Abstract
Plants recognize environmental information as external signals, which they use to coordinate developmental processes for their survival and reproduction. In the agricultural production of rice, the primary staple crop worldwide, soil nitrogen (N) is an important environmental factor. Heading date, a key trait for rice quality and yield, is modulated by N fertilisation; however, the molecular mechanisms underlying N-dependent flowering regulation remain unclear. Here, we conduct a genome-wide association study using differences in heading dates of Japanese rice cultivars grown under different N conditions. We identify Hd6, which is known to be involved in photoperiodic flowering regulation, as a key signalling component in the phenotypic variation observed under various N conditions. Further analyses using near-isogenic lines reveal that not only Hd6 but also Hd2 and Hd1 are required for the delayed flowering caused by N fertilisation. We also discover that Hd6 regulates floral inducer genes by stabilizing Hd2 through phosphorylation in response to N conditions and that the Hd6-Hd2 module can counteract the transcriptional regulation of Hd1. This study elucidates the molecular pathway directly linking N responses to flowering regulation in rice, providing insights for novel breeding and cultivation strategies.
Similar content being viewed by others
Introduction
Plants sense and interpret environmental cues as external signals that help them adjust their growth and development to optimise survival and reproductive success. Flowering regulation determines seasonal and regional adaptability, which in cereal crops is highly associated with yield1,2,3. In rice, a model crop of short-day plants, photoperiod (i.e., day length) sensitivity influences critical day length to initiate phase transition by interacting with circadian clocks. The two major floral inducers, Heading date 1 (Hd1) and Early heading date 1 (Ehd1), promote the expression of the florigen genes, Hd3a and rice flowering locus T1 (RFT1). These florigens are preferentially induced in leaves under short-day conditions and act as mobile, long-distance hormonal signals that trigger flowering4.
The identification of quantitative trait loci affecting flowering time (heading date in rice) under field conditions has revealed genes involved in the photoperiodic pathway of floral induction in rice, known as heading date (HD) genes. One remarkable HD gene is Hd1, a rice ortholog of Arabidopsis floral activator CONSTANS, and was the first HD gene to be cloned5,6. Hd1 is a bifunctional floral regulator which promotes the expression of floral inducer genes and flowering under short-day conditions and represses them under long-day condition in coordination with other flowering regulatory genes such as Hd2, Ghd7 and DTH87,8,9,10. Hd2 (also named pseudo response regulator 37 (PRR37)/DTH7/Ghd7.1) encoding a pseudo-response regulator protein that harbours the CCT domain significantly represses heading and increases grain yield under long-day conditions11,12,13,14. In addition, dozens of HD genes such as Grain number, plant height and heading date 7 (Ghd7)/Hd4, Ghd8/Hd5/DTH8, and rice indeterminate 1 (RID1)/Ehd2/OsID1 have been functionally analysed and positioned in the large genetic network that regulates rice flowering (reviewed in Vicentini et al.15). Furthermore, the HD genes include not only transcription factor genes but also post-transcriptional regulator genes. Hd6 and Hd16 encode two distinct casein kinases, CKII α subunit (CK2α) and CKI, respectively16,17,18. Hd16 can act as an inhibitor in flowering by directly phosphorylation of Ghd717. While Hd6 was identified long before Hd16, there is only genetic evidence that Hd6 can repress flowering and florigen gene expression in cooperation with Hd1 and Hd219,20. A biochemical study demonstrated that a recombinant Hd6 protein could phosphorylate a recombinant Hd2 protein in vitro21. However, the physiological and molecular functions of the Hd6-Hd2 module remain elusive.
As mentioned above, rice flowering is mainly controlled by the photoperiod, but other environmental factors are also involved. It has been well characterized that the environmental temperature perception pathway is strongly intertwined with the photoperiod pathway and essentially converges on the same genes to modify flowering time15,22. More studies have revealed that some flowering genes are responsive to abiotic stress23. Nitrogen (N), one of the most important nutrients for plants, is also a key regulatory factor that controls plant development and growth to ensure plant survival and reproduction24. In rice cultivation, excessive N fertilisation can delay flowering via reducing florigen expression23,25. Recently, it was reported that there are multiple types of flowering time responding to N among rice varieties, suggesting that genetic factor(s) determine the response26. N fertilisation-induced flowering delay is an important physiological phenomenon that is often exploited in agricultural practices, for example, to control the flowering time of two parents during seed production of F1 hybrid varieties.
N-dependent flowering delay has been reported in various plant species, such as Arabidopsis thaliana, maize, wheat and barley26. In Arabidopsis, nitrate can modulate the expression of floral inducer genes, including FT, which is an ortholog of Hd3a. Furthermore, changes in the expression of specific transcription factors and/or their interactions correlate well with variation in the expression of floral integrators, which may contribute to the genotype- and photoperiod-specific flowering time responses to nitrate supply change27,28. Another study showed that FLOWERING BHLH 4 and its downstream gene CO positively regulate flowering under low nitrogen conditions29. In rice, the MYB transcription factor Nhd1 and nitrate transporter OsNRT1.1A are involved in flowering regulation in response to N25,30. However, the molecular mechanisms underlying N-dependent flowering regulation in rice, particularly the relationship between N concentrations and the photoperiod-regulatory network consisting of HD genes, remain largely unknown.
In this study, we conduct genome-wide association study (GWAS) and find that Hd6 is a mediator of flowering regulation by N in rice. In addition, we discover that the Hd6-Hd2 module directly regulates the expression of flowering-inducing genes by counteracting Hd1 protein.
Results
GWAS of days to heading (DTH) under different N concentration conditions
To evaluate the effect of N fertiliser on flowering, we measured DTH in 149 Oryza sativa japonica varieties that were not highly genetically structured and interrelated (Supplementary Fig. 1a and Supplementary Data 1)31 to avoid excessive allelic heterogeneity and increase the sensitivity of signal detection in GWAS32. We cultivated the rice population in the field, with three levels of N fertilization (low, normal and high; 0, 9 and 18 kgN/10 a) and measured DTH in 2016. A comparative analysis of DTH among the three N conditions revealed that 64.8% of plants showed delayed flowering under normal versus low conditions, and 78.3% and 82.7% of plants in high showed delayed flowering when compared with normal and low plants, respectively (Fig. 1a). Further, DTH in each variety varied in response to the N condition, suggesting that this population was suitable for the isolation of genetic factor(s) related to flowering time responses to N levels.
a Violin plots showing day to heading (DTH) of 149 Japanese cultivars in 2016 under low, normal and high N fertilizer condition. Each dot connected by lines indicates DTH of the same cultivars. The DTH data was shown in Supplementary Data 1. b Genome-wide association study (GWAS) using the difference of DTH of the cultivars under low and high N conditions. Horizontal red lines indicate 5% genome-wide significance threshold after Bonferroni-correction. Blue lines indicate -log10P values = 5. c Local Manhattan plot (top) and LD heatmap (bottom) surrounding the candidate peak on Chr. 3. Red dashed lines indicate the close up of the peak region shown in this figure (b). d Exon-intron structure of Hd6 with polymorphisms inducing amino acid exchanges. e Violin plots showing DTH of 149 Japanese cultivars that have loss-of-function (haplotype [Hap.] A) and gain-of-function (Hap. B). The data corresponds to that shown in (a). Haplotype information is shown in Supplementary Data 1.
Next, we performed GWASs of DTH under the three conditions and observed similar pattern to the previous GWASs using DTH of Japanese rice cultivars, and some peaks were related to previously identified genes, HESO1 and Hd1 (Supplementary Fig. 1b–g)33,34. However, there was a significant peak on the terminal end of the long arm of chromosome (Chr.) 3 under the high N condition (Supplementary Fig. 1d). To further clarify the genomic region related to the response to N levels, we conducted additional GWASs considering the difference and ratio of DTH among the conditions (Fig. 1b, Supplementary Figs. 2a–e and 3a–f). We detected a prominent peak, which corresponded to that found in the GWAS under the high N condition, in the GWASs using the difference and ratio of DTH between low and high, and normal and high conditions (Fig. 1b and Supplementary Fig. 2a, c, d), but not in the results for that between low and normal (Supplementary Fig. 2b, e). These results highlighted that the candidate region was significantly correlated to N-responsive flowering regulation. We additionally performed GWASs of principal component (PC) scores derived from PCA with the DTH data (Supplementary Figs. 2f, g and 3g, h). We found a slight but prominent peak on Chr. 3 in a GWAS using PC2, which corresponded to that found in the above GWASs (Supplementary Fig. 2g), suggesting that PC2 mainly represented a response to N levels. Actually, the contribution of PC1 of the PCA was higher than 98% and that of PC2 was less than 2% and the factor loadings of the N conditions were different in PC2 but not in PC1 (Supplementary Fig. 2f).
Similar analyses for DTH data of 128 varieties grown under the same three N levels in 2017 also revealed a non-significant but prominent association between the candidate region and DTH difference and ratio under low vs. high and normal vs. high conditions (Supplementary Figs. 4a–j and 5a–i and Supplementary Data 2). The GWASs of PC scores from the analyses using the DTH data of 2017 roughly replicated those in 2016 (Supplementary Figs. 4k–m and 5j, k). These results strongly implied that the peak on Chr. 3 is mainly associated region with delayed flowering under high N levels.
