Abstract
Limited therapeutic options are available for pulmonary fibrosis because its molecular pathogenesis remains unclear. Here, we find that chemokine CCL20 expression is increased in both murine models and patients with pulmonary fibrosis. Type 2 alveolar epithelial cells are identified as the major producers of CCL20, and increased CCL20 expression results from decreased expression of the transcription factor JUN. AEC2-specific deletion of CCL20 protects mice from bleomycin-induced pulmonary fibrosis. Mechanistic studies reveal that CCL20 interacts with integrin α5β1, but not the classical receptor CCR6, on fibroblasts and subsequently enhances TGF-β/Smad signaling, which promotes the differentiation of lung fibroblasts into myofibroblasts. Antibody blockade of CCL20 or disruption of the CCL20–integrin α5β1 interaction attenuates established pulmonary fibrosis. Overall, our study highlights the CCL20–integrin α5β1–TGF-β signaling cascade as a potential therapeutic target for pulmonary fibrosis.
Similar content being viewed by others
Introduction
Pulmonary fibrosis (PF) is the final outcome of most diffuse parenchymal lung diseases and is characterized by alveolar wall damage, excessive extracellular matrix (ECM) deposition, and ultimately respiratory failure1. It not only includes idiopathic PF (IPF) with unknown causes but also includes many fibroproliferative lung diseases with identifiable triggers2,3. New antifibrotic agents (nintedanib and pirfenidone) have been approved for the treatment of PF but have limited efficacy and notable toxicity4,5. PF treatment remains an unmet clinical need because its pathogenesis is unclear. Therefore, exploring the underlying cellular and molecular mechanisms of PF and discovering new therapeutic targets are urgently needed.
One common pathological feature of PF is the presence of fibroblast foci containing myofibroblasts in the lung parenchyma; myofibroblasts are considered the main source of ECM protein deposition in PF lungs, as well as in fibrosis in other organs6,7. The myofibroblast pool is heterogeneous and is derived from multiple sources, such as resident fibroblasts, perivascular mesenchymal cells, or circulating fibrocytes, in response to stimulation with fibrotic factors, such as chemokine (C-C motif) ligand 1 (CCL1)8, CCL179, transforming growth factor-β1 (TGF-β1)10, connective tissue growth factor (CTGF)11 and platelet-derived growth factor (PDGF)12. Resident fibroblasts, the major source of myofibroblasts, are responsible for excessive collagen deposition and the progressive expansion of fibrotic lung lesions in PF13. Identifying mediators that induce the activation of resident fibroblasts into myofibroblasts might pave the way for preventive or therapeutic strategies to treat PF patients.
Chemokines are a family of small secreted proteins that includes nearly 50 members14. Chemokines can be classified into four subfamilies based on the position of the first two cysteine (C) residues in those proteins: CC chemokines, CXC chemokines, C chemokines, and CX3C chemokines15. One chemokine can bind to different chemokine receptors, and vice versa16. These factors play crucial roles in embryonic development, homeostasis, and pathological processes such as inflammation, tumor growth, and autoimmune diseases17. Recently, our team reported that CCL1, which is a CC chemokine, promoted lung fibrosis by activating the transition of lung fibroblasts into myofibroblasts through interactions with the autocrine motility factor receptor (AMFR) on fibroblasts, which increased the synthesis of profibrotic proteins8. In the above study, the levels of several chemokines, including chemokine (C-C motif) ligand 20 (CCL20), in the bronchoalveolar lavage fluid (BALF) of PF mice were significantly altered. CCL20, which is also known as macrophage inflammatory protein (MIP)−3α, belongs to the CC chemokine subfamily. Previously, CCL20 was shown to interact with its receptor C-C chemokine receptor 6 (CCR6) to recruit immune cells, such as Th17 cells18, dendritic cells19, and regulatory T (Treg) cells20, to sites of injury, contributing to the progression of various inflammatory diseases. The various target cells of CCL20 suggest that this chemokine has diverse functions in different pathological processes. Although studies have highlighted the importance of the pathogenic CCL20‒CCR6 axis in tissue fibrosis, such as liver fibrosis21,22, the role of this chemokine in PF remains unknown.
In this study, we observed increased CCL20 levels in the lung tissues of PF patients and in a bleomycin (BLM)-induced PF mouse model. Type 2 alveolar epithelial cells (AEC2s) were identified as the source of CCL20 during PF pathogenesis. The loss of CCL20 in AEC2s significantly protected mice from BLM-induced lung fibrosis. Furthermore, we found that CCL20 promoted lung fibrosis by driving the differentiation of lung fibroblasts into myofibroblasts. Mechanistically, CCL20 interacted with integrin α5β1 on fibroblasts and subsequently enhanced TGF-β/Smad signaling, which activated the transition of lung fibroblasts to myofibroblasts. Therefore, the treatment of mice with a CCL20 antibody or a peptide that blocks the interaction of CCL20 with integrin α5β1 significantly reversed established PF. Our data highlight the functional importance of CCL20 in PF and suggest a conceptual framework for understanding the interconnectivity between AEC2s and fibroblasts during the progression of PF.
Results
Increased CCL20 expression promotes the development of PF
Chemokines, which are the main factors involved in the inflammatory response, are closely involved in PF pathogenesis23,24. We used a cytokine array to measure the expression of chemokines in the BALF of mice with PF. Elevated CCL20 protein levels were observed in the BALF of BLM-challenged PF mice (Fig. 1a). The protein levels of CCL20 were measured in the BALF of mice instilled with one to six doses of BLM, and we found that the level of CCL20 increased with the progression of PF (Fig. 1b). Additionally, we observed that Ccl20 mRNA expression was upregulated in both the SiO2- and paraquat-induced PF models (Supplementary Fig. 1a, b).
a Heatmap showing the chemokine contents in BALF from PBS- or BLM-challenged mice detected with a cytokine array. b CCL20 concentrations in the BALF of mice subjected to multiple BLM exposures (n = 5 samples for Vehicle, 1BLM, 4BLM, 5BLM; n = 7 samples for 2BLM; n = 3 samples for 3BLM; n = 6 samples for 6BLM). c Schematic overview of the experimental design for (d–g). Masson’s trichrome staining (d), Crs (e) (n = 6 samples for PBS + Vehicle, PBS + BLM, CCL20 + BLM; n = 5 samples for CCL20 + Vehicle), hydroxyproline content (f) (n = 6 samples for PBS + Vehicle, CCL20 + Vehicle, CCL20 + BLM; n = 7 samples for PBS + BLM), and the expression levels of Col1a1, Col3a1, Fibronectin and Acta2 in the lung tissues (g) (n = 5 samples per group) of PBS- or BLM-challenged mice treated with or without CCL20. Scale bar, 200 μm (d). h Schematic overview of the experimental design for (i, j). Masson’s trichrome staining (i) and Crs (j) of PBS- or BLM-challenged mice treated with or without the CCL20 Ab (n = 6 samples per group). Scale bar, 1 mm (i). k Hydroxyproline content in PBS- or BLM-challenged mice treated with or without the CCL20 Ab (n = 6 samples per group). The data are presented as the means ± SEM. The data in (b) were analyzed by two-tailed Student’s t-test. The data in e, f, j, k were analyzed by one-way ANOVA. *p < 0.05; **p < 0.01; ***p < 0.001. Source data are provided as a Source data file.
Vehicle- or BLM-challenged mice were intratracheally administered recombinant CCL20 or PBS to determine whether CCL20 is involved in PF pathogenesis in vivo (Fig. 1c). CCL20 treatment in vehicle-challenged mice induced fibrotic changes, while the administration of CCL20 to BLM-challenged mice exacerbated PF, as indicated by increased collagen deposition, thickening of the alveolar septa (Fig. 1d), and decreased lung function (Fig. 1e). Additionally, lung hydroxyproline levels significantly increased with the upregulation of fibrosis-related genes (Fig. 1f, g). These data indicate a profibrotic effect of CCL20 on mouse lungs.
We examined the therapeutic effect of a CCL20-neutralizing antibody on mice with PF to confirm whether CCL20 is a therapeutic target in PF. After repeated BLM exposure, the mice were administered a CCL20-neutralizing antibody or control IgG by intratracheal instillation twice a week for 30 days (Fig. 1h). BLM-challenged mice exhibited a typical PF phenotype characterized by increased collagen deposition, decreased lung function, and reduced hydroxyproline levels (Fig. 1i–k). Treatment with the CCL20-neutralizing antibody ameliorated these detrimental changes (Fig. 1i–k). Taken together, these data show that anti-CCL20 therapy promotes the resolution of lung fibrosis in mice, further confirming the profibrotic role of CCL20 in PF pathogenesis.
CCL20 is secreted mainly by AEC2s in PF
We next sought to determine which cell type was the main source of CCL20. Compared with hematopoietic cells, nonhematopoietic cells from PF mice expressed higher levels of Ccl20 than did those from control mice (Fig. 2a). We then isolated various types of primary nonhematopoietic cells, including AECs, fibroblasts, and endothelial cells, from PBS- or BLM-challenged mice. As shown in Fig. 2b, Ccl20 was upregulated in AECs from fibrotic lungs but not in fibroblasts or endothelial cells (Fig. 2b). A reanalysis of a published microarray dataset also revealed a significant increase in the mRNA expression of Ccl20 in AECs from PF lungs compared with that in AECs from control lungs (Fig. 2c). BLM treatment in vitro dose-dependently increased Ccl20 expression in MLE-12 cells (a mouse lung epithelial cell line) (Fig. 2d). Consistently, Ccl20 expression was significantly elevated in primary mouse AECs treated with TGF-β or BLM (Fig. 2e, f). We performed immunostaining for CCL20 and SFTPC, a marker of type 2 AECs (AEC2s), in lung sections from control mice and PF mice. Similarly, CCL20 predominantly colocalized with AEC2s in mice with PF (Fig. 2g). Previous studies have shown that a population of aberrant basaloid cells (KRT5-/KRT17+) is highly enriched in PF lungs25,26. We performed multiplex immunofluorescence staining for CCL20, KRT17, and KRT5 in lung tissues from PF patients and healthy controls to determine whether the upregulated CCL20 is produced by these aberrantly expressed basaloid cells. CCL20 did not colocalize with KRT5-/KRT17+ basaloid cells (Supplementary Fig. 1c), suggesting that these aberrantly expressed basaloid cells were not significant producers of CCL20 in fibrotic lung tissues.
a Ccl20 expression in hematopoietic and nonhematopoietic cells from the lung tissues of PBS- or BLM-challenged mice (n = 4 samples per group). b Ccl20 expression in epithelial cells, fibroblasts and endothelial cells in lung tissues from PBS- or BLM-challenged mice (n = 3 samples per group). c Ccl20 expression in alveolar epithelial cells from PBS- or BLM-challenged mice. The data were obtained from an open-source dataset (GSE109913) (n = 3 samples per group). d Ccl20 expression in MLE-12 cells following treatment with different doses of BLM (n = 3 technical replicates per group). Ccl20 expression in AECs treated with TGF-β (e) or BLM (f) (n = 3 technical replicates per group). g IF staining for SFTPC and CCL20 in lung tissues from BLM-induced PF mice and controls. Scale bars, 25 μm. h Schematic of the method for generating Ccl20 conditional knockout mice. i Scheme for generating mice with Ccl20 genetically deficient in AEC2s. j Masson staining of lung tissues from the indicated mice. Scale bar, 100 μm. k Hydroxyproline content in lung tissues from the indicated mice (n = 7 per group). l Expression of fibrosis-related genes in lung tissues from the indicated mice (n = 5 samples per group). m IHC staining of α-SMA and collagen in lung tissues from the indicated mice. Scale bar, 50 μm. The data are presented as the means ± SEM. The data in a–c, e, f were analyzed by two-tailed Student’s t-test. The data in d, k were analyzed by one-way ANOVA. *p < 0.05; **p < 0.01; ***p < 0.001. Source data are provided as a Source data file.
