Abstract
The synergistic Cu0-Cuδ+ sites are found as the active sites for NH3 synthesis through nitrate electroreduction reaction, but still face significant challenges in stabilizing the Cuδ+ due to its self-reduction. Here we propose an Ohmic contact interface engineering strategy by loading copper nano-islands on indium hydroxide nanocubes. Attributed to the lower work function of Cu than that of In(OH)3 with n-type semiconductor nature, the electrons in Cu can transfer unimpededly to In(OH)3 at the interface of Ohmic junction, triggering and stabilizing polarized Cu0-Cuδ+ active sites. Cu@In(OH)3 sustains both high NH3 yield rate (4.28 mmol h−1 mgcat.−1) and Faradaic efficiency (97.35%) at −0.6 V vs. RHE, while maintaining stability for at least 120 h under an Ampere-level of 800 mA cm−2. Such Ohmic contact interface engineering approach allows for simultaneously constructing and stabilizing the Cu0-Cuδ+ for the electrosynthesis of ammonia, as well as other value-added chemicals relying on above active sites.
Similar content being viewed by others
Introduction
Ammonia is a widely utilized industrial chemical in pharmaceuticals, synthetic fibers, fertilizer manufacturing, and energy storage with significant worldwide relevance1,2,3. It is critical to the advancement of human society4. Currently, the Haber-Bosch process is the principal technique for generating ammonia, which demands high temperature (400–500 °C) and pressure (130–170 bar)5. This process uses around 1.4% of the world’s energy and accounts for roughly 1% of global CO2 emissions from energy consumption6. Producing NH3 from NO3− driven by renewable power is gaining popularity as an alternative method7,8,9,10. This technology has sparked great attention and shown promise as an eco-friendly alternative to the classic Haber-Bosch process11,12,13. Nonetheless, the electrochemical nitrate reduction reaction (NO3RR) process is a complex nine-proton coupled eight-electron transfer pathway with intricate reaction pathways and slow kinetics14,15. Exploring catalysts that can facilitate the rapid hydrogenation of nitrogen-containing intermediates is the major goal for realizing selective NH3 electrosynthesis with high Faradaic efficiency (FE).
Due to the susceptibility of Copper (Cu) sites to adsorb NO3−, originating from the similarity in the energy levels between the d-orbitals on Cu and the lowest unoccupied molecular orbital of NO3−, copper-based electrocatalysts are emerging as the most promising candidates for electrocatalytic reduction of nitrate to ammonia16,17,18,19,20. Throughout the NO3RR process, the Cu0-Cuδ+ sites are commonly recognized as the active species involved21,22,23,24. For example, CuO nanowire arrays underwent the in situ electrochemical reconstruction procedure to construct a mixed Cu/Cu2O active phase, which could act as the key component for NH3 synthesis23. Albeit the Cu0-Cuδ+ active sites can be derived from Cu2O or CuO though the abovementioned procedure, it still remains obstacles in rationally preventing the Cuδ+ from the continuous reduction into Cu0 at industrial-level current densities25.
Recently, interface engineering has attracted considerable attention because of the improvement in the catalytic performance for various reactions10,26,27,28. The precise regulation of interfacial chemistry is conducive to the modulation of geometric structure, electronic structure, interfacial bonding and/or lattice strain, which have been demonstrated to substantially promote the reaction kinetics and even durability29,30,31. Ohmic contact interface engineering, which relies on the combination of metal with semiconductor, can significantly eliminate the resistance of charge transfer in the catalysis process due to the low interfacial energy barrier32,33,34. Therefore, Ohmic contact with low energy barrier is suitable for acting as a bridge to integrate the active sites with adjustable electronic structure35,36,37. Inspired by the characteristics of Ohmic contact, the construction of an Ohmic junction by interfacing Cu metal with an appropriate semiconductor. This approach is expected to generate Cu0-Cuδ+ active sites not through in situ self-reduction but via spontaneous charge transfer. Even under negative potential conditions, the rapid charge transfer and the Fermi levels match are anticipated to address the issue of Cuδ+ reconstruction, thereby preventing the disappearance of Cu0-Cuδ+ active sites that often occurs in electrocatalytic reduction reactions.
Herein, we developed a Cu@In(OH)3 electrocatalyst through loading Cu with lower work function on the n-type semiconductor In(OH)3 nanocubes, in which the Cu0-Cuδ+ active sites were successfully triggered and stabilized due to the Ohmic contact interface engineering. Driven by the difference in the work function, the interfacial charge transfer from Cu to In(OH)3 is facilitated to maintain the presence of Cuδ+, which is evidenced by the X-ray absorption fine structure spectroscopic analysis. In such elegant catalyst, In(OH)3 not only serves as suitable n-type semiconductor to induce the polarized Cu0-Cuδ+ sites at Ohmic contact interface, but also hinder the competitive hydrogen evolution reaction (HER) process. Electron paramagnetic resonance (EPR) analysis and density functional theory (DFT) calculations elucidate that the formation of active hydrogen (*H) for the hydrogenation of *NO3 to *NH3 is promoted by low-polarization Cu0-Cuδ+, while the high-polarization Cu0-Cuδ+ at the Ohmic contact interface can absorb *NO2 efficiently for subsequent transformation into NH3. As designed Cu@In(OH)3 achieves excellent NO3RR performance, in terms of high NH3 yield rate of 4.28 mmol h−1 mgcat.−1 and FE of 97.35% at −0.6 V vs. RHE, as well as excellent stability for at least 120 h under −800 mA cm−2. This Ohmic contact interface engineering strategy provides a guidance for stabilizing the active sites, which paves the way for exploring advanced catalysts to synthesize ammonia and other indispensable chemicals.
Results
Design and characterization of catalysts
The Ohmic contact engineering is realized by matching the work functions (WF) of metal, semiconductor and the type of semiconductor (Fig. 1a). To elucidate the formation mechanism of the Ohmic contact interface in Cu@In(OH)3, initial calculations were conducted. The WF of Cu and In(OH)3 were determined to be 4.571 eV and 7.101 eV, respectively (Fig. 1b). In contrast with In(OH)3, the lower WF of Cu indicates a greater propensity for its electrons to escape the surface. Furthermore, Mott−Schottky (M − S) measurements were employed to ascertain the semiconductor type of In(OH)3. A positive slope in the M − S plot confirms its intrinsic n-type semiconducting behavior of In(OH)3 (Supplementary Fig. 1)38,39. This implies that electrons are more likely to migrate from Cu to In(OH)3 at the Cu@In(OH)3 interface. Based on the characterized work functions and the semiconductor type, a reliable band structure is illustrated for In(OH)3 (Fig. 1c). After contacting with each other, the difference in the work functions of Cu and In(OH)3 induces charge transfer and energy band bending, which hence establishes a typical Ohmic contact configuration as depicted in Fig. 1d. The accumulation of electrons at the Ohmic contact interface results in a significantly lower surface resistance than that of the semiconductor itself 40. Consequently, such interface engineering approach creates a low-barrier pathway in the Ohmic junction for migrating the electrons in Cu into In(OH)3 without being influenced by rectification effects, which might trigger the construction of polarized Cu0-Cuδ+ sites.
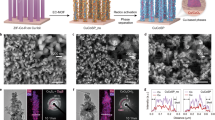
On the basis of the above theoretical prediction, we developed an Ohmic junction electrocatalyst by loading Cu on In(OH)3 (for synthesis details, please see experimental section). Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) characterization of Cu@In(OH)3 display the well-defined nano-cubes with an approximate size of 50 nm (Fig. 2a and Supplementary Fig. 2). The pristine Cu and In(OH)3 were also prepared as the counterparts (Supplementary Fig. 3). The elemental mapping presented by high-angle annular dark field scanning transmission electron microscopy (HAADF-STEM) confirms the coexistence of Cu, In, and O (Fig. 2b). The construction of Cu@In(OH)3 heterostructure was further substantiated by high-resolution transmission electron microscopy (HRTEM). The interplanar spacings of 0.39 nm and 0.18 nm can be observed on either side of the interface, corresponding to the In(OH)3 (200) and the Cu (200) planes, respectively (Fig. 2c). Selected-area electron diffraction (SAED) displays a square symmetrical pattern with clear diffraction spots of the (110) and (200) lattice planes of In(OH)3 (Supplementary Fig. 4), indexed to the [001] zone axis diffraction of In(OH)3, as well as the clear diffraction rings of the (111) and (200) lattice planes of Cu38. X-ray diffraction (XRD) analysis confirms the presence of cubic indium hydroxide and copper with no detectable impurity phases (Supplementary Figs. 5–7). Above all, these results prove the construction of Cu@In(OH)3 heterostructured catalyst with abundant interface.
a TEM image of Cu@In(OH)3. b HAADF-STEM image and corresponding elemental mapping images of Cu@In(OH)3. c HRTEM image of Cu@In(OH)3. d UPS spectra of Cu and In(OH)3 for work function and valance band edge calculation. e Cu 2p XPS spectra of Cu and Cu@In(OH)3. f Cu LMM XAES spectra of Cu and Cu@In(OH)3. g XAFS spectra of Cu foil, Cu2O, CuO, and Cu@In(OH)3. h FT-EXAFS spectra at Cu K-edge of Cu foil, Cu2O, CuO, and Cu@In(OH)3. i WT-EXAFS of Cu for Cu@In(OH)3. The color bars represent intensity with arb. units. Source data are provided as a Source Data file.
