Abstract
Wild rodent’s gut microbiota serves as a crucial reservoir of antibiotic resistance genes (ARGs), where antimicrobial-resistant bacteria interact with mobile genetic elements (MGEs) to facilitate horizontal gene transfer. This study analyzed 12,255 gut-derived bacterial genomes from wild rodents to characterize the distribution of ARGs and virulence factor genes (VFGs), and to identify their bacterial hosts. A total of 8119 ARGs and 7626 VFGs were identified. The most prevalent ARGs conferred resistance to elfamycin, followed by those associated with multi-class antibiotic resistance. Enterobacteriaceae, particularly Escherichia coli, harbored the highest numbers of ARGs and VFGs. A strong correlation between the presence of MGEs, ARGs, and VFGs was observed, highlighting the potential for co-selection and mobilization of resistance and virulence traits. These findings underscore the importance of expanded surveillance to monitor and mitigate the risk of transmission of resistant and potentially pathogenic bacteria from wild rodents to human and animal populations.
Similar content being viewed by others
Introduction
Antimicrobial resistance (AMR) poses a significant and escalating threat to public health globally1. This phenomenon is largely attributed to the acquisition of antibiotic resistance genes (ARGs), which can spread rapidly among bacterial populations through horizontal gene transfer mechanisms, such as bacteriophages, plasmids, and transposable genetic elements2,3. The pervasive nature of ARGs has been observed across diverse microbial communities, including those found in humans, animals, and environmental settings4,5.
The increasing prevalence of antibiotic-resistant bacteria (ARB) is closely linked to the medical use of antibiotics, which has led to a surge of resistance in bacteria associated with both human health and livestock6. Understanding the dynamics of ARB dissemination is critical from a One Health perspective, which emphasizes the interconnectedness of human, animal, and environmental health. Identifying the factors that contribute to the spread of ARB across these domains is paramount for developing effective strategies to mitigate AMR7,8.
In addition to ARGs, virulence factor genes (VFGs) play a crucial role in the colonization and pathogenicity of microorganisms within host environments9. Bacterial toxins and other virulence determinants can significantly impact the development of infectious diseases, causing tissue damage and triggering local and systemic inflammatory responses10. Although the evolution of antibiotic resistance and bacterial virulence occurs on different timescales, there is a recognized interplay-termed coselection-between ARGs and VFGs under selective pressure11. Recent studies have demonstrated that ARGs can enable pathogens to withstand treatment12,13,14, underscoring the importance of comprehensively understanding the relationship between microbial virulence and antibiotic resistance.
Animal manure has been identified as a significant reservoir for ARGs and VFGs, contributing to their rapid contamination of natural ecosystems and posing a serious threat to human health15,16,17. While manure is a known environmental source of these genes, wildlife-particularly rodents-also represent an important, but underexplored, reservoir. Due to their proximity to human settlements, wild rodents play a critical role in the dissemination of ARGs and VFGs. These rodents can harbor and spread antibiotic-resistant bacteria (ARB), contributing to the environmental transmission of resistance and virulence factors18,19. Their interactions with anthropogenic environments, including frequent contact with human dwellings, elevate the risk of introducing resistant pathogens into surrounding ecosystems, making them a key focus for understanding environmental AMR transmission. Certain species, such as the Norway rat (Rattus norvegicus) and the house mouse (Mus musculus), are recognized carriers of ARB19,20,21, further highlighting the need to address wildlife in AMR surveillance.
This study aims to elucidate the mechanisms underlying the dissemination of ARGs and the emergence of pathogenic ARB in natural environments. In this study, we analyzed 12,256 genomes from wild rodents to characterize the composition and distribution of ARGs and VFGs within their gut microbiota. Our research further explores the antibiotic resistance profiles within these microbial communities, the transfer of ARGs among different bacterial species via mobile genetic elements (MGEs), and the co-host relationships between ARGs and VFGs. By advancing our understanding of these critical interactions, this study contributes to the broader discourse on antimicrobial resistance and its implications for both ecological and public health.
Results
Comprehensive genome catalog of wild rodent gut microbiome
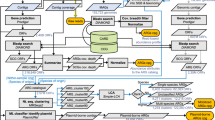
Using culture-dependent methods and metagenomic sequencing, this study constructed a strain-level genome catalog from the gut microbiota of wild rodents. Initially, eight types of culture media were utilized under both aerobic and anaerobic conditions, resulting in the isolation of 2198 pure bacterial cultures. After clustering the 16S rRNA gene sequences using CD-HIT with a 99% similarity threshold, 314 representative isolates were selected for whole genome sequencing and de novo assembly. Following quality control, 33 genomes were excluded due to high genomic contamination.
Subsequently, metagenome assembly and binning were performed on 610 metagenomic samples, generating 13,780 MAGs. These MAGs and the 281 isolate genomes were clustered at 99% ANI, yielding a total of 12,255 strain-level genomes (Fig. 1A). Genome sizes ranged from 0.26 to 22.21 Mbp (average 2.14 Mbp/genome), with GC count ranging from 22.21% to 74.09% (mean: 49.35%). The genomes had a mean completeness of 80.06%, and a mean contamination of 1.94% (Fig. 1B, C; Supplementary Data 1). Of these, 3522 genomes (28.74%) were classified as high-quality, exhibiting ≥90% completeness and <5% contamination.
A Workflow illustrating the steps involved in constructing the bacterial genome catalogue. B Distribution of genome length and GC count for each assembled genome. C Genome completeness and contamination metrics, representing the percentage of genome recovery and the corresponding contamination level. D Taxonomic classification of genomes across hierarchical levels. Rectangles represent different taxonomic ranks, with their lengths proportional to the number of genomes assigned to each level.
Taxonomic classification assigned 12,255 genomes to 25 phyla, 33 classes, 80 orders, 166 families, and 744 genera. Additionally, 17 genomes were classified as archaea. At the phylum level, Bacillota_A (n = 5501, 44.89%) and Bacteroidota (n = 3961, 32.32%) were dominant. Markedly, 9246 genomes (75.45%) could not be classified at the species level, indicating the presence of potentially novel species. These unclassified genomes belonged to 11 different phyla, with most assigned to Bacillota_A (n = 4352), followed by Bacteroidota (n = 3034) and Actinomycetota (n = 533) (Fig. 1D and Supplementary Data 1).
