Abstract
SOX2 is a potent oncodriver for various squamous cancers, but the underlying mechanism is largely unknown. Here we uncover a role of SOX2 in promoting global histone acetylation in esophageal squamous cancer cells (ESCCs). Mechanistic studies reveal that SOX2 promotes global histone acetylation in an AKT-independent manner, and does so by promoting histone acetylation at both SOX2 binding and non-SOX2 binding sites, and accounts for the formation of about half of the super-enhancers. Combined metabolic and transcriptional analyses reveal two mechanisms by which SOX2 enhances global histone acetylation: promoting the expression of multiple histone acetyltransferases and reducing acetyl-CoA consuming fatty acid synthesis in part by repressing the expression of ACSL5. Finally, SOX2 expression correlates negatively with ACSL5 and positively with histone acetylation in clinical esophageal squamous tumors. Altogether, our study uncovers a role of SOX2 in reprogramming lipid metabolism and driving histone hyperacetylation and super-enhancer function, providing mechanistic insights of SOX2 acting as a potent oncodriver.
Similar content being viewed by others
Introduction
Squamous cell carcinomas (SCCs) including esophageal SCC (ESCC) are aggressive malignancies with a squamous epithelium origin and no effective treatments1,2,3. SOX2 is a key pioneer transcription factor (TF) that plays a vital role in embryonic stem cells and marks bona fide stem cells in adult tissues4. Notably, SOX2 is also a squamous lineage-specific TF that is overexpressed in various SCCs and promotes tumor cell proliferation, cancer stem-cell activity, invasion, metastasis and therapeutic resistances5,6,7,8,9,10,11,12. Genetic studies in mice revealed that SOX2 is not only required for the initiation and progression of SCCs, but also can drive the initiation and progression of lung squamous cell carcinomas when overexpressed together with other genetic lesions13,14. The molecular mechanism by which SOX2 exerts as a potent oncogenic determinant for SCCs, however, is not fully understood15,16.
Reprogramming cellular metabolic pathways such as augmenting glycolysis to meet the enhanced needs for rapid proliferation is a hallmark of tumor cells17,18. Acetyl-CoA is a central metabolite that links glucose, glutamine and lipid metabolism and cellular signaling together19. Acetyl-CoA is produced by glucose metabolism, fatty acid β-oxidation and other metabolic processes, and serves as the substrate for de novo fatty acid and cholesterol biosynthesis and the donor for acetylation of histone and nonhistone proteins19,20. Histone acetylation catalyzed by lysine acetyltransferases (KATs) has critical roles in regulation of chromatin accessibility and formation of super-enhancers21. Super-enhancers are required for driving expression of cell-type-specific genes through interacting with master lineage-specific TFs, coregulators, RNA polymerase II as well as non-coding RNAs22,23. The global level of histone acetylation is sensitive to the production and availability of acetyl-CoA. In this regard, AKT has been shown to elevate acetyl-CoA level and drive histone acetylation and pancreatic cancer progression24,25. As a master TF of various stem cells and squamous lineage cells, SOX2 has been well documented for recruitment of various KATs for local histone acetylation in chromatin and transcriptional regulation26,27. Whether SOX2 reprograms metabolism and global histone acetylation to drive ESCCs, however, is not known.
Here, we found that SOX2’ oncogenic activity in ESCCs is associated with its ability to potently enhance global histone acetylation. SOX2 controls squamous lineage transcription program and, strikingly, is required for the formation of nearly half of the super-enhancers in ESCC cells. We elucidate the molecular mechanisms by which SOX2 enhances global histone acetylation and super-enhancer function and demonstrated that SOX2 expression positively correlates with histone acetylation status in clinical ESCC specimens.
Results
SOX2 enhances global histone acetylation in ESCC cells
We have previously demonstrated that in mouse embryonic stem cells and ESCCs, AKT stabilizes and drives SOX2 overexpression by protecting SOX2 from proteasome degradation27,28. Given that AKT has been shown to promote pancreatic tumor progression by enhancing histone acetylation25, we attempted to investigate whether AKT also promotes histone acetylation in ESCC cells. We thus examined the status of AKT, SOX2 and histone acetylation among a panel of ESCC-derived KYSE cell lines (K cell lines hereafter). As shown in Fig. 1a, Western blot (WB) analysis did not reveal a significant correlation between the levels of AKT and that of histone acetylation. Instead, we noticed an imperfect correlation between the levels of SOX2 and that of histone acetylation. To test whether AKT promotes histone acetylation in ESCC cells, we treated both SOX2-positive and negative ESCC cells with a potent AKT inhibitor, MK220629. As expected, addition of MK2206 markedly inhibited AKT kinase activity, as evidenced by a significant reduction of serine 473 -phosphorylated, active AKT (pS473-AKT1) but with no change of total levels of AKT protein in all cell lines tested (Fig. 1b). Notably, we observed that inhibition of AKT resulted in reduction of acetylation of both histones H3 and H4 only in SOX2-positive but not in SOX2-negative ESCC cells (Fig. 1b). Consistent with our previous studies27,28, inhibition of AKT by MK2206 markedly reduced the SOX2 protein level in SOX2-positive cells (Fig. 1b). Furthermore, MK2206 reduced the levels of both SOX2 and histone acetylation in a dose-dependent manner (Supplementary Fig. 1a). These results raised the possibility that SOX2 but not AKT may control global histone acetylation in ESCC cells.
a Western blot analyses of the levels of AKT1, SOX2, and histone acetylation in ESCC cell lines (Kyse) and cervical cancer cell line C33A. Simple linear regression analysis was performed to assess the relationship between SOX2 and H3ac/H4ac levels, along with correlation analysis. Antibodies against AKT1, SOX2, ACTIN, and H3 were probed on the same gel. The samples originate from the same experiment, but H3ac and H4ac were processed on separate gels in parallel. b Western blot analyses showing the effect of AKT inhibitor MK2206 on histone acetylation level in SOX2- positive and negative cells. c Western blot analyses showing the effect of SOX2 knockdown on histone acetylation level in various cells. In b, c antibodies against AKT1, SOX2, ACTIN, and H3 were probed on the same gel. The samples originate from the same experiment, but pS473-AKT1, H3ac and H4ac were processed on separate gels in parallel. d Western blot analyses showing the effect of SOX2 knockdown on site-specific histone acetylation K30 and K450 cells. Three biological replicates were analyzed. Antibodies against SOX2, ACTIN, and H3 were probed on the same gel. The samples originate from the same experiment, but H3K9ac, H3K27ac, H3ac, H4K5ac, H4K16ac and H4ac were processed on separate gels in parallel. e Knockout of SOX2 also downregulated histone acetylation in K450 cells. Antibodies against SOX2, H3 were probed on the same gel. The samples originate from the same experiment, H3ac and H4ac were processed on separate gels in parallel. f Ectopic expression of FLAG-SOX2 restored histone acetylation level in SOX2 knockdown K30 cells. Antibodies against SOX2, ACTIN, and H3 were probed on the same gel. The samples originate from the same experiment, H3ac and H4ac were processed on separate gels in parallel. g Ectopic expression of FLAg-SOX2 elevated both histone and non-histone acetylation in SOX2-negative K410 cells. Antibodies against AKT1, SOX2, ACTIN, and H3 were probed on the same gel. The samples originate from the same experiment, pS473-AKT1, H3ac and H4ac were processed on separate gels in parallel. h Immunofluorescence staining assay showing that ectopic expression of FLAG-SOX2 led to substantial elevation of histone acetylation level in K30 cells. Scale bar 20 μm. All quantification of histone acetylation levels were performed using Image J program. In a–c, e–h, experiments were repeated independently at least 3 times with similar results. Representative images/data from one experiment are shown. Data in d are from three biological replicates.
To assess whether SOX2 regulates global histone acetylation, we performed shRNA-mediated SOX2 knockdown (KD) in multiple ESCC cells. SOX2 KD had no effect on AKT1 protein level and its active marker S473 phosphorylation, but resulted in reduction of histone H3 and H4 acetylation in all SOX2-positive ESCC cells (Fig. 1c), and reduction of histone acetylation upon SOX2 KD was confirmed in biological replicates by WB analysis using H3 and H4 acetylation site-specific antibodies (Fig. 1d). Similarly, knockout of SOX2 by three distinct SOX2-specific guide RNAs via CRISPR/Cas9 resulted in reduction of global histone acetylation (Fig. 1e). Notably, ectopic expression of SOX2 restored the histone acetylation level in SOX2 KD K30 cells (Fig. 1f), confirming a role of SOX2 in regulating global histone acetylation. The K30 cells were used in this rescue experiment because this cell line allowed us to do both SOX2 KD and overexpression in the same cell line, own to its moderate level of SOX2 expression. Furthermore, ectopic expression of SOX2 in SOX2-negative K410 cells resulted in an obvious increase of acetylated histones and acetylated non-histone proteins (Fig. 1g). By immunofluorescent staining, we also observed that ectopic expression of SOX2 resulted in substantial elevation of histone acetylation in K30 cells (Fig. 1h). Regulation of histone acetylation by SOX2 is likely independent of AKT kinase activity, because inhibition of AKT did not further reduce histone acetylation level in SOX2 KD K450 cells (Supplementary Fig. 1b) and ectopic overexpression of SOX2 did not alter AKT1 protein level and activation (Fig. 1g). Furthermore, regulation of histone acetylation by SOX2 is unlikely an ESCC specific event, as SOX2 KD in two lung cancer cell lines also resulted in a similar global reduction of histone acetylation (Supplementary Fig. 1c). We also validated by histone acetylation site-specific antibodies that SOX2 KD in K450 cells resulted in global reduction of H3K9ac and H3K27ac (Supplementary Fig. 1d). Notably, regulation of global histone acetylation is likely a unique feature of SOX2, because neither ectopic expression of MYC nor OCT4, two other ES core TFs, was able to enhance global histone acetylation in K30 cells (Supplementary Fig. 1e). Altogether these data reveal a role of SOX2 in promoting global level of histone acetylation in esophageal cancer cells.
