Abstract
Meiotic crossovers, which exchange DNA between homologous chromosomes, are vital for accurate segregation and generate genetic diversity. In plant breeding, they help create new haplotypes by combining beneficial alleles. In Arabidopsis, heterozygous regions in an otherwise homozygous background attract more crossovers than in full F1 hybrids—a phenomenon so far observed only in this self-fertilizing species. Here we report a similar effect in outcrossing maize: local crossover rates increase up to threefold in regions where polymorphism is spatially confined compared to full hybrids. This stimulation occurs in both male and female meiosis and is strongest when heterozygous regions fully span the measured area, likely due to crossover redistribution. As Arabidopsis and maize represent distantly related plant lineages (eudicots and monocots), this shared phenomenon suggests a conserved mechanism. Importantly, it provides a tool for breeding, offering a way to boost recombination and accelerate the introgression of desired traits using interhomologue polymorphism.
Similar content being viewed by others
Main
Variation in eukaryotic populations arises through sexual reproduction, which combines genetic information from two parents1,2,3. However, mating is only the final step in creating variation; much of it is established during gamete formation through a specialized cell division process known as meiosis. During meiosis, chromosomes segregate randomly into daughter cells, which contributes to genetic diversity. A key mechanism for further shuffling genetic combinations inherited from parents is the reciprocal exchange of chromosome fragments, known as crossovers4,5,6. Crossovers are essential for crop breeding, and the ability to enhance them locally remains a key challenge in applied genetics7,8.
The distribution of crossovers along chromosomes is influenced by a range of factors, many of which remain incompletely understood9,10,11,12. One such factor is local DNA sequence differences between homologous chromosomes, known as cis-acting genetic polymorphism. In most studied species, local polymorphism tends to inhibit crossovers13,14,15. However, in the plant Arabidopsis thaliana, crossovers are stimulated in heterozygous regions if these are juxtaposed to homozygous regions on the same chromosome16,17,18,19. This effect has been most thoroughly studied in Arabidopsis, where it was attributed to its compact genome, distinctive recombination landscape and predominantly self-pollinating nature—features that may enable the plant to maximize the genetic benefits of rare outcrossing events20,21,22,23,24.
In this Article, through extensive breeding work, we developed a set of nearly isogenic maize lines (NILs) with distinct polymorphism patterns and phenotypic markers, enabling precise measurement of recombination frequency (Rf). Building on a high-quality PacBio genome assembly and high-coverage Illumina sequencing, we discovered that crossover stimulation in heterozygous domains adjacent to homozygous regions occurs even more effectively in maize, showing up to a threefold increase in crossover frequency. Given that maize is naturally outcrossing, with a genome ~20 times larger than Arabidopsis and a strikingly different crossover pattering, this finding suggests that the heterozygosity juxtaposition effect may be conserved among flowering plants25,26,27. These results indicate that there may be aspects of plant evolution worth revisiting. Furthermore, as maize is one of the world’s most important crops, our findings offer valuable insights for breeders, presenting a method to direct recombination to specific locations through the simple selection of optimal parental plants. This approach promises a universal strategy for efficiently transferring advantageous traits between crop varieties.
Results
Local stimulation of meiotic crossover in polymorphic regions occurs in both Arabidopsis and maize
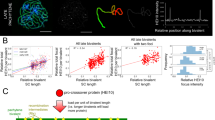
Segregation of linked markers can be used to measure crossover rates within a defined interval28,29,30. To demonstrate the phenomenon of crossover stimulation in the heterozygous region in Arabidopsis, we developed the R2-BT line (Recombinant × Recombinant for BT; Extended Data Fig. 1). This was achieved by crossing a recombinant from Col-BT × Ler cross, which carried a seed-expressed reporter encoding the Discosoma red fluorescent protein (dsRed), with another recombinant carrying an enhanced green fluorescent protein (eGFP) reporter, both selected based on previously identified crossover breakpoints18. Double-reporter individuals were selected in the F2 generation and backcrossed to the Col background, resulting in the R2-BT line. This line contained a 35 kb segment between the fluorescent markers derived from the Landsberg erecta (Ler) accession, while the remainder of the chromosome was from Columbia (Col), which is polymorphic to Ler (Extended Data Fig. 1 and Supplementary Table 1). We then crossed the R2-BT line with Col, creating a heterozygous BT interval in an otherwise homozygous background (hereafter ‘Juxtaposed’), and with Ler, generating a reverse combination, where the BT interval is homozygous and the rest of the chromosome is heterozygous (‘Reverse juxtaposed’) (Fig. 1a,b). In parallel, we generated Col-BT × Col inbred and Col-BT × Ler hybrid variants as controls (Fig. 1a,b). The crossover frequency in the BT interval was 2.22 times higher in the R2-BT × Col Juxtaposed cross compared to the Col-BT × Ler hybrid (Fig. 1c–e and Supplementary Tables 2 and 3). By contrast, in the R2-BT × Ler Reverse juxtaposed cross, BT crossover frequency was only 0.6 times that observed in the Col-BT × Col inbred (Fig. 1c–e). These results confirm that local heterozygosity within a homozygous background promotes crossovers, whereas local homozygosity within a heterozygous background inhibits them.
a, Interhomologue polymorphism in F1 plants representing ‘Inbred’, Hybrid, Juxtaposed and Reverse juxtaposed combinations. Each shows a pair of A. thaliana chromosome 3 (At_Chr 3; not to scale), with colour flags representing reporters used to measure crossover rates in the interval. b, Ideogram of At_Chr 3, indicating the BT interval location. The dashed rectangle highlights the 1 Mb section shown in c. c, SNP distribution per 5 kb along a 1 Mb section of At_Chr 3 including the BT interval in the Hybrid and Juxtaposed combinations. Green and red vertical lines show reporter positions. Grey rectangles below the graphs denote heterozygous regions in each configuration. d, Rf in the BT interval across the four backgrounds. The P values were calculated using Welch’s one-way ANOVA followed by the Games–Howell post hoc test; *P = 0.05, **P = 4.0 × 10−3, ***P = 9.9 × 10−4, ****P = 4.12 × 10−4. Each data point represents a single plant. Box plots: centre line, median; bounds, 25th and 75th percentiles. e, Segregation of eGFP and dsRed reporters in F2 seeds from Col-BT × Ler. Arrowheads indicate individual green and red recombinants, corresponding to Ler/Ler to Col/Ler and Col/Ler to Ler/Ler crossover events, respectively. Scale bar, 2 mm. f, Marker positions forming the ws3–lg1–gl2 and c1–sh1–wx1 intervals on maize chromosomes 2 (Chr 2) and 9 (Chr 9), respectively. Genetic distances (cM) from MaizeGDB. g, Maize ears in Ku and M backgrounds showing segregation at c1 and sh1 markers. h, Rf across five intervals in F1 plants (data partly based on Mikhailov and Chernov31). Each point is the mean Rf of 4–12 F1 families grown in 1 year. Green, M × 2-9m; navy, Ku × 2-9m. Plant numbers indicated below box plots (M/Ku). Box plot centre, median; bounds, 25th and 75th percentiles (see Supplementary Table 5 for details). Expected values from MaizeGDB using Kosambi (blue) and Haldane (orange) models are shown as horizontal bars. i, Scheme for generating NILs to measure Rf in maize with different heterozygosity patterns, illustrated with the Ku(c1,sh1,R1) line. R1 marker, required for c1 phenotype, not shown. Ku was crossed with 2-9m carrying linked c1–sh1–wx1 alleles. F3 plants homozygous for c1, sh1 and WX1 were selected and backcrossed seven times to Ku, selecting c1,sh1/++ heterozygotes. Final plants were selfed and selected for c1/sh1 homozygosity. Crosses to Ku produced Juxtaposed combinations; crosses to M produced fully heterozygous Hybrids. Chromosome 9 configurations are depicted. BC, backcross. j, Assembly contiguity is represented as an Nx plot (the length of the shortest contig that, along with longer and equal-length contigs, represents x% of the assembly) for the Ku123 line. k, SNP density across the 10 maize chromosomes for Ku(c1,sh1,R1) aligned to pure Ku (top) and M (bottom). Regions of polymorphism reflect introgressions from 2-9m. Orange and blue lines mark c1 and sh1 loci. l, SNP distribution along chromosome 9 in M(c1,sh1,R1) crosses. Grey bars, SNP density per 10 kb; burgundy line, SNP density per 1 Mb; grey horizontal bars below, estimated heterozygous regions. Coloured lines indicate the c1–sh1 interval used for Rf. The navy blue square on the x axis shows the centromere location. Inset zooms in on interval; SNP density plots partly trimmed. m, Rf (%) based on c1–sh1 marker segregation in male and female backcross generations from Hybrid M(c1,sh1,R1) × Ku(R1) and Juxtaposed M(c1,sh1,R1) × M(R1) crosses. Each data point denotes one plant. Sample sizes are shown below box plots. The two-sided P values were estimated by Welch’s t-test; *P = 0.0305, ***P = 4.2 × 10−4. Box plot centre, median; bounds, 25th and 75th percentiles.
To determine whether crossover stimulation in heterozygous regions adjacent to homozygous regions is a general phenomenon in plants, we decided to investigate similar genetic combinations in maize. For this purpose, we used the 2-9m line, which carries a set of linked mutant alleles for phenotypic traits on chromosomes 2 (ws3, lg1 and gl2) and 9 (c1, sh1 and wx1) (Fig. 1f,g, Supplementary Fig. 1 and Supplementary Table 4). The c1 marker requires the presence of a dominant R1 allele on chromosome 10 to show anthocyanin colouration of the kernels. We crossed the 2-9m line with two divergent inbred maize lines: MK01 and Ku123 (hereafter M and Ku, respectively).