Composition of ARGs in the gut microbiota of wild rodents
To investigate ARGs in the gut metagenomes of wild rodents, this study identified 8119 putative ARG Open Reading Frames (ORFs) by comparing sequences against the CARD (Comprehensive Antibiotic Resistance Database). These ARGs were associated with resistance to antibacterial agents spanning 107 drug resistance categories. Among them, 5817 (71.65%) conferred resistance to a single drug class, while 2302 (28.35%) showed resistance to multiple classes. Interestingly, 28 ARGs conferred resistance to macrolides-lincosamides-streptogramin B (MLS) antibiotics (Supplementary Data 2).
In total, 518 distinct ARGs were identified, including tet(Q), tet(W), and vanG, which were associated with 30 resistance types. The most prevalent were genes conferring multidrug resistance (39.19%), followed by those targeting peptide antibiotics (7.14%) and tetracyclines (7.14%) (Fig. 2A). Additionally, elfamycin resistance genes were the most abundant (49.88%), with multitype resistance genes accounting for 11.54%, glycopeptide resistance for 9.07%, and tetracycline resistance for 7.88% (Fig. 2B).
A Proportion of unique ARG types relative to the total number of ARGs identified. B Relative abundance of ARGs across different resistance types, expressed as a proportion of total ARG abundance. C Prevalence and abundance distribution of all detected ARGs across 610 samples. ARG abundance is represented as the average abundance per gene across all. Each dot represents an individual ARG, with color indicating its the distribution based on abundance and prevalence.
Regarding the resistance mechanisms, the majority of ARG-mediated resistance through antibiotic target alteration (78.93%), followed by target protection (7.47%), multitype resistance mechanisms (6.50%), and antibiotic efflux (5.65%) (Supplementary Fig. 1A). Ten ARGs were detected in over 90% of samples, suggesting widespread presence in the rodent gut microbiome. Among the most prevalent were Cdif_EFTu_ELF, Ecol_EFTu_KIR, Efac_EFTu_GE2A, Saur_fusA_FA, and Cdif_rpoB_RIF, which conferred resistance to various drugs, often due to point mutations or sequence variations in specific regions. Conversely, 65.82% of ARGs were found in fewer than 10% of the samples, indicating a low prevalence across the population (Fig. 2C and Supplementary Data 3).
Host bacteria and mobile genetic elements related to ARGs in the gut of wild rodents
ARGs enable antibiotic resistance in their host bacteria, potentially contributing to the development of multidrug-resistant (MDR) strains. In this study, host bacteria carrying ARGs were identified by assigning taxonomic classifications to genome containing ARG-associated ORFs. A total of 8119 ARG ORFs were distributed across 2118 genomes, with 2117 belonging to bacteria and one to archaea (Fig. 3A). These genomes had an average size of 2.45 Mbp/genome and an average GC content of 47.48% (Fig. 3B).
A Phylogenetic tree of 2,118 bacterial genomes. The inner rings indiacte taxonomic classification at the phylum level, while subsequent outer rings represent genus and species levels. Bar height corresponds to the number of ARGs identified in each genome. B Genome size and GC count distribution across bacterial genome. C Genome completeness and contamination levels, showing the percentage of genome recovery and degree of contamination. D Abundance of different f MGE types across the analyzed samples. E Procrustes analysis illustrating the association between ARGs (Green) and MGEs (Purple) in multidimensional space. F Spearman’s correlation between the richness indices of ARGs and MGEs across samples.
Among these, 703 genomes (33.19%) were classified as high-quality (>90% completeness and <5% contamination), while genomes 1415 (66.81%) were of medium-quality (50%–89% completeness and <10% contamination) (Fig. 3C). Out of the 2118 genomes, 702 genomes contained more than two ARGs, and 177 harbored more than five (Supplementary Fig. 1B). Among the 518 distinct ARGs, 148 were found in only one bacterial species, whereas 370 ARGs were shared across multiple species. Additionally, 44 ARGs were identified in over 50 genomes (Supplementary Fig. 1C).
To further explore the relationship between gut microbiome composition and antibiotic resistance, host bacteria were examined at the strain level. Bacteria from the Pseudomonadota phylum, mainly Enterobacteriaceae, were the dominant ARG carriers, accounting for 56.48% of all ARGs. Escherichia coli (E. coli) carried the highest number of ARGs (1540 ARG ORFs), followed by Enterococcus faecalis (E. faecalis) (225 ARG ORFs) and Citrobacter braakii (C. braakii) (210 ARG ORFs) (Supplementary Data 4).
MGEs play a crucial role in facilitating the horizontal transfer of ARGs within and between bacterial populations. Understanding their distribution and association with ARGs is essential to elucidate how antibiotic resistance spreads. In this study, 1196 MGE-associated ORFs were identified across 12,255 genomes by aligning protein sequences against the MGE database. These ORFs corresponded to 370 MGEs, classified into 15 types, including transposase, ISCR, integrase, and others (Supplementary Data 5). Transposable elements (TEs), marked by transposase genes, were the most abundant MGE type, accounting for 49.24% of total MGE abundance, followed by IS common region (ISCR) (26.08%) and integrase (11.84%). Additionally, 78 plasmid-associated ORFs were identified, but these represented only 1.37% of total MGE abundance (Fig. 3D).
Procrustes analysis revealed a strong association between the cecal mobilome and antibiotic resistance (PROTEST, M² = 0.3012, p = 0.001) (Fig. 3E). A significant positive correlation between MGE and ARG profiles was observed using the Richness index (Spearman’s correlation: R = 0.35, p < 2.2e-16) (Fig. 3F). Co-abundance analysis of the top 20 ARGs and MGEs revealed significant associations between these ARGs and 11 MGEs (Spearman’s correlation: R ≥ 0.08, p < 0.05) (Supplementary Fig. 1D and Supplementary Data 6).
Given that gene mobility can affect the distribution of ARGs across various sources-and recognizing the pivotal role of MGEs in antimicrobial resistance surveillance-this study further examined ARG-MGE co-localization. ARGs located within 5 kilobases (kb) of an MGE were classified as potentially mobile. In total, 130 distinct MGE-ARG combinations were identified, involving 69 unique ARGs across 60 genomes (Supplementary Data 7). Interestingly, Acinetobacter idrijaensis (MF16) contained five contigs with MGE-ARG combinations, while Enterobacter hormaechei_A (MFY43) had two such contigs (Supplementary Fig. 2).
