Abstract
Vacuolar sorting receptors (VSRs) are involved in sorting soluble vacuolar proteins during normal plant growth and development, but their role in plant stress responses remains largely unexplored. Here we report that a subgroup of the Arabidopsis thaliana VSR genes are transcriptionally induced during infection with avirulent Pseudomonas syringae strains, leading to higher VSR protein accumulation. We demonstrate that the pathogen-responsive VSR1, VSR5, VSR6 and VSR7 genes function redundantly in sorting vacuolar death-related enzymes induced during bacterial infection. Moreover, VSRs are required for fusion of the tonoplast with the plasma membrane and the subsequent release of vacuolar contents into the apoplast, where bacterial pathogens reside. Indeed, dysfunction of this subgroup of VSRs blocks hypersensitive cell death and leads to stronger disease symptoms and higher bacterial loads, revealing their essential role in defence against avirulent bacterial infection. Intriguingly, their disruption also leads to defects in autophagy, impairing autophagosome-mediated degradation of bacterial effector proteins. Collectively, our results show that VSR1, VSR5, VSR6 and VSR7 are key regulators of plant effector-triggered immunity (ETI) by orchestrating receptor-mediated vacuolar sorting of immunity-related proteins, tonoplast to plasma membrane fusion, and autophagic degradation of effector proteins.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All study data are included in the article and/or its Supplementary Information. The raw Illumina reads generated from RNA-seq experiments were deposited at NCBI Sequence Read Archive (BioProject ID: PRJNA1139386). This article does not contain datasets, code or materials in addition to those included. Source data are provided with this paper.
References
Zhang, H. W. et al. Two tonoplast MATE proteins function as turgor-regulating chloride channels in Arabidopsis. Proc. Natl Acad. Sci. USA 114, E2036–E2045 (2017).
Cui, Y. et al. MONENSIN SENSITIVITY1 (MON1)/CALCIUM CAFFEINE ZINC SENSITIVITY1 (CCZ1)-mediated Rab7 activation regulates tapetal programmed cell death and pollen development. Plant Physiol. 173, 206–218 (2017).
Wang, X. et al. The roles of endomembrane trafficking in plant abiotic stress responses. J. Integr. Plant Biol. 62, 55–69 (2020).
Gu, Y. N., Zavaliev, R. & Dong, X. N. Membrane trafficking in plant immunity. Mol. Plant 10, 1026–1034 (2017).
Zhu, D., Zhang, M., Gao, C. & Shen, J. Protein trafficking in plant cells: tools and markers. Sci. China Life Sci. 63, 343–363 (2020).
Jamet, E., Canut, H., Boudart, G. & Pont-Lezica, R. F. Cell wall proteins: a new insight through proteomics. Trends Plant Sci. 11, 33–39 (2006).
Kim, S. J. & Brandizzi, F. The plant secretory pathway: an essential factory for building the plant cell wall. Plant Cell Physiol. 55, 687–693 (2014).
Robinson, D. G. & Neuhaus, J. M. Receptor-mediated sorting of soluble vacuolar proteins: myths, facts, and a new model. J. Exp. Bot. 67, 4435–4449 (2016).
Robinson, D. G. Retromer and VSR recycling: a red herring? Plant Physiol. 176, 483–484 (2018).
Fruholz, S., Fassler, F., Kolukisaoglu, U. & Pimpl, P. Nanobody-triggered lockdown of VSRs reveals ligand reloading in the Golgi. Nat. Commun. 9, 643 (2018).
Kunzl, F., Fruholz, S., Fassler, F., Li, B. B. & Pimpl, P. Receptor-mediated sorting of soluble vacuolar proteins ends at the trans-Golgi network/early endosome. Nat. Plants 2, 16017 (2016).
Zouhar, J., Cao, W. H., Shen, J. B. & Rojo, E. Retrograde transport in plants: circular economy in the endomembrane system. Eur. J. Cell Biol. 102, 151309 (2023).
Wang, H., Zhuang, X. H., Hillmer, S., Robinson, D. G. & Jiang, L. W. Vacuolar sorting receptor (VSR) proteins reach the plasma membrane in germinating pollen tubes. Mol. Plant 4, 845–853 (2011).
Shen, J. et al. An in vivo expression system for the identification of cargo proteins of vacuolar sorting receptors in Arabidopsis culture cells. Plant J. 75, 1003–1017 (2013).
Shimada, T. et al. Vacuolar sorting receptor for seed storage proteins in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 100, 16095–16100 (2003).
Delgadillo, M. O. et al. MTV proteins unveil ER- and microtubule-associated compartments in the plant vacuolar trafficking pathway. Proc. Natl Acad. Sci. USA 117, 9884–9895 (2020).
Hu, S. et al. Plant ESCRT protein ALIX coordinates with retromer complex in regulating receptor-mediated sorting of soluble vacuolar proteins. Proc. Natl Acad. Sci. USA 119, e2200492119 (2022).
