Abstract
The development of highly active and CO-tolerant hydrogen oxidation reaction (HOR) electrocatalysts is of great significance for alkaline exchange membrane fuel cells (AEMFCs). Here, the designed atomically Ni anchored Ru/RuO2 heterostructure nanosheets (NiSA-Ru@RuO2 NSs/C) exhibit enhanced activity and stability for HOR in alkaline media. The optimized electrocatalyst delivers a high CO-tolerant durability with 92.3% retention in the 1000 ppm CO concentration after 5000 s test. Moreover, the anode catalyst NiSA-Ru@RuO2 NSs/C assembled AEMFCs output a peak power density (PPD) and specific PPD of 1.76 W cm−2 and 17.6 W mgPGM−1 under the H2/O2 condition and performed a long-term stability with negligible decay for 100 h at 0.5 A cm−2 for the AEMFCs. The relative mechanism studies reveal that the Ru/RuO2 heterostructure nanosheet and dispersed Ni single atoms have optimized the *H and *OH adsorption simultaneously and weaken the *CO adsorption. Our work may offer a significant guideline on the rational design of high-performance HOR electrocatalyst for energy-related applications.
Similar content being viewed by others
Introduction
Hydrogen technology, with its great potential for alleviating environmental impacts and craft novel energy opportunities, has attracted tremendous attention recently1,2,3,4,5,6,7. In comparison to advanced proton-exchange-membrane fuel cell (PEMFC) technology, alkaline exchange membrane fuel cells (AEMFCs) have unique advantages, including rapid development in AEMFC membranes8,9,10and rapid progress of non-noble metal oxygen reduction reaction cathode electrocatalysts11,12,13, making them the most promising candidate in the field of hydrogen energy conversion14,15,16,17. However, the anode hydrogen oxidation reaction (HOR) catalyst still relies on the use of Pt-based noble metal. In addition, the sluggish kinetics of HOR are 2-3 orders of magnitude lower in an alkaline environment than in an acidic one, greatly hindering the development for the AEMFCs18,19,20. Moreover, the most extensive hydrogen source applied recently is from reforming gas, while the source of this method inevitably contains a trace of CO, which severely limits the activity of the catalyst21,22. Hence, it is of great significance to develop highly active HOR catalysts resistant to CO species, yet it is still facing enormous challenges.
In the past decades, many efforts have been devoted on the morphology, electronic structure or atom doping strategy to excavate the highly active and CO-tolerant HOR catalyst. For example, Zhang et al. have reported that lattice-confined iridium (Ir) clusters on palladium (Pd) nanosheets have boosted the hydrogen oxidation activity23. Zhan et al. have claimed that the platinum (Pt) based high-entropy alloy nanowire has demonstrated high activity for the HOR process24. Ruthenium (Ru), despite being categorized as a precious metal, is far less expensive than Pt or Ir25,26,27. This, together with the huge potential for accelerating hydrogen oxidation, makes Ru a suitable choice for AEMFCs28,29,30,31,32. Nevertheless, the capacity to tolerate CO for Ru-based catalysts, especially when operated in high CO concentrations, is still lacking33,34,35. In addition, the pure metallic Ru has displayed much higher oxophilicity, which has reduced the H bonding sites, leading to largely reduced activity at high potentials. In general, the key notes to enhance the HOR activity of Ru-based catalysts is to optimize the H binding energy (HBE) and OH binding energy (OHBE). Therefore, it is crucial yet still a major challenge to precisely design a robust catalyst that can achieve a balance for optimizing the HBE and OHBE adsorption.
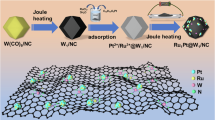
In this work, we have designed a Ni single-atom-anchored Ru/RuO2 heterostructure nanosheet catalyst (NiSA-Ru@RuO2 NSs/C), which shows competitive activity and high CO-tolerant ability for HOR in the alkaline electrolyte. The scheme comparison of the catalyst design strategy for pure Ru and NiSA-Ru@RuO2 NSs/C has demonstrated in Fig. 1a, b, respectively. The mass activity of NiSA-Ru@RuO2 NSs/C, increased 10.1 times after Ni doping, is 20.8 times greater than that of commercial Pt/C. The NiSA-Ru@RuO2 NSs/C has also demonstrated high CO-tolerant performance, with the activity sustaining 92.3% after operating at 1000 ppm CO environment for 5000 s test. The anode catalyst NiSA-Ru@RuO2 NSs/C assembled membrane electrode assembly (MEA) has delivered peak power densities of 1.76 W cm−2 and 1.25 W cm−2 under the H2/O2 and H2/Air conditions, which indicates that it has a competitive AEMFCs performance. Additionally, the MEA has performed negligible decay after 100 h stability test at the current density of 0.5 A cm−2. Detailed investigation and density functional theory (DFT) calculations have revealed that the Ni single atom and Ru/RuO2 heterostructure jointly modulate the HBE and OHBE and substantially boost the HOR kinetic, enabling it with high activity, robust CO-tolerant capability and huge application potential for the MEA device.
