Abstract
In adult tissues, stem cells (SCs) reside in specialized niches, where they remain relatively stationary state until activated by injury. While migration is essential for regeneration, mechanisms guiding SCs towards injury sites remain poorly understood due to challenges in tracking them in vivo. Here, we present an experimental framework to monitor intestinal SC (ISC) movement in real time during early gut regeneration. We identify the Drosophila PDGF-VEGF-related receptor, Pvr, as a critical regulator of this process, with ISC-specific Pvr depletion strongly impairing migration and regeneration. The ligand, Pvf1, produced by gut-associated trachea after damage, serves as a guidance cue directing ISCs towards injury sites. Our work highlights a critical role of gut-trachea crosstalk in guiding ISC migration during regeneration. As neovascularization of injury sites is a key feature of tissue repair in both flies and mammals, these findings could have broader implications for regenerative processes across diverse adult tissues.
Similar content being viewed by others
Introduction
The inherent ability of adult stem cells (SCs) to assume a migratory behavior in response to appropriate stimuli has gained a lot of attention in recent years due to the potential of exploiting such behavior for wound repair and regenerative medicine. Through their capacity to self-renew and generate committed daughter cells, SCs produce a continuous source of differentiated cells essential for the maintenance and repair of adult tissues. While the regulations underpinning SC divisions and differentiation are increasingly well understood, the signals that guide SCs towards injured areas and ensure their correct positioning within the tissue remain relatively unexplored.
Importantly, active migration of somatic SCs is thought to constitute an important component of the regenerative response in almost all adult tissues and has been particularly well studied in the context of skin and bone fracture healing processes1,2. Studies in mice coupling intravital imaging with SC labeling have shown that hair follicle SCs and interfollicular SCs are actively recruited from different regions of the skin to the wounded area and participate in epidermal repair3,4. Likewise, efficient repair of bone fractures depends on the recruitment and expansion of perivascular mesenchymal skeletal stem and progenitor cells, which constitute a source of mature osteoblasts5,6,7.
To date, most signals reported to regulate cell migration were identified based on their capacity to stimulate or inhibit migration in vitro and by manipulating one component at a time8. However, SC migration is likely to be regulated by the coordinated action of mechanical cues and biochemical signals released from the SC niche and nearby organs. Furthermore, the migratory modes of SCs in vivo deviate substantially from those observed in vitro highlighting the importance of studying the migration of SCs in their native environment8,9.
The Drosophila midgut provides a powerful model for studying adult SC-mediated regenerative processes. The fly intestine is made up of an epithelial monolayer of enterocytes (ECs), which are aligned on their basal sides on an extracellular matrix referred to as the basement membrane. The only dividing cells in the gut are the intestinal SCs (ISCs), which are embedded basally throughout the epithelium and divide either symmetrically, producing ISC-ISC or EB-EB pairs, or asymmetrically, giving rise to one daughter ISC and one transient progenitor cell, an enteroblast (EB) or enteroendocrine progenitor cell, that are destined to differentiate into an EC or EE cell (EEC), respectively10,11,12,13,14.
To maintain intestinal homeostasis, a complex network of signals emanating from the ISC niche (ECs, EECs, EBs, and visceral muscles (VMs)) ensures that cell loss is tightly coupled with ISC-dependent proliferation and cell fate decisions15,16,17,18. In response to intestinal infections, increased ISC divisions and accelerated tissue turnover rates ensure that dying epithelial cells are replaced. In addition to gut resident cells, the tracheal system, an oxygen-delivering network functionally equivalent to the mammalian blood vessels, constitutes an important component of the regenerative response19,20. The terminal tracheal cells (TTC; equivalent to the mammalian tip cells) infiltrate the adult gut and respond to reactive oxygen species (ROS) emanating from damaged cells by triggering increased tracheole branching, which is essential for driving ISC divisions and intestinal repair19,20.
As well as extrinsic signals from the surrounding niche, intrinsic SC properties have also been shown to contribute to the regenerative response. Notably, a recent study showed that inhibiting actin dynamics in ISCs, and hence their capacity to migrate, impairs damage induced ISC divisions, suggesting that ISC migration and divisions are intrinsically linked processes21. Here, we use transient exposure to DSS or the highly pathogenic Pseudomonas entomophila (P.e.) Gram-negative bacteria to introduce localized damage of the gut epithelium and live imaging of whole-mount guts to track the migration of ISCs towards affected areas. Our data show that ISC migration is rapidly induced following exposure to DSS or P.e. and results in the formation of ISC clusters at damaged areas of the gut epithelium. We further show that PDGF-VEGF-related factor 1 (Pvf1), produced by the trachea in response to intestinal damage, acts as a guidance signal to stimulate ISC migration and gut regeneration.
In mammals, PDGF and VEGF both act as chemoattractants with essential roles in tissue repair. The PDGF-BB/PDGFR axis functions as a potent stimulator of a variety of cells in vitro, and the direct application of PDGF-BB to wounds has been shown to recruit fibroblasts and improve healing5,22,23,24,25,26,27,28,29,30. Nevertheless, the physiologically relevant source of PDGF-BB and its regulation during tissue repair are not known. In flies, the PDGFR-VEGFR-related receptor, Pvr, is a critical regulator of developmentally controlled cell migrations31,32,33,34,35,36. Interestingly, Pvr and its ligand, PDGF-VEGF-related factor 2 (Pvf2), are both expressed in ISCs, albeit the role of Pvf2-Pvr signaling in intestinal regeneration remains contentious. While one study showed that Pvf2 is required for the regenerative response triggered by administration of paraquat37, another found that Pvf2 and Pvr are dispensable for the proliferative response triggered by intestinal infections38.
Here we show that Pvr, but not Pvf2, is required for ISC migration and divisions triggered by intestinal infections and localized damage. We find that intestinal damage triggers Pvf1 expression in the gut-associated trachea, and that Pvf1 derived from tracheal cells acts as a chemoattractant to guide ISC migration during tissue repair. Knocking down Pvf1 in tracheal cells impairs the ISC-driven regenerative response, highlighting the coupling between ISC migration and divisions. As neovascularization is a hallmark of both tissue repair in all adult organs, these findings are likely applicable to repair processes in a broad range of adult tissues.
Results
Pvr promotes actin-based ISC motility and tissue regeneration
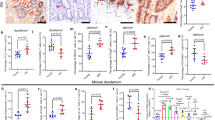
To shed light on the role of Pvr signaling in mediating regenerative growth, we knocked down Pvr in ISCs and their immediate progenitors, the EBs, using the ISC/EB specific driver, esgts>, or in ISCs alone using esgts> in combination with EB-specific expression of the Gal4 inhibitor Gal80 (esgts::Gal4; Su(H)::Gal80; hereafter referred to as ISCts>), and examined the effect on infection-induced ISC divisions and tissue turnover rates. Consistent with a role of Pvr in regulating ISC dynamics, ISC-specific knockdown of Pvr strongly suppressed the increase in ISC proliferation associated with both DSS treatment and enteric infections (Fig. 1a, b and Supplementary Fig. 1a, b). To analyze the effect of ISC-specific Pvr knockdown on tissue turnover rates, we used TransTimer, a genetic tool that allows ISC-specific expression of a short-lived GFP (half-life ∼2 h) and a long-lived RFP (half-life ∼18.5 h), to follow the maturation of ISCs (green and red) into EBs, ECs, and EECs (red only, Fig. 1c)39. Knockdown of Pvr in ISCs strongly suppressed the infection induced acceleration in tissue turnover rates (Fig. 1d–h). We next used the ReDDM system to deplete Pvr and Pvf2 in both ISCs and EBs and follow the effect on tissue turnover rates40. Notably, while ISC/EB-specific (esg>) Pvr knockdown strongly suppressed the infection-induced increase in tissue turnover rates (Supplementary Fig. 1c–i), Pvf2 depletion did not (Supplementary Fig. 1h). Consistent with a role of Pvr in mediating regenerative growth, knockdown of Pvr in ISCs using two different RNAis increased the sensitivity to oral infection with the highly pathogenic Pseudo entomophila (Pe) bacterium (Fig. 1i). As previously mentioned, ISC migration and divisions are thought to be closely interlinked processes. To analyze how ISC-specific Pvr knockdown affects actin dynamics in a regenerative context, we expressed RFP-tagged LifeAct in ISCs. As previously reported, global damage triggered the formation of highly dynamic actin-based lamellipodia within 2 h of an oral infection (Fig. 1j, k’ and Movies 1 and 2)21. Strikingly, ISC-specific Pvr knockdown completely abolished the infection-induced formation of actin-based protrusion quantified as an increase in circularity and a decrease in protrusion formation (Fig. 1j–m and Supplementary Fig. 1j, k and Movie 3). Altogether, these data support an ISC-specific role of Pvr in promoting damage-induced actin-based protrusions previously reported to be essential for ISC migration.