Hd6 is a candidate gene for N-responsive flowering regulation
To identify the candidate region on Chr. 3, we calculated pairwise linkage disequilibrium (LD) correlations around the peak and found an LD block from 30.30 Mb to 31.8 Mb (Fig. 1c). The candidate region of the peak included three polymorphisms with significant associations and amino acid exchanges or stop-loss variants (Supplementary Data 3). One of the polymorphisms was a stop-loss variant in LOC_Os03g55389 (Hd6), which encodes CK2α16,20. Because Hd6 reportedly plays a major role in the photoperiodic control of flowering, we focused on this gene as a candidate gene of N-responsive flowering regulation. There were two haplotypes in the coding region of Hd6 in our population: haplotype (Hap) A and Hap B. Hap A reportedly is a loss-of-function type because of a premature stop codon16,33, whereas Hap B is functional (Fig. 1d). We compared DTH at the different N levels between the Hd6 haplotypes (Fig. 1e). In all N conditions, DTH was shorter for Hap A than for Hap B. DTH was longer under high than under low or normal in Hap B, while differences among the various N conditions were not observed in Hap A, which was consistent with the GWAS results. These results suggested that the functional Hd6 allele is required for N-responsive flowering regulation.
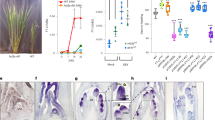
Studies have demonstrated that Hd6 requires functional Hd1 and Hd2 to control flowering time in rice19,20. Therefore, we investigated the epistatic interactions among Hd1, Hd2 and Hd6 in N-responsive flowering regulation by comparing DTH among functional (+/+) and non-functional (−/−) alleles in our population (Fig. 2a–c). Apparent differences were not observed among the three N levels, except for a combination of all functional alleles (Hd1(+/+) Hd2(+/+) Hd6(+/+)). This result supported that an epistatic interaction among Hd1, Hd2 and Hd6 is essential for N-responsive flowering regulation. To confirm this genetic relationship, we compared the DTH of five near-isogenic lines (NILs; NIL-Hd1/Hd2/Hd6, NIL-Hd2/Hd6, NIL-Hd1/Hd6, NIL-Hd1 and NIL-Hd2) and Nipponbare (Hd1/Hd2) under normal and high conditions (Fig. 2d, e). NIL-Hd1/Hd2/Hd6 (Hd1(+/+) Hd2(+/+) Hd6(+/+)) showed a delay in DTH under H compared to N, whereas the other NILs did not show such N response. These results demonstrated that Hd6 was a causal gene of the peak detected in GWAS for delayed flowering under high-N condition, and that the epistatic interaction among Hd1, Hd2 and Hd6 is a prerequisite for N-responsive flowering regulation.
a, b Exon-intron structure of Hd with polymorphisms inducing amino acid exchanges in our population. c Box plots showing days to heading of 149 Japanese cultivars in three N levels between eight functional and non-functional alleles combinations in Hd6, Hd1 and Hd2. The data corresponds to that shown in Fig. 1a. Haplotype information of each cultivar is shown in Supplementary Data 1. +/+, functional haplotype. −/−, non-functional haplotype. Letters indicate significant differences in each pattern of haplotype (P < 0.05, Tukey’s HSD test). The Tukey–Kramer’s HSD tests were separately conducted among the haplotype combinations. N means the number of cultivars having the haplotype pattern. d NIL-Hd1, NIL-Hd2 and NIL-Hd6 were introgressed with Kasalath segment carrying Hd1 (−/−), Hd2 (−/−) and Hd6 (+/+), respectively. White and red segments indicate Nipponbare and Kasalath with the names of the selection markers, respectively. e Boxplot for comparison of DTH of the NILs and Nipponbare (hd6/Hd1/Hd2) under normal and high N condition. P-values were calculated by two-tailed paired t-tests. The DTH date were obtained from more than four biological replicates. The box plot boundaries reflect the interquartile range; the centre line is the median and the whiskers represent 1.5× the interquartile range from the lower and upper quartiles. Source data are provided as a Source Data file.
Linkage between Hd6 and N concentration
To substantiate the genetic evidence at the molecular level, we first analysed the effect of N application on Hd6 expression. Consistent with previous observations35, exogenous application of various concentrations of NH4Cl as an N source to rice seedlings in hydroponic culture induced the expression of known N metabolic genes, including GS1;2 and AS1 (Supplementary Fig. 6a, b). However, Hd6 expression did not respond to the N treatment, and mRNA levels of Hd1 and Ghd7 tended to be reduced after N supply (Fig. 3a and Supplementary Fig. 6c, d). To clarify the interaction between Hd6 protein levels and N, we generated an anti-Hd6 antibody to detect endogenous Hd6 protein expression (Supplementary Fig. 7). Hd6 protein levels in NIL (Hd6) seedlings harbouring the functional Hd6 allele clearly showed a N dose-dependent increase, which peaked around 1.6 mM NH4Cl in this experimental condition (Fig. 3b). These results indicated that N regulates the amount of Hd6 protein, not its expression.
a Profile of Hd6 transcripts in response to different N concentrations in shoots. NIL(Hd6) seedlings were treated with different concentrations of NH4Cl as indicated after pretreatment of 0.4 mM NH4Cl for 3 days. Shoot parts were harvested as indicated hours (hr) after the N treatment for RNA extractions. mRNA levels were normalized by the copy numbers of Actin1 transcript. The mean values of 0.1 mM sample were set as 1. qPCR was performed in triplicate, and values with the SD are shown. There was no significant difference (P > 0.05) based on Tukey’s HSD test. b Profile of Hd6 protein in response to different N concentrations for 24 h. Sample preparation was essentially same as that in (a). Representative image was shown. Relative amounts of Hd6 protein were normalized to the values of the loading control, β-tubulin (β-Tub) and set value of 0.1 mM sample as 1 in the bottom of image. c Profile of Hd2 transcripts in response to different N concentrations in shoots. Samples correspond to that in (a). d Accumulation of 3FLAG-Hd2 in response to different concentrations of N source. Immunoblotting of 3FLAG-Hd2 was performed with crude extracts from 35S:Hd6/Ubq:Ω:3FLAG-Hd2 and Ubq:Ω:3FLAG-Hd2 seedlings treated with NH4Cl. Seedlings were treated with the indicated N concentrations for 24 h after pretreatment of a lower concentration for N starvation. Immunoblotting with anti-β-Tub antibody was used for the loading control. Each experiment was repeated independently for at least 3 times with similar results. e, f mRNA levels of Hd6 (e) and Hd2 (f) in transgenic plants. The mRNA levels were normalized by that of Ubq. Mean values with the SD were obtained by three independent qPCR reactions. Complementation of Hd6 and Hd2 function by introduction of functional Hd6 and Hd2 into NIL (hd6 hd16). Source data are provided as a Source Data file.
N influences the activity of the Hd6-Hd2 module
Since, as mentioned above, a genetic interaction between Hd6 and Hd2 has been revealed and our results indicated that Hd2 is required for N-responsive flowering regulation along with Hd6, we hypothesised that N may modulate the Hd6-Hd2 module. Like Hd6 expression, endogenous Hd2 expression is not affected by different N concentrations (Fig. 3c)11,12. To investigate Hd2 protein function in vivo, we analysed transgenic rice plants harbouring either a Ubq:Ω:3FLAG–Hd2 or a Ubq:Ω:3FLAG–Hd2 construct in tandem with CaMV35S–Hd6 (35S:Hd6/Ubq:Ω:3FLAG–Hd2) in a NIL (hd6 hd16) with natural variation for defective alleles of both Hd6 and Hd16 in the Nipponbare background. The presence of Hd6 protein significantly enhanced the immunological detection of 3FLAG-Hd2 in transgenic plants using anti-FLAG antibody, indicating that Hd6 protein enhances 3FLAG-Hd2 protein accumulation in vivo (Fig. 3d). 3FLAG-Hd2 clearly accumulated in response to N dosage in the presence of Hd6 (Fig. 3d). Artificial overexpression of Hd6 did not increase 3FLAG-Hd2 transcript levels (Fig. 3e, f). These results indicate that the turnover of 3FLAG-Hd2 protein is attenuated by N in an Hd6-dependent manner.