Li, H. et al. A plant-unique protein BLISTER coordinates with core retromer to modulate endosomal sorting of plasma membrane and vacuolar proteins. Proc. Natl Acad. Sci. USA 120, e2211258120 (2023).
Gao, C. et al. Dual roles of an Arabidopsis ESCRT component FREE1 in regulating vacuolar protein transport and autophagic degradation. Proc. Natl Acad. Sci. USA 112, 1886–1891 (2015).
Shimada, T., Takagi, J., Ichino, T., Shirakawa, M. & Hara-Nishimura, I. Plant vacuoles. Annu. Rev. Plant Biol. 69, 123–145 (2018).
Yamada, K., Shimada, T., Nishimura, M. & Hara-Nishimura, I. A VPE family supporting various vacuolar functions in plants. Physiol. Plant. 123, 369–375 (2005).
Hara-Nishimura, I. & Hatsugai, N. The role of vacuole in plant cell death. Cell Death Differ. 18, 1298–1304 (2011).
Hatsugai, N. & Hara-Nishimura, I. Two vacuole-mediated defense strategies in plants. Plant Signal. Behav. 5, 1568–1570 (2010).
Hatsugai, N. et al. A novel membrane fusion-mediated plant immunity against bacterial pathogens. Genes Dev. 23, 2496–2506 (2009).
Carter, C. et al. The vegetative vacuole proteorne of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 16, 3285–3303 (2004).
Jones, J. D. G., Staskawicz, B. J. & Dangl, J. L. The plant immune system: from discovery to deployment. Cell 187, 2095–2116 (2024).
Leong, J. X. et al. A bacterial effector counteracts host autophagy by promoting degradation of an autophagy component. EMBO J. 41, e110352 (2022).
Banfield, M. J. Perturbation of host ubiquitin systems by plant pathogen/pest effector proteins. Cell. Microbiol. 17, 18–25 (2015).
Lal, N. K. et al. Phytopathogen effectors use multiple mechanisms to manipulate plant autophagy. Cell Host Microbe 28, 558–571 (2020).
Hafrén, A. et al. Selective autophagy limits cauliflower mosaic virus infection by NBR1-mediated targeting of viral capsid protein and particles. Proc. Natl Acad. Sci. USA 114, E2026–e2035 (2017).
Zouhar, J., Muñoz, A. & Rojo, E. Functional specialization within the vacuolar sorting receptor family: VSR1, VSR3 and VSR4 sort vacuolar storage cargo in seeds and vegetative tissues. Plant J. 64, 577–588 (2010).
Wang, Z. Y. et al. The Arabidopsis Vacuolar Sorting Receptor1 is required for osmotic stress-induced abscisic acid biosynthesis. Plant Physiol. 167, 137–152 (2015).
Wang, D., Weaver, N. D., Kesarwani, M. & Dong, X. N. Induction of protein secretory pathway is required for systemic acquired resistance. Science 308, 1036–1040 (2005).
Zavaliev, R. & Dong, X. NPR1, a key immune regulator for plant survival under biotic and abiotic stresses. Mol. Cell 84, 131–141 (2024).
Glazebrook, J., Rogers, E. E. & Ausubel, F. M. Isolation of Arabidopsis mutants with enhanced disease susceptibility by direct screening. Genetics 143, 973–982 (1996).
Tse, Y. C. et al. Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 16, 672–693 (2004).
Miao, Y., Yan, P., Kim, H., Hwang, I. & Jiang, L. Localization of green fluorescent protein fusions with the seven Arabidopsis vacuolar sorting receptors to prevacuolar compartments in tobacco BY-2 cells. Plant Physiol. 142, 945–962 (2006).
Cui, Y. et al. Biogenesis of plant prevacuolar multivesicular bodies. Mol. Plant 9, 774–786 (2016).
Rojo, E. et al. VPE gamma exhibits a caspase-like activity that contributes to defense against pathogens. Curr. Biol. 14, 1897–1906 (2004).
Zhang, B. et al. PIRIN2 stabilizes cysteine protease XCP2 and increases susceptibility to the vascular pathogen Ralstonia solanacearum in Arabidopsis. Plant J. 79, 1009–1019 (2014).
Ahmed, S. U. et al. The plant vacuolar sorting receptor AtELP is involved in transport of NH(2)-terminal propeptide-containing vacuolar proteins in Arabidopsis thaliana. J. Cell Biol. 149, 1335–1344 (2000).
Shen, J., Ding, Y., Gao, C., Rojo, E. & Jiang, L. N-linked glycosylation of AtVSR1 is important for vacuolar protein sorting in Arabidopsis. Plant J. 80, 977–992 (2014).
Miao, Y., Li, K. Y., Li, H. Y., Yao, X. & Jiang, L. The vacuolar transport of aleurain-GFP and 2S albumin-GFP fusions is mediated by the same pre-vacuolar compartments in tobacco BY-2 and Arabidopsis suspension cultured cells. Plant J. 56, 824–839 (2008).
Kim, H. et al. Homomeric interaction of AtVSR1 is essential for its function as a vacuolar sorting receptor. Plant Physiol. 154, 134–148 (2010).
