Abstract
In many flowering plants, male and female reproductive organs mature at different times to avoid self-pollination, a phenomenon termed dichogamy. Most dichogamous species are either protandrous or protogynous, making this strategy difficult to study genetically. However, in the ginger Alpinia mutica, protandrous and protogynous floral morphs co-occur within populations, and the synchronized rhythmic movement of styles and dehiscence of stamens promotes cross-pollination between morphs. Here we demonstrate that a single Mendelian locus with a dominant allele governing protogyny controls sexual polymorphism. We used haplotype-resolved genomes and population genomics to identify the dichogamy-determining region, revealing a large deletion in the protandrous morphotype. We found that the key gene SMPED1, located adjacent to the deletion, governs the timing of anther dehiscence and style movement. SMPED1 is widespread among angiosperms and probably has conserved function. Our findings represent a new genetic characterization of a key mating system gene controlling the synchrony of sex organs in flowering plants.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive at the National Genomics Data Center, China National Center for Bioinformation, Beijing Institute of Genomics, Chinese Academy of Sciences and are publicly accessible at https://ngdc.cncb.ac.cn/gsa. Among them, the HiFi reads, Hi-C reads and full-length transcriptome reads can be found under accession number CRA020599 (reviewer link: https://ngdc.cncb.ac.cn/gsa/s/Su7E6mDl), and the resequencing data are under accession number CRA020857 (reviewer link: https://ngdc.cncb.ac.cn/gsa/s/Z3T7vDQ6). Transcriptome reads of different time points can be shared on request from the corresponding author J.-L.Z. because these data are under further explored. Source data are provided with this paper.
Code availability
All analysis tools used in this study are publicly available, as described in Methods and Reporting Summary.
References
Harder, L. D. & Barrett, S. C. H. Ecology and Evolution of Flowers (Oxford Univ. Press, 2006).
Darwin, C. The Different Forms of Flowers on Plants of the Same Species (John Murray, 1877).
Barrett, S. C. H. The evolution of plant sexual diversity. Nat. Rev. Genet. 3, 274–284 (2002).
Charlesworth, D. & Charlesworth, B. Inbreeding depression and its evolutionary consequences. Annu. Rev. Ecol. Evol. Syst. 18, 237–268 (1987).
Morran, L. T., Parmenter, M. D. & Phillips, P. C. Mutation load and rapid adaptation favour outcrossing over self-fertilization. Nature 462, 350–352 (2009).
Bertin, R. I. & Newman, C. M. Dichogamy in angiosperms. Bot. Rev. 59, 112–152 (1993).
Lloyd, D. G. & Webb, C. J. The avoidance of interference between the presentation of pollen and stigmas in angiosperms I. Dichogamy. N. Z. J. Bot. 24, 135–162 (1986).
Bertin, R. I. Incidence of monoecy and dichogamy in relation to self-fertilization in angiosperms. Am. J. Bot. 80, 557–560 (1993).
Darwin, C. On the Various Contrivances by Which British and Foreign Orchids Are Fertilized by Insects, and on the Good Effects of Intercrossing (John Murray, 1862).
Darwin, C. The Effects of Cross- and Self-Fertilization in the Vegetable Kingdom (Murray, 1876).
Renner, S. S. & Muller, N. A. Plant sex chromosomes defy evolutionary models of expanding recombination suppression and genetic degeneration. Nat. Plants 392, 392–402 (2021).
Boualem, A. et al. A conserved mutation in an ethylene biosynthesis enzyme leads to andromonoecy in melons. Science 321, 836–838 (2008).
Huu, C. N. et al. Presence versus absence of CYP734A50 underlies the style-length dimorphism in primroses. eLife 5, e17956 (2016).
Xue, H., Gong, Y., Wright, S. I. & Barrett, S. C. H. The genomic basis of the tristylous floral polymorphism—evidence for a role of gene duplications in a region of restricted recombination. Mol. Biol. Evol. 42, msaf170 (2025).