Consistent with the roles of Hd6 and Hd2 in the rice photoperiodic flowering pathway, the magnitude of floral repression was significantly and additively enhanced in the presence of Hd2 and Hd6 (Fig. 4a and Supplementary Fig. 8). Among these lines, only 35S:Hd6/Ubq:Ω:3FLAG–Hd2 plants showed the effect of high-N on the delay of flowering (Fig. 4a and Supplementary Fig. 9), confirming that Hd1, Hd2 and Hd6 are essential requisites for N-responsive flowering regulation as suggested by the experimental results obtained with the Japanese varieties and NILs (Fig. 2c, e), because Nipponbare harbours functional Hd1 and Hd2 and non-functional Hd6 (Supplementary Data 1).
a Comparison of the DTH between NIL and the transgenic plants. n = 3, P-values were calculated by one-tailed paired t-tests. The box plot boundaries reflect the interquartile range; the centre line is the median and the whiskers represent 1.5× the interquartile range from the lower and upper quartiles. b Venn diagram of the genes downregulated by high N; adjusted P value < 0.05, logFC ≥ −1. c Visualization of GO enrichment analysis using 103 DEGs (low N > high N only in 35S:Hd6/Ubq:Ω:3FLAG-Hd2). All detected 20 GO terms are shown. d mRNA levels of Ehd1, Hd3a and RFT1 in NIL (hd6, hd16) and transgenic plants grown under different N conditions. Total RNAs were extracted from 55-day-old plants of T2 generation. Mean values with the SD. n ≥ 5 independent experiments. **P value < 0.01, *P value < 0.05 and n.s indicates no significant difference based on two-sided student’s t-test. e Effects of kinase inhibitors on 3FLAG-Hd2 accumulation. 35S:Hd6/Ubq:Ω:3FLAG-Hd2 seedlings were treated with different types of casein kinase inhibitor as the indicated concentration for 6 h. Each experiment was repeated independently for at least 3 times with similar results. f CIP treatment to crude extracts prepared from 35S:Hd6/Ubq:Ω:3FLAG-Hd2 seedlings. Crude extracts were applied to SDS-PAGE after incubation with or without CIP. Each experiment was repeated independently for at least 3 times with similar results. g Profile of 3FLAG-Hd2 protein in immunoblotting using SDS-PAGE containing phos-tag. Asterisk with the bracket indicates a smearing pattern of the 3FLAG-Hd2 signal under high N sample. 60 μg of crude extracts were loaded per lane for immunoblotting, reacted with anti-FLAG antibody. Each experiment was repeated independently for at least 3 times with similar results. Source data are provided as a Source Data file.
To further analyse the repressive action of the Hd6-Hd2 module in N-responsive flowering, we conducted RNA-sequencing analyses of the transgenic plants under low and high conditions at ZT0 when the transcripts of the floral inducer genes are most abundant (Fig. 4b and Supplementary Fig. 10). We identified 409 differentially expressed genes (DEGs) that were unique to 35S:Hd6/Ubq:Ω:3FLAG–Hd2 plants in response to the N condition. Among these, 306 DEGs were significantly upregulated, and 103 were significantly downregulated in the transgenic plants under the high condition. Gene Ontology (GO) analysis of the unique DEGs in each transgenic line revealed that the 6 genes among the 103 DEGs downregulated in 35S:Hd6/Ubq:Ω:3FLAG–Hd2 plants were related to flowering and photoperiodism, whereas these GO terms were not found for genes upregulated in 35S:Hd6/Ubq:Ω:3FLAG–Hd2 plants and for DEGs in NIL or Ubq:Ω:3FLAG–Hd2 plants (Fig. 4c and Supplementary Fig. 11). Transcriptome profiling and quantitative reverse transcription (RT-q)PCR assays revealed significant decreases in the transcript levels of genes related to flower development under high condition only in 35S:Hd6/Ubq:Ω:3FLAG–Hd2 plants (Fig. 4d and Supplementary Data 4). These genes included Ehd1, Hd3a, RFT1, OsMADS14, OsMADS18 and LHS1/OsMADS1, which are major floral inducer and floral development genes. Based on these results, we concluded that N signalling uses the Hd6-Hd2 module to repress floral induction in the rice photoperiodic flowering pathway.
To clarify the biochemical relationship between Hd6 and Hd2, we further analysed the biochemical properties of 3FLAG-Hd2 protein using 35S:Hd6/Ubq:Ω:3FLAG–Hd2 plants. 3FLAG-Hd2 accumulation was cancelled in the presence of the CK2 inhibitor benzimidazole but not the CK1 inhibitor PF60462 (Fig. 4e), indicating that Hd6-dependent phosphorylation is essential for the stabilisation of the Hd2 protein. 3FLAG-Hd2 protein tended to produce a broad band, suggesting the detection of the phosphorylated Hd2 during this growth stage. As expected, the upper portion of the 3FLAG-Hd2 signal in immunoblots disappeared after protein treatment with alkaline phosphatase, confirming the phosphorylation (Fig. 4f). Furthermore, immunoblotting analysis using Phos-tag, which specifically captures phosphate residues, demonstrated that phosphorylated 3FLAG-Hd2 protein was super-shifted under the high N condition (Fig. 4g), suggesting 3FLAG-Hd2 phosphorylation under this condition. Mass spectrometric analysis of a purified recombinant Hd2 (rHd2) pre-incubated with or without recombinant Hd6 (rHd6) revealed a phosphorylation site in rHd2 by rHd6 on the serine at position 369 (S369) (Supplementary Fig. 12a–c), which is consistent with a previous study showing that the middle region of Hd2 can be phosphorylated by Hd621. These results demonstrated that N-induced Hd6 protein accumulation enhances the phosphorylation of S369 of Hd2 protein.
Repression of Hd2 integrated into the regulation of Hd1-dependent Hd3a expression
Given that Hd1 function is epistatic to Hd2 function in the rice photoperiodic flowering pathway9,36, floral repression conferred by Hd2 protein stabilised by Hd6 protein accumulated under high-N condition may be integrated into Hd1 action. The genetic relationships among Hd6, Hd2 and Hd1 have been revealed, but the molecular relationships remain unknown. Therefore, we analysed the physical relationships among these proteins. In rice mesophyll protoplasts, transiently expressed GFP-Hd6, GFP-Hd2 and GFP-Hd1 were observed in the nucleus (Fig. 5a). Hd6 was highly accumulated in the nucleolus, whereas Hd2 and Hd1 were not observed in the nucleolus (Fig. 5a and Supplementary Fig. 13a). A bimolecular fluorescence complementation (BiFC) assay revealed the presence of Hd6-Hd2 and Hd6-Hd1 complexes in the nucleus, and YFP signals were accumulated in the nucleolus (Fig. 5b, c and Supplementary Fig. 13b). The interaction of Hd2 and Hd1 was not observed in the presence or absence of Hd6. We confirmed the direct association of N-induced Hd6 protein produced in NIL plants expressing Hd6 with a recombinant Hd2 protein (rHd2) synthesised by E. coli, which indicated that the capacity of Hd6 proteins to form a complex with Hd2 in plants increases in response to exogenous N levels (Fig. 5d). We also performed domain analysis of Hd2 for interaction with Hd6 by BiFC assay. The Hd2 protein has two conserved domains: pseudo receiver (PR) and CCT (Supplementary Fig. 12a)21. Truncated Hd2 protein containing PR but not CCT (Nterm and ΔMCCT) interacted with Hd6 in the cytosol and truncated Hd2 containing the middle region (300–480) (Mreg and ΔCCT) interacted in the nucleus, whereas truncated Hd2 containing only the C-terminal region, including the CCT domain (Cterm), could no longer interact (Supplementary Fig. 13c). These results indicated that the PR domain and the middle region of Hd2 are essential for Hd6-Hd2 interaction and determine the subcellular localisation of the complex. In vitro pull-down assays using recombinant proteins produced in E. coli confirmed the Hd6-Hd2 interaction, whereas only a faint signal was observed for Hd1–Hd2 interaction and none for Hd1–Hd6 interaction (Supplementary Fig. 13d). Therefore, the physical interaction of the Hd6-Hd2 complex may be stable, whereas that of other combinations may be not or depend on the conditions.
a Subcellular localisation of GFP-Hd6, GFP-Hd1 and GFP-Hd2. Scale bars, 10 μm. Each experiment was repeated independently for at least 3 times with similar results. Individual and merged images of differential interference contrast images of protoplasts YFP (green) and chlorophyll autofluorescence (red) are shown. Scale bars, 10 μm. b BiFC assay for physical interaction among Hd6, Hd1 and Hd2. Each experiment was repeated independently for at least 3 times with similar results. Scale bars, 10 μm. c Quantification of BiFC assay in (b). Percentage of cells showing BiFC signal in nuclei is indicated by yellow bars with the total number of cells observed on the right. d Association of endogenous Hd6 protein with a recombinant Hd2 protein (rHd2). CT indicates Nipponbare. Immunologically detected Hd6 proteins were normalized with the digitalized values of the band in SDS-PAGE gel corresponding to the Rubisco large subunit (RbcL) as a loading control. Relative amounts of binding protein were set to the value of 0.1 mM NH4Cl sample as 1. Each experiment was repeated independently for at least 3 times with similar results. e Schematic presentation of reporter and internal control constructs used for our transient assay. Oligonucleotide sequence containing the reported Hd1 binding motif (CORE2) was shown in the middle of panel. f Effects of Hd2 and Hd6 on Hd1-dependent induction of CORE2 reporter expression. The activity by vector control (far left bar) was set as 1. P-values were calculated by two-tailed paired t-tests. Data are presented as mean values +/− SD. n = 3 independent experiments. g Hd2 repressive activity on the transactivation activity of Hd1 in dose-dependent manner. Letters indicate significant differences in each pattern of haplotype (P < 0.05, Tukey’s HSD test, n = 3 independent experiments). Data are presented as mean values +/− SD. h N-dependent regulation of the expression of the floral inducer genes through the photoperiodic flowering regulators in rice under LD condition. Source data are provided as a Source Data file.