Hayashi, Y. et al. A proteinase-storing body that prepares for cell death or stresses in the epidermal cells of Arabidopsis. Plant Cell Physiol. 42, 894–899 (2001).
Rojo, E., Zouhar, J., Carter, C., Kovaleva, V. & Raikhel, N. V. A unique mechanism for protein processing and degradation in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 100, 7389–7394 (2003).
Richau, K. H. et al. Subclassification and biochemical analysis of plant papain-like cysteine proteases displays subfamily-specific characteristics. Plant Physiol. 158, 1583–1599 (2012).
Misas-Villamil, J. C., van der Hoorn, R. A. & Doehlemann, G. Papain-like cysteine proteases as hubs in plant immunity. New Phytol. 212, 902–907 (2016).
Zeng, Y. L. et al. Unique COPII component AtSar1a/AtSec23a pair is required for the distinct function of protein ER export in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 112, 14360–14365 (2015).
Takeuchi, M. et al. A dominant negative mutant of sar1 GTPase inhibits protein transport from the endoplasmic reticulum to the Golgi apparatus in tobacco and Arabidopsis cultured cells. Plant J. 23, 517–525 (2000).
Niemes, S. et al. Sorting of plant vacuolar proteins is initiated in the ER. Plant J. 62, 601–614 (2010).
Kwon, Y. et al. AtCAP2 is crucial for lytic vacuole biogenesis during germination by positively regulating vacuolar protein trafficking. Proc. Natl Acad. Sci. USA 115, E1675–E1683 (2018).
Lee, Y. et al. Functional identification of sorting receptors involved in trafficking of soluble lytic vacuolar proteins in vegetative cells of Arabidopsis. Plant Physiol. 161, 121–133 (2013).
Hauck, P., Thilmony, R. & He, S. Y. A Pseudomonas syringae type III effector suppresses cell wall-based extracellular defense in susceptible Arabidopsis plants. Proc. Natl Acad. Sci. USA 100, 8577–8582 (2003).
Jones, J. D. & Dangl, J. L. The plant immune system. Nature 444, 323–329 (2006).
Saucet, S. B. et al. Two linked pairs of Arabidopsis TNL resistance genes independently confer recognition of bacterial effector AvrRps4. Nat. Commun. 6, 6338 (2015).
Tornero, P. et al. RAR1 and NDR1 contribute quantitatively to disease resistance in Arabidopsis, and their relative contributions are dependent on the R gene assayed. Plant Cell 14, 1005–1015 (2002).
Mackey, D., Belkhadir, Y., Alonso, J. M., Ecker, J. R. & Dangl, J. L. Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell 112, 379–389 (2003).
Munch, D. et al. Retromer contributes to immunity-associated cell death in Arabidopsis. Plant Cell 27, 463–479 (2015).
Wang, Z. P. et al. Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 16, 144 (2015).
Hunter, P. R., Craddock, C. P., Di Benedetto, S., Roberts, L. M. & Frigerio, L. Fluorescent reporter proteins for the tonoplast and the vacuolar lumen identify a single vacuolar compartment in Arabidopsis cells. Plant Physiol. 145, 1371–1382 (2007).
Kriegel, A. et al. Job sharing in the endomembrane system: vacuolar acidification requires the combined activity of V-ATPase and V-PPase. Plant Cell 27, 3383–3396 (2015).
Hofius, D. et al. Autophagic components contribute to hypersensitive cell death in Arabidopsis. Cell 137, 773–783 (2009).
Minina, E. A. et al. Transcriptional stimulation of rate-limiting components of the autophagic pathway improves plant fitness. J. Exp. Bot. 69, 1415–1432 (2018).
Jung, H. et al. Arabidopsis cargo receptor NBR1 mediates selective autophagy of defective proteins. J. Exp. Biol. 71, 73–89 (2020).
Zhuang, X. H. et al. A BAR-domain protein SH3P2, which binds to phosphatidylinositol 3-phosphate and ATG8, regulates autophagosome formation in Arabidopsis. Plant Cell 25, 4596–4615 (2013).
Leary, A. Y., Savage, Z., Tumtas, Y. & Bozkurt, T. O. Contrasting and emerging roles of autophagy in plant immunity. Curr. Opin. Plant Biol. 52, 46–53 (2019).
Tamura, K. et al. Why green fluorescent fusion proteins have not been observed in the vacuoles of higher plants. Plant J. 35, 545–555 (2003).
Oliviusson, P. et al. Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. Plant Cell 18, 1239–1252 (2006).
Gershlick, D. C. et al. Golgi-dependent transport of vacuolar sorting receptors is regulated by COPII, AP1, and AP4 protein complexes in tobacco. Plant Cell 26, 1308–1329 (2014).
Fuji, K. et al. The adaptor complex AP-4 regulates vacuolar protein sorting at the trans-Golgi network by interacting with VACUOLAR SORTING RECEPTOR1. Plant Physiol. 170, 211–219 (2016).
