Abstract
Dietary consumption of lysine in humans leads to the biosynthesis of Δ1-piperideine-6-carboxylic acid (P6C), with elevated levels linked to the neurological disorder epilepsy. Here we demonstrate that P6C biosynthesis is also a critical component of lysine catabolism in Arabidopsis thaliana. P6C regulates vitamin B6 homeostasis, and increased P6C levels deplete B6 vitamers, resulting in compromised plant immunity. We further establish a key role for pyridoxal and pyridoxal-5-phosphate biosynthesis in plant immunity. Our analysis indicates that P6C metabolism probably evolved through combining select lysine and proline metabolic enzymes horizontally acquired from diverse bacterial sources at different points during evolution. More generally, certain enzymes from the lysine and proline metabolic pathways were probably recruited in evolution as potential guardians of B6 vitamers and for semialdehyde detoxification.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Source data are provided with this paper. All other data that support the findings of this study are available from the corresponding author upon request.
Change history
03 March 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41477-025-01960-8
References
Civitelli, R. et al. Dietary l-lysine and calcium metabolism in humans. Nutrition 8, 400–405 (1992).
Matthews, D. E. Review of lysine metabolism with a focus on humans. J. Nutr. 150, 2548S–2555S (2020).
Návarová, H., Bernsdorff, F., Döring, A.-C. & Zeier, J. Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 24, 5123–5141 (2012).
Ding, P. et al. Characterization of a pipecolic acid biosynthesis pathway required for systemic acquired resistance. Plant Cell 28, 2603–2615 (2016).
Hartmann, M. et al. Biochemical principles and functional aspects of pipecolic acid biosynthesis in immunity. Plant Physiol. 174, 124–153 (2017).
Song, J. T., Lu, H. & Greenberg, J. T. Divergent roles in Arabidopsis thaliana development and defense of two homologous genes, ABERRANT GROWTH AND DEATH2 and AGD2-LIKE DEFENSE RESPONSE PROTEIN1, encoding novel aminotransferases. Plant Cell 16, 353–366 (2004).
Chen, Y. C. et al. N-hydroxy-pipecolic acid is a mobile metabolite that induces systemic disease resistance in Arabidopsis. Proc. Natl Acad. Sci. USA 115, E4920–E4929 (2018).
Hartmann, M. et al. Flavin monooxygenase-generated N-hydroxypipecolic acid is a critical element of plant systemic immunity. Cell 173, 456–469.e416 (2018).
Kachroo, A., Liu, H., Yuan, X., Kurokawa, T. & Kachroo, P. Systemic acquired resistance-associated transport and metabolic regulation of salicylic acid and glycerol-3-phosphate. Essays Biochem. 66, 673–681 (2022).
Chaturvedi, R. et al. Plastid ω3‐fatty acid desaturase‐dependent accumulation of a systemic acquired resistance inducing activity in petiole exudates of Arabidopsis thaliana is independent of jasmonic acid. Plant J. 54, 106–117 (2008).
Vlot, A. C., Dempsey, D. M. A. & Klessig, D. F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 47, 177–206 (2009).
Yu, K. et al. A feedback regulatory loop between G3P and lipid transfer proteins DIR1 and AZI1 mediates azelaic-acid-induced systemic immunity. Cell Rep. 3, 1266–1278 (2013).
Gao, Q.-M. et al. Mono- and digalactosyldiacylglycerol lipids function nonredundantly to regulate systemic acquired resistance in plants. Cell Rep. 9, 1681–1691 (2014).
Jung, H. W., Tschaplinski, T. J., Wang, L., Glazebrook, J. & Greenberg, J. T. Priming in systemic plant immunity. Science 324, 89–91 (2009).
Chanda, B. et al. Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants. Nat. Genet. 43, 421–427 (2011).
Shine, M. et al. Glycerol-3-phosphate mediates rhizobia-induced systemic signaling in soybean. Nat. Commun. 10, 5303 (2019).
Wang, C. et al. Free radicals mediate systemic acquired resistance. Cell Rep. 7, 348–355 (2014).