As Hd1 protein can bind directly to CO response element 2 (CORE2) found in the Hd3a promoter proximal region37, we developed an in vivo transient reporter assay system using rice protoplasts prepared from rice seedlings to investigate the potential roles of Hd6 and Hd2 protein in CORE2-dependent Hd3a transcription in the presence of Hd1 protein. We found that Hd1 significantly activated firefly luciferase (LUC) expression driven by an artificial promoter, i.e., the 35S promoter inserted four times of CORE2-containing oligonucleotide in front of the minimal promoter (MP) (Fig. 5e, f). The repressive effect of Hd2 on the CORE2-containing promoter was observed upon single transfection of Hd2 (Supplementary Fig. 14), and co-transfection of Hd1 and Hd2 significantly reduced the Hd1 solo activation of the CORE2-containing promoter (Fig. 5f). The counteractive effect of Hd2 on Hd1 was dose-dependent (Fig. 5g). Therefore, Hd2 protein antagonises the CORE2-dependent action of Hd1 protein. In other words, the regulation of Hd3a expression via Hd1 and Hd2 is mutually exclusive. Furthermore, Hd6 can enhance the repressive effect of Hd2 (Fig. 5f), which is consistent with the accumulation of Hd2 in the presence of Hd6 (Fig. 3d). These results demonstrated that N-induced Hd6 protein accumulation enhances the phosphorylation of Hd2 protein, thus stabilising it for the regulation of floral inducer gene expression together with Hd1 (Fig. 5h).
Discussion
N fertilisation is closely related to flowering in plants, but studies in various crops, including rice have shown that the types of responses are not entirely consistent24,26. Multiple type of flowering time among rice varieties to N-response was reported26. However, the molecular mechanism, in particular the relationship between N and the photoperiod-regulatory network consisting of HD genes, remained elusive. We showed that N at a high dosage significantly delayed heading of rice in the natural field (Fig. 1a). A GWAS using DTH data of Japanese rice varieties under different N conditions identified a region on Chr. 3 containing the gene(s) responsible for N-responsive flowering regulation (Fig. 1b). Subsequent genetic analysis revealed that this response requires Hd6 in the candidate region, and Hd2 and Hd1, which have been previously identified as regulators in the photoperiodic flowering pathway of rice (Figs. 1c–e and 2). Near this candidate region, another important HD gene, Hd16, is located, but all polymorphism,s including the functional one on this gene had low -log10(p) (Supplementary Fig. 15a)16,17. Excluding varieties carrying the non-functional Hd16 (Hap. B) had no effect on the observed relationship between N and the haplotypes of Hd6, Hd2 and Hd1 in flowering regulation (Fig. 2c and Supplementary Fig. 15b). Furthermore, our GWAS did not detect the peaks around the regions where Hd2 and Hd1 are located (Supplementary Fig. 15c, d). The reason may be that the allelic diversity in our population is not suitable for the detection of the two genes by our GWAS system (Fig. 2a, b and Supplementary Data 1). Our results demonstrated that Hd6 protein accumulation induced under high-N conditions accelerated the phosphorylation and subsequent stabilisation of Hd2 protein in the nucleus (Figs. 3b, d, 4e–g and 5b, c and Supplementary Figs. 12 and 13b), leading to strong repression of floral inducer genes (Fig. 4b–d). The diurnal regulation of Hd1, Hd2 and Ghd7 expression by circadian clocks orchestrates primarily the timing and duration of action of these flowering and floral regulators. Hd1 and Ghd7 acted as floral repressors with certain critical day length thresholds if external light signals were given at appropriate timings under a non-inductive photoperiod38,39,40. Contrary to such daily environmental stimuli, changes in N concentrations in paddy field are a long-term environmental stimuli which plants have to acclimatise to. The responsiveness of Hd6 protein accumulation and subsequently, Hd2 levels, to a broad range of N concentrations can confer a dosage-dependent regulation to repress floral inducer gene expression in parallel with day-length recognition (Figs. 3b, d and 4d), suggesting that the integration of N regulation into day length regulation of flowering may allow modifying the critical day-length response in local environments for rice cultivation area expansion.
We observed the suppression of Hd1-dependent Hd3a transactivation by Hd2 and no obvious stable physical interaction between Hd2 and Hd1 in vivo (Fig. 5b, c, f, g). This implies that, as expected in the previous study37, Hd2 accumulated in the nucleus interferes with Hd3a transcriptional activation at the CORE2 region to which the transcriptional activator Hd1 binds. In conclusion, we proposed the model underling the N-induced flowering regulation, in which N induce Hd6 protein accumulation and the increased Hd6 enhances the phosphorylation and stabilization of Hd2 protein. The increased Hd2 modulates the expression of the floral inducer genes together with Hd1 (Fig. 5h). However, the additional factors and mechanisms should also be related to this regulation, because Hd6/hd1/Hd2 varieties also exhibited the N-insensitive flowering phenotype (Figs. 2c, e and 5h). Arabidopsis PRR protein acts as a core transcriptional repressor by recruiting a corepressor component, such as the TOPLESS protein41,42, therefore, Hd2, one of the PRR protein, likely forms another protein complex to repress florigen expression and can act antagonistically against the Hd1 complex on the promoter. It was suggested that the phenotypic and transcriptional functions of Hd2 for flowering time and floral inducer gene expression strongly depend on the light condition, i.e., repression under long days and activation under short days9. Du et al.43 reported a tight association between an enrichment of histone marks (H3K27me3) around the CORE region of the Hd3a promoter and Hd1-DTH8 complex formation on the same Hd3a promoter region. Chromatin regulation at the Hd3a promoter in various environmental and genetic contexts will be key for understanding the mutually exclusive functional coordination between Hd1 and Hd2 proteins, which at present remains an open question. We detected the transcriptional activation of Hd1 and the repression of Hd6-Hd2 module by our transient reporter analyses with rice mesophyll protoplasts (Fig. 5f, g), while the transcriptional activity of Hd2 and Hd1 has been revealed to depend on photoperiodic condition and genetic background, such as the haplotype of Ghd7 9,38. Therefore, our transient reporter assays might represent one of several possible regulatory scenarios among Hd6-Hd2 module, Hd1, other HD genes, and the downstream genes, but even so, the molecular relationship between the Hd6-Hd2 module and Hd1 on the promoter of the floral inducer genes should be the key mechanism for integrating multiple internal and external cues into flowering regulation. We also detected the interaction between Hd6 and Hd1 in rice cells, suggesting Hd1 phosphorylation by Hd6 (Fig. 5b). A previous study demonstrated that Hd1 can be phosphorylated by OsK4 kinase associated with HDR144. A more recent study showed that Hd1 protein homeostasis is regulated by autophagy to control rice flowering45. Therefore, it is possible that Hd6 regulates Hd1 protein stability by modulating its phosphorylation state. It has been reported that the expression of Ghd7, which regulates the transcriptional activity of Hd1 can be respond to the change of N condition9,46, but in the present study, such response was not observed under our conditions (Supplementary Fig. 6d and Supplementary Data 4). Therefore, under our experimental conditions, including the rice varieties and the growth conditions, the importance of Ghd7 for the N-dependent flowering regulation could not be clarified.
Among Japanese rice cultivars, many modern varieties frequently inherit single or double non-functional hd6 and hd1 alleles (Fig. 2c)33. Another CK mutation, namely in Hd16 (a CKI), appeared after the occurrence of the hd6 mutation17. Our study suggests that selection towards reduced N sensitivity could be required to optimise flowering time. Indeed, the increase proportion of the establishment of the Hd1/Hd2 varieties carrying the loss-of-function hd6 haplotype in our GWAS population follows the increase of the N application in Japanese paddy fields from 1940s to 1960s (Supplementary Fig. 16). Studies are investigating the mechanisms underlying the relation between N transport/assimilation and flowering in rice. Wang et al.25 showed that overexpression of the nitrate transporter gene OsNRT1.1A improved rice N utilisation and conferred early maturation associated with a reduction in florigen expression. Further, the expression of MYB-type transcription factor Nhd1 was induced by N treatment and Nhd1 can upregulate Hd3a expression30. In terms of the crosstalk between the HD genes and N responses, several HD factors, including Ghd7 and Hd17/OsELF3−1, regulate N uptake by regulating ABC1 REPRESSOR146,47. Non-functional Hd16 allele contributes to remaining rice yield under low-nutrient conditions48. However, it remains unclear how N input regulates the flowering-regulatory network of HD genes. Although our study revealed that the Hd6-Hd2 module is the key crosstalk point between N signalling and the photoperiodic network involved in regulating rice flowering (Fig. 5h), the molecular pathway between N and Hd6 accumulation is still unclear. The relationship between N availability and protein phosphorylation has been well studied. Studies performed more than 25 years ago suggested that protein phosphorylation and dephosphorylation might be involved in nitrate signaling49,50. Calcineurin B-like-interacting protein kinases (CIPKs) have been characterized to be involved in the protein phosphorylation in response to N status51. Recently, mitogen-activated protein kinase (MAPK) cascade was shown to underlie the complex regulatory network controlling responses to nitrate availability, such as nitrate uptake and senescence in Arabidopsis thaliana52. In rice, OsCIPK3 was shown to be involved in flowering under LD condition through phosphorylation of OsFD1, which is a transcriptional factor regulating the floral development genes in SAM with Hd3a53. The study showed that the expression of OsCIPK3 is abundant in SAM and young leaves. Therefore, this kinase and/or related kinase cascades might modulate the accumulation and the kinase activity of Hd6 in leaves. To increase crop sustainably, the amounts of N-based fertilisers used should be reduced, which may have unexpected effects on various physiological traits, including flowering. Therefore, reconsidering the modulation of plant growth and metabolism by incorporating flowering time gene variation will provide us with novel breeding and cultivation strategies for future sustainable agriculture.