To corroborate the previously calculated work functions, which plays the determinant role in constructing Ohmic junction, the electronic structures of Cu and In(OH)3 were further examined using ultraviolet photoelectron spectroscopy (UPS). The UPS spectra reveal the metallic characteristics for Cu, as evidenced by the curve at the low-energy onset regions, whereas In(OH)3 exhibits the semiconducting properties. The work functions of Cu and In(OH)3 are determined based on the secondary electron cutoff edge (Ecut off) and the Fermi edge (Ef). The work function relative to the vacuum level are computed using the formula WF = 21.22 − |Ecut off − Ef | 35,40,41. According to the UPS results, the work functions are found to be 4.02 eV and 7.82 eV (vs. Vac.) of Cu and In(OH)3, respectively, which align with the calculated values in Fig. 1b. The UPS findings also shed light on the charge redistribution that occurs upon the interface between Cu and In(OH)3. Driven by the disparity in work functions, the electrons transfer is promoted from the metal with the lower work function to the semiconductor with the higher work function. This process continues until the Fermi levels on both sides of the heterojunction reach an equal position, which further results in the electron depletion on Cu to generate polarized Cu0-Cuδ+ near the interface of Ohmic junction37.
The chemical composition and valence states of the elements in Cu@In(OH)3 were further elucidated through X-ray photoelectron spectroscopy (XPS). As depicted in Supplementary Fig. 8, the XPS survey spectrum clearly shows the characteristic peaks for Cu 2p, In 3 d, and O 1 s. The O 1 s peak observed at 531.5 eV (Supplementary Fig. 9) belongs to the typical hydroxide group42. The high-resolution In 3 d spectra for Cu@In(OH)3 and In(OH)3 (Supplementary Fig. 10) reveal two signals at 444.8 and 452.6 eV assignable to In 3d5/2 and In 3d3/2, respectively43. Notably, the In 3d5/2 signal in Cu@In(OH)3 shifts towards lower binding energy compared to that in In(OH)3, suggesting the charge transfer at the interface. Regarding the Cu 2p spectra (Fig. 2e), the deconvoluted peak at 931.6 eV corresponds to Cu0/Cu+44. The Cu LMM X-ray Auger electron spectroscopy (XAES) fitting results (Fig. 2f) further indicate the emergence of a characteristic peak for Cu+45, which is attributed to the electron depletion after the interface engineering of Ohmic contact with In(OH)3.
To further substantiate the above findings, X-ray absorption fine structure (XAFS) measurements were conducted to probe the detailed structure of the heterostructured electrocatalyst with Ohmic contact interface engineering. X-ray absorption near-edge structure (XANES) analysis (Fig. 2g) reveals the presence of Cu+ species in Cu@In(OH)3, with an absorption energy between that of Cu foil and Cu2O references. Linear combination fitting (LCF) was employed to quantify the ratio of Cu0 and Cuδ+ based on the XANES spectrum of Cu@In(OH)3. The LCF results indicate that the ratio of Cu0 to Cuδ+ is approximately 4:1 (Table S1). Extended X-ray absorption fine structure (EXAFS) data (Fig. 2h) show a distinct peak at 2.2 Å for Cu@In(OH)3, which corresponds to the first coordination shell of Cu–Cu scattering8. Additionally, a weak peak for Cu–O bonds (1.6 Å) is observed, which manifests the anchoring of Cu to In(OH)3 via copper–oxygen bonds. Wavelet transform (WT) spectrum (Fig. 2i) further visually confirms the presence of Cu–O and Cu–Cu shell layers46,47. Indicated by the above analysis, the Ohmic contact indeed can facilitate the electron transfer from Cu to In(OH)3, which triggers the polarized Cu0-Cuδ+ site.
Electrocatalytic NO3RR evaluation
To highlight the activity of as-engineered Cu0-Cuδ+ sites, the electrochemical NO3RR performance of the Cu@In(OH)3 catalyst was evaluated using a standard three-electrode H-type cell with the 0.1 M KOH + 0.1 M KNO3 electrolyte under ambient conditions. The colorimetric method was employed to quantify NH3 and NO2− with ultraviolet-visible (UV-vis) spectrophotometry (Supplementary Figs. 11, 12)48. As shown in the linear sweep voltammetry (LSV) curves (Fig. 3a), the current density of Cu@In(OH)3 increases markedly upon the addition of 0.1 M NO3− to the electrolyte. In sharp contrast with individual Cu and In(OH)3, Cu@In(OH)3 might deliver superior NO3RR performance as reflected in its higher current densities (Supplementary Figs. 13–15). The ammonia-producing FE of Cu@In(OH)3, calculated for potentials ranging from −0.4 to −0.8 V vs. RHE (Fig. 3b), exhibit a volcanic trend, peaking at −0.6 V vs. RHE with an FE of 97.35% after 2 hours of catalysis. Such value is relatively higher than those of Cu (67.86%) and In(OH)3 (38.96%). The NH3 yield rate of Cu@In(OH)3 is calculated to be 4.28 mmol h−1 mgcat−1 at −0.6 V (Fig. 3c), outperforming the counterpart samples (Supplementary Figs. 16–18). The control experiment has also excluded the impact of carbon paper on the performance of ammonia or nitrite synthesis (Supplementary Fig. 19).
a LSV curves of Cu@In(OH)3 at a scan rate 50 mV s−1 in 0.1 M KOH with/without 0.1 M NO3−. b NH3 FEs of Cu, In(OH)3, and Cu@In(OH)3. c NH3 yield rate of Cu, In(OH)3, and Cu@In(OH)3. d in situ XANES spectra of Cu@In(OH)3. e LCF results of the corresponding XANES spectra. f FT Cu K-edge EXAFS spectra of Cu@In(OH)3 at different time. g The cyclic stability test of Cu@In(OH)3. Experimental conditions: electrocatalytic NO3RR was conducted in an H-cell separated by a Nafion 117 membrane under ambient conditions (room temperature). Catalyst loading: 0.1 mg cm−2 on TGP-H-090 carbon paper (1 × 1 cm2). Electrolyte: 30 mL of 0.1 M KOH + 0.1 M KNO3 (pH ≈ 13). The catholyte was continuously stirred at 400 rpm. All potentials are not iR corrected. Error bars denote the standard deviations calculated from three independent measurements. Source data are provided as a Source Data file.
Additionally, the main electrocatalysis products include ammonia, nitrite ions, and hydrogen gas (Supplementary Figs. 20–22). With increasing potential, nearly all nitrite ions are converted to ammonia, and the FE of hydrogen increased due to the competitive HER. And almost all the electrons may participate in the NO3RR electrocatalytic process rather than the reduction of the catalyst. Furthermore, the FE distribution of the products for pristine copper and indium hydroxide indicates that the individual components prefer the two-electron NO3−-to-NO2− process, especially for In(OH)3 (Supplementary Figs. 23 and 24). The NO2− produced by the two individual components may be transferred to the high-polarization Cu0-Cuδ+ pairs at the Ohmic interface for subsequent six-electron transfer process to realize the tandem catalysis process. The results of electrocatalytic ammonia synthesis over Cu@In(OH)3 catalysts at various NO2− concentrations demonstrate that the NH3-producing FE consistently exceeds 90% at −0.4 V vs. RHE (Supplementary Fig. 25). This indicates that Cu@In(OH)3 enables effective conversion of NO2− into ammonia with sufficient nitrite reactants. Consequently, the above proposed tandem route, from individual components to the Ohmic contact interface, should be reasonable. Nitrogen species concentrations were determined using colorimetric methods (Supplementary Fig. 26). After continuous NO3RR measurement on the Cu@In(OH)3 electrocatalyst for 10 h, the NO3− removal efficiency reach 80.6% (Supplementary Fig. 27). To confirm the origin of the products, the proton nuclear magnetic resonance (1H-NMR) analysis was implemented on ammonium after electrocatalysis in 15NO3−, 14NO2−, and 15NO3−/14NO2− mixed solutions (Supplementary Fig. 28)49. Notably, both 14NH4+ and 15NH4+ are detected in the mixed electrolyte, indicating the simultaneous reduction of nitrite and nitrate over the Cu@In(OH)3 electrocatalyst. The electrochemical active surface area (ECSA) was determined by measuring the electrochemical double-layer capacitance (Supplementary Fig. 29). The similar results exclude the contribution of ECSA in the NO3RR activity, highlighting the decisive role of polarized Cu0-Cuδ+ sites induced by the Ohmic contact in enhancing the electrocatalytic performance.