To further confirm that AEC2s are the cellular source of CCL20 in PF development in vivo, we generated AEC2-specific Ccl20 knockout (KO) mice (Fig. 2h). The severity of PF was assessed in these mice following BLM challenge. We found that BLM-induced lung fibrosis was markedly suppressed in AEC2-specific Ccl20 KO mice (Ccl20fl/flSftpccre mice), as revealed by decreased collagen deposition, reduced hydroxyproline levels, and downregulated expression of fibrosis‑related genes in lung tissues (Fig. 2i–l). Similar results were observed in AEC2-specific Ccl20 knockout (KO) mice generated via intratracheal injection of AAV-Sftpc-Cre into mice, in which the Ccl20 gene floxed at exons 2–3 (Supplementary Fig. 1d–g).
Several studies have reported that CTHRC1 (collagen triple helix repeat containing 1) is a marker for pathological fibroblasts in pulmonary fibrosis27,28. In line with these findings, we reported that the CTHRC1 protein level was significantly increased in the lung tissues of mice with BLM-induced pulmonary fibrosis. Conditional deletion of Ccl20 in AEC2s suppressed BLM-mediated upregulation of the CTHRC1 protein in mouse lungs (Supplementary Fig. 1h). In addition, we isolated primary lung fibroblasts from WT and Ccl20fl/flSftpccre mice following BLM treatment. RT‒PCR analysis revealed that Ccl20 deficiency in mice significantly reduced Cthrc1 mRNA expression in primary lung fibroblasts (Supplementary Fig. 1i). Taken together, these data indicate that the depletion of CCL20 in AEC2s attenuates PF development.
We further examined the mechanism underlying CCL20 regulation in AEC2s during PF pathogenesis. An analysis of a public dataset revealed 1449 genes whose expression was significantly changed in AECs from BLM-treated mice compared with those from control mice. Among them, 28 genes were transcription factors that were predicted to bind to the Ccl20 promoter (Supplementary Fig. 2a). We subsequently performed a correlation analysis between CCL20 concentrations in BALF and the expression of these 28 transcription factors in primary AECs isolated from PBS- and BLM-treated mice (Supplementary Fig. 2b). CCL20 protein levels were negatively correlated with the expression of Jun and Nr3c2 but were positively correlated with the expression of Tfdp1 (Supplementary Fig. 2c–e). Jun overexpression in MLE-12 cells induced a marked decrease in Ccl20 mRNA expression (Supplementary Fig. 2f). However, neither Tfdp1 overexpression nor Nr3c2 silencing affected Ccl20 mRNA expression (Supplementary Fig. 2g, h). Additionally, Jun overexpression reduced the luciferase activity of the CCL20 promoter (Supplementary Fig. 2i), suggesting that JUN may serve as a transcriptional repressor of CCL20. The negative correlation between Ccl20 and Jun expression was verified by analyzing a public dataset (Supplementary Fig. 2j). Compared with that in control lungs, Jun expression in AECs from PF lungs was lower (Supplementary Fig. 2k). Immunofluorescence (IF) staining verified that JUN expression was significantly lower in AEC2s from mice with PF than in those from control mice (Supplementary Fig. 2l). Furthermore, we intratracheally injected a Jun‑overexpressing lentivirus into mice to determine the effects of Jun overexpression on the production of CCL20 and the pathogenesis of PF in vivo (Supplementary Fig. 2m). The expression of Jun was upregulated, whereas the expression of Ccl20 was downregulated, in the lung tissues of the Lenti‑Jun‑treated mice (Supplementary Fig. 2n). Accordingly, the degree of fibrosis in Jun-overexpressing mice was significantly reduced, as revealed by reduced collagen deposition and decreased hydroxyproline levels (Supplementary Fig. 2o, p), and this result was further confirmed by the decreased mRNA levels of fibrosis‑related genes, including Acta2, Col1a1, Col3a1, and Fibronectin, in Jun‑overexpressing mice (Supplementary Fig. 2q). Taken together, these results indicate that the reduced expression of JUN in AEC2s following chronic lung injury contributed to the increase in CCL20 levels during PF pathogenesis.
CCL20 activates lung fibroblasts in a CCR6-independent manner
We next aimed to determine how CCL20 contributes to the development of PF. CCL20 interacts with the well-known receptor CCR6 and contributes to various inflammatory diseases by recruiting immune cells to injury sites29,30,31. To confirm whether CCL20 influences immune cells in PF, we analyzed a public single-cell RNA-Seq dataset from the lungs of the bleomycin cohort and found that Ccr6 was expressed mainly in CD4+ T cells and B cells in the BLM group. Subsequent flow cytometry analysis of the BLM-induced PF model confirmed a significant increase in total CD4+ T cells (Supplementary Fig. 3a, b). Intracellular staining further revealed significant expansions in Th1, Th2, and Th17 subsets, alongside a non-significant trend toward increased Tregs (Supplementary Fig. 3c, d). Compared with those in the BLM group, Th17 cells were reduced in the lungs of the CCL20-neutralizing antibody group, whereas no statistically significant alterations were observed in the Th1 and Th2 subpopulations (Supplementary Fig. 3c, d). While total B-cell percentages remained unchanged in fibrotic lungs, plasma cells expanded markedly 3-fold compared with those in control lung tissue. However, compared with the BLM group, the CCL20-neutralizing antibody group did not significantly reduce plasma cell accumulation (Supplementary Fig. 3c, d). Taken together, these results demonstrated that the CD4+ cell population, specifically the Th17 subset, is an immune cell type that may correlate with the profibrotic function of CCL20.
To examine whether CCL20 promotes PF by targeting nonhematopoietic cells, we first obtained Ccr6-deficient (Ccr6−/−) mice, performed reciprocal bone marrow (BM) transplantation, and generated (Ccr6−/−) → WT chimeric mice (Supplementary Fig. 3e). Compared with BLM alone, the administration of recombinant CCL20 to these mice significantly exacerbated BLM-induced PF, as indicated by increased collagen deposition and hydroxyproline levels (Supplementary Fig. 3f, g). These data indicate that CCL20 exacerbates BLM-induced fibrosis by targeting nonhematopoietic cells. CCL20 interacts with CCR6 and has been reported to induce epithelial-to-mesenchymal transition (EMT) in epithelial cells32,33. Owing to the important role of this biological process in a variety of fibrotic diseases34, we examined whether CCL20 exerts profibrotic effects by regulating EMT in AECs. Unexpectedly, neither MLE-12 cells nor primary AEC2s expressed CCR6 (Supplementary Fig. 3h, i). Moreover, CCL20 treatment did not induce EMT in these cells (Supplementary Fig. 3g), indicating that CCL20 has no effect on AECs.
We then examined the effect of CCL20 on the activation of primary lung fibroblasts, which are effector cells in PF. These isolated primary mouse lung fibroblasts were spindle shaped, stained positively for the mesenchymal marker vimentin, contained actin stress fibers that were positive for phalloidin, and were negative for CD31 (endothelial cells), EpCAM (epithelial cells), and CD45 (immune cells) (Supplementary Fig. 3k). Indeed, the migration assay revealed that CCL20 treatment increased the migration of primary mouse lung fibroblasts in a dose-dependent manner (Fig. 3a). Collagen contraction was also increased in primary fibroblasts after CCL20 treatment (Fig. 3b). Additionally, we found that recombinant CCL20 increased the mRNA levels of fibrosis-related genes, the degree of collagen secretion, and the number of α-SMA-positive cells, as measured by RT‒PCR, a collagen assay, and IF staining, respectively (Fig. 3c–e). Additionally, we obtained similar results using MRC-5 human lung fibroblasts (Supplementary Fig. 3l–n). We did not observe significant changes in the proliferation or senescence of MRC-5 cells after CCL20 stimulation (Supplementary Fig. 3o–q). Collectively, these data demonstrate that CCL20 drives the activation of fibroblasts.
a Migration analysis of primary mouse lung fibroblasts treated with different doses of CCL20 (n = 3 technical replicates per group). b–e Activation analysis of primary mouse lung fibroblasts treated with or without CCL20 (n = 6 technical replicates per group). Scale bars, 50 μm (e). f Schematic overview of the experimental design for (g, h). IF staining for α-SMA (g) and PCR analysis of the mRNA levels of genes associated with fibrosis (h) (n = 3 samples per group) in fibroblasts cocultured with AECs from PF mice in the presence of IgG or CCL20 Ab. Scale bar, 50 μm (g). Schematic overview (i) and representative fluorescence images (j) of Tomato-positive cells in the lung tissues of Col1a2-Tomato mice treated with i.t. injections of Ad-Ccl20 (2 × 109 pfu/mouse) compared with Ad-Ctrl-injected controls (n = 3 samples per group). Scale bar, 100 μm. The data are presented as the means ± SEM. The data in a, c, d, h were analyzed by two-tailed Student’s t-test. *p < 0.05; **p < 0.01; ***p < 0.001. Source data are provided as a Source data file.
We next examined the functional role of CCL20 from PF AEC2s in fibroblast activation. AEC2s from mice with PF were cocultured with primary lung fibroblasts in the presence of IgG or a CCL20-neutralizing antibody (Fig. 3f). Fewer α-SMA-positive cells were observed in the CCL20-neutralizing antibody-treated group than in the IgG-treated group (Fig. 3g). Moreover, compared with IgG treatment, CCL20-neutralizing antibody treatment suppressed the expression of fibrosis-related genes (Fig. 3h), suggesting that the CCL20-neutralizing antibody blocked the activation of fibroblasts caused by the AEC2s from mice with PF. To examine the CCL20-dependent regulation of fibroblast activation in vivo, we intratracheally administered CCL20-carrying adenoviruses into transgenic mice expressing the fluorescent protein tdTomato under the control of the mouse collagen type I alpha 2 (Col1a2) promoter and analyzed the lungs 3 days after the second injection (Fig. 3i). IF analysis revealed that CCL20 induced Col1a2+ fibroblast accumulation (Fig. 3j). Taken together, these results suggest that AEC2s from mice with PF secrete CCL20 to activate lung fibroblasts.
Given that CCR6 is the only known receptor for CCL20, we hypothesized that CCL20 could induce fibroblast activation via CCR6. Flow cytometry revealed that primary mouse lung fibroblasts expressed CCR6 (Supplementary Fig. 4a). We tested whether CCL20 activates lung fibroblasts by binding to CCR6 via isolation of primary mouse lung fibroblasts from Ccr6−/− mice (Supplementary Fig. 4b). The profibrotic effects of CCL20, including increasing the number of α-SMA-positive cells and increasing the expression of fibrosis-related genes, were not affected by CCR6 knockout in these cells (Supplementary Fig. 4c–e). We further verified this observation by knocking out CCR6 in MRC-5 cells via lentiviral-mediated delivery of the CRISPR‒Cas9 system (Supplementary Fig. 4f). CCR6 deletion did not suppress the activation of MRC-5 cells driven by CCL20 (Supplementary Fig. 4g, h). These results suggest that CCL20 activates lung fibroblasts in a CCR6-independent manner.