Wenig, M. et al. Systemic acquired resistance networks amplify airborne defense cues. Nat. Commun. 10, 3813 (2019).
Riedlmeier, M. et al. Monoterpenes support systemic acquired resistance within and between plants. Plant Cell 29, 1440–1459 (2017).
Wang, C. et al. Extracellular pyridine nucleotides trigger plant systemic immunity through a lectin receptor kinase/BAK1 complex. Nat. Commun. 10, 4810 (2019).
Shine, M. B., Xiao, X., Kachroo, P. & Kachroo, A. Signaling mechanisms underlying systemic acquired resistance to microbial pathogens. Plant Sci. 279, 81–86 (2018).
Gao, Q.-M., Kachroo, A. & Kachroo, P. Chemical inducers of systemic immunity in plants. J. Exp. Bot. 65, 1849–1855 (2014).
Cecchini, N. M., Steffes, K., Schlappi, M. R., Gifford, A. N. & Greenberg, J. T. Arabidopsis AZI1 family proteins mediate signal mobilization for systemic defence priming. Nat. Commun. 6, 7658 (2015).
Lim, G.-H. et al. The plant cuticle regulates apoplastic transport of salicylic acid during systemic acquired resistance. Sci. Adv. 6, eaaz0478 (2020).
Lim, G.-H. et al. Plasmodesmata localizing proteins regulate transport and signaling during systemic acquired immunity in plants. Cell Host Microbe 19, 541–549 (2016).
Huang, D. et al. Salicylic acid-mediated plasmodesmal closure via Remorin-dependent lipid organization. Proc. Natl Acad. Sci. USA 116, 21274–21284 (2019).
Wang, C. et al. Pipecolic acid confers systemic immunity by regulating free radicals. Sci. Adv. 4, eaar4509 (2018).
Shine, M. et al. Phased small RNA–mediated systemic signaling in plants. Sci. Adv. 8, eabm8791 (2022).
Carr, J. P., Lewsey, M. G. & Palukaitis, P. in Advances in Virus Research Vol. 76 (eds Carr, J. P. & Loebenstein, G.) 57–121 (Academic Press, 2010).
Broquist, H. P. Lysine–pipecolic acid metabolic relationships in microbes and mammals. Annu. Rev. Nutr. 11, 435–448 (1991).
Goyer, A. et al. Characterization and metabolic function of a peroxisomal sarcosine and pipecolate oxidase from Arabidopsis. J. Biol. Chem. 279, 16947–16953 (2004).
Soda, K., Misono, H. & Yamamoto, T. l-lysine:alpha-ketoglutarate aminotransferase. I. Identification of a product, delta-1-piperideine-6-carboxylic acid. Biochemistry 7, 4102–4109 (1968).
Dodt, G. et al. The human l-pipecolic acid oxidase is similar to bacterial monomeric sarcosine oxidases rather than d-amino acid oxidases. Cell Biochem. Biophys. 32, 313–316 (2000).
Mills, P. B. et al. Mutations in antiquitin in individuals with pyridoxine-dependent seizures. Nat. Med. 12, 307–309 (2006).
Plecko, B. et al. Biochemical and molecular characterization of 18 patients with pyridoxine‐dependent epilepsy and mutations of the antiquitin (ALDH7A1) gene. Hum. Mutat. 28, 19–26 (2007).
Stockler, S. et al. Pyridoxine dependent epilepsy and antiquitin deficiency: clinical and molecular characteristics and recommendations for diagnosis, treatment and follow-up. Mol. Genet. Metab. 104, 48–60 (2011).
Kaminiów, K., Pająk, M., Pająk, R. & Paprocka, J. Pyridoxine-dependent epilepsy and antiquitin deficiency resulting in neonatal-onset refractory seizures. Brain Sci. 12, 65 (2021).
Funck, D., Sinn, M., Forlani, G. & Hartig, J. S. Guanidine production by plant homoarginine-6-hydroxylases. eLife 12, RP91458 (2024).
Mooney, S., Leuendorf, J.-E., Hendrickson, C. & Hellmann, H. Vitamin B6: a long known compound of surprising complexity. Molecules 14, 329–351 (2009).