We first examined the Rf in M × 2-9m and Ku × 2-9m F1 crosses31. For five intervals—lg1–gl2, ws3–lg1 and ws3–gl2 on chromosome 2, and c1–sh1 and sh1–wx1 on chromosome 9—the Rf values were consistent with genetic distances reported by the Maize Genetics and Genomics Database (MaizeGDB)32, and no deviations from Mendelian segregation were observed (Fig. 1h and Supplementary Tables 5 and 6). This suggests that the regions of chromosome 2 and 9 covering the examined intervals do not contain extensive chromosomal rearrangements that could affect formation of meiotic crossovers. We then introgressed individual marker combinations into M and Ku through 5–7 backcrosses (Fig. 1i and Extended Data Fig. 2), resulting in the following NILs: M(c1,sh1,R1), Ku(c1,sh1,R1), M(sh1,wx1), Ku(sh1,wx1), M(lg1,gl2), Ku(lg1,gl2), M(ws3,lg1), M(ws3,gl2), M(c1,sh1,wx1,R1) and M(ws3,lg1,gl2). In parallel, the M(R1) and Ku(R1) lines were generated as R1-containing variants of the M and Ku lines.
We used PacBio high-fidelity technology to sequence the Ku parent (at 30× depth) and performed a de novo genome assembly (Fig. 1j and Supplementary Fig. 2). The genome assembly had an N50 value of 241.8 Mb, a total assembly size of 2.432 Gb and a BUSCO (Benchmarking Universal Single-Copy Orthologs) completeness of 98.57% (Fig. 1j, Supplementary Fig. 2 and Supplementary Table 7). Chromosome 9 was represented by two contigs and chromosome 2 by five contigs. In addition, we also identified centromeric regions, genes, repeats and organellar integrants within the Ku assembly (Extended Data Figs. 3–6 and Supplementary Tables 8–11). The region containing the markers ws3–lg1–gl2 is located at the very end of chromosome 2, at positions 0.78, 3.85 and 9.59 Mb. The ws3–lg1 interval has a gene density of 24.1 genes per Mb, which is more than twice the genome-wide average of 10.5 genes per Mb, while transposable elements account for 69.1% of this interval compared to the genome average of 81.4%. The lg1–gl2 interval shows a gene density of 15.5 genes per Mb, with transposable elements comprising 72.9% of the region. The c1–sh1–wx1 region is also located in a subtelomeric area, although further from the chromosome end, at positions 10.90, 12.52 and 25.14 Mb. The c1–sh1 and sh1–wx1 intervals have nearly identical gene densities (16.6 and 16.8 genes per Mb, respectively), but the former contains slightly fewer transposable elements (79.5% versus 82.2%) (Extended Data Figs. 3–5 and Supplementary Table 8).
We then compared the genome assembly of Ku to the reference genome assemblies of the lines B73 and Mo17 (Extended Data Fig. 7)33,34. Chromosomes 2 and 9 showed a high collinearity with both reference genomes, with only a few minor structural variations, which should not affect our data. The largest rearrangement detected was a ~4.9 Mb inversion spanning the centromere of chromosome 9. Conversely, Ku has six large heterochromatic knobs with a total length of 84.03 Mb—almost four times the length found in Mo17 (23.47 Mb)34. As these knobs are not located directly within the measurement intervals and remain recombinationally inactive, they do not affect our results.
To investigate the effectiveness of introgression within the obtained materials, we sequenced all NILs and their parents using Illumina technology to an average depth of 20× (Supplementary Table 12). The obtained reads were mapped to the Ku assembly, allowing us to examine single-nucleotide polymorphisms (SNP) (Fig. 1k and Extended Data Fig. 8). Chromosome 2 showed a relatively even SNP distribution between the Ku and M lines. By contrast, chromosome 9 contained a large block of approximately 40 Mb with a reduced density of polymorphisms, reflecting a previously described selective sweep35. A similar low-density block was also observed when comparing it to the 2-9m line (orange arrows in Extended Data Fig. 8). Importantly, the regions containing the intervals for measuring Rf on both chromosomes were fully polymorphic between M, Ku and 2-9m parents. The analysis confirmed that the NILs carried introgressed regions precisely at the sites encompassing the markers, with additional, unintended introgressions present on other chromosomes (for details, see descriptions of the introgressed regions in the following sections).
Furthermore, each of the NILs was crossed to both the M and Ku parental lines or their R1-containing variants. Depending on the parent used, the resulting F1 plants were fully heterozygous (for example, Ku(c1,sh1,R1) × M(R1)) or heterozygous only for the measurement interval within an otherwise homozygous background (for example, Ku(c1,sh1,R1) × Ku(R1)) (Fig. 1i). Hence, these crosses corresponded to heterozygosity/homozygosity combinations equivalent to ‘Hybrid’ or Juxtaposed contexts used in our Arabidopsis experiment (Fig. 1a,d). The average SNP densities for the Ku × M and M × 2-9m hybrids were 0.581 and 0.952 SNPs per kb, respectively (Supplementary Table 13). This is substantially lower than in Arabidopsis Col × Ler cross (4.44 SNPs per kb; Supplementary Table 1). As Rf for these F1 plants was measured in bidirectional backcrosses—calculated as the ratio of recombinant to total offspring—male and female meiosis was assessed separately.
By analysing the segregation of phenotypic markers in kernels from the progeny of NIL × M and NIL × Ku crosses, we measured the Rf on chromosome 9. We first examined the Rf in the c1–sh1 interval, located between 16 (c1) and 20 (sh1) cM of the chromosome 9 genetic map (Fig. 1f). For the M(c1,sh1,R1) line, we observed a nearly twofold increase in Rf, from 5.7 ± 0.5% (n = 6, male) and 5.2 ± 0.3% (n = 10, female) in Hybrid configuration to 10.0 ± 1.7% (n = 6, male) and 10.9 ± 2.1% (n = 7, female) in the Juxtaposed configuration (Fig. 1l,m and Supplementary Tables 14 and 15). This result shows that the effect of recombination stimulation in heterozygous regions, when located within an otherwise homozygous chromosome, is not unique to Arabidopsis but also occurs in maize, despite differences in polymorphism density.
The heterozygosity juxtaposition effect occurs in both male and female meiosis and requires adjacent recombinationally active regions
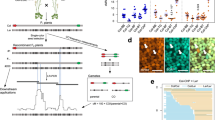
To assess whether the effect is specific to the M background, we measured recombination in crosses for the Ku(c1,sh1,R1) line. We observed similar increases, from 5.3 ± 0.3% (n = 5, male) and 5.2 ± 0.3% (n = 8, female) in the Hybrid configuration to 9.6 ± 1.8% (n = 6, male) and 10.1 ± 1.9% (n = 10, female) in the Juxtaposed configuration (Fig. 2a,b and Supplementary Tables 14 and 15). Next, we examined the Rf for the sh1–wx1 interval, located between 20 and 48 cM (Fig. 1f). Here, we observed an even greater increase in Rf in Juxtaposed combinations compared to Hybrid: For the M(sh1,wx1) lines, the frequency increased from 16.6 ± 1.0% (n = 8, male) and 11.2 ± 0.7% (n = 10, female) to 38.3 ± 1.0% (n = 6, male) and 30.2 ± 1.1% (n = 11, female) (Fig. 2c,d and Supplementary Tables 14 and 15). For the Ku(sh1,wx1) line, the increase was from 16.8 ± 0.9% (n = 6, male) and 15.3 ± 0.6% (n = 11, female) to 37.2 ± 1.7% (n = 7, male) and 37.3 ± 1.2% (n = 9, female) (Fig. 2f,g and Supplementary Tables 14 and 15). These results show that, regardless of the genetic background (M or Ku), locally occurring polymorphism between homologues stimulates crossover, leading to a substantial increase in Rf. Importantly, we reveal that the stimulation of crossover due to local interhomologue occurs in both male and female meiosis (Fig. 2h).
a, Interhomologue SNP distribution in the Ku(c1,sh1,R1) crosses along chromosome 9. Grey vertical bars represent SNP density per 10 kb, and the burgundy trend line shows SNP density per 1 Mb. Grey horizontal bars beneath the graph indicate estimated heterozygous regions based on SNPs. Coloured lines mark the positions of the c1–sh1 interval used to measure Rf. The navy blue square on the x axis shows the centromere location. The inset offers a close-up of the interval, with the SNP density plots below partially trimmed. b, Rf (%) measured by segregation of c1–sh1 markers in male and female backcross generations for Hybrid Ku(c1,sh1,R1) × M(R1) and Juxtaposed Ku(c1,sh1,R1) × Ku(R1) crosses. The numbers of individuals are indicated below the box plots. Each data point represents measurements from one plant. The two-sided P values were estimated by Welch’s t-test; ***P = 3.4 × 10−4, **P = 2.07 × 10−3. The centre line of the box plot shows the median, and the upper and lower bounds show the 75th and 25th percentiles. c,d, As in a and b, but for the M(sh1,wx1) crosses. ***P = 3.6 × 10−9, ****P = 9.5 × 10−11. e, The interhomologue polymorphism to the right of the sh1–wx1 interval in the Juxtaposed M(sh1,wx1) × M cross does not affect the Rf within this interval, as it lies within a recombinationally inactive chromosomal region. The grey area shows the distribution of SNPs per Mb, while blue and red lines indicate the crossover frequency (cM Mb−1) along the short chromosome arm for Flint × Flint and Dent × Dent crosses, respectively, as reported by ref. 37. Solid, dashed and dotted lines represent populations with the highest, median and lowest genome-wide recombination rates among 23 populations within each group37. f,g, As in a and b, but for the Ku(sh1,wx1) crosses. ***P = 2.9 × 10−6, ****P = 9.2 × 10−10. h, Model of crossover stimulation in heterozygous regions (shaded) when juxtaposed with homozygous regions (light blue). In maize, this effect is observed in both male and female meiosis. i, Polymorphism-triggered crossover stimulation relies on remodelling from recombinationally active chromosomal regions. The increase in crossover frequency in heterozygous region 1 (blue-grey shaded area) is primarily driven by the presence of the upstream homozygous region (large arrow) rather than the downstream homozygous region (smaller arrow), as the former is highly recombinationally active (light-grey shaded area). It remains unknown whether crossovers can be redistributed from recombinationally active regions to inactive regions, such as heterozygous region 2.