CCL20 binds to integrin α5β1 to increase the activity of the TGF-β signaling pathway and induce lung fibroblast activation
To identify the receptor mediating CCL20-induced fibroblast activation, we performed mass spectrometry (MS) to analyze the CCL20 immunocomplex in membrane proteins extracted from CCL20-treated fibroblasts. A total of 440 proteins were found to bind to CCL20, and the top 10 proteins interacting with CCL20 identified by MS analyses are listed in Fig. 4a. In addition, cell lysates were extracted from His-tagged CCL20-treated fibroblasts and immunoprecipitated with anti-His antibody or anti-IgG in triplicates. Quantitative analysis of the pulldown fractions was performed using high-resolution microscale proteomic sequencing. Statistical analysis of the proteomic data revealed 260 differentially expressed proteins across the experimental groups (Fig. 4b). Our proteomic sequencing analysis identified integrin α5 and β1 as the most significantly enriched candidates among the top 10 proteins interacting with CCL20 identified by MS analyses. Given the well-known roles of integrin α5β1 in tissue fibrosis, we hypothesized that CCL20 induces fibroblast activation via integrin α5β1. Surface plasmon resonance (SPR) analysis revealed a high-affinity interaction between CCL20 and integrin α5β1 (Fig. 4c, d). Coimmunoprecipitation (coIP) assays further confirmed the interaction between CCL20 and integrin α5 or integrin β1 in HEK293T cells (Fig. 4e, f). Additionally, the recombinant CCL20 protein could interact with endogenous integrin α5 and integrin β1 on the membrane of fibroblasts, as revealed by IF staining (Fig. 4g, h). Immunoblotting and qPCR analyses revealed that CCL20 increased the expression of integrin α5 and integrin β1 in MRC-5 cells (Supplementary Fig. 5a, b). Moreover, integrin α5 and integrin β1 levels were significantly increased in the lungs of mice following BLM injection (Supplementary Fig. 5c). To further confirm whether CCL20 activates fibroblasts by binding to integrin α5β1, we used ATN-161, a novel integrin α5β1 antagonist, to inhibit the activity of integrin α5β1. Compared with CCL20 treatment alone, the combination of ATN-161 plus CCL20 significantly inhibited the activation of primary mouse lung fibroblasts, as indicated by the reduced expression of α-SMA and COL1 (Fig. 4i, j), suppressed migratory ability (Fig. 4k), and reduced number of α-SMA-positive cells (Supplementary Fig. 5d). Similar results were obtained for MRC-5 human lung fibroblasts (Fig. 4l–n and Supplementary Fig. 5e). Furthermore, CCL20 failed to induce fibroblast activation after integrin α5 knockdown (Supplementary Fig. 5f, g) or integrin β1 knockdown (Supplementary Fig. 5h, i). These data suggest that CCL20 activates lung fibroblasts by binding to integrin α5β1.
a List of the 10 proteins that interact with CCL20 identified by MS analyses. b Volcano plot of enriched proteins that interact with CCL20 (n = 3 technical replicates per group). Significantly altered proteins are shown in red (false discovery rate (FDR) < 0.05, log2FC > 1) and blue (FDR < 0.05, log2FC < −1). c Identification of integrin α5 and integrin β1 from CCL20 immunoprecipitates in fibroblasts by MS analysis. d Surface plasmon resonance (SPR) analysis of the kinetic interaction between CCL20 and integrin α5β1. CoIP analysis of the interaction between CCL20 and integrin α5 (e) or integrin β1 (f) in HEK293T cells. IF images showing the colocalization of CCL20 with integrin α5 (g) or integrin β1 (h) in MRC-5 cells. Scale bars, 10 μm. i Expression of Col1a1, Col3a1, Fibronectin and Acta2 in primary fibroblasts treated with or without ATN-161 in the presence of CCL20. j Immunoblots showing COL1 and α-SMA levels in primary fibroblasts treated with or without ATN-161 in the presence of CCL20. k Migratory ability of primary fibroblasts treated with or without ATN-161 in the presence of CCL20. Scale bars, 100 μm. l Expression of ACTA2 in MRC-5 cells treated with or without ATN-161 in the presence of CCL20. m Immunoblots showing the levels of COL1 and α-SMA in MRC-5 cells treated with or without ATN-161 in the presence of CCL20. n Migratory ability of MRC-5 cells treated with or without ATN-161 in the presence of CCL20. Scale bars, 100 μm. The data are presented as the means ± SEM. For (i–n), n = 3 technical replicates per group. The data in i–n were analyzed by one-way ANOVA. *p < 0.05; **p < 0.01; ***p < 0.001. Source data are provided as a Source data file.
We performed molecular docking to determine the binding pattern of CCL20 with integrin α5 or integrin β1 and predicted the possible binding sites on the basis of their protein structures (Supplementary Fig. 6a). Two amino acid sites in integrin α5 (T341 and H345), two amino acid sites in integrin β1 (R6 and K378) and six amino acid sites in CCL20 (D38, D59, K78, K91, K94 and N95) were identified as potential binding sites (with predicted interaction numbers greater than three). Next, we generated a series of integrin α5, integrin β1, and CCL20 point mutants. Only the CCL20 D59A and K94A/N95A mutations reduced the interaction with integrin α5 (Supplementary Fig. 6b). Only the D38A mutation resulted in a reduced interaction with integrin β1 (Supplementary Fig. 6c). These data suggest that the amino acid sites D59, K94, and N95 in CCL20 are critical for the binding of CCL20 to integrin α5 and that the amino acid site D38 is responsible for the binding of CCL20 to integrin β1. The integrin α5 T341A and H345A mutants failed to bind to CCL20, suggesting that T341 and H345 serve as the key sites for CCL20 binding (Supplementary Fig. 6d). In addition, the integrin β1 R6A mutant had a reduced interaction with CCL20, indicating that R6 in integrin β1 serves as the key site for CCL20 binding (Supplementary Fig. 6e).
Given that integrins play crucial roles in the activation of TGF-β35, which is a central mediator of fibrogenesis, we hypothesized that the CCL20–integrin α5β1 interaction could promote the differentiation of fibroblasts into myofibroblasts via the activation of the TGF-β signaling pathway. The activation of TGF-β requires the binding of integrin to an arginine–glycine–aspartic acid (RGD) sequence in the latency-associated peptide (LAP) of TGF-β, leading to the dissociation of LAP and the release of active TGF-β. Indeed, integrin α5 could interact with LAP (Fig. 5a), and higher levels of active TGF-β were observed in the culture medium of CCL20-treated MRC-5 cells than in that of control cells (Fig. 5b). Additionally, we examined the levels of active TGF-β in the supernatants of CCL20-treated MRC-5 cells by measuring its effect on Smad-mediated transcription. As shown in Fig. 5c, the supernatants from CCL20-treated MRC-5 cells significantly increased Smad binding element (SBE4)-luciferase reporter activity. The phosphorylation of Smad2 and Smad3 (p-Smad2 and p-Smad3), which are canonical downstream transcription factors that mediate the effects of TGF-β, was significantly increased in CCL20-treated mouse primary lung fibroblasts and MRC-5 human lung fibroblasts, as determined by immunoblotting (Fig. 5d, e). Once the TGF-β signaling pathway is activated, p-Smad3 translocates to the nucleus to regulate target gene expression. IF staining revealed that p-Smad3 accumulated in the nuclei of primary lung fibroblasts and MRC-5 cells after CCL20 stimulation (Fig. 5f, g). In primary lung fibroblasts pretreated with ATN-161, CCL20 failed to activate the TGF-β signaling pathway, as indicated by the decreased levels of p-Smad2 and p-Smad3 and the reduced accumulation of p-Smad3 in the nucleus (Fig. 5h, i). Similar results were obtained in MRC-5 human lung fibroblasts (Fig. 5j, k). These data verify that the CCL20–integrin α5β1 interaction in lung fibroblasts activates the TGF-β signaling pathway.
a CoIP analysis of the interaction between LAP and integrin α5. b Ratio of active TGF-β to total TGF-β in the culture medium of CCL20-treated MRC-5 cells, as determined by ELISA (n = 3 technical replicates per group). c TGF-β-reporter luciferase activity in HEK293T cells in response to the cell supernatant of fibroblasts treated with or without CCL20 (n = 4 technical replicates per group). d Immunoblots showing the levels of phosphorylated and total Smad3 or Smad2 in primary fibroblasts following CCL20 stimulation (n = 3 technical replicates per group). e Immunoblots showing the levels of phosphorylated and total Smad3 or Smad2 in MRC-5 cells following CCL20 stimulation (n = 3 technical replicates per group). f IF staining for phosphorylated Smad3 in primary fibroblasts stimulated with CCL20. Scale bars, 20 μm. g IF staining for phosphorylated Smad3 in MRC-5 cells stimulated with CCL20. Scale bars, 20 μm. h Immunoblots showing the levels of phosphorylated and total Smad3 or Smad2 in primary fibroblasts treated with or without ATN-161 in the presence of CCL20 (n = 3 technical replicates per group). i IF staining for phosphorylated Smad3 in primary fibroblasts treated with or without ATN-161 in the presence of CCL20. Scale bars, 20 μm. j Immunoblots showing the levels of phosphorylated and total Smad3 or Smad2 in MRC-5 cells treated with or without ATN-161 in the presence of CCL20 (n = 3 technical replicates per group). k IF staining for phosphorylated Smad3 in MRC-5 cells treated with or without ATN-161 in the presence of CCL20. Scale bars, 20 μm. l IF staining for α-SMA in primary fibroblasts treated with or without SIS3 in the presence of CCL20. Scale bars, 20 μm. m IF staining for α-SMA in MRC-5 cells treated with or without SIS3 in the presence of CCL20. Scale bars, 20 μm. n Expression of Acta2 in primary fibroblasts treated with or without SIS3 in the presence of CCL20 (n = 3 technical replicates per group). o Expression of ACTA2 in MRC-5 cells treated with or without SIS3 in the presence of CCL20 (n = 3 technical replicates per group). p Schematic diagram of the method used to evaluate the therapeutic effects of ATN-161 on the PF model. q Hydroxyproline levels in lung tissues from the indicated mice after BLM exposure (n = 7 samples for BLM + PBS, BLM + PBS + ATN161, BLM + CCL20 + ATN161, n = 6 samples for BLM + CCL20 + PBS). r Masson’s trichrome staining of lung tissues from the indicated mice after BLM exposure. Scale bar, 100 μm. s The expression levels of Fibronectin and Acta2 in lung tissues from the indicated mice after BLM exposure (n = 6 mice per group). t Immunoblots showing the levels of phosphorylated and total Smad3 or Smad2 in primary lung fibroblasts isolated from the indicated mice (n = 3 samples per group). The data are presented as the means ± SEM. The data in b–e, s, t were analyzed via two-tailed Student’s t-test. The data in h–j, n–o, q were analyzed via one-way ANOVA. n.s., not significant; *p < 0.05; **p < 0.01; ***p < 0.001. Source data are provided as a Source data file.