Results
Morphological and structural characterizations
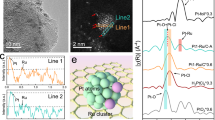
The Ni, Ru assembled nanosheets (Ni-Ru ANSs) were prepared through a wet-chemical method. Briefly, ruthenium (III) acetylacetonate (Ru(acac)3), nickel (II) acetylacetonate (Ni(acac)2), citric acid (CA) and 1-dodecanethiol were dissolved in benzyl alcohol and heated at 180 °C for 5 h. After centrifuging and cleaning, the product was collected. The characterization of this product is shown in Supplementary Fig. 1. The morphology is exhibited as nanosheet assembly structure (Supplementary Fig. 1a, b). In addition, the X-ray diffraction (XRD) pattern (Supplementary Fig. 1c) exhibits no diffraction peaks, indicating its amorphous character. The high-resolution transmission electron microscopy (HRTEM) image (Supplementary Fig. 1d) has shown no lattice space, further verifying the amorphous structure of this product. After loading the product with carbon black, the mixture was heated at 350 °C for 1 h, leading to NiSA-Ru@RuO2 NSs/C (see details in the Experimental section). The Ru@RuO2 NSs/C sample has been prepared without adding Ni precursor. As depicted in Fig. 2a and Supplementary Fig. 2, the nanosheet morphology of NiSA-Ru@RuO2 NSs/C is depicted from TEM and the high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images. The XRD pattern exhibits that there exists RuO2 (No. 43-1027) and Ru (No. 06-0663) in NiSA-Ru@RuO2 NSs/C (Fig. 2b). The scanning electron microscope energy-dispersive spectroscopy (SEM-EDS) result has revealed that the atomic ratio of Ru/Ni was measured to be 88.8/11.2 (Fig. 2c). The HRTEM image has demonstrated that the lattice spaces of 0.318 nm and 0.214 nm are assigned to the (110) facet of RuO2, and (002) facet of Ru, respectively (Fig. 2d). For the marked zone with orange square in Fig. 2d, the enlarged HRTEM in Fig. 2e, f indicates the formed lattice dislocation. In addition, more HRTEM images shown in Supplementary Fig. 3 has verified the lattice dislocation existed in the NiSA-Ru@RuO2 NSs/C. The line scan profile of Fig. 2g has shown that the Ru and Ni elements have existed in different proportions on the nanosheets (Fig. 2h). Besides that, Ru and Ni elements have uniformly dispersed across the entire nanosheet, according to HAADF-STEM-EDS elemental mappings in Fig. 2i. Detailed information about different Ni contents contained samples have also been supplied, named as NiSA1-Ru@RuO2 NSs/C (with adding 3.5 mg Ni(acac)2 in the catalyst preparation) and NiSA2-Ru@RuO2 NSs/C (with adding 6.5 mg Ni(acac)2 in the catalyst preparation) (Supplementary Figs. 4, 5). The morphology of NiSA1-Ru@RuO2 NSs/C and NiSA2-Ru@RuO2 NSs/C is shown as nanosheet structures. The XRD pattern has indicated the existence of Ru and RuO2 in these samples.
HOR performance
The HOR activity was thoroughly evaluated through linear sweep voltammetry (LSV) in a three-electrode system using a H2-saturated 0.1 M KOH electrolyte. As shown in Fig. 3a, the relevant catalysts are shown with distinct anode current density. Among them, the NiSA-Ru@RuO2 NSs/C has possessed the highest current density at 0.1 V vs RHE, indicating the higher hydrogen oxidation activity. In comparison, the NiSA-Ru@RuO2 NSs/C has shown no oxidative current in the N2-saturated 0.1 M KOH electrolyte (Supplementary Fig. 6), indicating the current density in H2 for the NiSA-Ru@RuO2 NSs/C was ascribed to the hydrogen oxidation. In addition, the NiSA1-Ru@RuO2 NSs/C and NiSA2-Ru@RuO2 NSs/C have performed poor activity (Supplementary Fig. 7). The polarization curve with different rotation rates has been conducted (Supplementary Fig. 8a), which shows the slope of 4.51 cm2 mA−1 s−1/2, closing to the theoretical value of 4.87 cm2 mA−1 s−1/2 for two-electron transfer of HOR (Supplementary Fig. 8b). In Fig. 3b, the exchange current (j0) of the developed catalysts could be further extracted from the Butler-Volmer equation. The j0 of NiSA-Ru@RuO2 NSs/C is 0.76 mA, which is 2.2 and 3.6 times higher than those of Ru@RuO2 NSs/C (0.35 mA) and Pt/C (0.21 mA), as shown in Supplementary Table 1. The kinetic current (jk) and j0 of testing catalysts were normalized to the mass of noble metal (denoted as jk,m and j0,m, respectively) for quantitative comparison. The jk,m and j0,m are summarized in Fig. 3c. As depicted, the NiSA-Ru@RuO2 NSs/C has performed the highest jk,m (mass activity) of 3.96 ± 0.02 A mgRu−1, which is 10.1 and 20.8 times higher than the Ru@RuO2 NSs/C (0.39 ± 0.04 A mgRu−1) and Pt/C (0.19 ± 0.05 A mgPt−1). The mass activity comparison is listed in Supplementary Table 2, which has indicated that the competitive HOR performance for the NiSA-Ru@RuO2 NSs/C. The mass activities of NiSA1-Ru@RuO2 NSs/C (0.36 ± 0.04 A mgRu−1) and NiSA2-Ru@RuO2 NSs/C (0.72 ± 0.06 A mgRu−1) are shown in Supplementary Fig. 9, presenting a lower value than that of NiSA-Ru@RuO2 NSs/C.
a HOR polarization curves of NiSA-Ru@RuO2 NSs/C, Ru@RuO2 NSs/C and Pt/C in H2-saturated 0.1 M KOH, the resistances of NiSA-Ru@RuO2 NSs/C, Ru@RuO2 NSs/C and Pt/C are 31.4 ± 0.2, 30.8 ± 0.2, and 31.9 ± 0.1 Ω, respectively, b Tafel plots with the Butler−Volmer fitting curves, c Mass activity of NiSA-Ru@RuO2 NSs/C, Ru@RuO2 NSs/C, and Pt/C, HOR polarized curves of (d) NiSA-Ru@RuO2 NSs/C, e Pt/C recorded in the pure H2 (solid line) and H2 with 1000 ppm CO (dot line), f Histogram comparison of different catalysts towards CO poisoning tolerance with recording LSV curves value at 50 mV vs. RHE. g Relative current–time chronoamperometry response of NiSA-Ru@RuO2 NSs/C and Pt/C in H2/1000 ppm CO-saturated 0.1 M KOH solution at 0.1 V vs RHE. These data are obtained with no iR corrected.
