Abstract
DICER-LIKE 1 (DCL1), a plant-specific RNase III enzyme, is fundamental to post-transcriptional gene regulation mediated by microRNAs (miRNAs). DCL1 processes precursor miRNAs into mature miRNAs, typically 20–22 nucleotides long. Despite its crucial role, the RNA elements that guide DCL1’s cleavage site selection have remained largely uncharacterized. In this study, we employed a high-throughput sequencing approach to analyse Arabidopsis thaliana DCL1 cleavage patterns on over 46,000 short hairpin RNA sequences previously studied with human DICER. Our analyses revealed that DCL1 cleavage preferences are governed by specific secondary RNA structures and sequence motifs, among which a particular RNA element, designated the GHR motif, emerged as pivotal. This motif remarkably influences cleavage site selection independently of the double-stranded RNA-binding domains and helicase domains of DCL1, operating primarily through the RNase IIIDa domain. The GHR motif is evolutionarily conserved across plant species and is essential for the precise cleavage of various plant precursor miRNAs. Our findings also suggest a role for the GHR motif in the biogenesis of non-canonical 22-nucleotide miRNAs, expanding its functional impact. These insights deepen our understanding of the molecular mechanisms underlying DCL1’s specificity and highlight its integral role in miRNA maturation and gene regulatory networks in plants.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
A. thaliana miRNA sequences were collected from miRbase v.22 (ref. 55). Orthologous pri-miRNAs from 29 plant species were collected from a previous paper23. Protein structures were obtained from the Protein Data Bank (7XW2 for human DICER and 7ELE for DCL1). Structure analysis figures were illustrated using Chimera v.1.17.3 and PyMol-2.5.4 (Schrödinger). The sequencing and processed data from this study have been submitted to the Gene Expression Omnibus (GSE278156). Source data are provided with this paper. All other data are available from the corresponding author upon request.
Code availability
The source code for data analysis is available via Zenodo at https://doi.org/10.5281/zenodo.13828938 (ref. 58).
References
Ha, M. & Kim, V. N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 15, 509–524 (2014).
Bartel, D. P. Metazoan microRNAs. Cell 173, 20–51 (2018).
Park, W., Li, J., Song, R., Messing, J. & Chen, X. CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr. Biol. 12, 1484–1495 (2002).
Kurihara, Y. & Watanabe, Y. Arabidopsis micro-RNA biogenesis through Dicer-like 1 protein functions. Proc. Natl Acad. Sci. USA 101, 12753–12758 (2004).
Margis, R. et al. The evolution and diversification of Dicers in plants. FEBS Lett. 580, 2442–2450 (2006).
Achkar, N. P., Cambiagno, D. A. & Manavella, P. A. miRNA biogenesis: a dynamic pathway. Trends Plant Sci. 21, 1034–1044 (2016).
Yu, Y., Jia, T. & Chen, X. The ‘how’ and ‘where’ of plant microRNAs. N. Phytol. 216, 1002–1017 (2017).
Song, X., Li, Y., Cao, X. & Qi, Y. MicroRNAs and their regulatory roles in plant–environment interactions. Annu. Rev. Plant Biol. 70, 489–525 (2019).
Zhan, J. & Meyers, B. C. Plant small RNAs: their biogenesis, regulatory roles, and functions. Annu. Rev. Plant Biol. 74, 21–51 (2023).
Xie, Z. et al. Expression of Arabidopsis MIRNA genes. Plant Physiol. 138, 2145–2154 (2005).
Dong, Z., Han, M. H. & Fedoroff, N. The RNA-binding proteins HYL1 and SE promote accurate in vitro processing of pri-miRNA by DCL1. Proc. Natl Acad. Sci. USA 105, 9970–9975 (2008).
Wei, X. et al. Structural basis of microRNA processing by Dicer-like 1. Nat. Plants 7, 1389–1396 (2021).
Baumberger, N. & Baulcombe, D. C. Arabidopsis ARGONAUTE1 is an RNA Slicer that selectively recruits microRNAs and short interfering RNAs. Proc. Natl Acad. Sci. USA 102, 11928–11933 (2005).
Rajagopalan, R., Vaucheret, H., Trejo, J. & Bartel, D. P. A diverse and evolutionarily fluid set of microRNAs in Arabidopsis thaliana. Genes Dev. 20, 3407–3425 (2006).