To examine whether the CCL20–integrin α5β1 interaction activates fibroblasts by enhancing the TGF-β signaling pathway, we treated primary lung fibroblasts and MRC-5 cells with CCL20 with or without SIS3, which is an inhibitor of Smad3 phosphorylation. IF staining and PCR revealed that inhibiting TGF-β/Smad signaling activation with SIS3 completely abolished the upregulation of α-SMA caused by CCL20 (Fig. 5l–o). Furthermore, we tested the effect of integrin α5β1 inhibition on the CCL20-induced aggravation of PF in vivo (Fig. 5p), and the intraperitoneal injection of ATN-161 reduced the changes in PF induced by CCL20 treatment (Fig. 5q–s). Additionally, the inhibition of integrin α5β1 almost completely abolished the effect of CCL20 on the activation of TGF-β/Smad signaling in primary lung fibroblasts (Fig. 5t). Taken together, our data show that by binding to integrin α5β1, CCL20 increases active TGF-β levels and enhances the TGF-β/Smad signaling pathway, leading to the activation of fibroblasts.
Disturbing the CCL20 and integrin α5β1 interaction protects mice from BLM/HCl-induced PF
The interaction of CCL20 with integrin α5β1 activates fibroblasts and contributes to lung fibrogenesis; thus, disrupting this interaction may be a therapeutic strategy for PF. We tried to interrupt this interaction by screening for α-helical peptides that can inhibit this protein–protein interaction (PPI). An α-helix peptide (Pep-CCL20) at residues 79–93 in the CCL20 domain is the closest α-helical region to the CCL20–integrin α5β1 interaction site according to the homology modeling results. Pep-CCL20 exhibited a high affinity for integrin α5β1 (Fig. 6a). We performed CoIP analysis to determine whether Pep-CCL20 could disturb the interaction between CCL20 and integrin α5β1 and found that Pep-CCL20 reduced the interaction between CCL20 and integrin α5 but did not affect the interaction between CCL20 and integrin β1 (Fig. 6b). Additionally, Pep-CCL20 had no effect on the interaction of CCL20 with CCR6 (Supplementary Fig. 7a). Pep-CCL20 reduced the abundance of active TGF-β in MRC-5 cells (Fig. 6c). Accordingly, the phosphorylation of Smad3 was inhibited by Pep-CCL20 in primary lung fibroblasts and MRC-5 cells (Supplementary Fig. 7b, c). Moreover, Pep-CCL20 suppressed the activation of primary lung fibroblasts induced by CCL20, as revealed by the reduced expression of α-SMA and COL1 and decreased migratory ability (Fig. 6d–f and Supplementary Fig. 7d). Similar results were observed in MRC-5 cells (Fig. 6g–I and Supplementary Fig. 7e). The therapeutic effect of Pep-CCL20 was subsequently assessed in mice following BLM induction. The mice were treated with Pep-CCL20 on day 10 after the last BLM exposure and were sacrificed on day 40 (Fig. 6j). The administration of Pep-CCL20 significantly alleviated BLM-induced lung injury and fibrosis, as indicated by reduced collagen deposition and decreased hydroxyproline levels (Fig. 6k, l). The mice that were administered Pep-CCL20 exhibited significant reductions in the expression of α-SMA, fibronectin, COL1, Col3a1, and p-Smad3 in the lung (Fig. 6m–o). Taken together, our data suggest that Pep-CCL20 inhibits PF in a BLM-induced mouse model. Finally, the therapeutic effect of Pep-CCL20 was assessed in a hydrochloric acid (HCl)-induced PF model (Supplementary Fig. 7f). Consistently, mice administered Pep-CCL20 presented reduced collagen deposition (Supplementary Fig. 7g), which was coupled with significant reductions in the expression of fibrosis-related genes (Acta2, Fibronectin, Col1a1 and Col3a1) (Supplementary Fig. 7h). These data indicate the antifibrotic effects of Pep-CCL20 on the HCl-induced PF model. Collectively, these data show that Pep-CCL20 accelerates fibrosis resolution in mouse models and highlight the potential for the use of this agent to treat PF patients.
a SPR analysis of the kinetic interaction of Pep-CCL20 and integrin α5β1. b IP analysis of the interaction between CCL20 and integrin α5 or integrin β1 in the presence of Pep-CCL20. c TGF-β-reporter luciferase activity in HEK293T cells exposed to the supernatant of fibroblasts subjected to the indicated treatments. d Expression of ACTA2 in primary fibroblasts treated with or without Pep-CCL20 in response to CCL20 stimulation. e Immunoblot showing the levels of COL1 in primary fibroblasts treated with or without Pep-CCL20 in response to CCL20 stimulation. f Migration analysis of primary fibroblasts treated with or without Pep-CCL20 in response to CCL20 stimulation. Scale bars, 100 μm. g Expression of ACTA2 in MRC-5 cells treated with or without Pep-CCL20 in response to CCL20 stimulation. h Immunoblot showing the levels of COL1 in MRC-5 cells treated with or without Pep-CCL20 in response to CCL20 stimulation. i Migration analysis of fibroblasts treated with or without Pep-CCL20 in response to CCL20 stimulation. Scale bars, 100 μm. j Schematic diagram of the method for evaluating the therapeutic effects of Pep-CCL20 on the PF model. Masson’s trichrome staining (k), hydroxyproline content (l) (n = 6 samples per group), protein and mRNA expression of genes associated with fibrosis (m, n), and IHC staining for phosphorylated Smad3 (o) in lung tissues from PBS- or Pep-CCL20-treated PF mice (n = 6 samples per group). Scale bars, 100 μm (k) and 50 μm (m, o). The data are presented as the means ± SEM. For (c–i), n = 3 technical replicates per group. For (m–o), n = 3 samples per group. The data in m, o were analyzed via two-tailed Student’s t-test. The data in c–i, l were analyzed by one-way ANOVA. *p < 0.05; **p < 0.01; ***p < 0.001. Source data are provided as a Source data file.
CCL20 and clinical PF
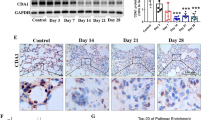
To examine the correlation between our findings and human PF progression, we first examined CCL20 expression in lung sections from PF patients and healthy controls. Immunohistochemical (IHC) staining revealed that CCL20 was highly expressed in the lung tissues of PF patients compared with those of healthy controls (Fig. 7a). Consistently, higher CCL20 protein levels were detected in the serum of IPF and non-IPF ILD patients than in the serum of healthy controls (Fig. 7b). To explore the correlation between serum CCL20 levels and disease severity in IPF patients, associations between serum CCL20 levels and lung function parameters were analyzed. Notably, CCL20 levels were negatively correlated with the percentage of predicted forced vital capacity (FVC%) and the percentage of predicted carbon monoxide diffusing capacity (DLCO%) in IPF patients (Fig. 7c, d). We further categorized the IPF cohort into 4 stages based on DLCO% predicted: stage I (≥80%), II (60%–80%), III (40%–60%), and IV (20%–40%). CCL20 levels gradually increased with increasing IPF severity (stage I to IV) (Supplementary Fig. 7i). These findings showed that higher serum CCL20 levels are correlated with disease severity. We also performed IHC analysis of two previously reported pivotal chemokines associated with PF, CCL18 and CCL2136,37, in human lung tissue samples. IHC analysis revealed marked upregulation of both CCL18 and CCL21 in pulmonary tissues from PF patients compared with those from controls (shown in Revised Supplementary Fig. 7j, k). Furthermore, we detected elevated serum CCL18 levels in both IPF and non-IPF ILD patients, as determined by ELISA (shown in Revised Supplementary Fig. 7l). The serum level of CCL18 was negatively associated with the percent-predicted forced vital capacity (FVC% predicted) but not the percent-predicted carbon monoxide diffusing capacity (DLCO% predicted) (shown in Supplementary Fig. 7m, n). Moreover, the average serum CCL18 concentration increased as the disease stage increased (shown in Revised Supplementary Fig. 7o). The serum levels of CCL18 in IPF patients were significantly greater than those of CCL20 (CCL18 vs CCL20: 140.434 ± 64.327 ng/ml vs 3.979 ± 2.482 pg/ml), indicating the potential role of CCL18, but not CCL20, as a biomarker for IPF. CCL20 should be regarded as a therapeutic target for IPF. We performed IF staining for CCL20 and E-Cad, a marker of AECs, in lung sections from control individuals and patients with PF. CCL20 predominantly colocalized with AECs from PF patients (Fig. 7e). The reanalysis of data from another study further confirmed that CCL20 levels were increased in AEC2s from IPF lungs compared with those from control lungs (Fig. 7f). Consistently, lower JUN expression was detected in AEC2s from PF patients than in those from controls (Fig. 7g). Furthermore, primary human lung fibroblasts were isolated from fresh human lung tissues and treated with CCL20 in the presence or absence of Pep-CCL20 (Fig. 7h). The results revealed that CCL20 treatment activated primary human lung fibroblasts, as indicated by the increased expression of fibrosis-related genes, increased number of α-SMA-positive cells, and increased migration ability (Fig. 7i–k). Moreover, we obtained human precision-cut lung slices (PCLSs), which constitute an ex vivo culture system that mimics in vivo conditions in humans38, from fresh human lung tissues to clarify the therapeutic effect of Pep-CCL20 on a human fibrosis model (Fig. 7l). PCLSs from control lungs were stimulated with BLM, which resulted in pathological structural changes and increases in collagen deposition and α-SMA expression, and these effects were reduced in Pep-CCL20-treated PCLSs (Fig. 7m, n). Moreover, we treated PCLSs derived from fibrotic lung tissues with Pep-CCL20. The results revealed that collagen deposition and the expression of fibrosis-related genes were significantly reduced following Pep-CCL20 treatment (Fig. 7o, p). These data further confirm that CCL20 plays a critical role in fibroblast activation in humans.
a IHC staining for CCL20 in lung tissues from patients with PF (n = 56 samples per group) and age-matched controls (n = 24 samples per group). Scale bar, 50 μm. b ELISA of the CCL20 content in the serum of patients with IPF (n = 44 samples per group) and non-IPF ILD (n = 17 samples per group) compared with healthy controls (n = 13 samples per group). Analysis of the correlation between CCL20 levels and lung function in IPF patients (n = 44 samples per group), as measured by the FVC% (c) and DLCO% (d). e IF staining for E-cad and CCL20 in lung tissues from patients with PF and age-matched controls. Scale bars, 100 μm. f CCL20 expression in AEC2s from the lung tissue of patients with IPF and age-matched controls (n = 3 samples per group). Data were obtained from an open-source dataset (GSE94555). g IF staining for JUN and CCL20 in lung tissues from patients with PF and age-matched controls. Scale bars, 10 μm. h Schematic overview of the experimental design for (i–k). i IF staining for α-SMA in fibroblasts treated with or without Pep-CCL20 and stimulated with CCL20. Scale bars, 20 μm. j PCR analysis of fibrogenic genes in fibroblasts treated with or without Pep-CCL20 and stimulated with CCL20 (n = 3 technical replicates per group). k Migration analysis of fibroblasts treated with or without Pep-CCL20 and stimulated with CCL20 (n = 6 technical replicates per group). Scale bars, 100 μm. l Schematic representation of the experimental setup. m HE and Masson’s trichrome staining of PCLSs prepared from healthy lungs treated with Pep-CCL20 in the presence of BLM for 5 days. Scale bars, 100 μm. n α-SMA immunostaining of PCLSs prepared from healthy lungs treated with Pep-CCL20 in the presence of BLM for 5 days. Scale bars, 50 μm. o HE and Masson staining of PCLSs prepared from PF lungs treated with Pep-CCL20 for 5 days. Scale bars, 100 μm. p Relative mRNA expression of the indicated genes in PCLSs prepared from PF lungs treated with PBS or Pep-CCL20 (n = 4 samples per group). The data are presented as the means ± SEM. The data in a, b, f, p were analyzed by two-tailed Student’s t-test. The data in c, d were analyzed by Two-tailed Pearson correlation analysis. The data in j, k were analyzed by one-way ANOVA. *p < 0.05; **p < 0.01; ***p < 0.001. Source data are provided as a Source data file.
