Abstract
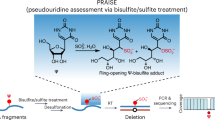
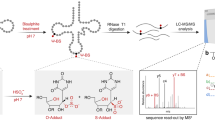
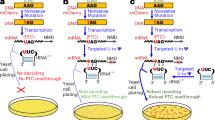
Pseudouridine (Ψ) is the most abundant RNA modification, yet studies of Ψ have been hindered by a lack of robust methods to profile comprehensive Ψ maps. Here we utilize bisulfite-induced deletion sequencing to generate transcriptome-wide Ψ maps at single-base resolution across various plant species. Integrating ribosomal RNA, transfer RNA and messenger RNA Ψ stoichiometry with mRNA abundance and polysome profiling data, we uncover a multilayered regulation of translation efficiency through Ψ modifications. rRNA pseudouridylation could globally control translation, although the effects vary at different rRNA Ψ sites. Ψ in the tRNA T-arm loop shows strong positive correlations between Ψ stoichiometry and the translation efficiency of their respective codons. We observed a general inverse correlation between Ψ level and mRNA stability, but a positive correlation with translation efficiency in Arabidopsis seedlings. In conclusion, our study provides critical resources for Ψ research in plants and proposes prevalent translation regulation through rRNA, tRNA and mRNA pseudouridylation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available in the main text or the Supplementary tables. The BID-seq and RNA-seq data reported in this study have been deposited in the Gene Expression Omnibus database (https://www.ncbi.nlm.nih.gov/geo) under accession numbers GSE262373, GSE262374, GSE262375, GSE262376, GSE277198 and GSE277201. Source data are provided with this paper.
Change history
21 January 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41477-025-01913-1
References
Cohn, W. E. 5-Ribosyl uracil, a carbon–carbon ribofuranosyl nucleoside in ribonucleic acids. Biochim. Biophys. Acta 32, 569–571 (1959).
Hamma, T. & Ferre-D’Amare, A. R. Pseudouridine synthases. Chem. Biol. 13, 1125–1135 (2006).
Spenkuch, F., Motorin, Y. & Helm, M. Pseudouridine: still mysterious, but never a fake (uridine)! RNA Biol. 11, 1540–1554 (2014).
Li, X., Ma, S. & Yi, C. Pseudouridine: the fifth RNA nucleotide with renewed interests. Curr. Opin. Chem. Biol. 33, 108–116 (2016).
De Zoysa, M. D. & Yu, Y. T. Posttranscriptional RNA pseudouridylation. Enzymes 41, 151–167 (2017).
Adachi, H., De Zoysa, M. D. & Yu, Y. T. Post-transcriptional pseudouridylation in mRNA as well as in some major types of noncoding RNAs. Biochim. Biophys. Acta Gene Regul. Mech. 1862, 230–239 (2019).
Westhof, E. Pseudouridines or how to draw on weak energy differences. Biochem. Biophys. Res. Commun. 520, 702–704 (2019).
Carlile, T. M. et al. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature 515, 143–146 (2014).
Lovejoy, A. F., Riordan, D. P. & Brown, P. O. Transcriptome-wide mapping of pseudouridines: pseudouridine synthases modify specific mRNAs in S. cerevisiae. PLoS ONE 9, e110799 (2014).
Schwartz, S. et al. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell 159, 148–162 (2014).
Li, X. et al. Chemical pulldown reveals dynamic pseudouridylation of the mammalian transcriptome. Nat. Chem. Biol. 11, 592–597 (2015).
Roovers, M. et al. Formation of the conserved pseudouridine at position 55 in archaeal tRNA. Nucleic Acids Res. 34, 4293–4301 (2006).
Zhang, L. S. et al. BID-seq for transcriptome-wide quantitative sequencing of mRNA pseudouridine at base resolution. Nat. Protoc. 19, 517–538 (2024).