Methods
Plant growth conditions
Two sets of 149 and 128 Oryza sativa japonica varieties were collected from various places in Japan and maintained in the Togo Field, Field Science Center, Nagoya University (Supplementary Data 1 and 2). Information on the breeding years was obtained from the Rice Variety Database (https://ineweb.narcc.affrc.go.jp/hinsyu_top.html). DNA was prepared and genotyped in the previous study54; we identified 379,521 polyorphisms in the 149-variety set and 381,691 polymorphisms in the 128-variety set. The sequence data have been deposited in the DDBJ Sequence Read Archive (DRA) under accession numbers DRA004358 and DRA008452. Agronomic trait phenotyping was performed in 2016 and 2017 at a paddy field of the Togo Field. N fertiliser was applied at 0, 9, 18 kg N/10 a in total to represent low-, normal- and high-N conditions, respectively. Plants in the normal- and high-N conditions received 5 and 8 kg N/10 a at the time of transplantation, followed by 4 and 10 kg N/10 a in two equally split doses: one at the tilling stage (14 days after transplantation) and the second at the panicle initiation stage. DTH was recorded as the number of days from sowing to the appearance of the first panicle on three randomly selected plants.
We used a set of five NILs for Hd6, Hd1 and Hd2 in the Nipponbare background. We could guarantee that the genomic fragment had a single gene mutation affecting flowering time because the fragment had been fine-mapped in the Kasalath or Koshihikari variety when the genes had been cloned16,17,19,20. Nipponbare was used as a control cultivar. Plants were grown in a growth chamber at 70% humidity under long-day conditions with daily cycles of 14.5 h of light at 28 °C and 9.5 h of dark at 24 °C. Light was provided by a metal halide lamp (photosynthetic photon flux density of 450 μmol/m2/s).
For NH4Cl treatment, we prepared a nutrient solution55 without N source. Seedlings were grown for 11 days under half-strength nutrient solution with 1 mM NH4Cl and further grown for 3 days in a nutrient solution containing 0.4 mM NH4Cl. For gene expression and protein analyses, seedlings were transferred to nutrient solutions containing 0.1–3.2 mM NH4Cl and sampled at 6 and 24 h after treatment. For the analyses of the heading of transgenic plants, we prepared Yoshida’s nutrient solution (pH 5.5)56 without N source. Seedlings were transplanted to pots with soil containing no nutrients, and half-strength Yoshida’s solutions with different NH4NO3 concentrations (0.7 and 2.4 mM for LH and HN, respectively) were used for N treatment. The nutrient solution was changed weekly. For pot cultivation tests of NILs, seedlings were transplanted into pots with soil containing 28 and 112 mg N fertiliser. Fertiliser was added every 30 days until flowering. The sample sizes for analysing flowering time phenotypes were empirically determined.
GWAS
GWASs were performed using the “GWAS” function in the R package rrBLUP with default parameter settings, and Manhattan plots and quantile–quantile (Q–Q) plots with –log10 P-values determined using linear mixed model were generated using the R package qqman57.
Linkage disequilibrium analysis
Linkage disequilibrium (LD) analysis using the R package “LD heatmap”58 was used to define LD blocks surrounding significant peaks based on confidence intervals.
Candidate gene isolation
The positions of polymorphisms in the rice genome were determined according to the information files of the rice gene locus (all.locus_brief_info.7.0) and genomic features (all.gff3) that were obtained from the rice genome annotation project (https://rice.uga.edu/download_osa1r7.shtml). Genes annotated as “retrotransposon protein” or “hypothetical protein” were omitted.
Plasmid construction
The sequences of the primers used in this study were purchased from FASMAC (Japan) and are listed in Supplementary Data 5. PCR fragments for cloning were sequenced to confirm that no mutations were induced. For transgenic plant production, a full-length CDS fragment encoding Hd2 were introduced into BamHI/SpeI site of pUbi-Ω-FLAG/pCAMBIA59. To generate 35S:Hd6, Hd6 CDS were amplified using PrimeSTR GXL DNA polymerase (TaKaRa) from cDNA of Hap.B cultivar leaves (Yamadanishiki) as the template, and introduced into pGWB502 via TOPO-D/pENTR (Invitrogen) by LR Gateway reaction. 35S:Hd6 sequence was amplified from 35S:Hd6/pGWB502 plasmid and introduced into AscI site of pUbi-omega-FLAG-Hd2/pCAMBIA to generate 35S:Hd6/pUbq: Ω:FLAG-Hd2 plasmid.
For antigen production and in vitro pull-down assays, Hd6 fragment was PCR-amplified, and was subjected to a fill-in reaction using Klenow Fragment (Large Fragment E. coli DNA polymerase I; TaKaRa) after digestion with XhoI and subsequently, BamHI, and finally cloned into the filled-in EcoRI and BamHI sites of the pMAL-c5X vector.
To construct the reporter construct for the transient reporter assay, we used the internal control plasmid hRluc-nosT/pUC19 as a backbone. The firefly LUC gene was derived from a binary construct, OsPRR1:LUC pBINPLUS20. LUC was PCR-amplified as described above with appropriate primers having NcoI and SacI sites, and hRluc was replaced with the amplified LUC fragment after restriction enzyme digestion. The fragment was also cloned into MP from 35S with SmaI and NcoI sites to create MP-LUC-nosT/pUC19. An oligonucleotide sequence corresponding to −185 to −137 before the transcription start site of Hd3a with two appropriate restriction enzymatic sites was produced and annealed (EcoRV-2xHd3a-SmaI: GGGATATCCTCTCAACCACAGCTCGATCCAGCAGCCCTGCACCACACACAGTTCTCTCAACCACAGCTCGATCCAGCAGCCTGCACCACACACAGTTCCCGGGCC). The CaMV35S promoter without MP was PCR-amplified with appropriate primers having HindIII and SmaI sites and cloned into the HindIII/SmaI site of MP-LUC-nosT/pUC19 to create CaMV35S-MP-LUC-nosT/pUC19. After digestion of the Hd3a oligonucleotide fragment with EcoRV and SmaI, it was cloned into the SmaI site of CaMV35S-MP-LUC-nosT/pUC19, and this was repeated once more to create four Hd3a oligonucleotide sequences in the middle of the CaMV35S-MP fragment. For effector constructs, Hd6, Hd1 and Hd2 cDNA fragments were PCR-amplified with appropriate primers having a SmaI site and cloned into the SmaI site of pUbq-omega-nosT/pUC19.
For constructs used to identify the subcellular localisation of Hd6, Hd2 and Hd1, we amplified CDS of these gene and introduced into the XbaI/SpeI site of pE2113_GW_sGFP60. The CDS of AtFIB1 (AT5G52470) amplified with DNA fragments synthesized by Azenta Corporation (Japan) as template was introduced into the XbaI/SpeI site of pE2113_GW_mScarlet vector57 to produce the subcellular marker constructs for nucleous61.
To construct the construct for BiFC assay in rice mesophyll protoplasts, the coding regions of the N- and C-terminal halves of YFP with linker sequence were amplified from pGWnY and pGWcY as the template and inserted into the SpeI/StuI site of OREB1/pE2113_GW_sGFP57 to generate the expression vector for the protein fused with nYFP and cYFP, OREB1/pE2113_GW_nYFP and OREB1/pE2113_GW_cYFP, respectively. Hd6, Hd1, and full-length and truncated Hd2 were introduced into XbaI/SpeI site of OREB1/pE2113_GW_nYFP and OREB1/pE2113_GW_cYFP to replace OREB1 with these HD gene CDS fragments.
RT-qPCR-based gene expression analysis
cDNA was synthesized from 1 μg of total RNA extracted from the seedling or the leaves of 55-day-old rice plants using ReverTra Ave qPCR RT Master Mix with gDNA Remover (TOYOBO). qPCRs were run using TaqMan (NipponGene) or TB Green Prime Mix ExTaq (Tli RNaseH Plus; TaKaRa) on an ABI PRISM 7900 Sequence Detection System according to the manufacturers’ instructions. The gene-specific primers used are listed in Supplementary Data 5.