a, b Quantification of PH3+ cells in midguts from control ISC-specific Pvr RNAi flies (ISCts>Pvr RNAi) following oral infection with Ecc15 (a), n = 7, 17, 15, 16, 19, 18) or P.e. (b), n = 20, 20, 18, 18, 19, 19). c–h ISC-specific Pvr knockdown suppresses infected-induced acceleration of tissue turnover. c Schematics of the TransTimer system. dGFP and RFP are co-expressed in ISCs via the Dlts> driver. Differentiation into EBs or pre-EECs terminates further dGFP/RFP production. Differential stability of the fluorophores results in rapid degradation of dGFP, which has a half-life of 2 h, whereas RFP persists for about 20 h in differentiated cells. RFP+/GFP- cells were scored as newly produced progenitor (EBs) and mature cells (EECs/ECs, n = 19, 18, 20, 20) (h). i ISC-specific Pvr knockdown reduces survival to oral P.e. infection (n = 150/genotype). j–m Actin dynamics in ISCs monitored post-P.e. infection using ISCts > -driven YFP to label ISCs (green) and LifeAct to visualize F-actin dynamics (red). Controls without (j–j’) or with 2-h P.e. infection (k–k’) show infection-induced actin-based protrusions. Pvr RNAi (l–l’) disrupts protrusions, quantified as an increase in circularity (m, n = 9/683, 14/954, 8/909, 8/842 guts/cells). n–n’ High-powered infrared two-photon laser was used to generate localized ~50 mm wounds (red stippled circle). Confocal images of the R4 region from control flies at ablation (T0, o) and 4 h later (T4, o’) with ISCs labeled in white. Migrated ISCs equals number of ISCs present within 100 μm radius of the wound at T4 subtracted number of ISCs present at T0. o–q Confocal images of control (o–o’) and Pvr RNAi (p–p’) guts at 0 and 4 h post-ablation with ISCs labeled in green. Quantification (q, n = 9, 9 guts) shows reduced migration with Pvr knockdown. Statistical tests: two-tailed Mann–Whitney test (q), Mantel–cox Log-rank test (i), or Kruskal–Wallis with post-hoc multiple comparison analysis. Data are presented as mean values ± SD or ± SEM (m). Source data are provided as a Source data file.
Localized damage triggers rapid directional ISC migration
To obtain a condition for studying the directed movement of ISCs towards a localized area of damage, we next used pulsed two-photon infrared lasers to generate a localized wound of about 50 μm and live imaging of whole-mount guts to monitor ISC migration. As previously reported, the toxicity associated with live imaging causes the guts to deteriorate within a few hours of wounding. Therefore, it was not compatible with monitoring ISC migration in real time21. Instead, we defined a radius of 100 μm around the ablation site and counted the number of ISCs at the time of ablation and 4 h later (Fig. 1n–n’). We observed an accumulation of ISCs 4 h post-ablation around the wound site. According to previous reports and our own observations, ISCs do not divide within the first 4 h of epithelial wounding21. ISC-specific Pvr knockdown prevented the accumulation of ISCs at the wound, consistent with the role of Pvr in promoting ISC migration in response to epithelial damage (Fig. 1o–q).
To device an alternative strategy allowing ISC migration to be followed in real time, we introduced a GFP-tagged E-Cadherin (E-Cad-GFP) in the background. By applying short-term damaging conditions—either a 1-h exposure to the chemical agent, DSS, or a 2-h infection with Pe—we could observe localized epithelial wounds, as evident by the loss of E-Cad-GFP and the leakage of ingested fluorescent dextran through epithelial breaches (Fig. 2a–c’). On average these damaging conditions produced one hole per R4 region (Fig. 2d). By using perturbation of membrane-associated E-Cad staining as an indicator of epithelial damage induced by DSS (see yellow star in Fig. 2e–e”’), we observed directed movements of ISCs towards areas with disrupted E-Cad “patterns”, resulting in the formation of ISC clusters within 2–6 h (Fig. 2e–e”’, g, h and Movie 4). By contrast, ISCs remained quiescent in undamaged regions with regular membrane-associated E-Cad staining (Fig. 2f–h and Movie 5). Similarly, we found that a transient 2-h infection with the pore-forming Pe bacteria caused localized damage, evident through the loss of E-Cad-GFP and leakage of fluorescent dextran and triggered directed ISC movement of ISCs towards these damaged sites within 2–7 h (Fig. 2i–i”’, Movie 6). Quantification of tissue renewal over the subsequent 24 h confirmed that brief, 2-h exposure to Pe induces localized regeneration, seen as small, discrete patches of renewed tissue (black areas, Fig. 2j, k, m, n). In contrast, prolonged 16-h infection, a condition commonly used to induce gut damage, resulted in extensive regeneration, with approximately 40% of the total R4 region showing renewed tissue on average (Fig. 2j, l–n). Lastly, using optogenetics to induce targeted damage by expressing a light-activatable pro-apoptotic gene, Dronc (Optodronc), in small EC clones, we could observe the migration of ISCs toward apoptotic cells (Fig. 2o–o”’ and Movie 7)41. Together, these findings reveal that localized wounding of the gut epithelium triggers a rapid regenerative response, where ISCs migrate over distances of several cell perimeters towards damaged areas forming distinct ISC clusters (Fig. 2e–e”’, g–i”’, o–o”’ and Movies 4, 6, 7). Notably, we did not detect ISC divisions within this time frame, suggesting that migration precedes ISC proliferation in the early phase of the regenerative response.
a–d Guts expressing E-Cad::GFP and Moesin::GFP from endogenous promoters were used to visualize adherens junctions (AJs) and the apical epithelial surface in control (a–a’), DSS-treated (b–b’), and P.e.-infected (c–c’) flies. In controls, ingested fluorescent dextran (red) remains luminal. DSS and P.e. cause epithelial disruption indicated by loss of E-Cad::GFP/Moesin::GFP signals and dextran leakage (white arrows in (b–c’), n = 16, 14, 14 guts). e–f”’ Guts with E-Cad::GFP (white) and ISCs (cytoplasmic white, ISCts > YFP) after 1 h DSS feeding. Loss of membrane E-Cad was used as a proxy for epithelial damage. Live imaging over 6 h revealed ISCs migrating towards an area with disrupted E-Cad “pattern” (orange stippled circle, e–e”’, g, h, n = 5/11, 5/19 guts/ISCs). In intact E-Cad areas (green stippled circle), ISCs remained static (f–h). i–i”’ Tracked trajectories following a 2 h P.e. infection show directed ISC (white) migration toward a damaged region, identified by the loss of E-Cad-GFP signal and dextran leakage. j–n Guts with EC-specific expression of the apoptotic reporter, Apoliner, infected for 2 (k) or 16 (l) hours to induce localized or widespread damage. To induce Apoliner/fluorophore expression in all ECs, mexts>Apoliner flies were shifted to the permissive temperature (29 °C) for 24 h prior to infection, then returned to room temperature (RT, non-permissive) and infected with PE for 2 h (OD200) or overnight (OD100). As Gal80ts is reactivated at RT, newly regenerated ECs are marked by the loss of fluorophore signal (black areas, stippled lines). A 2-h infection led to small, localized renewal zones (k, m, n), while a 16-h infection caused widespread renewal (l–n; m: n = 5/26, 12/189, 13/46 guts/renewed regions, n: n = 5, 11, 13 guts). o–o”’ Optogenetics damage was induced by expressing a light-activatable OptoDronc in small EC clones. ISC trajectories show directed movement towards apoptotic ECs (marked by yellow stars). Significance was tested with a two-tailed Mann–Whitney test (g, h), or Kruskal–Wallis with post-hoc multiple comparison analysis (for the rest). Data are presented as mean values ± SD. Source data are provided as a Source data file.
