Abstract
We aimed to investigate the differences in prognosis between patients with HER2-low and HER2-zero status. This retrospective cohort study conducted at multi-institution included 1627 patients diagnosed with HER2-low or HER2-zero breast cancer (stages I–III). Survival analysis after propensity score matching was used. In total, 445 patients with HER2-low and 707 patients with HER2-zero status were included. The median follow-up was 92.7 months. Locoregional and distant recurrence-free survival were comparable between patients with HER2-low and HER2-zero status (p = 0.872, p = 0.746, respectively). HER2-low status did not affect overall survival. However, in subgroups with lymph node metastases, patients with HER2-low status showed better recurrence-free survival compared with that of patients with HER2-zero status (p = 0.033). In conclusion, survival outcomes were comparable between patients with HER2-low and HER2-zero breast cancer. More studies are needed to validate our findings and examine the biological mechanism underlying these prognostic differences.
Similar content being viewed by others
Introduction
Breast cancer with the low expression of human epidermal growth factor receptor 2 (HER2) has been previously classified as a luminal or triple-negative subtype, but the low HER2 expression has not been considered in treatment selection. With the emergence of novel anti-HER2 antibody-drug conjugates (trastuzumab deruxtecan, T-Dxd), clinical trial results1 have suggested that HER2-low positive breast cancer may be a clinically and biologically distinct disease entity2,3,4.
Some previous studies have reported on the prognostic implication of low HER2 expression; however, their findings were inconsistent, and the evidence remains insufficient to draw any conclusions5,6,7,8. Pooled analysis using previous prospective data by Denkert et al. showed that patients with HER2-low positive breast cancer had significantly better survival outcomes than those with HER2-zero (immunohistochemistry score of 0, no staining) breast cancer9. However, another nationwide study of more than 30,000 patients reported comparable overall survival (OS) outcomes for patients with HER2-low and HER2-zero breast cancer10.
It remains unclear how low HER2 expression affects survival outcomes in patients with breast cancer. Additional evidence is required to elucidate the contribution of this factor to prognostication in this patient group. This study aimed to investigate the differences in survival outcomes between patients with HER2-low and HER2-zero breast cancer types, using propensity-score matching. The secondary aim of this study was to examine factors associated with survival in patients with HER2-low status.
Results
We retrospectively collected information on 1627 patients with primary breast cancer with HER2-low or HER2-zero status. Before propensity score matching, there was no difference in age at diagnosis or TNM stage between two groups. However, patients with HER2-zero showed lesser HR positive tumor (p = 0.010) and higher histologic tumor grade (p = 0.005); they received more chemotherapy (p < 0.001) and less hormone therapy (p = 0.012), compared with their counterparts (Supplementary Table 1). After cox regression analysis of HER2 expression for survival outcomes in unmatched patients showed that HER2-low status was not prognostic factor associated with any survival outcome (Supplementary Table 2).
After adjusting for confounding factors using 1:2 propensity score matching, 445 (38.6%) patients with HER2-low status and 707 (61.4%) patients with HER2-zero status were included in the analysis (Table 1). Clinicopathologic variables including age, tumor stage, nodal stage, HR status, and histologic grade were well-balanced between the two groups. Furthermore, the rates of chemotherapy, radiotherapy, and hormone therapy were comparable between the groups. The mean age of the patients in the HER2-low status group was 51.4 years, and most of them had a tumor size <2 cm; additionally, a quarter of them had a poor tumor grade, and 89.9% received chemotherapy.
In the matched cohort, 914 (79.3%) patients had HR-positive breast cancer, and 238 (20.7%) had HR-negative cancer. The clinicopathological characteristics of the patients in the HER2-low and HER2-zero groups, stratified by HR status, were comparable (Supplementary Table 3). In the HR-positive/HER2-low subgroup (n = 357), approximately 40% of patients had a tumor size >2 cm, and 30.5% had nodal metastasis, while 89.1% and 91.0% received chemotherapy and hormone therapy, respectively. In the HR-negative / HER2-low subgroup (n = 88), approximately 46.6% of patients had a tumor size >2 cm, and 35.2% of patients had a nodal metastasis, while nearly all of them (93.2%) received chemotherapy. The use of adjuvant treatments did not differ between patients according to HER2 status and either HR-positive or HR-negative breast cancer.