Antibody production
Overexpressed recombinant protein was purified using Talon Metal Affinity Resin (Clontech) according to the manufacturer’s instructions. The purified soluble recombinant protein was used directly to produce rabbit polyclonal antibodies. To exclude non-specific binding, anti-Hd6 antibodies were affinity-purified from crude antisera. GST-Hd6 was subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). Stripped GST-Hd6 recombinant protein was incubated with crude anti-Hd6 antiserum at room temperature for 2 h. Bound anti-Hd6 antibodies were eluted with 0.1 M glycine-HCl (pH 2.5) and neutralized with 1 M Tris-HCl.
Immunoblotting analysis
Harvested plant materials were ground in liquid N2, and proteins were extracted by adding two volumes of 2× buffer A (1× is 20 mM Tris-HCl pH 8.0, 150 mM NaCl, 0.5% Tween-20, DNase (10 μg/mL), RNase (10 μg/mL), 1 × Complete tablet [Roche]). The extracts were centrifuged at 19,000 × g at 4 °C for 20 min. Protein was quantified with the Bradford assay using Bio-Rad Protein Assay Dye Reagent Concentrate (Bio-Rad), mixed with an equal volume of 2 × sample buffer (1 × is 67.5 mM Tris-HCl pH 6.8, 2% SDS, 10% glycerol, 0.01% bromophenol blue, and 0.1 M dithiothreitol), and boiled for 5 min. The protein samples were separated by 12.5% or 8% SDS-PAGE and transferred to an Immobilon-P membrane (Merck Millipore) in a tank transfer system with electro-transfer buffer (25 mM Tris, 192 mM glycine, 20% methanol). The blots were incubated with anti-Hd6 or anti-FLAG M2 (Sigma-Aldrich; F3165) monoclonal antibody-peroxidase conjugate (Sigma-Aldrich, Merck Millipore) and then with goat anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody (only after anti-Hd6 incubation). To obtain a loading control, monoclonal anti-tubulin antibody produced in mouse clone TUB.2.1 (Sigma-Aldrich, T5201) was used. Peroxidase activity was detected using ECL Prime Western Blotting Detection Reagent (Amersham, GE Healthcare) according to the manufacturer’s instructions.
Benzimidazole, a CK2 inhibitor (ab120939; Abcam Biochemicals), and PF670462 (4-[1-cyclohexyl-4-(4-fluorophenyl)−1H-imidazol-5-yl]-2-pyrimidinamine dihydrochloride), a mammalian CK1ε and CK1σ inhibitor (Tokyo Chemical Industry), were dissolved in DMSO to generate 100 × stock solutions. Transgenic rice seedlings in hydroponic culture were treated with 100 μM CK inhibitor or 1% DMSO as a mock control for 6 h. Then, proteins were extracted using buffer A. The crude extracts were incubated with 100 units of alkaline phosphatase at 37 °C for 15 min. As a control, equal units of alkaline phosphatase were first boiled for 5 min and then mixed with the samples62. The treated proteins were separated by SDS-PAGE and subjected to immunoblotting analyses. Proteins prepared from 35:Hd6/Ubq:Ω:3FLAG–Hd2 plants were separated by 6% SDS-PAGE containing 50 μM Phos-tag (Wako) and subjected to immunoblotting.
Plant transformation
Binary vector harbouring Ubq:Ω:3FLAG–Hd2 and/or 35:Hd6 was introduced into Agrobacterium tumefaciens strain EHA10163 through electroporation. Nipponbare and NILs (hd6 and hd16), substitution lines harbouring double-defective natural variations for Hd6 and Hd16 from cultivar Koshihikari17, were used for analyses. These EHA101 stains were grown for 3 days on the AB medium containing 50 mg/L hygromycin and 50 mg/L kanamycin, solidified with 1.5% agar. The bacterial cells were resuspended in AAM medium. The calli were soaked in this suspension for 2 min and blotted dry, using sterile Kimwipes. Then these calli were transferred on filter paper placed on a co-culture medium, which was prepared by spreading 5.35 mL of a liquid 2N6-AS medium on a bottom medium. After co-cultivation, the calli were washed three times with water containing 0.6 mg/L acetosyringone. After washing, the transgenic calli were selected on a medium containing 25 mg/L meropenem and 50 mg/l hygromycin. Hygromycin-resistant plants were transplanted into soil and grown as described above.
RNA-sequencing
RNA-sequencing analysis was conducted as previously described in Huang et al.64 with some modifications. In brief, total RNA was extracted from leaves of 55-day-old rice plants using Sepasol-RNA Super G (Nacalai Tesque). cDNA library construction and sequencing were carried out at the Beijing Genomics Institute (BGI-Shenzhen, China; http://www.genomics.cn/en/index). The 150-bp paired-end reads were aligned with the Nipponbare reference genome (IRGSP1.0) using HISAT2. Uniquely mapped reads were counted using featureCounts (v1.6.4)65 DEGs were extracted using iDEP1.1 (|logFC| ≥ 1, Padjusted < 0.05) (http://bioinformatics.sdstate.edu/idep11/). The GO term enrichment analyses of the DEGs were conducted using ShinyGo v0.80 (http://bioinformatics.sdstate.edu/go/)66.
In vitro pull-down assay
GST-Hd6 and GST-Hd1 recombinant proteins were expressed in E. coli (strain BL21 [DE3])20. MBP-Hd6 and MBP-HIS-Hd2 recombinant proteins were also expressed in BL21 (DE3) cells. Two hundred micrograms of crude lysates expressing each of the recombinant proteins described above were mixed and allowed to interact. MBP and MBP-fusion proteins were collected using Amylose resin (New England Biolabs) according to the manufacturer’s instructions after gentle agitation at 4 °C for 2 h. After washing of the resin, 20 μL of 2 × sample buffer was added, the mixture was heat-denatured, and the sample was subjected to 10% SDS-PAGE. MBP fusion proteins were visualized with Coomassie Brilliant Blue staining. After preparing a duplicated SDS-PAGE gel, co-precipitated GST fusion proteins were analysed using immunoblotting with a GST·Tag monoclonal antibody (Sigma-Aldrich; 71097). As negative controls, individual GST and MBP were prepared. The MBP-HIS-Hd2/OsPRR37 plasmid was kindly donated by Prof. Nam-Chon Paek.
Mesophyll protoplast preparation
We used an efficient rice green tissue protoplast system67. Dehulled seeds of Koshihikari were sterilized with 75% ethanol for 1 min and then with 2.5% sodium hypochlorite for 20 min. The seeds were washed with sterile water and incubated on MS medium under continuous light (~150 μmol/m2/s) at 28 °C for 7–9 days. Green tissues from the stem and sheath of ~100 rice seedlings were used. A bundle of rice plants was cut with a razor blade into ~0.5-mm strips using propulsive force. The strips were incubated in an enzyme solution (1.5% Cellulase RS, 0.75% Macerozyme R−10, 0.6 M mannitol, 10 mM MES at pH 5.7, 10 mM CaCl2 and 0.1% BSA) in the dark for 3–4 h under gentle shaking. Then, an equal volume of W5 solution (154 mM NaCl, 125 mM CaCl2, 5 mM KCl and 2 mM MES at pH 5.7) was added, followed by shaking with the hand. The released protoplasts were filtered through a nylon mesh into round-bottom tubes and washed two times using W5 solution. The mesophyll protoplasts were pelleted by centrifugation at 500 × g for 5 min in a swinging bucket. After a wash with W5 solution, the pellets were resuspended in MMG solution (0.4 M mannitol, 15 mM MgCl2 and 4 mM MES at pH 5.7) and prepared at a concentration of 2 × 106 cells/mL, as determined by using a haematocytometer. Plasmid DNA was prepared using NucleoBond Xtra Midi (U0412, Takara) per the manufacturer’s instructions. PEG-mediated transfections were carried out. Briefly, for each sample, ~15 μg of plasmid DNA was mixed with 100 μL of protoplasts (∼ 2 × 105 cells) in a 1.5-mL tube. Then, 110 μL of freshly prepared PEG solution (40% [w/v] PEG 4000 [81240; Sigma], 0.2 M mannitol and 0.1 M CaCl2) was added, and the mixture was incubated at room temperature in the dark for 20 min. After incubation, 500 μL W5 solution was added and mixed well by gently inverting the tube, and the protoplasts were pelleted by centrifugation at 500 × g for 5 min. The protoplasts were resuspended gently in 1 mL of WI solution (0.5 M mannitol, 20 mM KCl and 4 mM MES at pH 5.7). Finally, the protoplasts were cultured in the dark at 28 °C for 16–18 h. Cultured protoplasts were harvested by centrifugation at 500 × g for 5 min.
BiFC assay
Mesophyll protoplasts (2 × 105 cells) were co-transfected with constructs expressing split-EYFP fused with each of Hd6, Hd1 and Hd2 for 12–16 h. EYFP and chlorophyll fluorescence signals in protoplasts were observed through a Zeiss LSM 700 confocal laser scanning microscope using laser light with a wavelength of 488–555 nm.
Transient reporter assay
fLUC and hRLUC activities were measured using a dual-luciferase reporter assay system. Firefly luciferase and Renilla luciferase activities were measured using the Dual-Luciferase Reporter Assay System (E1910; Promega) according to the manufacturer’s protocol and Infinite 200 PRO (Tecan Life Sciences). The relative ratio was determined by comparing the activity ratio with that obtained with the empty vector. Mean relative ratios were calculated based on data derived from three independent experiments.