Wu, L. et al. DNA methylation mediated by a microRNA pathway. Mol. Cell 38, 465–475 (2010).
Jacobsen, S. E., Running, M. P. & Meyerowitz, E. M. Disruption of an RNA helicase/RNAse III gene in Arabidopsis causes unregulated cell division in floral meristems. Development 126, 5231–5243 (1999).
Ray, A., Lang, J. D., Golden, T. & Ray, S. SHORT INTEGUMENT (SIN1), a gene required for ovule development in Arabidopsis, also controls flowering time. Development 122, 2631–2638 (1996).
Tagami, Y., Motose, H. & Watanabe, Y. A dominant mutation in DCL1 suppresses the hyl1 mutant phenotype by promoting the processing of miRNA. RNA 15, 450–458 (2009).
Liu, C., Axtell, M. J. & Fedoroff, N. V. The helicase and RNaseIIIa domains of Arabidopsis Dicer-Like1 modulate catalytic parameters during microRNA biogenesis. Plant Physiol. 159, 748–758 (2012).
Yan, X. et al. Parallel degradome-seq and DMS-MaPseq substantially revise the miRNA biogenesis atlas in Arabidopsis. Nat. Plants 10, 1126–1143 (2024).
Moro, B. et al. Efficiency and precision of microRNA biogenesis modes in plants. Nucleic Acids Res. 46, 10709–10723 (2018).
Bologna, N. G. et al. Multiple RNA recognition patterns during microRNA biogenesis in plants. Genome Res. 23, 1675–1689 (2013).
Chorostecki, U., Moro, B., Rojas, A. M. L., Debernardi, J. M. & Schapire, A. L. Evolutionary footprints reveal insights into plant microRNA biogenesis. Plant Cell 29, 1248–1261 (2017).
Addo-Quaye, C. et al. Sliced microRNA targets and precise loop-first processing of MIR319 hairpins revealed by analysis of the Physcomitrella patens degradome. RNA 15, 2112–2121 (2009).
Bologna, N. G., Mateos, J. L., Bresso, E. G. & Palatnik, J. F. A loop-to-base processing mechanism underlies the biogenesis of plant microRNAs miR319 and miR159. EMBO J. 28, 3646–3656 (2009).
Zhu, H. et al. Bidirectional processing of pri-miRNAs with branched terminal loops by Arabidopsis Dicer-like1. Nat. Struct. Mol. Biol. 20, 1106–1115 (2013).
Gonzalo, L. et al. R-loops at microRNA encoding loci promote co-transcriptional processing of pri-miRNAs in plants. Nat. Plants 8, 402–418 (2022).
Song, L., Axtell, M. J. & Fedoroff, N. V. RNA secondary structural determinants of miRNA precursor processing in Arabidopsis. Curr. Biol. 20, 37–41 (2010).
Werner, S., Wollmann, H., Schneeberger, K. & Weigel, D. Structure determinants for accurate processing of miR172a in Arabidopsis thaliana. Curr. Biol. 20, 42–48 (2010).
Mateos, J. L., Bologna, N. G., Chorostecki, U. & Palatnik, J. F. Identification of microRNA processing determinants by random mutagenesis of Arabidopsis MIR172a precursor. Curr. Biol. 20, 49–54 (2010).
Tian, Y. et al. A phosphate-binding pocket within the platform-PAZ-connector helix cassette of human Dicer. Mol. Cell 53, 606–616 (2014).
Liu, Z. et al. Cryo-EM structure of human Dicer and its complexes with a pre-miRNA substrate. Cell 173, 1191–1203 (2018).
Lee, Y., Lee, H., Kim, H., Kim, V. N. & Roh, S. Structure of the human DICER–pre-miRNA complex in a dicing state. Nature 615, 331–338 (2023).
Re, D. A. et al. Alternative use of miRNA-biogenesis co-factors in plants at low temperatures. Development 146, dev172932 (2019).
Kurihara, Y., Takashi, Y. & Watanabe, Y. The interaction between DCL1 and HYL1 is important for efficient and precise processing of pri-miRNA in plant microRNA biogenesis. RNA 12, 206–212 (2006).