SOX2 controls histone acetylation levels at promoter, enhancer and super-enhancer regions
To investigate further that SOX2 regulates global histone acetylation, we carried out chromatin immunoprecipitation followed by sequencing (ChIP-seq) assay to determine the genomic binding profile of SOX2 and the effect of transient SOX2 KD on genomic histone acetylation landscape in K450 cells. K450 cells were chosen because it showed a mid-high level of SOX2 protein expression among SOX2-positive ESCC cell lines (Fig. 1a). A total of 23,621 SOX2 chromatin binding events were identified in K450 cells. Further, 21,437 of the SOX2 binding events (90.8%) showed more than 1.5-fold reduction in intensities in the SOX2 KD cells (Fig. 2a), indicating that these were genuine SOX2 binding events. Notably, 38.7% and 31.1% of SOX2 binding events occurred at enhancers and promoters, respectively (Fig. 2b, upper panel), consistent with its role in gene expression reported in embryonic stem cells and ESCCs30,31. In consistence with SOX2 being a sequence specific DNA binding TF, the identified SOX2 binding regions were significantly enriched for SOX2 motif (Fig. 2b, lower panel). The SOX2 binding regions were also enriched of the binding motifs of P63 and bZIP type TFs, such as Jun B and AP1, and the motifs for other SOX family members (Fig. 2b and Supplementary Fig. 2). This is in agreement with the previous findings that SOX2 and p63 exhibited significant genomic co-occupancy in SCCs31, and suggests that they may act together with SOX2 in transcriptional regulation in K450 cells.
a Heatmap comparison of SOX2 ChIP-seq signals in the control and SOX2 knockdown K450 cells, ranked by SOX2 intensities in control K450 cells. b Genomic distribution of SOX2 ChIP-seq peaks in control K450 cells. Promoter (TSS ± 1000 bp). Statistics of ChIP-seq peaks showing significant enrichment of SOX family transcription factor binding motifs in SOX2 ChIP-seq peaks. c Heatmap analyses of H4ac, H3K27ac and H3K4me3 levels on SOX2 binding regions, ranked by SOX2 ChIP-seq intensities in control K450 cells. d Profiles of SOX2, H4ac, H3K27ac and H3K4me3 ChIP-seq signals from control and SOX2 knockdown K450 cells. e Heatmap comparison of SOX2 binding intensities over ChIP-seq peaks showed reduced levels of H4ac, H3K27ac and H3K4me3 (1.5-fold change) upon SOX2 knockdown, ranked by SOX2 ChIP-seq signals from control K450 cells. All ChIP-seq signals are displayed from −3 kb to +3 kb surrounding the peak center. f Venn diagram analyses of super-enhancers identified in control and SOX2 knockdown K450 cells. g Normalized H3K27ac, H4ac and SOX2 ChIP-seq signals of control and SOX2 knockdown K450 cells over the lost, shared and gained super-enhancers upon SOX2 depletion. In a–g one ChIP-seq replicate was performed, and data are shown from two analytical replicates.
ChIP-seq analyses of H4ac, H3K27ac and H3K4me3 revealed that in the control shRNA treated K450 cells, 10,639 (45.0%) and 11,112 (47.0%) of the SOX2 binding sites were accompanied with histone H4ac and H3K27ac, while to a lesser extent, 9,348 (39.6%), with H3K4me3 (Fig. 2c). Notably, upon SOX2 KD, all the three active histone marks showed reduced chromatin occupancies, with H4ac showing the greatest reduction (Fig. 2d), consistent with the observation that SOX2 promotes global histone acetylation. Furthermore, among the ChIP-seq peaks showing reduced histone marks, 4,624 (60.3%) H4ac, 6,767 (64.1%) H3K27ac and 6,732 (55.2%) H3K4me3 peaks were directly bound by SOX2 (Fig. 2e, upper panels), while the rest peaks showed relatively weaker to non-SOX2 binding (P-value < 0.05, Fig. 2e bottom panels), indicating that SOX2 promotes histone acetylation beyond the regions it binds. As histone acetylation is known to promote H3K4 methylation32, the substantial reduction of H3K4me3 in the SOX2 KD cells could be a consequence of SOX2 on histone acetylation. Together these epigenomic analyses established not only a close correlation between SOX2 binding and histone acetylation but also confirmed a role of SOX2 in promoting global histone acetylation.
Since a substantial portion of SOX2 peaks occurred at enhancers, we wanted to further investigate the relationship between SOX2 chromatin binding and super-enhancers (SEs). Using ROSE algorithm (https://hpc.nih.gov/apps/ROSE.html) mainly based on the ranking of H3K27ac intensities23,33, a well-recognized enhancer marker, we identified a total of 258 SEs in the control K450 cells and 163 SEs in the SOX2 KD cells. Among the 163 SEs identified in the SOX2 KD cells, 128 SEs were also presented in the control cells and 35 SEs were unique to the SOX2 KD cells (Fig. 2f). Notably, although H3K27ac showed comparable levels at the 128 SEs shared between the control and shSOX2 treated cells, the average H4ac level at these SEs was clearly reduced in the SOX2 KD cells, suggesting that the activities of the 128 shared SEs might also be reduced upon SOX2 loss (Fig. 2g) and H4ac might serve as a more sensitive marker of enhancer activity compared to H3K27ac. We found that the group of gained SEs in SOX2 KD cells showed the lowest SOX2 binding intensities and the group of lost SEs showed a modest level of SOX2 bindings intensities compared to the group of shared SEs in the control K450 cells (Fig. 2g). These results suggest that the shared SEs are more resilient to incomplete SOX2 KD since they exhibit the strongest SOX binding, whereas the lost SEs are modest in SOX2 binding and thus more sensitive to SOX2 dosage. Altogether, the ChIP-seq analyses revealed a crucial role of SOX2 in control of histone acetylation at thousands of the regulatory regions, including most super-enhancers, in ESCC cells.
Rapid degradation of SOX2 confirms its role in both local and global histone acetylation
The above epigenomic analyses based on SOX2 KD could be complicated by the potential off target effect of shRNAs and/or the potential secondary effect of 2-day SOX2 KD. To rigorously test the role of SOX2 in regulation of histone acetylation, we made use of the dTAG system to rapidly and specifically degrade SOX2 in ESCC cells34,35,36. To this end, we engineered a K450 SOX2-dTAG cell line by knocking in the FKBP12F36V degrader in-frame at the SOX2 N-terminus (Fig. 3a). The successful knock-in of FLAG-FKBP12F36V into SOX2 was verified by DNA sequencing and WB results (Fig. 3b) showing an up-shifted SOX2 band with the expected molecular weight that was also overlapped with the band detected by anti-FLAG antibody. We noticed that knock-in of FLAG-FKBP12F36V reduced the SOX2 fusion protein expression level by ~70% when compared to the SOX2 level in parental cells (Fig. 3b, lane 5 vs lane 1). We also noticed that the reduced expression of SOX2 fusion protein was accompanied by a reduced level of acetylated H3 and acetylated H4 (Fig. 3b), further confirming a role of SOX2 in promoting global histone acetylation. We confirmed that dTAG treatment had no effect on SOX2 in parental K450 cells, but the same treatment induced essentially complete degradation of SOX2 fusion protein within 3 h (Fig. 3b, c). Note that, although dTAG treatment did effectively eliminate the SOX2 fusion protein, it did not appear to reduce further the global level of histone acetylation (Fig. 3b, c), presumably because knock-in of FLAG-FKBP12F36V already significantly down-regulated the level of SOX2 fusion protein and global histone acetylation.
a Diagram illustrating the construction of SOX2-dTAG gene with an in-frame FLAG-FKBP12F36V degron knocked in at the SOX2 N-terminus. b, c WB analysis showing the levels of SOX2, SOX2-dTAG, histone acetylation, and EP300 in parental and SOX2-dTAG K450 cells without and with dTAG treatment for 3 h, 12 h, and 24 h. Quantification of histone acetylation levels were performed using Image J program. In b antibodies against SOX2, ACTIN were probed on the same gel. The samples originate from the same experiment, but FLAG, H3ac and H4ac were processed on separate gels in parallel. In c antibodies against SOX2, H3 were probed on the same gel. The samples originate from the same experiment, but FLAG, EP300, H3K27ac and H4ac were processed on separate gels in parallel. d Heatmap comparison of histone H4 acetylation ChIP-seq signals in SOX2-dTAG K450 cells treated without or with dTAG for 3 h and 24 h, ranked by acH4 intensities in SOX2-dTAG K450 cells without dTAG treatment. e, f UCSC genome browser snapshots of a representative KRT cluster containing KRT5 and KRT6A (e) and GOLT1B (f) with normalized signals of H4ac from SOX2-dTAG K450 cells treated without or with dTAG for 3 h and 24 h. g Profiles of H4ac ChIP-seq signals from SOX2-dTAG K450 cells treated without or with dTAG for 3 h and 24 h. h Profiles of H4ac ChIP-seq signals on SOX2 containing peaks from SOX2-dTAG K450 cells treated without or with dTAG for 3 h and 24 h. i Profiles of H4ac ChIP-seq signals on SOX2 absent peaks from SOX2-dTAG K450 cells treated without or with dTAG for 3 h and 24 h. j ChIP-qPCR analysis of the SOX2 binding on KRT6A and KRT5 genes in SOX2-dTAG K450 cells treated without or with dTAG for 3 h and 24 h. k ChIP-qPCR analysis of the EP300 binding on KRT6A and KRT5 genes in SOX2-dTAG K450 cells treated without or with dTAG for 3 h and 24 h. ChIP-qPCR data (j, k) are represented as mean +/- SD from 4 biological replicates, n = 4. P values were determined by two-sided unpaired t test. *, P < 0.05, ***, P < 0.001. In b, c experiments were repeated independently at least 2 times with similar results. Representative data from one experiment is shown. In d-i one ChIP-seq replicate was performed, and data are shown from two analytical replicates. Data in j, k are from three biological replicates.
To more precisely evaluate how rapid degradation of SOX2-dTAG affected histone acetylation, we carried out ChIP-seq analysis by comparing the samples treated with dTAG for 0, 3 h and 24 h. As shown in Fig. 3d, ChIP-seq analysis revealed that histone H4 acetylation was globally reduced upon 3 h dTAG treatment in comparison to the untreated control cells, and that it was further reduced upon 24 h treatment. The reduction of H4 acetylation upon 3 h and 24 h dTAG treatment was exemplified with keratin family genes (Fig. 3e) and GOLT1B (Fig. 3f). Overall, the average H4ac peak level was reduced upon 3 h dTAG treatment and further reduced upon 24 h dTAG treatment (Fig. 3g). When average H4 acetylation peaks were sorted by with or without overlapping SOX2 peaks, it is clear that reduction of histone acetylation is more severe at the peaks with SOX2 than that without SOX2 (Figs. 3h, i). These results indicate that SOX2 binding promotes local histone acetylation, in agreement with the previous reports that SOX2 can recruit EP300 and other KATs. By ChIP-qPCR analysis, we confirmed SOX2 binding of both KRT6A and KRT5 genes, and this binding was lost upon 3 h dTAG treatment (Fig. 3j), consistent with our WB results that by this time point SOX2 was already effectively degraded. Notably, ChIP-qPCR analysis also revealed that EP300 was associated with both KRT6A and KRT5 genes, and EP300 binding was reduced upon 3 h dTAG treatment and there was no further reduction of EP300 binding at 24 h time point (Fig. 3k). Altogether, these results not only confirmed a role of SOX2 in recruitment of EP300 that is likely to contribute to local histone acetylation, but also supported a role of SOX2 in promoting global histone acetylation, because reduction of histone acetylation was also observed at no SOX2 binding regions and further reduction of histone acetylation at 24 h was observed long after loss of SOX2-dependent local recruitment of EP300 and presumably other KATs as well.
SOX2 does not significantly regulate the expression and subcellular localization of acetyl-CoA producing enzymes, glucose and glutamine metabolism, but may slightly promote fatty acid β-oxidation
To investigate how SOX2 enhances global histone acetylation in ESCC cells, we first hypothesized that SOX2 could drive global histone acetylation by promoting the expression of acetyl-CoA producing enzymes. The expression and subcellular localization of enzymes, namely ATP-citrate lyase (ACLY)20, acyl-CoA synthetase short-chain family, member 2 (ACSS2)37, and pyruvate dehydrogenase complex (PDC)38 have been implicated to account for acetyl-CoA synthesis in the cytosolic-nucleus compartment and drive histone acetylation. We found that knockdown or knockout of SOX2 affected neither the levels of their proteins nor their cytoplasm and nucleus distribution (Supplementary Fig. 3), suggesting that SOX2 is unlikely to enhance global histone acetylation by regulating the expression and subcellular localization of the acetyl-CoA producing enzymes, although we could not exclude the possibility that their activities could be affected.