For cathodic electroreduction processes at the negative potentials, the Cu0-Cuδ+ sites are generally unstable when they are derived from copper oxides. But in this work, the polarized Cu0-Cuδ+ sites are developed by regulating the electron transfer from metallic Cu to In(OH)3 at the interface of Ohmic junction, which is totally contrary to the previous strategies relying on the dynamic reconstruction process. The presence of In(OH)3 component with a high work function can continuously accept the electrons from Cu during the electrocatalysis process, which may play a vital role in stabilizing the Cu0-Cuδ+ active sites. To verify this, the local atomic structure and chemical state of Cu in Cu@In(OH)3 during the NO3RR process were recorded by the in situ XAFS (Supplementary Fig. 30). As illustrated in Fig. 3d, the Cu K-edge XANES exhibit no significant alteration throughout the prolonged electrocatalytic ammonia synthesis process, signifying the stability of Cu0-Cuδ+ sites22. Moreover, the linear combination fitting (LCF) analysis of the Cu K-edge XANES data (Fig. 3e, Table S2) reveals that the Cuδ+ species constitute nearly 80% even after long-time electrocatalysis. In situ EXAFS analysis (Fig. 3f) further validates the XANES results, which suggests the maintenance of polarized Cu0-Cuδ+ sites. Furthermore, the structural stability of the Cu@In(OH)3 catalyst after the NO3RR was assessed using XRD and TEM. There are no significant changes in the morphology or the crystal structure of the catalyst after the electrocatalysis reaction (Supplementary Figs. 31 and 32). This stability is also reflected in the consistent electrocatalytic performance, as evidenced by repeated cyclic measurements at −0.6 V for each 2-hour cycle. Over 12 cycles, no obvious decrease in FE is observed (Fig. 3g, Supplementary Figs. 33 and 34). The maintenance of stability is primarily attributed to the Ohmic contact in the Cu@In(OH)3, which markedly mitigates charge transfer resistance by the low interfacial energy barrier. In addition, the maintenance of the Cu0-Cuδ+ sites avoid the dynamic reconstruction to consume electrons, which can actively participate in the electrocatalytic reduction reaction to guarantee the NH3 yield rate and FE. To further determine the maintenance of the Ohmic contact during the electrocatalytic NO3RR process, the work function and d-band center of the Cu@In(OH)3 were calculated with additional electrons introduced. As the number of added electrons increases, the work function of the system decreases (Table S3 and Supplementary Fig. 35). In contrast, the d-band center of copper basically remains unchanged, whereas the d-band center of indium decreases with the addition of more electrons (Table S4 and Supplementary Fig. 36). As indicated by the above results, only the indium hydroxide component in Cu@In(OH)3 will be influenced at negative potentials. The minimal impact on copper manifests the maintenance of electron flow from Cu to In(OH)3 at the Ohmic interface. These results are consistent with those of in situ XAFS analysis (Fig. 3d-e), suggesting the stability of the polarized Cu0-Cuδ+ sites. As illustrated in Table S5, Cu@In(OH)3 exhibits a competitive ammonia yield rate and FE compared with reported catalysts. This manifests that Cu@In(OH)3 can serve as a promising electrocatalyst for efficient nitrate reduction and resource recovery.
In situ spectroscopic analysis
To investigate the NO3RR reaction process and mechanism determined by the Cu0-Cuδ+ sites on Cu@In(OH)3, advanced operando SR-FTIR spectroscopy and in situ Raman spectroscopy were employed during the electrocatalytic NO3RR process (Supplementary Figs. 37, 38). In situ Raman analysis of the Cu@In(OH)3 surface reveals the persistent emergence of the peak at 1050 cm−1 assignable to NO3− upon the addition of NO3− (Fig. 4a)50. As the electrolysis time increases, the antisymmetric bending vibration of the NH3 at 1620 cm−1 rise continuously. Notably, the bands at 1528 and 1175 cm−1 correspond to the N = O stretching vibration of *HNO and the asymmetric vibration of NH2, respectively51. Additionally, the peaks at 1325 cm−1 are assigned to antisymmetric vibrations of the NO2 group in nitrate52.
a In situ Raman spectra of Cu@In(OH)3 for NO3RR test. b Infrared signals in the range of 4000–1000 cm−1 under various potentials for Cu@In(OH)3 during electrocatalytic NO3RR. c in situ DEMS signals of Cu@In(OH)3 for NO3RR test. OCP denotes open-circuit potential. Source data are provided as a Source Data file.
As recorded by the operando SR-FTIR spectra captured within the wavenumber range of 1000 to 4000 cm−1 during a negative scan from 0 to −1.0 V vs. RHE, the infrared signals trends can be clearly visualized (Fig. 4b). In the range of 3000 to 3600 cm−1, it reveals distinct spectral features corresponding to the symmetrical and asymmetrical stretching vibration modes of N − H. The infrared band at 1650 cm−1 can be indexed to the rocking mode of −NH218,53. Furthermore, the spectral bands indicative of NO2− production at approximately 1280 cm−1 and the symmetrical stretching vibration mode of N − O at around 1450 cm−1 are recognized in Fig. 4b54.
Additionally, in situ differential electrochemical mass spectrometry (DEMS) was further implemented to capture the volatile intermediates and products generated by Cu@In(OH)3 during three cycles of it measurements (−1.5 V vs. RHE; rest for 200 s, active for 200 s). Signals at m/z of 2, 14, 15, 16, 17, 30, 31 and 46 can be identified in Fig. 4c, corresponding to H2, N, NH2, NH3, NO, NOH, NO2 and NOOH intermediate products or molecular fragments, respectively55. The abovementioned molecular fragments can also be detected in the additional control experiments of isotopic labeling with K15NO3− (Supplementary Fig. 39). These clearly observed signals at different m/z values are in line with the results from the above in situ spectroscopic analysis. Accordingly, the main reaction pathway of NO3RR on Cu@In(OH)3 can be inferred as follows: *NO3 → *NO2 → *NOOH → *NO → *NOH → *N → *NH → *NH2 → *NH3. This pathway elucidates the sequential reduction of nitrate to ammonia on the Cu0-Cuδ+ sites, laying a foundation for the subsequent theoretical calculation.
Theoretical calculations and mechanistic analysis
To uncover the role of the Ohmic contact in triggering the Cu0-Cuδ+ sites and enhancing the ammonia production performance of Cu@In(OH)3, DFT calculations were conducted over Cu, In(OH)3, and Cu@In(OH)3, of which the optimized models were presented in Supplementary Fig. 40 and Supplementary Data 1. The electrostatic potential along the z-axis at the interface and the difference charge density analysis (Fig. 5a) demonstrate the charge distribution between Cu and In(OH)3. The Bader charge coloring diagram clearly shows that the copper atoms adjacent to indium hydroxide are in a state of losing electrons, indicating the presence of Cuδ+ (Fig. 5b and Table S6). As a result, the construction of polarized Cu0-Cuδ+ sites can be confirmed by the charge depletion in Cu and accumulation in In(OH)3 at the Ohmic contact interface, which is in accordance with the above theoretical analysis and experimental measurements.
a The electrostatic potential distribution along z-axis and charge density difference profile of Cu@In(OH)3. Yellow and blue colors represent electron accumulation and depletion regions. Atom color-coding: purple, In; orange, Cu; pink, O; white, H. b The Bader charge coloring diagram of Cu@In(OH)3. (red denotes positive; blue denotes negative. The atomic size order: In>Cu>O > H). c Gibbs free energy of reaction pathways of NO3RR for Cu, In(OH)3, and Cu@In(OH)3. d Gibbs free energy of nitrite desorption for Cu, In(OH)3, and Cu@In(OH)3. e Gibbs free energy of HER for Cu, In(OH)3, and Cu@In(OH)3. f *NO2 adsorption energy (Eads-NO2) and ΔG*H versus polarization of the adjacent Cu sites. Schematic illustrations of water dissociation and NO3RR on different catalysts: g Cu, h Cu@In(OH)3. Source data are provided as a Source Data file.