Xie, D. et al. Phase separation of SERRATE drives dicing body assembly and promotes miRNA processing in Arabidopsis. Nat. Cell Biol. 23, 32–39 (2021).
Rojas, A. M. L. et al. Identification of key sequence features required for microRNA biogenesis in plants. Nat. Commun. 11, 5320 (2020).
Hirata, R. & Makabe, T. Unpaired nucleotides on the stem of microRNA precursor are important for precise cleavage by Dicer-like 1 in Arabidopsis. Genes Cells 27, 280–292 (2022).
Cuperus, J. T. et al. Unique functionality of 22-nt miRNAs in triggering RDR6-dependent siRNA biogenesis from target transcripts in Arabidopsis. Nat. Struct. Mol. Biol. 17, 997–1003 (2010).
Chen, H. M. et al. 22-nucleotide RNAs trigger secondary siRNA biogenesis in plants. Proc. Natl Acad. Sci. USA 107, 15269–15274 (2010).
Lee, Y., Kim, H. & Kim, V. N. Sequence determinant of small RNA production by DICER. Nature 615, 323–330 (2023).
Nguyen, T. D., Trinh, T. A., Bao, S. & Nguyen, T. A. Secondary structure RNA elements control the cleavage activity of DICER. Nat. Commun. 13, 2138 (2022).
Le, C. T., Nguyen, T. D. & Nguyen, T. A. Two-motif model illuminates DICER cleavage preferences. Nucleic Acids Res. 52, 1860–1877 (2024).
Gu, S. et al. The loop position of shRNAs and pre-miRNAs is critical for the accuracy of dicer processing in vivo. Cell 151, 900–911 (2012).
Le, T. N. Y., Le, C. T. & Nguyen, T. A. Determinants of selectivity in the dicing mechanism. Nat. Commun. 15, 8989 (2024).
Lorenz, R. et al. ViennaRNA package 2.0. Algorithms Mol. Biol. 6, 26 (2011).
Chen, X. A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303, 2022–2025 (2004).
Aukerman, M. J. & Sakai, H. Regulation of flowering time and floral organ identity by a microRNA and its Apetala2-like target genes. Plant Cell 15, 2730–2741 (2003).
Jung, J. et al. The GIGANTEA-regulated MicroRNA172 mediates photoperiodic flowering independent of CONSTANS in Arabidopsis. Plant Cell 19, 2736–2748 (2007).
Zhao, L., Kim, Y., Dinh, T. T. & Chen, X. miR172 regulates stem cell fate and defines the inner boundary of APETALA3 and PISTILLATA expression domain in Arabidopsis floral meristems. Plant J. 51, 840–849 (2007).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Aronesty, E. Comparison of sequencing utility programs. Open Bioinform. J. 7, TOBIOIJ-7-1 (2013).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Cock, P. J. A. et al. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 25, 1422–1423 (2009).
Kozomara, A., Birgaoanu, M. & Griffiths-Jones, S. miRBase: from microRNA sequences to function. Nucleic Acids Res. 47, 155–162 (2019).
Image Lab v.6.0.1 (BioRad, 2017).
Lian, H. et al. Redundant and specific roles of individual MIR172 genes in plant development. PLoS Biol. 19, e3001044 (2010).
Nguyen, T. D. The high-throughput shRNA cleavage assays of DCL1 on 46000 variants. Zenodo https://doi.org/10.5281/zenodo.13828938 (2025).
Acknowledgements
We thank our laboratory colleagues at HKUST and Peking University for their invaluable technical assistance, engaging discussions and insightful critiques of the manuscript. We also thank the Biosciences Central Research Facility at HKUST (Clear Water Bay) for their high-quality instruments and services. We thank the National Center for Protein Sciences at Peking University in Beijing, China, for providing technical support and instrumentation. This research was funded by the Research Grants Council of Hong Kong under grant number 16103023, Hong Kong University of Science and Technology, the National Natural Science Foundation of China (grant number 32488102), the Chinese Ministry of Science and Technology (grant number 2023YFC3402200) and the Qidong-SLS Innovation Fund.
