in 0.1 M HClO4 electrolyte based on the calibration results (Supplementary Fig. 39a). To prepare the catalyst ink, 6.0 mg FeCoNiCuPt HEA-NPs/G was dispersed into a mixed solution containing 1.0 mL deionized H2O, 1.0 mL isopropanol and 60 μL Nafion (5 wt%) and sonicated for 1 h to obtain a homogeneous ink. Then, 8.0 μL of the catalyst ink was drop-cast onto a glassy carbon electrode (5 mm in diameter, 0.196 cm2 in area), resulting in a catalyst loading of 122.3 μg cm−2 (that corresponds to a Pt loading of 13.2 μgPt cm−2). For the Pt/C electrode, 6.0 mg Pt/C (20 wt% Pt/C, Johnson Matthey) was dispersed in a mixed solution containing 1.0 mL deionized H2O, 1.0 mL isopropanol and 60 μL Nafion (5 wt%), and 8.0 μL of the catalyst ink was drop-cast on the glassy carbon electrode, resulting in a catalyst loading of 122.3 μg cm−2 (Pt loading of 24.5 μgPt cm−2). Before catalyst coating, the glassy carbon electrode was polished with alumina polishing slurries (50 nm), cleaned with deionized water, and dried naturally. The catalysts were activated by CV measurements at a scan rate of 500 mV s−1 from 0.05 V to 1.10 V (versus RHE) until stable states were reached. The LSV curves were collected at a sweep rate of 10 mV s−1 from 0.05 V to 1.10 V at a rotating rate of 1600 r.p.m. and were processed with both iR (100%) and background corrections. The solution resistance (i.e., R) was determined by the high frequency intercept with the real axis in the Nyquist plot obtained from electrochemical impedance spectroscopy (EIS) measurement, which was conducted in the frequency range of 100 kHz to 0.1 Hz with a 5 mV amplitude at open circuit potential. The R value was determined to be 25.15 ± 2.29 Ω. For accelerated durability tests, the catalyst was subjected to CV sweeps between 0.6 V and 1.0 V in an O2-saturated 0.1 M HClO4 solution at a scan rate of 500 mV s−1. The mass activity and specific activity were determined by normalizing the kinetic currents (calculated from the Koutecký-Levich equation) with mass loading and ECSA of Pt, respectively. The ECSA was determined by integrating the hydrogen underpotential deposition area (Hupd) on the CV curve by taking a value of 210 μC cm−2 (Qref) for the adsorption of one monolayer of hydrogen, according to the following equation:
where SH, V, and mPt represent the integral area of hydrogen underpotential deposition, scan speed, and Pt loading, respectively.
The electrochemical measurements for the HER were conducted in N2-saturated 0.5 M H2SO4 electrolyte. The electrolyte was freshly prepared and promptly utilized (pH = 0.33 ± 0.0082), which was prepared by adding 28.05 mL of H2SO4 (95%) into 500 mL of deionized water, followed by adjusting the volume to 1000 mL in a volumetric flask. A Hg/Hg2SO4 (filled with saturated K2SO4) electrode and a Pt wire were used as the reference electrode and counter electrode, respectively. The measured potential against reference electrode was converted to RHE by \({E}_{{\mbox{RHE}}}=\,{E}_{{\mbox{Hg}}/{{\mbox{Hg}}}_{2}{{\mbox{SO}}}_{4}}{\mbox{+}}0.682\) in 0.5 M H2SO4 electrolyte based on the calibration results (Supplementary Fig. 39b). The working electrodes were prepared following a similar protocol to the ORR measurements except that the loading of FeCoNiCuPt HEA-NPs/G on the RDE was controlled at 15.3 μg cm−2 (Pt loading of 1.7 μgPt cm−2). The LSV curves were collected at a sweep rate of 10 mV s−1 from 0.05 V to − 0.35 V at a rotating rate of 1600 r.p.m to remove the bubbles generated during the HER process and were iR-compensated. The EIS-determined R value was determined to be 3.43 ± 1.51 Ω. The HER mass activity was obtained by normalizing the total currents with the mass loading of Pt.