To highlight the contribution of the Cu0-Cuδ+ sites to the NO3RR activity, the Gibbs free energies of the intermediates were further analyzed. Figure 5c presents the diagrams of typical NO3RR processes, where the optimal adsorption configurations of the reaction intermediates are presented in Supplementary Figs. 41–43. Notably, the hydrogenation of *NO2 into *NOOH is the rate-determining step (RDS) for Cu@In(OH)3, with an extremely low energy barrier of 0.16 eV, while the pristine Cu surface exhibits the same RDS with an energy barrier of 0.54 eV. This validates that the Cu0-Cuδ+ sites can effectively facilitate the NO3RR process. As depicted in Fig. 5d, Cu@In(OH)3 with the Cu0-Cuδ+ sites enables the strongest *NO2 adsorption in stark contrast with individual Cu and In(OH)3, which conduces to the favorable *NO2 hydrogenation to produce ammonia rather than the formation of NO2− byproduct. The projected density of states (PDOS) of Cu@In(OH)3 upon the adsorption of *NO2 was further analyzed. When *NO2 is absorbed on the Cu0-Cuδ+ sites at the interface, the charge transfer between *NO2 and Cu atoms results in stronger binding with the intermediates, which is reflected in the overlap between the O 2p orbital and the Cu 3 d orbitals (Supplementary Fig. 44)56.
Significantly, the NO3RR process encompasses not only the adsorption and deoxygenation of NO3−, but also a continuous hydrogenation process. This implies that the NO3RR process is highly dependent on the active hydrogen (*H) generated via the Volmer process during water splitting57,58, which facilitates the reduction of NO3−-to-NH3 by assisting nitrogenous intermediates. To identify this, the quenching experiments were conducted with tertiary butyl alcohol (t-BuOH) as the scavenger to capture active *H59,60. With 50 mM TBA in the electrolyte (Supplementary Fig. 45), the decrease in the NH3 yield rates suggests the active *H-mediated mechanism. To further evidence the active H*-mediated mechanism and identify the specific active sites responsible for *H generation, the EPR technique was carried out61,62. Without adding NO3− in the catholyte, the sequence of DMPO-H intensities for nine typical signals with the ratio of 1:1:2:1:2:1:2:1:1, is Cu@In(OH)3 ≈ Cu > In(OH)3 (Supplementary Fig. 46). This indicates that the Cu species are more likely to produce sufficient active *H, while In(OH)3 fails in providing *H38,63,64. The above signals disappear after adding NO3− into the solution (Supplementary Figs. 47–49), indicating the consumption of active H* by the nitrogen-containing intermediates originated from the electrocatalytic NO3RR process. To further elucidate the behavior of *H during the catalytic process, we performed an in situ EPR experiment on the Cu@In(OH)3 catalyst. The almost consistent signal intensities reveal that the catalyst can continuously generate a certain amount of active hydrogen throughout the electrolysis process (Supplementary Fig. 50). Thus, the catalytic activity and stability are basically guaranteed. EPR results further affirm the active *H-mediated mechanism over Cu@In(OH)3. According to the experimental results, although individual indium hydroxide and copper indeed exhibits comparable performance in NO3RR, there catalysis mechanisms are different. Because of the high energy increase for *NO2 → *NOOH step (Fig. 5c), as-produced active hydrogen on the individual copper (non-polarized Cu sites) will be transformed into H2 at more negative potentials (Fig. 5e). This explains its low NH3-producing FE for pristine Cu (Supplementary Fig. 23). For individual In(OH)3, the *NO hydrogenation process (the RDS for NO3RR) will be suppressed owing to the insufficient *H supply (Fig. 5c, e), which results in high NO2−-producing FE and low NH3-producing FE (Supplementary Fig. 24). Due to the Ohmic contact at the interface between copper and indium hydroxide in Cu@In(OH)3, the interfacial charge transfer triggers the high-polarization Cu0-Cuδ+ pairs, which are defined on the basis of the Bader charge difference of the adjacent Cu (Fig. 5b and Table S8). Suggested by the DFT calculations, the linear scaling relationships of the adsorption energy for *NO2 intermediates and the adsorption Gibbs free energy of H atoms (ΔG*H) with the Bader charge difference are established65. In detail, as the Bader charge difference increases (the polarization degree increases), the Δ*H shows an upward trend, while the adsorption energy of *NO2 gradually declines (Tables S6–S8 and Fig. 5f). The Cu0-Cuδ+ pairs with the higher polarization degree near the interface enable strong adsorption for *NO2 intermediates to serve as the active sites for further NH3 production. Meanwhile, the copper atoms with lower polarization are more likely to provide sufficient active hydrogen for intermediates hydrogenation on the high-polarization Cu0-Cuδ+ pairs through spillover (Fig. 5h). Based on the above DFT calculations and experimental results, the high-polarization Cu0-Cuδ+ pairs play the critical role of real active sites in NO3RR. Although individual In(OH)3 has shown comparable performance like pristine Cu (Fig. 5g), it cannot serve as the highly active component because of its weaker *NO2 adsorption, higher energy barrier for *NO hydrogenation and insufficient *H supply, in sharp contrast with the high-polarization Cu0-Cuδ+ pairs at the Ohmic interface. As for Cu@In(OH)3, the NO2− produced by the two individual components may be transferred to the high-polarization Cu0-Cuδ+ pairs at the Ohmic interface for subsequent six-electron transfer process to realize the tandem catalysis process. However, the DFT calculations and experimental results have confirmed the high-polarization Cu0-Cuδ+ pairs as the real active sites at the Ohmic contact interface, which themselves enable direct eight-electron nitrate-to-ammonia process attributed to the stronger adsorption of *NO3 and *NO2 intermediates, as well as the lower energy barrier for the RDS. Unlike the state-of-the-art Cu-based catalysts66,67,68,69,70, which generally followed the tandem [two+six]-electron pathway relying on other active components or received *H from other metal-based “active hydrogen pump” substrates, as-proposed Ohmic contact interface engineering strategy endows the Cu@In(OH)3 catalyst with high- and low-polarization Cu0-Cuδ+ pairs simultaneously to lower the energy barrier for *NO2 hydrogenation and guarantee the *H supply, respectively, for facilitating the NH3 electrosynthesis through a direct eight-electron pathway. Albeit the tandem route is reasonable and should not be ruled out, in this work, the key novelties are summarized as the construction and stabilization of Cu0-Cuδ+ pairs and the definition of a general descriptor based on the Bader charge difference of the adjacent Cu pairs to explain the catalysis mechanism. To further investigate the reaction kinetics of different Cu sites, the nitrite reduction experiments were performed at different NO2− concentrations. For the pristine Cu catalyst without polarized sites, it indicates the quasi-first-order kinetics (the fitted reaction order is ~0.72) as reflected by the linear relationship between the reaction rates and the NO2− concentrations (Supplementary Fig. 51). As for Cu@In(OH)3, the reaction order is fitted as ~0.83 at low NO2− concentrations (Supplementary Fig. 52), while the corresponding value decreases to ~0.09 at high NO2− concentrations. Compared with the pristine Cu with weaker *NO2 adsorption (Fig. 5d), the reaction order transition in Cu@In(OH)3 is ascribed to its stronger *NO2 adsorption on the high-polarization Cu0-Cuδ+ sites, which enable high *NO2 coverage. More importantly, this suggests the high-polarization Cu0-Cuδ+ sites will not be poisoned by the *NO2 (or NO2−) as the reaction order is not decreased to negative value with much higher nitrite concentration. Attributed to the sufficient supply of *H on the low-polarization Cu0-Cuδ+ sites, the hydrogenation of the adsorbed *NO2 on the high-polarization Cu0-Cuδ+ sites can be promoted for ammonia synthesis, which also avoids the competitive desorption of *NO2 into the electrolyte as nitrite by-products.