Phosphopeptide identification by MS/MS analysis
Protein samples were separated by SDS-PAGE followed by CBB (Coomassie Brilliant Blue) staining. Tryptic peptide preparation and mass spectrometry have been essentially described previously68. In brief, Hd2 protein bands derived from the MBP-HIS-Hd2 samples incubated with (n = 1) or without (n = 1) Hd6 were cut into pieces and destained with 30% acetonitrile/ammonium bicarbonate buffer at room temperature. Gel pieces were dehydrated by centrifuge concentrator and rehydrated by 10 ng/µL trypsin/Lys-C mixture (Promega) containing 0.01% ProteaseMAX surfactant (Promega). Additional ProteaseMAX surfactant solution was added to the gel pieces and in-gel digestion was performed at 50 °C for 1 hour. After digestion, the solution surrounding the gel pieces was collected. Remaining peptides were extracted from the gel piece by adding the ammonium bicarbonate buffer. All peptide-containing solutions were combined and desalted using MonoSpin C18 spin columns (GL Sciences). Mass spectrometric analysis was qualitatively performed using a triple quadrupole mass spectrometer (QTRAP5500, Sciex) equipped with nanoLC system (Eksigent LC425, Sciex). Desalted peptides were loaded onto a C18 NANO HPLC CAPILLARY COLUMN (NTCC-360/75-3−125, Nikkyo Technos Co., Ltd.) and eluted with a linear gradient (2–35% acetonitrile with 0.1% formic acid) over 30 min, followed by 60% acetonitrile with 0.1% formic acid over 10 min at a flow rate of 300 nL min−1. Nanospray ion source (Sciex) settings were as follows: Curtain Gas (CUR), 20; Collision Gas (CAD), High; IonSpray Voltage (IS), 2300; Ion Source Gas 1 (GS1), 20; Ion Source Gas 2 (GS2), 0; Interface Heater Temperature 150. MS was performed in the m/z range of 400–1000, and MS/MS in the m/z range of 100–1000. MS/MS was performed by selecting three precursor ions with the highest peak intensity at each elution time. Charge state of precursor ions was determined by Enhanced Resolution (ER) measurement. Precursor ions for MS/MS were treated as separate ions if their m/z differed by 250 mDa. Dynamic exclusion was applied for 30 s after MS/MS acquisition. The MS/MS results obtained from each sample were analyzed using ProteinPilot software (Sciex) with the following parameters: Sample type, Identification; Cys Alkylation, MMTS; Digestion, Trypsin; Instrument, 5500 QTRAP ESI; Search Effort, Through; FDR Analysis, No. To ensure the detection of phosphopeptides, “Phosphorylation emphasis” was used as special factor. We performed the database search against a custom database containing ~400 randomly selected protein sequences including Hd2 in ProteinPilot. All MS/MS spectra were manually verified to confirm the specific phosphopeptides.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The DNA sequence data has been deposited in DNA Data Bank of Japan (DDBJ) under accession DRA004358 and DRA008452. RNA-sequencing data generated in this paper has been deposited in DDBJ under accession PRJDB35993. Mass spectrometric data has been deposited with the Japan ProteOme STandard Repository (jPOST) under ProteomeXchange Consortium ID PXD057884. Source data are provided with this paper.
References
Izawa, T. Adaptation of flowering-time by natural and artificial selection in Arabidopsis and rice. J. Exp. Bot. 58, 3091–3097 (2007).
Lu, S. et al. Natural variation at the soybean J locus improves adaptation to the tropics and enhances yield. Nat. Genet. 49, 773–779 (2017).
Navarro, J. A. R. et al. Corrigendum: a study of allelic diversity underlying flowering-time adaptation in maize landraces. Nat. Genet. 49, 970 (2017).
Zhou, S. et al. Transcriptional and post-transcriptional regulation of heading date in rice. N. Phytol. 230, 943–956 (2021).
Yano, M. et al. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12, 2473–2484 (2000).
Li, Y. P. & Xu, M. L. CCT family genes in cereal crops: a current overview. Crop J. 5, 449–458 (2017).
Nemoto, Y., Nonoue, Y., Yano, M. & Izawa, T. Hd1,a CONSTANS ortholog in rice, functions as an Ehd1 repressor through interaction with monocot-specific CCT-domain protein Ghd7. Plant J. 86, 221–233 (2016).
Zhang, Z. et al. Alternative functions of Hd1 in repressing or promoting heading are determined by Ghd7 status under long-day conditions. Sci. Rep. 7, 5388 (2017).
Gao, H. et al. Days to heading 7, a major quantitative locus determining photoperiod sensitivity and regional adaptation in rice. Proc. Natl. Acad. Sci. USA 111, 16337–16342 (2014).
Zong, W. et al. Strong photoperiod sensitivity is controlled by cooperation and competition among Hd1, Ghd7 and DTH8 in rice heading. N. Phytol. 229, 1635–1649 (2021).
Koo, B. H. et al. Natural variation in OsPRR37 regulates heading date and contributes to rice cultivation at a wide range of latitudes. Mol. Plant 6, 1877–1888 (2013).
Liu, T., Liu, H., Zhang, H. & Xing, Y. Validation and characterization of Ghd7.1, a major quantitative trait locus with pleiotropic effects on spikelets per panicle, plant height, and heading date in rice (Oryza sativa L.). J. Integr. Plant Biol. 55, 917–927 (2013).
Yan, W. et al. Natural variation in Ghd7.1 plays an important role in grain yield and adaptation in rice. Cell Res. 23, 969–971 (2013).
Vicentini, G. et al. Environmental control of rice flowering time. Plant Commun. 4, 100610 (2023).
Takahashi, Y., Shomura, A., Sasaki, T. & Yano, M. Hd6, a rice quantitative trait locus involved in photoperiod sensitivity, encodes the alpha subunit of protein kinase CK2. Proc. Natl. Acad. Sci. USA 98, 7922–7927 (2001).
Hori, K. et al. Hd16, a gene for casein kinase I, is involved in the control of rice flowering time by modulating the day-length response. Plant J. 76, 36–46 (2013).
Nemoto, Y., Hori, K. & Izawa, T. Fine-tuning of the setting of critical day length by two casein kinases in rice photoperiodic flowering. J. Exp. Bot. 69, 553–565 (2018).
Yamamoto, T., Lin, H., Sasaki, T. & Yano, M. Identification of heading date quantitative trait locus Hd6 and characterization of its epistatic interactions with Hd2 in rice using advanced backcross progeny. Genetics 154, 885–891 (2000).
Ogiso, E., Takahashi, Y., Sasaki, T., Yano, M. & Izawa, T. The role of casein kinase II in flowering time regulation has diversified during evolution. Plant Physiol. 152, 808–820 (2010).
Kwon, C. T., Koo, B. H., Kim, D., Yoo, S. C. & Paek, N. C. Casein kinases I and 2α phosphorylate Oryza sativa pseudo-response regulator 37 (OsPRR37) in photoperiodic flowering in rice. Mol. Cells 38, 81–88 (2015).
Chen, R. et al. Rice functional genomics: decades’ efforts and roads ahead. Sci. China Life Sci. 65, 33–92 (2022).
Nagalla, A. D., Nishide, N., Hibara, K. I. & Izawa, T. High ambient temperatures inhibit Ghd7-mediated flowering repression in rice. Plant Cell Physiol. 62, 1745–1759 (2021).
Withrow, A. P. The interrelationship of nitrogen supply and photoperiod on the flowering, growth and stem anatomy of certain long and short day plants. Butl. Univ. Bot. Stud. 7, 40–64 (1945).
Wang, W. et al. Expression of the nitrate transporter gene. Plant Cell 30, 638–651 (2018).
Zhang, S. et al. Nitrogen as a regulator for flowering time in plant. Plant Soil 480, 1–29 (2022).
Olas, J. J. et al. Nitrate acts at the Arabidopsis thaliana shoot apical meristem to regulate flowering time. N. Phytol. 223, 814–827 (2019).
Yan, F. H., Zhang, L. P., Cheng, F., Yu, D. M. & Hu, J. Y. Accession-specific flowering time variation in response to nitrate fluctuation in. Plant Divers 43, 78–85 (2021).
Sanagi, M. et al. Low nitrogen conditions accelerate flowering by modulating the phosphorylation state of FLOWERING BHLH 4 in Arabidopsis. Proc. Natl. Acad. Sci. USA 118, e2022942118 (2021).
Zhang, S. et al. Nitrogen mediates flowering time and nitrogen use efficiency via floral regulators in rice. Curr. Biol. 31, 671–683.e675 (2021).
Yano, K. et al. GWAS with principal component analysis identifies a gene comprehensively controlling rice architecture. Proc. Natl. Acad. Sci. USA 116, 21262–21267 (2019).
Brachi, B., Morris, G. P. & Borevitz, J. O. Genome-wide association studies in plants: the missing heritability is in the field. Genome Biol. 12, 232 (2011).
Yano, K. et al. Genome-wide association study using whole-genome sequencing rapidly identifies new genes influencing agronomic traits in rice. Nat. Genet. 48, 927–934 (2016).