Survival outcomes according to HER2 status
The median follow-up time was 92.7 months (range, 0.3–273.1 months). During the follow-up period, 79 and 68 cases of recurrence and death from any cause, respectively, were observed in the matched cohort. The 5-year OS rates for patients with HER2-low and HER2-zero were 95.4% and 97.6%, respectively. The corresponding 5-year disease-free survival rates were 96.6% and 95.0%, respectively. The Kaplan–Meier survival curves revealed no differences in OS, RFS, LRRFS, or DRFS rates between the groups (p = 0.293, p = 0.080, p = 0.872, p = 0.746, respectively) (Fig. 1). The Cox regression analysis results showed that HER2-low status did not affect survival outcomes (OS: hazard ratio, 1.298; 95% CI, 0.798–2.112; p = 0.294, RFS: hazard ratio, 0.639; 95% CI, 0.386–1.059; p = 0.082) (Table 2).
The HR-status was not associated with survival outcomes in either the HER2-low or HER2-zero group. Among patient with HR-positive subtype, the Kaplan–Meier survival curves revealed no differences in OS, RFS, LRRFS, or DRFS rates between the groups (p = 0.484, p = 0.065, p = 0.996, p = 0.630, respectively, Supplementary Fig. 1). Among patient with HR-negative subtype, there was no statistical difference in survival curves as well (Supplementary Fig. 2).
Similarly, in Cox regression analyses, the HER2-low status did not affect prognosis in subgroups according to the HR-status (Supplementary Table 4).
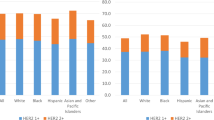
Additionally, the cohort was divided into subgroups based on the lymph node metastasis. Among 369 patients with lymph node metastasis, patients with HER2-low status received more axillary lymph node dissection and lesser chemotherapy than HER2-zero subgroup (p < 0.001 and p = 0.006, respectively). Interestingly, the RFS rates of patients with HER2-low status were higher than those of patients with HER2-zero status (p = 0.033, Fig. 2). However, there was no differences in other survival outcomes such as OS, LRRFS, and DRFS after cox regression analysis.
Discussion
Since the phase 3 clinical trial DESTINY-Breast 04 demonstrated the efficacy of T-Dxd in the treatment of metastatic HER2-low breast cancer, several studies have investigated the prognostic implications of HER2-low status11,12,13,14. However, evidence has been inconsistent, and the impact of HER2-low status on outcomes remains unclear. Thus, this study aimed to examine the survival outcomes of patients with HER-low status after propensity score matching, showing comparable long-term outcomes in patients with HER2-low and HER2-zero status. Additionally, patients with HER2-low status showed better survival outcomes compared with those with HER2-zero in the subgroup with lymph node metastasis.
Our findings are in line with those of previous studies. A nationwide study using a large retrospective registry dataset reported that OS rates were comparable between these groups10. However, some evidence suggests that breast cancer-specific survival rates may be better in patients with HER2-low status, especially those in the HR-negative subgroup, than in their counterparts. Nevertheless, this nationwide study lacked recurrence data and did not adjust for confounders, despite the risk of selection bias. Additionally, it had missing data on some prognostic variables included in multivariate analysis. In contrast, the present study analyzed long-term survival and disease recurrence data after adjusting several confounding factors.
Subgroup analysis among patients with lymph node metastasis demonstrated that patients with HER2-low status had better RFS than patients with HER2-zero, suggesting that the clinical impact of low HER2 expression may differ depending on lymph node status. These results are consistent with those of previous studies. Mutai et al., in a study of 608 patients with HR-positive breast cancer, reported that HER2-low status was associated with improved OS and RFS rates compared with those associated with HER-zero status in patients with high genomic risk13. Other studies have suggested that HER2-low status may be associated with a better prognosis compared with that associated with HER2-negative status in high-risk tumors, such as HR-negative tumors without a pathological complete response after neoadjuvant therapy, or those treated without any adjuvant therapy15. The mechanisms of these effects remain unclear and may be linked to some biological differences. However, improved outcomes may be achieved without anti-HER2 therapy, suggesting that HER2-low status may be a biological marker rather than an oncogenic driver in these subgroups. Gene expression analysis by Schettini et al. showed that proliferation-related genes were significantly downregulated, and luminal-related genes were upregulated in HER2-low breast cancer4. In addition, several pathologic characteristics related to reduced tumor aggressiveness, such as low Ki-67 labeling index, low tumor grade, and absence of lymphatic invasion, have been associated with HER2-low breast cancer16,17,18. As we lacked the relevant data, we could not test this hypothesis. Further research, including studies using in-depth molecular profiling and functional genomic analysis, is required to elucidate these effects.
In a study by Park et al., oncologic outcomes differed according to menopausal status among patients with HR-positive/HER2-low status19. Among postmenopausal patients with breast cancer, RFS and OS rates were higher in the HER2-low subgroup than in the HER2-zero subgroup. The authors proposed that the main cause of this difference in prognosis was the late recurrence in the luminal subtype. However, herein, after adjusting for patient age and endocrine therapy using propensity score matching, no difference in survival outcomes was observed between patients with the HER2-low and HER2-zero status. Moreover, the HER2-low status did not seem to affect disease prognosis in the subgroups defined by HR expression. Further studies are needed to understand outcome patterns according to HER2-low status.