Renner, S. S. How common is heterodichogamy?. Trends Ecol. Evol. 16, 595–597 (2001).
Gleeson, S. K. Heterodichogamy in walnuts: inheritance and stable ratios. Evolution 36, 892–902 (1982).
Groh, J. S. et al. Ancient structural variants control sex-specific flowering time morphs in walnuts and hickories. Science 387, eado5578 (2025).
Li, Q.-J. et al. Flexible style that encourages outcrossing. Nature 410, 432 (2001).
Li, Q.-J. et al. Study on the flexistyly pollination mechanism in Alpinia plants (Zingiberaceae). Acta Bot. Sin. 43, 364–369 (2001).
Cui, X., Wei, R. & Huang, R. A preliminary study on the genetic system of Amomum tsao-ko. J. Yunnan Univ. 17, 290–297 (1995).
Li, Q.-J. et al. Mating system and stigmatic behaviour during flowering of Alpinia kwangsiensis (Zingiberaceae). Plant Syst. Evol. 232, 123–132 (2002).
Liu, M., Sun, S. & Li, Q. The relation between stigma position and receptivity in two flexistylous gingers. Biodivers. Sci. 15, 639–644 (2007).
Chen, S. et al. Genetic and genomic insights into dichogamy in Zingiberaceae. Plant Commun. 6, 101352 (2025).
Thompson, T. E. & Romberg, L. D. Inheritance of heterodichogamy in pecan. J. Hered. 76, 456–458 (1985).
Huang, J. et al. Stigma receptors control intraspecies and interspecies barriers in Brassicaceae. Nature 614, 303–308 (2023).
Zhang, L. et al. FERONIA receptor kinase-regulated reactive oxygen species mediate self-incompatibility in Brassica rapa. Curr. Biol. 31, 3004–3016 (2021).
Sass, C., Iles, W. J. D., Barrett, C. F., Smith, S. Y. & Specht, C. D. Revisiting the Zingiberales: using multiplexed exon capture to resolve ancient and recent phylogenetic splits in a charismatic plant lineage. PeerJ 4, e1584 (2016).
Carlsen, M. M. et al. Resolving the rapid plant radiation of early diverging lineages in the tropical Zingiberales: pushing the limits of genomic data. Mol. Phylogenet. Evol. 128, 55–68 (2018).
Cheng, S. P. et al. Haplotype-resolved genome assembly and allele-specific gene expression in cultivated ginger. Hortic. Res. 8, 188 (2021).
D’Hont, A. et al. The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 488, 213–217 (2012).
Li, H.-T. et al. Origin of angiosperms and the puzzle of the Jurassic gap. Nat. Plants 5, 461–470 (2019).
Willemstein, S. C. An Evolutionary Basis for Pollination Ecology Leiden Botanical Series 10 (Brill, 1987).
Routley, M. B., Bertin, R. I. & Husband, B. C. Correlated evolution of dichogamy and self-incompatibility: a phylogenetic perspective. Int. J. Plant Sci. 165, 983–993 (2004).
Charlesworth, B. & Charlesworth, D. A model for the evolution of dioecy and gynodioecy. Am. Nat. 112, 975–997 (1978).
Harkess, A. et al. The asparagus genome sheds light on the origin and evolution of a young Y chromosome. Nat. Commun. 8, 1279 (2017).
Kappel, C., Huu, C. N. & Lenhard, M. A short story gets longer: recent insights into the molecular basis of heterostyly. J. Exp. Bot. 68, 5719–5730 (2017).
Haldane, J. B. S. A mathematical theory of natural and artificial selection. Math. Proc. Camb. 23, 363–372 (2008).
Orr, H. A. & Betancourt, A. J. Haldane’s sieve and adaptation from the standing genetic variation. Genetics 157, 875–884 (2001).
Maydeu-Olivares, A. & García-Forero, C. in International Encyclopedia of Education 3rd edn (eds Peterson, P. et al.) 190–196 (Elsevier, 2010).