Pvr drives ISC migration and clustering at injury sites
We next investigated the role of Pvr in mediating ISC migration following a 2-h Pe infection. Consistent with a requirement for Pvr signaling in this process, we found that Pvr-depleted ISCs remained static and did not migrate or form large ISC clusters in response to epithelial damage induced by Pe infection (Fig. 3a–e and Movies 8 and 9). Of note, we observed that strong (PvrRNAi KK), but not intermediate (PvrRNAi GD), ISC-specific Pvr depletion suppressed the loss of E-Cad-GFP typically seen within hours of a Pe infection (Supplementary Fig. 2a, b). This effect occurred despite normal bacterial ingestion, as indicated by the presence of fluorescent dextran in the gut lumen and a marked reduction in host survival following Pe infection (Fig. 1i and Supplementary Fig. 2a, b). These findings imply that ISC dynamics may non-autonomously facilitate the extrusion of damaged ECs, and that disruption of this process has detrimental consequences for the host. To analyze the order of events involved in tissue regeneration, we used TransTimer to follow the maturation of ISCs (green and red) into EBs, ECs and EECs (red only, Fig. 3f, i)39. Notably, ISC-to-EB differentiation primarily occurred between 12 and 17 h after epithelial damage (Fig. 3g”, h”–i and Movie 10), indicating that ISC migration and the formation of ISC clusters precedes ISC division and maturation during regenerative growth. In agreement with this, ISC-specific expression of Dacapo, which arrests cells in G1 and inhibits ISC divisions42, did not prevent the formation of ISC clusters produced by a 2-h Pe infection (Supplementary Fig. 2c, d).
a–e Guts with ISC-specific expression of YFP to label ISCs (in green) and LiveAct-RFP to monitor actin dynamics (in red) dissected from uninfected flies (a–a”, control) or flies exposed to P.e. for 2 h (b–e”). Representative time points from real time recordings showing that ISC-specific Pvr knockdown (c–d”) suppresses ISC migration and the formation of ISC clusters (b–b”), as quantified in (e) by counting the number of cells per cluster (n = 17, 17, 20, 20 guts). The acquired movies were aligned in Fiji using “rigid body transformation to reduce movement related to muscle contractions. f–i The TransTimer system was used to follow the differentiation of ISCs into progenitors and mature cells from 12 to 17 h after infection. At 12 h only a few ISCs (red and green) have differentiated into EBs/EECs/ECs (red only, labeled by while stars), while 5 h later many more ISCs have either differentiated or are in the process of transitioning (more red than yellow) as depicted in (i, n = 5 guts). Significance was tested with Kruskal–Wallis with post-hoc multiple comparison analysis. Data are presented as mean values ± SD. Source data are provided as a Source data file.
Pvr activation triggers Rac1-mediated actin remodeling
To decipher how Pvr signaling regulates actin dynamics, we ectopically expressed an activated form of Pvr, PvrCA, and RFP-LifeAct in ISCs and monitored the effects on cell shape and actin-based protrusions. ISC-specific Pvr activation resulted in large-flattened ISCs that formed actin-based protrusions in a non-coordinated fashion, suggesting that Pvr signaling is sufficient to trigger lamellipodia formation (Fig. 4a–c). A previous study showed that infection induced lamellipodia formation, which is required for ISC displacement, is mediated through Rac1 and its downstream effectors, Dia and the Arp2/3 complex, which drives the nucleation and branching of actin filaments21. Interestingly, Pvf1-Pvr signaling was previously reported to activate Rac1-mediated protrusions at the leading-edge during border cell migration32,34,43,44,45. To monitor the effect of Pvr signaling on Rac1 activity, we co-expressed a Rac1 activity reporter, pak3RBD::GFP44,46, with PvrCA specifically in the in ISCs. We found that ligand-independent Pvr activation is sufficient to trigger Rac1 signaling and that blocking Rac1 function is sufficient to suppress the cellular spreading and formation of protrusions triggered by ISC-specific PvrCA expression (Fig. 4d–j). Moreover, ISC-specific Pvr knockdown suppressed the infection associated increase in Rac1 activity, demonstrating that Rac1 acts downstream of Pvr to promote actin-based protrusion in this condition (Fig. 4k–n). Supporting the notion that ISC migration precedes proliferation, cell cycle arrest did not impair the phenotypical changes in cell shape and the cellular flattening of ISCs triggered by ectopic Pvr activation (Supplementary Fig. 2c, e–i). A previous study reported that PvrCA in ISCs and EBs resulted in a Ras-dependent expansion of the ISC/EB pool and intestinal dysplasia38. To investigate this phenotype in more details, we used the Transtimer system to activate Pvr signaling in ISCs only and analyze the effect on tissue turnover rates. Consistent with a critical role of Pvr in promoting tissue turnover (Fig. 1a–h), ISC-specific Pvr activation is sufficient to accelerate tissue turnover rates in the absence of an infection (Fig. 4o–q). Altogether, our results show that ectopic Pvr signaling is both required and sufficient to activate ISCs and promote tissue turnover.
a–c Confocal images of guts expressing E-Cad::GFP under its endogenous promoter to label cell-cell adhesion junctions (in green) and ISCts-driven expression of LifeAct (to label F-actin in red) alone (a–a”’, control) or with ISC-specific Pvr activation (b–b”’, ISCts>PvrCA). Ectopic Pvr activation results in large-flattened ISCs that form actin-based protrusions in a non-coordinated fashion, quantified as an increase in cell size in (c, n = 13/333, 11/139 guts/cells). (d–f) The effect of ISC-specific Pvr activation on Rac1 activity was followed using a Rac1 sensor (pak3RBD::GFP). Confocal images of posterior midguts from control flies d and flies with ISC-specific Pvract expression e showing an increase in Rac activity levels upon activation of Pvr in ISCs as quantified in (f) (n = 16, 15 guts). g–j The large-flattened morphology and formation of actin-based protrusions triggered by ISC-specific Pvr activation (h compared to g) are suppressed upon knockdown of Rac (i). Confocal images of dissected posterior midguts from uninfected (k, control) and P.e. infected control flies (l) and flies with ISC-specific Pvr knockdown m showing that the increase in Rac activity associated with oral P.e. infection (l compared to k) is suppressed upon Pvr knockdown m as quantified in (n) (n = 9/68, 15/124, 15/100 guts/ROI). o–q The TransTimer system was used to follow the differentiation of ISCs into progenitors (EBs) and mature EECs and ECs in control guts (o–o”) and guts with ISC-specific Pvr activation (p–p”). Confocal images of posterior midguts from control flies (o–o”) and flies expressing Pvract (p–p”) in ISCs using ISCTransTimer tracing for 2 days. Pvr activation results in accelerated tissue turnover visualized by an increase in the number of EBs and mature EECs and ECs (red only cells, p–p”) compared with control guts (o–o”) as quantified in (q) (n = 22, 25 guts). Significance was tested with a two-tailed Mann–Whitney test (c, f,q) or Kruskal–Wallis with post-hoc multiple comparison analysis (n). Data are presented as mean values ± SD or ± SEM (c, n). Source data are provided as a Source data file.