Discussion
CCL20 has been reported to participate in the occurrence of human lung diseases, such as asthma39,40,41,42,43. However, the role of CCL20 in PF is poorly understood. In this study, we first showed that the CCL20–integrin α5β1–TGF-β axis was crucial for modulating fibroblast activation during PF. The principal findings were as follows: (i) CCL20 protein levels in BALF were elevated and contributed to lung fibrogenesis; (ii) the reduced expression of JUN in AEC2s following chronic lung injury led to an increase in CCL20 levels in BALF during PF pathogenesis; (iii) CCL20 bound to integrin α5β1, increased active TGF-β levels and enhanced the activity of the TGF-β/Smad signaling pathway, leading to the activation of fibroblasts; and (iv) genetic knockout of CCL20 in AEC2s, neutralization of CCL20, or disruption of the CCL20 and integrin α5β1 interaction by a screened α-helix peptide alleviated PF. Our findings highlight the importance of CCL20 as a key regulator of integrin α5β1 function in fibroblasts during PF.
Several chemokines, such as CCL18 and CCL21, have been reported to be associated with PF36,37. Our results align with prior evidence identifying CCL18 and CCL21 as significantly elevated chemokines in PF. Given the higher serum levels of CCL18 than those of CCL20 in IPF patients, our data indicate the potential role of CCL18, but not CCL20, as a biomarker for IPF, which is consistent with the findings of Elhai et al., which reported that CCL18 could be used as a serum biomarker for interstitial lung disease diagnosis and prognosis37. Notably, while these chemokines exhibit strong pathophysiological associations, persistent knowledge gaps remain regarding their clinical utility in PF diagnosis and prognosis as standalone or combinatorial serum biomarkers. This validation would require a large cohort with longitudinal follow-up—a scale not yet achieved in our existing biorepositories. Accumulating evidence has shown that CCL20 plays important roles in tissue fibrosis, such as hepatic fibrosis, renal fibrosis, and cystic fibrosis22,44,45,46. Increased CCL20 levels in airways promote cystic fibrosis by inducing CCR6-mediated lung homing of circulating human group 2 innate lymphoid cells (ILC2s)44. CCL20 interacts with CCR6 and contributes to the recruitment of γδ T cells into the injured liver, which protects the liver from inflammation and fibrosis45. CCL20 production, which is stimulated by soluble biglycan, is responsible for CCR6-positive Th17 cell recruitment and renal fibrogenesis46. These observations show that the role of CCL20 in fibrotic diseases relies on its interaction with CCR6, its only known receptor, and the subsequent recruitment of immune cells to injury sites. In this study, we observed that the CCL20-neutralizing antibody suppressed the expansion of Th17 cells in the BLM-induced PF model. Given the crucial roles of Th17 cells in lung fibrosis, whether T cells also act as direct targets of CCL20, as well as the inner mechanism underlying this process, remains to be determined in a future study, which will provide an in-depth understanding of the pathogenesis of PF. A reduction in the number of myofibroblasts, which are effector cells in the PF, was observed in CCL20 antibody-treated mice. This discovery prompted us to assess the impact of CCL20 on fibroblasts. We found that CCL20 acted as a profibrotic cytokine for fibroblast activation. The sole receptor for CCL20 is CCR6, and we further examined whether CCL20 could drive fibroblast activation in a CCR6-dependent manner. Interestingly, CCR6 deficiency did not abolish the activation of fibroblasts caused by CCL20, suggesting that the effect of CCL20 on fibroblast activation was independent of CCR6. Using CoIP–MS analysis, we identified integrin α5β1 as a receptor for CCL20. CCL20 treatment failed to activate fibroblasts after integrin α5β1 silencing or inhibition. The interaction of CCL20 with integrin α5β1 on lung fibroblasts increased the levels of active TGF-β, which is a typical profibrotic factor47, and thus facilitated TGF-β-induced fibroblast activation. Therefore, our findings identify the unknown receptor and mechanism by which CCL20 regulates fibroblast activation.
The next important issue was how CCL20 expression was upregulated in AEC2s. Given that the increase in CCL20 expression occurred at the transcriptional level, we attempted to identify the transcription factor responsible for this upregulation. Among all the transcription factors whose expression was significantly altered in AECs during PF, only the expression of Jun, Tfdp1, and Nr3c2 correlated with CCL20 protein levels in the BALF. Modulating the expression of Tfdp1 or Nr3c2 in lung epithelial cells did not affect CCL20 expression. We showed that JUN, whose expression was reduced in AEC2s, contributed to the upregulation of CCL20. Several transcription factors can bind to the CCL20 promoter, including NF-κB, CCAAT/enhancer-binding proteins, ESE-1, SP1, and AP-148,49,50,51. Most of them act as activators to increase the expression of CCL20. Interestingly, in this study, we identified JUN as a novel transcriptional repressor of CCL20, and a reduction in JUN expression in AEC2s following lung injury was shown to be responsible for the increased levels of CCL20 in BALF during PF.
Integrins, which typically function as signaling proteins, are transmembrane glycoprotein receptors that consist of an α-subunit and a β-subunit, and they include 18 and 8 isoforms, respectively, resulting in 24 known heterodimers52. Accumulating evidence has revealed that several integrin heterodimers play crucial roles in organ fibrosis, including lung fibrosis, hepatic fibrosis, kidney fibrosis, and skin fibrosis53. Integrins that contain the αv subunit, such as αvβ1 and αvβ6, have been shown to be crucial in PF54,55. These αv-containing integrins contribute to PF by directly binding to the RGD motif in the LAP of TGF-β and mediating its activation56. Several inhibitors of αvβ6 and/or αvβ1 integrins have progressed to clinical trials for patients with PF53. Integrin α5β1 was also reported to play a role in PF by binding to the RGD motif in fibronectin, contributing to fibroblast migration or EMT57,58,59. However, whether integrin α5β1 can induce TGF-β activation by binding to the RGD motif in the LAP of TGF-β has not been assessed. In this study, higher expression of the integrin α5β1 was detected in the lung tissues of mice with PF than in those of control mice. We confirmed that integrin α5β1 interacts with CCL20, can bind to LAP in latent TGF-β, and induces the release of active TGF-β, which increases the phosphorylation of Smad2/3, contributing to fibroblast activation. Volociximab, a chimeric monoclonal antibody that specifically binds to integrin α5β1, has advanced to clinical trials for several diseases, including cancer and age-related macular degeneration60,61. Further exploration of the therapeutic effects of volociximab on lung fibrosis will be of interest.
Although two drugs (pirfenidone and nintedanib) have been approved for the treatment of IPF, neither is known to be curative. Peptide drugs, which exhibit obvious advantages such as high biological activity and specificity, have become a research hotspot and important direction in the current international new drug research and development field. Our previous study, along with others, confirmed that α-helical peptides designed to disrupt the protein‒protein interactions involved in PF pathogenesis effectively reduce PF pathology62,63. Because we investigated the critical role of the CCL20–integrin α5β1–TGF-β axis in PF development, we screened and generated an α-helical peptide consisting of a fifteen-amino acid deletion fragment of CCL20 that disrupts the interaction of CCL20 and integrin α5β1 and exerts potent therapeutic effects on PF by reducing the activity of the TGF-β signaling pathway and suppressing fibroblast activation. Our study offers a potential candidate for the treatment of fibrosing interstitial lung diseases.
In summary, our study revealed that CCL20 is involved in the progression of PF. Increased levels of CCL20 in BALF, which result from the reduced expression of JUN in AEC2s, contribute to PF pathogenesis by binding to integrin α5β1 on the membrane of lung fibroblasts, from which active TGF-β is released and drives the activation of fibroblasts. Therefore, our findings provide new insights into lung fibrogenesis and show that the CCL20–integrin α5β1 signaling pathway may be a target for treating fibroproliferative diseases.
Limitations of the study
Our current study has several limitations. Our study focused on the role of the CCL20–integrin α5β1 interaction in fibroblast activation during PF progression. However, we cannot rule out the possibility that other integrin α5β1+ cells interact with CCL20 and contribute to lung fibrogenesis. In addition, our study revealed that the CCL20–integrin α5β1 interaction activated the TGF-β/Smad signaling pathway, and whether other downstream signaling pathways are activated by CCL20–integrin α5β1 remains to be determined in a future study. Moreover, the stability of peptide drugs is relatively poor. Further studies are needed to improve the stability and increase the druggability of peptide drugs. Follow-up studies are needed to clarify whether the peptide has any side effects.
Methods
Human subjects
Fresh human lung explants and serum were obtained from PF patients and healthy controls. Patients with PF were diagnosed through multidisciplinary discussion. Control subjects were recruited from individuals undergoing routine health check-ups at the health examination center. The clinical characteristics of the serum donors are presented in Supplementary Table 1.
PF lung tissues were collected from PF patients undergoing transplantation. Control lung samples obtained from patients who underwent surgery for pulmonary nodules were at least 10 cm away from the lesion. The clinical characteristics of the donors for the lung tissues are detailed in Supplementary Table 2. All participants provided written informed consent, and ethics approval was obtained from the Human Ethics Committee of the Second Xiangya Hospital of Central South University (approved no. 2022‒052). The study conformed to the principles outlined in the Declaration of Helsinki.
A human pulmonary interstitial fibrosis tissue array (LC561) was purchased from Tissue Array Com LLC (Derwood, MD, USA) and contained 24 pulmonary fibrosis tissues and 2 normal lung tissues. A human normal lung tissue microarray (LCN241) was purchased from Tissue Array. The Com LLC contained 24 samples of lung tissues.
Precision-cut lung slices
PCLSs from human lungs were prepared as previously described38. PCLSs from PF lungs were cultured in DMEM supplemented with 10% FBS and incubated with the peptide Pep-CCL20 (5 μM) for 5 days. The PCLSs were subjected to HE, Masson’s trichrome, or gene expression analysis. PCLSs from normal lungs were treated with BLM (7.5 mU/ml) (Selleck, Shanghai, China) in the presence or absence of the peptide Pep-CCL20 (2.5 μM or 5 μM). Five days later, the PCLSs were subjected to HE, Masson’s trichrome, and IF staining. The media (±stimulation) was replenished every other day.