As mentioned before, the introgression of sh1–wx1 to M was much less efficient than into Ku (bottom panels in Fig. 2c,f): Along with the sh1–wx1 interval, a segment of approximately 6.7 Mb extending to the right from wx1 was also introgressed in M(sh1,wx1). In addition, the entire middle part of the chromosome and a fragment of the right arm originated from 2-9m (Fig. 2c). By contrast, in the Ku(sh1,wx1) line, the introgression is very precise with the end of the region from 2-9m perfectly coinciding with the wx1 marker (Fig. 2f). Unexpectedly, we observed an equally strong increase in Rf in both cases, with Juxtaposed configurations showing a 2.2- to 2.7-fold increase compared to Hybrid (Fig. 2d,g and Supplementary Tables 14 and 15).
In Arabidopsis, the heterozygosity juxtaposition effect depends on the redistribution of crossovers from adjacent homozygous regions into the heterozygous region16,19. In maize the crossover frequency, as estimated by chiasma counts, does not differ between hybrids and their parental inbred lines36, indicating that the presence of heterozygosity alone does not induce additional crossovers. Therefore, we consider it likely that the juxtaposition effect in maize also results from crossover redistribution. Based on this assumption, one might expect stronger stimulation of recombination in the Ku(sh1,wx1) line than in M(sh1,wx1), which, however, is not observed.
We propose that this is because the several-megabase region immediately to the right of wx1 shows almost no recombination, as evidenced by the marker distribution on the genetic map (Fig. 1f) and the published crossover data37,38 (Fig. 2e and Extended Data Fig. 9). We argue that, due to the low Rf in this region, there is limited potential for its redistribution into the heterozygous region. Consequently, the observed increase in the Rf in the juxtaposed M(sh1,wx1) and Ku(sh1,wx1) lines is primarily caused by the homozygous regions located to the left side of sh1–wx1, which spans nearly 20 cM genetically, despite covering only ~12 Mb (insets in bottom panels in Fig. 2c,f). This result suggests that the juxtaposition effect requires adjacent recombinationally active regions, whereas homozygosity in neighbouring recombinationally silent regions does not contribute to crossover stimulation (Fig. 2i). However, we currently lack the tools to determine whether this mechanism can also stimulate crossovers within inactive regions (Fig. 2i).
The increase in crossover frequency within the measured interval depends on the distance from the boundary with the homozygous region
Based on the segregation of phenotypic markers observed in seedlings, we measured the Rf in intervals located on chromosome 2 (Fig. 1f). In the lg1–gl2 interval, we again observed an increase in the Rf in Juxtaposed compared to Hybrid configurations (Fig. 3a–d). For M(lg1,gl2) line, this increase was from 20.0 ± 2.3% (n = 5, male) and 18.8 ± 0.8% (n = 12, female) to 28.1 ± 1.4% (n = 8, male) and 26.4 ± 1.8% (n = 8, female), respectively. However, this increase was much more pronounced in the Ku(lg1,gl2) line, rising from 17.3 ± 1.1% (n = 3, male) and 16.7 ± 0.7% (n = 10, female) to as high as 34.5 ± 1.4% (n = 8, male) and 42.3 ± 1.4% (n = 11, female), respectively (Fig. 3a–d and Supplementary Tables 14 and 15).
a, Interhomologue SNP distribution in the M(lg1,gl2) crosses along chromosome 2. Grey vertical bars represent SNP density per 10 kb, and the burgundy trend line shows SNP density per 1 Mb. Grey horizontal bars beneath the graph indicate estimated heterozygous regions based on SNPs. Coloured lines mark the positions of the lg1–gl2 interval used to measure Rf. The navy blue square on the x axis shows the centromere location. The inset offers a close-up of the interval, with the SNP density plots below partially trimmed. b, Rf (%) measured by segregation of lg1–gl2 markers in male and female backcross generations for Hybrid M(lg1,gl2) × Ku and Juxtaposed M(lg1,gl2) × M crosses. The two-sided P values were estimated by Welch’s t-test; *P = 0.0196, **P = 0.0029. c,d, As in a and b, but for the Ku(lg1,gl2) crosses. ***P = 1.2 × 10−5, ****P = 9.2 × 10−11. e, Genetic structure of the combinations used in crosses for the intervals ws3–lg1–gl2 (chromosome 2) and c1–sh1–wx1 (chromosome 9). For chromosome 2, Juxtaposed A corresponds to the cross M × M(ws3,lg1), Juxtaposed B to M × M(ws3,lg1,gl2), and Juxtaposed A* to M × M(lg1, gl2). For the Hybrid category, combinations A, B and A* are the same as for the Juxtaposed category but crossed with Ku. For chromosome 9, Juxtaposed A, B and A* correspond to M × M(c1,sh1,R1), M × M(c1,sh1,wx1,R1) and M × M(sh1,wx1), respectively, while the Hybrid combinations are analogous but involve crosses with Ku. The diagram indicates only the approximate positions of heterozygous regions. Detailed polymorphism patterns for the Juxtaposed A and A* configurations are shown in a, and in Figs. 1l and 2c. For the Juxtaposed B combinations, precise sequence data are not available; however, the homozygous regions are expected to span large portions of the chromosomes outside the measurement intervals, as the lines were generated through six to eight rounds of backcrossing. f, Rf in the ws3–lg1 and lg1–gl2 intervals for the combinations shown in e. Statistical significance was determined using two-sided Welch’s ANOVA, followed by the Games–Howell post hoc test (see Supplementary Table 17 for details). g, Similar to f, but depicting the c1–sh1 and sh1–wx1 intervals for the genotype combinations presented in e. For b, d, f and g, the number of individuals is indicated below the box plots. Each data point represents measurements from a single plant. Different colours within the same group indicate data collected in different years. The centre line of each box plot represents the median, and the upper and lower bounds correspond to the 75th and 25th percentiles, respectively.
To understand this difference, we examined the heterozygosity pattern in M(lg1,gl2) and Ku(lg1,gl2). We observed that while the introgression in Ku almost perfectly coincides with the interval, the introgression in M extends approximately 7 Mb beyond the lg1–gl2 region (bottom panels in Fig. 3a,c). Consequently, the crossover redistribution from homozygous to heterozygous regions also encompasses the area outside the measurement interval, leading to a lower increase in recombination rates within this interval in the M background compared to the Ku background. Thus, our results show that the stimulation of crossover recombination is more pronounced when the measurement region closely coincides with the heterozygous region.
To further investigate this relationship, we used a series of three-point crosses in which Rf was measured in two adjacent intervals on the same chromosome and compared the results with data from two-point crosses (Fig. 3e). Based on the markers, we classified the crosses into three Juxtaposed types—A, A* and B—where A and A* have the shortest and B the longest heterozygous region. Similarly, hybrid crosses were categorized into types A, A* and B, although they remained fully heterozygous and differed only in the parental origin of the region containing the markers (from genotype M or 2-9m). On this occasion, we also compared the kernel set between the Juxtaposed and Hybrid combinations and found no differences indicative of reduced fertility (Supplementary Table 16). Rf was measured for all marker combinations (see Methods for details).
In all cases, ‘Juxtaposed A’ or ‘Juxtaposed A*’ combinations showed significantly higher Rf than ‘Juxtaposed B’ and both Hybrid types (Fig. 3f,g and Supplementary Tables 14 and 17). Moreover, for the longer intervals (lg1–gl2 and sh1–wx1), Juxtaposed B combinations also showed significantly higher Rf than at least one of the Hybrid types, indicating that extending the heterozygous region beyond the measurement interval reduces the magnitude of crossover stimulation (Fig. 3f,g and Supplementary Table 17). By contrast, for the shorter intervals ws3–lg1 and c1–sh1, where the heterozygous region is several times longer than the measurement interval, the Juxtaposed B combinations showed recombination frequencies that did not differ significantly from those observed in the hybrids (Supplementary Table 17). These findings suggest that crossover stimulation is stronger when the measurement interval is closer to the boundary with a homozygous region. However, an alternative hypothesis remains plausible: that the juxtaposition effect increases as the length of the heterozygous block decreases.