Trachea-derived Pvf1 guides ISC movement toward injury sites
Pvr signaling is activated upon binding of one of its three ligands, Pvf1-3. According to single-cell RNAseq data of the adult gut available at the FlyCellAtlas (https://scope.aertslab.org/#/FlyCellAtlas)47, Pvf3 is poorly expressed in the gut, while Pvf1 and Pvf2 show distinct expression patterns, with Pvf2 being highly expressed in ISCs/EBs and Pvf1 in mature ECs. Consistent with this, a previous study found that Pvf2 expression is restricted to ISCs and EBs in the adult gut37. As knockdown of Pvf2 in ISCs/EBs did not suppress the infection-induced acceleration in tissue turnover rates (Supplementary Fig. 1h), we focused our attention on Pvf1. Notably, Pvf1 was previously reported to act as a guidance signal for Pvr-mediated border cell migration32,34,43,44,45. Consistent with a role of Pvf1 in promoting regenerative growth, Pvf1 expression (Fig. 5e, f) and production (Fig. 5a–d”) were induced in several conditions associated with epithelial damage, including oral infections with P.e. (Fig. 5a–b”, d–d”) or following ingestion of DSS (Fig. 5c–c”). While 16 h of oral infection resulted in widespread induction of Pvf1-HA in VMs, ECs, and gut-associated trachea (Fig. 5a–b”), a shorter exposure of 6 h to DSS or Pe induced Pvf1 production in the gut-associated trachea and in a subset of ECs that likely correspond to damaged areas of the epithelium (Fig. 5c–d”). Consistent with Pvf1 acting as the ligand for Pvr in promoting regenerative growth, ubiquitous Pvf1 depletion, but not Pvf2 depletion, significantly impaired the proliferative response to Pe infection (Fig. 5g and Supplementary Fig. 3a). To identify the source of Pvf1, we knocked it down in ISCs/EBs, ECs, VMs and trachea. While knockdown of Pvf1 in ISCs/EBs, ECs or VMs had little or no effect on proliferative response to oral infections (Supplementary Fig. 3b–e), trachea-specific Pvf1, but not Pvf2 or Pvf3, depletion significantly reduced Erwinia carotovora carotovora 15, Ecc15, and Pe induced ISC divisions (Fig. 5h–i and Supplementary Fig. 3f). This reduction was comparable to that obtained by simultaneous knockdown of Pvf1 in ECs, VMs (Fig. 5j), suggesting that trachea-derived Pvf1 might guide ISC migration during regeneration. Consistent with this, we found that trachea-specific knockdown of Pvf1 suppressed the infection induced increase Rac1 sensor activity within ISCs and slowed down their migratory response to oral infection (Fig. 5k–n), although ISC clustering was eventually observed at later stages (Supplementary Fig. 3g–i). We noticed that trachea-specific knockdown of Pvf1 triggered a morphological change in ISCs that resulted in large-flattened cells that formed cellular protrusions in a non-coordinated fashion (Fig. 5m–m”” and Movies 11 and 12), reminiscent of that observed upon ISC-specific Pvr activation (Fig. 4b–b”’). This contrasts with ISC-specific Pvr depletion, which completely abolished the infection-induced formation of actin-based protrusion, suggesting that residual Pvf1, possibly originating from non-tracheal sources, may still be sufficient to activate ISCs under these conditions. Supporting this idea, ubiquitous Pvf1 knockdown impaired regenerative growth more strongly than trachea-specific Pvf1 depletion (Fig. 5g–i). Nevertheless, taken together, our data show that trachea-derived Pvf1 provides ISCs with a directional cue that is required for efficient migration of ISCs towards damaged areas. Two recent studies have shown that the tracheal remodeling associated with damage of the gut epithelium is required to support the associated proliferative response19,20. Importantly, knockdown of Pvf1 or Pvr in the trachea did not affect the infection induced increase in tracheal coverage (Supplementary Fig. 3j, k). On the other hand, ectopic expression of Pvf1 in ECs, but not in tracheal cells, was sufficient to trigger surplus divisions under homeostatic conditions (Supplementary Fig. 3l, m), suggesting that tracheal remodeling is necessary to render Pvf1 accessible to ISCs, thereby enabling their migration and proliferation. Altogether, our results show that damage of the gut epithelium triggers the expression of Pvf1 in the gut-associated trachea, which then acts as a chemoattractant to guide the migration of ISCs towards damaged areas (Supplementary Fig. 3n).
a–b” Confocal images of posterior midguts from mock-treated or P.e. infected (16 h) flies expressing Pvf1-HA from its endogenous promoter, showing a widespread induction of Pvf1-HA in the gut epithelium, VMs, and trachea (b’–b”). Confocal images of posterior midguts treated with DSS (c–c”) or infected with P.e. (d–d”) for 6 h, showing that Pvf1-HA is induced in a subset of ECs and tracheal cells (white arrows) at this stage. RT-qPCR of dissected midguts showing that Pvf1 expression is induced at 6 and 16 h after Ecc15 (e, n = 5, 6, 5 replicates) and P.e. (f, 5, 5, 5 replicates) infection. g Quantification of PH3+ cells in control midguts or midguts with global RNAi-mediated depletion of Pvf1, Pvf2, or both (n = 18, 15, 20, 19, 20 guts). h–i PH3+ quantification in control midguts or midguts with trachea-specific Pvf1 knockdown following oral Ecc15 (g, n = 16, 15, 15, 16, 16, 15 guts) or P.e. (h, n = 16, 15, 15, 16, 16, 15 guts) infection. j Quantification of PH3+ cells in control midguts or midguts with RNAi-mediated depletion of Pvf1 in trachea, VM, and ECs following a 16-h oral Ecc15 infection (n = 15, 13, 14, 12 guts). k Rac1 activity within ISCs was followed using a Rac1 sensor (pak3RBD::GFP). Quantification of sensor activity in posterior midguts from uninfected flies and P.e. infected control flies and flies with trachea-specific Pvf1 knockdown shows that the infection-induced increase in Rac activity is suppressed by Pvf1 depletion (n = 8/10, 8/10, 8/10 guts/ROI). l–n Guts expressing GFP under the control of ISCts-lexA>LexAop system to label ISCs (white) were dissected from control flies (l–l””) flies or flies with trachea-specific Pvf1 knockdown (dsrf>Pvf1 RNAi; m–m””) following a 2-h P.e. exposure. Time-lapse imaging showing that Pvf1 knockdown suppresses ISC migration (l–m””), quantified as reduced velocity in (n, n = 4/44, 10/143 guts/cells). Significance was tested with a two-tailed Mann–Whitney test (n), or Kruskal–Wallis with post-hoc multiple comparison analysis (for the rest). Data are presented as mean values ± SD or ±SEM (n). Source data are provided as a Source data file.