For the PEMWE test, the cathode catalyst ink was prepared by dispersing 30 mg FeCoNiCuPt HEA-NPs/G in a mixture of water/isopropanol (1:1 v/v, 10 mL) and Nafion solution (5 wt%, 216 µL), and the anode catalyst ink was prepared by dispersing 30 mg commercial Ir/IrOx (SS-Ir-1001, Anhui Contango New Energy Technology Co., Ltd) in a mixture of 10 mL isopropanol and 10 µL Nafion. After being sonicated to form uniform dispersions, the inks were separately sprayed onto the two sides of a proton exchange membrane (Nafion 115; thickness: 127 μm; area: 4 cm2) to obtain the catalyst-coated membrane (CCM). The noble metal loadings were kept at 0.50 mgIr cm−2 for the anode and 0.04 mgPt cm−2 for the cathode. A porous Ti foam (Anhui Contango New Energy Technology Co., Ltd) and carbon paper (HCP120, Shanghai Hesen Electric Co., Ltd) served as the anodic and cathodic gas diffusion layers (GDL), respectively. The CCM was placed between two layers of polytetrafluoroethylene (PTFE) and hot-pressed at 80 °C for 3 min under a pressure of 0.5 MPa to ensure a strong bond. Then, the CCM, GDL, and Ti foam were integrated into the PEMWE. The performance of the PEMWE was evaluated at 80 °C using deionized H2O as the reactant with a flow rate of 100 mL min−1. Steady-state polarization tests were carried out under galvanostatic conditions over a range of current densities from 0 to 3 A cm−2 at 80 °C and ambient pressure. The stability was assessed by conducting a galvanostatic test at a current density of 1 A cm−2.
Data availability
The data that support the findings and conclusions generated in this study are provided in the main article and the Supplementary Information. Source data are provided with this paper.
References
Yao, Y. et al. High-entropy nanoparticles: synthesis-structure-property relationships and data-driven discovery. Science 376, eabn3103 (2022).
Schweidler, S. et al. High-entropy materials for energy and electronic applications. Nat. Rev. Mater. 9, 266–281 (2024).
Han, X. et al. Nanoscale high-entropy alloy for electrocatalysis. Matter 6, 1717–1751 (2023).
Glasscott, M. W. et al. Electrosynthesis of high-entropy metallic glass nanoparticles for designer, multi-functional electrocatalysis. Nat. Commun. 10, 2650 (2019).
George, E. P., Raabe, D. & Ritchie, R. O. High-entropy alloys. Nat. Rev. Mater. 4, 515–534 (2019).
Wu, D. et al. On the electronic structure and hydrogen evolution reaction activity of platinum group metal-based high-entropy-alloy nanoparticles. Chem. Sci. 11, 12731–12736 (2020).
van Deelen, T. W., Hernández Mejía, C. & de Jong, K. P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2, 955–970 (2019).
Mori, K. et al. Hydrogen spillover-driven synthesis of high-entropy alloy nanoparticles as a robust catalyst for CO2 hydrogenation. Nat. Commun. 12, 3884 (2021).
Zhang, W., Feng, X., Mao, Z. X., Li, J. & Wei, Z. Stably immobilizing sub-3 nm high-entropy Pt alloy nanocrystals in porous carbon as durable oxygen reduction electrocatalyst. Adv. Funct. Mater. 32, 2204110 (2022).
Hu, Y. et al. Hollow-carbon confinement annealing: a new synthetic approach to make high-entropy solid-solution and intermetallic nanoparticles. Nano Lett. 23, 10765–10771 (2023).
Su, D. S., Perathoner, S. & Centi, G. Nanocarbons for the development of advanced catalysts. Chem. Rev. 113, 5782–5816 (2013).
Ren, J.-T., Chen, L., Wang, H.-Y. & Yuan, Z.-Y. High-entropy alloys in electrocatalysis: from fundamentals to applications. Chem. Soc. Rev. 52, 8319–8373 (2023).
Zhan, C. et al. Subnanometer high-entropy alloy nanowires enable remarkable hydrogen oxidation catalysis. Nat. Commun. 12, 6261 (2021).