To differentiate between these possibilities, we reanalysed data from three A. thaliana Col-0 × Ct-1 F2 populations for which more detailed information was available16. In these datasets, each F2 individual was genotyped along the chromosome, allowing us to more precisely define the length and position of the heterozygous region encompassing the measurement interval. Crossover frequency was quantified for each individual based on the segregation of fluorescent markers in pollen (I2f and CEN3 populations) or seeds (420 population) (Supplementary Table 18). We then grouped individuals according to the distance between the measurement interval and the boundary with the adjacent homozygous region and plotted crossover frequencies for each F2 plant within these groups (Fig. 4a–c). For the I2f and 420 populations, where the measurement intervals are located at the ends of chromosomes, we performed Spearman correlation analysis using group-wise average recombination frequencies (Fig. 4d). We found that the closer the interval was to the homozygous region, the stronger the crossover stimulation effect (Spearman Rho = −0.92, P = 6.29 × 10−4, and −0.87, P = 1.01 × 10−3, for 420 and I2f intervals, respectively).
a–c, Crossover frequency in F2 individuals from Col-0 × Ct-1 populations for the I2f (a), 420 (b) and CEN3 (c) intervals, plotted as a function of the distance between the heterozygous measurement interval and adjacent homozygous regions. The top panels show Arabidopsis chromosomes with the positions of all three intervals and illustrate how the distance from the boundary is calculated. Chromosomes 2 and 3 in a and c were inverted to match the orientation of the data shown in the bottom panels. The bottom panels show Rf (%) for plants grouped by distance between the interval and the boundary. For CEN3 (c), only the effect on the long chromosome arm was analysed. Each data point represents an individual F2 plant. The centre line of each box plot indicates the median, and the box edges represent the 25th and 75th percentiles. Whiskers indicate the data range within 1.5× the interquartile range from the lower and upper quartiles. The trend line represents a third-degree polynomial fit generated using linear regression. Grey area shows 95% confidence interval of the fitted polynomial model. d, Spearman rank correlation between Rf (cM) and the distance of the heterozygous measurement interval (I2f or 420) from the boundary with the adjacent homozygous region. Each data point represents the mean Rf for a group of samples sharing the same distance from the boundary. A linear regression model was applied to illustrate the overall trend, with the grey area showing the 95% confidence interval. e, As in d, but for the correlation between Rf and the length of the adjacent homozygous region. f, The influence of the genetic structure of the studied region on Rf. Heterozygous regions are shown as light-grey shaded areas in otherwise homozygous chromosome. The intervals used for recombination measurements are indicated by coloured dashed lines, and the average Rf in these intervals for full hybrids is represented by the grey bars. When the studied interval overlaps with the heterozygous region (situation 1), the increase in the interval crossover frequency will be higher than when the heterozygous region extends beyond the studied area (situation 2).
Next, we regrouped individuals based on the length of the homozygous block directly adjacent to the heterozygous region containing the measurement interval. While we observed that longer homozygous segments were associated with stronger recombination stimulation within the neighbouring heterozygous region, the correlations were notably weaker (Rho = −0.45, P = 0.02 for 420; Rho = −0.33, P = 0.042 for I2f; Fig. 4e). Based on these results, we conclude that the key determinant of the juxtaposition effect is the physical proximity of the heterozygous region to the homozygous boundary (Fig. 4f). These findings also indicate that even a relatively short homozygous segment adjacent to a heterozygous region can substantially enhance recombination in the latter.
For the CEN3 population, correlation analysis was not feasible because the measurement interval is located in the middle of the submetacentric chromosome, meaning that the heterozygous region can extend toward both chromosome arms (Fig. 4c). However, when plotting crossover frequency as a function of distance to the boundary for a subset of individuals with a partially or fully fixed short arm, we observed that the juxtaposition effect also operates within centromeric regions. It is important to note that the data presented here are from A. thaliana, and it remains an open question whether heterozygosity-juxtaposition-triggered crossover stimulation also occurs in pericentromeric regions in maize.
Short homozygous blocks interrupting the heterozygous region suppress local crossover stimulation
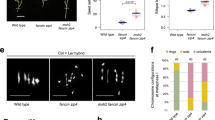
Finally, we examined recombination in maize by investigating segregation of ws3, lg1 and gl2 markers in the F2 generation (Figs. 1f and 5a–f). For the M(ws3,lg1) and M(lg1,gl2) introgressions, we observed a statistically significant increase in Rf in the Juxtaposed combination (cross with M) compared to Hybrid combination (cross with Ku) (P = 7.1 × 10−5 and P = 1.3 × 10−5, respectively, Welch’s test; Fig. 5a–d and Supplementary Tables 14 and 15). However, when we introduced a homozygous block of approximately 3 Mb within the heterozygous ws3–gl2 interval, forming the ‘Interrupted juxtaposed’ combination (top panel in Fig. 5e), the Rf did not differ from that observed in the full hybrid (Fig. 5e,f).
a, Interhomologue SNP distribution in the M(ws3,lg1) × M cross along chromosome 2. Grey vertical bars represent SNP density per 10 kb, with the burgundy trend line showing SNP density per 1 Mb. Grey horizontal bars beneath the graph indicate estimated heterozygous regions based on SNPs. Coloured lines mark the positions of the ws3–lg1 interval used to measure Rf. The navy blue square on the x axis shows the centromere location. The inset offers a close-up of the interval, with the SNP density plots below partially trimmed. b, Rf (%) measured by segregation of ws3–lg1 markers in F2 generations for Hybrid M(ws3,lg1) × Ku(R1) and Juxtaposed M(ws3,lg1) × M crosses (male/female averaged). The two-sided P values were estimated by Welch’s t-test; ****P = 7.1 × 10−5. The numbers of individuals are indicated below the box plots. Each data point represents measurements from one plant. Different colours within the same group indicate data collected in different years. The centre line of the box plot shows the median, and the upper and lower bounds show the 75th and 25th percentiles. c,d, As in a and b, but for the M(lg1,gl2) crosses. ****P = 1.3 × 10−5. e, As in a, but for the Hybrid M(ws3,gl2) × Ku(R1) and Interrupted juxtaposed M(ws3,gl2) × M crosses, where the heterozygous region contains a short homozygous block (gap). f, As in b, but measured by segregation of ws3–gl2 markers. NS, not significant. g, The effect of genetic structure on Rf. Heterozygous regions are shown as light-grey shaded areas in otherwise homozygous chromosome. The intervals used for recombination measurements are indicated by coloured dashed lines, and the average Rf in these intervals for full hybrids is represented by the grey bars. Small violet arrows denote expected subsegmental crossover changes based on homozygous/heterozygous state. Crossover frequency is lower when heterozygosity is interrupted by homozygosity (compare chromosome configurations 1 and 2).
As both the ws3–lg1 and lg1–gl2 intervals show increased recombination when juxtaposed with homozygous regions (Fig. 5b,d), the ws3–gl2 interval would also be expected to show elevated recombination. The absence of this effect in the Interrupted juxtaposed configuration likely reflects the mechanism observed in Arabidopsis, where the juxtaposition effect relies on crossover redistribution from homozygous to neighbouring heterozygous regions (see also the comparison between Juxtaposed with Reverse juxtaposed in Fig. 1d). In the Interrupted juxtaposed configuration, crossover events may be redirected from the intervening homozygous block toward neighbouring heterozygous regions within the same measuring interval, ultimately yielding no significant change in the ws3–gl2 Rf (Supplementary Table 15). This outcome is therefore consistent with findings in Arabidopsis16.
Discussion
In yeast and mammals, local interhomologue polymorphism inhibits crossover recombination, leading to repair via alternative mechanisms13,39,40,41. By contrast, crossover stimulation in heterozygous regions adjacent to homozygous chromosome segments has been well documented in Arabidopsis16,17,18,19. It is important to note that A. thaliana predominantly reproduces through self-pollination, resulting in a high frequency of homozygous loci in natural populations42,43. Therefore, this stimulation of crossover in heterozygous regions is often interpreted as a strategy to maximize the use of remaining genetic variation and enhance population variability44,45. In addition, Arabidopsis has several unique features, including short life cycle, a small, compact genome, low densities of DNA methylation and transposable elements, a structurally conserved genome and a high correlation between SNPs and recombination as well as a crossover distribution concentrated toward chromosome centres17,20,46. These characteristics sharply contrast with those of larger crop genomes, such as wheat, barley and maize26,47,48,49. Surprisingly, in this study, we provide direct evidence that this crossover stimulation effect also occurs in maize, which is naturally outcrossing.
Maize has long served as an excellent model for studying meiotic recombination, owing to its remarkable phenotypic variability and the pioneering work of many researchers, most notably Barbara McClintock. For over a century, research using maize has revealed that most crossovers occur within genes, while the abundant retrotransposons present in its genome are largely recombinationally inactive50. Due to the high variability of retrotransposons and their dominant contribution to the physical length of chromosomal regions, much of the research has focused on their suppressive effect on recombination51,52. For instance, heterozygosity for a 26 kb retrotransposon cluster insertion leads to a twofold reduction in Rf between the bz1 and stc1 markers53.
Importantly, intragenic recombination studies have enabled the separation of the effects of repetitive elements from those of SNP54,55. The most extensive investigation was carried out by Dooner within the highly recombinogenic Bronze (Bz) hotspot, which spans approximately 1.5 kb (ref. 55). By using flanking markers on both sides of the Bz locus, the experimental system enabled the detection of both crossover and non-crossover recombination events (that is, short, non-reciprocal exchanges). Comparing different crosses involving bz alleles with varying degrees of polymorphism, Dooner observed that crossovers predominated in polymorphic allele pairs, whereas both crossover and non-crossover events were observed when sequence polymorphism was minimal. This suggests that polymorphism within a single hotspot may bias repair toward the crossover pathway—an observation consistent with recent findings in Arabidopsis18. Unfortunately, the available data do not allow a direct comparison of crossover frequency between the different sets of alleles.
An early indication that heterozygosity flanked by homozygous regions might stimulate crossover frequency in maize came from the study by Mikhailov and Chernov31. They examined F2 populations and measured Rf between pairs of markers, with the extent of heterozygosity inferred using a third marker. Recombination was higher in crosses where heterozygosity was confined to the intervals defined by the two markers. These results served as a foundation for the current study.