To illustrate how the heterostructure affects the HOR performance, pure metallic Ru and RuO2 were also prepared, respectively. As shown in Supplementary Fig. 10, the sample shows a metallic Ru phase (JCPDS No. 06-0663) after proceeding the NiSA-Ru@RuO2 NSs/C in the H2/Ar condition, and it has depicted extremely poor HOR activity (Supplementary Fig. 11). In comparison, the RuO2 phase (JCPDS No. 43-1027) can be obtained when processing the NiSA-Ru@RuO2 NSs/C in the air condition at high temperature (Supplementary Fig. 12), and this sample demonstrated limited HOR activity (Supplementary Fig. 13). These data have collectively verified the vital role of the Ru/RuO2 heterostructure for boosting the high performance HOR process. The CO tolerance stability was evaluated by measuring HOR polarization curves in H2 and a mixture of 1000 ppm CO/H2. As depicted in Fig. 3d, the NiSA-Ru@RuO2 NSs/C has shown no obvious activity change at 50 mV vs. RHE after testing in the 1000 ppm CO/H2 condition. Ru@RuO2 NSs/C has shown 6.5% declining comparing with its original activity (Supplementary Fig. 14), while the Pt/C has declined severely (Fig. 3e). The CO poisoning tolerance for different catalysts was summarized with recording LSV curves value in Fig. 3f. The histogram comparison has indicated the NiSA-Ru@RuO2 NSs/C owing the best CO poisoning capability with no activity decline, while the Ru@RuO2 NSs/C, and Pt/C has declined 6.5% and 60.9%, respectively. In addition, the current–time chronoamperometry for the CO tolerance capability has been evaluated. For the 1000 ppm CO/H2 condition, the NiSA-Ru@RuO2 NSs/C even can preserve 92.3% of its initial performance after 5000 s test (Fig. 3g). However, the commercial Pt/C nearly has no activity after 2000 s test. The CO-tolerant capability of various catalysts was listed in Supplementary Table 3, which has verified the competitive CO-tolerant performance for NiSA-Ru@RuO2 NSs/C. The stability test in the pure H2 condition has indicated that NiSA-Ru@RuO2 NSs/C can sustain 92.1% of its initial performance (Supplementary Fig. 15). In addition, the NiSA-Ru@RuO2 NSs/C after the stability test has also been characterized. As demonstrated in Supplementary Fig. 16, the morphology has been maintained as the nanosheet structure. The Ru and Ni elements were detected, which is consistent with the HAADF-STEM-EDS elemental mappings of NiSA-Ru@RuO2 NSs/C after stability test (Supplementary Fig. 17). In order to further demonstrate the high stability of our prepared catalyst, we have analyzed the XRD and XPS after the accelerated durability testing (ADT) with scanning the CV from 0.1 V − 0.4 V (vs. RHE) with 2000 cycles and H2-saturated i-t stability test for 3000 s. As depicted in Supplementary Fig. 18, the phases of RuO2 and Ru were detected for NiSA-Ru@RuO2 NSs/C, which indicates that the catalyst has maintained as the initial state after hydrogen oxidation reaction. For XPS analysis, the Ru has exhibited the metallic and oxidative state after the ADT process, and the Ni species has contained as the oxidative state, which are the same to those of the original state (Supplementary Fig. 19). In addition, we have also tested the XPS spectrum of the NiSA-Ru@RuO2 NSs/C after i-t stability test, where the metallic and oxidative Ru state existed. The Ni species has also depicted the oxidative state (Supplementary Fig. 20). All of these have confirmed the high stability of NiSA-Ru@RuO2 NSs/C.
Mechanism investigation
The X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) of the Ru-K edge were then applied to study the local configuration. As shown in Fig. 4a, the XANES spectrum has depicted that the K-edge position of NiSA-Ru@RuO2 NSs/C has shifted to a higher energy than that of the Ru foil, while lower than that of RuO2, indicating the partial oxidation of Ru for the NiSA-Ru@RuO2 NSs/C. EXAFS was then carried out to analyze the local coordination environment of Ru. The Ru foil and RuO2 samples are applied as the comparison samples. As illustrated in Fig. 4b, the peaks of NiSA-Ru@RuO2 NSs/C located at 1.45, and 2.35 Å are assigned to the Ru-O, and Ru-Ru bond, respectively. For the Ru@RuO2 NSs/C, the dominant peak at 1.41 Å is attributed to the Ru-O bond. The Ru-Ru bond at 2.34 Å can also be detected. Furthermore, the wavelet transform (WT) of Ru K-edge EXAFS was then carried out to analysis the local coordination environment of NiSA-Ru@RuO2 NSs/C, Ru@RuO2 NSs/C, Ru foil and RuO2 (Fig. 4c–f). These images give a clear visualization that the Ru-Ru, and Ru-O bond were presented in the NiSA-Ru@RuO2 NSs/C. This result is according with the EXAFS analysis. In Supplementary Fig. 21, it has shown the fitted K-space data of the Ru edge. The corresponding FT-EXAFS curves in the R space of Ru K-edge for NiSA-Ru@RuO2 NSs/C were fitted (Supplementary Fig. 22). Furthermore, the XANES and EXAFS were applied to study the Ni state and electronic structure of NiSA-Ru@RuO2 NSs/C. As illustrated in Fig. 4g, h, the Ni in NiSA-Ru@RuO2 NSs/C has bonded with O to form Ni-O bonding compared with Ni foil and NiO normalized absorbance, while no Ni-Ni bond has been observed for the NiSA-Ru@RuO2 NSs/C, indicating that the existence of Ni single atom for the NiSA-Ru@RuO2 NSs/C. In Fig. 4i–k, the WT of Ni K-edge has indicated that the Ni-O bond is the main bond for the NiSA-Ru@RuO2 NSs/C. In addition, the fitting result also verify the Ni has bonded with O (Fig. 4l). The relative fitting parameters of Ru and Ni bonds are summarized in Supplementary Table 4, which is useful for constructing the simulation calculation model. Moreover, for the Ru 3p XPS spectra (Fig. 4m), the peaks for NiSA-Ru@RuO2 NSs/C located at 461.1, 462.9 and 465.9 eV are assigned to the metallic Ru, Ru4+ and Run+(n > 4), respectively36, which have almost no shift for binding energy compared with the Ru@RuO2 NSs/C. The full scan and C 1 s of XPS spectra of NiSA-Ru@RuO2 NSs/C are depicted in Supplementary Fig. 23. As shown in Fig. 4n, the Ni 2p XPS spectrum has shown the oxidative state for NiSA-Ru@RuO2 NSs/C. The Ni 2p XPS spectrum can be split into two peaks, at which the peaks at 856.4 and 861.8 eV are corresponding to the Ni2+ and satellite peak of Ni. Following the above analysis, the atomic model of NiSA-Ru@RuO2 NSs/C is depicted in Fig. 4o.
a Ru K-edge XANES spectra, b the corresponding FT-EXAFS and (c–f) Ru K-edge WT-EXAFS of NiSA-Ru@RuO2 NSs/C, Ru@RuO2 NSs/C, Ru foil and RuO2. g Ni K-edge XANES spectra, (h) the corresponding FT-EXAFS and (i–k) Ni K-edge WT-EXAFS of NiSA-Ru@RuO2 NSs/C, Ni foil and NiO. l Fitting result of Ni K-edge for NiSA-Ru@RuO2 NSs/C. m Ru 3p and (n) Ni 2p XPS spectrum of NiSA-Ru@RuO2 NSs/C. o The atomic model of NiSA-Ru@RuO2 NSs/C.