Tarailo-Graovac, M. & Chen, N. Using RepeatMasker to identify repetitive elements in genomic sequences. Curr. Protoc. Bioinform. 25, 4.10.11–14.10.14 (2009).
Jurka, J. et al. Repbase update, a database of eukaryotic repetitive elements. Cytogenet. Genome Res. 110, 462–467 (2005).
Ou, S. et al. Benchmarking transposable element annotation methods for creation of a streamlined, comprehensive pipeline. Genome Biol. 20, 275 (2019).
Brůna, T., Hoff, K. J., Lomsadze, A., Stanke, M. & Borodovsky, M. BRAKER2: automatic eukaryotic genome annotation with GeneMark-EP+ and AUGUSTUS supported by a protein database. NAR Genom. Bioinform. 3, lqaa108 (2021).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37, 907–915 (2019).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Li, H. L. et al. Haplotype-resolved genome of diploid ginger (Zingiber officinale) and its unique gingerol biosynthetic pathway. Hortic. Res. 8, 189 (2021).
Gabriel, L., Hoff, K. J., Bruna, T., Borodovsky, M. & Stanke, M. TSEBRA: transcript selector for BRAKER. BMC Bioinform. 22, 566 (2021).
Aramaki, T. et al. KofamKOALA: KEGG ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics 36, 2251–2252 (2020).
Zdobnov, E. M. & Apweiler, R. InterProScan—an integration platform for the signature-recognition methods in InterPro. Bioinformatics 17, 847–848 (2001).
The Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408, 796–815 (2000).
Shang, L. et al. A complete assembly of the rice Nipponbare reference genome. Mol. Plant 16, 1232–1236 (2023).
Wang, Z. et al. A chromosome-level reference genome of Ensete glaucum gives insight into diversity and chromosomal and repetitive sequence evolution in the Musaceae. GigaScience 11, giac027 (2022).
Droc, G. et al. The banana genome hub: a community database for genomics in the Musaceae. Hortic. Res. 9, uhac221 (2022).
Martin, G. et al. Improvement of the banana “Musa acuminata” reference sequence using NGS data and semi-automated bioinformatics methods. BMC Genom. 17, 243 (2016).
Wang, Z. et al. Musa balbisiana genome reveals subgenome evolution and functional divergence. Nat. Plants 5, 810–821 (2019).
Belser, C. et al. Chromosome-scale assemblies of plant genomes using nanopore long reads and optical maps. Nat. Plants 4, 879–887 (2018).
Li, Z. et al. The Musa troglodytarum L. genome provides insights into the mechanism of non-climacteric behaviour and enrichment of carotenoids. BMC Biol. 20, 186 (2022).
Emms, D. M. & Kelly, S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 20, 238 (2019).
Yang, Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 24, 1586–1591 (2007).
Li, L., Stoeckert, C. J. Jr. & Roos, D. S. OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res. 13, 2178–2189 (2003).
Ronquist, F. et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 (2012).
Camacho, C. et al. BLAST+: architecture and applications. BMC Bioinform. 10, 421 (2009).
Wang, Y. et al. MCScanX: a toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 40, e49 (2012).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Zhang, Z. et al. KaKs_Calculator: calculating Ka and Ks through model selection and model averaging. Genom. Proteom. Bioinform. 4, 259–263 (2006).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Vasimuddin, M., Misra, S., Li, H. & Aluru, S. Efficient architecture-aware acceleration of BWA-MEM for multicore systems. In 2019 IEEE International Parallel and Distributed Processing Symposium (IPDPS) 314–324 (IEEE, 2019).
McKenna, A. et al. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164 (2010).
Zhou, X. & Stephens, M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 44, 821–824 (2012).
Danecek, P. et al. The variant call format and VCFtools. Bioinformatics 27, 2156–2158 (2011).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408 (2001).
Javelle, M. & Timmermans, M. C. P. In situ localization of small RNAs in plants by using LNA probes. Nat. Protoc. 7, 533–541 (2012).
Kramer, E. M. Methods for studying the evolution of plant reproductive structures: comparative gene expression techniques. Methods Enzymol. 395, 617–636 (2005).