Virulence–resistance interplay in rodent gut microbiota
To analyze the VFGs in the gut metagenomes of wild rodents, this study identified 7626 VFG ORFs by comparing the metagenomes with the VFDB (Virulence Factor Database). These ORFs showed homology with 796 distinct VFGs, which were classified into 176 virulence factors, including elongation factor thermos unstable (EF-Tu, 49.13%), Heat Shock Protein 60 (GroEL, 14.02%), and Capsule (11.45%) (Fig. 4A and Supplementary Data 8). The predicted VFGs were categorized into 13 functional groups, with the largest being adherence (66.24%), followed by immune modulation (14.50%) and motility (6.92%) (Fig. 4B). The study designated genomes as potential pathogens if they contained at least one VFG, resulting in 1529 genomes being classified as pathogens (Supplementary Data 8). E. coli carried the highest number of VFGs. In terms of VFG-hosting bacteria, E. coli carried 1699 VFGs, most of which were related to nutritional/metabolic factors, adherence, and the effector delivery system (Fig. 4C).
A Overall abundance of VFs across the examined samples. B Relative abundance of different VFG categories identified in the samples. C Strain-level distribution of VFGs among different host species. D Procrustes analysis illustrating the co-occurrence patterns between ARGs (green) and VFGs (purple). E Spearman’s correlation analysis of Richness indices for ARGs and VFGs across samples.
Procrustes analysis also indicated a correlation between ARGs and VFGs (PROTEST, M² = 0.298, p = 0.001) (Fig. 4D). The study revealed a significant positive correlation between f VFG and ARG profiles based on the Richness index (Spearman’s correlation: R = 0.9, p < 2.2e-16) (Fig. 4E). Further analysis of co-hosted ARGs and VFGs revealed that 659 genomes contained at least one ARG and one VFG, indicating the presence of potentially pathogenic ARB. These genomes spanned 16 phyla, 20 classes, 43 orders, 88 families, 218 genera, with 321 genomes remaining unannotated at the species level. The genome of Escherichia albertii carried the most VFGs, while an E. coli genome carried the highest number of ARGs. (Supplementary Fig. 3 and Supplementary Data 9). Additionally, five genomes were found to harbor both VFGs and MGE–ARG combinations. These genomes were assigned to Klebsiella quasipneumoniae (RN10.bin.106), Acinetobacter pittii (RN12.bin.45), Acinetobacter geminorum (HJ5.bin.26), Escherichia coli (RN3.bin.26), and Acinetobacter oleivorans (HJX8.bin.54) (Supplementary Fig. 4).
Rodent-specific gut microbial ARGs
The specificity of ARGs in microorganisms from the intestines of wild rodents was assessed by comparing the 12,255 genomes obtained in this study with those of laboratory mice (4760 MAGs)22, humans (36,467 MAGs)23, and pigs (16,394 MAGs)24 reported in previous studies. Functional annotation revealed 4491 ARGs in the 4760 laboratory mice genomes, 64,546 ARGs in 36,467 human genomes, and 10,130 ARGs in 16,394 pig genomes (Supplementary Data 10). In comparison to the 8119 ARGs identified in wild rodent genomes, 254 resistance genes were found to be shared across all four hosts, while 88 ARGs were specific to wild rodents. Remarkably, the human genome carried 235 unique ARGs not found in other animals (Fig. 5A).
Ecol_EFTu_KIR, Cdif_EFTu_ELF, and Efac_EFTu_GE2A were the predominant ARGs present in the gut microbiota across different animals. Among these, Ecol_EFTu_KIR was most prevalent in wild rodents (6.55%) and humans (4.56%), while Cdif_EFTu_ELF was most abundant in laboratory mice (5.88%) and pigs (7.46%) (Fig. 5B).
Taxonomy annotations revealed that 1111 genomes from laboratory mice, 12,895 human genomes, and 3622 pig genomes harbored ARGs. At the family level, the ARGs-carrying MAGs were primarily assigned to Enterobacteriaceae in wild rodent (n = 4200), laboratory mice (n = 2455), humans (n = 38,919), and pigs (n = 4708) (Supplementary Data 10).
Discussion
While significant progress has been made in understanding AMR dynamics in humans and domestic animals, far less attention has been given to its occurrence and impact in wildlife25. In the present study, we aimed to fill this knowledge gap by providing a comprehensive overview of the resistome, virulome, and mobilome within the gut microbiome of wild rodents, highlighting their potential role as reservoirs for AMR and virulence factors. Wild rodents harbor resistance genes that can spread through HGT26, contributing to the emergence of MDR pathogens27,28. The co-presence of virulence factors further raises concerns over cross-species transmission, posing risks to wildlife, livestock, and humans29. Therefore, a deeper understanding of ARG and VFG dynamics in wild rodents is essential for assessing One Health-related public health threats.
Traditional AMR surveillance has predominantly focused on clinically relevant isolates of ARB cultured from human samples, potentially overlooking the broader resistome within uncultured bacteria and non-pathogenic bacterial hosts30. To overcome this limitation, we employed both culture-dependent and culture-independent (metagenomic) approaches to examine the gut microbiota of wild rodents. This dual approach enabled the identification of 8043 putative ARGs, classified into 517 unique types across 107 resistance classes, demonstrating the extensive diversity of resistance mechanisms within these microbial communities. Among these ARGs, a distinction emerged between those conferring resistance to a single antibiotic class and those associated with multiple classes. Specifically, 34.5% of the ARGs were predicted to confer resistance to two or more antibiotic classes, highlighting the widespread presence of MDR genes. This has adverse implications, as MDR strains can persist in environments with varied antimicrobial exposure, posing a major threat both human and veterinary heath31.
Despite the advantages of culture-based methods, they may underrepresent fastidious or unculturable microbes. To counteract this limitation, we incorporated metagenomic sequencing, which enabled the detection of a broader spectrum of microbial taxa and resistance determinants32,33. Robust bioinformatic pipelines and stringent quality control ensured high-confidence identification of ARGs. A frequent concern in resistome studies is whether detected ARGs reflect intrinsic rather than acquired resistance. Indeed, many resistance genes, including those identified here, may have evolved from genes with other functions and could be native to certain bacterial taxa34. For example, β-lactamases are involved not only in antibiotic resistance but also in cell wall remodeling35,36. While intrinsic resistance cannot be ruled out, the widespread distribution of these genes across diverse bacterial hosts in our study suggests strong environmental selection pressures.