Our study had some limitations. First, this was a retrospective study, likely subject to selection bias. To overcome the limitations of retrospective analyses, we conducted propensity score matching by adjusting for several important prognostic factors, helping address the limitations of some previous studies. Second, data on the HER2 status were based on local pathology reports, and no central confirmation of pathological assessment was performed. Although all participating institutions adhered to the guidelines of the American Society of Clinical Oncology/College of American Pathologists, the reproducibility of the reported results, especially those for immunohistochemistry scores of 0 or 1+, is not guaranteed. Third, in the matched sample, over 85% of all patients had received systemic chemotherapy. This skew may have affected the reported estimates, affecting generalizability of the findings to patients that do not undergo chemotherapy.
In conclusion, our study reported comparable survival outcomes between patients with HER2-low and HER2-zero breast cancer. In the subgroup with lymph node metastasis, the HER2-low status was associated with better survival outcomes than those with HER2-zero status. More studies are needed to validate our findings and examine the biological mechanism underlying these prognostic differences.
Methods
Patients with primary breast cancer who underwent curative surgery between January 2012 and December 2017 were retrospectively identified from four institutions in South Korea. The inclusion criteria comprised patients aged 18–75 years with invasive breast cancer (pT1-3, N0-3). The exclusion criteria were patients with de novo metastasis and those with pregnancy-associated breast cancer. The primary outcomes of interest in this study were locoregional recurrence-free survival (LRRFS), distant recurrence-free survival (DRFS), recurrence-free survival (RFS), and OS, which were compared between the patient groups. LRRFS was defined as the duration from diagnosis until the development of a recurrence in the breast or chest wall and/or regional lymph nodes on the side previously affected by cancer. DRFS was defined as the duration from diagnosis until the development of a recurrence in a distant organ. RFS was defined as the duration from diagnosis until any type of disease recurrence was detected. OS was defined as the duration from diagnosis until death.
Data on demographics such as age at the time of surgery, menopause status, date of diagnosis, and family history were collected. Pathologic information, including disease stage, tumor size, number of metastatic lymph nodes, histologic grade, Ki-67, hormone receptor (HR) status, and HER2 status, was documented. Surgical details, such as surgery date, the type of surgery performed (breast conserving surgery or mastectomy), any axillary surgery (no axillary surgery, sentinel lymph node biopsy alone, or axillary lymph node dissection), and post-operative complications, were recorded. Furthermore, we documented additional treatments received, including neoadjuvant or adjuvant chemotherapy, hormone therapy, target therapy, and radiation therapy. All instances of breast cancer recurrence, including locoregional recurrence, distant recurrence, and recurrence-free survival, were recorded during the follow-up period.
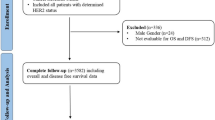
The patient selection process is summarized in Fig. 3. HER2-low breast cancer was defined as the breast cancer with immunohistochemistry (IHC) 1+, or 2+ and in-situ hybridization (ISH)-negative status. Among 1794 patients diagnosed with primary breast cancer between January 1, 2012, and December 31, 2017, those with ductal carcinoma in situ and insufficient survival data were excluded. Finally, 1152 patients were included in the analysis, including 707 patients with HER2-zero status and 445 patients with HER2-low status, after 1:2 propensity score matching.
This study was approved by the Institutional Review Board of Hanyang University Hospital (No. HYUH 2022-12-034), and followed the Declaration of Helsinki that protect human subjects in medical research.
Statistical analyses
The clinicopathological characteristics were compared between two groups using the Student’s t-test, chi-square test, and Fisher’s exact test. To mitigate the effects of any selection bias due to the retrospective nature of the study, propensity score matching with the greedy nearest neighbor method was used for all covariates to adjust for confounding factors in each group. The covariates were age, pathologic tumor stage, pathologic nodal stage, hormone receptor status, histologic grade, and adjuvant treatment (hormone therapy, radiation therapy, and chemotherapy. Each treated unit was matched with one control unit (1:2 greedy nearest neighbor matching method). After propensity score matching, the chi-square test, Fisher exact test, and t-test were performed to assess the balance between the two groups. The caliper was set to 0.01, maintaining the absolute value of the difference in logits of propensity scores at ≤0.01.
Survival curves were estimated using the Kaplan–Meier method, and the log-rank test was used to compare LRRFS, DRFS, RFS, and OS rates between the two groups. To calculate the hazard ratio for survival outcomes, along with the corresponding 95% confidence interval (CI), we conducted the Cox proportional hazards regression analysis. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, USA).