Comparable phenomena have not been documented in other plant species, with the possible exception of lima bean (Phaseolus lunatus), as reported by Allard56. In that study, five generations of inbreeding were carried out while maintaining heterozygosity in three marker-defined intervals. Although the author aimed to select for plants with altered recombination frequencies in both positive and negative directions, only selection for increased recombination was successful, while attempts to decrease Rf were ineffective. This outcome aligns with the juxtaposition effect, as inbreeding would have progressively increased homozygosity in genomic regions outside the monitored intervals.
The separation of dicotyledonous Arabidopsis and monocotyledonous maize by approximately 140 million years of evolution57, coupled with the conservation of key features of this process—such as its dependence on polymorphism patterns along the chromosome and independence from genetic background—suggests that the juxtaposition effect may be universal among all flowering plants. From an evolutionary perspective, this mechanism may be beneficial in populations with a high level of inbreeding, where a large portion of the genome is homozygous. Given the typically low number of crossovers in most eukaryotes58, stimulating crossovers in heterozygous regions enhances the generation of novel allelic combinations within the population. In the extreme scenario where only a single chromosomal region is heterozygous, any crossover within that region will consistently produce new allele combinations, whereas crossovers within homozygous regions will invariably recreate parental haplotypes (Fig. 6a). Importantly, in Arabidopsis, the chromosomal distribution of crossovers has been shown to be highly similar between hybrids and inbred lines19,59, indicating that a heterogeneous pattern of polymorphism along the chromosome is necessary to elicit this effect.
a, Stimulating crossovers in heterozygous regions enables the creation of more allelic combinations in populations with high levels of homozygosity (that is, with high inbreeding coefficient). When large chromosomal segments are homozygous, crossovers within these regions produce gametes that retain the parental allele configuration (top panel). By contrast, crossovers in heterozygous regions always result in recombinant gametes, generating novel allelic combinations (bottom panel). b, Potential application of the juxtaposition effect in commercial crop breeding. The diagram illustrates a scenario in which a breeder aims to transfer valuable variation (QTL1) from one parental line into another. After crossing, doubled haploids are generated, each representing a unique recombinant product of the two parents. Due to the short genetic distance between QTL1 (from parent 1) and QTL2 (from parent 2), the likelihood of obtaining a recombinant is very low. Selection of doubled haploid (DH) lines based on polymorphism patterns allows identification of ideal partners for subsequent crosses in the breeding program. The resulting progeny show a Juxtaposed configuration in the region encompassing QTL1 and QTL2, substantially increasing the chances of obtaining a recombinant. Alternative doubled haploid lines are shown in grey; these facilitate the QTL1/QTL2 combination while retaining a genetic background predominantly from parent 1. A second round of doubled haploid production facilitates the development of a new elite line enriched with QTL1.
Meiotic crossover is crucial for plant breeding, enabling the development of novel haplotypes that combine beneficial alleles during the pre-breeding of elite crop varieties7,60. While practical crossover frequency can be globally increased through genetic modifications60,61, the benefits of this approach for breeding remain debated62,63. Moreover, such strategies typically require mutations in DNA repair genes, which often lead to chromosomal segregation defects, particularly in cereals64,65. An alternative strategy, widely considered optimal for breeding, involves directing crossover events to specific chromosomal regions7,8. However, no such method has yet been developed for plants. In this study, we demonstrate that a similar outcome can be achieved without genetic modifications by selecting parental lines that show specific heterozygosity patterns around target genomic regions (Fig. 6b). Because the effect manifests only when a large proportion of the chromosome is homozygous, this strategy is particularly complementary to doubled-haploid technology, which is widely used for the rapid production of inbred lines. We see the juxtaposition effect as especially useful when linkage prevents recombination between two closely linked quantitative trait loci (QTLs) in a single genotype (Fig. 6b)—selecting optimal doubled haploid lines for crossing can multiply the probability of obtaining the desired genotype. As the effect likely relies on crossover redistribution from adjacent homozygous regions (see conceptual models on Figs. 2h,i and 5g), it could potentially be also exploited to locally suppress recombination and preserve linkage between beneficial QTLs. An open question remains whether the juxtaposition effect can also be harnessed to unlock genetic variation in recombination-suppressed pericentromeric regions.
In maize, this approach increased local crossover frequency by up to an unprecedented threefold. We did not observe differences in Rf stimulation between two maize backgrounds differing with the polymorphism density, suggesting that the strength of the effect is not affected by the polymorphism density. Hence, we propose that this phenomenon should be integrated into the design of breeding strategies. In the era of high-throughput sequencing, where thousands of fully characterized lines are readily available, this discovery offers a powerful tool for breeders to accelerate the transfer of beneficial traits and enhance selection efficiency (Fig. 6b).
Methods
Generation of lines and assessment of Rf in Arabidopsis
The R2-BT line was achieved by crossing a recombinant from Col-BT × Ler-0 cross, which carried a dsRed marker expressed in seeds, with another recombinant carrying an eGFP reporter, both selected based on previously identified crossover breakpoints18. Double-reporter individuals homozygous for one transgene and hemizygous for the other (GR/-R or GR/G-) were selected using an epifluorescence stereomicroscope (Lumar ver. 12, Zeiss) and backcrossed to the Col background, resulting in the R2-BT line. The scheme illustrating the generation of the R2-BT line is presented in Supplementary Fig. 1.
Recombination within the R2-BT lines was assessed using a seed-based system as outlined in the published protocol16,18. Homozygous fluorescently tagged lines were crossed with the non-colour accessions, and images of F2 seeds derived from entire plants were captured using the epifluorescence stereomicroscope. Each image set included brightfield as well as ultraviolet images through red and green fluorescence filters. Seed counting was performed using the CellProfiler v2.1.1., following the established protocol66. Due to low recombination rates within the intervals, single-colour recombinant seeds were identified manually. To improve the accuracy of crossover measurements within these short intervals, all seeds from each plant were analysed, yielding an average sample size of ~3,900–10,000 seeds per biological replicate. The Rf in centimorgans (cM) was calculated using the following formula:
where g represents the number of green-only seeds, r the number of red-only seeds, and n the total number of seeds per plant. Raw seed scoring data for all measurements are presented in Supplementary Table 2.
Generation of phenotypic reporter lines for Rf assessment in maize
To develop maize NILs, the inbred line 2-9m, characterized by nine linked mutant alleles for phenotypic traits (Supplementary Table 4), was used as the male parent in crosses with two inbred lines, Ku123 and MK01. The multimarker line 2-9m was developed in the early 1980s in Moldova from a cross between two previously established lines carrying markers on chromosome 2 (ws3,lg1,gl2), and chromosomes 9 and 10 (c1,sh1,wx1,R1). These parental lines carried dominant alleles at the loci of interest, except for the recessive r1 allele31. After two rounds of self-pollination with phenotypic selection, four to six backcrosses were performed using either MK01 or Ku123 as the recurrent parent (Fig. 1i and Supplementary Table 12).
At each generation, phenotypic selection was based on grain or seedling traits, and only plants retaining the target marker pair in the genome (for example, c1–sh1 in Fig. 1i or lg1–gl2 in Extended Data Fig. 2) were advanced to the next backcrossing cycle. NILs were systematically labelled: ‘M’ or ‘Ku’ indicated the MK01 or Ku123 genetic background, respectively, and transferred mutant alleles were listed in parentheses.
This strategy generated 12 NILs, including the following: M(c1,sh1,R1), M(sh1,wx1), M(c1,sh1,wx1,R1), M(lg1,gl2), M(ws3,lg1), M(ws3,gl2), M(ws3,lg1,gl2), M(R1), Ku(c1,sh1,R1), Ku(sh1,wx1), Ku(lg1,gl2), Ku(R1). In addition, the recessive c1 and dominant R1 alleles were incorporated to enable anthocyanin pigmentation in kernels, simplifying the tracking of segregation at the c1 locus. Consequently, M(R1) and Ku(R1) lines were developed for hybridization with NILs instead of MK01 or Ku123 when analyses specifically targeting the c1 locus were required.
Assessment of Rf in maize NILs
The Rf in F1 hybrid plants of NILs, carrying intervals c1–sh1, sh1–wx1 and lg1–gl2, was assessed using backcross families derived from crossing these NIL F1 hybrids with their respective NIL parental lines. These backcross families were generated with the NIL F1 hybrids serving as either the female or male parent. The presence of the marker (mutant allele) was determined either by visual inspection of kernels on the ear—c1 (colourless aleurone layer), sh1 (shrunken endosperm) and wx1 (waxy endosperm)—or by observing phenotypes in seedlings grown from the plant’s seeds—ws3 (white leaf sheath and husk), lg1 (liguleless leaves) and gl2 (glossy leaves that retain water droplets).
The Rf between two markers in the backcross families was calculated using the following formula:
where b and c represent the number of recombinant progenies, and n represents the total progeny number in the family (n = a + b + c + d). a and d are the numbers of non-recombinant progenies. The error associated with the Rf was calculated using the following formula:
For the NILs M(ws3,lg1), M(lg1,gl2) and M(ws3,gl2), Rf was estimated using F2 populations and maximum likelihood estimation, which reduces estimation error when certain genotypic classes are missing or very small67,68. The Rf was calculated using the following formula:
where \(\theta\) is the product of non-recombination probabilities in male and female meiosis, determined from the following quadratic equation:
which can be solved as follows:
This estimate corresponds to the geometric mean of recombination in male and female meiosis, where
The error in this estimate was calculated as follows:
The average Rf for each genotype was obtained by averaging the Rf values of families (replicates) containing more than 50 individuals.