Given that glucose and glutamine metabolism and fatty acid β-oxidation can all produce acetyl-CoA, we next investigated whether SOX2 regulates these metabolic processes to produce more acetyl-CoA. To this end, we carried out stable isotope tracing experiments by using [U-13C6]-glucose, [1-13C1]-glutamine, [5-13C1]-glutamine and [U-13C16]-palmitate as the tracers, respectively39 (Supplementary Fig. 4a). Subsequent gas chromatography coupled with mass spectrometry analyses revealed that SOX2 KD slightly reduced the levels of pyruvate-derived citrate and isocitrate, but did not affect the levels of succinate, malate, fumarate and α-ketoglutarate (Supplementary Fig. 4b). Similarly, SOX2 KD also did not significantly affect glutamine reductive carboxylation (Supplementary Fig. 4c) or oxidative metabolism (Supplementary Fig. 4d). Furthermore, no significant changes in M2 isotopomers derived from [U-13C16]-palmitate was observed upon SOX2 KD or SOX2 overexpression (Supplementary Fig. 4e), suggesting that SOX2 did not significantly impact fatty acid β-oxidation. We also measured the change of acetyl-CoA levels in K30 cells upon SOX2 KD by mass spectrometry. A trend of slight reduction of overall cellular acetyl-CoA was observed upon SOX2 KD, although in multiple experiments this change was not statistically significant (Supplementary Fig. 4f). We also carried out real-time and live cell measurement of mitochondrial respiration by Seahorse assay, and the results revealed insignificant difference between control and SOX2 KD K450 cells (Supplementary Fig. 4g). Similar analyses with K30 cells also revealed that SOX2 KD did not significantly alter glucose and glutamine metabolism and mitochondrial respiration (Supplementary Fig. 4h–j).
The above mitochondrial respiration assay was conducted with the full culture medium. To better assess if SOX2 regulates fatty acid β-oxidation, we carried out Seahorse assay in the presence of sodium palmitate but lack of glucose, glutamine, and pyruvate. Under this palmitate only condition, a consistent modest reduction of mitochondrial respiration was observed in SOX2 KD K450 cells (Supplementary Fig. 5). We therefore conclude that SOX2 can slightly enhance fatty acid β-oxidation in ESCC cells when fatty acid becomes the major nutrient.
SOX2 enhances the expression of multiple lysine acetyltransferases (KATs)
To further investigate the mechanism(s) by which SOX2 promotes global histone acetylation, we performed RNA sequencing (RNA-seq) analysis to determine the differentially expressed genes (DEGs) in K450 cells with and without SOX2 KD. In three biological replicates (Supplementary Fig. 6a), with a 3-fold change as a cut-off and adjusted-P value < 0.05, we identified 277 up-regulated genes and 2165 down-regulated genes upon SOX2 depletion in K450 cells (Fig. 4a), demonstrating a prevailing role of SOX2 in driving transcriptional activation in ESCC cells. Along this line, we also noticed a global reduction at about 2.1-fold of total mRNA transcripts in the SOX2 KD cells compared with the control KD cells, using spike-in mRNA reads as control (Supplementary Fig. 6b). Among the top down-regulated genes are multiple keratin family members and epithelial lineage-specific genes (Fig. 4b), highlighting a key role of SOX2 in programming squamous lineage gene expression31,40. The regulation of keratin family genes by SOX2 was further supported by the presence of SOX2 peaks within multiple keratin gene loci and the reduced intensities of H3K27ac, H4ac peaks and H3K4me3 upon SOX2 KD (Fig. 4c). Gene ontology (GO) analysis indicates that SOX2-activated genes are enriched in RNA biogenesis, epidermis development and DNA replication (Supplementary Fig. 6c), in agreement with a key role of SOX2 in promoting ESCC proliferation.
a Number of up-regulated and down-regulated, differentially expressed genes (DEGs, adjusted P value < 0.05, fold-change > 3) upon SOX2 depletion in K450 cells. The statistical test used in RNA-seq data was two-sided (also in Fig. 4b). b Volcano plot displaying DEGs (adjusted P value < 0.05, fold-change > 3) upon SOX2 depletion in K450 cells. Top 10 up- and down-regulated genes, KATs and ACSL5 were indicated. c UCSC genome browser snapshots of a representative KRT cluster containing KRT5 and KRT6A, and normalized signals of RNA, SOX2, H4ac, H3K27ac and H3K4me3 from control and SOX2 knockdown K450 cells were shown. d Heatmap analyses showing mRNA expression levels of the indicated KAT genes in control and SOX2 knockdown K450 cells, signals were z-scaled and DESeq2 normalized. e UCSC genome browser snapshots of a representative HAT gene, KAT7, and normalized signals of RNA, SOX2, H4ac, H3K27ac and H3K4me3 from control and SOX2 knockdown K450 cells. f, g RT-PCR analyses of the transcription levels of multiple KATs in SOX2 knockdown (f) and SOX2 overexpression K30 cells (g), primers were used as indicated in Supplementary Data 1. h Western blot showing the protein levels of multiple KATs and HDAC1, HDAC2, and HDAC3 in SOX2 knockdown and overexpression K30 and SOX2 knockout K450 cells. In h antibodies against SOX2, ACTIN KAT3B were probed on the same gel. The samples originate from the same experiment, KAT2A, KAT2B, KAT3A, KAT7, KAT8, KAT9, HDAC1, HDAC2 and HDAC3 were processed on separate gels in parallel. RT-PCR data (f, g) are represented as mean values +/- SD from 3 biological replicates, n = 3. P values were determined by two-sided unpaired t test. *, P < 0.05, *, P < 0.01,***, P < 0.001. Source data are provided in the Source Data file. In a, b, d three RNA-seq replicates were performed, and data are shown from two analytical replicates. In c, e one ChIP-seq replicate was performed, and data are shown from two analytical replicates. Data in f, g are from 3 biological replicates. In h experiments were repeated independently at least 2 times with similar results. Representative data is shown.
We also inquired our RNA-seq data whether SOX2 may promote histone acetylation by regulating the expression of KATs and HDACs. We found that SOX2 KD slightly reduced the expression of multiple KAT genes, including the major KATs as EP300 (p300), CREBBP (CBP) and KAT7 (HBO1) (Fig. 4d and Supplementary Fig. 6d). Of note, these KATs were also SOX2 binding targets identified by ChIP-seq, and KAT7 was shown as an example (Fig. 4e). It is noteworthy that SOX2 KD also downregulated the expression of multiple members of HDAC family and SIRT family histone deacetylases (Supplementary Fig. 6e–g). We confirmed by qRT-PCR analysis that multiple KAT mRNA levels decreased after SOX2 KD and increased upon ectopic SOX2 over-expression in K30 cells (Fig. 4f, g). We validated by WB analysis that SOX2 KD also resulted in reduced protein levels of KAT3A, KAT3B, KAT7 and KAT8, whereas ectopic overexpression of SOX2 had an opposite effect on these KATs (Fig. 4h, left panel). Similarly, we observed that knockout of SOX2 also resulted in a similar reduction of KAT proteins (Fig. 4h, right panel). Interestingly, although RNA-seq analysis suggested that SOX2 also enhanced HDAC1, HDAC2, and HDAC3 expression (Supplementary Fig. 6e, f), such changes did not alter HDAC1/2/3 protein levels (Fig. 4h). Together, these results demonstrated a role of SOX2 in global transcription activation, including keratin genes and multiple KATs in ESCCs and suggested that SOX2 may promote histone acetylation in part via its ability to promote multiple KAT expression. Consistently, knockdown of KAT3B/EP300, KAT3A/CBP or KAT7/HBO1 downregulated global histone acetylation in K450 cells (Supplementary Fig. 6h), in support that up-regulated KAT expression could contribute to elevated global histone acetylation in SOX2-positve ESCC cells.
SOX2 inhibits lipid anabolism in part by suppressing ACSL5 expression
Mining our RNA-seq data from K450 cells revealed that SOX2 KD resulted in down-regulation of a large number and up-regulation of fewer genes involved in fatty acid β-oxidation and lipid metabolism (Fig. 5a and Supplementary Fig. 7). RNA-seq analysis of SOX2 KD in K30 cells also revealed a similar regulation of lipid metabolic genes by SOX2 (Supplementary Fig. 8). We next focused on K450 cells since the regulation on lipid metabolic genes is more robust, likely due to a higher level of SOX2 expression than K30 cells. The RNA-seq results were confirmed by qRT-PCR analysis (Figs. 5b–d). This observation raised the possibility that SOX2 may regulate lipid metabolism in ESCC cells. Because fatty acid synthesis or β-oxidation can either consume or produce significant portion of acetyl-CoA, we hypothesized that SOX2 may also indirectly regulate global histone acetylation by controlling fatty acid metabolism. To this end, we examined the level of lipid droplets in control and SOX2 KD K30 and K140 cells by Oil-Red O staining. As shown in Fig. 5e, SOX2 KD resulted in significantly increased accumulation of lipid droplets in K30 and K140 cells. Similarly, Oil-Red O staining revealed that SOX2 knockout led to lipid droplet accumulation in K450 cells (Fig. 5f). In the contrary, ectopic overexpression of SOX2 diminished the level of lipid droplets in K30 cells (Fig. 5g). Furthermore, comparison of lipid droplet level in SOX2-positive and SOX2-negative ESCC cells by Oil-Red O staining revealed an inverse correlation between SOX2 protein level and lipid droplet level (Fig. 5h).
a Heatmap analyses showing SOX2 regulates the expression of various lipid metabolism-related enzymes in K450 cells, signals were z-scaled and DESeq2 normalized. b RT-PCR analysis confirming the regulation of various fatty acid metabolism-related genes by SOX2 in K450 cells. c RT-PCR analysis confirming that SOX2 knockdown led to significant upregulation of ACSL5 and ACSL6 in K450 cells. d RT-PCR analysis showing that ectopic SOX2 overexpression led to significant downregulation of ACSL5 and ACSL6 in K450 cells. e, f Oil red staining showing elevated lipid droplet level in SOX2 knockdown K30 and K140 cells (e) and in SOX2 knockout K450 cells (f). g Oil red staining showing substantial reduction of lipid droplet level in SOX2 overexpressing K30 cells. h Oil red staining comparing the levels of lipid droplets in SOX2-positive K70 and K450 cells and SOX2-negative K150 and K410 cells. i Western blot analysis showing that SOX2 also negatively controlled ACSL5 protein level but had no effect on ACC1 and FASN. Antibodies against SOX2, ACTIN, ACSL5, ACC1 were probed on the same gel. The samples originate from the same experiment, FASN were processed on separate gels in parallel. j Oil red staining showing accumulation of lipid droplets in K450 cells with ectopic overexpression of ACSL5 and marked reduction of lipid droplet level in K410 cells with knockdown of ACSL5. k Western blot analysis showing upregulation of histone acetylation level in K450 and K410 cells with ACSL5 knockdown. Note that ACSL5 knockdown upregulated SOX2 protein level in SOX2 positive K450 cells. Antibodies against SOX2, ACTIN, ACSL5, H3 were probed on the same gel. The samples originate from the same experiment, H3ac and Kac were processed on separate gels in parallel. l Western blot analysis showing that overexpression of ACSL5 downregulated histone acetylation levels in both SOX2-positive K30 and K450 cells and SOX2-negative K150 and K410 cells. Note that overexpression of ACSL5 slightly downregulated SOX2 protein level in SOX2 positive K30 and K450 cells. m Western blot analysis showing that overexpression of ACSL5 downregulated global non-histone acetylation level in cells SOX2-positive K450 cells and SOX2-negative K410 cells. In k, l antibodies against SOX2, ACTIN, ACSL5, H3 were probed on the same gel. The samples originate from the same experiment, H3ac, H4ac and AcK were processed on separate gels in parallel. n RT-PCR analysis showing that overexpression of ACSL5 downregulated the expression of multiple SOX2 target genes. RT-PCR data (b, c, d, n) are represented as mean +/- SD from 3 biological replicates, n = 3. Western blot data (l, m) were repeated independently at least 3 times with similar results. Quantifications of Oil red staining are shown in (e, f, g, h, j), 3 independent experiments. Scale bar, 20 μm. Data represented as mean ± SD; ns, P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001. Student’s t test (unpaired, two-tailed). Source data are provided as a Source Data file.