Discussion
Identification of the signals that guide ISC migration during tissue repair has been hampered by the difficulty of following this process in real time in vivo. Here, we provide the first real time recordings of ISC migration showing directed movements towards damaged areas of the gut epithelium. Taking advantage of the restricted/localized damage caused by brief exposure to the highly pathogenic Pe bacterium or damaging agents, we employed live imaging on cultured gut explants to monitor spatiotemporal ISC dynamics in the early phase of tissue regeneration. Our data show that ISC migration takes place within 2–6 h of damaging the gut epithelium and precedes ISC divisions and differentiation. ISC-to-EB differentiation is mainly observed at later stages when ISCs have already formed clusters. We further show that Pvr is a critical regulator of the migratory response associated with tissue regeneration, as ISC-specific Pvr depletion inhibits the formation of actin-based cellular extrusions and ISC migration in this condition. While Pvr knockdown impairs ISC migration, it also suppresses regenerative ISC divisions and maturation, highlighting the intimate link between ISC migration and divisions. This aligns with previous findings showing that inhibiting ISC migration impairs their proliferative capacity during regeneration21. Notably, our results reveal that the reverse is not true, as cell cycle arrest does not block ISC motility during the early stages of regeneration. Consistent with a role of Pvr in regulating actin dynamics, ectopic Pvr activation is sufficient to induce actin-based protrusions and a flattened morphology of ISCs in the absence of epithelial damage. Interestingly, Pvr activation was recently reported to trigger spreading and a similar lamellar morphology of hemocytes adhering to the surface of epidermal wounds in the Drosophila larvae48. One of the best characterized developmental functions of Pvr, is its role in collective border cell migration32,34,43,44,45. Pvf1 released from the oocyte acts as a guidance signal to activate Pvr signaling in the leading cells, and ectopic Pvf1 expression is sufficient to redirect the migration of border cells35. Pvr activation generates protrusions at the leading edge through the activation of Rac1 and its downstream effectors, Scar/Wave and the Arp2/3 complex44. Consistent with a similar pathway operating in ISCs, we find that Pvr is required to activate Rac1-dependent lamellipodia formation and ISC migration in response to enteric infections. Our data also aligns with previous observations showing that ISC-specific knockdown of Rac1 or Arp3 suppresses lamellipodia formation triggered by enteric infections21.
Crosstalk between the trachea and the gut is essential for mediating tissue regeneration. Two recent studies showed that damage of the gut epithelium triggers ROS-dependent remodeling of the trachea, which in turn is required to promote regenerative growth19,20. Intriguingly, reducing global levels of ROS by feeding flies the antioxidant, N-acetyl cysteine, was reported to suppress the infection induced increase in tracheal Pvf149. Hence, it is tempting to speculate that ROS emanating from damaged epithelial cells induces Pvf1 expression in the gut-associated trachea, which subsequently guides ISCs towards injured sites. Interestingly, while Pvf1 is required in the trachea to promote regenerative growth, ectopic expression of Pvf1 in the trachea is not sufficient to trigger ISC divisions, suggesting that remodeling of the TTC might be necessary to deliver Pvf1 at damaged areas of the gut.
The same stimuli that trigger SC migration during tissue healing are often subverted into promoting migration and dissemination of cancer cells. Not surprisingly, abnormal PDGF and VEGF activities have been reported in a wide range of human tumors, and expression levels of PDGFRs/PDGFs correlate with invasiveness, tumors size, chemoresistance and poor clinical outcome50. Furthermore, studies on cancer cell lines have shown that PDGF-PDGFR signaling contributes to cancer cell dissemination in vitro. However, as the native microenvironment differs considerably from that of in vitro cell culture systems, it is essential to address the contribution of the PDGF/PDGFR axis to the dissemination of cancer cells in vivo. Here we show that ectopic Pvr activation triggers a partial epithelial-mesenchymal transition (EMT) characterized by internalization and accumulation of the cell-cell adhesion proteins in the cytoplasm, altered F-actin dynamics and the formation of cellular protrusions accompanied by changes in cell morphology. The observation that Yorkie transformed ISC tumors express high levels of Pvf151, suggest that tumor-derived Pvf1 might potentiate cancer SC dissemination.
The acquisition of mesenchymal traits by SCs, essential for their mobility and for tissue regeneration, has been observed in a number of adult tissues, including the respiratory epithelium, a tissue that in structure and composition is similar to the fly gut52,53. Hence, it is possible that crosstalk between the respiratory epithelium and the vasculature plays an identical role in guiding SC migration towards affected areas in this tissue. The partial EMT of ISCs during tissue regeneration must be complemented and balanced by the reverse process, mesenchymal-epithelial transition (MET), upon return to homeostasis. Understanding the mechanisms underpinning MET and the resolution of regenerative ISC clusters upon return to homeostasis will be an exciting future area of research.
Methods
Fly stocks and husbandry
Animals were maintained on a standard cornmeal diet (containing: 82 g/L cornmeal, 60 g/L sucrose, 34 g/L yeast, 8 g/L agar, 4.8 mL/L propionic acid and 1.6 g/L methyl-4- hydroxybenzoate) at 25 °C and 60% relative humidity under 12-h light/dark cycle conditions. Females were used for all experiments. Temporal control of transgene expression using tubGal80ts in adult flies was achieved by raising flies at 18 °C through development until 4 days after eclosion to allow maturation of the digestive system. Then, flies were shifted to 29 °C to induce Gal4 mediated transgene expression in a cell-type specific manner. Duration of UAS induction was 4–8 days unless otherwise stated. Flies were flipped onto fresh medium every second day. Mated females were used for experiments.
The following lines were generous gifts from the colleagues in the fly community: Esg-ReDDM UAS-CD8::GFP; UAS-H2B::RFP, tubGal80ts/TM6b (Maria Dominguez, IN, Spain). w-; DSRF.Term-Gal4, UAS-CD8::GFP/CyO; tub-Gal80ts and w-, UAS-Dcr-2; DSRF.Term-Gal4/CyO (Irene Miguel-Aliaga, Crick institute, United Kingdom), endo ecad 3x GFP (Yohanns Bellaiche, Institut Curie, France), UAS-transtimer: UAS-dGFP-2A-RFP (Norbert Perrimon, Harvard Medical School, USA), ISCts: esgGal4, UAS-YFP; Su(H)Gal80, tubGal80ts (Heinrich Jasper, Genentech, USA), esglexA, lexOGFP; Su(H)Gal80, tubGal80ts (J. Cordero, Beatson Institute, United Kingdom); OptoDronc (Romain Levayer, Institut Pasteur, France). UAS-pvf1-RNAi v102699, UAS-pvf1-RNAi v46875, UAS-pvf1-RNAi v6173, UAS-Pvr-RNAi v105353, UAS-Pvr-RNAi v43461, UAS-pvf2-RNAi v102072, UAS-pvf2-RNAi v7629 were obtained from the Vienna Drosophila RNAi center. Esg-Gal4 BL93857, Mef2-Gal4 BL27390, tub-Gal80ts BL7107 and BL7108, Mex-Gal4 BL91368, Delta-Gal4 BL77753, UAS-liveAct-RFP BL58362, Pvf1-HA BL92471, UAS-Pvf1 BL58425, UAS-Pvr-CA BL58496, UAS Rac-DN BL6292, Rac sensor BL52304, UAS-Dacapo BL83334, UAS-Wee1 BL65389, da-Gal4 BL95282, tub-Gal4 BL5138, Apoliner BL59023, UAS-pvf3 RNAi BL38962, FRT-GAL80-FRT BL62103, esg::YFP BL78333, hs-Flp, UAS-mCD8::GFP BL28832 were obtained from the Bloomington Drosophila stock center.