The identification of ARGs conferring resistance to clinically important antibiotics-such as elfamycin, glycopeptides, and tetracyclines-further supports a link to anthropogenic pollution. These antibiotics differ in their usage patterns: tetracyclines are commonly used in both medicine and agriculture, while glycopeptides (e.g., vancomycin) are typically restricted to human healthcare37,38. The detection of resistance genes for both suggests that wild rodents are exposed to a broad range of environmental antibiotic contaminants, likely through agricultural runoff, medical waste, or contaminated habitats.
By categorizing ARGs into specific resistance classes, our study provides insight into the antibiotics most affected by these mechanisms. The most abundant ARG types were linked to resistance against elfamycin and multiple antibiotic classes. These findings reflect the adaptive capabilities of rodent gut microbiota in resisting various antimicrobials and raise concerns about the potential for ARG transmission to pathogenic bacteria39. Further studies are needed to assess how these resistance mechanisms might contribute to the evolution and spread of AMR in natural ecosystems. Genomic analysis revealed the presence of several ARGs in over 90% of the samples, underscoring their pervasive presence in the wild rodent gut microbiome. ARGs such as Cdif_EFTu_ELF, Ecol_EFTu_KIR, and Efac_EFTu_GE2A were among the most common, suggesting that these ARGs play critical roles in gut microbial ecology. These genes confer resistance to clinically significant antibiotics, such as elfamycin, glycopeptide, and tetracycline, again indicating a potential link to environmental antibiotic exposure40. Their presence suggests that these genes provide an adaptive advantage, especially in habitats influenced by human activity. Interestingly, a large proportion (~66.3%) of identified ARGs were of low prevalence (present in <10% of samples). While these may seem less immediately concerning, they could represent a latent reservoir of resistance that may expand under selective pressure, such as increased antibiotic use in agriculture or healthcare41,42. Monitoring both common and rare ARGs is thus crucial for capturing the full scope of AMR potential in wildlife.
Analysis of ARG-hosting bacteria revealed a broad range of carriers, with E. coli emerging as the dominant host, harboring 92 ARGs—significantly more than any other species. This finding is consistent with previous studies that identified E. coli as the major ARG carrier in magpies (Pica pica), rabbits (Oryctolagus cuniculus), and Andean condors (Vultur gryphus)43,44. Other carriers included E. faecalis and C. braakii. E. coli’s prominence as an ARG host is concerning due to its high HGT potential and its frequent association with human and animal infections45,46. This reinforces its role as a key vector of AMR within the One Health framework. MGEs are essential drivers of HGT and play a pivotal role in the dissemination of AMR47,48. MGEs-including plasmids, TEs, ISCR elements, and integrons-enable rapid bacterial adaptation to selective pressures such as antibiotic exposure49. In this study, we identified 1251 MGE ORFs across 381 unique MGEs, spanning 17 types. TEs were the most abundant (51.7%), followed by ISCR elements (20.1%) and integrase genes (12.55%). This is consistent with prior findings in pig gut microbiomes, where TEs predominated the mobilome in E. coli (77.7% of all MGEs)50.
While plasmids are critical AMR vectors, plasmid-associated genes accounted for only 1.5% of MGEs in the present study, suggesting that other mechanisms dominate in this environment51. The predominance of transposases and other MGEs, highlights their central role in ARG mobilization, driving the spread of AMR52, among rodent gut bacteria. Supporting this, we found significant positive correlations between MGE and ARG profiles, reinforcing the hypothesis that MGEs drive ARG dissemination53. These results highlight the need to monitor MGEs alongside ARGs to better understand AMR evolution in wildlife. A previous large-scale study showed that MGEs carrying ARGs in the human microbiome have broad host ranges, suggesting that effective AMR mitigation strategies must extend beyond pathogens to include commensal species39. Further ecological studies are essential to unravel the roles of MGEs in wildlife and their potential spillover into human populations, especially in regions with frequent human-animal interactions. In addition to ARGs and MGEs, we identified 7586 VFG ORFs, corresponding to 790 distinct genes grouped into 176 virulence factors. These were classified into 13 functional categories, reflecting the diverse strategies used by bacteria to colonize hosts and evade immune defenses49. The most prevalent categories included immune modulation (20.6%), adherence (19.7%), motility (16.0%), and metabolic factors (14.7%). This diversity underscores the pathogenic potential embedded within the gut microbiota of wild rodents.
The co-existence of ARGs and VFGs in the rodent gut bacteria raises significant public health concerns. These microbes could serve as reservoirs of MDR pathogens, capable of transmitting both resistance and virulence traits to other animals or humans54. Such bacteria, equipped for both survival and pathogenicity, may cause hard-to-treat infections21,28. Consequently, surveillance of wild rodent populations should be prioritized as part of global AMR monitoring, particularly under the One Health paradigm. Tackling these risks will require interdisciplinary collaboration, environmental stewardship, and proactive AMR containment strategies.
In summary, we characterized the ARGs and VFGs in the cecal microbiota of wild rodents through a comprehensive genomic analysis that integrated newly generated data with publicly available datasets. A total of 8119 putative AMR protein-coding genes were identified, representing 518 unique ARG types across 107 resistance classes, highlighting the extensive diversity of resistance mechanisms in these ecosystems. The most abundant ARGs were associated with elfamycin resistance, followed by those conferring resistance to multiple antibiotic classes. In addition, we detected 7626 putative VFGs, corresponding to 176 distinct virulence factors grouped into 13 categories, reflecting the pathogenic potential harbored within the rodent gut microbiota. E. coli emerged as the predominant bacterial host for both ARGs and VFGs, reinforcing its role as a key reservoir and potential vector of AMR and virulence. Furthermore, significant correlations between MGEs and the presence of both VFGs and ARGs, suggest a potential for localization and horizontal gene transfer, which could facilitate the dissemination of these traits within microbial communities. These findings expand our understanding of the resistome and virulome in wildlife and emphasize the ecological role of wild rodents as reservoirs of AMR. They also provide a foundation for future research and the development of targeted surveillance and mitigation strategies to reduce the public health risks associated with antimicrobial resistance emerging from wildlife sources.