Avila, E. L. et al. Expression analysis of Arabidopsis vacuolar sorting receptor 3 reveals a putative function in guard cells. J. Exp. Bot. 59, 1149–1161 (2008).
Aarts, N. et al. Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc. Natl Acad. Sci. USA 95, 10306–10311 (1998).
Axtell, M. J. & Staskawicz, B. J. Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 112, 369–377 (2003).
van Doorn, W. G. Classes of programmed cell death in plants, compared to those in animals. J. Exp. Bot. 62, 4749–4761 (2011).
Happel, N. et al. Arabidopsis mu A-adaptin interacts with the tyrosine motif of the vacuolar sorting receptor VSR-PS1. Plant J. 37, 678–693 (2004).
Hatsugai, N. et al. Involvement of adapter protein complex 4 in hypersensitive cell death induced by avirulent bacteria. Plant Physiol. 176, 1824–1834 (2018).
Marquardt, L. et al. Vacuole fragmentation depends on a novel Atg18-containing retromer-complex. Autophagy 19, 278–295 (2023).
Zavodszky, E. et al. Mutation in VPS35 associated with Parkinson’s disease impairs WASH complex association and inhibits autophagy. Nat. Commun. 5, 3828 (2014).
Boutouja, F. et al. Vps10-mediated targeting of Pep4 determines the activity of the vacuole in a substrate-dependent manner. Sci. Rep. 9, 10557 (2019).
Üstün, S., Hafrén, A. & Hofius, D. Autophagy as a mediator of life and death in plants. Curr. Opin. Plant Biol. 40, 122–130 (2017).
Üstün, S. & Hofius, D. Anti- and pro-microbial roles of autophagy in plant–bacteria interactions. Autophagy 14, 1465–1466 (2018).
Yuan, M. et al. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 592, 105–109 (2021).
Lenz, H. D. et al. Autophagy differentially controls plant basal immunity to biotrophic and necrotrophic pathogens. Plant J. 66, 818–830 (2011).
Mohr, P. G. & Cahill, D. M. Suppression by ABA of salicylic acid and lignin accumulation and the expression of multiple genes, in Arabidopsis infected with Pseudomonas syringae pv. tomato. Funct. Integr. Genomics 7, 181–191 (2007).
O’Brien, J. A., Daudi, A., Butt, V. S. & Bolwell, G. P. Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 236, 765–779 (2012).
Schlößer, M. et al. Localization of four class I glutaredoxins in the cytosol and the secretory pathway and characterization of their biochemical diversification. Plant J. 118, 1455–1474 (2024).
Koestel, J. & Batoko, H. A plant-specific bridging adaptor for amphisome biogenesis. J. Cell Biol. 221, e202210011 (2022).
Hu, S., Li, Y. & Shen, J. A diverse membrane interaction network for plant multivesicular bodies: roles in proteins vacuolar delivery and unconventional secretion. Front. Plant Sci. 11, 425 (2020).
Shen, J. et al. A plant Bro1 domain protein BRAF regulates multivesicular body biogenesis and membrane protein homeostasis. Nat. Commun. 9, 3784 (2018).
Ohad, N., Shichrur, K. & Yalovsky, S. The analysis of protein–protein interactions in plants by bimolecular fluorescence complementation. Plant Physiol. 145, 1090–1099 (2007).
Krebs, M. et al. Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc. Natl Acad. Sci. USA 107, 3251–3256 (2010).
Grant, M. R. et al. Structure of the Arabidopsis RPM1 gene enabling dual specificity disease resistance. Science 269, 843–846 (1995).
Mindrinos, M., Katagiri, F., Yu, G. L. & Ausubel, F. M. The A. thaliana disease resistance gene RPS2 encodes a protein containing a nucleotide-binding site and leucine-rich repeats. Cell 78, 1089–1099 (1994).
Wirthmueller, L., Zhang, Y., Jones, J. D. & Parker, J. E. Nuclear accumulation of the Arabidopsis immune receptor RPS4 is necessary for triggering EDS1-dependent defense. Curr. Biol. 17, 2023–2029 (2007).
Miao, Y. & Jiang, L. Transient expression of fluorescent fusion proteins in protoplasts of suspension cultured cells. Nat. Protoc. 2, 2348–2353 (2007).
Shen, J. et al. AtBRO1 functions in ESCRT-I complex to regulate multivesicular body protein sorting. Mol. Plant 9, 760–763 (2016).
Wang, L. et al. The genetic network controlling the Arabidopsis transcriptional response to Pseudomonas syringae pv. maculicola: roles of major regulators and the phytotoxin coronatine. Mol. Plant Microbe Interact. 21, 1408–1420 (2008).
Guan, R. et al. Multilayered regulation of ethylene induction plays a positive role in Arabidopsis resistance against Pseudomonas syringae. Plant Physiol. 169, 299–312 (2015).