Zhang, X., Henriques, R., Lin, S.-S., Niu, Q.-W. & Chua, N.-H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 1, 641–646 (2006).
Peterson, R., Slovin, J. P. & Chen, C. A simplified method for differential staining of aborted and non-aborted pollen grains. Int. J. Plant Biol. 1, e13 (2010).
Acknowledgements
Q.-J.L. was the principal investigator of this project but passed away on 1 December 2022. This paper is in memory of the important contributions that he made to our understanding of the biology of gingers and the evolution of reproductive strategies in plants. This research was supported by a Joint Project between Yunnan Provincial Science and Technology Department and the ‘Double First-Class’ University Project of Yunnan University (grant no. 2019FY003001 to Q.-J.L.), the Ministry of Science and Technology of the PRC, the State Key Research Plan (grant no. 2019YFC1711100 to W.C.), the National Natural Science Foundation of China (grant no. U1602263 to Q.-J.L.; grant no. 41871047 to J.-L.Z.), a ‘Young Talent Project’ of Yunnan (grant no. YNWR-QNBJ-2019-214 to J.-L.Z.; grant no. C619300A101 to J.-J.H.) and the Postgraduate Research and Innovation Foundation of Yunnan University (grant no. 2021Z021 to A.-D.H.). We thank Y.-M. Xia, F.-C. Wu, H.-Z. Lu, H.-P. Xi, J. Gao, L.-J. Jiang and others from Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, for their kind help in collecting samples. We appreciate Q.-H. Duan and L. Yang from the College of Horticulture Science and Engineering, Shandong Agricultural University, Tai’an, China, for their help in the experiments on silencing by AS-ODNs. We thank D. Charlesworth, M. Lenhard and S. Wright for valuable discussions on hemizygosity.
Author information
Authors and Affiliations
Contributions
J.-L.Z., Y.D., J.-J.H., S.C.H.B., W.C. and Q.-J.L. designed, conceived and supervised the research. A.-D.H., S.-C.D., X.-C.P., J.-L.Z., Q.-J.L., H.L., J.-H.C., Y.D., Y.-L.W., W.-J.W. and Q.-Y.L. collected the samples. J.-L.Z., Q.-J.L. and Y.-L.L. conducted the Mendelian inheritance experiments and the corresponding data analysis. S.-C.D., W.C., Y.D., X.-C.P. and J.-L.Z. performed the genome assembly, annotation, GWAS and evolutionary analysis. X.-C.P. and W.C. performed the SMPED1 protein prediction. A.-D.H., J.-J.H., X.-C.P., X.-M.Z., P.-W.L., X.X. and J.-H.C. validated SMPED1 and conducted the RT-qPCR experiments in transgenic plants. A.-D.H. and H.L. conducted the RNA in situ hybridizations. A.-D.H., X.-C.P., Y.D., J.-J.H. and J.-L.Z. conducted the experiments using AS-ODNs. J.-L.Z., A.-D.H., S.-C.D. and W.-J.W. analysed the expression data. The paper was drafted by J.-L.Z., Y.D., S.C.H.B., W.C., Q.-J.L., J.-J.H., W.J.K. and B.L. All authors contributed to the review of the paper before submission for publication and approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Takashi Akagi, Roberta Bergero and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Style and anther behavior of Alpinia mutica flowers under natural light conditions and continuous illumination.
Progression of daily flower maturation of the PG and PA morphs under natural light conditions (top) and continuous illumination (bottom). The small circles show close-up images of the stigmas (left circles) and anthers (right circles). Images at the time of anther dehiscence and style rotation are highlighted by blue borders. The scale bar is 1 cm.