Methods
Field collection and biological sampling of wild rodents
A total of 128 wild rodents were captured, including Apodemus agrarius (n = 18), Rattus norvegicus (n = 30), Rattus flavipectus (n = 20), Mus musculus (n = 20), and Microtus fortis (n = 40), using peanuts or sunflower seeds as bait. Detailed sample information is provided in Supplementary Data 11. The specific trapping and collection methods were conducted as previously described40. This study was approved by Institutional Animal Care and Use Committee of Qingdao Agricultural University (Approval No. QAU-AEW-20210701001).
Additionally, publicly available datasets, including 482 gut metagenomes samples from wild rodents (Table 1), were obtained from the NCBI SRA database, with corresponding sample information listed in Supplementary Data 12.
Isolation and whole genome sequencing of bacterial isolates
Culturomics analysis was conducted on the cecal contents of Lasiopodomys brandtii (n = 43), Apodemus agrarius (n = 4), Spermophilus dauricus (n = 1), and Microtus fortis (n = 40) (Supplementary Data 13). The samples were serially diluted (1 × 10 to 1 × 104) and plated on six different culture media, including Brain Heart Infusion (BHI), Bifidobacterium BS Medium (BS), Gifu Anaerobic Medium (GAM), Lactose Bile Broth (LBB), Columbia Blood Agar Base (CBB), and Reinforced Medium for Clostridium (RCM), all provided by Qingdao Hope Bio-Technology Co., Ltd, China. The cultures were incubated at 37 °C for 1–7 days under both aerobic and anaerobic conditions. Selected colonies were subjected to 2–3 rounds of purification via the plate marking method. All bacterial isolates were subsequently inoculated into liquid medium in Hungate tubes and incubated on a shaker at 37 °C for 1–3 days under aerobic and anaerobic conditions. All isolated bacterial strains were stored at −80 °C in 25% glycerol.
While culture-based methods are valuable for obtaining viable isolates, they are inherently limited in their ability to detect fastidious or unculturable microorganisms. To mitigate this limitation and achieve a more comprehensive representation of microbial diversity, we combined culture-dependent techniques with culture-independent metagenomic sequencing approaches (see “Metagenomic sequencing and data analysis”). Additionally, we applied quality control measures and selected bioinformatic thresholds, including minimum abundance cutoffs and contamination/completeness filters, to reduce spurious reads and sequencing artifacts. These steps helped ensure that both isolate and metagenomic data were robust and representative of the underlying microbial communities.
Bacteria from overnight cultures were pelleted by centrifugation, and genomic DNA was extracted using the CTAB protocol. The bacterial 16S rRNA genes were amplified using universal primers 27 F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492 R (5′-TACGGCTACCTTGTACGACTT-3′). Amplicons were confirmed by 1% agarose gel electrophoresis and subjected to Sanger sequencing. The resulting sequences were aligned against SILVA rRNA database55 (release 138) using BLAST (v2.13.0) to assign taxonomic identities.
The 16S rRNA genes from the isolated strains were clustered using CD-HIT with a 99% similarity threshold, and one representative isolate from each cluster was selected for whole genome sequencing. DNA library construction, conducted by Shanghai Personalbio Technology Co., Ltd, China, followed key steps: the extracted DNA was randomly fragmented by ultrasonic waves and end-repaired. MGIEasy PF adapters were ligated, followed by magnetic bead purification using MGIEasy DNA Purification Magnetic Beads.
PCR was used to enrich the library templates, and final fragment selection (~450 bp) was performed by agarose gel electrophoresis. Quality control of the library was assessed using an Agilent Bioanalyzer to ensure a single peak and absence of adapter dimers. The library concentration was quantified, mixed in the appropriate ratio, and denatured prior to paired-end sequencing (2 × 150 bp) on a DNBSEQ-T7 sequencer. Basic quality assessment of raw reads was performed using FASTP56 (v0.23.0), and filtered reads were de novo assembled using SPAdes57 (v3.15.5) with default parameters. CheckM258 (v1.0.1) was applied to have ≥50% completeness and ≤10% contamination retained.
Metagenomic sequencing and data analysis
Cecal contents were collected from 128 wild rodents and preserved at −80 °C until analysis. DNA was extracted using the OMEGA Mag-Bind Soil DNA Kit (M5635-02, Omega Bio-Tek, Norcross, GA, USA), following the manufacturer’s protocol. The concentration and purity of the extracted DNA were evaluated using a QubitTM 4 Fluorometer and 1% agarose gel electrophoresis, respectively. DNA samples that passed quality control were prepared for library construction at a concentration of 10 nM. Sequencing libraries were prepared by Shanghai Personalbio Technology Co., Ltd, and sequenced on the Illumina HiSeq platform with 150 bp paired-end sequencing.
The 128 samples collected in this study, along with 482 publicly available samples retrieved from NCBI, were processed using the same standardized data analysis pipeline. Fastp (v0.23.0) was used to filter for high-quality reads, which were then processed with Bowtie259 (v2.5.0) to remove host genomic DNA contamination. Details of the host reference genomes used in this study are included in Supplementary Data 12. The contigs were assembled using MEGAHIT60 (v1.2.9). Sequencing depth files for the contigs were generated using BWA61 (v0.7.17-r1198), SAMtools62 (v1.18), and the script jgi_summarize_BAM_contig_depths. Binning was performed with MetaBAT263 (v2.15), using options “-m 2000 -s 200000 --seed 2023”. The completeness and contamination of each bin from the superior bin set were assessed using CheckM258 (v1.0.1), with bins having ≥50% completeness and ≤10% contamination retained. Finally, all metagenome-assembled genomes (MAGs) were dereplicated at 99% Average Nucleotide Identity (ANI) using dRep64 (v3.4.3) with options “-pa 0.9 -sa 0.99”.
Genome collection, gene prediction, and functional annotation
Isolate genomes and MAGs were re-clustered using dRep64 (v3.4.3) with an ANI threshold of 99% with options “-pa 0.9 -sa 0.99”. Taxonomic assignment for the genomes was carried out using GTDB-Tk65 (v2.3.2). Gene prediction was performed with Prodigal66 (v2.6.1), and the predicted proteins were clustered using MMseqs267 (v7e284) with parameters set to 90% identity and 90% overlap, after removing incomplete genes shorter than 100 bp.