Carlile, T. M. et al. mRNA structure determines modification by pseudouridine synthase 1. Nat. Chem. Biol. 15, 966–974 (2019).
Sun, L. et al. Transcriptome-wide analysis of pseudouridylation of mRNA and non-coding RNAs in Arabidopsis. J. Exp. Bot. 70, 5089–5600 (2019).
Krutyholowa, R., Zakrzewski, K. & Glatt, S. Charging the code – tRNA modification complexes. Curr. Opin. Struct. Biol. 55, 138–146 (2019).
Nakamoto, M. A., Lovejoy, A. F., Cygan, A. M. & Boothroyd, J. C. mRNA pseudouridylation affects RNA metabolism in the parasite Toxoplasma gondii. RNA 23, 1834–1849 (2017).
Sloan, K. E. et al. Tuning the ribosome: the influence of rRNA modification on eukaryotic ribosome biogenesis and function. RNA Biol. 14, 1138–1152 (2017).
Jack, K. et al. rRNA pseudouridylation defects affect ribosomal ligand binding and translational fidelity from yeast to human cells. Mol. Cell 44, 660–666 (2011).
Pederiva, C. et al. Control of protein synthesis through mRNA pseudouridylation by dyskerin. Sci. Adv. 9, eadg1805 (2023).
Basu, A. et al. Requirement of rRNA methylation for 80S ribosome assembly on a cohort of cellular internal ribosome entry sites. Mol. Cell. Biol. 31, 4482–4499 (2011).
Zhao, Y., Rai, J. & Li, H. Regulation of translation by ribosomal RNA pseudouridylation. Sci. Adv. 9, eadg8190 (2023).
Eyler, D. E. et al. Pseudouridinylation of mRNA coding sequences alters translation. Proc. Natl Acad. Sci. USA 116, 23068–23074 (2019).
Niu, Y. et al. The Arabidopsis mitochondrial pseudouridine synthase homolog FCS1 plays critical roles in plant development. Plant Cell Physiol. 63, 955–966 (2022).
Lu, S., Li, C., Zhang, Y., Zheng, Z. & Liu, D. Functional disruption of a chloroplast pseudouridine synthase desensitizes Arabidopsis plants to phosphate starvation. Front. Plant Sci. 8, 1421 (2017).
Wang, Z. et al. Pseudouridylation of chloroplast ribosomal RNA contributes to low temperature acclimation in rice. New Phytol. 236, 1708–1720 (2022).
Fleming, A. M. et al. Structural elucidation of bisulfite adducts to pseudouridine that result in deletion signatures during reverse transcription of RNA. J. Am. Chem. Soc. 141, 16450–16460 (2019).
Khoddami, V. et al. Transcriptome-wide profiling of multiple RNA modifications simultaneously at single-base resolution. Proc. Natl Acad. Sci. USA 116, 6784–6789 (2019).
Dai, Q. et al. Quantitative sequencing using BID-seq uncovers abundant pseudouridines in mammalian mRNA at base resolution. Nat. Biotechnol. 41, 344–354 (2023).
Taoka, M. et al. Landscape of the complete RNA chemical modifications in the human 80S ribosome. Nucleic Acids Res. 46, 9289–9298 (2018).
Taoka, M. et al. A mass spectrometry-based method for comprehensive quantitative determination of post-transcriptional RNA modifications: the complete chemical structure of Schizosaccharomyces pombe ribosomal RNAs. Nucleic Acids Res. 43, e115 (2015).
Ofengand, J. & Bakin, A. Mapping to nucleotide resolution of pseudouridine residues in large subunit ribosomal RNAs from representative eukaryotes, prokaryotes, archaebacteria, mitochondria and chloroplasts. J. Mol. Biol. 266, 246–268 (1997).
Mergner, J. et al. Mass-spectrometry-based draft of the Arabidopsis proteome. Nature 579, 409–414 (2020).
Arribere, J. A. & Gilbert, W. V. Roles for transcript leaders in translation and mRNA decay revealed by transcript leader sequencing. Genome Res. 23, 977–987 (2013).