Operando electrochemical impedance spectroscopy (EIS) at various voltages was applied to detect the adsorption and desorption kinetics of reaction intermediates for the Ru HOR process. The Nyquist plots of the NiSA-Ru@RuO2 NSs/C have depicted a smaller semicircular than the Ru@RuO2 NSs/C, which has indicated that the NiSA-Ru@RuO2 NSs/C surface is inclined to promote the reactants adsorption during the HOR process (Fig. 5a, b). Comparing with Ru@RuO2 NSs/C, the rapid decrease of the phase angle peak in the low frequency has indicated that the NiSA-Ru@RuO2 NSs/C has endowed an enhanced charge transfer rate, further accelerating the kinetics for the HOR (Fig. 5d, e). As shown in Fig. 5c, the underpotential deposited hydrogen (Hupd) stripping peak of NiSA-Ru@RuO2 NSs/C has appeared at 0.079 V vs. RHE, which is negative than the Ru@RuO2 NSs/C (0.105 V vs. RHE), and Pt/C (0.277 V vs. RHE), indicating the weakened HBE for NiSA-Ru@RuO2 NSs/C. The enhanced HOR activity of Ru@RuO2 NSs/C to NiSA-Ru@RuO2 NSs/C was ascribed to the Ni single atom modification, as verified by the weaker HBE. Furthermore, the CO-stripping experiment was conducted to reflect the anti-CO ability during the HOR process. Comparing with the Ru@RuO2 NSs/C, the CO-stripping peak of NiSA-Ru@RuO2 NSs/C has negatively shift of 19 mV. The NiSA-Ru@RuO2 NSs/C has also negatively shifted the peak of 95 mV in comparison with Pt/C, demonstrating the enhanced CO anti-poisoning capability (Fig. 5f). This has further verified the enhanced HOR activity and CO-tolerant capability for NiSA-Ru@RuO2 NSs/C. Besides that, we have supplied the in-situ Raman and in-situ FTIR spectra to illustrate the catalysis mechanism, the in-situ testing devices were shown in Supplementary Fig. 24. As depicted in Supplementary Fig. 25, the in-situ Raman technique was applied to investigate the presence of the OH intermediate adsorption on the catalyst surface. Within the potential range from −0.1 V–0.4 V (vs. RHE), a dominant peak for NiSA-Ru@RuO2 NSs/C at 718 cm−1 increases gradually with increasing potential, which was assigned as the *OH adsorption peak37. The *OH adsorption peak of Ru@RuO2 NSs/C is shown at 733 cm−1, indicating that NiSA-Ru@RuO2 NSs/C has a weaker *OH adsorption38. In addition, the in-situ FTIR technique was applied to investigate the catalytic mechanism for the HOR process. The in-situ FTIR spectra of NiSA-Ru@RuO2 NSs/C and Ru@RuO2 NSs/C were analyzed to discuss the H2O bonds (at about 3200 cm−1) in NiSA-Ru@RuO2 NSs/C and Ru@RuO2 NSs/C (Supplementary Figs. 26, 27). The H2O stretching peaks can be deconvoluted into three peaks, which were symmetric H-bonded H2O vibration (~3170 cm−1), asymmetric H-bonded H2O vibration (~3400 cm−1), and weakly H-bonded H2O vibration (~3590 cm−1). As reported, the symmetric H-bonded H2O has strengthened the hydrogen-bond network in the interfacial water, which is favorable for the proton-coupled electron transfer process with reducing energy barrier from the gap39. Hence, the higher concentration of symmetric H-bonded H2O has accelerated the hydrogen transfer, thus promoting the HOR process. For details, we have chosen two FTIR curves at 0 V and 0.1 V (vs. RHE), respectively, to demonstrate the symmetric H-bonded H2O stretching intensity. The comparison of specific symmetric H-bonded H2O stretching intensity for NiSA-Ru@RuO2 NSs/C and Ru@RuO2 NSs/C at 0 V and 0.1 V (vs. RHE) are shown in Supplementary Fig. 28a, b and Supplementary Fig. 29a, b, respectively. NiSA-Ru@RuO2 NSs/C with a stronger symmetric H-bonded H2O stretching intensity indicates a fast hydrogen transfer ability. The specific statements of in-situ Raman and in-situ FTIR analyzed above have further illustrated the catalysis mechanism of NiSA-Ru@RuO2 NSs/C for HOR. In addition, the electrochemical in-situ attenuated total reflection surface enhanced infrared absorption spectroscopy (ATR-SEIRAS) was conducted to clarify the mechanism of enhanced CO-tolerant behavior on NiSA-Ru@RuO2 NSs/C. Generally, there are two types of CO adsorption on the catalyst, which has divided into bridge adsorption (COB) and linear adsorption (COL). The COL has tended to acquire high potential to be oxidized in the comparison with the COB, while the COL adsorption was regarded as the main cause for the catalyst poisoning. As depicted in Fig. 5g, the ATR-SEIRAS was conducted with increasing the sweep voltage from 0 V vs. RHE to 0.8 V vs. RHE. The weak peaks at 1950 cm−1 and 2080 cm−1 have assigned to the COB and COL of NiSA-Ru@RuO2 NSs/C, while the Ru@RuO2 NSs/C has shown a stronger COB and COL adsorption (Fig. 5h). These spectra have further verified that Ni single atom anchored NiSA-Ru@RuO2 NSs/C has demonstrated the better CO-tolerant ability for the HOR process.
Nyquist plots of (a) NiSA-Ru@RuO2 NSs/C and (b) Ru@RuO2 NSs/C at different voltages. c CV curves of NiSA-Ru@RuO2 NSs/C, Ru@RuO2 NSs/C, and Pt/C tested in 0.1 M KOH. Bode phase plots of (d) NiSA-Ru@RuO2 NSs/C and (e) Ru@RuO2 NSs/C. f CO-stripping curves of NiSA-Ru@RuO2 NSs/C, Ru@RuO2 NSs/C, and Pt/C. Electrochemical in-situ ATR-SEIRAS spectra of (g) NiSA-Ru@RuO2 NSs/C and (h) Ru@RuO2 NSs/C, bridge adsorption (COB) and linear adsorption (COL) peaks were labeled with green.