Ampere-level NO3RR performance evaluation
To further investigate the industrialization potential of the catalyst, a two-electrode anion exchange membrane (AEM) flow electrolytic cell was developed, integrating the NO3RR with oxygen evolution reaction (OER) to enhance energy utilization efficiency. In this AEM flow electrolytic cell, Cu@In(OH)3 served as the cathode, while commercial Ni foam acted as the anode. The anodic electrolyte consisted of 1.0 M KOH, and the cathodic electrolyte contained 1.0 M KOH and 1 M NO3−. A detailed scheme of the two-electrode flow cell device is presented in Fig. 6a, b. The LSV curves in Fig. 6c demonstrate that the addition of 1 M NO3− significantly reduced the voltage required to achieve an industrial-grade current density of 1 A cm−2 to only 2.00 V, a remarkable decrease of 500 mV. To assess the ammonia production performance with this device, a transformation experiment was conducted using 200 mL of electrolyte, with periodic updates every 24 hours. As depicted in Fig. 6d, at a low cell voltage of −2.03 V, the NO3− concentration decreases progressively during electrolysis, along with a reduction in current density from 1.2 A·cm−2 to 0.8 A·cm−2. Notably, the current density will return to a consistent level once the electrolyte is refreshed. Attributed to the stabilized Cu0-Cuδ+ in the catalyst, the device can be continuously operated for 120 hours at a current density of 1.2 ~ 0.8 A cm−2. We further implemented the XPS and TEM characterizations on the Cu@In(OH)3 after the high-current test. As displayed in the In 3 d spectra, only the In3+ 3d5/2 and 3d3/2 signals can be observed at 444.0 and 453.0 eV, respectively, while no peaks of loss features for In metal emerges at higher binding energy (Supplementary Fig. 53). It indicates that indium hydroxide is not reduced to the indium metal. The TEM images after the reaction also support the above conclusion. The Cu@In(OH)3 maintains a uniform cubic morphology, of which the interplanar spacings of In(OH)3 also can be clearly observed (Supplementary Fig. 54). Furthermore, The in situ Raman was performed on Cu@In(OH)3 to prove the stability of the indium hydroxide component. As the electrolysis time increases, the characteristic Raman peaks of In(OH)3 at 207 cm−1, 306 cm−1, 353 cm−1, 387 cm−1, and 652 cm−1 remain virtually unchanged without broadening or disappearance (Supplementary Figs. 55–57)71,72,73. This consistency in the Raman spectra indicates that the In(OH)3 component maintains its structural integrity and stability throughout the electrolysis process. All above results confirm the stability of indium hydroxide after the high-current reduction, which plays the determinant role in stabilizing the Cu0-Cuδ+ active sites. As illustrated in Fig. 6e, the NH3 production rate and corresponding FE of the Cu@In(OH)3 catalyst remain stable even after 120 hours. This demonstrates the potential of the Cu@In(OH)3 catalyst for practical industrial applications, offering a promising approach for efficient nitrate reduction and ammonia synthesis in an integrated electrochemical system. Apart from the issue of catalyst stability, maintaining high performance at industrial-scale current densities, reducing energy consumption, ensuring reaction selectivity, and overcoming system integration challenges are all critical issues. The integration of electrocatalytic ammonia synthesis systems with existing industrial processes and infrastructure also poses significant challenges, such as heat management and mass transport limitations. To overcome these limitations, further research and development are required to improve the catalyst durability, optimize the reaction conditions, design achievable systems, and address the technical and economic challenges associated with large-scale electrocatalytic ammonia production.
a, b Scheme and digital photo of the electrolyzer coupling NO3RR with OER. c The LSV curves of overall water splitting systems and NO3RR//OER paired-electrolysis system (scan rate 100 mV s−1 in 1 M KOH with/without 1 M NO3−). d Stability measurement of NO3RR//OER paired-electrolysis system at the industrial current density. e Time-dependent Faradaic efficiency and yield rate of NH3. (AEM flow electrolytic cell separated by a 2 × 2 cm2 Nafion 117 membrane, ambient conditions, 0.1 mg cm−2 catalyst mass loading: on 2 × 2 cm2 TGP-H-090 carbon paper, 200 mL of 1 M KOH + 1 M KNO3, flow rate: 10 mL min−1.) Source data are provided as a Source Data file.
Discussion
In summary, we developed a Cu@In(OH)3 heterostructured catalyst through Ohmic contact interface engineering, which triggered and stabilized the Cu0-Cuδ+ sites for ammonia electrosynthesis from nitrate. The Cu@In(OH)3 achieves a high NH3 yield rate of 4.28 mmol h−1 mgcat.−1 with the FE of 97.35% at −0.6 V vs. RHE. The electrocatalytic NO3RR can stably proceed for 120 h under an Ampere-level current density of 800 mA cm−2. The n-type semiconductor In(OH)3 with high work function played the determinant role in forming Ohmic contact interface and stabilizing Cu0-Cuδ+, which was comprehensively understood by theoretical calculations and in situ XAFS analysis. The Cu0-Cuδ+ sites near the Ohmic interface have exhibited high polarization degree for strong adsorption of *NO2, of which the hydrogenation process was assisted by sufficient *H stemmed from the low-polarization Cu0-Cuδ+ sites far from such interface. Advanced in situ techniques, including operando SR-FTIR spectroscopy, in situ Raman spectroscopy, and in situ DEMS, along with DFT calculations, revealed the reaction mechanism and highlighted the decisive role of the Ohmic contact in improving the catalysis performance. This work not only provides fresh understanding into stabilizing the Cu0-Cuδ+ sites for ammonia electrosynthesis, but also suggests an appealing strategy for developing catalysts based on this interface chemistry.
Methods
Materials synthesis
All chemical reagents used in this study were purchased from Shanghai Aladdin Chemical Reagent Co., Ltd. and utilized without further purification. For the isotope-labelling experiment, K15NO3 ( ≥ 99 atom%) was specifically employed.
The Cu@In(OH)3 catalyst was synthesized via a one-step hydrothermal method. Initially, 1.0 mmol of In(NO3)3·xH2O and 1.0 mmol of Cu(NO3)2·3H2O were dissolved in 10 mL of ethylene glycol under continuous stirring to form solution A. Subsequently, 2 g of NaOH was dissolved in 5 mL of H2O to prepare solution B, which was then mixed with solution A after complete dissolution of NaOH. The resulting precursor solution was transferred into a 25 mL Teflon-lined stainless-steel autoclave and maintained at 200 °C for 24 hours in an oven.
For the synthesis of pure Cu and In(OH)3, the procedure was similar to that of Cu@In(OH)3. However, pure Cu was obtained without In(NO3)3·xH2O, while pure In(OH)3 was synthesized without adding Cu(NO3)2·3H2O.
Material characterization
The surface morphology of the samples was characterized using a Supra 55 Zeiss field emission scanning electron microscope (SEM) and a Tecnai G2 F30 transmission electron microscope (TEM) operating at an accelerating voltage of 300 kV. X-ray diffraction (XRD) was obtained using a Bruker D8 Advance diffractometer with a Cu Kα radiation source (λ = 0.15406 nm). The chemical state of the catalysts was examined using a Thermo SCIENTIFIC ESCALAB 250Xi X-ray photoelectron spectroscope (XPS) with Al Kα radiation. Ultraviolet photo-electron spectroscopy (UPS, Thermo SCIENTIFIC Nexsa, Hel (21.22 eV)) was used to characterize the work function and valence band position. The specific surface area and pore structure were analyzed by the Brunauer–Emmett–Teller (BET) nitrogen adsorption-desorption isotherms measured on an ASAP 2460 Micromeritics Tristar II instrument. Electron paramagnetic resonance (EPR) spectroscopy was performed using an EPR 200 M spectrometer (Guoyi Quantum Technology (Hefei) Co., LTD). Ultraviolet-visible (UV-Vis) absorption spectroscopy was recorded using a HITACHI UH5300 spectrophotometer. The Cu K-edge X-ray absorption fine structure (XAFS) spectroscopy was conducted at the 14 W beamline of the Shanghai Synchrotron Radiation Facility (SSRF).
Electrochemical tests
Electrochemical studies were conducted using a Biologic VMP-3 electrochemical workstation. Typically, 10 mg catalyst was dispersed in a solution containing 1800 μL ethanol and 200 μL Nafion solution by sonication for at least 30 min to form catalyst ink. This ink (20 μL) was evenly sprayed onto carbon paper (TGP-H-090) to serve as the working electrode and then dried under ambient conditions. The mass loading of the catalyst on the carbon paper was 0.1 mg cm−2.