Tambasco-Studart, M. et al. Vitamin B6 biosynthesis in higher plants. Proc. Natl Acad. Sci. USA 102, 13687–13692 (2005).
Fitzpatrick, T. B. et al. Two independent routes of de novo vitamin B6 biosynthesis: not that different after all. Biochem. J. 407, 1–13 (2007).
Tambasco-Studart, M., Tews, I., Amrhein, N. & Fitzpatrick, T. B. Functional analysis of PDX2 from Arabidopsis, a glutaminase involved in vitamin B6 biosynthesis. Plant Physiol. 144, 915–925 (2007).
Titiz, O. et al. PDX1 is essential for vitamin B6 biosynthesis, development and stress tolerance in Arabidopsis. Plant J. 48, 933–946 (2006).
Thompson, M. G. et al. Massively parallel fitness profiling reveals multiple novel enzymes in Pseudomonas putida lysine metabolism. MBio 10, 02577–02518 (2019).
Pena, I. A. et al. Pyridoxine-dependent epilepsy in zebrafish caused by Aldh7a1 deficiency. Genetics 207, 1501–1518 (2017).
Percudani, R. & Peracchi, A. A genomic overview of pyridoxal‐phosphate‐dependent enzymes. EMBO Rep. 4, 850–854 (2003).
Zhang, Y. et al. Vitamin B6 contributes to disease resistance against Pseudomonas syringae pv. tomato DC3000 and Botrytis cinerea in Arabidopsis thaliana. J. Plant Physiol. 175, 21–25 (2015).
Chandrasekaran, M., Paramasivan, M. & Chun, S.-C. Bacillus subtilis CBR05 induces vitamin B6 biosynthesis in tomato through the de novo pathway in contributing disease resistance against Xanthomonas campestris pv. vesicatoria. Sci. Rep. 9, 6495 (2019).
Fitzpatrick, T. B. Vitamin B6 in plants: More than meets the eye. In Advances in Botanical Research Vol. 59 (eds Rébeillé, F. & Douce, R.) 1–38 (Elsevier, 2011).
Wagner, S. et al. Analysis of the Arabidopsis rsr4-1/pdx1-3 mutant reveals the critical function of the PDX1 protein family in metabolism, development, and vitamin B6 biosynthesis. Plant Cell 18, 1722–1735 (2006).
Wondrak, G. T. & Jacobson, E. L. Vitamin B6: Beyond coenzyme functions. In Water Soluble Vitamins: Clinical Research and Future Application (ed. Stanger, O.) 291–300 (Springer, 2012).
Wang, S. et al. Fusarium‐produced vitamin B6 promotes the evasion of soybean resistance by Phytophthora sojae. J. Integr. Plant Biol. 65, 2204–2217 (2023).
Liu, H. et al. The bacterial effector AvrRxo1 inhibits vitamin B6 biosynthesis to promote infection in rice. Plant Commun. 3, 100324 (2022).
Mills, P. B. et al. Epilepsy due to PNPO mutations: genotype, environment and treatment affect presentation and outcome. Brain 137, 1350–1360 (2014).
Huang, J. & Gogarten, J. P. Did an ancient chlamydial endosymbiosis facilitate the establishment of primary plastids? Genome Biol. 8, R99 (2007).
Wolf, Y. I., Aravind, L. & Koonin, E. V. Rickettsiae and Chlamydiae: evidence of horizontal gene transfer and gene exchange. Trends Genet. 15, 173–175 (1999).
Fischer, S., Michnick, S. & Karplus, M. A mechanism for rotamase catalysis by the FK506 binding protein (FKBP). Biochemistry 32, 13830–13837 (1993).
Van Duyne, G. D., Standaert, R. F., Karplus, P. A., Schreiber, S. L. & Clardy, J. Atomic structures of the human immunophilin FKBP-12 complexes with FK506 and rapamycin. J. Mol. Biol. 229, 105–124 (1993).