Extended Data Fig. 2 Genome assembly and annotation of the PA and PG morphs in the Alpinia mutica genome.
a, Genome survey of the PA morph based on k-mer distribution of Illumina data. The k-mer distributions were constructed on the basis of 31-mers. b, Assembly and annotation pipeline of all sequencing data into the four haplotypes of the PA and PG morphs. Red-dashed square indicates the procedure of gene annotation. c, Hi-C contact map showing the 24 pseudochromosomes for the PA morph haplotype 2 (H2) genome using Hi-C reads. Numbers along diagonal line are chromosome numbers. d, Gene synteny among the four haplotypes of PA and PG morphs.
Extended Data Fig. 3 Comparison of genetic diversity (π) between the two dichogamous morphs in and around the DDR.
a, Genetic diversity (π) of PA and PG morphs across the DDR and adjacent region. Box plot in the upper right corner indicates the general π of PG is significantly lower than the π of PA with ****P < 0.0001 estimated by two-tailed Mann-Whitney U test. Number of samples are indicated on bar. The central lines within the box plots represent the medians, the box represents the interquartile range. The data are presented as the mean ± SEM and the whiskers extend to minima and maxima. b, Difference of π between PA Vs. PG. The gray vertical line indicates the position of SMPED1.
Extended Data Fig. 4 Relative expression level of AmSMPED1 and allele-specific expression analysis.
a, Relative expression level of AmSMPED1 in anthers (left) and styles (right) under natural conditions and continuous illumination (with light exposure after sunset), as determined by RT-qPCR data. The transition point of style reciprocal movement is indicated by the directional arrowheads (see temporal details in Extended Data Fig. 1). The data are presented as the mean ± SEM and the whiskers extend to minima and maxima. Biological replicates (n) of each time point are indicated. b, A synonymous mutation (T Vs. C) is the only difference between recessive allele d and dominant allele D in the coding region. RNA-seq reads are mapped to different alleles to explore the pattern of allele-specific expression of the recessive allele d and dominant allele D. Because expressions of the two alleles are extracted from the same RNA sequences (RNA-seq), the amount of gene expression can be calculated by the number of reads (counts) mapped to the reference allele. c, Allele-specific expressions of recessive allele d and dominant allele D at different times in the PA (recessive homozygosity d/d) and PG (dominant heterozygosity d/D) morphs under different illumination. Allele-specific expression of PA and PG are indicated by different colors. The recessive d and dominant allele D are represented by different dashed lines. Solid lines are the expression of recessive allele d plus dominant allele D.
Extended Data Fig. 5 Expression of other genes related to the DDR.
a, AmCOR27 expression levels in anthers and styles over the course of one day. The left/right panels are from RT-qPCR/ RNA-seq for anthers and styles, respectively. AmCOR27 was not expressed in the PA morph. b, Expression levels of other genes in the DDR. The gray shading indicates the night before sunrise at the location where the plants were cultivated. The data are presented as the mean ± SEM and the whiskers extend to minima and maxima. Biological replicates (n) of each time point are indicated.
Extended Data Fig. 6 Sequence of SMPED, overexpression/knockdown of SMPEDs in Arabidopsis thaliana and validation of promoters.
a, Sequence of AmSMPED1 and its two homologous proteins in Arabidopsis thaliana (At5g67020 and At3g50340). b, Confirmation of overexpression of AmSMPED1 (35S:AmSMPED1), AtSMPED1 (35S:AtSMPED1), and AtSMPED2 (35S:AtSMPED2), as well as simultaneous knockdown of AtSMPED1 and AtSMPED2 (ami-AtSMPED1/2) in A. thaliana. Full-length amplification of AmSMPED1 was then performed, and the results showed that, under identical starting RNA concentrations, the amplified bands from overexpression lines were notably brighter compared to those from wild-type and positive control plants, indicating successful overexpression of AmSMPED1 in the transgenic lines. Overexpression of AmSMPED1 (35S:AmSMPED1) was confirmed by RT-PCR. Total RNA from wild-type plants, positive controls, and transgenic lines was quantified and normalized to the same initial concentration. The data are presented as the mean ± SEM and the whiskers extend to minima and maxima. Biological replicates (n) of each treatment are indicated. The blank space on the bar indicates contracted bar due to large values. The asterisks denote significant differences between samples using two-sided Mann–Whitney U-test. ***, P < 0.0001. c, Validation of promoters in the DDR. The upper diagram is the location of promoters in the DDR. “ATG” is the initiation codon of AmSMPED1. The lower is a diagram for validation. LUC is luciferase and REN is Renilla luciferase. The results of validation are indicated in Fig. 3b.