The inverse correlation between SOX2 and lipid accumulation suggests a role of SOX2 in either promoting lipid catabolism such as fatty acid β-oxidation and/or inhibiting lipid anabolism. Because our stable isotope tracing assay using [U-13C16]-palmitate failed to detect significant alteration of fatty acid β-oxidation in SOX2 KD K450 cells (Supplementary Fig. 4e), we surmised that SOX2 may not significantly affect this pathway despite its regulation of multiple fatty acid β-oxidation related genes (Fig. 5a). This could be due to the fact that majority of fatty acid β-oxidation related genes were only slightly downregulated upon SOX2 KD (Fig. 5b). We then focused on whether SOX2 has a role in inhibiting fatty acid anabolism. We noticed that SOX2 KD resulted in significant up-regulation of ACSL5 and ACSL6 and down-regulation of ACSL1, ACSL3 and ACSL4 (Fig. 5a). The ACSL family proteins are the long-chain acyl-CoA synthetases that catalyze the activation of long-chain fatty acids of 12–22 carbons41. This reaction is required for channeling fatty acids to either anabolic pathways including the synthesis of phospholipids and triacylglycerols (TGs) or catabolic pathway such as β-oxidation. Previous studies indicate that different ACSL family isoforms direct fatty acids to different metabolic fates, with ACSL5 and ACSL6 mainly channeling long-chain acyl-CoA to synthesis of TGs and lipid droplets, ACSL4 to phospholipid biosynthesis, and ACSL1 and ACSL3 to β-oxidation42,43,44,45,46,47. We confirmed by qRT-PCR analysis that SOX2 KD led to elevated expression of ACSL5 and ACSL6 and modest reduction of ACSL1 and ACSL3 (Fig. 5c). On the contrary, overexpression of SOX2 in K450 cells led to modest increased levels of ACSL1 and ACSL3 mRNAs and marked reduction of ACSL5 and ACSL6 mRNAs (Fig. 5d). It is not clear how SOX2 regulates ACSL4 expression, as both SOX2 KD and overexpression led to modest reduction of ACSL4 expression (Fig. 5c, d). These results suggest that by repression of ACSL5 and ACSL6 and activation of ACSL1 and ACSL3, SOX2 could turn fatty acid metabolism from anabolic to catabolic pathway, in agreement with the inverse correlation between SOX2 expression and lipid droplet level we observed in ESCC cells.
To substantiate that SOX2 may enhance histone acetylation by controlling ACSL family gene expression, we focused on ACSL5, because it is the most significantly regulated ACSL gene by SOX2. We first confirmed by WB analysis that SOX2 KD led to increased ACSL5 protein level (Fig. 5i), which could be reversed by ectopic expression of SOX2 in SOX2 KD cells (Fig. 5i). Furthermore, we observed that ectopic overexpression of ACSL5 was sufficient to significantly elevate lipid droplet level in SOX2-positive K450 cells, whereas ACSL5 KD resulted in marked reduction of lipid droplets in SOX2-negative K410 cells (Fig. 5j), highlighting a key role of ACSL5 in controlling lipid droplet level in ESCC cells. Importantly, we observed that knockdown of ACSL5 resulted in substantial increase of histone acetylation in both SOX2-positive K450 cells and SOX2-negative K410 cells (Fig. 5k). In contrast, ectopic overexpression of ACSL5 in various ESCC cells all led to marked down-regulation of histone acetylation (Fig. 5l) and non-histone acetylation (Fig. 5m). Ectopic overexpression of ACSL5 also slightly down-regulated SOX2 protein level in K30 and K450 cells (Fig. 5l). We suggest this downregulation on SOX2 could be due to reduced histone acetylation by ACSL5 expression. Consistent with this idea, ectopic overexpression of ACSL5 downregulated the expression of multiple SOX2-enhanced keratin gene expression (Fig. 5n). Taken together, our study revealed a critical role of SOX2 in regulation of lipid metabolism, particularly lipid anabolism, possibly by repressing ACSL5 expression. Our study also provided evidence that SOX2 may enhance global histone acetylation in part by its ability to inhibit lipid anabolism by repressing ACSL5 expression.
Lipidomics analysis confirmed a role of SOX2 in lipid metabolism
To independently assess the role of SOX2 in regulation of lipid metabolism, we established an experimental framework to examine the lipid composition in SOX2 overexpression or KD cells48. As it is more convenient to knockdown and overexpress SOX2 in K30 cells than K450 cells, we primarily used K30 cells to analyze the effect of SOX2 on lipid metabolism. PCA analysis revealed that the lipid profiling data from three control and SOX2 KD samples were well separated (Supplementary Fig. 9a). As shown in Fig. 6a and Fig. 6b, long-chain TGs were profoundly elevated while phosphatidylcholine (PC), phosphatidylethanolamine (PE) and ether phospholipids decreased upon SOX2 KD. Because TGs are generally stored fats whereas phospholipids are crucial to the formation of lipid bilayers, these results suggest a role of SOX2 in suppressing fat storage and promoting synthesis of phospholipids required for membrane formation in proliferating cancer cells. SOX2 KD also resulted in decreased levels of various acylcarnitines (CARs) and increased levels of long-chain fatty acids (Fig. 6c and Supplementary Fig. 9b), in line with our observation that SOX2 inhibits fatty acid anabolism to promote catabolism.
a Volcano plot of lipidomic results showing fatty acids with significant alteration upon SOX2 knockdown in K30 cells. Group comparisons were performed using unpaired two-tailed Student’s t-tests, with false discovery rate (FDR) adjustment for multiple comparisons (Benjamini-Hochberg procedure). Significance threshold was set at q < 0.05. b, c Heatmap of dysregulated TGs (b) and carnitines (c) in SOX2 knockdown vs control K30 cells. d-f Scatter plots showing alteration of very long chain TGs in SOX2 KD K30 cells (d), SOX2 overexpressing K30 cells (e), and ACSL5 overexpressing K30 cells (f). g Oil-red staining assay comparing lipid droplets in control DMSO- treated and C646 (20 μM) treated K450 cells. h Western blot showing that C646 treatment markedly downregulated histone H3 and H4 acetylation in K30 and K450 cells. Antibodies against SOX2, ACTIN, EP300 and H3 were probed on the same gel. The samples originate from the same experiment, H3ac and H4ac were processed on separate gels in parallel. Quantifications of Oil red staining are shown in (g), 3 independent experiments. Scale bar, 20 μm. Data represented as mean ± SD; ns, P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001. Student’s t test (unpaired, two-tailed). Data in a–f are from four biological replicates, n = 4. In h experiments were repeated independently at least 2 times with similar results. Representative data is shown. Source data are provided in the Source Data file.
We next focused our lipidomics on TGs and compared the effect of SOX2 KD, SOX overexpression, and ACSL5 overexpression on TGs in K30 cells according to their chain length. While SOX2 KD led to a consistent upregulation of very long chain TGs (Fig. 6d), SOX2 overexpression in K30 cells markedly reduced their relative abundance (Fig. 6e). ACSL5 overexpression also increased the overall level of very long chain TGs (Fig. 6f), supporting our notion that SOX2 likely regulates lipid metabolism, at least in part, by regulating ACSL5 expression.
Our observation that ACSL5 overexpression drives a simultaneous lipid droplet accumulation and reduction of histone acetylation and that ACSL5 KD diminishes lipid droplet and elevates histone acetylation suggest that ACSL5 promotes long chain fatty acid synthesis/elongation and lipid droplet formation in the expense of histone acetylation. The most straight forward explanation of these results is that long chain fatty acid activation by ACSL5 (and others) drives fatty acid synthesis/elongation to generate various very long chain fatty acids and TGs, which is in competition with histone acetylation for acetyl-CoA. In support of this idea, we found that inhibition of histone acetylation by C646, a potent CBP/p300 inhibitor, resulted in a marked accumulation of lipid droplets in K30 and K450 cells (Fig. 6g). As expected, C646 markedly downregulated histone acetylation as revealed by WB analysis (Fig. 6h). However, C646 treatment did not significantly altered the protein levels of several key enzymes involved in fatty acid de novo synthesis or β-oxidation (Supplementary Fig. 9c).
We also carried out lipodomic analysis in K450 cells with or without SOX2 knockdown and confirmed that SOX2 KD led to a significant accumulation of very long fatty acids (Supplementary Fig. 10). Altogether, we concluded that SOX2 promotes global histone acetylation in part by its ability to repress ACSL5 and possible other fatty acid anabolic gene expression, which spares acetyl-CoA for histone acetylation rather than incorporates acetyl-CoA into the synthesis of long chain fatty acids and lipids.