For the three-point crosses, the average Rf between male and female was calculated and plotted alongside the F2 data for the genotypes M(R1) × M(c1,sh1,wx1,R1), Ku(R1) × M(c1,sh1,wx1,R1), M × M(ws3,lg1,gl2) and Ku(R1) × M(ws3,lg1,gl2) (Fig. 3f,g). When more data were available for one sex, a random subset was selected from the larger group to match the sample size of the other, ensuring a balanced calculation of the average Rf.
High-molecular-weight DNA extraction and long-read sequencing
Four 10-day-old Ku123 seedlings, grown in the dark at 21 °C and 70% humidity, were collected and ground in liquid nitrogen. High-molecular-weight DNA was extracted using the Nucleobond HMW DNA kit (Macherey-Nagel, 740160.20) according to the manufacturer’s protocol. DNA quality was checked on a 0.6% agarose gel, and samples were outsourced for library preparation and sequencing using a PacBio HiFi Revio.
Ku123 genome assembly
The draft genome assembly of the maize line Ku123 was generated using hifiasm v0.19.8 with default parameters69 from PacBio HiFi long reads. Collinearity of the resulting contigs was assessed against the reference genome assemblies B73 v5 (ref. 33) and Mo17 (ref. 34) using D-Genies v1.5.0 (ref. 70), with ‘Minimap2 v2.28’ and ‘many repeats’ settings. N90 contigs were oriented and assembled into pseudomolecules with 100 ‘N’ gaps based on their collinearity with B73 v5 and Mo17 v2 genomes. The final de novo genome assembly, including pseudomolecules, was subsequently compared to the B73 v5 and Mo17 v2 reference genomes using SyRI v1.7.0 (ref. 71), after masking the Ku123 genome for repetitive sequences, using bedtools v2.30.0 with the parameters ‘maskfasta -mc N’72.
Assessment of DNA polymorphism by Illumina sequencing
For each genotype, a single plant was grown for 10 days in a controlled environment chamber set to 21 °C, under long-day conditions (16 h light/8 h dark), with 70% humidity and a light intensity of 150 μmol. In the case of certain maize NILs, the subsequent generation was used, as the generation originally assessed for Rf was not available. DNA extraction was performed following the protocol outlined by ref. 73, and DNA quality was verified on a 1% agarose gel. Library preparation was conducted as described by ref. 19. Briefly, tagmentation was carried out by mixing 1 µl of 5 ng µl−1 DNA with 1 µl of tagmentation buffer (40 mM Tris–HCl, pH 7.5; 40 mM MgCl2), 0.5 µl of DMF (Sigma, 68-12-2), 2.35 µl of nuclease-free water (Thermo Fisher, R0581) and 0.05 µl of pre-loaded, in-house-produced Tn5 transposase. The loading of Tn5 with annealed linker oligonucleotides was previously described. The tagmentation reaction was incubated at 55 °C for 2 min and terminated by adding 1 µl of 0.1% SDS, followed by a 10 min incubation at 65 °C.
Amplification of the tagmented DNA was performed using the KAPA2G Robust PCR kit (Sigma, 2GRKB) with custom P5 and P7 indexing primers, ensuring each sample was amplified with a unique set of primers, as described by ref. 73. Successfully amplified libraries were pooled and size-selected using 2% agarose gel electrophoresis. DNA fragments between 400 and 700 bp were excised and extracted using the Gel Extraction Kit (ZymoResearch, D4008). Paired-end sequencing was then carried out on a NovaSeq X Plus (Illumina). Sequencing quality was assessed using FastQC v0.12.1 with default parameters.
Reads were mapped to the Ku123v1 assembly using Bowtie2 v2.2.3 (ref. 74) with the parameter ‘—trim5 20’ to remove 20 low-quality nucleotides from the 5′ ends of each read. Duplicate reads were removed from the resulting BAM file using Samtools v1.3.1 ‘rmdup’. SNP calling was performed with Samtools v1.3.1 ‘mpileup -u -f’ and Bcftools v1.13 ‘-v -c’75, and SNP densities were calculated using VCFtools v0.1.17 (ref. 76). All plotting and data visualizations were done using R software v4.1.2 (ref. 77).
Centromere location determination
The positions of centromere-specific repeats, including CentC, CRM1, CRM2, CRM3 and CRM4, were identified using BLASTn v2.9.0 (ref. 78) based on the high-quality maize transposable element library, maizeTE02052020 from the Maize TE Consortium. The centromere borders were determined visually by analysing the density of these specific repeats per megabase along the chromosomes.
Annotation of repetitive elements and genes
Transposable elements and repetitive elements annotation was conducted using the EDTA v2.2.1 software79 and the curated library from the Maize TE Consortium (maizeTE02052020), with the parameter ‘–species Maize’ and classified according to ref. 80. Any ‘unknown’ entries in the transposable element categories were excluded from further analysis. Gene prediction was performed ab initio using Augustus v3.5.0 online software81 with the pre-existing Zea mays parameters. Any gene predictions overlapping with transposable elements were removed using bedtools v2.30.0 ‘intersect -v’72.
Annotation of nuclear mitochondrial DNA (NUMTs) and nuclear plastid DNA (NUPTs) integration
Organellar DNA sequence of chloroplasts and mitochondria from B73 v5 (ref. 71) were aligned on the Ku123 v1 genome assembly using minimap2 v2.28.-r1209 (ref. 82) with default parameters. For mitochondria and chloroplasts, alignments of at least 5 kb and 95% identity and alignments of at least 3 kb and 95% identity were kept, respectively, as described in ref. 83. Contiguous alignment results with a distance of less than 100 kb were merged to generate regions of integrated organellar genome (Supplementary Table 11).
Genomic position of the phenotypic markers
Gene sequences corresponding to the phenotypic markers were obtained from MaizeGDB32, and their positions on the Ku123 v1 assembly were identified using BLASTn v2.9.0 (ref. 78). As no gene model was available for the ws3 phenotypic marker, the two genetically closest genes, mlo9 (Zm00001eb065900) and pco061701 (Zm00001eb065940), located upstream and downstream of ws3 on the MaizeGDB composite genetic map, were used. The midpoint between these two genes was estimated as the approximate physical position of ws3. All positions are available in Supplementary Table 4.
Statistical analysis
Rf differences between genotypes in Arabidopsis and maize were analysed by first assessing normality and homoscedasticity using the Shapiro–Wilk test and F-test, respectively. For datasets meeting these assumptions, a two-tailed Welch’s t-test was used for mean comparison. If normality was not satisfied, the non-parametric Mann–Whitney U-test was applied. For multiple comparisons of Rf in Arabidopsis (Fig. 1d) and maize (Fig. 3f,g), Welch’s analysis of variance (ANOVA) followed by the Games–Howell post hoc test was used, as the assumption of homoscedasticity was not met for all groups. All statistical analyses were conducted with a significance threshold of α = 0.05, and detailed results are provided in Supplementary Tables 3, 15 and 17.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated for this study are included in the published version of the article or its Supplementary Information. The Ku123 genome assembly and the genotyping-by-sequencing data have been deposited in the National Center for Biotechnology Information (NCBI) Sequence Read Archive under the BioProject accession code PRJNA1186655. Raw genotyping-by-sequencing data for the wild-type Col × Ler F2 population were downloaded from ArrayExpress E-MTAB-816541. The Col-0 TAIR10 reference genome is downloaded from the TAIR database. The sequence polymorphism data for the Col/Ler cross used in this study were downloaded from 1001 Genomes. Rf raw data generated in this study are provided in Supplementary Tables 2, 5 and 14. The genome assembly of maize B73 v5 was downloaded from MaizeGDB. The maize Mo17v2 genome assembly was downloaded from the NCBI BioProject PRJNA751841. The genome assembly of maize A188 was downloaded from MaizeGDB. The genome assembly of the maize variety W22 was downloaded from the NCBI accession number GCA_001644905.2. The source data file is available via Zenodo at https://doi.org/10.5281/zenodo.15862594 (ref. 84). All unique materials generated in this study are available from the authors.
References
Barton, N. H. & Charlesworth, B. Why sex and recombination? Science 281, 1986–1990 (1998).
McDonald, M. J., Rice, D. P. & Desai, M. M. Sex speeds adaptation by altering the dynamics of molecular evolution. Nature 531, 233–236 (2016).
Goddard, M. R., Godfray, H. C. J. & Burt, A. Sex increases the efficacy of natural selection in experimental yeast populations. Nature 434, 636–640 (2005).
Villeneuve, A. M. & Hillers, K. J. Whence meiosis? Cell 106, 647–650 (2001).
Jones, G. H. & Franklin, F. C. H. Meiotic crossing-over: obligation and interference. Cell 126, 246–248 (2006).
Hunter, N. Meiotic recombination: the essence of heredity. Cold Spring Harb. Perspect. Biol. 7, a016618 (2015).
Taagen, E., Bogdanove, A. J. & Sorrells, M. E. Counting on crossovers: controlled recombination for plant breeding. Trends Plant Sci. 25, 455–465 (2020).
Reynolds, M. et al. Addressing research bottlenecks to crop productivity. Trends Plant Sci. 26, 607–630 (2021).
Lam, I. & Keeney, S. Mechanism and regulation of meiotic recombination initiation. Cold Spring Harb. Perspect. Biol. 7, a016634 (2015).
Baudat, F., Imai, Y. & de Massy, B. Meiotic recombination in mammals: localization and regulation. Nat. Rev. Genet. 14, 794–806 (2013).
Dluzewska, J., Szymanska, M. & Ziolkowski, P. A. Where to cross over? Defining crossover sites in plants. Front. Genet. 9, 609 (2018).
Zelkowski, M., Olson, M. A., Wang, M. & Pawlowski, W. W. P. Diversity and determinants of meiotic recombination landscapes. Trends Genet. 35, 359–370 (2019).