Guo, H. et al. Polymer-confined pyrolysis promotes the formation of ultrafine single-phase high-entropy alloys: a promising electrocatalyst for oxidation of nitrogen. Adv. Funct. Mater. 33, 2308229 (2023).
Chen, T. et al. An ultrasmall ordered high-entropy intermetallic with multiple active sites for the oxygen reduction reaction. J. Am. Chem. Soc. 146, 1174–1184 (2024).
Li, M. et al. High-entropy alloy electrocatalysts go to (sub-)nanoscale. Sci. Adv. 10, eadn2877 (2024).
Li, H. et al. Fast site-to-site electron transfer of high-entropy alloy nanocatalyst driving redox electrocatalysis. Nat. Commun. 11, 5437 (2020).
Wu, D. et al. Platinum-group-metal high-entropy-alloy nanoparticles. J. Am. Chem. Soc. 142, 13833–13838 (2020).
Feng, G. et al. Sub-2 nm ultrasmall high-entropy alloy nanoparticles for extremely superior electrocatalytic hydrogen evolution. J. Am. Chem. Soc. 143, 17117–17127 (2021).
Ai, Y. et al. Ultra-small high-entropy alloy nanoparticles: efficient nanozyme for enhancing tumor photothermal therapy. Adv. Mater. 35, 2302335 (2023).
Feng, G., Pan, Y., Su, D. & Xia, D. Constructing fully-active and ultra-active sites in high-entropy alloy nanoclusters for hydrazine oxidation-assisted electrolytic hydrogen production. Adv. Mater. 36, 2309715 (2024).
Liang, J. et al. Metal bond strength regulation enables large-scale synthesis of intermetallic nanocrystals for practical fuel cells. Nat. Mater. 23, 1259–1267 (2024).
Song, J.-Y. et al. Generation of high-density nanoparticles in the carbothermal shock method. Sci. Adv. 7, eabk2984 (2021).
Cao, G. et al. Liquid metal for high-entropy alloy nanoparticles synthesis. Nature 619, 73–77 (2023).
Liu, Y.-H. et al. Toward controllable and predictable synthesis of high-entropy alloy nanocrystals. Sci. Adv. 9, eadf9931 (2023).
Qiao, H. et al. Scalable synthesis of high entropy alloy nanoparticles by microwave heating. ACS Nano 15, 14928–14937 (2021).
Wang, B. et al. General synthesis of high-entropy alloy and ceramic nanoparticles in nanoseconds. Nat. Synth. 1, 138–146 (2022).
Gao, S. et al. Synthesis of high-entropy alloy nanoparticles on supports by the fast moving bed pyrolysis. Nat. Commun. 11, 2016 (2020).
Yao, Y. et al. Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science 359, 1489–1494 (2018).
Guo, C. et al. Laser precise synthesis of oxidation-free high-entropy alloy nanoparticle libraries. J. Am. Chem. Soc. 146, 18407–18417 (2024).
Jiang, H. et al. Nanoalloy libraries from laser-induced thermionic emission reduction. Sci. Adv. 8, eabm6541 (2022).
Zheng, X. et al. Hydrogen-substituted graphdiyne-assisted ultrafast sparking synthesis of metastable nanomaterials. Nat. Nanotechnol. 18, 153–159 (2023).
Cha, J.-H. et al. Flash-thermal shock synthesis of high-entropy alloys toward high performance water splitting. Adv. Mater. 35, 2305222 (2023).
Li, Y. et al. Laser annealing-induced phase transformation behaviors of high entropy metal alloy, oxide, and nitride nanoparticle combinations. Adv. Funct. Mater. 33, 2211279 (2023).
Shi, W. et al. Transient and general synthesis of high-density and ultrasmall nanoparticles on two-dimensional porous carbon via coordinated carbothermal shock. Nat. Commun. 14, 2294 (2023).
Minamihara, H. et al. Continuous-flow reactor synthesis for homogeneous 1 nm-sized extremely small high-entropy alloy nanoparticles. J. Am. Chem. Soc. 144, 11525–11529 (2022).