Acknowledgements
We thank Y. Xia (Hong Kong Baptist University) for providing Pto DC3000 expressing avrRpm1 or avrRps4, and Pto DC3000 hrcC− strain; Y. Liang (Zhejiang University) for providing the rpm1 mutant plant; Y. Xiang (Lanzhou University) for providing the vha-a2 vha-a3 double mutant plant; Q.-J. Chen (China Agricultural University) for providing the CRISPR-Cas9 system; R. W. Innes (Indiana University) for providing the pTA7002-DEX backbone; X. Deng for the initial pathology tests; and S. Zhang (University of Missouri) for insightful suggestions and technical advice. This work was supported by grants from the National Natural Science Foundation of China (32170342 and 31970181), the Zhejiang Provincial Natural Science Foundation of China (LR20C020001), the Zhejiang A&F University Starting Funding (2024LFR053), and the 111 Project (D18008) to J.S.; the National Natural Science Foundation of China (32100286) to S.H.; and the Spanish Ministry for Science and Innovation MCIN/AEI/10.13039/501100011033/FEDER grant PID2021-128078NB-I00 to E.R.
Author information
Authors and Affiliations
Contributions
J.S., S.H. and E.R. designed the research. D.Z., S.H., W.C., Y.G., Y.L. and B.L. performed experiments. D.Z., S.H., E.R., J.X., L.J. and J.S. analysed the data. Y.G., Y.L. and B.L. contributed to material preparation. J.S., E.R. and L.J. wrote the paper with comments from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Takashi Ueda and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
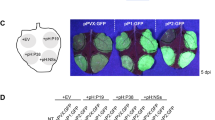
Extended Data Fig. 1 Phenotype analysis of different vsr mutant combinations.
(a and b) Phenotype of 24-day-old plants grown under long-day (a) or short-day (b) growth condition of the indicated vsr mutants and complemented lines with indicated GFP fusions. Note that homozygous vsr4vsr6vsr7 triple mutant are not identified from the self-pollination of vsr4(+/-)vsr6vsr7. All photographs are at the same magnification. The wet weights of shoot per plant of the indicated genotypes were quantified (Right). Data are presented as means ± SD from three independent experiments. Scale bar, 1 cm. (c) Seven-day-old seedlings of indicated genotypes were photographed. The root length of seedlings of the indicated genotypes was quantified (Right). Data are presented as means ± SD of 10 seedlings from three independent experiments. Scale bar, 1 cm. Different letters above bars in (a-c) indicate a significant difference at the P < 0.05 level by one-way ANOVA with Fisher’s LSD multiple comparisons test. Exact P-values of statistic tests in (a-c) are provided in the Source data file.
Extended Data Fig. 2 Vacuolar localization of pathogen upregulated proteins as putative VSR cargo proteins.
(a) Transcript levels (log2) of genes, function as putative VSR cargo proteins, in WT plants after inoculation of the Pto DC3000 avrRpm1 or Pto DC3000 at indicated time of post inoculation. Heatmap displays the relative expression (log2FC) of the genes in WT plants after inoculation with Pto DC3000 avrRpm1 or Pto DC3000 at different times points after inoculation relative to their expression before inoculation (0 h). (b) Subcellular localization of secYFP and Aleurain-GFP as controls. secYFP showed ER pattern, without any fluorescence signal in the vacuole. Vacuolar localized protein Aleurain-GFP colocalized with the PVC/MVB marker mRFP-VSR2 in punctae and showed diffused fluorescence signals in vacuole. Separated images of each channel in the white outline area are shown on the right side (from top to bottom: GFP/YFP, mRFP, and merged). Scale bars, 10 μm. (c) Colocalization of GFP/YFP-tagged putative VSR cargo in Papain-Like Cysteine Proteases family with the PVC/MVB marker mRFP-VSR2 was analyzed with a confocal microscope in protoplasts of Arabidopsis suspension cells. Separated images of each channel in the white outline area are shown on the right side (from top to bottom: GFP/YFP, mRFP, and merged). Scale bars, 10 μm. (d) Subcellular localization analysis of two Pathogenesis-related proteins (PR3 and PR4), three peroxidases (PRX33, PRX34, and PRX37), CPY, PEP4, and GRX. Colocalization of GFP/YFP-tagged proteins with the PVC/MVB marker mRFP-VSR2 was analyzed with a confocal microscope in protoplasts of Arabidopsis suspension cells. Separated images of each channel in the white outline area are shown on the right side (from top to bottom: GFP/YFP, mRFP, and merged). Scale bars, 10 μm. Similar confocal imaging results to those in (b–d) were obtained from three independent experiments.
Extended Data Fig. 3 Extracellular localization of pathogen-induced VSR cargo proteins in vsr mutants.