The NO3RR test was performed in a three-electrode setup within an H-type electrolytic cell (30 Ml, Gaoss Union) separated by a Nafion 117 ion exchange membrane (DuPont N117, thickness of 183 µm). The prepared catalyst-coated carbon paper, Hg/HgO (filled in 1 M KOH solution, Gaoss Union), and Pt plate (1 × 1 cm2, Gaoss Union) were used as the working, reference, and counter electrodes, respectively. The electrolyte consisted of a 30 mL solution of 0.1 M KOH and 0.1 M KNO3. The 0.1 M KOH electrolyte was prepared by dissolving 0.1 mol of KOH in 1 L of deionized water under stirring until complete dissolution. After cooling to room temperature, the solution was transferred to a plastic container and stored under ambient conditions. To obtain the mixed electrolyte (0.1 M KOH with KNO3), the same procedure was followed, with the addition of a predetermined amount of KNO3. The working electrode area was maintained at 1 cm2, and the electrolyte in the cathode chamber was continuously stirred at a rate of 400 rpm throughout the experiment. The reference electrode was converted to RHE according to the Nernst equation:
The double-layer capacitance (Cdl) was determined from cyclic voltammograms recorded at different scan rates (20, 40, 60, 80, and 100 mV s−1) in 0.1 M KOH containing 0.1 M KNO3. Measurements were conducted within the non-Faradaic potential window (0.0-0.1 V vs. RHE). The obtained Cdl values were then used to estimate the electrochemically active surface area (ECSA) according to the following relation:
Determination of NH3
The produced NH3 was quantified using the indophenol blue method, as previously reported48. Specifically, the diluted electrolyte (50-100 times 2 mL) was mixed with a solution containing salicylic acid (5 wt%) and sodium citrate (5 wt%) in NaOH (2 mL), followed by the addition of NaClO (1 mL) and Na[Fe(NO)(CN)5] (0.2 mL) solution consecutively. After allowing sufficient time for color development (at least 2 hours), the absorbance of the mixture at 655 nm was measured using UV-vis spectroscopy in the range of 500 to 800 nm. A calibration curve for concentration-absorbance was established using a series of standard ammonium sulfate solutions. Background correction was performed by subtracting the absorbance of NH3-free samples from the peak absorbances of all tested samples.
The Faradaic efficiency (FE) and yield rate were calculated based on the standard curve using the following formulas:
Where cNH3 represents the concentration of the NH3 production (mg mL−1), n is the transfer electron number, V represents the volume of electrolyte (30 mL), t represents the electrolysis time (2 h), and mcat represents mass of catalysts on the working area of cathode (0.1 mg). F represents the Faraday constant (96485 C mol−1), and Q represents the total charge transfer during electrolysis.
Determination of NO2 –
The Griess test was employed to determine the NO2 content in the electrolytes following chronoamperometry tests48. Typically, the Griess reagent was prepared by dissolving N-(1-naphthyl)ethylenediamine dihydrochloride (0.1 g), p-aminobenzene sulfonamide (2 g), and H3PO4 (5 mL) in DI water (25 mL). The diluted electrolyte (n times, 5 mL) was mixed with the Griess reagent (100 μL) and allowed to rest for approximately 10 minutes at room temperature before conducting UV-vis spectroscopy. The absorbance at around 540 nm on the UV-vis curves (400–650 nm) was used to quantify the NO2 concentration in the electrolyte. A calibration curve was generated using several aqueous solutions of KNO2 as reference samples.
Determination of NO3 –
A specific volume of electrolyte was withdrawn from the cell and diluted to bring it within the detectable range. Then, 20 μL of 0.8 w% sulfamic acid and 0.2 mL of 1 M HCl were added, followed by thorough mixing and a 20-min incubation at room temperature. Absorbance was measured at 220 and 275 nm using a UV-Vis spectrophotometer. The absorbance value was calculated as A = A220 nm - 2A275 nm. A calibration curve was generated using standard KNO3 solutions with known NO3−-N concentrations.
Determination of H2 gas
Gaseous products during the nitrate reduction reaction were quantified by online gas chromatography (GC 9790 II, Fuli analytical instrument Co, Ltd., China) equipped with a flame ionization detector (FID) and a thermal conductivity detector (TCD). The Faradaic efficiency (FE) was calculated based on the standard curve using the following formulas:
15N isotope-labeling experiment
Isotope-labeling nitrate reduction experiments were performed using a Bruker 400 MHz Nuclear Magnetic Resonance (NMR) System. For these experiments, 0.1 M K15NO3 or/and K14NO2 was used as the nitrogen source, while 0.1 M KOH served as the electrolyte. Following the electroreduction process, the resulting electrolytes containing 15NH4+ or/and 14NH4+ were collected and adjusted to a weakly acidic condition using H2SO4. Subsequently, 50 µL of deuterium oxide (D2O) was added to 0.5 mL of the adjusted solution for NMR detection.
Mott−Schottky measurements
The Mott-Schottky (M − S) experiments were performed using an Autolab PGSTAT204 in a three-electrode configuration, which included a working electrode, a Pt foil as the counter electrode, and an Hg/HgO as the reference electrode. The potential range was set from −2.5 V to 0 V versus Hg/HgO, and the frequency was varied from 2000 Hz to 4000 Hz.
In situ XAFS measurements
In this investigation, in situ X-ray absorption fine structure (XAFS) measurements at the Cu K-edge were carried out using a laboratory-based spectrometer (RapidXAFS 2 M, Anhui Absorption Spectroscopy Analysis Instrument Co., Ltd.). The X-rays were produced from a Mo target source operated at 20 kV and 20 mA. Energy selection was achieved with a Cu (533) spherically bent crystal analyzer (SBCA, 500 mm radius of curvature), which provided a diffraction geometry close to 90° backscattering at the Cu K-edge. After monochromatization, the beam was transmitted through the sample and detected by a high-resolution silicon drift detector (SDD) to record the intensity. All XAFS spectra were collected in transmission mode. The absorption edge position (E0) was calibrated against a Co foil standard, and the entire dataset was acquired within a single measurement session.
Operando SR-FTIR measurements
The operando SR-FTIR experiments were conducted using a custom-fabricated three-electrode electrochemical cell. A platinum wire functioned as the counter electrode, while a saturated Ag/AgCl electrode was utilized as the reference electrode. The Cu@In(OH)3 was deposited onto an Au-coated silicon prism to facilitate the measurements. Spectral data were collected by averaging 64 scans at a resolution of 4 cm−1 using a Bruker IFS 66 V spectrometer equipped with a liquid nitrogen-cooled mercury cadmium telluride (LN-MCT) detector. The background spectrum was acquired under open-circuit voltage conditions, and subsequent spectra were recorded stepwise by systematically varying the applied voltage.
In situ Raman measurements
In situ Raman spectroscopy was conducted using Renishaw’s inVia Raman microscopes with a 532 nm laser source in a custom cell designed for the experiment. The working electrode (1 × 1 cm²) was immersed in the electrolyte solution consisting of 0.1 M KOH and 0.1 M KNO3. The microscope’s lens was positioned perpendicular to the laser to ensure optimal interaction. A long-focus objective with 8 mm focal length and 50x magnification was used to minimize the risk of corrosion during the experiment. Silver/silver chloride/saturated potassium chloride (Ag/AgCl/saturated KCl) served as the reference electrode, while a platinum wire functioned as the counter electrode. The electrochemical measurements at −0.6 V vs. RHE were performed intermittently, and in situ Raman experiments were carried out continuously throughout the procedure.
In situ DEMS measurements
In situ DEMS measurements were employed to detect reaction intermediates using differential electrochemical mass spectrometry (PM-DEMS, Shanghai Jing-protech Co., LTD). The in situ DEMS experiments were conducted in a custom-designed cell containing 10 mL of electrolyte (0.1 M KOH + 0.1 M KNO3). Prior to measurement, Ar gas was introduced into the electrolyte at a flow rate of 10 mL min−1 to purge air until baseline stabilization. Carbon paper loaded with the catalyst served as the working electrode, while Ag/AgCl/saturated KCl and a graphite rod were used as the reference and counter electrodes, respectively. Chronoamperometry measurements (−1.5 V vs. RHE) were performed intermittently (rest for 200 s, active for 200 s). Each test was concluded when the mass signal returned to the baseline, after which the next test was initiated under identical conditions. This DEMS measurement consisted of three cycles. Additional control experiments of isotopic labeling with 15NO3− are consistent with the above. Just replaced KNO3 with K15NO3.
Computational details
The spin-polarized density functional theories (DFT) were carried out by using the Vienna Ab initio Simulation Package (VASP)74. The Perdew-Burke-Ernzerhof (PBE) generalized-gradient approximation (GGA) functional was employed to describe electron interactions75. To address the GGA functional’s limitations in describing van der Waals interactions, Grimme’s D3 correction was utilized76. An energy cutoff of 450 eV was set to ensure computational accuracy. A Monkhorst-Pack k-point mesh of 3 × 3 × 1 was used for standard calculations, while for density of states (DOS) calculations, this was increased to 5 × 5 × 1 to improve resolution. A vacuum region of 15 Å along the z-axis was established to prevent interactions between adjacent surfaces. During simulations, the atomic positions of the bottom layer were kept fixed. Bader charge analysis was applied to examine the behavior of electronic charge transfer on the outer surface atoms77,78.