Davyt, M., Bharti, N. & Ignatova, Z. Effect of mRNA/tRNA mutations on translation speed: implications for human diseases. J. Biol. Chem. 299, 105089 (2023).
Cui, W. et al. tRNA modifications and modifying enzymes in disease, the potential therapeutic targets. Int. J. Biol. Sci. 19, 1146–1162 (2023).
Liu, Y. et al. tRNA-m(1)A modification promotes T cell expansion via efficient MYC protein synthesis. Nat. Immunol. 23, 1433–1444 (2022).
Zaborske, J. M. et al. A nutrient-driven tRNA modification alters translational fidelity and genome-wide protein coding across an animal genus. PLoS Biol. 12, e1002015 (2014).
Song, J. et al. Differential roles of human PUS10 in miRNA processing and tRNA pseudouridylation. Nat. Chem. Biol. 16, 160–169 (2020).
Agris, P. F. Bringing order to translation: the contributions of transfer RNA anticodon-domain modifications. EMBO Rep. 9, 629–635 (2008).
Schultz, S. K. et al. Modifications in the T arm of tRNA globally determine tRNA maturation, function, and cellular fitness. Proc. Natl Acad. Sci. USA 121, e2401154121 (2024).
Kariko, K. et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 16, 1833–1840 (2008).
Szabo, E. X. et al. Metabolic labeling of RNAs uncovers hidden features and dynamics of the Arabidopsis transcriptome. Plant Cell 32, 871–887 (2020).
Shi, H. et al. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 27, 315–328 (2017).
He, P. C. & He, C. m(6) A RNA methylation: from mechanisms to therapeutic potential. EMBO J. 40, e105977 (2021).
Hu, J. et al. N(6)-Methyladenosine mRNA methylation is important for salt stress tolerance in Arabidopsis. Plant J. 106, 1759–1775 (2021).
Hsu, P. J., Shi, H. & He, C. Epitranscriptomic influences on development and disease. Genome Biol. 18, 197 (2017).
Liang, X. H., Liu, Q. & Fournier, M. J. Loss of rRNA modifications in the decoding center of the ribosome impairs translation and strongly delays pre-rRNA processing. RNA 15, 1716–1728 (2009).
Mignone, F., Gissi, C., Liuni, S. & Pesole, G. Untranslated regions of mRNAs. Genome Biol. 3, REVIEWS0004 (2002).
Hoernes, T. P. et al. Eukaryotic translation elongation is modulated by single natural nucleotide derivatives in the coding sequences of mRNAs. Genes 10, 84 (2019).
Hoernes, T. P. et al. Nucleotide modifications within bacterial messenger RNAs regulate their translation and are able to rewire the genetic code. Nucleic Acids Res. 44, 852–862 (2016).
Acknowledgements
We thank the Margot and Tomas Pritzker Family Foundation for the Pritzker Plant Biology Center at the University of Chicago, and the Bill & Melinda Gates Agricultural Innovations (Gates Ag One). C.H. is an investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
C.H., H.L., G.W. and C.Y. conceived the original idea and project. H.L. and G.W. performed the experiments. H.L. and C.Y. analysed the data. C.H. oversaw the study. G.W., H.L., C.Y. and C.H. wrote the paper. Z.Z., B.J., F.Y., K.H., C.J., L.Z., B.G., S.L., Y.C. and J.Z. participated in experiment design and discussions. All authors approved the paper.
Corresponding author
Ethics declarations
Competing interests
The authors have filed a provision patent application of the method reported in this paper through the University of Chicago. C.H. is a scientific founder, a member of the scientific advisory board and equity holder of Aferna Bio and Ellis Bio, a scientific cofounder and equity holder of Accent Therapeutics, and a member of the scientific advisory board of Rona Therapeutics and Element Biosciences. The other authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Brian Gregory and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The rRNA Ψ sites in the rRNAs of nuclear 18S, nuclear 5.8S and nuclear 25S identified in Arabidopsis using BID-seq.