Kusada, K. & Kitagawa, H. Continuous-flow syntheses of alloy nanoparticles. Mater. Horiz. 9, 547–558 (2022).
Sui, J., Yan, J., Liu, D., Wang, K. & Luo, G. Continuous synthesis of nanocrystals via flow chemistry technology. Small 16, 1902828 (2020).
Kim, K. S. et al. Continuous synthesis of high-entropy alloy nanoparticles by in-flight alloying of elemental metals. Nat. Commun. 15, 1450 (2024).
Phakatkar, A. H. et al. Ultrafast synthesis of high entropy oxide nanoparticles by flame spray pyrolysis. Langmuir 37, 9059–9068 (2021).
Hanabata, S. et al. Denary high-entropy oxide nanoparticles synthesized by a continuous supercritical hydrothermal flow process. J. Am. Chem. Soc. 146, 181–186 (2023).
Kusada, K. et al. Nonequilibrium flow-synthesis of solid-solution alloy nanoparticles: from immiscible binary to high-entropy alloys. J. Phys. Chem. C. 125, 458–463 (2021).
Yang, Y. et al. Aerosol synthesis of high entropy alloy nanoparticles. Langmuir 36, 1985–1992 (2020).
Ma, X., Zachariah, M. R. & Zangmeister, C. D. Crumpled nanopaper from graphene oxide. Nano Lett. 12, 486–489 (2012).
Zhao, P. et al. Facile and general method to synthesize Pt-based high-entropy-alloy nanoparticles. ACS Nano 16, 14017–14028 (2022).
Yang, C.-L. et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells. Science 374, 459–464 (2021).
Kar, N. et al. Retrosynthetic design of core-shell nanoparticles for thermal conversion to monodisperse high-entropy alloy nanoparticles. Nat. Synth. 3, 175–184 (2023).
Wang, X. et al. Continuous 2000 K droplet-to-particle synthesis. Mater. Today 35, 106–114 (2020).
Wang, X. et al. Continuous synthesis of hollow high-entropy nanoparticles for energy and catalysis applications. Adv. Mater. 32, 2002853 (2020).
Yao, Y. et al. Extreme mixing in nanoscale transition metal alloys. Matter 4, 2340–2353 (2021).
De Marco, M. L. et al. High-entropy-alloy nanocrystal based macro- and mesoporous materials. ACS Nano 16, 15837–15849 (2022).
Tao, L. et al. Precise synthetic control of exclusive ligand effect boosts oxygen reduction catalysis. Nat. Commun. 14, 6893 (2023).
Marcano, D. C. et al. Improved synthesis of graphene oxide. ACS Nano 4, 4806–4814 (2010).
Yao, Y. et al. Computationally aided, entropy-driven synthesis of highly efficient and durable multi-elemental alloy catalysts. Sci. Adv. 6, eaaz0510 (2020).
Hao, J. et al. Unraveling the electronegativity-dominated intermediate adsorption on high-entropy alloy electrocatalysts. Nat. Commun. 13, 2662 (2022).
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant no. 22479044) and Major Program of the Natural Science Foundation of Hunan Province (Grant no. 2021JC0006) awarded to H.F. We acknowledge the Analytical Instrumentation Center of Hunan University for TEM characterizations.
Author information
Authors and Affiliations
Contributions
H.F. oversaw the research in different phases and provided regular guidance and suggestions throughout the research. G.H. and X.Z. conducted the materials synthesis, structural characterizations of the prepared catalysts, and electrochemical measurements, interpreted the data, and drafted the paper. J.L. and J.C. participated in discussions about the experimental results and providing valuable suggestions for the research. G.H., X.Z., J.C., J.L., G.Y., and H.F. co-wrote the paper. All authors had an opportunity to comment on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Chuanxin He, Keun Su Kim, and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
He, G., Zhang, X., Liu, J. et al. Hydrocarbothermal flow synthesis of carbon-supported small and dense high-entropy alloy nanoparticles as electrocatalysts. Nat Commun 16, 8172 (2025). https://doi.org/10.1038/s41467-025-63527-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-63527-7