Farrant, R. D., Walker, V., Mills, G. A., Mellor, J. M. & Langley, G. J. Pyridoxal phosphate de-activation by pyrroline-5-carboxylic acid: increased risk of vitamin B6 deficiency and seizures in hyperprolinemia type II. J. Biol. Chem. 276, 15107–15116 (2001).
Zhao, J., Missihoun, T. D. & Bartels, D. The role of Arabidopsis aldehyde dehydrogenase genes in response to high temperature and stress combinations. J. Exp. Bot. 68, 4295–4308 (2017).
Dingler, F. A. et al. Two aldehyde clearance systems are essential to prevent lethal formaldehyde accumulation in mice and humans. Mol. Cell 80, 996–1012. e1019 (2020).
Darwin, K. H. & Stanley, S. A. The aldehyde hypothesis: metabolic intermediates as antimicrobial effectors. Open Biol. 12, 220010 (2022).
Fujii, T., Mukaihara, M., Agematu, H. & Tsunekawa, H. Biotransformation of l-lysine to l-pipecolic acid catalyzed by l-lysine 6-aminotransferase and pyrroline-5-carboxylate reductase. Biosci. Biotechnol. Biochem. 66, 622–627 (2002).
İpek, R., Çavdartepe, B. E., Kor, D. & Okuyaz, Ç. Pyridoxine-dependent epilepsy caused by a novel homozygous mutation in PLPBP gene. Metab. Brain Dis. 37, 3027–3032 (2022).
Liang, X., Zhang, L., Natarajan, S. K. & Becker, D. F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 19, 998–1011 (2013).
Nakagawa, T. et al. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104, 34–41 (2007).
Zhang, D.-X., Nagabhyru, P. & Schardl, C. L. Regulation of a chemical defense against herbivory produced by symbiotic fungi in grass plants. Plant Physiol. 150, 1072–1082 (2009).
Xia, Y. et al. An intact cuticle in distal tissues is essential for the induction of systemic acquired resistance in plants. Cell Host Microbe 5, 151–165 (2009).
Daudi, A. & O’Brien, J. A. Detection of hydrogen peroxide by DAB staining in Arabidopsis leaves. Bio Protoc. 2, e263 (2012).
Yu, K., Liu, H. & Kachroo, P. Pipecolic acid quantification using gas chromatography-coupled mass spectrometry. Bio Protoc. 10, e3841 (2020).
Dührkop, K., Scheubert, K. & Böcker, S. Molecular formula identification with SIRIUS. Metabolites 3, 506–516 (2013).
Schäffer, A. A. et al. Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nucleic Acids Res. 29, 2994–3005 (2001).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Deorowicz, S., Debudaj-Grabysz, A. & Gudyś, A. FAMSA: fast and accurate multiple sequence alignment of huge protein families. Sci. Rep. 6, 33964 (2016).
Price, M. N., Dehal, P. S. & Arkin, A. P. FastTree 2—approximately maximum-likelihood trees for large alignments. PLoS ONE 5, e9490 (2010).
Minh, B. Q. et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 37, 1530–1534 (2020).
Hauser, M., Steinegger, M. & Söding, J. MMseqs software suite for fast and deep clustering and searching of large protein sequence sets. Bioinformatics 32, 1323–1330 (2016).
Johnson, L. S., Eddy, S. R. & Portugaly, E. Hidden Markov model speed heuristic and iterative HMM search procedure. BMC Bioinform. 11, 431 (2010).
Schäffer, A. A. et al. IMPALA: matching a protein sequence against a collection of PSI-BLAST-constructed position-specific score matrices. Bioinformatics 15, 1000–1011 (1999).
Paysan-Lafosse, T. et al. InterPro in 2022. Nucleic Acids Res. 51, D418–D427 (2023).
Acknowledgements
We thank T. Missihoun for aldh3 seeds and ABRC for aldh, pdx and sos4 seeds. We thank L. Song for help with analytical analysis and W. Havens and A. Crume for technical help. The chemical analysis reported in this study was carried out at the Center for Agricultural and Life Sciences Metabolomics (https://plantpathology.ca.uky.edu/lab/Analytical-CORE). This work was supported by grants from the National Science Foundation (MCB#0421914, MCB#2435880, IOS#051909 and IOS#0817818) and the USDA National Institute of Food and Agriculture (GRANT13323564 and Hatch project no. 1014539). L.M.I. and L.A. were supported by the funds of the Division of Intramural Research of the National Library of Medicine at the National Institutes of Health. This Article is based on work supported by (while serving at) the National Science Foundation for A.K.