ARGs were identified by aligning protein sequences to the Comprehensive Antibiotic Resistance Database (CARD, v3.2.7) using DIAMOND68 (v2.1.8.162), applying criteria of >80% sequence identity and >80% query coverage with an e-value of 1e-5, and a minimum alignment score was 60. ARGs conferring resistance to at least two drug classes were categorized as multidrug resistance genes, and those conferring resistance to at least two mechanisms were grouped into the multitype mechanism category.
MGEs were identified by aligning gene sequences against the MGE Database69 using BLASTN (v2.13.0) with parameters “-evalue 1e-5 -perc_identity 80 -qcov_hsp_perc 80”. VFGs were identified through alignments with the Virulence Factor Database (VFDB)70 using DIAMOND (v2.1.8.162) with criteria of >80% sequence identity and >80% query coverage.
Potential pathogenic ARB were characterized based on the co-occurrence of ARGs and VFGs within the same genomes. To quantify the abundance of ARGs, MGEs, and VFGs, clean reads (20 million reads per sample) were aligned to the reference genes using Bowtie2 (v2.5.0) with default parameters. The read counts were normalized to transcripts per kilobase million (TPM).
Statistical analysis
The phylogenetic tree was annotated and visualized using the iTOL tool. Procrustes association analysis was conducted on the profiles of ARGs, MGEs, and VFGs, utilizing the “procrustes” function from the “vegan” package. Taxonomic assignments of genomes containing ARGs were identified, and Sankey plots were generated using the “ggsankey” package (version 0.0.9). The Richness index was calculated from the relative abundance of functional genes. Spearman’s correlation analysis was employed to explore the relationships between ARGs, MGEs, and VFGs, using the “corr.test” function from the psych package (version 2.4.6.26). Gene arrow maps were constructed using the “gggenes” (v0.4.1) package. GCView services (https://proksee.ca/) were used to visualize genome and to mark ARGs, MGEs, and VFGs. All other visualizations were produced with the ggplot2 package version 3.3.6. Statistical analyses were performed using R version 4.4.1.
Data availability
The sequencing reads from each library have been deposited in the NCBI under the accession number: PRJNA1175865. The genomes are available in the Figshare repository (https://doi.org/10.6084/m9.figshare.28752050). All supplementary figures and tables are provided as additional files.
References
Pal, C., Bengtsson-Palme, J., Kristiansson, E. & Larsson, D. G. The structure and diversity of human, animal and environmental resistomes. Microbiome 4, 54 (2016).
Redondo-Salvo, S. et al. Pathways for horizontal gene transfer in bacteria revealed by a global map of their plasmids. Nat. Commun. 11, 3602 (2020).
von Wintersdorff, C. J. et al. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front. Microbiol. 7, 173 (2016).
Hu, Y. et al. The bacterial mobile resistome transfer network connecting the animal and human microbiomes. Appl. Environ. Microbiol 82, 6672–6681 (2016).
Larsson, D. G. J. & Flach, C. F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 20, 257–269 (2022).
MacLean, R. C. & San Millan, A. The evolution of antibiotic resistance. Science 365, 1082–1083 (2019).
Hernando-Amado, S., Coque, T. M., Baquero, F. & Martínez, J. L. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat. Microbiol. 4, 1432–1442 (2019).
Baker, S., Thomson, N., Weill, F. X. & Holt, K. E. Genomic insights into the emergence and spread of antimicrobial-resistant bacterial pathogens. Science 360, 733–738 (2018).
Beceiro, A., Tomás, M. & Bou, G. Antimicrobial resistance and virulence: a successful or deleterious association in the bacterial world?. Clin. Microbiol. Rev. 26, 185–230 (2013).
Wu, H. J., Wang, A. H. & Jennings, M. P. Discovery of virulence factors of pathogenic bacteria. Curr. Opin. Chem. Biol. 12, 93–101 (2008).
Geisinger, E. & Isberg, R. R. Interplay between antibiotic resistance and virulence during disease promoted by multidrug-resistant bacteria. J. Infect. Dis. 215, S9–S17 (2017).
Moya, B., Juan, C., Albertí, S., Pérez, J. L. & Oliver, A. Benefit of having multiple ampD genes for acquiring beta-lactam resistance without losing fitness and virulence in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 52, 3694–3700 (2008).
Renzoni, A., Huggler, E., Kelley, W. L., Lew, D. & Vaudaux, P. Increased uptake and improved intracellular survival of a teicoplanin-resistant mutant of methicillin-resistant Staphylococcus aureus in non-professional phagocytes. Chemotherapy 55, 183–188 (2009).
Livermore, D. M. Bacterial resistance: origins, epidemiology, and impact. Clin. Infect. Dis. 36, S11–S23 (2003).
Lin, Q., Li, L., Fang, X. & Li, X. Substrate complexity affects the prevalence and interconnections of antibiotic, metal and biocide resistance genes, integron-integrase genes, human pathogens and virulence factors in anaerobic digestion. J. Hazard Mate 438, 129441 (2022).
Mu, M., Yang, F., Han, B., Tian, X. & Zhang, K. Manure application: a trigger for vertical accumulation of antibiotic resistance genes in cropland soils. Ecotoxicol. Environ. Saf. 237, 113555 (2022).
Shen, Q. et al. Fate of antibiotic resistance genes and metal resistance genes during the thermophilic fermentation of solid and liquid swine manures in an ectopic fermentation system. Ecotoxicol. Environ. Saf. 213, 111981 (2021).
Skarżyńska, M. et al. Salmonella and antimicrobial resistance in wild rodents-true or false threat?. Pathogens 9, 771 (2020).
Williams, S. H. et al. New York city house mice (Mus musculus) as potential reservoirs for pathogenic bacteria and antimicrobial resistance determinants. MBio 9, e00624-18 (2018).
Himsworth, C. G. et al. Avian pathogenicity genes and antibiotic resistance in Escherichia coli isolates from wild Norway rats (Rattus norvegicus) in British Columbia, Canada. J. Wildl. Dis. 52, 418–421 (2016).
Himsworth, C. G. et al. Carriage of methicillin-resistant Staphylococcus aureus by wild urban Norway rats (Rattus norvegicus). PLoS ONE 9, e87983 (2014).