Animal studies
C57BL/6J mice (18‒20 g, 6‒8 weeks of age) were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, Hunan). Ccl20fl/fl mice were generated by Cyagen Biosciences (Suzhou, China). Sftpc-iCre mice, Rosa26-tdTomato mice, and Ccr6-/- mice were purchased from GemPharmatech Co., Ltd. (Nanjing, China). To generate mice with CCL20 conditional knockout in AEC2 cells, Ccl20fl/flSftpccre mice were generated by intercrossing Ccl20fl/fl transgenic mice with Sftpc-iCre mice. Eight-week-old Ccl20fl/fl mice were intratracheally administered the AAV-Sftpc-Cre virus (1.5 × 1010 vg/mouse, HanBio Biotechnology, Shanghai, China) 14 days before the induction of the PF model. Genetically targeted mice were genotyped via standard PCR. All animals were housed under pathogen-free conditions in a temperature-controlled environment at 22‒24°C with a 12-h light/dark cycle. The mice had free access to food and water.
For the animal studies, the mice were earmarked and randomly separated into groups by an independent person. We ensured that the experimental groups were almost identical in terms of animal age and weight. The sample size was predetermined empirically according to our previous experience using the same strains and treatments. In general, we used n ≥ 6 mice per group. For all the experiments, young adult (6‒8-week-old) male mice were used. The animal experiments were approved by the Ethics Committee of the Second Xiangya Hospital of Central South University. All procedures were conducted according to the guidelines of the Institutional Animal Care and Use Committees of the Second Xiangya Hospital of Central South University. All animal procedures were consistent with the ARRIVE guidelines64.
Animal models
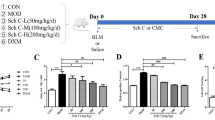
The mice were repeatedly administered BLM to establish the BLM-induced PF mouse model, as previously described61. The mice were anesthetized via an intraperitoneal injection of 400 mg/kg avertin (Sigma‒Aldrich, St. Louis, MO, USA). BLM in 50 μL of LPS-free PBS (1 U/kg) was intratracheally instilled 6 times at 14-day intervals. The mice were sacrificed 10 days after the last BLM injury. A total of 20 μL of an iso-osmolar solution of 0.1 M HCl was administered to the mice via intratracheal injection for a total of 6 treatments at intervals of 14 days to establish the HCl model.
For CCL20 administration in vivo, the mice were subjected to intratracheal injections of 100 μg/kg recombinant mouse CCL20 (Peprotech, NJ, USA) or an identical volume of saline once per week for a total of 6 injections. The mice were sacrificed 10 days after the last CCL20 administration.
For the Pep-CCL20 experiments, the mice were intraperitoneally injected with Pep-CCL20 twice per week beginning on day 10 after the last BLM or HCI administration. The mice in the control group were treated with PBS. The mice were sacrificed by the administration of excess anesthesia on day 40 after the last BLM or HCI administration.
For CCL20-neutralizing antibody administration in vivo, the mice were intratracheally administered the CCL20 neutralizing antibody (5 μg/mouse, R&D Systems, Minneapolis, MI, USA) twice per week for 4 weeks beginning on day 10 after the last BLM administration. An IgG antibody was injected into the mice in the control group.
To evaluate the effect of CCL20 on fibroblast activation in vivo, Rosa26-LSL-Cas9-tdTomato (Rosa26-tdTomato) mice were crossed with Col1a2-CreERT2 mice to generate Col1a2-CreERT2Rosa26-tdTomato mice. For Cre induction, the mice were intraperitoneally injected with 50 mg/kg tamoxifen (Sigma‒Aldrich) for 7 consecutive days. On days one and four after the last injection, the mice were intratracheally injected with Ad-Ccl20 (2 × 109 pfus/mouse; Beijing Likely Biotechnology, China). The mice were sacrificed three days after receiving Ad-Ccl20, and their lung tissues were harvested.
Cell lines and primary cultures
Human embryonic kidney 293T cells were purchased from Shanghai Bioleaf Biotech Co., Ltd., and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, NY, USA) supplemented with 10% fetal bovine serum (FBS) (Excell, Shanghai, China). Mouse alveolar epithelial MLE-12 cells were purchased from Miaolingbio (Wuhan, China) and cultured in DMEM/F12 (Gibco) supplemented with 10% FBS. Human fibroblast MRC-5 cells were purchased from Procell (Wuhan, China) and cultured in Minimum Essential Medium (MEM) (Procell) supplemented with 10% FBS. Primary mouse lung fibroblasts were cultured in DMEM supplemented with 15% FBS and 1% penicillin‒streptomycin. The cells were authenticated by morphological examination and confirmed to be mycoplasma free. All the cells were cultured at 37°C in a 5% CO2 incubator.
Mass spectrometry
To identify CCL20-interacting proteins, MRC-5 cells were treated with 100 ng/mL CCL20 for 30 min. Cell lysates were extracted from CCL20-treated MRC-5 cells and immunoprecipitated with protein A/G beads coupled to mouse anti-human monoclonal CCL20 antibodies (R&D Systems) or IgG at 4°C overnight, followed by five washes with lysis buffer. The immunoprecipitates were subjected to LC‒MS/MS sequencing and data analysis by Oebiotech Co., Ltd. (Shanghai, China). Briefly, the proteins were digested in the gel and extracted, followed by separation of the digestion products via a C18 column on the EASY-nLCTM 1200 system. The peptides were identified via a Q Exactive mass spectrometer equipped with a Nanospray Flex source (Thermo, USA). The MS/MS data were analyzed with the human fasta from UniProt via an in-house Proteome Discoverer (V.2.4, Thermo, USA). Unique peptides were defined as those exclusively assigned to a specific protein group in the database search. The entire list of proteins identified by MS analyses are shown in Supplementary Data 1.
High-resolution microscale proteomic sequencing
The immunoprecipitates were prepared as described above for mass spectrometry and subjected to high-resolution microscale proteomic sequencing and data analysis by the Majorbio Proteomic Service (Shanghai, China). Briefly, protein extraction and Peptides were prepared using Majorbio microprotein kit. Peptides from the preceding step were desalted with C18 tips; the eluate was dried in a vacuum concentrator, resuspended in 0.1% FA, and quantified by UV spectrophotometry on a NanoDrop One instrument (Thermo Scientific). The peptides were analyzed by an VanquishNeo coupled with a timsTOF Ultra2 mass spectrometer (Bruker, Germany). Chromatography was limited to an 8-min gradient. Data-independent acquisition (DlA) spectra were recorded on an Orbitrap Astral mass spectrometer in DIA mod. The detection was carried out over a mass range of 380-980m/z(MS1), and 150‒2000 m/z(MS2). The raw data were analyzed with Spectronaut software (Version 19). Parameters were configured as follows:: The peptide length range was set to 7‒52; Enzyme cutting site was trypsin/P. The maximum missed cleavage site was carbamidomethylation of cysteines as fixed modification, and oxidation of methionines and protein N-terminal acetylation as variable modifications, Protein FDR ≤0.01, Peptide FDR ≤0.0 l, Peptide Confidence ≥99%, XIC width ≤75 ppm. The protein quantification method was MaxLFQ. The entire list of differentially expressed proteins identified by high-resolution microscale proteomic sequencing are shown in Supplementary Data 2.
ELISA
BALF was obtained from control mice or mice with PF via lavage with 1 mL of saline per mouse. To remove debris and cells, the BALF samples were centrifuged at 1000 × g for 10 min at 4°C. The levels of CCL20 in the BALF were measured using a commercial ELISA kit (Multi Science, Hangzhou, China) according to the manufacturer’s instructions.
Immunohistochemistry
The left lobes of the lungs were fixed with 4% paraformaldehyde for 48 h at room temperature. After paraffin embedding, the mouse lungs were sliced into 5 μm sections and subjected to IHC staining. In brief, the sections were incubated with antibodies specific for CCL20 (Bioss, Beijing, China), α-SMA (Boster, Wuhan, China), integrin α5 (Proteintech, Wuhan, China), integrin β1 (Proteintech), or p-Smad3 (Affinity, Jiangsu, China) at 4°C overnight, followed by staining with secondary antibodies (ZSGB-BIO, Beijing, China). The sections were then stained with horseradish peroxidase (HRP)-conjugated streptavidin and developed using DAB solution (ZSGB-BIO). The nuclei were stained with hematoxylin (Biosharp). After dehydration, the slides were mounted with resinene. Histological images were acquired with an optical microscope (Olympus IX71; Olympus Optical, Tokyo, Japan).
Immunostaining
For IF staining, cells cultured on coverslips or frozen tissue sections were fixed with 4% paraformaldehyde, followed by permeabilization with 0.5% Triton X-100 at room temperature for 20 min. After being blocked with 3% BSA at 37°C for 1 h, the samples were incubated with the indicated antibodies at 4°C overnight. After three washes with PBS, the samples were incubated with the corresponding secondary antibodies for 2 h at room temperature. 4,6-Diamidino-2-phenylindole (DAPI) was used to stain the nuclei. Images were captured using a Zeiss LSM 780 laser scanning confocal microscope.
Lung function measurement
The mice were anesthetized and placed on a flexiVent pulmonary system (SCIREQ Inc., Montreal, Canada). The mechanical ventilation condition was set as a tidal volume of 10 mL/kg and a respiratory rate of 150 breaths/min. A positive end expiratory pressure (PEEP) of 3 cmH2O was used for lung function measurement. Dynamic pulmonary compliance was evaluated by Snapshot-150 perturbation.
RNA extraction and real-time PCR
Total RNA was isolated from the cell samples using an RNA extraction kit (Promega, Madison, WI, USA). The RNA was reverse transcribed into cDNA using the TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix Kit (TransGen, Beijing, China) according to the manufacturer’s instructions. PCR amplification was performed in triplicate; each reaction contained 1x SYBR FAST qPCR Master Mix (KAPA BIOSYSTEMS, Boston, MA, USA), 1 μL of mixed primers, and 1 μL of template cDNA and was run for 40 cycles using an Applied Biosystems® ViiA™ 7 Real-Time PCR System. GAPDH was used as the housekeeping gene. The primers used for qPCR are listed in Supplementary Table 3.
Hydroxyproline assay
Commercial kits were used to measure the hydroxyproline levels in mouse lungs (BIV-K555-100; BioVision Technologies Inc., San Francisco, CA, USA) according to the manufacturer’s instructions.
Isolation of hematopoietic cells and nonhematopoietic cells
Mouse lungs were perfused sequentially with 10 mL of PBS through the right ventricle, followed by perfusion with 1 mL of dispase (1 mg/mL; Roche, Basel, Switzerland) and 0.5 mL of warm 1% low melting point agarose (Thermo Fisher, Waltham, MA, USA). To obtain a single-cell suspension, the lung lobes were separated, minced, and digested with dispase (1 mg/mL) and DNase I (50 U/mL) in DMEM for 40 min at 37°C with frequent agitation before being filtered through 100- and 40-μm strainers. The cells were then suspended in ACK lysis buffer to lyse RBCs. After three washes with PBS and centrifugation at 600 × g for 10 min, the cells were incubated with anti-CD45-FITC (BioLegend, San Diego, CA, USA). Hematopoietic cells (CD45+) and nonhematopoietic cells (CD45-) were sorted using a BD FACSAria III cell sorter.