ACSL5 exerts as an ESCC tumor suppressor
We next wished to assess the role of SOX2-mediated ACSL5 repression and global histone acetylation in tumorigenesis. Analysis of TCGA database revealed that the expression of ACSL5 was inversely correlated with that of SOX2 expression (Fig. 7a). We thus investigated if SOX2 controls ESCC tumorigenesis in part by regulation of histone acetylation and ACSL5 expression. To this end, we used K450 cells, which produced tumors in nude mice by subcutaneous transplantation. We validated that knockdown of SOX2 and ectopic overexpression of FLAG-ACSL5 similarly reduced the global level of histone acetylation in K450 cells (Fig. 7b). Ectopic overexpression of FLAG-ACSL5 also impaired K450 cell proliferation, although to a less extent than SOX2 KD (Fig. 7c and Supplementary Fig. 11). Subsequent subcutaneous tumor xenograft assay demonstrated while SOX2 KD drastically impaired the growth of transplanted tumor cells in nude mice (Figs. 7d–f), ectopic expression of FLAG-ACSL5 also impaired the growth of transplanted tumors (Figs. 7g–i), although to a less extent compared to that of SOX2 KD. Oil-Red O staining of the resulting tumors respectively confirmed that SOX2 KD and FALG-ACSL5-expressing tumors contained a higher level of lipid droplets (Fig. 7j) than control tumors. In addition, immunohistochemistry (IHC) analysis revealed that SOX2 KD and FLAG-ACSL5-expressing tumors had a lower level of histone acetylation (Fig. 7k) than control tumors. We also confirmed that xenografts derived from SOX2 KD K450 cells showed higher level of ACSL5 mRNA (Fig. 7l). Thus, we conclude that ACSL5-driven fatty acid and lipid synthesis can suppress ESCC histone acetylation and tumor proliferation, and SOX2 drives tumorigenesis in part by suppressing ACSL5 expression.
a The expression profiling and expression correlation analysis of SOX2 and ACSL5 in ESCC tumors from TCGA database. Box plots showing distributions of the normal group (n = 11) and tumor group (n = 95). Box limits indicate IQR, midline represents median. Student’s t test (unpaired, two-tailed). Source data are provided as a Source Data file. b Western blot showing the effect of SOX2 knockdown and ACSL5 ectopic overexpression on histone acetylation in K450 cells. Antibodies against SOX2, ACTIN, ACSL5, H3 were probed on the same gel. The samples originate from the same experiment, FLAG, H3ac and H4ac were processed on separate gels in parallel. c Analysis of the cell proliferation in SOX2 knockdown and ACSL5 overexpressing K450 cells by CCK8 assay. d–f Subcutaneous tumor xenograft assay evaluating the tumorigenic activities of control and SOX2 knockdown K450 cells in nude mice. The tumor growth curves (d), the image of dissected xenografted tumors (e), and the weights of xenografted tumors (f) of shVector-infected and shSOX2 -infected K450 cell xenografts were shown. g–i Subcutaneous tumor formation assay evaluating the tumorigenic activities of control and ACSL5 overexpressing K450 cells. The tumor growth curves (g), the image of dissected xenografted tumors (h), and the weights of xenografted tumors (i) were shown. j Oil-red staining showing the levels of lipid droplets in xenograft tumors derived from representative shVec control, shSOX2 knockdown, OE-Vec, and OE-ACSL5 K450 cell-derived xenograft tumors. Scale bar 100 μm. k IHC staining showing the levels of acetylated H3 or H4 in xenograft tumors derived from shVec control, shSOX2, OE-Vec, and OE-ACSL5 overexpressing K450 cells. Scale bar 50 μm. l RT-PCR analysis showing elevated ACSL5 mRNA level in SOX2 knockdown K450-derived xenograft tumors. RT-PCR data (l) and CCK8 assay data (c) are represented as mean +/- SD from 3 biological replicates, n = 3. Tumor Volume and weight assay (d, g, f, i) data are represented as mean +/- SD, n = 8 (shSOX2 group n = 6). *P < 0.05, **P < 0.01, ***P < 0.001. Student’s t test (unpaired, two-tailed). Source data are provided in the Source Data file.
SOX2 expression positively correlates with histone acetylation in ESCC tumors
To assess whether SOX2 regulates histone acetylation in human ESCC patients, we analyzed SOX2 and histone acetylation in clinical specimens by IHC using tissue microarrays. SOX2 expression levels varied among ESCC tumors but were highly expressed in ESCC tumors compared to the adjacent normal tissues, hyperplasia and dysplasia tissues, as shown in Fig. 8a and a representative image in Fig. 8b. Similarly, histone H4 acetylation was elevated in most of the ESCC tumors compared to the adjacent normal tissues, hyperplasia and dysplasia tissues (Figs. 8c, d). Liner regression analysis revealed a positive correlation between SOX2 and acetylated H4 in ESCC tumor samples (Fig. 8e). Notably, a 10-year follow up showed that SOX2 expression correlated with better overall survival (HR, 0.710; 95% CI, 0.521-0.967; p = 0.028; Fig. 8f and Supplementary Table 1). Although high SOX2 expression appears to represent a better disease-free survival rate (Fig. 8g), this data lacks statistical significance. When SOX2 and acetylated H4 were analyzed together, we found that both high SOX2 and high acetylated H4 predicts better overall survival (HR, 0.694; 95% CI, 0.538-0.895; p = 0.052; Fig. 8h and Supplementary Table 1) and disease-free survival (Fig. 8i). Therefore, a positive correlation between SOX2 and histone acetylation was observed in ESCC tumors and, interestingly elevated SOX2 expression and histone acetylation may correlate with a better prognosis.
a Scatter plots of IHC analysis of SOX2 protein levels in tissue microarrays containing normal, hyperplasia, dysplasia and ESCC tumors. Statistical significance was assessed using one-way ANOVA. b The representative IHC images of SOX2 in normal, hyperplasia, dysplasia and tumor tissues (or SOX2 low and high tumors). c Scatter plots of IHC analysis of acetylated H4 (acK5/K8/K12/K16) levels in tissue microarrays containing normal, hyperplasia, dysplasia and ESCC tumors. Statistical significance was assessed using one-way ANOVA. d The representative IHC images of low and high level of acetylated H4 in normal, hyperplasia, dysplasia and tumor tissues. e Linear regression plot showing the correlation between SOX2 and acetylated H4 (K5, K8, K12, K16) levels in tumor tissues. Acetylated H4, acH4. Statistical significance was evaluated using paired-sample Pearson correlation analysis. f, g Overall survival (f) and disease-free survival (g) of low-SOX2 group and high SOX2 group ESCC patients. h, i Overall survival (h) and disease-free survival (i) of low-SOX2/AcH4 and high-SOX2/AcH4 expression ESCC patients (SOX2, low, score ≤11; high, score > 11; acetylated H4 (K5, K8, K12, K16), low, score ≤218; high, score > 218). Statistical significance was assessed using the log-rank test (f, g, h, i) (j) Working model illustrating how SOX2 promotes global histone acetylation and super-enhancer formation in ESCCs. (b, d) Scale bar, upper panel 1000 μm, lower panel 100 μm. In a, c, f–i, “n” represents the number of patients. Source data are provided in the Source Data file.
Discussion
In this study, we unravel a role of SOX2 in promoting global histone acetylation and super-enhancer formation, which drives squamous specific lineage transcriptional programs in ESCCs. We provide evidence that SOX2 promotes global histone acetylation by promoting local histone acetylation via DNA binding and recruitment of KATs such as EP300, by enhancing the expression of multiple KATs, and by repressing the expression of ACSL5, which diverts acetyl-CoA away from long chain fatty acid synthesis/elongation to histone acetylation (Fig. 8j). We showed that ectopic overexpression of ACSL5 resulted in accumulation of lipid droplets, downregulation of histone acetylation, and suppression of ESCC cell proliferation and tumor growth in mouse xenograft models, suggesting that SOX2 promotes ESCC cell proliferation and tumorigenesis in part via reprogramming ESCC lipid metabolism.
We were particularly interested in the molecular mechanisms by which SOX2 functions as a driver of squamous carcinoma. This study was inspired by the report that AKT can promote histone acetylation by stimulating the activity of acetyl-CoA producing enzyme ACLY25. We and others have reported that AKT can stabilize and drive SOX2 overexpression in mouse ES cells27, ESCCs and large B lymphoma28,49, raising the question whether AKT also promotes histone acetylation in ESCCs. As shown in Fig. 1, we surprisingly discovered that SOX2 rather than AKT has a unique role in enhancing global histone acetylation in ESCCs. The ability for SOX2 to drive up histone acetylation is unlikely limited only to ESCCs, as SOX2 also promotes histone acetylation in lung cancer cells (Supplementary Fig. 1c). The effect of SOX2 on global histone acetylation was further confirmed by our ChIP-seq analysis, which revealed that SOX2 KD and rapid degradation both led to reduction of histone acetylation not only in SOX2-binding regions but also in non SOX2-binding regions (Figs. 2 and 3).
Our ChIP-seq analysis also revealed a predominant role of SOX2 binding in promoting local histone acetylation, as the majority of down-regulated H4 acetylation and H3K27ac occurred at the regions with SOX2 binding (4626 SOX2 binding vs 3039 non or weak SOX binding) (Fig. 2e and Figs. 3g, h). Furthermore, our data revealed a critical role of SOX2 in driving the formation of super-enhancers in ESCC cells, as SOX2 binding was observed in most of the super-enhancers (Fig. 2g) and SOX2 KD impaired about half of the super-enhancers (Fig. 2f, g). For example, SOX2 binding is detected in the super-enhancers of multiple keratin gene loci, and keratins represented the most down-regulated genes upon SOX2 KD (Fig. 4b, c), consistent with its role as an oncogenic determinant of SCCs. This unusual ability to enhance local histone acetylation and super-enhancer formation is likely linked to SOX2 ability to recruit KATs such as CBP/p300 (Fig. 3k).
Metabolism rewiring is tightly linked to epigenetic regulation and has been recognized as a hallmark of cancer17,18. Mammalian chromatin contains at least 10 billion potential acetylation sites50, implying that a global change in histone acetylation would require rewiring of acetyl-CoA metabolic flux. In the search for molecular mechanism(s) by which SOX2 enhances global histone acetylation in ESCCs, we found that SOX2 regulates neither ACLY, ACSS2 and PDC-E2 expression nor their subcellular localization (Supplementary Fig. 3), although we could not rule out the possibility that SOX2 might regulate their enzymatic activities. Metabolic tracing analyses revealed that SOX2 KD did not significantly alter the metabolism of glucose and glutamine and fatty acid β-oxidation (Supplementary Fig. 4). However, under palmitate only condition, we did observe that SOX2 KD slightly reduces fatty acid β-oxidation in K450 cells, suggesting that SOX2 may have a minor role in promoting fatty acid β-oxidation (Supplementary Fig. 4i). Notably, by RNA-seq analysis we uncovered that SOX2 regulates the expression of a large number of fatty acid metabolism-related genes (Fig. 5a and Supplementary Fig. 6), raising the possibility that SOX2 may play a role in regulation of fatty acid metabolism. Along this line, we demonstrated that SOX2 has a critical role in suppressing lipid storage in ESCCs (Figs. 5e–i). As our metabolic tracing experiment suggested that SOX2 did not significantly regulate fatty acid β-oxidation, we focused our attention on whether SOX2 regulates lipid anabolism. We noticed from RNA-seq data and confirmed by RT-PCR analysis that SOX2 markedly represses ACSL5 and ACSL6 and moderately enhances ACSL1, ACSL3 and ACSL4 expression (Fig. 5a–d). This observation is intriguing because ACSL5 and ACSL6 are known to more likely channel activated long chain fatty acyl-CoA toward fatty acid synthesis/elongation and lipid storage42,45,46, whereas ACSL1, ACSL3 and ACSL4 tend to channel activated fatty acyl-CoA more toward fatty acid β-oxidation43,44,47,51,52. Indeed, we demonstrated that ectopic overexpression of ACSL5 resulted in a simultaneous accumulation of lipids (Fig. 5j) and downregulation of global histone acetylation (Fig. 5l), whereas ACSL5 KD diminished lipid storage (Fig. 5j) and elevated the global histone acetylation level in ESCCs (Fig. 5k). How SOX2 represses ACSL5 expression is currently largely unknown, we believe this repression is unlikely direct, because we failed to identify any SOX2 binding peak in the ACSL5 genomic locus (Supplementary Fig. 7c). It is noteworthy that our lipidomic analysis revealed a profound effect of SOX2 in lipid metabolism, consistent with our RNA-seq data that many lipid metabolic related genes are regulated by SOX2.