Author information
Authors and Affiliations
Contributions
T.N.-Y.L., T.D.N., Y.Y., W.X., X.C. and T.A.N. formulated the concept and design of the study. T.N.-Y.L. carried out the biochemical assays. T.D.N. analysed the data. Y.Y. and W.X. were involved in the transgenic plant experiments. All authors played a role in analysing the data, interpreting the results and drafting the manuscript. X.C. and T.A.N. oversaw the entire research project and were instrumental in obtaining the necessary funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Brian Gregory and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 High-throughput cleavage assays for DCL1.
(a) The biogenesis of animal and plant miRNAs. The blue arrowheads indicate the cleavage sites of enzymes on pri-miRNAs or pre-miRNAs. (b) The two directions of DCL1 cleavage on pri-miRNAs. The blue and red arrowheads indicate the first and second cleavages, respectively. (c) Schematic diagram of DCL1. The numbers indicate the amino acid positions. DExD helicase domain; DUF283, an unknown function domain; PAZ (Piwi/Argonaut/Zwille); RIIIDa and RIIIDb, two RNase III domains; and dsRBDs, double-stranded RNA-binding domains. (d) The purity of DCL1 was assessed by SDS-PAGE. The DCL1 purification experiments were repeated two times with similar results. (e) Diagrammatic structures of pri-miR166f and pri-miR166c. The blue arrowheads indicate DCL1 cleavage sites. (f) In vitro DCL1 cleavage assays were performed for the two pri-miRNAs shown in (e). The assays were repeated two times with similar results. (g) Diagrammatic structures of shRNAs with or without a 32-nt barcode. The blue arrowheads indicate the DCL1 cleavage sites. (h) In vitro DCL1 cleavage assays were performed for the two shRNAs shown in (g). The blue arrowheads indicate the DCL1 cleavage sites. The alternative cleavages of DCL1 are marked by asterisks. The assays were repeated two times with similar results. (i) A schematic cloning scheme was used for randomized shRNA substrates and DCL1-cleaved products. RA3 and RA5 represent sequencing adapters. CirRTP refers to a reverse transcription primer that incorporates both RA3 and RA5 adapter sequences. (j, m) Structural diagrams of shRNA and pre-miRNAs without or with a bulge at different positions. (k, n) In vitro DCL1 cleavage assays for shRNAs in (j), pre-miR166c variants in (m). Grey, blue and red arrowheads indicate DC20, D21, and D22, respectively. The alternative cleavages of DCL1 are marked by asterisks. (l, o) A quantitative analysis of cleavage accuracy at different sites, based on three repeated assays from (k) and (n). Bars indicate the precision of cleavage at each position, data values are shown as dots and error bars represent mean ± SD.
Extended Data Fig. 3 Conservation of the GHR motif in plant pri-miRNAs.
(a, d) Structural diagrams of various WT and mutant pre-miRNAs with high GHR scores at different positions. The arrowheads indicate the cleavage sites of DCL1. Red letters indicate the mutant nucleotides. F1, F2, and F3 are three fragments resulting from DCL1 cleavage. (b, e) In vitro DCL1 cleavage assays were performed for pre-miRNAs with varying GHR scores, as shown in (a) and (d). The red, blue, grey and white arrowheads indicate DC22, DC21, DC20 and DC19 cleavage sites, respectively. (c, f) Quantitative analysis of cleavage accuracy at different sites from three repeated assays in (b) and (e). Bars represent the precision of cleavage at each position, data values are shown as dots and error bars represent mean ± SD. (g) Purified recombinant HYL1 and SE proteins were assessed by SDS-PAGE. The purification of HYL1 and SE were repeated two times with similar results. (h, j) In vitro DCL1 cleavage assays were performed in the presence of HYL1 or SE proteins for the pre-miRNAs shown in (a) and (d). (i, k) Quantitative analysis of cleavage accuracy at different sites from three repeated assays in (h) and (j). Bars represent the precision of cleavage at each position, data values are shown as dots and error bars represent mean ± SD.
Extended Data Fig. 4 Role of GHR in the production of 22-nt miRNAs.