(a) Confocal images of cotyledon cells from Arabidopsis seedlings of the indicated genotypes transiently transformed with RD21A-mRFP, RDL1-mRFP, PAP1-mRFP, AALP-mRFP, RD19B-mRFP, mRFP-PR3, PR4-mRFP, mRFP-PRX33, mRFP-PRX34, mRFP-PRX37, CPY-mRFP, PEP4-mRFP, and GRX-mRFP. Arrows and asterisks indicate the fluorescence signal in extracellular spaces and vacuoles, respectively. Note that in the vsr5vsr6vsr7 or vsr1vsr6vsr7 triple mutants, much stronger fluorescent signal in the extracellular space (arrows), compared to that in WT. The vacuole protein VPEγ-RFP was not secreted in vsr mutant cells, suggesting the specificity of VSR sorting. Scale bar, 10 μm. (b) Quantification of relative extracellular (EC) to intracellular (IC) fluorescence intensity values for the indicated RFP tagged proteins. The region of interest (ROI) was kept constant for each measurement. Data are presented as means ± SD of 10 cells from three independent experiments. Different letters above bars indicate a significant difference at the P < 0.05 level by one-way ANOVA with Fisher’s LSD multiple comparisons test. Exact P-values of statistic tests are provided in the Source data file.
Extended Data Fig. 4 VSRs are weakly involved in PTI.
(a) VSR proteins level after inoculation with virulent strains Pto DC3000 EV. Seedlings of WT plants after inoculation with virulent strains of Pto DC3000 EV (OD600 = 0.02) at the indicated time points (0, 3, 6, and 12 h) were applied for protein extraction and immunoblotting with anti-VSR antibodies. The anti-actin was used as a loading control. The VSR protein level in the immunoblot was quantified (Right). Intensity was normalized by the loading control of anti-actin, and the first lane (0 h) in each experiment was arbitrarily set to 1. Data are presented as means ± SD from three independent experiments. (b) Disease symptoms on representative infected leaves. Leaves of indicated genotype plants 3 d after inoculation with Pto DC3000 EV (OD600 = 0.001). Leaves of vsr5vsr6vsr7 triple mutant inoculated exhibit chlorotic symptoms. Scale bars, 1 cm. (c) Bacterial growth 3 d after inoculation with Pto DC3000 EV (OD600 = 0.001) in the leaves of indicated genotype plants. Each bar represents log10-transformed values of the mean and SD of three biological replicates. Experiments were repeated three times with similar results. (d) Disease symptoms on representative infected leaves. Leaves of indicated genotype plants 3 d after inoculation with Pto DC3000 hrcC− (OD600 = 0.001). Scale bars, 1 cm. (e) Bacterial growth 3 d after inoculation with Pto DC3000 hrcC− (OD600 = 0.001) in the leaves of indicated genotype plants. Each bar represents log10-transformed values of the mean and SD of three biological replicates. c.f.u., colony-forming units. (f) Callose deposition in leaves of the indicated plant lines inoculated with Pto DC3000 hrcC− (OD600 = 0.1) and water (mock) detected by aniline blue staining at 12 h after inoculation. Quantification of callose staining intensity is shown on Right. Data are presented as means ± SD of 10 stained regions from three independent repeats. Scale bar, 500 µm. Different letters above bars in (a, c, e and f) indicate a significant difference at the P < 0.05 level by one-way ANOVA with Fisher’s LSD multiple comparisons test. Exact P-values of statistic tests in (a, c, e, and f) are provided in the Source data file.
Extended Data Fig. 5 Phenotype analysis of vsr2VSR3Cas9VSR4Cas9-1 mutant.
(a and b) Sequencing analysis of CRISPR-Cas9 edited line of vsr2VSR3Cas9VSR4Cas9-1. CRISPR-Cas9 editing of VSR3 (a) and VSR4 (b) was carried out using vsr2 T-DNA insertion mutant as the background. VSR3 and VSR4 share closely related sequences thus with identical gRNA1 and gRNA2 targets. Below each gene model, the nucleotide sequences are shown alongside the corresponding amino acid translations. The nucleotides highlighted in red in the sequences are specific changes from WT sequences. The stop codes are marked with stars. (c and d) Phenotype of 24-day-old plants grown under long-day (c) or short-day (d) growth condition. The experiments were repeated independently three times with similar results. (e and f) Disease symptoms analysis of vsr2VSR3Cas9VSR4Cas9-1 mutant. Leaves of WT and vsr2VSR3Cas9VSR4Cas9-1 triple mutant after 3 d inoculation with the Pto DC3000 EV (e) or Pto DC3000 expressing avrRpm1 (OD600 = 0.001) (f). Loss-of-function mutant of the corresponding resistance gene RPM1 (rpm1) served as an additional control. Scale bars, 1 cm. (g and h) Bacterial growth 3 d after inoculation with Pto DC3000 expressing Pto DC3000 EV (g) or avrRpm1 (h) in the leaves. Each bar represents log10-transformed values of the mean and SD of three biological replicates. Experiments were repeated three times with similar results. cfu, colony-forming units. (i) Trypan blue staining of dead cells in the leaves of WT, vsr2VSR3Cas9VSR4Cas9-1, and rpm1 mutants at 12 h after inoculation with Pto DC3000 avrRpm1 (OD600 = 0.001). Quantifications of trypan blue staining intensity are shown on the Right. Data are presented as means ± SD of three independent experiments. Scale bars, 5 mm. Different letters above bars in (g-i) indicate a significant difference at the P < 0.05 level by one-way ANOVA with Fisher’s LSD multiple comparisons test. Exact P-values of statistic tests in (h and i) are provided in the Source data file.