Borts, R. H. & Haber, J. E. Meiotic recombination in yeast: alteration by multiple heterozygosities. Science 237, 1459–1465 (1987).
Elliott, B. & Jasin, M. Repair of double-strand breaks by homologous recombination in mismatch repair-defective mammalian cells. Mol. Cell. Biol. 21, 2671–2682 (2001).
Cole, F., Keeney, S. & Jasin, M. Comprehensive, fine-scale dissection of homologous recombination outcomes at a hot spot in mouse meiosis. Mol. Cell 39, 700–710 (2010).
Ziolkowski, P. A. et al. Juxtaposition of heterozygous and homozygous regions causes reciprocal crossover remodelling via interference during Arabidopsis meiosis. Elife 4, e03708 (2015).
Blackwell, A. R. et al. MSH2 shapes the meiotic crossover landscape in relation to interhomolog polymorphism in Arabidopsis. EMBO J. 39, e104858 (2020).
Szymanska-Lejman, M. et al. The effect of DNA polymorphisms and natural variation on crossover hotspot activity in Arabidopsis hybrids. Nat. Commun. 14, 33 (2023).
Dluzewska, J. et al. MSH2 stimulates interfering and inhibits non-interfering crossovers in response to genetic polymorphism. Nat. Commun. 14, 6716 (2023).
The Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408, 796–815 (2000).
Kim, S. et al. Recombination and linkage disequilibrium in Arabidopsis thaliana. Nat. Genet. 39, 1151–1155 (2007).
Choi, K. et al. Arabidopsis meiotic crossover hot spots overlap with H2A.Z nucleosomes at gene promoters. Nat. Genet. 45, 1327–1336 (2013).
Brazier, T. & Glémin, S. Diversity and determinants of recombination landscapes in flowering plants. PLoS Genet. 18, e1010141 (2022).
Ziolkowski, P. A. & Henderson, I. R. Interconnections between meiotic recombination and sequence polymorphism in plant genomes. N. Phytol. 213, 1022–1029 (2017).
Yu, J. & Buckler, E. S. Genetic association mapping and genome organization of maize. Curr. Opin. Biotechnol. 17, 155–160 (2006).
Rodgers-Melnick, E. et al. Recombination in diverse maize is stable, predictable, and associated with genetic load. Proc. Natl Acad. Sci. USA 112, 3823–3828 (2015).
He, Y. et al. Genomic features shaping the landscape of meiotic double-strand break hotspots in maize. Proc. Natl Acad. Sci. USA 114, 12231–12236 (2017).
Sturtevant, A. H. The linear arrangement of six sex-linked factors in Drosophila, as shown by their mode of association. J. Exp. Zool. 14, 43–59 (1913).
Griffiths, A. J. F., Gelbart, W. M., Miller, J. H. & Lewontin, R. C. in Modern Genetic Analysis (ed. Griffiths, A. J. F.) 125–130 (W.H. Freeman and Company, 1999).
Melamed-Bessudo, C., Yehuda, E., Stuitje, A. R. & Levy, A. A. A new seed-based assay for meiotic recombination in Arabidopsis thaliana. Plant J. 43, 458–466 (2005).
Mikhailov, M. E. & Chernov, A. A. The effect of heterozygosity for marker loci on recombination fraction in adjacent chromosomal regions in maize. Russ. J. Genet. 40, 751–755 (2004).
Woodhouse, M. R. et al. A pan-genomic approach to genome databases using maize as a model system. BMC Plant Biol. 21, 385 (2021).
Hufford, M. B. et al. De novo assembly, annotation, and comparative analysis of 26 diverse maize genomes. Science 373, 655–662 (2021).
Chen, J. et al. A complete telomere-to-telomere assembly of the maize genome. Nat. Genet. 55, 1221–1231 (2023).
Swanson-Wagner, R. et al. Reshaping of the maize transcriptome by domestication. Proc. Natl Acad. Sci. USA 109, 11878–11883 (2012).
Sidhu, G. K. et al. Recombination patterns in maize reveal limits to crossover homeostasis. Proc. Natl Acad. Sci. USA 112, 15982–15987 (2015).
Bauer, E. et al. Intraspecific variation of recombination rate in maize. Genome Biol. 14, R103 (2013).
Kianian, P. M. A. et al. High-resolution crossover mapping reveals similarities and differences of male and female recombination in maize. Nat. Commun. 9, 2370 (2018).
Cooper, T. J. et al. Mismatch repair disturbs meiotic class I crossover control. Preprint at bioRxiv https://doi.org/10.1101/480418 (2021).
Cole, F. et al. Mouse tetrad analysis provides insights into recombination mechanisms and hotspot evolutionary dynamics. Nat. Genet. 46, 1072–1080 (2014).
Peterson, S. E., Scott, K. & Jasin, M. Mechanistic insight into crossing over during mouse meiosis. Mol. Cell 78, 1252–1263.e3 (2020).
Barrett, S. C. H. The evolution of plant sexual diversity. Nat. Rev. Genet. 3, 274–284 (2002).
Charlesworth, D. & Wright, S. I. Breeding systems and genome evolution. Curr. Opin. Genet. Dev. 11, 685–690 (2001).
Ziolkowski, P. A. Why do plants need the ZMM crossover pathway? A snapshot of meiotic recombination from the perspective of interhomolog polymorphism. Plant Reprod. 36, 43–54 (2022).
Henderson, I. R. & Bomblies, K. Evolution and plasticity of genome-wide meiotic recombination rates. Annu. Rev. Genet. 55, 23–43 (2021).
Lian, Q. et al. A pan-genome of 69 Arabidopsis thaliana accessions reveals a conserved genome structure throughout the global species range. Nat. Genet. 56, 982–991 (2024).
The International Wheat Genome Sequencing Consortium. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science 361, eaar7191 (2018).
Mayer, K. F. X. et al. A physical, genetic and functional sequence assembly of the barley genome. Nature 491, 711–716 (2012).
Jiao, Y. et al. Improved maize reference genome with single-molecule technologies. Nature 546, 524–527 (2017).
Dooner, H. K., Hsia, A.-P. & Schnable, P. S. in Handbook of Maize: Genetics and Genomics (eds Bennetzen, J. L. & Hake, S.) 377–403 (Springer, 2009); https://doi.org/10.1007/978-0-387-77863-1_19
Yao, H. et al. Molecular characterization of meiotic recombination across the 140-kb multigenic a1-sh2 interval of maize. Proc. Natl Acad. Sci. USA 99, 6157–6162 (2002).
Yao, H. & Schnable, P. S. Cis-effects on meiotic recombination across distinct a1-sh2 intervals in a common Zea genetic background. Genetics 170, 1929–1944 (2005).
Dooner, H. K. & He, L. Maize genome structure variation: interplay between retrotransposon polymorphisms and genic recombination. Plant Cell 20, 249–258 (2008).
Dooner, H. K. & Martínez-Férez, I. M. Recombination occurs uniformly within the bronze gene, a meiotic recombination hotspot in the maize genome. Plant Cell 9, 1633–1646 (1997).
Dooner, H. K. Extensive interallelic polymorphisms drive meiotic recombination into a crossover pathway. Plant Cell 14, 1173–1183 (2002).
Allard, R. W. Evidence for genetic restriction of recombination in the lima bean. Genetics 48, 1389–1395 (1963).
Moore, M. J., Bell, C. D., Soltis, P. S. & Soltis, D. E. Using plastid genome-scale data to resolve enigmatic relationships among basal angiosperms. Proc. Natl Acad. Sci. USA 104, 19363–19368 (2007).
Mercier, R., Mézard, C., Jenczewski, E., Macaisne, N. & Grelon, M. The molecular biology of meiosis in plants. Annu. Rev. Plant Biol. 66, 297–327 (2015).
Lian, Q. et al. The megabase-scale crossover landscape is largely independent of sequence divergence. Nat. Commun. 13, 3828 (2022).
Mieulet, D. et al. Unleashing meiotic crossovers in crops. Nat. Plants https://doi.org/10.1038/s41477-018-0311-x (2018).
Arrieta, M. et al. An induced mutation in HvRECQL4 increases the overall recombination and restores fertility in a barley HvMLH3 mutant background. Front. Plant Sci. 12, 706560 (2021).
Taagen, E., Jordan, K., Akhunov, E., Sorrells, M. E. & Jannink, J. L. If it ain’t broke, don’t fix it: evaluating the effect of increased recombination on response to selection for wheat breeding. G3: Genes, Genomes, Genet. 12, jkac291 (2022).
Epstein, R. et al. Exploring impact of recombination landscapes on breeding outcomes. Proc. Natl Acad. Sci. USA 120, e2205785119 (2023).
Desjardins, S. D. et al. FANCM promotes class I interfering crossovers and suppresses class II non-interfering crossovers in wheat meiosis. Nat. Commun. 3, 3644 (2022).
Bazile, J. et al. TaRECQ4 contributes to maintain both homologous and homoeologous recombination during wheat meiosis. Front. Plant Sci. 14, 1342976 (2023).
Kbiri, N., Dluzewska, J., Henderson, I. R. & Ziolkowski, P. A. Quantifying meiotic crossover recombination in Arabidopsis lines expressing fluorescent reporters in seeds using SeedScoring pipeline for CellProfiler. Methods Mol. Biol. 2484, 121–134 (2022).
Allard, R. W. The analysis of genetic-environmental interactions by means of diallel crosses. Genetics 41, 305–318 (1956).
Lynch, M. & Walsh, B. Genetics and Analysis of Quantitative Traits (Sinauer Associates, 1998).
Cheng, H. et al. Haplotype-resolved assembly of diploid genomes without parental data. Nat. Biotechnol. 40, 1332–1335 (2022).