Author information
Authors and Affiliations
Contributions
H.L. performed the majority of the experiments with help from P.N., R.L., P.K., M.G., A.K. and K.Y. L.M.I. and L.A. evaluated phylogenetic relationships. P.K. wrote the paper with edits from all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Xinhua Ding and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 A simplified model illustrating lysine catabolic pathway.
(a) In the saccharopine pathway, lysine is first converted to saccharopine by lysine ketoglutarate reductase (LKR), which is then converted to glutamate and AASA by saccharopine dehydrogenase (SDH). AASA can spontaneously convert to P6C, thus joining the Pip-pathway. AASA is metabolized to AAA by aldehyde dehydrogenase (AASAdh/Antiquitin) encoded by the ALDH7B4 gene in Arabidopsis. (b) Lysine undergoes oxidative deamination to form ε-amino-α-caproric acid, followed by cyclization to piperideine-2 carboxylic acid (P2C) and subsequent reduction to pipecolic acid (Pip). Lysine to P2C and P2C to Pip steps are catalyzed by ALD1 and SARD4, respectively. Pip is also oxidized to piperideine-2 carboxylic acid (P6C) by SOX (PipOX) or to N-hydroxypipecolic acid (NHP) via the FMO1 flavin monooxygenase. Low level accumulation of Pip in sard4 plants suggest involvement of additional unknown enzymes (indicated by?). Enzymes are shown in green.
Extended Data Fig. 2 SOX converts Pip to P6C.
(a) SDS-PAGE gel showing purity of E. coli expressed and purified SOX-HIS and FMO1-HIS proteins. (b) Schematic representation of the SOX catalyzed enzymatic reaction that converts Pip to P6C. (c) In vitro enzymatic catabolism of Pip. Purified SOX enzyme (20 µg) was incubated with 1 or 10 mM of Pip followed by GC-MS analysis. (d) MS and MS/MS fragmentation of Pip (upper panel) and P6C (lower panel) using 10 V collision energy (CE). (e) Absorption spectrum of P6C-o-aminobenzaldehyde-derivative. P6C was synthesized using an in vitro SOX-catalyzed enzymatic reaction. (f) A comparison of SOX and FMO1 in vitro enzymatic activities towards their common substrate Pip. Purified SOX (10 µg) and FMO1 (30 µg) enzymes were incubated with 1 mM Pip followed by GC-MS analysis. The error bars represent SD (n = 4 biological replicates). Asterisks denote a significant difference (multiple unpaired t test, ****P < 0.0001). The experiments described here were repeated at least twice with similar results.
Extended Data Fig. 3 In planta expression of SOX converts Pip to P6C.
(a) Confocal micrographs showing transient expression of ALD1, FMO1, SOX, or co-expression of ALD1 + FMO1 or ALD1 + SOX in Nicotiana benthamiana plants. (b-d) Levels of Pip (b), NHP (c) and P6C (d) in N. benthamiana plants transiently expressing ALD1, SOX, FMO1, ALD1 + SOX or ALD1 + FMO1. Plants transiently expressing empty vector were used as controls. (e-f) UHPLC-MS analysis showing extracted ion-chromatograms e) and corresponding mass spectra (f) of P2C and P6C. Tissue extracts were prepared from mock- and Pst-avrRpt2 inoculated Col-0 plants sampled 24 h post inoculation. P6C standard (std) used in this analysis was synthesized using an in vitro SOX-catalyzed enzymatic reaction. The samples were run on a Kinetex F5, 1.7 µm (2.1 mm x 150 mm) column. The error bars represent SD (n = 3 or 4 biological replicates). Asterisks denote a significant difference (multiple unpaired t test, **P < 0.002; ***P < 0.0002; ****P < 0.0001). The experiments described here were repeated at least twice with similar results.