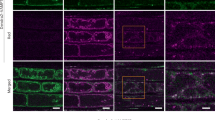
Extended Data Fig. 6 Vacuole morphology and vacuolar pH analysis in vsr mutants.
(a) Confocal microscopic images of the vacuolar membrane marker YFP-VAMP711 expressed in leaf epidermal cells of WT and vsr mutants. FM4-64 was used to label and visualize the cell PM. No obvious vacuole fragmentation was observed in leaf cells of the indicated vsr mutants. Scale bar, 10 μm. (b) Lysotracker Red staining indicates aberrant aggregation (arrows) of lytic compartments in root cells of vsr5vsr6vsr7 and vsr1vsr6vsr7 mutants in comparison with WT plants (Top). The morphological phenotype was further aggravated in vsr5vsr6vsr7 and vsr1vsr6vsr7 upon treatment with 100 µM BTH (bottom). Control and BTH-treated 5-d-old seedlings were stained with 2 µM Lysotracker Red for 15 min before imaging. The green signals of YFP-VAMP711 show the tonoplast. Imaging conditions were identical across all genotypes. Scale bar, 10 μm. (c) The pH of vacuole is not altered in vsr mutants. Representative pseudo‐colored images of the vacuole in WT, vsr5vsr6vsr7, and vsr1vsr6vsr7. The rainbow scale correlates to pH value. The vacuolar H+-ATPase mutant vha-a2 vha-a3 was used as a control. Quantification of vacuole pH (Right). Compared to WT, no significant difference (P > 0.05) in vacuole pH in vsr5vsr6vsr7 or vsr1vsr6vsr7 mutant, while loss of the tonoplast VATPase increases the vacuolar pH in root epidermal cells. Data are presented as means ± SD of 10 seedlings from three independent experiments. Different letters above bars indicate a significant difference at the P < 0.05 level by one-way ANOVA with Fisher’s LSD multiple comparisons test. Scale bar, 10 μm. Similar confocal imaging results to those in (a and b) were obtained from three independent experiments. Exact P-values of statistic tests are provided in the Source data file.
Extended Data Fig. 7 Autophagic phenotype analysis of vsr mutants.
(a) Photographs of the seedlings from the indicated genotypes after maintaining them for 7 d in complete darkness. Seedlings were grown 7 d on half-strength MS under long-day conditions before transfer to dark. Note that starvation-induced chlorosis is enhanced in vsr5vsr6vsr7 or vsr1vsr6vsr7, compared with WT or complemented lines (GFP-VSR7/vsr5vsr6vsr7 and GFP-VSR1/vsr1vsr6vsr7). Scale bar, 1 cm. (b) Quantification analysis of the total chlorophyll content of seedlings in (a). Data are presented as means ± SD of three independent experiments. (c) Quantification analysis of the NBR1 proteins level upon infection with Pto DC3000 avrRpm1. The immunoblot intensity was normalized by the loading control of anti-actin, and the first lane (0 h) in each experiment was arbitrarily set to 1. Data are presented as means ± SD from three independent experiments. The differences were compared among WT and vsr mutants at the indicated time points. (d) Immunoblot analysis of NBR1 accumulation upon infection with Pto DC3000 avrRpm1. Total proteins were extracted from 5-day-old WT, vsr5vsr6vsr7, vsr5vsr6vsr7atg5, and atg5 seedlings at the indicated time points (0 and 6 h), followed by immunoblot analysis with anti-NBR1 antibody. The cytoplasmic marker anti-cFBPase is used as a loading control. The immunoblot intensity was normalized by the loading control of anti-cFBPase, and the first lane (WT, 0 h) was arbitrarily set to 1. Quantification analysis of the NBR1 proteins level is shown on Right. Data are presented as means ± SD from three independent experiments. (e) Quantification analysis of the ratio of free eYFP to eYFP-ATG8e proteins level upon infection with Pto DC3000 avrRpm1. Data are presented as means ± SD from three independent experiments. Different letters above bars indicate a significant difference at the P < 0.05 level by one-way ANOVA with Fisher’s LSD (b, d, and e) or Tukey’s (c) multiple comparisons test. Exact P-values of statistic tests in (b-e) are provided in the Source data file.
Extended Data Fig. 8 Colocalization analysis of GFP-VSR1 with endosomal markers after infection with Pto DC3000 avrRpm1.