The Ψ sites were accurately marked and compared with previously published sites. Yellow color marks the Ψ sites identified by BID-seq only; Grey color marks the Ψ sites only previously published, but not identified in this study; Blue color marks the Ψ sites that not only identified in this study but reported previously.
Extended Data Fig. 2 The conserved Ψ sites in the rRNAs of nuclear 18S, nuclear 5.8S and nuclear 25S in rice and Arabidopsis.
The Ψ sites were accurately marked and compared with previously published sites. Yellow color marks the Ψ sites only in rice. Blue color marks the Ψ sites conserved in both rice and Arabidopsis.
Extended Data Fig. 3 The conserved Ψ sites in the rRNA of nuclear 18S, nuclear 5.8S and nuclear 25S in maize and Arabidopsis.
The Ψ sites were accurately marked and compared with previously published sites. Yellow color marks the Ψ sites only in maize. Blue color marks the Ψ sites conserved in both maize and Arabidopsis.
Extended Data Fig. 4 The conserved Ψ sites in the rRNAs of nuclear 18S, nuclear 5.8S and nuclear 25S in soybean and Arabidopsis.
The Ψ sites were accurately marked and compared with previously published sites. Yellow color marks the Ψ sites only in soybean. Blue color marks the Ψ sites conserved in both soybean and Arabidopsis.
Extended Data Fig. 5 rRNA sequence conservation and rRNA Ψ sites among plant species and mammals.
a, The number of total- and conserved Ψ sites in rRNAs of nuclear 18S, nuclear 5.8S, nuclear 25S, chloroplast 23S, and mitochondria 26S identified in the four plants. b, c, The mutation types of the ossvr1 and osrlua3 mutant lines, in the background of ZH11 using CRISPR-Cas9. d, Diagram comparing the rRNA sequence conservation and Ψ sites for nuclear encoded 18S, 5.8S and 25S/28S rRNAs in four plant species as well as mouse and human. The yellow to purple colors represent the rRNA sequence conservation scores ranging from low to high. Each spot represents a rRNA Ψ site in different species. The Ψ site numbers are labeled along with the species names. Number in red color stands for the number of Ψ sites aligned to human rRNA among the total number of nuclear encoded rRNA Ψ sites identified in corresponding species. A total of 105 Ψ sites are identified in nuclear encoded rRNA in human. The BID-seq datasets of mouse and human were downloaded from the Gene Expression Omnibus database under the accession number of GSE238245 and GSE179798 respectively.
Extended Data Fig. 6 Correlation analysis of Ψ level with translation efficiency of genes across multiple tissues in Arabidopsis.
a, Correlation analysis between Ψ level on each rRNA site and 977 transcripts’ translation efficiency at transcript level (Etranscript). The median correlation value was shown by the yellow or the blue dot. The error bars represent the interquartile range (IQR). Etranscript were calculated by normaling ribosome-bound RNA (with polysome footprinting data) to the whole-cell mRNA level. b, Examples of Ψ sites on Nu-18S: 913 and Nu-18S: 1195 that calculated with the correlations between Ψ levels and translation efficiency (Etranscript) among nine Arabidopsis tissues are shown. Etranscript were calculated by normaling ribosome-bound RNA (with polysome footprinting data) to the whole-cell mRNA level. Examples of correlations regarding to specific genes were also showed in b. Nu-18S: 913 belongs to the initiation region, and Nu-18S: 1195 belongs to the decoding region. c, Heatmap showing all the correlation value between the Ψ level of each rRNA site and the translation efficiency (Etranscript) of each transcript. The correlation matrix was clustered by the similarities score among Ψ sites and 977 transcripts.