Oral infections
For oral infections with bacteria (Ecc15 or Pe), overnight cultures were grown from single colonies (Ecc15) or directly from a glycerol stock kept at −80 °C (Pe). Cultures were grown in conical flasks containing LB broth at 29 °C (Ecc15) or at 30 °C with rifampicin supplied (Pe). The next day, optical density OD600 was measured, cultures were spun down, and the remaining bacterial pellet was resuspended in an appropriate volume of 5% sucrose such that OD600 was adjusted to either OD100 for measurement of PH3+ in response to PE infection or 200 for all other experiments. The concentrated bacterial solution was then, in a volume of 50 μl, added directly onto Whatman filter paper disks resting on the surface of normal fly food with reduced yeast content (containing: 82 g/L cornmeal, 60 g/L sucrose, 17 g/L yeast, 8 g/L agar, and 4.6 g/L methyl-4-hydroxybenzoate). To ensure efficient intake of bacteria, flies were sorted 10 by 10 in empty vials and starved for 2–3 h before transfer to the prepared vials containing bacteria for the indicated amount of time. To assess survival to bacterial infection with Pe, mated female flies raised at 18 °C through development were sorted 10 flies per vial and transferred to 29 °C for 7 days before initiating the first infection. Flies were repeatedly infected once every 2 days by transferring them to fresh vials with newly prepared Pe solution added.
DSS treatment
For DSS treatment, 2 h starved flies were fed on Whatman filter paper disks with 5% sucrose solution supplemented with 5% dextran sodium sulfate (DSS) salt (Colitis grade, MP Biomedicals, Lachine, QC, Canada) for the indicated amount of time.
Fluorescent dextran treatment
Flies were fed on a filter disc containing 50 µl of fluorescent dextran 647 (thermofisher D22914) diluted to a final concentration of a 40 µg/ml in water containing 5% sugar (control), 5% sugar + Pe (OD200), or 5% sugar + 5% DSS. Flies were exposed to Pe for 2 h or DSS for 1 h prior to dissection. Dissected guts were imaged on an Andor Dragonfly 200 spinning disk confocal on a Leica 25x water immersion objective to identify areas of basal dextran leaks. A maximum intensity project (MIP) of the basal-most 5-µm stack from the dextran fluorescence channel was merged with a MIP of the E-Cad-GFP channel. The number of dextran leaks per R4 region was quantified. 3D rendering of longitudinal sections of the midgut was made using Imaris software. Dextran leaks correlated with loss of E-Cad::GFP and Moe::GFP, labeling the AJs and the apical brush border of ECs, respectively.
Tissue renewal area
In healthy cells, the caspase activity reporter, Apoliner, displays a diffuse cytosolic GFP signal. Upon activation of caspase-dependent apoptosis, Apoliner is cleaved, causing the GFP fragment to translocate into the nuclei of dying cells. Transgenic flies expressing Apoliner/fluorophores under the EC-specific driver, Mexts>, were maintained at 18 °C to keep the temperature-sensitive repressor of Gal4, Gal80ts, active. To achieve sustained Apoliner/fluorophore expression in all ECs, flies were shifted to the permissive temperature (29 °C) for 24 h prior to infection. Subsequently, flies were transferred to room temperature (RT, non-permissive) and infected with PE for 2 h (OD200) or overnight (OD100). For short-term infection (2 h), flies were transferred back onto normal adult fly food and kept there overnight at RT. As Gal80ts is reactivated at RT, newly regenerated ECs are therefore marked by the loss of fluorophore signal. Midguts were imaged using an Andor Dragonfly 200 spinning disk confocal microscope with a Leica 25x water immersion objective. The total area of nonfluorescent (Apoliner-negative) regions per midgut was quantified using ImageJ and divided by the total area of the R4 region to calculate the proportion of renewed tissue.
Dissections and immunohistochemistry
Midguts were dissected in PBS and immediately transferred into fixative consisting of 4% paraformaldehyde in PBS for 1 h at RT. Fixed midguts were washed twice with PBS and further two times 15 min with PBS + 0,1% Trition (PBS-T) with agitation. Midguts that were not stained with antibodies (EsgReDDM and dltstranstimer) were immediately washed once with PBS and mounted on microscopy slides at this stage. Midguts that were stained with antibodies were incubated for 2 h in blocking solution (PBS-T containing 10% FCS), followed by incubation with primary antibodies prepared in blocking solution at 4 °C overnight. The next day, midguts were washed three times, 15 min each, with PBS-T and incubated with secondary antibodies for 3 h at RT or overnight at 4 °C. At last, the stained midguts were washed two times, 15 min each, with PBS-T, followed by a single wash with PBS, before being mounted on glass slides in Vectashield mounting media with or without DAPI. All midguts were mounted with 0,12 mm SecureSeal spacers (Grace Bio-Labs) except for experiments assessing the number of mitotic (PH3+) cells per gut. Primary antibodies were used with the following dilutions: rabbit anti-PH3 1:1000 (Milipore 06-570) and rat anti HA 1:1000 (Merck 11867423001). Secondary antibodies were used with the following dilutions: Alexa Fluor 488-conjugated goat anti-rabbit 1:1000 (Thermofisher #A-11008) and Cy3-conjugated goat anti-rat 1:1000 (Thermofisher #A-10522)
Imaging of fixed tissues
For fixed tissues, guts were imaged on an inverted Zeiss LSM-900 confocal microscope with Zen Blue software using 5x and 20x objectives. Confocal stacks were acquired with an interval of 1 μm spanning the intestinal epithelium from the base to the lumen. All images, including the ones for quantifications, were acquired either covering the entire posterior gut R4 region or single tiles of the posterior gut in region R4BC. Images were processed using Fiji and Adobe Photoshop software.
Live imaging of gut explants
To monitor ISC migration and clustering following insult, live imaging was performed on flies fed on Whatman filter paper discs with 5% sucrose solution supplemented with either PE OD200 or 5% DSS prepared as described above. The feeding window was limited to 2 h to produce restricted damage. To ensure that flies fed during this short feeding window, eriogalucine (brilliant blue R,FD& C Blue No.1 from Sigma) was added at a final concentration of 0.05% w/v. Only flies which had clearly fed as indicated by blue midguts were dissected and used for further analysis. Whole midguts were then dissected into modified Schneider’s medium as described in ref. 54. After dissection, midguts were mounted on a 35 mm coverslip imaging dish (Ibidi, 80136) and embedded in 0.5% low melting agarose prepared using modified Schneider’s medium. One hundred microlitres of medium supplemented with calcium blocker Isradipine (10 μg/ml, Sigma Aldrich, I6658) was added prior to imaging. Live movies of ISCs following insult were acquired on a Nikon spinning disc microscope with 60 × 1.2 NA objective or a Leica Dmi8 microscope coupled with ANDOR Dragonfly 200 spinning disk scanner using 25 × 0.95 NA objective and using 488 nm and 561 nm lasers. Confocal stacks were acquired with an interval of 1 μm with an interval of 10 min for 10 h.
Laser ablation
For laser ablation, live ex vivo mounted guts were imaged on a Leica SP5-X MP UV using a 40x objective. A 50 μm diameter region within the epithelium was then ablated using a 900 nm multiphoton laser (MaiTai), and the ablated gut was imaged immediately post ablation. Ablated guts were left at the incubator for recovery for 4 h. After recovery, the entire R4 region was reimaged. The number of SCs was counted within a 100 μm diameter of athe blated region at both timepoints. The number of migrated cells was then calculated as the number of stem cells at 4 h post ablation minus the number present immediately after ablation. This was then normalized to the total number of SCs within the region.
Optogenetics
OptoDronc experiment was performed by inducing OptoDronc expression in clones of ECs using the heat-shock FLP/FRT system and tracking ISC movement towards the dying ECs. The crosses for optogenetic experiments were kept in at 18 °C in foil-wrapped vials to prevent premature light activation. After eclosion, flies were kept at 18 °C for 5 days to allow midguts maturation. Mated females were then heat-shocked at 37 °C for 10 min to induce clone formation and subsequently returned to 18 °C for 2 days to allow OptoDronc expression. Flies were then dissected in live imaging media and imaged using an Andor Dragonfly 200 Spinning Disk confocal microscope with a Leica 25x water immersion objective. OptoDronc activation, and thus apoptosis, was triggered using 488 nm laser line, which was also used to visualize EC clones and ISCs. Midguts containing EC clones of 6–8 ECs were selected, and SC movements were manually tracked using the TrackMate plugin on ImageJ.