Our RNA-seq analysis revealed that SOX2 regulates the expression of a panel of KATs, HDACs and SIRTs (Fig. 4 and Supplementary Fig. 6). We confirmed by WB analysis that SOX2 enhanced the protein levels of multiple KATs including KAT3B/p300, KAT3A/CBP and KAT7/HBO1 (Figs. 4f–h), the major KATs involved in histone acetylation. Knockdown of KAT3B/p300, KAT3A/CBP and KAT7/HBO1 could downregulate the global histone acetylation in K450 cells (Supplementary Fig. 6h), in support that SOX2 could modulate histone acetylation by enhancing their expression. Interestingly, WB analysis showed that SOX2 KD did not significantly affect the protein levels of HDAC1/2/3, the major HDACs responsible for histone deacetylation (Fig. 4h). We have not yet to investigate if altered expression of SIRTs contributes to SOX2-driven histone acetylation, because SIRTs are not the major enzymes for histone deacetylation. Thus, our data favor the idea that SOX2 is likely to drive global histone acetylation in part by its ability to enhance the transcriptional activation of multiple KATs.
We present evidence that SOX2 expression positively correlates histone acetylation in clinical ESCC tumors (Fig. 8), in agreement with our finding that SOX2 promotes global histone acetylation. BET bromodomain inhibitors, which displace BET proteins such as BRD4 from chromatin by competing with their acetyl-lysine recognition modules, have shown promising efficacy in several models of cancer53,54,55. In accordance with the histone hyperacetylation phenotype, we found that SOX2-positive ESCC cells were less sensitive to BET inhibitors than SOX2-negative ESCC cells (Supplementary Fig. 12), suggesting that elevated histone acetylation may render ESCC insensitivity to BET inhibition. Thus, it remains an interesting question for future study whether direct targeting histone acetyltransferase(s) could provide selectively therapeutic efficacy to SOX2 positive ESCCs.
Interestingly, despite of a strong role of SOX2 in promoting ESCC proliferation and graft growth, higher SOX2 expression and histone acetylation appears to correlate with a better clinic prognosis (Fig. 8f–i). A possible explanation is that other subtypes of ESCCs may acquire more aggressive phenotypes through different oncogenic mechanisms56. However, it is also plausible that, through the mechanism we report here, SOX2 suppresses long chain fatty acid synthesis and storage, a process that has been implicated in cancer progressiveness and malignancy57,58.
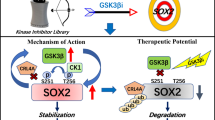
In sum, our study revealed a role of SOX2 in promoting global histone acetylation and super-enhancer function in ESCCs (Fig. 8J). Interestingly, Myc has been previously shown to influence global histone acetylation in part through upregulation of KAT2A/GCN5 expression59. SOX2 is likely to enhance global histone acetylation in part by promoting KAT expression and by repressing ACSL5 expression, which in turn inhibits long chain fatty acid synthesis/elongation and channels acetyl-CoA to histone acetylation. This unique ability for regulating histone acetylation and lipid metabolism is functionally linked to potent SOX2 transcriptional activity and likely provides an explanation why SOX2 is powerful oncogenic determinant in ESCCs and possibly other tumors.
Methods
Ethical regulations
All research complies with relevant ethical regulations. All human participants provided written informed consent. Ethical approval was obtained from the ethical committee of the Central Hospital of Shantou City and the ethical committee of the Medical College of Shantou University (Protocol No. SUMC-2021-61). All mouse experiments were performed according to the guidelines of the animal care and use. Ethical approval was obtained from committees of Experimental Animal Center, Institute of Biomedical Sciences in East China Normal University (Protocol No. m20200331). All methods were performed in line with the ethical guidelines of the 1975 Declaration of Helsinki.
Cell culture
The ESCC and non-small cell lung cancer cells were routinely cultured in RPMI1640 medium (Invitrogen) with supplementation of 10% fetal bovine serum (Sigma, F8318) and 1% penicillin/streptomycin, whereas C33A cells were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% Fetal Bovine Serum (Sigma, F8318) and 1% penicillin/streptomycin solution (Invitrogen). All cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2.
Generation of knockdown and ectopic expression cell lines
For stable cell line generation, lentiviruses were produced by Lipofectamine 2000 (Invitrogen) transfection of HEK293T cells (Invitrogen) with pLKO shRNA-carrying plasmids and helper plasmids pMD2.G and psPAX2. Viral supernatants were collected 48 h after transfection, and filtered through a 0.45-μm filter. The stable cell lines were obtained by puromycin selection for 1 week. All shRNA sequences are provided in the Supplementary Data 1.
Generation of SOX2-dTAG knock-in cell lines using CRISPR–Cas9 genome editing
Endogenous knock-in of dTAG degron into SOX2 was generated by CRISPR–Cas9-mediated genome editing as previously described36. Briefly, we used an online CRISPR design tool (https://chopchop.cbu.uib.no/) to select suitable guide RNA sequence. We construct a donor vector (pUC57-SOX2-dTAG donor) containing the knockin sequence, the pX459-sgSOX2 plasmid with a gene-specific sgRNA targeting the SOX2 N-terminal genomic locus, and the pX459-sgPITCh plasmid with the PITCh-specific sgRNA targeting the pPUC57-SOX2-dTAG donor construct. We set up transfection system: pX459-sgPITCh: pX459-sgSOX2: pUC57-SOX2-dTAG donor = 1: 2: 2 by using LipoFilter (HB-TRLF-1000) in K450. At 48 h after transfection, the cells were cultured with the medium containing 1 μg/mL puromycin (MCE, Cat. #HY-B1743) for another 48 h to select puromycin-positive single cells into the 96-well plates and then allowed them to expand for 2 weeks with basic culture medium. The correct SOX2-dTAG knock-in cell line was verified by both Sanger sequencing of genomic DNA and western blot by DMSO or 1 μM dTAG-V1 (Tocris, Cat. #6914/5) treatment. The SOX2 sgRNA sequences are provided in the Supplementary Data 1.
Animal studies
Mice were maintained on a standard rodent chow diet as specified with 12-h light and dark cycles. The ambient temperature was maintained at 22 ± 1 °C, and relative humidity was kept at 50 ± 10%. Standard rodent cages with corn cob bedding were provided, along with ad libitum access to food and water. For assessing the effect of SOX2 knockdown and ACSL5 overexpression on tumor xenograft proliferation, 2 × 106 control, shSOX2, and OE-ACSL5 K450 cells in a 0.1 mL volume of serum-free medium were mixed with an equal amount of Matrigel (BD Biosciences) and inoculated s.c. into the right flank of 4- to 5-week-old female BALB/c nude mice (6 for each group). Tumor sizes were monitored twice weekly with digital callipers and volumes were calculated with the formula: (mm3)=length × width2 × 0.5. Humane endpoints were triggered by: (1) tumor volume ≥2000 mm3 or (2) > 20% body weight loss sustained for 48 hr.
We confirm that the maximal tumor size/burden was not exceeded in this study.
Western blot and immunofluorescence staining
The western blot and immunofluorescence staining were performed according to standard protocols as described previously28. Briefly, Western blotting was performed by lysing cells or tissues in RIPA buffer supplemented with protease/phosphatase inhibitors, followed by protein quantification using BCA assay. Proteins (20-50 μg) were separated by SDS-PAGE on 8-12% gels, transferred to nitrocellulose membranes, and blocked with 5% milk in TBST. Membranes were incubated overnight at 4 °C with primary antibodies, followed by secondary antibodies for 1 h at room temperature. Signals were detected with a Odyssey CLx system (Li-Cor), with β-actin serving as loading controls. Band intensities were quantified using ImageJ, and uncropped blot images are provided in Source Data. For immunofluorescence staining, cells grown on glass coverslips were fixed with 4% paraformaldehyde (15 min, RT), permeabilized with 0.1% Triton X-100 (10 min), and blocked with 5% BSA (1 h, RT). Primary antibodies were incubated overnight at 4 °C, followed by Alexa Fluor-conjugated secondary antibodies (2 h, RT) and DAPI counterstain (5 min). Images were acquired using a confocal microscope with consistent exposure settings across compared samples. For quantification, ≥3 random fields per condition were analyzed using ImageJ. Unprocessed raw images are provided in Source Data.
The following antibodies were used: anti-SOX2 antibody (Abcam, ab92494, 1:3000 for WB and 1:1000 for IF staining), anti-SOX2 antibody (Cell Signaling Technology, #23064, 1 μg for ChIP assay), anti-ACSL5 antibody (Abcam, ab104892, 1:1000 for WB), anti-AKT1 antibody (Cell Signaling Technology, #9272, 1:1000 for WB), anti-phospho-AKT(Ser473) antibody (Cell Signaling Technology, #12694, 1:1000 for WB), anti-β-Actin antibody (HUABIO, EM21002, 1:5000 for WB), anti-GAPDH antibody (HUABIO, EM1101, 1:5000 for WB), anti-Histone H3 antibody (HUABIO, EM30605, 1:5000 for WB), anti-FLAG M2 antibody (Sigma, F3165, 1:5000 for WB), anti-acetyl H3ac antibody (Homemade, 1:3000 for WB), anti-acetyl H4 (acetyl-K5/K8/K12/K16) antibody (Abcam, ab177790, 1:3000 for WB and 1:1000 for IF), anti-H4ac antibody (Millipore, #06-866, 1 μg for ChIP assay), anti-AcK antibody (PTM BIO, PTM-101, 1:3000 for WB), anti-H3K9ac antibody (PTM BIO, PTM-156, 1:3000 for WB), anti-H3K27ac antibody (Millipore, #07-360, 1 μg antibody for ChIP assay), anti-H3K4me3 antibody (Cell Signaling Technology, #9751, 2 μg antibody for ChIP assay), anti-KAT2A antibody (Abcam, ab217876, 1:1000 for WB), anti-KAT2B antibody (Cell Signaling Technology, #3378 T, 1:1000 for WB), anti-KAT3B antibody (Active Motif, #61401, 1:1000 for WB), anti-KAT7 antibody (Abcam, ab70183, 1:1000 for WB), anti-KAT8 antibody (Abcam, ab72056, 1:1000 for WB), anti-KAT9 antibody (Abways, CY7094, 1:1000 for WB), anti-ACC1 antibody (Cell Signaling Technology, #3676, 1:1000 for WB), anti-FASN antibody (Cell Signaling Technology, #3180, 1:1000 for WB), anti-ACSS2 antibody (Abcam, ab66038, 1:1000 for WB), anti-ACLY antibody (Abcam, ab40793, 1:1000 for WB), anti-PDC-E2 antibody (Abcam, ab110332,1:3000 for WB and 1:1000 for IF staining), anti-LaminA/C antibody (Abcam, ab108595, 1:3000 for WB and 1:1000 for IF staining), anti-HDAC1 antibody (ABclonal, A0238, 1:1000 for WB), and anti-HDAC2 antibody (ABclonal, A2084, 1:1000 for WB).