Extended Data Fig. 4 Maximum-likelihood phylogenetic tree of the aldehyde dehydrogenase superfamily.
(a-c Relative levels of G3P (a), SA (b) and SAG (c) in mock- and Pst- avrRpt2 inoculated Col-0 and 35S–PipOX plants. The leaves were sampled 24 h post treatment. The error bars represent SD (n = 3 or 4 biological replicates). Asterisks denote a significant difference (multiple unpaired t test, **P < 0.002; ***P < 0.0002; ****P < 0.0001). These experiments were repeated at least twice with similar results. (d) Phylogenetic analysis showing the major clades in the ALDH family. Several conserved clades were collapsed into filled triangles for convenience. Only key clades are labeled, and clades that contain Arabidopsis or human proteins are labeled as such with their gene names and biochemical activities where known. Additionally displayed are the metabolites, such as amino acids lignin and ethanol, whose pathways involve the functioning of the corresponding ALDH. The raw data for these trees can be accessed from the supplementary material (Supplementary Fig. 2). Clades with bootstrap values > 80% are marked with filled circles. The color of the collapsed triangle reflects the phyletic distribution of the branches in that clade, as given in the key.
Extended Data Fig. 5 Relationship between P6C and vitamin B6 biosynthesis pathway in plants.
Vitamin B6 vitamers comprise of PN (pyridoxine), PL (pyridoxal) and PM (pyridoxamine) and their phosphorylated forms PNP, PLP and PMP, respectively. PL and PLP form multiple complexes with P6C. De novo synthesis of PLP involves ribose 5-phosphate (R5P), glyceraldehyde-3-phosphate (Gl3P) and glutamine and is catalysed by PDX1 and PDX2 enzymes. Conversion of PN, PL and PM to their phosphorylated forms is mediated by SOS4. Pase indicates the phosphatase that converts PMP, PLP, and PNP to their non-phosphorylated forms. PDX3 is an oxidase that converts PMP and PNP to PLP. Tase refers to transaminase.
Extended Data Fig. 6 Vitamers are glycosylated and interconverted into PM, PN or PL forms.
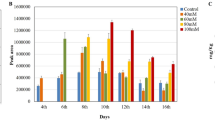
(a, b, e, f) Relative levels of vitamers in Col-0 plants treated with water or PN (a, c), PM (b, d), PL (e) or PLP (f). (c-d) These subpanels show relative levels of vitamers that are present at low levels in a and b. PNG, PMG, PLG, represent glycosylated derivatives of PN, PM, PL, respectively. The error bars represent SD (n = 3 or 4 biological replicates). Asterisks denote a significant difference (multiple unpaired t test, *P < 0.0033; **P < 0.0021; ***P < 0.0002; ****P < 0.0001). The experiments described here were repeated at least twice with similar results.
Extended Data Fig. 7 A mutation in PLP phosphatase restores sos4 phenotypes.
(a) Simplified scheme showing vitamer biosynthesis and their interconversion. Treatment with individual vitamers results in a significant increase in glycosylated derivatives of PN, PL and PM that are represented by PNG, PLG, PMG, respectively. Dashed green lines indicate that PL-PM and PM-PN are interconvertible and that PN can be converted to PL. (b) Relative levels of PM, PN, PL and PLP in four-week-old Col-0 and plpp1 plants. (c) Typical morphological phenotype of four-week-old indicated genotypes. Scale bar represents 1 cm. (d) SAR response in distal leaves of Col-0, sos4, plpp1 and sos4 plpp1 plants treated locally with mock (MgCl2) or Pst-avrRpt2. The error bars represent SD (n = 3 or 4 biological replicates). Asterisks denote a significant difference (multiple unpaired t test, *P < 0.0033; **P < 0.0021; ****P < 0.0001). The experiments described here were repeated at least twice with similar results.
Extended Data Fig. 8 Vitamin B6 homeostasis plays an important role in plant defense.