Clustering analysis
Clustering analysis was performed on flies starved and fed with sucrose solution supplemented with PE OD200 for 2 h Flies were then placed on normal food for a further 4 h before being imaged in modified Schneider’s medium as above.
Image analysis and quantifications
Image analysis and quantification were performed using the open-source FIJI software. For quantitative measurements of specific cell types per area, a z-stacks were acquired with a 20x objective in the R4bc region for each mounted gut. Acquired z-stacks were converted to maximum-intensity projections. For dlts>transtimer data, the background was subtracted in all channels using FIJI (rolling ball radius 50 pixels). Cell number was manually counted using the CellCounter Fiji plugin and normalized to the total epithelial area counted in. Cells per area is defined as cells per 10,000 μm2 in all cases.
For clustering quantification, imaged midguts were analysed using Cellpose. Acquired z-stacks were converted to maximum-intensity projections. These were then segmented using the cyto3 model. Cellpose generated masks of the cells were then manually corrected to improve the segmentation. After the finished mask was generated, it was imported into FIJI. SSIDC cluster indicator, a spatial clustering algorithm within the Biovoxxel toolbox, was then applied to the masks using an intercellular distance (epsilon) of 5 pixels, which corresponds to 2 μm.
For SC morphometric analysis following infection and PVR knockdown, masks of individual SCs were generated using Cellpose. These binary masks were then fed on to the extended particle analyzer under Biovoxxel toolbox on FIJI to obtain aspect ratio, cellular extent (ratio of cellular area to the bounding rectangle), and circularity. For measuring the mean Rac sensor GFP fluorescence intensity, MIP of confocal stacks of a volume of 5 μm from the basal-most surface of the epithelium. Ten random ROIs (100 × 100 pixels) were selected per midgut to calculate the mean sensor intensity from each midgut. Tracheal coverage was quantified as previously described in ref. 19. For cell tracking, time-lapse images were processed in FIJI. Individual timelapse movies were aligned using the Stack registration (rigid body) plugin in FIJI, to reduce movements due to VM twitching. The SCs were then manually tracked on these aligned movies using the TrackMate plugin, and mean velocity was calculated. Directionality index (DI) was calculated as the cosine of the angle (Ѳ) made by the initial position of an ISC relative to the injury site and its final position after migration. A cell migrating directly toward the injury site yields a Ѳ near 0°, resulting in a DI close to 1. Conversely, movement directly away from the injury site results in a Ѳ near 180°, giving a DI close to −1. For the control region, an arbitrary point in the middle of the midgut was selected, and the movement of ISCs relative to this point was used to calculate the corresponding DI.
RNA extraction and qPCR
Eight to ten dissected midguts per biological replicate were directly transferred into lysis buffer and flash frozen in liquid nitrogen. Total RNA was extracted using RNAeasy microkit (Qiagen) according to the manufacturer’s instructions. For cDNA synthesis, RNA was treated with DNase and reverse-transcribed using Superscript II reverse transcriptase (Invitrogen). The resulting cDNA was used for real-time RT-PCR on a QuantStudio 5 Real-Time PCR system using RealQ Plus 2x Master Mix Green (Ampliqon) with 8 ng of cDNA template. Samples were normalized to levels of ribosomal protein 49 expression levels and analyzed with QuantStudio 5 Real-Time PCR software using the delta-delta Ct method. Five to six biological replicates were used for each condition or genotype, and triplicate measurements were performed.
The following primers were used:
Pvf1_F 5′- AAT CAA CCG TGA GGA ATG CAA −3′
Pvf1_R 5′- GCA CGC GGG CAT ATA GTA GT −3′
Pvr_F 5′-GGT GCT AAA GCA AAA CGA AAG TT-3′
Pvr_R 5′-GGA TCG TGC GAT TGG CAT ATT C-3′
RP49_F 5′-TGA GCC ACC TCA TCC AAA TCA CCT-3′
RP49_R 5′-TCG ATC ACG ATG AAT GGC CGG TAA-3′
Statistics
Visualization of data in graphs and statistical analysis was performed using GraphPad Prism software. Each dataset was assessed for normal distribution using the Shapiro–Wilks normality test before analysis. Comparisons of three or more groups were analyzed by one-way ANOVA followed by Tukey’s multiple comparison tests if passing normality tests. If similar comparisons did not pass normality tests, Kruskal–Wallis test followed by Dunn’s multiple comparison tests were applied. Differences between control and one group were analyzed by Student’s t-test if passing normality tests or Mann–Whitney test if not. All error bars indicate standard deviation. Comparison of survival curves was analyzed using Mantel–Cox Log Rant test.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All source data needed to evaluate the conclusions are present in the paper as a Source Data file. Source data are provided with this paper.
References
Dekoninck, S. & Blanpain, C. Stem cell dynamics, migration and plasticity during wound healing. Nat. Cell Biol. 21, 18–24 (2019).
Gonzales, K. A. U. & Fuchs, E. Skin and its regenerative powers: an alliance between stem cells and their niche. Dev. Cell 43, 387–401 (2017).
Aragona, M. et al. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat. Commun. 8, 14684 (2017).
Park, S. et al. Tissue-scale coordination of cellular behaviour promotes epidermal wound repair in live mice. Nat. Cell Biol. 19, 155–163 (2017).
Bohm, A. M. et al. Activation of skeletal stem and progenitor cells for bone regeneration is driven by PDGFRbeta signaling. Dev. Cell 51, 236–254.e212 (2019).
Su, P. et al. Mesenchymal stem cell migration during bone formation and bone diseases therapy. Int. J. Mol. Sci. 19, https://doi.org/10.3390/ijms19082343 (2018).
Tang, Y. et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 15, 757–765 (2009).
de Lucas, B., Perez, L. M. & Galvez, B. G. Importance and regulation of adult stem cell migration. J. Cell Mol. Med. 22, 746–754 (2018).
Friedl, P., Sahai, E., Weiss, S. & Yamada, K. M. New dimensions in cell migration. Nat. Rev. Mol. Cell Biol. 13, 743–747 (2012).
Biteau, B. & Jasper, H. Slit/Robo signaling regulates cell fate decisions in the intestinal stem cell lineage of Drosophila. Cell Rep. 7, 1867–1875 (2014).
Guo, Z. & Ohlstein, B. Stem cell regulation. Bidirectional Notch signaling regulates Drosophila intestinal stem cell multipotency. Science 350, https://doi.org/10.1126/science.aab0988 (2015).
Zeng, X. & Hou, S. X. Enteroendocrine cells are generated from stem cells through a distinct progenitor in the adult Drosophila posterior midgut. Development 142, 644–653 (2015).
Micchelli, C. A. & Perrimon, N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439, 475–479 (2006).
Ohlstein, B. & Spradling, A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439, 470–474 (2006).
Biteau, B. & Jasper, H. EGF signaling regulates the proliferation of intestinal stem cells in Drosophila. Development 138, 1045–1055 (2011).
Jiang, H., Grenley, M. O., Bravo, M. J., Blumhagen, R. Z. & Edgar, B. A. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell 8, 84–95 (2011).
Liang, J., Balachandra, S., Ngo, S. & O’Brien, L. E. Feedback regulation of steady-state epithelial turnover and organ size. Nature 548, 588–591 (2017).
Lin, G., Xu, N. & Xi, R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature 455, 1119–1123 (2008).
Perochon, J. et al. Dynamic adult tracheal plasticity drives stem cell adaptation to changes in intestinal homeostasis in Drosophila. Nat. Cell Biol. 23, 485–496 (2021).