(a-c) Colocalization analysis of GFP-VSR1 with the MVB/PVC marker mCherry-Rha1 (a), Golgi marker mCherry-SYP32 (b), and TGN marker VHA-a1-RFP (c). Confocal images are representative of mesophyll cells in leaves of 4-week-old plants before (0) and after (3 h) inoculation of the Pto DC3000 avrRpm1. (d) Colocalization analysis of mCherry-Rha1 with the autophagosome marker eYFP-ATG8e in leaves of 4-week-old plants before (0) and after (3 h) inoculation of the Pto DC3000 avrRpm1. Separated images of each channel in the white outlined area are shown on the right side (from top to bottom: GFP/eYFP, RFP/mCherry, and merged). Arrows indicate the colocalized fluorescence. The colocalization percentage is included in the bottom. Colocalization was quantified from 10 individual leaves of three independent experiments. Data are presented as means ± SD. n, total numbers of analyzed punctae. ****P < 0.0001; n.s., P > 0.05 in two-tailed unpaired Student’s t-test. Scale bars, 10 μm.
Extended Data Fig. 9 Effector proteins colocalized and interacted with ATG8.
(a-c) FRET analysis of the colocalized puncta between YFP tagged avrRpm1-YFP (a), avrRpt2-YFP (b), or avrRps4-YFP (c) and cerulean tagged ATG8e in protoplasts of Arabidopsis suspension cells. The dashed circular outlines indicate examples of colocalized punctae for photobleaching. Arrows indicate the colocalized fluorescence. Separated images of each channel, both pre-bleach and post-bleach, are shown on the right side (from top to bottom: CFP, YFP, and merged). Scale bars, 10 μm. (d) Colocalization analysis of avrRpm1-YFP, avrRpt2-YFP, or avrRps4-YFP with the PVC/MVB marker RFP-Rha1 in protoplasts of Arabidopsis suspension cells. Separated images of each channel are shown on the right side (from top to bottom: YFP, RFP, and merged). Scale bars, 10 μm. (e) The pair of YFPc and YFPn-NBR1 was used as a negative control for NBR1 interaction with avrRpm1, avrRpt2, and avrRps4 in N. benthamiana plants using a BiFC assay. (f) ATG8e interaction with bacterial effectors avrRpm1, avrRpt2, and avrRps4 in N. benthamiana plants using a BiFC assay. ATG8e fused with a N-terminus of YFP (YFPn) was co-expressed with avrRpm1, avrRpt2, or avrRps4 fused with C-terminus YFP (YFPc). Reconstituted YFP fluorescence, colocalized with autophagosome marker mCherry-ATG8e, indicates a positive interaction. The pair of SH3P2-YFPc and YFPn- ATG8e was used as a positive control, while the pair of YFPc and YFPn-ATG8e was used as a negative control. The regions within the white outline are enlarged (Right) (magnification: 5×). Scale bar, 10 µm. The experiments in (d–f) were repeated independently three times with similar results.
Extended Data Fig. 10 The autophagic degradation of effectors was impaired in vsr mutant plant.
(a) Colocalization analysis of GFP/YFP fused effectors and mCherry-ATG8e. Five-day-old transgenic plants expressing autophagosome marker mCherry-ATG8e and the DEX::avrRpm1-GFP, DEX::avrRpt2-GFP, or DEX::avrRps4-YFP were treated with DEX (+) for 24 h, with (+) or without 0.5 μM Conc A for 6 h, followed by confocal analysis. The regions within the white outline are enlarged. Arrows and arrowheads indicate colocalized punctae in cytosol or vacuole, respectively. Scale bar, 10 μm. (b) VSR mutant impair the autophagy degradation of avrRps4-YFP. Confocal images of cotyledon cells from Arabidopsis seedlings of the vsr1vsr6vsr7 transformed with DEX::avrRps4-YFP after DEX induction for 24 h, with (+) or without 0.5 μM Conc A for 6 h, followed by confocal analysis. Note that the vsr1vsr6vsr7 triple mutants accumulated YFP and mCherry positive puncta (arrows) in the cytosol after ConcA treatment, whereas WT Conc A treated plants displayed mostly intravacuolar localization of the puncta (arrowheads). Scale bar, 10 μm. (c) Quantification of the number of avrRps4-YFP punctae in vacuole when plants were treated with DEX (+) and Conc A (+). Data are presented as means ± SD of 20 individual cells from three independent experiments. ****P < 0.0001 in two-tailed unpaired Student’s t-test. Scale bars, 10 μm. The experiments in (a and b) were repeated independently three times with similar results.
Supplementary information
Supplementary Information
Supplementary Figs. 1–5.
Supplementary Table 1
List of genes exhibiting increased transcript levels and meeting the criteria for VSR cargo after inoculation with virulent or avirulent strains of Pseudomonas syringae.
Supplementary Table 2
Primers used in this study.
Supplementary Data 1
Statistical source data for Supplementary Figs. 1, 3 and 4.
Source data
Source Data Figs. 1–6 and Extended Data Figs. 1, 3–8 and 10
Statistical source data.
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, D., Hu, S., Cao, W. et al. Vacuolar sorting receptors coordinate lytic vacuolar and autophagic transport for plant effector-triggered immunity. Nat. Plants 11, 1827–1846 (2025). https://doi.org/10.1038/s41477-025-02077-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41477-025-02077-8