Cabanettes, F. & Klopp, C. D-GENIES: dot plot large genomes in an interactive, efficient and simple way. PeerJ 6, e4958 (2018).
Goel, M., Sun, H., Jiao, W. B. & Schneeberger, K. SyRI: finding genomic rearrangements and local sequence differences from whole-genome assemblies. Genome Biol. 20, 277 (2019).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Rowan, B. A. et al. An ultra high-density Arabidopsis thaliana crossover map that refines the influences of structural variation and epigenetic features. Genetics 213, 771–787 (2019).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Danecek, P. et al. Twelve years of SAMtools and BCFtools. Gigascience 10, giab008 (2021).
Danecek, P. et al. The variant call format and VCFtools. Bioinformatics 27, 2156–2158 (2011).
R Core Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2021).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Ou, S. et al. Benchmarking transposable element annotation methods for creation of a streamlined, comprehensive pipeline. Genome Biol. 20, 275 (2019).
Wicker, T. et al. A unified classification system for eukaryotic transposable elements. Nat. Rev. Genet. 8, 973–982 (2007).
Keller, O., Kollmar, M., Stanke, M. & Waack, S. A novel hybrid gene prediction method employing protein multiple sequence alignments. Bioinformatics 27, 757–763 (2011).
Li, H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34, 3094–3100 (2018).
Lin, G. et al. Chromosome-level genome assembly of a regenerable maize inbred line A188. Genome Biol. 22, 175 (2021).
Mikhailov, M. E., Boideau, F., Szymanska-Lejman, M., Botnari, V. & Ziolkowski, P. Source data for “Enhancing local meiotic crossovers in Arabidopsis and maize through juxtaposition of heterozygous and homozygous regions” (Nature Plants). Zenodo https://doi.org/10.5281/zenodo.15862594 (2025).
Acknowledgements
This work was supported by the National Agency for Research and Development of the Republic of Moldova under State Program 20.80009.5107.03 (to V.B., Chief Investigator, and M.E.M., Chief Executive) and by the National Science Center, Poland (NCN), under grant 2020/39/I/NZ2/02464 to P.A.Z. The computations were performed at the Poznan Supercomputing and Networking Center (grant 312). Funding to pay the Open Access publication charges for this article was provided by Initiative of Excellence–Research University at Adam Mickiewicz University, Poznań, Poland. We express their gratitude to C. Underwood for his insightful comments on the manuscript and to J. Ziółkowska for creating the maize and chromosome illustrations. Special thanks are extended to P. Kopeć for his guidance on the Ku de novo genome assembly and to A. B. Korol for pioneering recombination research in Moldova.
Author information
Authors and Affiliations
Contributions
M.E.M. and P.A.Z. designed the research; M.E.M., M.S.-L. and F.B. performed the research; F.B. performed the computational analyses; V.B. coordinated the maize experiments; M.E.M., F.B., M.S.-L. and P.A.Z. analysed the data; and M.E.M., F.B. and P.A.Z. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Ruth Epstein and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Generation of the R2-BT line.
a, Schematic illustrating the creation of R2-BT through single recombinant crosses between Col-BT/Ler (R) carrying only one reporter gene. Successive backcrosses aim to clean up the Ler background. b, The genetic structure of the BT interval for recombinants #112 (RRG-/G-, left) and #130 (R−R/−R, right) used to create R2-BT, determined by targeted sequencing of the BT region. The plots show the percentage of Col alleles (blue) and Ler alleles (red) across the entire BT region. The dashed rectangles indicate the location of Col/Ler crossover breakpoints. c, The genetic structure of R2-BT determined through whole-genome sequencing based on short reads. Polymorphisms indicating the presence of Ler-derived fragments are marked in gray. The BT region on chromosome 3 is indicated by a red line.
Extended Data Fig. 2 Generation of maize NILs.
A detailed scheme illustrating the development of the M(lg1,gl2)6 NIL through successive backcrosses and phenotypic marker selection. ⊗ represents self-pollination. ‘+’ or unlabeled loci indicate homozygosity for the dominant allele, ‘±’ indicates heterozygosity, and ‘-’ indicates homozygosity for the recessive allele. ‘+/±/−’ indicates families with individuals of mixed genotypes. In any MK01 × M(lg±,gl±)n families, individuals with genotypes lg+gl+, lg+gl±, lg±gl+, and lg±gl± were identified by analyzing the self-pollinated progeny.
Extended Data Fig. 3 Gene and transposon density in the Ku123 genome regions containing the ws3-lg1-gl2 and c1-sh1-wx1 intervals.
a, Gene density within the ws3-lg1-gl2 interval, plotted in 250 kb windows. b, Transposable element (TE) density within the same interval, plotted in 500 kb windows. c, TE density excluding the most common LTRs, Ty1 (Copia) and Ty3 (Gypsy). d-f, Similar plots as in a-c, but for the c1-sh1-wx1 interval. Colored vertical lines represent the position of the markers used to measure recombination frequency (Rf).
Extended Data Fig. 4 Gene density across the chromosomes of the Ku123 genome assembly.
The graphs depict gene density for each chromosome, calculated in 0.5 Mb windows. Light-purple rectangles highlight the positions of heterochromatic knobs. Colored vertical lines represent the position of the markers used to measure Rf.
Extended Data Fig. 5 Repeat sequences in the Ku123 genome.
a, Proportions of different Class I and Class II transposable elements in the Ku123 genome assembly, calculated based on genomic sequence coverage. b, Proportions of other repeat types (non-transposable elements) in the Ku123 genome assembly, also calculated based on genomic sequence coverage. c, Distribution of various repeat types across the 10 chromosomes of Ku123 genome assembly. Colored vertical lines represent the position of the markers used to measure Rf.
Extended Data Fig. 6 Distribution of the centromeric specific repeats along the chromosomes of the Ku123 genome.
For each chromosome, the density per Mb for the centromeric repeats CentC, CRM1, CRM2, CRM3 and CRM4 are represented by the curves colored in light red, light brown, green, blue and purple, respectively. The insets show close-ups of individual centromeric regions. Colored vertical lines represent the position of the markers used to measure Rf.
Extended Data Fig. 7 Synteny plots showing the alignment of the Ku123 de novo genome assembly with the B73 and M017 reference genomes for every chromosome.
Extensive duplication in Mo17 on chromosome 6 corresponds to an expansion of 45S rDNA arrays. Ku123-specific large insertions on chromosomes 1, 3, 5, 6, 7 and specific to M017 on chromosome 8 correspond to knob insertions (see Extended Data Fig. 4). The position of the markers used for recombination frequency (c1, sh1 and wx1 on chromosome 9 and ws3, lg1 and gl2 on chromosome 2) are indicated by black tick marks on the Ku123 respective chromosomes.
Extended Data Fig. 8 Genome-wide SNP density of the 12 maize lines used in this study.
SNP density is represented by gray histograms of 10 kb windows along the 10 chromosomes of each maize line. Arrowheads in blue and green present below the SNP plots indicate the locations of Rf measurement intervals on chromosomes 2 and 9, respectively. The position of the marker R1 is indicated by a pink arrowhead under the SNP plots on chromosome 10. Orange arrows highlight regions with a reduced density of polymorphisms.
Extended Data Fig. 9 The polymorphism pattern-dependent redistribution of crossovers encompasses only recombinationally active regions.
a, The interhomolog polymorphism in the ‘Juxtaposed’ M(sh1,wx1) × M cross, located to the right of the sh1-wx1 interval, does not affect the recombination frequency within this interval because it encompasses a recombinationally inactive chromosomal region. The gray area shows the distribution of SNPs per Mb, while blue and red lines indicate the crossover frequency (cM/Mb) along the short chromosome arm for Flint × Flint and Dent × Dent crosses, respectively, as reported by Bauer et al.37 Solid, dashed, and dotted lines represent populations with the highest, median, and lowest genome-wide recombination rates among 23 populations within each group37. The vertical lines represent the borders of the sh1-wx1 interval used for measuring Rf. b, The distribution of interhomolog SNPs in the M(sh1,wx1)7 × M cross is shown for the first 35 Mb of chromosome 2. The gray plot shows SNP density per 10 kb and horizontal bars show the estimated distribution of heterozygous regions based on SNPs. The red and blue curves represent the distribution of crossovers as in a. Colored vertical lines indicate the positions of the sh1-wx1 interval used to measure Rf. c, As in a, combining the distribution of crossovers (in cM/Mb, dark blue curves) for 23 different population from Bauer et al.36.
Extended Data Fig. 10 Short homozygous blocks interrupting the heterozygous region suppress local crossover stimulation.
a, Interhomolog SNP distribution in the M(ws3,lg1) × Ku(R1) cross along chromosome 2. Gray vertical bars represent SNP density per 10 kb, with the burgundy trend line showing SNP density per 1 Mb. Gray horizontal bars beneath the graph indicate estimated heterozygous regions based on SNPs. Colored lines mark the positions of the ws3-lg1 interval used to measure Rf. The navy blue square on the x-axis shows the centromere location. The inset offers a close-up of the interval, with the SNP density plots below partially trimmed. b, As in (a), but for the M(lg1,gl2) × Ku(R1) cross.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2.
Supplementary Tables
Supplementary Tables 1–18.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mikhailov, M.E., Boideau, F., Szymanska-Lejman, M. et al. Enhancing local meiotic crossovers in Arabidopsis and maize through juxtaposition of heterozygous and homozygous regions. Nat. Plants 11, 1769–1784 (2025). https://doi.org/10.1038/s41477-025-02085-8
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41477-025-02085-8
This article is cited by
-
Diversity favoured: heterozygosity attracts crossovers
Nature Plants (2025)