Kieser, S., Zdobnov, E. M. & Trajkovski, M. Comprehensive mouse microbiota genome catalog reveals major difference to its human counterpart. PLoS Comput. Biol. 18, e1009947 (2022).
Nayfach, S., Shi, Z. J., Seshadri, R., Pollard, K. S. & Kyrpides, N. C. New insights from uncultivated genomes of the global human gut microbiome. Nature 568, 505–510 (2019).
Gaio, D. et al. A large-scale metagenomic survey dataset of the post-weaning piglet gut lumen. GigaScience 10, giab039 (2021).
Khan, S. A., Imtiaz, M. A., Sayeed, M. A., Shaikat, A. H. & Hassan, M. M. Antimicrobial resistance pattern in domestic animal - wildlife - environmental niche via the food chain to humans with a Bangladesh perspective; a systematic review. BMC Vet. Res. 16, 302 (2020).
Rozwandowicz, M. et al. Plasmids carrying antimicrobial resistance genes in Enterobacteriaceae. J. Antimicrob. Chemother. 73, 1121–1137 (2018).
Silva, V. et al. Antimicrobial resistance and genetic lineages of Staphylococcus aureus from wild rodents: first report of mecc-positive methicillin-resistant S. aureus (MRSA) in Portugal. Animals 11, 1537 (2021).
Gilliver, M. A., Bennett, M., Begon, M., Hazel, S. M. & Hart, C. A. Antibiotic resistance found in wild rodents. Nature 401, 233–234 (1999).
Sonola, V. S., Katakweba, A., Misinzo, G. & Matee, M. I. Molecular epidemiology of antibiotic resistance genes and virulence factors in multidrug-resistant Escherichia coli isolated from rodents, humans, chicken, and household soils in Karatu, Northern Tanzania. Int. J. Environ. Res. Public Health 19, 5388 (2022).
Ruppé, E. et al. Prediction of the intestinal resistome by a three-dimensional structure-based method. Nat. Microbiol. 4, 112–123 (2019).
Hendriksen, R. S. et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat. Commun. 10, 1124 (2019).
de Abreu, V. A. C., Perdigão, J. & Almeida, S. Metagenomic approaches to analyze antimicrobial resistance: an overview. Front. Genet. 11, 575592 (2020).
Olsen, N. S. & Riber, L. Metagenomics as a transformative tool for antibiotic resistance surveillance: highlighting the impact of mobile genetic elements with a focus on the complex role of phages. Antibiotics14, 296 (2025).
Baquero, F. et al. Evolutionary pathways and trajectories in antibiotic resistance. Clin. Microbiol. Rev. 34, e0005019 (2021).
Sauvage, E., Kerff, F., Terrak, M., Ayala, J. A. & Charlier, P. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 32, 234–258 (2008).
Zeng, X. & Lin, J. Beta-lactamase induction and cell wall metabolism in gram-negative bacteria. Front. Microbiol. 4, 128 (2013).
Su, J. Q., Wei, B., Xu, C. Y., Qiao, M. & Zhu, Y. G. Functional metagenomic characterization of antibiotic resistance genes in agricultural soils from China. Environ. Int. 65, 9–15 (2014).
O’Driscoll, T. & Crank, C. W. Vancomycin-resistant enterococcal infections: epidemiology, clinical manifestations, and optimal management. Infect. Drug Resist 8, 217–230 (2015).
Forster, S. C. et al. Strain-level characterization of broad host range mobile genetic elements transferring antibiotic resistance from the human microbiome. Nat. Commun. 13, 1445 (2022).
Shang, K. M. et al. Metagenomic profiling of cecal microbiota and antibiotic resistome in rodents. Ecotoxicol. Environ. Saf. 286, 117186 (2024).
Kümmerer, K. The presence of pharmaceuticals in the environment due to human use-present knowledge and future challenges. J. Environ. Manag. 90, 2354–2366 (2009).
Sarmah, A. K., Meyer, M. T. & Boxall, A. B. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 65, 725–759 (2006).
Livermore, D. M. et al. Antibiotic resistance in bacteria from magpies (Pica pica) and rabbits (Oryctolagus cuniculus) from west Wales. Environ. Microbiol. 3, 658–661 (2001).
Fuentes-Castillo, D. et al. Genomic data reveal international lineages of critical priority Escherichia coli harbouring wide resistome in Andean condors (Vultur gryphus Linnaeus, 1758). Mol. Ecol. 29, 1919–1935 (2020).
Poirel, L. et al. Antimicrobial resistance in Escherichia coli. Microbiol. Spectr. 6 (2018).
Zhang, Q. et al. Metagenomic insight into the global dissemination of the antibiotic resistome. Adv. Sci.10, e2303925 (2023).
Fondi, M. & Fani, R. The horizontal flow of the plasmid resistome: clues from inter-generic similarity networks. Environ. Microbiol. 12, 3228–3242 (2010).
Ebmeyer, S., Kristiansson, E. & Larsson, D. G. J. A framework for identifying the recent origins of mobile antibiotic resistance genes. Commun. Biol. 4, 8 (2021).
Choi, M. et al. The diversity of lipopolysaccharide (O) and capsular polysaccharide (K) antigens of invasive Klebsiella pneumoniae in a multi-country collection. Front. Microbiol. 11, 1249 (2020).
Mencia-Ares, O. et al. Genomic insights into the mobilome and resistome of sentinel microorganisms originating from farms of two different swine production systems. Microbiol. Spectr. 10, e0289622 (2022).
Che, Y. et al. Conjugative plasmids interact with insertion sequences to shape the horizontal transfer of antimicrobial resistance genes. Proc. Natl. Acad. Sci. USA 118, e2008731118 (2021).
Johansson, M. H. K. et al. Detection of mobile genetic elements associated with antibiotic resistance in Salmonella enterica using a newly developed web tool: mobileElementFinder. J. Antimicrob. Chemother. 76, 101–109 (2021).
Partridge, S. R., Kwong, S. M., Firth, N. & Jensen, S. O. Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 31, e00088–17 (2018).
Himsworth, C. G. et al. Ecology of Leptospira interrogans in Norway rats (Rattus norvegicus) in an inner-city neighborhood of Vancouver, Canada. PLoS Negl. Trop. Dis. 7, e2270 (2013).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2013).