Suganami, M. et al. Effective use of legacy data in a genome-wide association studies improves the credibility of quantitative trait loci detection in rice. Plant Physiol. 191, 1561–1573 (2023).
Ohashi, M. et al. Reduction in sucrose contents by downregulation of fructose-1,6-bisphosphatase 2 causes tiller outgrowth cessation in rice mutants lacking glutamine synthetase1;2. Rice (N. Y) 11, 65 (2018).
Lin, H., Yamamoto, T., Sasaki, T. & Yano, M. Characterization and detection of epistatic interactions of 3 QTLs, Hd1, Hd2, and Hd3, controlling heading date in rice using nearly isogenic lines. Theo. Appl. Genet. 101, 1021–1028 (2000).
Zhang, B. et al. Genetic interactions among Ghd7, Ghd8, OsPRR37 and Hd1 contribute to large variation in heading date in rice. Rice (N. Y) 12, 48 (2019).
Goretti, D. et al. Transcriptional and post-transcriptional mechanisms limit heading date 1 (Hd1) function to adapt rice to high latitudes. PLoS Genet. 13, e1006530 (2017).
Izawa, T. et al. Phytochrome mediates the external light signal to repress FT orthologs in photoperiodic flowering of rice. Genes Dev. 16, 2006–2020 (2002).
Ishikawa, R. et al. Suppression of the floral activator Hd3a is the principal cause of the night break effect in rice. Plant Cell 17, 3326–3336 (2005).
Itoh, H., Nonoue, Y., Yano, M. & Izawa, T. A pair of floral regulators sets critical day length for Hd3a florigen expression in rice. Nat. Genet. 42, 635–638 (2010).
Nakamichi, N. et al. PSEUDO-RESPONSE REGULATORS 9, 7, and 5 are transcriptional repressors in the Arabidopsis circadian clock. Plant Cell 22, 594–605 (2010).
Wang, L., Kim, J. & Somers, D. E. Transcriptional corepressor TOPLESS complexes pseudoresponse regulator proteins and histone deacetylases to regulate circadian transcription. Proc. Natl. Acad. Sci. USA 110, 761–766 (2013).
Du, A. et al. The DTH8-Hd1 module mediates day-length-dependent regulation of rice flowering. Mol. Plant 10, 948–961 (2017).
Sun, X. et al. The Oryza sativa regulator HDR1 associates with the kinase OsK4 to control photoperiodic flowering. PLoS Genet. 12, e1005927 (2016).
Hu, Z. et al. Autophagy targets Hd1 for vacuolar degradation to regulate rice flowering. Mol. Plant 15, 1137–1156 (2022).
Wang, Q. et al. The Ghd7 transcription factor represses ARE1 expression to enhance nitrogen utilization and grain yield in rice. Mol. Plant 14, 1012–1023 (2021).
Sun, Q. et al. EARLY FLOWERING3-1 represses grain number, plant height, and heading date7 to promote ABC1 REPRESSOR1 and regulate nitrogen uptake in rice. Plant Physiol. 196, 1857–1868 (2024).
Tanaka, N. et al. Evidence for rice Heading date 16 contribution to yield increase under low-nutrient conditions. Soil Sci. Plant Nutr. 65, 589–597 (2019).
Sakakibara, H., Kobayashi, K., Deji, A. & Sugiyama, T. Partial characterization of the signaling pathway for the nitrate-dependent expression of genes for nitrogen-assimilatory enzymes using detached maize leaves. Plant Cell Physiol. 38, 837–843 (1997).
Sueyoshi, K. et al. Effects of inhibitors for signaling components on the expression of the genes for nitrate reductase and nitrite reductase in excised barley leaves. Soil Sci. Plant Nutr. 45, 1015–1019 (1999).
Yang, C. et al. Diverse roles of the CIPK gene family in transcription regulation and various biotic and abiotic stresses: a literature review and bibliometric study. Front. Genet. 13, 1041078 (2022).
Schenk, S. T. et al. Nitrate activates an MKK3-dependent MAPK module via NLP transcription factors in Arabidopsis. Plant J. 121, e70010 (2025).
Peng, Q. et al. Phosphorylation of OsFD1 by OsCIPK3 promotes the formation of RFT1-containing florigen activation complex for long-day flowering in rice. Mol. Plant 14, 1135–1148 (2021).
Suganami, M. et al. Redefining awn development in rice through the breeding history of Japanese awn reduction. Front. Plant Sci. 15, 1370956 (2024).
Makino, A., Mae, T. & Ohira, K. Photosynthesis and ribulose 1,5-bisphosphate carboxylase in rice leaves: changes in photosynthesis and enzymes involved in carbon assimilation from leaf development through senescence. Plant Physiol. 73, 1002–1007 (1983).
Yoshida, S., Forno, D. A., Cook, J. H. & Gomez, K. A. Routine procedures for growing rice plants in culture solution. In Laboratory Manual for Physiological Studies of Rice (eds. Yoshida, S. et al.) 61–66 (IRRI, Philippines, 1976).
Yoshida, H. et al. Genome-wide association study identifies a gene responsible for temperature-dependent rice germination. Nat. Commun. 13, 5665 (2022).
Shin, J. H., Blay, S., McNeney, B. & Graham, J. LDheatmap: an R function for graphical display of pairwise linkage disequilibria between single nucleotide polymorphisms. J. Stat. Softw. 16, 1–10 (2006).
Hirano, K. et al. SMALL ORGAN SIZE 1 and SMALL ORGAN SIZE 2/DWARF AND LOW-TILLERING form a complex to integrate auxin and brassinosteroid signaling in rice. Mol. Plant 10, 590–604 (2017).
Yoshida, H. et al. Integrated genome-wide differentiation and association analyses identify causal genes underlying breeding-selected grain quality traits in japonica rice. Mol. Plant 16, 1460–1477 (2023).
Khan, A. et al. The Arabidopsis STRESS RESPONSE SUPPRESSOR DEAD-box RNA helicases are nucleolar- and chromocenter-localized proteins that undergo stress-mediated relocalization and are involved in epigenetic gene silencing. Plant J. 79, 28–43 (2014).
Sasaki, A. et al. Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science 299, 1896–1898 (2003).
Hood, E. E., Helmer, G. L., Fraley, R. T. & Chilton, M. D. The hypervirulence of Agrobacterium tumefaciens A281 is encoded in a region of pTiBo542 outside of T-DNA. J. Bacteriol. 168, 1291–1301 (1986).
Huang, P. et al. OsIDD2, a zinc finger and INDETERMINATE DOMAIN protein, regulates secondary cell wall formation. J. Integr. Plant Biol. 60, 130–143 (2018).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
Ge, S. X., Jung, D. & Yao, R. ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics 36, 2628–2629 (2020).
Zhang, Y. et al. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 7, 30 (2011).
Usui, K. et al. Extracellular vesicle-mediated secretion of protochlorophyllide in the cyanobacterium Leptolyngbya boryana. Plants 11, 910 (2022).
Acknowledgements
The authors thank NARO Genebank and Kyushu University for providing rice seeds. We thank Masako Hattori and Kenji Yano (Nagoya University) for helpful advice and technical assistants, Daisuke Seshita (Nagoya University) and Yoichi Morinaka (Fukui Prefectural University) for helpful advice about statistical analyses, Kiyosumi Hori (NARO) and Toshio Yamamoto (Okayama University) for providing the NILs and their information, and Asako Kobayashi (Fukui Agricultural Experiment Station) and Soichi Kojima (Tohoku University) for helpful advice about N application to paddy field in Japan. This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (grant numbers 21K15120 and 24K01886 to H.Y., 19K05983 and 24K01735 to H.I. and 22H02294 and 23K23560 to MMatsuoka), by the Cabinet Office, Government of Japan, Moonshot Research and Development Program for Agriculture, Forestry and Fisheries (funding agency: Bio-oriented Technology Research Advancement Institution) grant no. JPJ009237 to H.Y., by the commissioned research fund provided by F-REI (JPFR24030101 to H.Y. and MMatsuoka and JPFR25020105 to H.Y.), by the Priority Research Project (foR-F) of Fukushima University (262Q001 to H.Y. and MMatsuoka) and by the Assisted Joint Research Program (Exploration Type) of the J-GlycoNet cooperative network, which is accredited by the Minister of Education, Culture, Sports, Science and Technology, MEXT, Japan, as a Joint Usage/Research Center.
Author information
Authors and Affiliations
Contributions
H.Y., H.I. and M.Matsuoka. designed the study. H.Y. and M.Mori conducted the GWAS analyses. H.I. conducted the phenotyping of the rice plants. E.K. produced the transgenic rice lines. H.Y., H.I. and M.K. elucidated the molecular mechanisms. H.I., S.N. and H.M. conducted the phosphorylation analyses. H.Y., H.I., S.N. and M.Matsuoka wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Shunan Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yoshida, H., Itoh, H., Mori, M. et al. Photoperiodic flowering regulators are required for nitrogen-dependent flowering delay in rice under long-day condition. Nat Commun 16, 9231 (2025). https://doi.org/10.1038/s41467-025-64299-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41467-025-64299-w