DFT calculations
To elucidate the origin of enhanced hydrogen oxidation reaction (HOR) activity in NiSA-Ru@RuO2 NSs/C, three distinct theoretical models are systematically evaluated through density functional theory (DFT) calculations. The Ni-Ru/RuO2 model, featuring the Ni-substituted RuO2 (110) surface supporting a Ru nanocluster, corresponds to the NiSA-Ru@RuO2 structure, while the other two models are introduced for comparative analysis. The freestanding Ru-Cluster model was used to analyze the significance of heterostructure, and the Ru/RuO2 model is for exploring the effect of Ni doping. Notably, the coordination numbers (CNs) for the Ru-Ru, Ru-O and Ni-O are 1.54, 4.03 and 5.00, respectively, which successfully approximate the actual coordination environment shown by XAS. The Gibbs free energies of *H/*OH adsorption on Ru-Cluster, Ru/RuO2, and Ni-Ru/RuO2 were studied first (Supplementary Fig. 30 and Fig. 6a, b). For the 3 theoretical models, the initially guessed *H/*OH adsorption configurations are shown in Supplementary Figs. 31, 34, 37, and all the corresponding optimized adsorption configurations are exhibited in Supplementary Figs. 32, 33, 35, 36, 38, 39, respectively. The free energies demonstrate that the Ni-Ru/RuO2 has weaker *H/*OH adsorption than Ru/RuO2. Especially for the *H intermediate, the Ni-Ru/RuO2 has shown a prominently weaker adsorption compared with Ru-Cluster and Ru/RuO2. The charge transfer can be observed with the charge density differences of *OH adsorption on Ru-Cluster, Ru/RuO2, and Ni-Ru/RuO2 (Fig. 6c–f, Supplementary Fig. 40), so the relation between charge transfer and adsorption is investigated (Supplementary Fig. 41). This generally weak relation has indicated the *H/*OH-adsorbent interactions may be mainly based on sharing electrons rather than interatom electron transfer. The d-band centers of the atoms adjacent to the adsorbate were also analyzed for all the investigated configurations on the 3 models, and the relation between them and adsorption free energy is shown in Fig. 6i. It shows a rough trend that a lower local d-band center corresponds to a weaker *H/*OH adsorption, which is consistent with the previous reports31. As shown in Fig. 6g, h, the lower d-band center of Ni-Ru/RuO2 (−2.85 eV) than Ru/RuO2 (−2.37 eV) and Ru-Cluster (−2.55 eV) (Supplementary Fig. 42) explains why it shows weaker *OH binding, which contributes to the better HOR performance31. The DFT analysis reveals that the synergetic effect of the heterostructure and the Ni adjusts the d-band center of the Ru-based nanosheet, thus achieving a reasonable adsorption energy of *H and *OH and facilitating the HOR performance. Besides, in order to figure out why the anti-poisoning capability of Ni-Ru/RuO2 is better to Ru/RuO2, the CO adsorption on Ni-Ru/RuO2 and Ru/RuO2 are also investigated (Supplementary Fig. 43–45). As shown in Supplementary Fig. 45, the weaker *CO adsorption on Ni-Ru/RuO2 accords with its anti-poisoning, which can be attributed to its lower d-band center (Supplementary Fig. 46) as well.
Gibbs free energy changes of the investigated (a) *H and (b) *OH adsorption configurations on the surface of Ru/RuO2, and Ni-Ru/RuO2, where the sphere representations of the adsorbate and loaded structure are enlarged. The configurations corresponding to the optimal adsorption are plotted in red, and the top view of their corresponding optimized geometries are exhibited. Top (left) and side (right) view of charge density difference of *OH adsorption on the surface of (c, d) Ru/RuO2, and (e, f) Ni-Ru/RuO2. Blue and yellow colors represent losing and gaining electrons, respectively. Projected DOS (PDOS) diagram of the Ru 4 d orbital for the local environment of optimal *OH adsorption on (g) Ru/RuO2, and (h) Ni-Ru/RuO2, annotated with the d-band center positions (purple). (i) Relation between the local d-band center and the Gibbs free energy of the optimal *H/*OH adsorption.
AEMFCs performance
To access the applicability for the NiSA-Ru@RuO2 NSs/C, the membrane electrode assembly (MEA) has been assembled with commercial Pt/C (0.4 mgPt cm−2) as the anode electrode and NiSA-Ru@RuO2 NSs/C (0.1 mgRu cm−2) or commercial Pt/C (0.1 mgPt cm−2) as the cathode electrode. The physical fuel cell picture contained MEA, and the runner plate is shown in Supplementary Fig. 47. As shown in Fig. 7a, the polarization and power density curves of the AEMFC have delivered a peak power density (PPD) of 1.76 W cm−2 for the NiSA-Ru@RuO2 NSs/C anode catalyst in the H2/O2 condition, and the commercial Pt/C has outputted a PPD of 0.84 W cm−2. The PPD comparison of NiSA-Ru@RuO2 NSs/C and some reported works has indicated the competitive AEMFC performance of NiSA-Ru@RuO2 NSs/C (Supplementary Fig. 48). In terms of the H2/Air condition, the commercial Pt/C merely outputs a PPD of 0.68 W cm−2, while the anode catalyst NiSA-Ru@RuO2 NSs/C assembled AEMFC has performed a PPD of 1.25 W cm−2 (Fig. 7b). In addition, the AEMFC assembled with commercial PtRu/C as an anode catalyst (0.1 mgPGM cm−2) has also been conducted. The performance has shown that PPDs of 1.31 and 0.95 W cm−2 have been achieved in the H2/O2 and H2/Air condition, respectively, unable to compete with NiSA-Ru@RuO2 NSs/C (Supplementary Fig. 49). In Fig. 7c, we have compared the histogram of PPD for the anode catalyst, which has indicated that NiSA-Ru@RuO2 NSs/C has shown higher power density than the commercial Pt/C in the H2/O2 and H2/Air condition. In addition, we have compared the specific PPD with that normalized by the anode noble metal (MetalA) loading of NiSA-Ru@RuO2 NSs/C, which has indicated that the NiSA-Ru@RuO2 NSs/C has depicted the highest value of 17.6 W mgPGM−1 among the other reported works (Supplementary Fig. 50a). The specific PPD tested under the H2/Air condition was also evaluated and summarized in Supplementary Fig. 50b, which further demonstrated the competitive AEMFC performance of NiSA-Ru@RuO2 NSs/C. Moreover, the stability test for the catalyst, especially in the harsh fuel cell condition, is extremely vital for the catalyst quality evaluation. As shown in Fig. 7d, the anode catalyst NiSA-Ru@RuO2 NSs/C assembled MEA has declined almost negligible cell voltage loss with a current density of 0.5 A cm−2 in the H2/Air condition, demonstrating the competitive durability and huge practical application compared with some previous works (Supplementary Table 5).