Tamamouna, V. et al. Remodelling of oxygen-transporting tracheoles drives intestinal regeneration and tumorigenesis in Drosophila. Nat. Cell Biol. 23, 497–510 (2021).
Hu, D. J., Yun, J., Elstrott, J. & Jasper, H. Non-canonical Wnt signaling promotes directed migration of intestinal stem cells to sites of injury. Nat. Commun. 12, 7150 (2021).
He, S. et al. Endothelial cells promote migration of mesenchymal stem cells via PDGF-BB/PDGFRbeta-Src-Akt in the context of inflammatory microenvironment upon bone defect. Stem Cells Int. 2022, 2401693 (2022).
Lin, R. Z. et al. Human endothelial colony-forming cells serve as trophic mediators for mesenchymal stem cell engraftment via paracrine signaling. Proc. Natl. Acad. Sci. USA 111, 10137–10142 (2014).
Massberg, S. et al. Activated platelets trigger an inflammatory response and enhance migration of aortic smooth muscle cells. Thromb. Res. 110, 187–194 (2003).
Mishima, Y. & Lotz, M. Chemotaxis of human articular chondrocytes and mesenchymal stem cells. J. Orthop. Res. 26, 1407–1412 (2008).
Nedeau, A. E. et al. A CXCL5- and bFGF-dependent effect of PDGF-B-activated fibroblasts in promoting trafficking and differentiation of bone marrow-derived mesenchymal stem cells. Exp. Cell Res. 314, 2176–2186 (2008).
Pierce, G. F. et al. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J. Cell Biol. 109, 429–440 (1989).
Pierce, G. F. et al. In vivo incisional wound healing augmented by platelet-derived growth factor and recombinant c-sis gene homodimeric proteins. J. Exp. Med. 167, 974–987 (1988).
Rajkumar, V. S. et al. Platelet-derived growth factor-beta receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healing. Am. J. Pathol. 169, 2254–2265 (2006).
Wang, S. et al. Platelet-derived growth factor receptor beta identifies mesenchymal stem cells with enhanced engraftment to tissue injury and pro-angiogenic property. Cell Mol. Life Sci. 75, 547–561 (2018).
Cho, N. K. et al. Developmental control of blood cell migration by the Drosophila VEGF pathway. Cell 108, 865–876 (2002).
Duchek, P., Somogyi, K., Jekely, G., Beccari, S. & Rorth, P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell 107, 17–26 (2001).
Heino, T. I. et al. The Drosophila VEGF receptor homolog is expressed in hemocytes. Mech. Dev. 109, 69–77 (2001).
Janssens, K., Sung, H. H. & Rorth, P. Direct detection of guidance receptor activity during border cell migration. Proc. Natl. Acad. Sci. USA 107, 7323–7328 (2010).
McDonald, J. A., Pinheiro, E. M. & Montell, D. J. PVF1, a PDGF/VEGF homolog, is sufficient to guide border cells and interacts genetically with Taiman. Development 130, 3469–3478 (2003).
Wood, W., Faria, C. & Jacinto, A. Distinct mechanisms regulate hemocyte chemotaxis during development and wound healing in Drosophila melanogaster. J. Cell Biol. 173, 405–416 (2006).
Choi, N. H., Kim, J. G., Yang, D. J., Kim, Y. S. & Yoo, M. A. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell 7, 318–334 (2008).
Bond, D. & Foley, E. Autocrine platelet-derived growth factor-vascular endothelial growth factor receptor-related (Pvr) pathway activity controls intestinal stem cell proliferation in the adult Drosophila midgut. J. Biol. Chem. 287, 27359–27370 (2012).
He, L., Binari, R., Huang, J., Falo-Sanjuan, J. & Perrimon, N. In vivo study of gene expression with an enhanced dual-color fluorescent transcriptional timer. Elife 8, https://doi.org/10.7554/eLife.46181 (2019).
Antonello, Z. A., Reiff, T., Ballesta-Illan, E. & Dominguez, M. Robust intestinal homeostasis relies on cellular plasticity in enteroblasts mediated by miR-8-Escargot switch. EMBO J. 34, 2025–2041 (2015).
Valon, L. et al. Robustness of epithelial sealing is an emerging property of local ERK feedback driven by cell elimination. Dev. Cell 56, 1700–1711.e1708 (2021).
Lane, M. E. et al. Dacapo, a cyclin-dependent kinase inhibitor, stops cell proliferation during Drosophila development. Cell 87, 1225–1235 (1996).
Fulga, T. A. & Rorth, P. Invasive cell migration is initiated by guided growth of long cellular extensions. Nat. Cell Biol. 4, 715–719 (2002).
Zhou, S. et al. Two Rac1 pools integrate the direction and coordination of collective cell migration. Nat. Commun. 13, 6014 (2022).
Bianco, A. et al. Two distinct modes of guidance signalling during collective migration of border cells. Nature 448, 362–365 (2007).
Sun, Z. et al. Basolateral protrusion and apical contraction cooperatively drive Drosophila germ-band extension. Nat. Cell Biol. 19, 375–383 (2017).
Li, H. et al. Fly cell atlas: a single-nucleus transcriptomic atlas of the adult fruit fly. Science 375, eabk2432 (2022).
Tsai, C. R. et al. Pvr and distinct downstream signaling factors are required for hemocyte spreading and epidermal wound closure at Drosophila larval wound sites. G3 12, https://doi.org/10.1093/g3journal/jkab388 (2022).
Medina, A. B. et al. Neuroendocrine control of intestinal regeneration through the vascular niche in Drosophila. Dev. Cell https://doi.org/10.1016/j.devcel.2025.06.036 (2025).
Pandey, P. et al. New insights about the PDGF/PDGFR signaling pathway as a promising target to develop cancer therapeutic strategies. Biomed. Pharmacother. 161, 114491 (2023).
Song, W. et al. Tumor-derived ligands trigger tumor growth and host wasting via differential MEK activation. Dev. Cell 48, 277–286.e276 (2019).
Forte, E. et al. EMT/MET at the crossroad of stemness, regeneration and oncogenesis: the Ying-Yang equilibrium recapitulated in cell spheroids. Cancers 9, https://doi.org/10.3390/cancers9080098 (2017).
Haller, S. et al. mTORC1 activation during repeated regeneration impairs somatic stem cell maintenance. Cell Stem Cell 21, 806–818.e805 (2017).
Marchetti, M., Zhang, C. & Edgar, B. A. An improved organ explant culture method reveals stem cell lineage dynamics in the adult Drosophila intestine. Elife 11, https://doi.org/10.7554/eLife.76010 (2022).
Acknowledgements
We are grateful to all members of the Colombani and Andersen laboratory for scientific discussion and for carefully reading the manuscript. J.C. and D.S.A. are funded by H2020 European Research Council grant number 803630, Novo Nordisk Foundation grant number NNF180C0033920, Danish Research Council grant number 4285-00064B. We thank the Carlsberg foundation for equipment grants CF19-0353 and CF23-1302. A.J. was funded by the Horizon-MSCA grant number 101109581.
Author information
Authors and Affiliations
Contributions
D.J.M., A.J., J.C., and D.S.A. designed the research, D.J.M. and A.J. conducted most experiments for the manuscript with the support of E.C., A.B.T., and M.R. supervised the initial real time imaging experiments, C.F.C., R.L, J.C., and D.S.A. performed the genetic screen that formed the basis for the Pvf1 project, D.J.M., A.J., J.C., and D.S.A. analyzed the data, J.C. and D.S.A. supervised the project, and D.S.A. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Chrysoula Pitsouli and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mackay, D.J., John, A., Christensen, C.F. et al. Pvf1-Pvr-mediated crosstalk between trachea and gut guides intestinal stem cell migration to promote gut regeneration. Nat Commun 16, 8597 (2025). https://doi.org/10.1038/s41467-025-63704-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63704-8