The reaction Gibbs free energy (ΔG) is defined as ΔG = ΔE + ΔEZPE - TΔS,
ΔE is the reaction energy, ΔEZPE is zero-point energies, T is the temperature (298.15 K), ΔS is the difference in entropy from vibrational frequency calculations. The zero point can also contain insignificant internal energy changes, which are uniformly corrected and processed by the VASPKIT79. The entropy of gas phase N2, H2, NH3 is obtained from the NIST database with standard condition80.
Data availability
All data that support the findings in this paper are available within the article and its Supplementary Information. Source data are provided in this paper. The computational structures are available on the open-source repository (https://iochem-bd.bsc.es/create/zul/main.zul). Source data are provided with this paper.
References
Erisman, J. W., Sutton, M. A., Galloway, J., Klimont, Z. & Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 1, 636–639 (2008).
Schlögl, R. Catalytic synthesis of ammonia—a “never-ending story”?. Angew. Chem. Int. Ed. 42, 2004–2008 (2003).
Zhang, C. et al. Enabling unconventional “alternating-distal” N2 reduction pathway for efficient ammonia electrosynthesis. Angew. Chem. Int. Ed. 64, e202502957 (2025).
MacFarlane, D. R. et al. A roadmap to the ammonia economy. Joule 4, 1186–1205 (2020).
van Langevelde, P. H., Katsounaros, I. & Koper, M. T. M. Electrocatalytic nitrate reduction for sustainable ammonia production. Joule 5, 290–294 (2021).
Wang, M. et al. Can sustainable ammonia synthesis pathways compete with fossil-fuel based Haber–Bosch processes?. Energ. Environ. Sci. 14, 2535–2548 (2021).
Wang, Y., Wang, C., Li, M., Yu, Y. & Zhang, B. Nitrate electroreduction: mechanism insight, in situ characterization, performance evaluation, and challenges. Chem. Soc. Rev. 50, 6720–6733 (2021).
Li, P. et al. Pulsed nitrate-to-ammonia electroreduction facilitated by tandem catalysis of nitrite intermediates. J. Am. Chem. Soc. 145, 6471–6479 (2023).
Chen, G.-F. et al. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy 5, 605–613 (2020).
Chen, F.-Y. et al. Efficient conversion of low-concentration nitrate sources into ammonia on a Ru-dispersed Cu nanowire electrocatalyst. Nat. Nanotechnol. 17, 759–767 (2022).
Zhou Y., Fu X., Chorkendorff I., Nørskov J. K. Electrochemical ammonia synthesis: the energy efficiency challenge. ACS Energy Lett. 10, 128-132 (2024).
Chen, W. et al. Emerging applications, developments, prospects, and challenges of electrochemical nitrate-to-ammonia conversion. Adv. Funct. Mater. 33, 2300512 (2023).
Xiong, Y. et al. Electrochemical nitrate reduction: ammonia synthesis and the beyond. Adv. Mater. 36, 2304021 (2024).
Liu, J.-X., Richards, D., Singh, N. & Goldsmith, B. R. Activity and selectivity trends in electrocatalytic nitrate reduction on transition metals. ACS Catal. 9, 7052–7064 (2019).
Liu, J. et al. Electrocatalytic upgrading of nitrogenous wastes into value-added chemicals: A review. Mater. Today 73, 208–259 (2024).
Zhang, S. et al. Fe/Cu diatomic catalysts for electrochemical nitrate reduction to ammonia. Nat. Commun. 14, 3634 (2023).
Liao, W. et al. Sustainable conversion of alkaline nitrate to ammonia at activities greater than 2 A cm−2. Nat. Commun. 15, 1264 (2024).
Zhang, B. et al. Defect-induced triple synergistic modulation in copper for superior electrochemical ammonia production across broad nitrate concentrations. Nat. Commun. 15, 2816 (2024).
Tang, S. et al. General synthesis of high-entropy single-atom nanocages for electrosynthesis of ammonia from nitrate. Nat. Commun. 15, 6932 (2024).
Wang, Y. et al. Ammonia electrosynthesis from nitrate using a stable amorphous/crystalline dual-phase Cu catalyst. Nat. Commun. 16, 897 (2025).
Shen, Z. et al. Cu/Cu+ Synergetic Effect in Cu2O/Cu/CF Electrocatalysts for Efficient Nitrate Reduction to Ammonia. ACS Sustain. Chem. Eng. 11, 9433–9441 (2023).
Bai, L. et al. Electrocatalytic nitrate and nitrite reduction toward ammonia using Cu2O nanocubes: active species and reaction mechanisms. J. Am. Chem. Soc. 146, 9665–9678 (2024).
Wang, Y., Zhou, W., Jia, R., Yu, Y. & Zhang, B. Unveiling the activity origin of a copper-based electrocatalyst for selective nitrate reduction to ammonia. Angew. Chem. Int. Ed. 59, 5350–5354 (2020).
Zhang, J. et al. Evidence for distinct active sites on oxide-derived Cu for electrochemical nitrate reduction. J. Am. Chem. Soc. 146, 30708–30714 (2024).
Yoon, A. et al. Revealing catalyst restructuring and composition during nitrate electroreduction through correlated operando microscopy and spectroscopy. Nat. Mater. 24, 762–769 (2025).
Zhao, G., Li, P., Cheng, N., Dou, S. X. & Sun, W. An Ir/Ni(OH)2 heterostructured electrocatalyst for the oxygen evolution reaction: breaking the scaling relation, stabilizing iridium(V), and beyond. Adv. Mater. 32, 2000872 (2020).
Zhao, G., Rui, K., Dou, S. X. & Sun, W. Heterostructures for electrochemical hydrogen evolution reaction: a review. Adv. Funct. Mater. 28, 1803291 (2018).
Gao, W. et al. Alloying of Cu with Ru enabling the relay catalysis for reduction of nitrate to ammonia. Adv. Mater. 35, 2202952 (2023).
Lao, M. et al. Platinum/nickel bicarbonate heterostructures towards accelerated hydrogen evolution under alkaline conditions. Angew. Chem. Int. Ed. 58, 5432–5437 (2019).
Zhang, J., Zhang, Q. & Feng, X. Support and interface effects in water-splitting electrocatalysts. Adv. Mater. 31, 1808167 (2019).
Wu, G. et al. In-plane strain engineering in ultrathin noble metal nanosheets boosts the intrinsic electrocatalytic hydrogen evolution activity. Nat. Commun. 13, 4200 (2022).
Zhang, S. et al. Piezoelectricity regulated ohmic contact in M/BaTiO3 (M = Ru, Pd, Pt) for charge collision and hydrogen free radical production in ammonia electrosynthesis. Mater. Today 66, 17–25 (2023).
Li, Z. et al. Metal-semiconductor oxide (WO3@W) induces an efficient electro–photo synergistic catalysis for MOR and ORR. Chem. Eng. J. 414, 128814 (2021).
Zhang, J.-J. et al. Constructing Ohmic contact in cobalt selenide/Ti dyadic electrode: the third aspect to promote the oxygen evolution reaction. Nano Energy 39, 321–327 (2017).
Yang, Y. et al. Hierarchical ohmic contact interface engineering for efficient hydrazine-assisted hydrogen evolution reaction. Adv. Mater. 36, 2307979 (2024).
Xu, H., Li, J. & Chu, X. Interfacial built-in electric-field for boosting energy conversion electrocatalysis. Nanoscale Horiz. 8, 441–452 (2023).
Chen, Z. et al. Work function-guided electrocatalyst design. Adv. Mater. 36, 2401568 (2024).
Lv, C. et al. Selective electrocatalytic synthesis of urea with nitrate and carbon dioxide. Nat. Sustain. 4, 868–876 (2021).
Xie, Q. et al. Copper-indium hydroxides derived electrocatalysts with tunable compositions for electrochemical CO2 reduction. J. Energy Chem. 63, 278–284 (2021).
Guo, P. et al. Heterojunction-induced rapid transformation of Ni3+/Ni2+ sites which mediates urea oxidation for energy-efficient hydrogen production. Adv. Mater. 36, 2311766 (2024).
Hou, Y. et al. A generic interface to reduce the efficiency-stability-cost gap of perovskite solar cells. Science 358, 1192–1197 (2017).
Yan, Q. et al. Enhancing Compatibility of Two-Step Tandem Catalytic Nitrate Reduction to Ammonia Over P-Cu/Co(OH)2. Adv. Mater. 36, 2408680 (2024).