Polarization and power density curves of AEMFC with Ni-Ru@RuO2/C (0.1 mgRu cm−2) in anode and Pt/C (0.4 mgPt cm−2) in cathode in the (a) H2/O2 condition and (b) H2/Air condition. c The PPD of NiSA-Ru@RuO2 NSs/C-based and commercial Pt/C-based MEA under H2/O2 and H2/Air media, d AEMFC stability test at the current density of 0.5 A cm−2 under the H2/Air condition with the NiSA-Ru@RuO2 NSs/C (0.1 mgRu cm−2) as the anode and the commercial Pt/C (0.4 mgPt cm−2) as the cathode.
Discussion
In conclusion, the Ni single atom anchored heterostructured Ru/RuO2 nanosheet HOR catalyst NiSA-Ru@RuO2 NSs/C has been rationally designed. Impressively, the Ni atom anchoring and Ru/RuO2 heterostructure dual engineering have collectively alleviated the HBE and OHBE process, which has boosted the HOR kinetics, resulting in competitive activity and stability towards hydrogen oxidation in alkaline electrolyte. The NiSA-Ru@RuO2 NSs/C has exhibited 10.1 and 20.8 folds of mass activity than that of Ru@RuO2 NSs/C and commercial Pt/C. In addition, the NiSA-Ru@RuO2 NSs/C has also performed long CO-tolerant capability with minor decay, even operated in the 1000 ppm CO condition for a 5000 s test. For a practical fuel cell, the NiSA-Ru@RuO2 NSs/C assembled AEMFCs has outputted an enhanced PPD of 1.76 W cm−2 and a specific PPD of 17.6 W mgRu−1 compared with commercial Pt/C and previous works. Our work may inspire more scientific interest in designing a highly active and CO-tolerant electrocatalyst for the hydrogen oxidation reaction and beyond.
Methods
Chemicals
Ruthenium(III) acetylacetonate (Ru(acac)3, 97%) and Nickel(II) acetylacetonate (Ni(acac)2, 97%) were purchased from Sigma-Aldrich. 1-dodecanethiol (>90%) was purchased from Aladdin. Citric acid (C6H8O7, (CA), AR), benzyl alcohol (C6H5CH2OH, AR) and potassium hydroxide (KOH, ≥85%) were purchased from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). Pt/C (20%, wt% Pt) was purchased from the Johnson Matthey (JM) Corporation. All chemicals were used without any purification. The pure water (18 MΩ cm−1) used in all experiments was obtained by an ultra-pure purification system.
Preparation of electrolytes
A 0.1 M KOH (pH=12.7 ± 0.02) electrolyte was prepared by dissolving 6.6 g of KOH with deionized water to a 1-liter volumetric flask and stored in a sealed polypropylene container. The prepared electrolytes are placed in a dry, and well-ventilated area to prevent deterioration.
Characterization
The phase structure was characterized on a Bruker D8 advance X-ray diffractometer operating with a Cu Kα radiation. The low-magnification transmission electron microscopy images were collected on a JEM 1400. The HRTEM images, HAADF-STEM images and HAADF-STEM-EDS elemental mappings were obtained on a FEI Tecnai F30 TEM at an accelerating voltage of 300 kV. The EDS analysis was operated on a scanning electron microscope (ZEISS Sigma). The chemical composition was investigated from XPS analysis performed on a Scientific K-Alpha spectrometer (Thermo Fisher). In-situ ATR-SEIRAS was conducted on a Nicolet 870 spectrometer. An EverGlo infrared light source and an MCT-A detector were equipped.
Electrocatalyst preparation
To prepare the catalyst, 15 mg Ru(acac)3, 5 mg Ni(acac)2, 40 mg CA, 50 µL 1-dodecanethiol, and 9 mL benzyl alcohol were ultrasonicated in a 30 mL vial for 40 min. The vital was then transferred to an oil bath and heated at 180 °C for 5 h. After the vial was cooled down to room temperature, the black product was collected by centrifugation and washed with the ethanol/acetone (v/v = 1/9) mixture for 3 times. After loading the product with carbon black, the NiSA-Ru@RuO2 NSs/C catalyst was obtained through 350 °C heat treatment for 1 h at the air atmosphere and then washed with pure water for two times. The NiSA1-Ru@RuO2 NSs/C and NiSA2-Ru@RuO2 NSs/C were prepared with the same procedure with NiSA-Ru@RuO2 NSs/C except adding 3.5 mg and 6.5 mg Ni(acac)2, respectively. The pure Ru catalyst with metallic phase was obtained with processing the NiSA-Ru@RuO2 NSs/C in the 5% H2/Ar condition at 250 °C for 1 h. The RuO2 phase catalyst was obtained with processing the NiSA-Ru@RuO2 NSs/C in the air condition at 500 °C for 3 h.
Electrochemical measurements
A typical three-electrode system was applied to perform the electrochemical measurements at CHI660E electrochemical station. The glassy carbon RDE electrode (5 mm diameter and 0.19625 cm2, Pine Research Instrumentation, USA), graphite rod and saturated calomel electrode (SCE) were used as the working electrode, counter electrode and reference electrode, respectively. To prepare the working electrode, 2 mg catalyst was dispersed in the mixture containing water (895 μL), isopropanol (100 μL) and Nafion (5%, 5 μL) to obtain the catalyst ink of 2 mg mL−1 by sonicating for 1 h at 15 °C. For details of catalyst loading calculation, 5.7 µL × 2 mg mL−1 × 26% (noble metal proportion) =3 μg. 3 μg noble metal was then cast onto an RDE (0.19625 cm2) and dried at room temperature. To ensure the accuracy of the RHE, we calibrated the reference electrode in H2-saturated 0.1 M KOH electrolytes periodically before conducting experiments. Before electrochemical testing, two platinum foils as the working electrode and counter electrode, a hydrogen-saturated KCl saturated solution as electrolyte, test the CV curve in the −0.95 to −1.10 V range and find the position where the current is 0 A, which was taken as the thermodynamic potential for the hydrogen electrode reactions. The potential is applied to convert to the RHE. The activation process was operated in a 0.1 M KOH at scan rate of 5 mV s−1. The LSV curves were performed in H2-saturated 0.1 M KOH at a scan rate of 5 mV s−1. The resistance measurement of the electrodes and electrolyte was measured using iR compensation. The current–time chronoamperometry curve was conducted with a noble metal loading of 4.5 μg at the 0.1 V vs RHE with saturated H2 and 1000 ppm CO/H2. All polarization curves were recorded without iR compensation.