Chromatin Immunoprecipitation (ChIP) assay
ChIP assay was performed as previously described60 with the following modifications. The cells were crosslinked with 1% formaldehyde for 10 min and then quenched by adding 125 mM glycine. Chromatin samples were sonicated in lysis buffer (high salt buffer with 0.1% SDS and protease inhibitors). Then 1 µg SOX2 (CST, #23064), 2 µg H3K4me3 (CST, #9751), 1 µg H3K27Ac (Millipore, #07-360) or 1 µg H4ac (Millipore, 06-866) antibody was individually incubated overnight with the chromatin samples derived from a mixture of 5 × 105 K450 cells and 1 × 105 mouse ES cells that served as a spike-in control. The protein-DNA complexes were immobilized on 10 uL protein A/G beads (Smart lifesciences, SA032005) and then washed three times with high salt buffer (500 mM NaCl, 10 mM Tris-HCl pH 8.0, 0.1% sodium deoxycholate, 1% Triton X-100, 1 mM EDTA), two times with low salt buffer (250 mM LiCl, 10 mM Tris-HCl, pH 8.0, 0.5% NP40, 0.5% Na-deoxycholate, 1 mM EDTA), and one time with TE Buffer (10 mM Tris-HCl pH 8.0, 1 mM EDTA). ChIP DNA samples were then decrosslinked in elution buffer (50 mM Tris-HCl, pH 8.0, 10 mM EDTA, 1% SDS, with Proteinase K and RNase A) at 65 °C for at least 5 h and purified by a PCR extraction kit (QIAGEN 28106). Finally, the DNA samples were directly used for library generation using a Vazyme kit (Vazyme, ND607), libraries were pooled and sequenced on the Illumina NovaSeq 6000 sequencer with paired-end sequencing.
ChIP-seq data processing and normalization
The ChIP-seq reads were aligned to the human genome build hg19 and mouse genome build mm10 using the bowtie2 (v2.3.4.1) with the default parameters and duplicate reads were removed by picard MarkDuplicates. The human ChIP-seq reads were multiplied by a scale factor which, when multiplied, equalized Mus musculus mapped reads and visualized in UCSC genome browser. The ChIP-seq peaks of SOX2 in control and SOX2 knockdown cells were called by MACS2 (v2.1.2) with the parameters --fix-bimodal -p 0.05 because SOX2 signals were weak, and the ChIP-seq peaks of H4ac, H3K27ac and H3K4me3 were called with the parameters -B -q 0.01 --nomodel -extsize 150. The differential ChIP-seq peaks were called with -p 1e-7, and further filtered to acquire at least 1.5-fold changed peaks. The genome distribution of SOX2 ChIP-seq peaks was calculated by R package ChIPseeker and plotted by EXCEL. The motifs of SOX2 binding sites were identified by Homer. The heatmaps and the emission profiles were plotted by Deeptools. Super-enhancers were identified using ROSE based on the ranking of H3K27ac intensities. The Veen Diagram and density plot were plotted by R package VennDiagram and ggplot2. The ChIP-seq data for control and SOX2 knockdown K450 cells were deposited in GSE256475: (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE256475), whereas the ChIP-seq data for SOX2-dTAG K450 cells were deposited in GSE300317: (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE300317).
RNA-seq data processing and analysis
RNA-seq analysis of gene expression profiles was performed with three biological replicates per sample. The data exhibited high reproducibility, as evidenced by Pearson’s correlation coefficients (> 0.98). Processing pipelines followed established methods (Xiao Yanhui et al.). Differentially expressed genes (DEGs) were identified using the fold change cutoff≥ 3 and adjusted P value < 0.05. Gene expression Z-scores were visualized in heatmaps generated by the R package pheatmap. Functional enrichment analysis (Gene Ontology) was conducted via clusterProfiler. Raw RNA-seq data for control and SOX2-knockdown K450 cells are available under GEO accession GSE256475.
Metabolite extraction for GC-MS measurement
Each cell sample (5.0 × 106 cells) was harvested by adding 1.6 mL of 80% (v/v) methanol/water solution in each tube. The samples were sonicated by 7 cycles with 2 min each cycle in ice-bath and stored at 4 °C for 1 h. The supernatants were collected after centrifugation at 14,000 g and 4 °C for 15 min, followed by evaporation to dryness with the vacuum centrifuge.
Chemical derivatization and isotope tracing in GC-MS analysis
The chemical derivatization for isotope tracing and GC-MS analysis have been reported previously39. Briefly, the dried samples were reconstituted with methoxyamine hydrochloride in anhydrous pyridine, and further reacted with N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide (MTBSTFA) with 1% tert-butyldimethylsilyl (TBDMS). The instrumental analysis was further performed on an Agilent GC-MS system. Helium (> 99.999%) was used as a carrier gas at a constant flow rate of 1 mL/min through the column for separation. The electron energy was 70 eV, and data was collected in a full scan mode (m/z 50–600).
Lipid extraction
300 µL of pre-cooled methanol with internal standards was added to each dish, cells were collected by scraping then freeze-thawed in liquid nitrogen 3 times, and sonicated for 3 min in ice bath. 1 mL MTBE and 300 μL water were added to each sample, separately, followed by vortex for 5 min. The mix was then centrifuged for 10 min at 12,000 g at 6 °C. After centrifugation, the upper layer was lipid enrichment (~1 mL). 400 μL of the upper layer’s supernatant was removed to a new tube, and a small amount of supernatant from each sample was mixed together as quality control (QC) sample. The supernatant was dried with a nitrogen stream at room temperature and the residues were stored at −80 °C till analysis. Before LC-MS analysis, lipids were resuspended in 50 µL of solvent mixtures, including 10 µL of CH2Cl2: MeOH (2:1, v/v) and 40 µL of acetonitrile: isopropanol: H2O with 5 mM ammonium acetate (65:30:5, v/v/v).
LC-MS data acquisition
LC-MS analyses were performed using an Ultimate 3000 HPLC coupled with Thermo Q-Exactive mass spectrometric system. 3 µL of sample were injected for each analysis. Lipid separation was conducted onto a Waters C8 column (2.1 × 100 mm, 1.7 µm) with pre-column in reversed-phase chromatography. Mobile phase A was composed of 40% water and 60% acetonitrile. Mobile phase B was composed of 90% isopropanol and 10% acetonitrile. 10 mM ammonium formate was added to both mobile phases to improve ionization. Gradient elution initiated with 32% B at 0.3 mL/min for 1.5 min, increased to 85% B in 14 min, then increased to 97% B in 0.1 min, held for 2.4 min. An electrospray ionization (ESI) source was used for MS measurement under alternating mode between positive and negative ionization, with a capillary voltage of 3000 V for positive and −2400 V for negative mode. The vaporizer temperature was 350 °C. Mass range was from m/z 150 to 2000.
Untargeted Lipidomics Data Analysis
Raw LC-MS data were imported into TraceFinder software for peak picking and integration. The lipids with peaks less than 80% of the total number of samples were not retained. The response values of different lipids in each sample were derived, and finally, protein quantification in cell samples and internal standards were used to normalize the data. For untargeted lipidomics analysis, lipids were identified in MS-DIAL online software. Lipid retention time drift correction was performed using QC samples.
Oil-Red O staining
For Oil-Red O staining, cultured cells were washed twice with ice-cold PBS and fixed with 4% paraformaldehyde for 20-30 min at room temperature. The fixed cells were gently washed 1-2 times with PBS, and stained with Oil-Red-O working solution (Oil-Red-O in 6:4 isopropanol: water solution) for 30 min at room temperature. After removing the staining solution, the cells were washed with PBS for 3 times and used for imaging acquisition.
Seahorse assay
Standard XF substrate oxidation stress test was performed by Seahorse XFe 96 Analyzer (Agilent Technologies, USA) using the XF Long Chain Fatty Acid Oxidation Stress Test Kit (p/n 103672-100) (Agilent Technologies) according to the manufacturer’s instructions. Briefly, lentiviral shVec and shSOX2 infected K30, K450 cells were seeded into Agilent Seahorse XFe96 well plates at 80% cell confluency in the complete growth medium and cultured for one day. One h before measurement, the cells were washed once with and then incubated with the Seahorse XF Assay Medium supplemented with 2 mM L-glutamine, 1 mM sodium pyruvate and 10 mM glucose for 1 h at 37 °C in a non-CO2 incubator. The assays with injected Etomoxir (4 μM), Oligomycin (1.5 μM), FCCP (1 μM) or Rotenone/Antimycin A (0.5 μM) were run with the Seahorse XF substrate oxidation stress assay template files of the Wave 2.4.2 software (Agilent Technologies). The protein concentration was measured using BCA kit (K3001, Shenergy Biocolor) and protein content was used to normalize the oxygen consumption rate (OCR). For Seahorse assay under the condition with sodium palmitate only, glucose, glutamine, and pyruvate were omitted from the medium.
RNA isolation and RT-PCR
Total RNAs were extracted from cells using TRIZOL (Invitrogen). The cDNAs were generated using the ReverTra Ace qPCR RT Kit (Toyobo Co., Ltd. Life Science Department. Osaka, Japan) according to the manufacturer’s manual. RT-qPCR analyses were performed by SYBR Premix Ex Taq II (TaKaRa), with 18S as the internal reference. The primers used are all shown in Supplementary Data 1 and the relative expressions of target genes were measured by the 2−ΔΔCT method.
Cell growth assay
Cells indicated were counted, plated in 96-well plates and the growth curve for 4 days were determined by CCK-8 assay as instructed by the manufacturer (Sigma-Aldrich). The 1:10 volume CCK-8 was added into medium for 2 h before OD450 was detected.
Immunohistochemical staining and evaluation
ESCC tissue microarrays (TMAs) were prepared according to established protocols61. Immunohistochemistry (IHC) was subsequently performed with the PV-9000 Polymer Detection System (ZSGB-BIO, China) and Liquid DAB Substrate Kit (Invitrogen, USA), adhering to manufacturer guidelines and published methodology62. Anti-SOX2 (abcam, ab92494) was used at 1:200 dilution, whereas anti-Acetyl-Histone H4 (abcam, ab177790) was used at 1:1000 dilution. The evaluation of IHC staining results was performed as described previously62. For statistical analysis, both SOX2 and acetyl-H4 were divided into 2 subgroups, high-expression and low-expression, on the basis of X-tile software analysis63.
Statistics & reproducibility
Statistical analysis and graphs were generated using GraphPad Prism 8.0 software and R (4.3.1). Statistical analyses were performed using an unpaired/paired two-sided t test. Kaplan-Meier survival curves and log-rank tests were used to estimate survival differences between groups. The level of significance was set at *, P < 0.05, **, P < 0.01, and ***, P < 0.001 for three independent experiments. The exact p values are provided in the Source Data file.
Software
The following software was used for data analysis: STAR (v2.6.0c), featureCounts (v2.0.3), DESeq2, clusterProfiler for RNA-seq analysis. FastQC v0.11.7, samtools 1.8, bowtie2 (v2.3.4.1), MACS2 (v2.1.2), ROSE (v3.3.4), DeepTools (v3.3.0) for ChIP-seq analysis. Image Studio Lite (V 5.2.5) for Western Blots imaging, Bio-Rad CFX Maestro 1.1(V4.1.2433.1219) for RT-PCR, OLYMPUS cellSens Standard (V1.14) for IF. GraphPad Prism 8.0 and R(4.3.1)
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The ChIP-seq and RNA-seq data for control and SOX2 knockdown K450 cells were deposited in the GEO database under accession code GSE256475, whereas the ChIP-seq data for SOX2-dTAG K450 cells were deposited in the GEO database under accession code GSE300317. All the data needed to evaluate the conclusions in the paper are presented in the paper and/or the expanded view content. The mass spectrometry data for metabolomics have been deposited at MetaboLights: (https://www.ebi.ac.uk/metabolights/editor/MTBLS7120/descriptors), and are accessible with the identifier MTBLS7120. Source data are provided with this paper.
References
Morgan, E. et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology 163, 649–658 e2 (2022).