Huang, Y. et al. Pulsed electroreduction of low-concentration nitrate to ammonia. Nat. Commun. 14, 7368 (2023).
Wang, R. et al. Tuning the acid hardness nature of Cu catalyst for selective nitrate-to-ammonia electroreduction. Angew. Chem. Int. Ed. 64, e202425262 (2025).
Liu, K. et al. Thermally enhanced relay electrocatalysis of nitrate-to-ammonia reduction over single-atom-alloy oxides. J. Am. Chem. Soc. 146, 7779–7790 (2024).
Qian, K. et al. Elucidating the strain–vacancy–activity relationship on structurally deformed Co@CoO nanosheets for aqueous phase reforming of formaldehyde. Small 17, 2102970 (2021).
Gong, S. et al. Electronic modulation of a single-atom-based tandem catalyst boosts CO2 photoreduction to ethanol. Energ. Environ. Sci. 16, 5956–5969 (2023).
Syal, B., Kumar, P. & Gupta, P. Recent advancements in the preparation and application of copper single-atom catalysts. ACS Appl. Nano Mater. 6, 4987–5041 (2023).
Wang, K. et al. Intentional corrosion-induced reconstruction of defective NiFe layered double hydroxide boosts electrocatalytic nitrate reduction to ammonia. Nat. Water 1, 1068–1078 (2023).
Niu, Z., Fan, S., Li, X., Duan, J. & Chen, A. Interfacial engineering of CoMn2O4/NC induced electronic delocalization boosts electrocatalytic nitrogen oxyanions reduction to ammonia. Appl. Catal. B Environ. 322, 122090 (2023).
Fan, Z. et al. Interfacial electronic interactions promoted activation for nitrate electroreduction to ammonia over Ag-modified Co3O4. Angew. Chem. Int. Ed. 63, e202410356 (2024).
Majhi K. C., et al. In-tandem electrochemical reduction of nitrate to ammonia on ultrathin-sheet-assembled iron–nickel alloy nanoflowers. Angew. Chem. Int. Ed. 64, e202500167 (2025).
Yao, Y., Zhu, S., Wang, H., Li, H. & Shao, M. A spectroscopic study on the nitrogen electrochemical reduction reaction on gold and platinum surfaces. J. Am. Chem. Soc. 140, 1496–1501 (2018).
Chen, X., Cheng, Y., Zhang, B., Zhou, J. & He, S. Gradient-concentration RuCo electrocatalyst for efficient and stable electroreduction of nitrate into ammonia. Nat. Commun. 15, 6278 (2024).
Liu, L., Zheng, S.-J., Chen, H., Cai, J. & Zang, S.-Q. Tandem nitrate-to-ammonia conversion on atomically precise silver nanocluster/MXene electrocatalyst. Angew. Chem. Int. Ed. 63, e202316910 (2024).
Zhang, P. et al. Unlocking bimetallic active centers via heterostructure engineering for exceptional phosphate electrosorption: internal electric field-induced electronic structure reconstruction. Environ. Sci. Technol. 58, 2112–2122 (2024).
Fan, K. et al. Active hydrogen boosts electrochemical nitrate reduction to ammonia. Nat. Commun. 13, 7958 (2022).
Zhou, J. et al. Regulating active hydrogen adsorbed on grain boundary defects of nano-nickel for boosting ammonia electrosynthesis from nitrate. Energ. Environ. Sci. 16, 2611–2620 (2023).
Cao, Y. et al. Metal–organic framework derived low-crystallinity cobalt–nitrogen–carbon electrocatalysts for nitrate reduction to ammonia. J. Mater. Chem. A 12, 30409–30419 (2024).
Zhang C., et al. Promoting the intermediates hydrogenation for urea electrosynthesis over an “active hydrogen pump” catalyst. Angew. Chem. Int. Ed., e202507869.
Zheng, S.-J. et al. Unveiling ionized interfacial water-induced localized H* enrichment for electrocatalytic nitrate reduction. Angew. Chem. Int. Ed. 64, e202413033 (2025).
Yu, J. et al. Influence of active hydrogen on pathway selection in electrochemical nitrate reduction. eScience 5, 100350 (2025).
Lv, C. et al. Emerging p-block-element-based electrocatalysts for sustainable nitrogen conversion. ACS Nano 16, 15512–15527 (2022).
Lv, C. et al. A defect engineered electrocatalyst that promotes high-efficiency urea synthesis under ambient conditions. ACS Nano 16, 8213–8222 (2022).
Zhou, Y. et al. Long-chain hydrocarbons by CO2 electroreduction using polarized nickel catalysts. Nat. Catal. 5, 545–554 (2022).
Hu, Q. et al. Ammonia electrosynthesis from nitrate using a ruthenium–copper cocatalyst system: a full concentration range study. J. Am. Chem. Soc. 146, 668–676 (2024).
Yu, W. et al. Laser-controlled tandem catalytic sites of CuNi alloys with ampere-level electrocatalytic nitrate-to-ammonia reduction activities for Zn–nitrate batteries. Energ. Environ. Sci. 16, 2991–3001 (2023).
Kang, B., Xu, B., Chen, Z., Li, F. & Wang, Y. Promoting active hydrogen supply for kinetically matched tandem electrocatalytic nitrate reduction to ammonia. Appl. Catal. B Environ. Energy 360, 124528 (2025).
Fu, Y. et al. Enhancing electrochemical nitrate reduction to ammonia over cu nanosheets via facet tandem catalysis. Angew. Chem. Int. Ed. 62, e202303327 (2023).
Li, Z. et al. Boosting ammonia electrosynthesis via interfacial tandem nitrate reduction enabled by an amorphous@crystalline electrocatalyst. Mater. Today 85, 49–59 (2025).
Yang, J., Frost, R. L. & Martens, W. N. Thermogravimetric analysis and hot-stage Raman spectroscopy of cubic indium hydroxide. J. Therm. Anal. Calorim. 100, 109–116 (2010).
Ma, L. et al. Operando unraveling the dynamic correlation between structure evolution and performance shift during CO2 electroreduction on In2O3 electrodes. Appl. Catal. B Environ. 369, 125153 (2025).
Li B., et al. Engineering crystalline/amorphous interfaces for enhanced CO2 electroreduction. Angew. Chem. Int. Ed., e202509502.
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Tang, W., Sanville, E. & Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens Matter 21, 084204 (2009).
Bader, R. F. W. A quantum theory of molecular structure and its applications. Chem. Rev. 91, 893–928 (1991).
Wang, V., Xu, N., Liu, J.-C., Tang, G. & Geng, W.-T. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 267, 108033 (2021).
Johnson R. NIST 101. Computational Chemistry Comparison and Benchmark Database. CCCBDBDComputational Chemistry Comparison and Benchmark Database (1999).
Acknowledgements
C.L. acknowledges the National Natural Science Foundation of China (52302231 and 22479034), and Key Research & Development Plan of Heilongjiang Province (2024ZXJ03C06). X.Z. acknowledges the National Natural Science Foundation of China (21871066). C.Y. acknowledges the National Natural Science Foundation of China (52101246) and the Natural Science Foundation of Heilongjiang Province, China (YQ2022B006). G.Y. acknowledges the support by the Welch Foundation Award F-1861 and the Camille Dreyfus Teacher-Scholar Award. We thank the BL14W1 beamline of Shanghai Synchrotron Radiation Facility (SSRF). We thank the staff members of the Infrared spectroscopy and microspectroscopy Beamline (https://cstr.cn/31131.02.HLS.IRSM) at the Hefei Light Source (https://cstr.cn/31131.02.HLS), for providing technical support and assistance in data collection and analysis. We thank the Anhui Absorption Spectroscopy Analysis Instrument Co, Ltd. for XAFS measurements and analysis. Thanks sci-go (www.sci-go.com) for the TEM characterization.
Author information
Authors and Affiliations
Contributions
G.Y., C.L. conceived and directed the project. Z.L. carried out the design of catalysts and key experiments. Z.L. and C.L. wrote the manuscript. M.Z. and X.Z. performed theoretical calculations. H.L. and Z.Q. conducted operando SR-FTIR measurements. C. Yan, D.Y., R.Y., C.Z., P.S., C. Yin, D.L and L.S. conducted part of the characterizations. Z.L., M.Z., H.L., C. Yan, and C.L. analysed the data. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Federico Dattila, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Z., Zheng, M., Yan, C. et al. Stabilizing Cu0-Cuδ+ sites via ohmic contact interface engineering for ampere-level nitrate electroreduction to ammonia. Nat Commun 16, 8940 (2025). https://doi.org/10.1038/s41467-025-63996-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63996-w