MEA test
For the preparation of the catalyst ink, the NiSA-Ru@RuO2 NSs/C, commercial Pt/C and ionomer solution were dispersed into the deionized water and isopropanol (v/v = 1/20) in an ultrasonic bath for 1 h. The catalyst-coated membrane (CCM) was prepared with spraying the ink into two sides of the alkaline polymer electrolytes (APEs, 25 ± 3 µm in thickness) with active area of 4 cm2. After that, the CCM was soaked into 1 M KOH at 60 °C for 24 h to change the anion of the membrane and ionomer into OH−. Then, the membrane electrode assembly (MEA) was prepared with assembling the rinsed CCM, carbon papers and the current collector. The fuel cell was tested on the 850E Multi Range fuel cell test station with a temperature of 80 °C. The flow rate of the H2/O2 or Air were set as 1000 mL min‒1 under a backpressure of 0.2 MPa. The durability tests were conducted with H2 and air (CO2-free) humidified at 80 °C at a flow rate of 300 mL/min and 500 mL/min, respectively.
Computational method
The density functional theory (DFT) calculations are performed by the Viena Ab initio Simulation Package (VASP)40,41,42, and the generalized gradient approximation of Perdew–Burke–Ernzerhof (GGA-PBE) is utilized to describe the exchange−correlation energy and electronic exchange43,44. We set the cutoff energy to 450 eV, and employ the energy convergence criterion of 10−4 eV and the force convergence criterion of 0.03 eV Å−1 for most calculations. As for the zero-point energy calculation, the energy convergence criterion is changed to 10−5 eV. The empirical correction, Grimme’s scheme (DFT-D3), is chosen to represent the van der Waals interactions45. The Γ-centered k-point grid of 1 × 1 × 1 was chosen for optimizing slab geometry first, and a Γ-centered k-point grid of 2 × 2 × 1 was used for analyzing energetics and electronic structures. For all the slabs, a vacuum distance of 15 Å was added along the z direction. All their corresponding initial nonequivalent *H/*OH/*CO configurations are searched by the adsorption analysis module of pymatgen46, with a near reduction threshold of 0.3 and a symmetry reduction threshold of 0.1. For the coordination number detection of the theoretical model, the cutoff is set to the 1.2x of the nearest distance of a specific element pair. The selection of relevant planes of RuO2 was based on these works47,48,49,50,51. The Gibbs free-energy change (ΔG) of *H adsorption and *OH adsorption is given as the difference between the initial and final states:
where \(\Delta {{{\rm{E}}}}\) denotes the energy difference between the reaction reactants and products, ΔZPE represents changes in the zero-point energy, ΔS represents changes in entropy and T is the temperature. Here, T is 298 K.
The d-band center was calculated by following formula:
where \(\epsilon\) and \(\rho \left(\epsilon \right)\) denote energy and density of states, respectively.
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information files. Source data are provided with this paper.
References
Wan, C. et al. Amorphous nickel hydroxide shell tailors the local chemical environment on the platinum surface for alkaline hydrogen evolution reaction. Nat. Mater. 22, 1022–1029 (2023).
Dotan, H. et al. Decoupled hydrogen and oxygen evolution by a two-step electrochemical–chemical cycle for efficient overall water splitting. Nat. Energy 4, 786–795 (2019).
Yang, C.-L. et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells. Science 374, 459–464 (2021).
Wang, Q. et al. Atomic metal–non-metal catalytic pair drives efficient hydrogen oxidation catalysis in fuel cells. Nat. Catal. 6, 916–926 (2023).
Chong, L. et al. Ultralow-loading platinum-cobalt fuel cell catalysts derived from imidazolate frameworks. Science 362, 1276–1281 (2018).
Hou, M. et al. Microenvironment reconstitution of highly active Ni single atoms on oxygen-incorporated Mo2C for water splitting. Nat. Commun. 15, 1342 (2024).
Yao, R. et al. Stable hydrogen evolution reaction at high current densities via designing the Ni single atoms and Ru nanoparticles linked by carbon bridges. Nat. Commun. 15, 2218 (2024).
Wang, J. et al. Poly(aryl piperidinium) membranes and ionomers for hydroxide exchange membrane fuel cells. Nat. Energy 4, 392–398 (2019).
Chen, N. et al. Poly(alkyl-terphenyl piperidinium) ionomers and membranes with an outstanding alkaline-membrane fuel-cell performance of 2.58 W cm−2. Angew. Chem. Int. Ed. 60, 7710–7718 (2021).
Fan, J. et al. Poly(bis-arylimidazoliums) possessing high hydroxide ion exchange capacity and high alkaline stability. Nat. Commun. 10, 2306 (2019).
Adabi, H. et al. High-performing commercial Fe–N–C cathode electrocatalyst for anion-exchange membrane fuel cells. Nat. Energy 6, 834–843 (2021).
Jiao, L. et al. Chemical vapour deposition of Fe–N–C oxygen reduction catalysts with full utilization of dense Fe–N4 sites. Nat. Mater. 20, 1385–1391 (2021).
Liu, K. et al. Insights into the activity of single-atom Fe–N–C catalysts for oxygen reduction reaction. Nat. Commun. 13, 2075 (2022).
Lu, S. & Zhuang, Z. Investigating the influences of the adsorbed species on catalytic activity for hydrogen oxidation reaction in alkaline electrolyte. J. Am. Chem. Soc. 139, 5156–5163 (2017).
Zhu, S. et al. The role of ruthenium in improving the kinetics of hydrogen oxidation and evolution reactions of platinum. Nat. Catal. 4, 711–718 (2021).
Xiao, F. et al. Recent advances in electrocatalysts for proton exchange membrane fuel cells and alkaline membrane fuel cells. Adv. Mater. 33, 2006292 (2021).
Ni, W. et al. An efficient nickel hydrogen oxidation catalyst for hydroxide exchange membrane fuel cells. Nat. Mater. 21, 804–810 (2022).
Xue, Y. et al. A highly-active, stable and low-cost platinum-free anode catalyst based on RuNi for hydroxide exchange membrane fuel cells. Nat. Commun. 11, 5651 (2020).
An, L., Zhao, X., Zhao, T. & Wang, D. Atomic-level insight into reasonable design of metal-based catalysts for hydrogen oxidation in alkaline electrolytes. Energy Environ. Sci. 14, 2620–2638 (2021).
Liu, E. et al. Unifying the hydrogen evolution and oxidation reactions kinetics in base by identifying the catalytic roles of hydroxyl-water-cation adducts. J. Am. Chem. Soc. 141, 3232–3239 (2019).