Extended Data Fig. 7 In situ hybridization of AmSMPED1 in cross sections of anthers and styles of Alpinia mutica.
The precise localization of AmSMPED1 in the anthers and styles was detected via digoxigenin (DIG)-labeled locked nucleic acid (LNA) oligonucleotide probes. Positive in situ hybridization results are characterized by deep purple signals. The miRCURY LNA miRNA detection probe was used as a negative control. Enlarged photographs are in red-dashed square at the bottom to illustrate detail of in situ hybridization. Red arrowheads indicated localization of AmSMPED1 in the anther and style. For each time point, samples were collected three times with two samples each for a total of six biological replicates at each time point for both anthers and styles. All replicates showed similar results. Each scale bar for anthers is 1 mm and for styles is 100 µm.
Extended Data Fig. 8 Growing status of wild-type (WT) and transgenic lines of Arabidopsis thaliana.
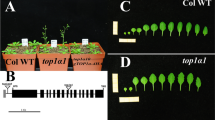
a, Representative photographs of wild-type (WT) Arabidopsis thaliana plants and lines overexpressing or knocked down for AtSMPED1 and/or AtSMPED2 or overexpressing AmSMPED1. WT is the A. thaliana Col-0 accession, 35S:AtSMPED1/2 and 35S:AmSMPED1 are the transgenic A. thaliana plants that overexpress AtSMPED1/2 or AmSMPED1, respectively, and ami-AtSMPED1/2 is a transgenic line in which AtSMPED1 and AtSMPED2 expression levels are knocked down by artificial microRNAs. The scale bar is 1 cm. b, Leaf number on the twenty-first day and bolting rates on the twenty-eighth day. There was no significant difference between WT and transgenic plants in their vegetative traits. The data are presented as the mean ± SEM and the whiskers extend to minima and maxima. Biological replicates (n) are indicated.
Extended Data Fig. 9 Whole-genome duplication events and the phylogenetic tree of dichogamy-determining regions in ginger and related families.
a, Phylogenetic tree of Musaceae and Zingiberaceae species with Oryza sativa and A. thaliana as outgroups. The blue boxes show the divergence time frames. b, Whole-genome duplication and divergence events revealed by Ks distribution. c, Unrooted phylogenetic tree of DDRs in Musaceae and Zingiberaceae species revealing three distinctive types of DDRs.
Extended Data Fig. 10 Numbers of homologous genes in the dichogamy-determining regions (DDR) and their distributions in angiosperms.
Numbers on the outer ring represent the numbers of homologous genes in the DDR indicated in Fig. 2b. Gene symbols (COR27/28, SMPED1, EREBP-like, Unknown, Monothiol glutaredoxin, DNAJB12, Asn/Gln amidotransferase) are represented by lowercases a-h (legend on the upper left). Darker color indicates more numbers of genes (legend on the lower left). The different color on the middle ring represents different plant groups (legend on the upper right). Dots on the node represent bootstrap values and the darker color higher bootstrap values (legend on the lower right). The bolds with underline above the legends indicate where the legend are.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2, Tables 1–9 and Methods.
Supplementary Video 1
Style movement of the PA morph in a day, related to Fig. 1.
Supplementary Video 2
Style movement of the PG morph in a day, related to Fig. 1.
Source data
Source Data Extended Data Fig. 6
Unprocessed gels for Extended Data Fig. 6b.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, JL., Dong, Y., Huang, AD. et al. Ginger genome reveals the SMPED1 gene causing sex-phase synchrony and outcrossing in a flowering plant. Nat. Plants (2025). https://doi.org/10.1038/s41477-025-02125-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41477-025-02125-3