Extended Data Fig. 7 Ψ modification motifs in Arabidopsis and rice.
a, b, All potential TΨG motif sequences with a sliding window of 1nt in Arabidopsis (a) and rice (b). Motifs with fractions over 20% were selected to plot the figure and the number of each motif (N) is shown. c, d, All potential TΨC motif sequences with a sliding window of 1nt in Arabidopsis (c) and rice (d). All the detected Ψ sites within tissues were combined to calculate the motifs. Motifs with fractions over 20% were selected to plot the figure and the number of each motif (N) is showed. The sliding window of 1 nt means 1 nucleotide upstream and 1 nucleotide downstream of the given motif. Combining with the motif length of 3 nt, the window size is 5 nt. e, f, Heatmap showing motif types and numbers of Ψ modifications in each tissue of Arabidopsis (e) and rice (f). All the detected Ψ sites within tissues were combined to calculate the motifs. 8DAY represents 8 days and 2 W represents 2 weeks. 10DAA represents 10 days after anthesis.
Extended Data Fig. 8 Overview of the quality of BID-seq data.
a–c, mRNA Ψ levels in the harvested samples were quantified using LC-MS/MS for rice (a), Arabidopsis (b), maize and soybean (c). Three biological replicates were used. Data are means ± SD, n = 3. d, e, Principal component analysis (PCA) of Ψ fractions in Arabidopsis (d) and in rice (e) across different tissues. 8DAY represents 8 days and 2 W represents 2 weeks. 10DAA represents 10 days after anthesis.
Extended Data Fig. 9 mRNA Ψ modification in various tissues of Arabidopsis and rice.
a, BID-seq revealed a large number of mRNA Ψ sites in ten rice tissues. b, Diverse average Ψ fractions on mRNA across different tissues in rice, including plumule (n = 1,468), radicle (n = 1,851), seedling 8DAY (n = 1,153), seedling 2 W (n = 1,781), straw heading (n = 1,710), flag leaf heading (n = 1,631), panicle (n = 1,604), flag leaf 10DAA (n = 1,699), embryo (n = 1,844), and endosperm (n = 1,128) shown in boxplot. The red dots mark the mean, the lines show the median, the boxes represent the interquartile range (IQR), and the whiskers extend to 1.5× of the IQR. c, Boxplot showing fractions of tissue common (n = 123) and unique mRNA Ψ sites in Arabidopsis, including seedling (n = 478), shoot (n = 400), root (n = 1,160), rossta leaf (n = 385), cauline leaf (n = 315), stem (n = 416), flower (n = 706), silique (n = 370), and seed (n = 712). d, Boxplot showing fractions of tissue common (n = 55) and unique mRNA Ψ sites in rice, including plumule (n = 399), radicle (n = 1,042), seedling 8DAY (n = 200), seedling 2 W (n = 552), straw heading (n = 408), flag leaf heading (n = 509), panicle (n = 440), flag leaf 10DAA (n = 482), embryo (n = 728), and endosperm (n = 401). The lines show the median, the boxes represent the interquartile range (IQR), and the whiskers extend to 1.5× of the IQR. 8DAY represents 8 days and 2 W represents 2 weeks. 10DAA represents 10 days after anthesis.
Supplementary information
Supplementary Tables 1–3
Supplementary Table 1. Number of tRNA Ψ sites among nine Arabidopsis tissues. Supplementary Table 2. Number of mRNA Ψ sites among nine Arabidopsis tissues. Supplementary Table 3. Number of mRNA Ψ sites among ten different rice tissues.
Source data
Source Data Fig. 1
Source data for Figs. 1, 2 and 5 and Extended Data Figs. 5, 7, 8 and 9.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, H., Wang, G., Ye, C. et al. Quantitative RNA pseudouridine maps reveal multilayered translation control through plant rRNA, tRNA and mRNA pseudouridylation. Nat. Plants 11, 234–247 (2025). https://doi.org/10.1038/s41477-024-01894-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41477-024-01894-7
This article is cited by
-
Pseudouridine is the hidden language of plant RNA translation
Nature Plants (2025)
-
Regulatory roles of RNA modifications in plant development and fruit ripening
aBIOTECH (2025)