Tang, T. et al. Synergistic electrocatalysts for alkaline hydrogen oxidation and evolution reactions. Adv. Funct. Mater. 32, 2107479 (2022).
Gao, Y. et al. A completely precious metal–free alkaline fuel cell with enhanced performance using a carbon-coated nickel anode. Proc. Natl. Acad. Sci. USA 119, e2119883119 (2022).
Zhang, B. et al. Lattice-confined Ir clusters on Pd nanosheets with charge redistribution for the hydrogen oxidation reaction under alkaline conditions. Adv. Mater. 33, 2105400 (2021).
Zhan, C. et al. X. Huang, Subnanometer high-entropy alloy nanowires enable remarkable hydrogen oxidation catalysis. Nat. Commun. 12, 6261 (2021).
Sun, S. et al. Bifunctional WC-supported RuO2 nanoparticles for robust water splitting in acidic media. Angew. Chem. Int. Ed. 61, e202202519 (2022).
Lin, C. et al. In-situ reconstructed Ru atom array on α-MnO2 with enhanced performance for acidic water oxidation. Nat. Catal. 4, 1012–1023 (2021).
Cai, C. et al. Optimizing hydrogen binding on Ru sites with RuCo alloy nanosheets for efficient alkaline hydrogen evolution. Angew. Chem. Int. Ed. 61, e202113664 (2022).
Han, L. et al. Design of Ru-Ni diatomic sites for efficient alkaline hydrogen oxidation. Sci. Adv. 8, eabm3779 (2022).
Huang, Z. et al. Implanting oxophilic metal in PtRu nanowires for hydrogen oxidation catalysis. Nat. Commun. 15, 1097 (2024).
Mao, J. et al. Isolated Ni atoms dispersed on Ru nanosheets: high-performance electrocatalysts toward hydrogen oxidation reaction. Nano Lett 20, 3442–3448 (2020).
Shi, H. et al. Local charge transfer unveils antideactivation of Ru at high potentials for the alkaline hydrogen oxidation reaction. J. Am. Chem. Soc. 146, 16619–16629 (2024).
Zhang, B. et al. A strongly coupled Ru–CrOx cluster–cluster heterostructure for efficient alkaline hydrogen electrocatalysis. Nat. Catal. 7, 441–451 (2024).
Zhou, Y. et al. Lattice-confined Ru clusters with high CO tolerance and activity for the hydrogen oxidation reaction. Nat. Catal. 3, 454–462 (2020).
Yang, X. et al. Ru colloidosome catalysts for the hydrogen oxidation reaction in alkaline media. J. Am. Chem. Soc. 144, 11138–11147 (2022).
Wang, T. et al. High CO-tolerant Ru-based catalysts by constructing an oxide blocking layer. J. Am. Chem. Soc. 144, 9292–9301 (2022).
Liu, L. et al. Trace lattice S inserted RuO2 flexible nanosheets for efficient and long-term acidic oxygen evolution catalysis. Small 19, 2208202 (2023).
Song, X. et al. Improving the hydrogen oxidation reaction rate of Ru by active hydrogen in the ultrathin Pd interlayer. J. Am. Chem. Soc. 145, 12717–12725 (2023).
Lin, F. et al. Synthesis of isolated Ru–O3 sites on hexagonal close-packed intermetallic penta-metallene for hydrogen oxidation electrocatalysis. Nat. Synth. https://doi.org/10.1038/s44160-024-00685-4 (2024).
Jin, Y. et al. The role of phosphorus on alkaline hydrogen oxidation electrocatalysis for ruthenium phosphides. Angew. Chem. Int. Ed. 63, e202406888 (2024).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-Initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 6, 15–50 (1996).
Hafner, J. Ab-initio simulations of materials using Vasp: density-functional theory and beyond. J. Comput. Chem. 29, 2044–2078 (2008).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Ong, S. P. et al. Python Materials Genomics (pymatgen): A robust, open-source Python library for materials analysis. Comp. Mater. Sci. 68, 314–319 (2013).
Xu, C. et al. Prediction on the surface phase diagram and growth morphology of nanocrystal ruthenium dioxide. J. Am. Ceram. Soc. 97, 3702–3709 (2014).
Zakaryan, H. A., Kvashnin, A. G. & Oganov, A. R. Stable reconstruction of the (110) surface and its role in pseudocapacitance of rutile-like RuO2. Sci. Rep. 7, 10357 (2017).
Zhu, Y. et al. Facilitating alkaline hydrogen evolution reaction on the hetero-interfaced Ru/RuO2 through Pt single atoms doping. Nat. Commun. 15, 1447 (2024).
Li, Y. et al. Arming Ru with oxygen-vacancy-enriched RuO2 sub-nanometer skin activates superior bifunctionality for pH-universal overall water splitting. Adv. Mater. 35, 2206351 (2023).
Zhang, J. et al. Interface engineering of snow-like Ru/RuO2 nanosheets for boosting hydrogen electrocatalysis. Sci. Bull. 67, 2103–2111 (2022).
Acknowledgments
This work was financially supported by the National Key R&D Program of China (2024YFA1509500, 2022YFA1504500), the National Natural Science Foundation of China (22025108, 22475143, U21A20327, 22121001), the Fundamental Research Funds for the Central Universities (20720240059, 20720240053), Young Elite Scientists Sponsorship Program by CAST (grant no. 2023QNRC001), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Collaborative Innovation Center of Suzhou Nano Science and Technology and Startup supports from Xiamen University. We acknowledge SOLEIL for provision osynchrotron radiation facilities and we would like to thank the assistance from SOLEIL beamline staff (the data of X-ray absorption spectroscopy were obtainedon beamline ODE).
Author information
Authors and Affiliations
Contributions
Q.S. and X.H. conceived and supervised the research. L.L., Q.S., and X.H. designed the experiments. Q.S. and L.L. performed most of the experiments and data analysis. W.Y. and N.F. performed in situ ATR-SEIRAS. Q.K. and Z.H. performed the XAS test. R.R and L.Z. performed the MEA test. L.J., Y.J., and Y.L. performed the DFT calculation. L.L., Q.S., and X.H. wrote the manuscript with support from all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Hongwen Huang, Yong-Tae Kim, Kyeounghak Kim and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, L., Jin, L., Ren, R. et al. A concurrently optimization of H and OH binding energies in atomically Ni anchored Ru/RuO2 nanosheet driving high CO-tolerant hydrogen oxidation catalysis. Nat Commun 16, 8951 (2025). https://doi.org/10.1038/s41467-025-63998-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-63998-8