Lau, S. C. M., Pan, Y., Velcheti, V. & Wong, K. K. Squamous cell lung cancer: current landscape and future therapeutic options. Cancer Cell 40, 1279–1293 (2022).
Johnson, D. E. et al. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 6, 92 (2020).
Sarkar, A. & Hochedlinger, K. The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell 12, 15–30 (2013).
Bass, A. J. et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat. Genet 41, 1238–1242 (2009).
Long, K. B. & Hornick, J. L. SOX2 is highly expressed in squamous cell carcinomas of the gastrointestinal tract. Hum. Pathol. 40, 1768–1773 (2009).
Maier, S. et al. SOX2 amplification is a common event in squamous cell carcinomas of different organ sites. Hum. Pathol. 42, 1078–1088 (2011).
Becher, O. J. & Holland, E. C. Sox2, a marker for stem-like tumor cells in skin squamous cell carcinoma and hedgehog subgroup medulloblastoma. EMBO J. 33, 1984–1986 (2014).
Tam, W. L. & Ng, H. H. Sox2: masterminding the root of cancer. Cancer Cell 26, 3–5 (2014).
Vanner, R. J. et al. Quiescent sox2(+) cells drive hierarchical growth and relapse in sonic hedgehog subgroup medulloblastoma. Cancer Cell 26, 33–47 (2014).
Liu, Y. et al. Targeting AKT as a promising strategy for SOX2-positive, chemoresistant osteosarcoma. Bone Res 13, 25 (2025).
Xue, M. et al. AKT1 as a therapeutic target for platinum-resistant SOX2 positive ovarian cancer cells. Sci. Rep. 15, 15096 (2025).
Boumahdi, S. et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature 511, 246–250 (2014).
Ferone, G. et al. SOX2 Is the determining oncogenic switch in promoting lung squamous cell carcinoma from different cells of origin. Cancer Cell 30, 519–532 (2016).
Metz, E. P. & Rizzino, A. Sox2 dosage: a critical determinant in the functions of Sox2 in both normal and tumor cells. J. Cell Physiol. 234, 19298–19306 (2019).
Porter, L. & McCaughan, F. SOX2 and squamous cancers. Semin Cancer Biol. 67, 154–167 (2020).
Ward, P. S. & Thompson, C. B. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell 21, 297–308 (2012).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Pietrocola, F., Galluzzi, L., Bravo-San Pedro, J. M., Madeo, F. & Kroemer, G. Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab. 21, 805–821 (2015).
Wellen, K. E. et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–1080 (2009).
Pott, S. & Lieb, J. D. What are super-enhancers?. Nat. Genet 47, 8–12 (2015).
Hnisz, D. et al. Super-enhancers in the control of cell identity and disease. Cell 155, 934–947 (2013).
Whyte, W. A. et al. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153, 307–319 (2013).
Carrer, A. et al. Acetyl-CoA metabolism supports multistep pancreatic tumorigenesis. Cancer Discov 9, 416–435 (2019).
Lee, J. V. et al. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab. 20, 306–319 (2014).
Chen, X. et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 133, 1106–1117 (2008).
Fang, L. et al. A methylation-phosphorylation switch determines Sox2 stability and function in ESC maintenance or differentiation. Mol. Cell 55, 537–551 (2014).
Wang, Z. et al. AKT drives SOX2 overexpression and cancer cell stemness in esophageal cancer by protecting SOX2 from UBR5-mediated degradation. Oncogene 38, 5250–5264 (2019).
Lindsley, C. W. The Akt/PKB family of protein kinases: a review of small molecule inhibitors and progress towards target validation: a 2009 update. Curr. Top. Med Chem. 10, 458–477 (2010).
Masui, S. et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat. Cell Biol. 9, 625–635 (2007).
Jiang, Y. et al. Co-activation of super-enhancer-driven CCAT1 by TP63 and SOX2 promotes squamous cancer progression. Nat. Commun. 9, 3619 (2018).
Jain, K. et al. An acetylation-mediated chromatin switch governs H3K4 methylation read-write capability. Elife 12, e82596 (2023).
Loven, J. et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 153, 320–334 (2013).
Nabet, B. et al. The dTAG system for immediate and target-specific protein degradation. Nat. Chem. Biol. 14, 431–441 (2018).
Abuhashem, A. & Hadjantonakis, A. K. Generation of knock-in degron tags for endogenous proteins in mice using the dTAG system. STAR Protoc. 3, 101660 (2022).
Ji, Y. X., Hu, S. & Chen, F. X. Protocol for rapidly inducing genome-wide RNA Pol II hyperphosphorylation by selectively disrupting INTAC phosphatase activity. STAR Protoc. 4, 102640 (2023).
Zlotorynski, E. Gene expression: ACSS2 boosts local histone acetylation. Nat. Rev. Mol. Cell Biol. 18, 405 (2017).
Sutendra, G. et al. A nuclear pyruvate dehydrogenase complex is important for the generation of acetyl-CoA and histone acetylation. Cell 158, 84–97 (2014).
Jin, N. et al. Identification of metabolic vulnerabilities of receptor tyrosine kinases-driven cancer. Nat. Commun. 10, 2701 (2019).
Jiang, Y. Y. et al. TP63, SOX2, and KLF5 establish a core regulatory circuitry that controls epigenetic and transcription patterns in esophageal squamous cell carcinoma cell lines. Gastroenterology 159, 1311–1327 (2020).
Quan, J., Bode, A. M. & Luo, X. ACSL family: the regulatory mechanisms and therapeutic implications in cancer. Eur. J. Pharm. 909, 174397 (2021).
Mashek, D. G., McKenzie, M. A., Van Horn, C. G. & Coleman, R. A. Rat long chain acyl-CoA synthetase 5 increases fatty acid uptake and partitioning to cellular triacylglycerol in McArdle-RH7777 cells. J. Biol. Chem. 281, 945–950 (2006).
Ellis, J. M. et al. Adipose acyl-CoA synthetase-1 directs fatty acids toward beta-oxidation and is required for cold thermogenesis. Cell Metab. 12, 53–64 (2010).
Ellis, J. M. et al. Mouse cardiac acyl coenzyme a synthetase 1 deficiency impairs fatty acid oxidation and induces cardiac hypertrophy. Mol. Cell Biol. 31, 1252–1262 (2011).
Bu, S. Y. & Mashek, D. G. Hepatic long-chain acyl-CoA synthetase 5 mediates fatty acid channeling between anabolic and catabolic pathways. J. Lipid Res 51, 3270–3280 (2010).
Teodoro, B. G. et al. Long-chain acyl-CoA synthetase 6 regulates lipid synthesis and mitochondrial oxidative capacity in human and rat skeletal muscle. J. Physiol. 595, 677–693 (2017).
Zhu, G. et al. Pharmacological inhibition of SRC-1 phase separation suppresses YAP oncogenic transcription activity. Cell Res 31, 1028–1031 (2021).
Parisi, L. R., Li, N. & Atilla-Gokcumen, G. E. Very long chain fatty acids are functionally involved in necroptosis. Cell Chem. Biol. 24, 1445–1454 e8 (2017).
Chen, J. et al. PI3K/AKT inhibition reverses R-CHOP resistance by destabilizing SOX2 in diffuse large B cell lymphoma. Theranostics 10, 3151–3163 (2020).
Kinnaird, A., Zhao, S., Wellen, K. E. & Michelakis, E. D. Metabolic control of epigenetics in cancer. Nat. Rev. Cancer 16, 694–707 (2016).
Li, L. O. et al. Liver-specific loss of long chain acyl-CoA synthetase-1 decreases triacylglycerol synthesis and beta-oxidation and alters phospholipid fatty acid composition. J. Biol. Chem. 284, 27816–27826 (2009).
Wu, M., Cao, A., Dong, B. & Liu, J. Reduction of serum free fatty acids and triglycerides by liver-targeted expression of long chain acyl-CoA synthetase 3. Int J. Mol. Med 27, 655–662 (2011).
To, K. K. W., Xing, E., Larue, R. C. & Li, P. K. BET bromodomain inhibitors: novel design strategies and therapeutic applications. Molecules 28, 3043 (2023).
Gajjela, B. K. & Zhou, M. M. Bromodomain inhibitors and therapeutic applications. Curr. Opin. Chem. Biol. 75, 102323 (2023).
Filippakopoulos, P. et al. Selective inhibition of BET bromodomains. Nature 468, 1067–1073 (2010).
Liu, Z. et al. Integrated multi-omics profiling yields a clinically relevant molecular classification for esophageal squamous cell carcinoma. Cancer Cell 41, 181–195 e9 (2023).
Petan, T., Jarc, E. & Jusovic, M. Lipid droplets in cancer: guardians of fat in a stressful world. Molecules 23, 1941 (2018).
Yue, S. et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 19, 393–406 (2014).
Knoepfler, P. S. et al. Myc influences global chromatin structure. EMBO J. 25, 2723–2734 (2006).
Lan, F. et al. Recognition of unmethylated histone H3 lysine 4 links BHC80 to LSD1-mediated gene repression. Nature 448, 718–722 (2007).
Liu, W. et al. Large-scale and high-resolution mass spectrometry-based proteomics profiling defines molecular subtypes of esophageal cancer for therapeutic targeting. Nat. Commun. 12, 4961 (2021).
He, J. Z. et al. Spatial analysis of stromal signatures identifies invasive front carcinoma-associated fibroblasts as suppressors of anti-tumor immune response in esophageal cancer. J. Exp. Clin. Cancer Res 42, 136 (2023).
Camp, R. L., Dolled-Filhart, M. & Rimm, D. L. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin. Cancer Res 10, 7252–7259 (2004).
Acknowledgements
We thank Dr. Tiebang Kang from SUN YAT-SEN University (Guangzhou, China) for providing K30, K140, K410 and K520 cells and Dr. Zhihua Liu at Cancer Hospital Chinese Academy of Medical Sciences (Beijing, China) for providing K70, K150, K450 and K510 cells. We also thank members of Wong’s lab for valuable discussion. This study is supported by grants from National Natural Science Foundation of China (82350710227 to J.W., 91957120 to S.H.L., 82002995 to Z.W., 82203355 to L.K, and 32430022 and 32525001 to F.L.), the National Key Research and Development Program of China (2023YFA1800402 to J. W. and 2021YFA1300100 to F. L.), and Taishan Scholar Project special funding to Z.W.
Author information
Authors and Affiliations
Contributions
Conceptualization, Z.W, S.H.L., F.L. and J.W.; methodology, Z.W., R.D., L.K., H.Y., J.H., Yt.Z., Y.J.; investigation, Z.W., R.D., L.K., H.Y., Z.C., L.S., Yf.Z., Z.H., Yy.H.; writing-original draft, Z.W., R.D.; writing-review and editing; L.X., S.H.L., F.L., and J.W.; supervision, L.X., J.L., F.L., S.L., and J.W.; funding acquisition, Z.W, L.K., L.X., S.H.L., F.L., and J.W.
Corresponding authors
Ethics declarations
Competing interests
Fei Lan is a scientific co-founder and stockholder of Active Motif Shanghai, Inc. and Alternative Bio, Inc. All other authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Brian Strahl, Benjamin Beck and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Z., Dai, R., Kang, L. et al. SOX2 drives esophageal squamous carcinoma by reprogramming lipid metabolism and histone acetylation landscape. Nat Commun 16, 8190 (2025). https://doi.org/10.1038/s41467-025-63591-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-025-63591-z