Isolation of primary fibroblasts
Primary fibroblasts were isolated from 6- to 8-week-old male C57BL/6 mice as previously described65. In brief, the mice were sacrificed by excessive anesthesia. The chest was subsequently opened, and the whole lung was removed. After being cut into 1 mm3 pieces in DMEM containing 15% FBS and 2% penicillin‒streptomycin, the tissues were spread evenly in a 10 cm dish. After 5–7 days of culture, the adherent fibroblasts were harvested for passage.
Isolation of primary AEC2s
AEC2s were isolated as described previously with minor modifications66. In brief, single-cell suspensions of mouse lungs were isolated. Primary biotinylated antibodies, including anti-CD45 (BioLegend), anti-CD16/32 (BioLegend), anti-CD34 (BioLegend), and anti-CD31 (BioLegend), were added to cells suspended in 500 μL of DMEM containing 50 U/mL DNase I. After being incubated on ice for 60 min, the cells were washed and incubated with streptavidin-labeled magnetic beads (2.5 μL of SA MyOne T1 beads/1 × 106 cells) for 30 min on a rotator, and the labeled cells were then removed with a magnetic separator. The cells were further stained with antibodies, including anti-CD24-PE (Abcam, Cambridge, UK), anti-Sca-1-APC (BioLegend), and anti-EpCAM-PE-Cy7 (BioLegend). AEC2s (PE-Cy7+APC-PE-) were sorted using a BD FACSAria III cell sorter. The data were analyzed using FCS Express 6 software.
Isolation of pulmonary capillary endothelial cells
Endothelial cells were isolated as previously described with minor modifications67. Briefly, single-cell suspensions were isolated from mouse lungs, and the cells were incubated with anti-CD31-PE and anti-intercellular cell adhesion molecule (ICAM)-2-APC antibodies (BioLegend). The CD31+ICAM2+ pulmonary capillary endothelial cells were sorted using a BD FACS Aria III cell sorter.
Cell migration assay
Migration assays were performed using 24-well transwell chambers with polycarbonate membranes with an 8 μm pore size (Corning, NY, USA). The chambers were precoated with 10 μg/mL fibronectin on the lower surface. MRC-5 cells or primary fibroblasts were seeded in the upper chamber in FBS-free culture medium, and the bottom chamber contained FBS-free medium supplemented with the indicated concentrations of recombinant CCL20 alone or in combination with ATN-161 (5 nM, Selleck) or Pep-CCL20 (5 μM). After 12 h of incubation at 37°C, the cells that remained on the upper side of the filters were removed with a cotton swab. The cells that migrated toward the bottom chamber were fixed with 4% paraformaldehyde and then stained with crystal violet solution (Beyotime Technology, Shanghai, China) for 2 h. Then, the cells in three to five random fields were counted via an optical microscope.
Senescence-associated β-galactosidase staining
Senescence-associated β-galactosidase (SA-β-gal) staining was performed using a Senescence β-Galactosidase Staining Kit (Beyotime Technology, Shanghai, China). MRC5 cells were seeded in 12-well plates at a density of 5 × 104 cells/well and treated with different concentrations of CCL20 or TGF-β for 24 h. Then, the cells were fixed at room temperature for 15 min. The staining mixture was subsequently added and incubated overnight at 37°C without CO₂. On the following day, the cells were washed with PBS and observed under a light microscope. SA-β-gal-positive cells, which exhibited a bluish-green color, were quantified by counting three randomly selected fields of view. The results are expressed as the percentage of SA-β-gal-positive cells relative to the total number of cells.
Sirius red collagen detection
Collagen levels were detected using a Sirius Red Total Collagen Detection Kit (Chondrex, Woodinville, WA, USA) according to the manufacturer’s instructions. In brief, culture medium from primary lung fibroblasts treated with or without CCL20 was collected and incubated with a concentrating solution at 4°C overnight. After centrifugation at 10,000 rpm for 3 min, the pellet was dissolved in 0.05 M acetic acid and incubated with Sirius Red solution. The sample solutions were then centrifuged and dissolved in extraction buffer. Optical density values were measured using a microplate reader, and the collagen levels were calculated by regression analysis.
Three-dimensional collagen gels
Primary lung fibroblast suspensions at a density of 2 × 105/mL in serum-free medium were mixed with 3 mg/mL neutralized rat tail collagen type 1 (Corning) at a ratio of 2:1. The mixture was subsequently seeded on 24-well plates and allowed to coagulate at 37°C for 1 h. Then, the edge of the gel was detached from the well walls, and 1 mL of medium was added. After 48 h, images of the gels were acquired, and the gel area was measured using ImageJ software (US National Institutes of Health).
Flow cytometry
To examine CCR6 expression in primary lung fibroblasts, PE-conjugated CCR6 antibody (BioLegend) or its isotype control was added to 100 mL (1 × 106 cells) cell suspensions and incubated on ice for 20 min. After being centrifuged at 350 × g for 5 min, the cells were washed twice with cell staining buffer before flow cytometric analysis. The data were collected using a Cytek NL-3000 flow cytometer (Cytek Biosciences, Fremont, CA, USA) and analyzed with FCS EXPRESS 6 software.
Immunoprecipitation
CoIP was performed to verify protein‒protein interactions. The cell lysates were gently mixed with the indicated antibodies at 4°C overnight, followed by incubation with Protein A/G Plus-Agarose (Santa Cruz Biotechnology, TX, USA) at 4°C for 2 h. Then, the immunocomplex was washed 4–5 times with lysis buffer and boiled in 2× SDS sample buffer for 10 min. The samples were resolved by denaturing SDS‒PAGE and blotted with the indicated antibodies.
Biacore test
SPR was conducted with a BIAcore T200 instrument (GE Health Science Inc., Marlborough, MA, USA). Integrin α5β1 was immobilized by amine coupling. After activation of a CM5 chip with N-hydroxysuccinimide and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide, the integrin α5β1 protein in PBS was passed over the chip until >500 response units (RU) were achieved. The activated free binding sites were blocked with ethanolamine. The analyte CCL20 or Pep-CCL20 was injected at a flow rate of 30 mL/min for a contact time of 180 s, followed by autonomous dissociation for 600 s. The kinetic curve and Kd value were calculated using BIAevaluation software.
Plasmid construction
Myc-DDK-tagged integrin α5 was obtained from Origene, Myc-tagged integrin β1 and GFP-tagged human CCL20 were obtained from YouBio (Changsha, China), and HA-tagged CCR6 was purchased from Sino Biological, Inc. (Beijing, China). CCL20 mutants (D38A, D59A, K78A, K91A, K94A, and N95A), integrin α5 mutants (T341A and H345A), and integrin β1 mutants (R26A and K398A) were generated using the Fast Mutagenesis System (TransGen).
Immunoblotting
Tissues and cells were lysed with RIPA lysis buffer (Beyotime Technology) containing PMSF and a protease inhibitor cocktail (MCE, HY-K0010). Total protein was then quantified using a BCA protein assay kit (Beyotime Technology). Equal quantities of the indicated proteins were separated by 10% SDS‒PAGE and transferred to PVDF membranes. After being blocked with skim milk, the PVDF membranes were incubated overnight with primary antibodies against GAPDH (Proteintech), α-SMA (Boster), COL1 (Invitrogen, CA, USA), Smad3 (Cloud-Clone Corp, Wuhan, China), Smad2 (Proteintech), p-Smad2 (Cell Signaling Technology, MA, USA), or p-Smad3 (Affinity) at 4°C, followed by incubation with a horseradish peroxidase-labeled secondary antibody. Images were visualized using ChemiDoxTM XRS+ with Image LabTM Software (Bio-Rad, Hercules, CA, USA) with a chemiluminescence detection reagent (Tanon, Shanghai, China).
Statistical analysis
The data were obtained from at least two independent experiments. Statistical analyses were performed using GraphPad Prism 8 (San Diego, CA, USA). Comparisons between two groups were performed using unpaired two-tailed Student’s t-test. Multiple-group comparisons were performed using one-way ANOVA followed by Tukey’s multiple comparisons test or Dunnett’s multiple comparisons test. The results are presented as the means ± standard errors of the means (SEM). In all the cases, a p-value < 0.05 was considered statistically significant. Asterisk coding is indicated in the figure legends as *p < 0.05, **p < 0.01, and ***p < 0.001. The sample sizes are presented in the figure legends.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All the data supporting the results of this study are presented within the article, the Supplementary Information, or the Source Data file. Additional information can be obtained from the corresponding authors upon reasonable request. The mass spectrometry proteomics data have been deposited in the ProteomeXchange Consortium with the dataset identifier PXD061156. The original data of high-resolution microscale proteomic sequencing have been deposited in the ProteomeXchange Consortium with the dataset identifier PXD064810. Source data are provided with this paper.
References
Wuyts, W. A. et al. The pathogenesis of pulmonary fibrosis: a moving target. Eur. Respir. J. 41, 1207–1218 (2013).
Lederer, D. J. & Martinez, F. J. Idiopathic pulmonary fibrosis. N. Engl. J. Med. 378, 1811–1823 (2018).
Wijsenbeek, M. & Cottin, V. Spectrum of fibrotic lung diseases. N. Engl. J. Med. 383, 958–968 (2020).
Margaritopoulos, G. A., Vasarmidi, E. & Antoniou, K. M. Pirfenidone in the treatment of idiopathic pulmonary fibrosis: an evidence-based review of its place in therapy. Core Evid. 11, 11–22 (2016).
Richeldi, L. et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N. Engl. J. Med. 370, 2071–2082 (2014).
Zabihi, M. et al. Understanding myofibroblast origin in the fibrotic lung. Chin. M. J. Pulm. Crit. Care Med. 2, 142–150 (2024).
Todd, N. W., Luzina, I. G. & Atamas, S. P. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenes. Tissue Repair 5, 11 (2012).
Liu, S. S. et al. The chemokine CCL1 triggers an AMFR-SPRY1 pathway that promotes differentiation of lung fibroblasts into myofibroblasts and drives pulmonary fibrosis. Immunity 54, 2042–2056 (2021).
Wang, Q. R. et al. CCL17 drives fibroblast activation in the progression of pulmonary fibrosis by enhancing the TGF-beta/Smad signaling. Biochem. Pharmacol. 210, 115475 (2023).
Boutanquoi, P. M. et al. TRIM33 prevents pulmonary fibrosis by impairing TGF-beta1 signalling. Eur. Respir. J. 55, 1901346 (2020).
Lee, C. H., Shah, B., Moioli, E. K. & Mao, J. J. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J. Clin. Investig. 120, 3340–3349 (2010).
Vij, N., Sharma, A., Thakkar, M., Sinha, S. & Mohan, R. R. PDGF-driven proliferation, migration, and IL8 chemokine secretion in human corneal fibroblasts involve JAK2-STAT3 signaling pathway. Mol. Vis. 14, 1020–1027 (2008).
Hoyles, R. K. et al. An essential role for resident fibroblasts in experimental lung fibrosis is defined by lineage-specific deletion of high-affinity type II. Am. J. Respir. Crit. Care Med. 183, 249–261 (2011).