Data availability
Research data that support the findings of this study are securely stored in an institutional repository and are available to share from the corresponding author upon reasonable request.
Abbreviations
- DRFS:
-
distant recurrence-free survival
- HER2:
-
human epidermal growth factor receptor 2
- HR:
-
hormone receptor
- IHC:
-
immunohistochemistry
- ISH:
-
in-situ hybridization
- LRRFS:
-
locoregional recurrence-free survival
- OS:
-
overall survival
- RFS:
-
recurrence-free survival
References
Modi, S. et al. Trastuzumab Deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387, 9–20 (2022).
Agostinetto, E. et al. HER2-low breast cancer: molecular characteristics and prognosis. Cancers 13, https://doi.org/10.3390/cancers13112824 (2021).
Zhang, G. et al. Distinct clinical and somatic mutational features of breast tumors with high-, low-, or non-expressing human epidermal growth factor receptor 2 status. BMC Med. 20, 142 (2022).
Schettini, F. et al. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 7, 1 (2021).
Tarantino, P. et al. Prognostic and biologic significance of ERBB2-low expression in early-stage breast cancer. JAMA Oncol. 8, 1177–1183 (2022).
Tan, R. et al. HER2 expression, copy number variation and survival outcomes in HER2-low non-metastatic breast cancer: an international multicentre cohort study and TCGA-METABRIC analysis. BMC Med. 20, 105 (2022).
Jacot, W. et al. Prognostic Value of HER2-low expression in non-metastatic triple-negative breast cancer and correlation with other biomarkers. Cancers 13, https://doi.org/10.3390/cancers13236059 (2021).
Gampenrieder, S. P. et al. Landscape of HER2-low metastatic breast cancer (MBC): results from the Austrian AGMT_MBC-Registry. Breast Cancer Res. 23, 112 (2021).
Denkert, C. et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 22, 1151–1161 (2021).
Won, H. S. et al. Clinical significance of HER2-low expression in early breast cancer: a nationwide study from the Korean Breast Cancer Society. Breast Cancer Res. 24, 22 (2022).
Peiffer, D. S. et al. Clinicopathologic characteristics and prognosis of ERBB2-low breast cancer among patients in the National Cancer Database. JAMA Oncol. 9, 500–510 (2023).
Almstedt, K. et al. Long-term prognostic significance of HER2-low and HER2-zero in node-negative breast cancer. Eur. J. Cancer 173, 10–19 (2022).
Mutai, R. et al. Prognostic impact of HER2-low expression in hormone receptor positive early breast cancer. Breast 60, 62–69 (2021).
Chen, M. et al. Prognostic values of clinical and molecular features in HER2 low-breast cancer with hormonal receptor overexpression: features of HER2-low breast cancer. Breast Cancer 29, 844–853 (2022).
Kang, S. et al. Pathological complete response, long-term outcomes, and recurrence patterns in HER2-low versus HER2-zero breast cancer after neoadjuvant chemotherapy. Eur. J. Cancer 176, 30–40 (2022).
Horisawa, N. et al. The frequency of low HER2 expression in breast cancer and a comparison of prognosis between patients with HER2-low and HER2-negative breast cancer by HR status. Breast Cancer 29, 234–241 (2022).
Xu, H. et al. Clinicopathological characteristics and prognosis of HER2-low early-stage breast cancer: a single-institution experience. Front Oncol. 12, 906011 (2022).
Ergun, Y., Ucar, G. & Akagunduz, B. Comparison of HER2-zero and HER2-low in terms of clinicopathological factors and survival in early-stage breast cancer: A systematic review and meta-analysis. Cancer Treat. Rev. 115, 102538 (2023).
Park, W. K. et al. The Impact of HER2-low expression on oncologic outcomes in hormone receptor-positive breast cancer. Cancers 15, https://doi.org/10.3390/cancers15225361 (2023).
Acknowledgements
This study was supported by the research fund of Hanyang University (HY- 02300000003523). The authors would like to thank Editage (www.editage.co.kr) for English language editing.
Author information
Authors and Affiliations
Contributions
Concept and design: C.D.C., M.S.C. Acquisition, analysis, or interpretation of data: K.E.K., J.K., E.U., J.L., G.G., J.I.K. Drafting of the manuscript: C.D.C., M.S.C. Statistical analysis: N.C. Supervision: C.D.C., M.S.C.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
The retrospective study design warranted a waiver for the requirement of written informed consent by the institutional review boards.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cha, C.D., Kim, K.E., Kim, J. et al. Prognostic difference between early breast cancer patients with HER2 low and HER2 zero status. npj Breast Cancer 11, 31 (2025). https://doi.org/10.1038/s41523-025-00737-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-025-00737-8