(a) Typical morphological phenotype of Arabidopsis plants treated with water or 1 mM vitamers (PN/PL/PM) prior to inoculation with Pst-avrRpt2 (106 CFU/ml). The plants were photographed 12 h post-inoculation. Arrows indicates pathogen inoculated leaves. (b) H2O2 levels in plants treated with water or 1 mM vitamers (PN/PL/PM) prior to inoculation with Pst-avrRpt2 (106 CFU/ml). Scale bar represent 0.5 cm. (c) Electrolyte leakage in Arabidopsis plants treated with water or 1 mM vitamers (PN/PL/PM) prior to inoculation with Pst-avrRpt2 (106 CFU/ml). Error bars represent SD (n = 5). (d) Plate assay showing growth of Pst-avrRpt2 on Kings B medium in the presence of 20 µM H2O2 or 1 mM vitamers (PN/PL/PM). (e-g) Relative levels of SA (left panel) and SAG (right panel) (e), Pip (f), NHP (left panel) and NHP-glucoside (right panel) (g) in Arabidopsis plants treated with water or 1 mM vitamers (PN/PL/PM) prior to inoculation with Pst-avrRpt2 (106 CFU/ml). (h) Growth of Pst-avrRpt2 on Arabidopsis plants treated with water or 1 mM vitamers (PN/PL/PM) prior to inoculation. The error bars represent SD (n = 3 or 4 biological replicates). Asterisks denote a significant difference (multiple unpaired t test, **P < 0.0021; ****P < 0.0001). The experiments described here were repeated at least twice with similar results.
Extended Data Fig. 9 Vitamin B6-treated plants exhibit a basal metabolomic profile.
(a) Heat map and grouping analyses of the chemical profiles in Arabidopsis plants treated with water or 1 mM vitamers (PN/PL/PM) prior to inoculation with Pst avrRpt2 (106 CFU/ml). The samples were analyzed in positive electrospray ionization (ESI) mode. Shades of blue and red colors indicate low to high values, respectively. (b) Principal component analysis of entities from Arabidopsis plants treated with water or 1 mM vitamers (PN/PL/PM) prior to inoculation with Pst-avrRpt2 (106 CFU/ml). The samples were analyzed in positive ESI mode. PCA was executed with MassHunter Mass Profiler Professional software on the log2-transformed data. (c) Leaf length of indicated genotypes grown on Murashige and Skoog (MS) medium with or without 5 mM Pip or 5 mM Pip + 200 μM vitamin B6. The error bars represent SD (n = 8 biological replicates). Asterisks denote a significant difference (multiple unpaired t test, **P < 0.0021; ****P < 0.0001). One-way ANOVA was also used to determine statistical significance in Fig. 9c. Different letters above bars indicate statistically significant differences among groups (Tukey’s post hoc test, p < 0.05). The experiments described here were repeated at least twice with similar results.
Extended Data Fig. 10 Phyletic distribution heatmap.
The heatmap represents the percentage of complete genomes that possess the ortholog of a specific protein family across different lineages. Each cell reflects the proportion of genomes within a particular lineage (columns) that contain the protein family (rows). The color key at the bottom ranging from grey to red indicates the percent distribution of a family within a given lineage.
Supplementary information
Supplementary Information
Supplementary text and figures.
Supplementary Tables
Supplementary Tables 1–8.
Source data
Source Data Fig. 1
Source data for Fig. 1.
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 3
Source data for Fig. 3.
Source Data Fig. 4
Source data for Fig. 4.
Source Data Fig. 5
Source data for Fig. 5.
Source Data Extended Data Fig. 2
Source data for Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Source data for Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Source data for Extended Data Fig. 4.
Source Data Extended Data Fig. 6
Source data for Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Source data for Extended Data Fig. 7.
Source Data Extended Data Fig. 8
Source data for Extended Data Fig. 8.
Source Data Extended Data Fig. 9
Source data for Extended Data Fig. 9.
Source Data Fig. 1e and 5a
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, H., Iyer, L.M., Norris, P. et al. Piperideine-6-carboxylic acid regulates vitamin B6 homeostasis and modulates systemic immunity in plants. Nat. Plants 11, 263–278 (2025). https://doi.org/10.1038/s41477-025-01906-0
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41477-025-01906-0