Jiang, B. C., Liu, T. & Gao, Y. J. Chemokines in chronic pain: cellular and molecular mechanisms and therapeutic potential. Pharmacol. Ther. 212, 107581 (2020).
Nagarsheth, N., Wicha, M. S. & Zou, W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat. Rev. Immunol. 17, 559–572 (2017).
Zlotnik, A. & Yoshie, O. The chemokine superfamily revisited. Immunity 36, 705–716 (2012).
Ozga, A. J., Chow, M. T. & Luster, A. D. Chemokines and the immune response to cancer. Immunity 54, 859–874 (2021).
Zijlstra, G. J. et al. Glucocorticoids induce the production of the chemoattractant CCL20 in airway epithelium. Eur. Respir. J. 44, 361–370 (2014).
Demedts, I. K. et al. Accumulation of dendritic cells and increased CCL20 levels in the airways of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 175, 998–1005 (2007).
Ito, M. et al. Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 565, 246–250 (2019).
Affo, S. et al. CCL20 mediates lipopolysaccharide induced liver injury and is a potential driver of inflammation and fibrosis in alcoholic hepatitis. Gut 63, 1782–1792 (2014).
Gao, B. & Xu, M. Chemokines and alcoholic hepatitis: are chemokines good therapeutic targets?. Gut 63, 1683–1684 (2014).
Mor, A. et al. Blockade of CCL24 with a monoclonal antibody ameliorates experimental dermal and pulmonary fibrosis. Ann. Rheum. Dis. 78, 1260–1268 (2019).
Hoffmann-Vold, A. M. et al. High level of chemokine CCL18 is associated with pulmonary function deterioration, lung fibrosis progression, and reduced survival in systemic sclerosis. Chest 150, 299–306 (2016).
Adams, T. S. et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci. Adv. 6, eaba1983 (2020).
Habermann, A. C. et al. Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci. Adv. 6, eaba1972 (2020).
Tsukui, T. et al. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat. Commun. 11, 1920 (2020).
Fang Y. et al. RUNX2 promotes fibrosis via an alveolar-to-pathological fibroblast transition. Nature 640, 221–230 (2025).
Kallal, L. E., Schaller, M. A., Lindell, D. M., Lira, S. A. & Lukacs, N. W. CCL20/CCR6 blockade enhances immunity to RSV by impairing recruitment of DC. Eur. J. Immunol. 40, 1042–1052 (2010).
Schutyser, E., Struyf, S. & Van Damme, J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 14, 409–426 (2003).
Wang, C., Kang, S. G., Lee, J., Sun, Z. & Kim, C. H. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal Immunol. 2, 173–183 (2009).
Marsigliante, S., Vetrugno, C. & Muscella, A. Paracrine CCL20 loop induces epithelial-mesenchymal transition in breast epithelial cells. Mol. Carcinog. 55, 1175–1186 (2016).
Kadomoto, S. et al. Tumor-associated macrophages induce migration of renal cell carcinoma cells via activation of the CCL20-CCR6 axis. Cancers 12, 89 (2019).
Yao, L. D. et al. Dysregulated bidirectional epithelial–mesenchymal crosstalk: A core determinant of lung fibrosis progression. Chin. M. J. Pulm. Crit. Care Med. 2, 27–33 (2024).
Nolte, M. & Margadant, C. Controlling immunity and inflammation through integrin-dependent regulation of TGF-beta. Trends Cell Biol. 30, 49–59 (2020).
Mothes, R. et al. Distinct tissue niches direct lung immunopathology via CCL18 and CCL21 in severe COVID-19. Nat. Commun. 14, 791 (2023).
Elhai, M. et al. Circulating lung biomarkers in idiopathic lung fibrosis and interstitial lung diseases associated with connective tissue diseases: Where do we stand?. Semin. Arthritis Rheum. 50, 480–491 (2020).
Uhl, F. E. et al. Preclinical validation and imaging of Wnt-induced repair in human 3D lung tissue cultures. Eur. Respir. J. 46, 1150–1166 (2015).
Weckmann, M. et al. Critical link between TRAIL and CCL20 for the activation of TH2 cells and the expression of allergic airway disease. Nat. Med. 13, 1308–1315 (2007).
Kuchibhotla, V. N. S. et al. Inhibition of beta-catenin/CBP signalling improves airway epithelial barrier function and suppresses CCL20 release. Allergy 75, 1786–1789 (2020).
Faiz, A. et al. Profiling of healthy and asthmatic airway smooth muscle cells following interleukin-1beta treatment: a novel role for CCL20 in chronic mucus hypersecretion. Eur. Respir. J. 52, 1800310 (2018).
Wypych, T. P. et al. Microbial metabolism of L-tyrosine protects against allergic airway inflammation. Nat. Immunol. 22, 279–286 (2021).
Post, S. et al. ADAM10 mediates the house dust mite-induced release of chemokine ligand CCL20 by airway epithelium. Allergy 70, 1545–1552 (2015).
Schulz-Kuhnt, A. et al. ILC2 lung-homing in cystic fibrosis patients: functional involvement of CCR6 and impact on respiratory failure. Front. Immunol. 11, 691 (2020).
Hammerich, L. et al. Chemokine receptor CCR6-dependent accumulation of gammadelta T cells in injured liver restricts hepatic inflammation and fibrosis. Hepatology 59, 630–642 (2014).
Nastase, M. V. et al. Biglycan, a novel trigger of Th1 and Th17 cell recruitment into the kidney. Matrix Biol. 68, 293–317 (2018).
Frangogiannis, N. Transforming growth factor-beta in tissue fibrosis. J. Exp. Med. 217, e20190103 (2020).
Hoffmann, A. & Baltimore, D. Circuitry of nuclear factor kappaB signaling. Immunol. Rev. 210, 171–186 (2006).
Kwon, J. H., Keates, S., Simeonidis, S., Grall, F., Libermann, T. A. & Keates, A. C. ESE-1, an enterocyte-specific Ets transcription factor, regulates MIP-3alpha gene expression in Caco-2 human colonic epithelial cells. J. Biol. Chem. 278, 875–884 (2003).
Zhao, L., Xia, J., Wang, X. & Xu, F. Transcriptional regulation of CCL20 expression. Microbes Infect. 16, 864–870 (2014).
Chang, L. H. et al. Role of macrophage CCAAT/enhancer binding protein delta in the pathogenesis of rheumatoid arthritis in collagen-induced arthritic mice. PLoS ONE 7, e45378 (2012).
Hynes, R. O. Integrins: a family of cell surface receptors. Cell 48, 549–554 (1987).
Slack, R. J., Macdonald, S. J. F., Roper, J. A., Jenkins, R. G. & Hatley, R. J. D. Emerging therapeutic opportunities for integrin inhibitors. Nat. Rev. Drug Discov. 21, 60–78 (2022).
Puthawala, K. et al. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am. J. Respir. Crit. Care Med. 177, 82–90 (2008).
Reed, N. I. et al. The alphavbeta1 integrin plays a critical in vivo role in tissue fibrosis. Sci. Transl. Med. 7, 279–288 (2015).
Margadant, C. & Sonnenberg, A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. Embo. Rep. 11, 97–105 (2010).
Cheng, W. H., Lee, K. Y., Yu, M. C., Chen, J. Y., Lin, C. H. & Chen, B. C. Pref-1 induced lung fibroblast differentiation by hypoxia through integrin alpha5beta1/ERK/AP-1 cascade. Eur. J. Pharmacol. 909, 174385 (2021).
Zhao, X. K. et al. Focal adhesion kinase regulates fibroblast migration via integrin beta-1 and plays a central role in fibrosis. Sci. Rep. 6, 19276 (2016).
Chen, Y. C. et al. Particulate matters increase epithelial-mesenchymal transition and lung fibrosis through the ETS-1/NF-kappaB-dependent pathway in lung epithelial cells. Part. Fibre Toxicol. 17, 41 (2020).
Besse, B. et al. Phase Ib safety and pharmacokinetic study of volociximab, an anti-alpha5beta1 integrin antibody, in combination with carboplatin and paclitaxel in advanced non-small-cell lung cancer. Ann. Oncol. 24, 90–96 (2013).
Kuwada, S. K. Drug evaluation: volociximab, an angiogenesis-inhibiting chimeric monoclonal antibody. Curr. Opin. Mol. Ther. 9, 92–98 (2007).
Liu, S. S. et al. TRIB3‒GSK-3β interaction promotes lung fibrosis and serves as a potential therapeutic target. Acta Pharm. Sin. B. 11, 3105–3119 (2021).
Liu, S. S. et al. Targeting degradation of the transcription factor C/EBPbeta reduces lung fibrosis by restoring activity of the ubiquitin-editing enzyme A20 in macrophages. Immunity 51, 522–534 (2019).
Kilkenny, C. et al. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br. J. Pharmacol. 160, 1577–1579 (2010).
Seluanov, A., Vaidya, A. & Gorbunova, V. Establishing primary adult fibroblast cultures from rodents. J. Vis. Exp. 44, 2033 (2010).
Liang, J. et al. Hyaluronan and TLR4 promote surfactant-protein-C-positive alveolar progenitor cell renewal and prevent severe pulmonary fibrosis in mice. Nat. Med. 22, 1285–1293 (2016).
Rafii, S. et al. Platelet-derived SDF-1 primes the pulmonary capillary y vascular niche to drive lung alveolar regeneration. Nat. Cell. Biol. 17, 123–136 (2015).
Acknowledgements
We are grateful to Professor Zhuowei Hu for his guidance and valuable suggestions on this work during his lifetime. We thank Dr. Jianfeng Wu (School of Life Sciences, Xiamen University, Fujian, China) for helping construct the Ccl20fl/flSftpccre mice. This work was supported by grants from the National Natural Science Foundation of China (82273997 and 82322002 to S.S.L.), the Hunan Natural Science Foundation (2022JJ30820 to S.S.L.), the Beijing Natural Science Foundation (7222260 to S.S.L.), the Hunan Provincial Health Commission Research Program (A202302045948 to S.S.L.), and the Central South University Innovation-Driven Research Program (2023CXQD026 to S.S.L.).
Author information
Authors and Affiliations
Contributions
S.S.L. (Shanshan Liu) and X.L. conceived and designed the project. S.S.L. (Suosi Liu) and Q.R.W. led the in vitro and mouse studies, with assistance from J.L.M., Z.Y.Z., and Y.Z. (Yu Zhang), J.H.Y., L.P.T., and X.N.T. S.S.L. (Suosi Liu), Q.R.W., M.Y., and Y.Z. (Yan Zhang) analyzed the data. H.P., Z.K.X., and Y.X.T. provided the human samples (lung tissues and serum) and supported the clinical interpretation of the data. Y.X. and Z.G.Z. provided scientific advice and expertise. S.S.L. (Shanshan Liu), X.L., Q.R.W., J.L.M., and S.S.L. (Suosi Liu) wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Leonard Foster, Argyrios Tzouvelekis, and the other, anonymous, reviewer for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, S., Wang, Q., Min, J. et al. The CCL20–integrin α5β1 interaction enhances TGF-β/Smad signaling to promote fibroblast activation in pulmonary fibrosis. Nat Commun 16, 9183 (2025). https://doi.org/10.1038/s41467-025-64211-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-64211-6