Chen, S., Zhou, Y., Chen, Y. & Gu, J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018).
Bankevich, A. et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19, 455–477 (2012).
Chklovski, A., Parks, D. H., Woodcroft, B. J. & Tyson, G. W. CheckM2: a rapid, scalable and accurate tool for assessing microbial genome quality using machine learning. Nat. Methods 20, 1203–1212 (2023).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, D. et al. MEGAHIT v1.0: a fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods 102, 3–11 (2016).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Danecek, P. et al. Twelve years of SAMtools and BCFtools. GigaScience 10, giab008 (2021).
Kang, D. D. et al. MetaBAT 2: an adaptive binning algorithm for robust and efficient genome reconstruction from metagenome assemblies. PeerJ 7, e7359 (2019).
Olm, M. R., Brown, C. T., Brooks, B. & Banfield, J. F. DRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication. ISME J. 11, 2864–2868 (2017).
Chaumeil, P. A., Mussig, A. J., Hugenholtz, P. & Parks, D. H. GTDB-Tk v2: memory friendly classification with the genome taxonomy database. Bioinformatics 38, 5315–5316 (2022).
Hyatt, D. et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinforma. 11, 119 (2010).
Mirdita, M., Steinegger, M., Breitwieser, F., Söding, J. & Levy Karin, E. Fast and sensitive taxonomic assignment to metagenomic contigs. Bioinformatics 37, 3029–3031 (2021).
Buchfink, B., Xie, C. & Huson, D. H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12, 59–60 (2015).
Pärnänen, K. et al. Maternal gut and breast milk microbiota affect infant gut antibiotic resistome and mobile genetic elements. Nat. Commun. 9, 3891 (2018).
Liu, B., Zheng, D., Zhou, S., Chen, L. & Yang, J. VFDB 2022: a general classification scheme for bacterial virulence factors. Nucleic Acids Res. 50, D912–D917 (2022).
Bowerman, K. L. et al. Effects of laboratory domestication on the rodent gut microbiome. ISME Commun. 1, 49 (2021).
Thomas, J. C. t. et al. Unveiling the gut microbiota and resistome of wild cotton mice, Peromyscus gossypinus, from heavy metal- and radionuclide-contaminated sites in the southeastern United States. Microbiol. Spectr. 9, e0009721 (2021).
Lavrinienko, A. et al. Two hundred and fifty-four metagenome-assembled bacterial genomes from the bank vole gut microbiota. Sci. Data 7, 312 (2020).
Lesker, T. R. et al. An integrated metagenome catalog reveals new insights into the murine gut microbiome. Cell Rep. 30, 2909–2922.e2906 (2020).
Youngblut, N. D. et al. Large-scale metagenome assembly reveals novel animal-associated microbial genomes, biosynthetic gene clusters, and other genetic diversity. MSystems 5, e01045–20 (2020).
Ammar, A. et al. Rodent gut bacteria coexisting with an insect gut virus in parasitic cysts: metagenomic evidence of microbial translocation and co-adaptation in spatially-confined niches. BioRxiv (2024).
Zhao, Y. et al. Exploring Alashan Ground Squirrel (Spermophilus alashanicus) diversity: metagenomic and transcriptomic datasets from the Helan Mountains. Sci. Data 11, 517 (2024).
Rosshart, S. P. et al. Wild mouse gut microbiota promotes host fitness and improves disease resistance. Cell 171, 1015–1028.e1013 (2017).
Rosshart, S. P. et al. Laboratory mice born to wild mice have natural microbiota and model human immune responses. Science 365, eaaw4361 (2019).
Milovic, A. et al. Lactobacilli and other gastrointestinal microbiota of Peromyscus leucopus, reservoir host for agents of Lyme disease and other zoonoses in North America. PLoS ONE 15, e0231801 (2020).
Kohl, K. D. et al. Metagenomic sequencing provides insights into microbial detoxification in the guts of small mammalian herbivores (Neotoma spp.). FEMS Microbiol. Ecol. 94, fiy184 (2018).
Stapleton, T. E., Lindsey, L. M., Sundar, H. & Dearing, M. D. Rodents consuming the same toxic diet harbor a unique functional core microbiome. Anim. Microbiome 6, 43 (2024).
Kuang, Z. et al. Host diet shapes functionally differentiated gut microbiomes in sympatric speciation of blind mole rats in Upper Galilee, Israel. Front. Microbiol. 13, 1062763 (2022).
Chen, C., Chen, S. & Wang, B. A glance at the gut microbiota and the functional roles of the microbes based on marmot fecal samples. Front. Microbiol. 14, 1035944 (2023).
Cabral, L. et al. Gut microbiome of the largest living rodent harbors unprecedented enzymatic systems to degrade plant polysaccharides. Nat. Commun. 13, 629 (2022).
Regan, M. D. et al. Nitrogen recycling via gut symbionts increases in ground squirrels over the hibernation season. Science 375, 460–463 (2022).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 32170538).
Author information
Authors and Affiliations
Contributions
K.M.S.: Conceptualization, Writing -original draft, Software, Visualization. H.M.: Supervision, Project administration, Writing—original draft. H.M.E.: Conceptualization, Writing—original draft, Validation. Y.J.W.: Formal analysis, Visualization. J.X.Z.: Data curation, Software. Y.Q.: Investigation. J.M.L.: Investigation; Resources. Z.Y.Z.: Investigation, Methodology. X.X.Z.: Conceptualization, Funding acquisition, Resources, Supervision. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shang, KM., Ma, H., Elsheikha, H.M. et al. Comprehensive genome catalog analysis of the resistome, virulome and mobilome in the wild rodent gut microbiota. npj Biofilms Microbiomes 11, 101 (2025). https://doi.org/10.1038/s41522-025-00746-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41522-025-00746-2
This article is cited by
-
Gut microbiota response to Enterocytozoon bieneusi infection in wild rodents: enhanced vitamin B and K2 biosynthesis pathways
BMC Genomics (2026)
-
Blastocystis infection enhances vitamins B and K2 biosynthesis in the Tibetan antelope (Pantholops hodgsonii) gut microbiota
BMC Genomics (2026)
-
Diet and environmental factors jointly drive the gut microbiome, resistome, and virulome of urban bats
npj Biofilms and Microbiomes (2026)