(a) The plots display the average GHR motif scores in 3-bp motifs at various positions within pri-miR828 from diverse plants. (b) Diagrammatic structures of pri-miR828. The blue arrowheads indicate DCL1 cleavage sites. (c) The diagram indicates the lengths of potential fragments derived from pri-miR828 processing by BTL or LTB directions. (d) In vitro DCL1 cleavage assays were performed for pri-miR828 using two mutant DCL1 enzymes. The assays were repeated two times with similar results. (e) The repeated assay from Fig. 4d. (f) Time-course in vitro DCL1 cleavage assays were conducted for pre-miRNAs with different GHR scores. (g) Quantitative analysis of relative cleavage efficiency was derived from three repeated assays in (f), calculated from the band density of the cleaved product (F2 fragment) normalized to the high-GHR pre-miR828 at 3 min. The grey and green lines correspond to lowGHR, and highGHR of pre-miR828, respectively. Shaded regions around the lines represent 95% confidence intervals for cleavage efficiency measurements. (h) The DCL1 cleavage accuracy for WT or mutant pre-miR828 was calculated from the sequencing results of purified F1, F2, or F3 fragments obtained from the gel in Fig. 4d.
Extended Data Fig. 5 GHR depends on RIIIDa to control DCL1 cleavage sites.
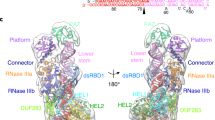
(a) Model of the structure and the pre-miRNA substrate containing GHR at DCL1 cleavage site. (b) Schematic diagram of DCL1 and its mutants used in this study. The numbers indicate the amino acid positions, the white boxes indicate deletion area. (c) The purity of proteins was assessed by SDS-PAGE. The purification of DCL1 variants was repeated once for each mutation. (d) A schematic representation illustrates the DCL1-pre-miR-166f complex during the dicing state (PDB: 7ELE). Residue E1507 interacts with the C-G pair situated within the minor groove by forming a hydrogen bond with the -NH2 group of the guanine base.
Extended Data Fig. 6 The GHR motif is crucial for miRNA expression and function.
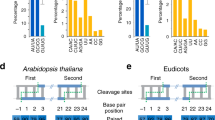
(a) Structural diagrams of various WT and mutant pre-miR172a with high or low GHR scores. The arrowheads indicate the cleavage sites of DCL1. Red letters indicate the mutant nucleotides. (b) In vitro DCL1 cleavage assays for pre-miRNAs with varying GHR scores. The blue and black arrowheads indicate the F1/3 and F2 fragments of DC21, respectively. (c) Quantitative analysis of relative cleavage efficiency for three high-GHR and three low-GHR pre-miRNAs compared to WT pre-miR172a, based on data from Fig. 6b and (b). Bars represent the relative cleavage efficiency of each mutant pre-miR172a normalized to pre-miR172a WT, data values for each pre-miRNAs are shown as dots and error bars represent mean ± SD. The p-values were calculated by a two-tailed t-test. (d) In vitro DCL1 cleavage assays for pre-miR172a variants with DCL1ΔdsRBD1. The blue and black arrowheads indicate the F1/3 and F2 fragments of DC21, respectively. The assays were repeated two times with similar results. (e) The relative expression of miR172a/b/e by qPCR in individual lines. (f) The relative expression of two genes, TOE1 and SMZ, by qPCR in individual lines. For (e) and (f), the number of plants (n = 10/8) for each line is indicated below the corresponding boxplot, the center line represents the median, the box’s lower and upper bounds correspond to the 25th and 75th percentiles, whiskers show 1.5x the interquartile extending from bounds of box, minima is the minimum value, and maxima is the maximum value. The p-values were calculated by a two-tailed t-test.
Supplementary information
Supplementary Tables
Supplementary Tables 1–12.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 1
Unprocessed gels.
Source Data Fig. 2
Unprocessed gels.
Source Data Fig. 3
Unprocessed gels.
Source Data Fig. 4
Unprocessed gels.
Source Data Fig. 5
Unprocessed gels.
Source Data Fig. 6
Unprocessed gels.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed gels.
Source Data Extended Data Fig. 3
Unprocessed gels.
Source Data Extended Data Fig. 4
Unprocessed gels.
Source Data Extended Data Fig. 5
Unprocessed gels.
Source Data Extended Data Fig. 6
Unprocessed gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Le, T.NY., Nguyen, T.D., Yu, Y. et al. Key RNA elements influencing DCL1 cleavage in plant microRNA biogenesis. Nat. Plants 11, 1528–1543 (2025). https://doi.org/10.1038/s41477-025-02067-w
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41477-025-02067-w