Abstract
Epidemiologic data, supported by experiments, suggest aspirin may improve survival in breast cancer patients. However, recent trials reported a lack of protection, though the length of intervention was limited. Among 10,705 stages I–III breast cancer patients in the Nurses’ Health Studies (NHS/NHSII), we examined the associations between post-diagnostic aspirin use and long-term breast cancer survival. During up to 34 years of follow-up, regular post-diagnostic aspirin use was associated with a 38% and 28% lower risk of breast cancer-specific and total mortality. Associations were more evident with longer duration of post-diagnostic aspirin use but attenuated with higher stage and older age at diagnosis. Pre-diagnostic long-term aspirin use was associated with the downregulation of tumor proliferation pathways in NHS/NHSII and the aspirin-gene-expression-signature predicted better survival in METABRIC. Our study highlighted the need for trials with longer duration and suggested that aspirin use before diagnosis may alter the tumor-microenvironment towards a less proliferative type.
Similar content being viewed by others
Introduction
Aspirin, a commonly used anti-inflammation and anti-coagulation medication inhibits breast tumor growth and proliferation in multiple experimental settings1,2,3,4. Large meta-analyses of randomized trials of daily aspirin use originally designed for vascular events prevention revealed reduced cancer mortality in individuals randomized to aspirin5,6,7,8, and the protective effect was only evident with ≥5 years duration of treatment and ≥5 years of follow-up5. In the Nurses’ Health Studies (NHS), we previously reported a dose-dependent inverse association between post-diagnostic aspirin use and breast cancer mortality over 20 years, with 64% reduced risk of death from breast cancer among women who used aspirin 6–7 days per week, compared to non-users9. However, two recent aspirin intervention trials—one among largely healthy older individuals (the ASPirin in Reducing Events in the Elderly (ASPREE) trial)10,11, one conducted in breast cancer patients (the Aspirin after Breast Cancer (ABC) trial)12—reported null findings for daily aspirin use and death due to breast cancer or invasive disease-free survival (iDFS)10,12,13. These discrepancies may be attributable to differences in the duration of aspirin use and length of follow-up and may reflect differences in study population characteristics. In these trials, the median length of intervention (19.5 months–4.7 years) and follow-up (33.8 months–4.7 years) was short for both trials10,11,12,13. Short-term trials may miss the long-term and late benefits of adjuvant therapy14, which is particularly salient for hormone receptor-positive breast cancers where recurrence and breast cancer-specific death often occur a decade later or longer. There is increasing evidence from breast cancer patient trials that the effect of some adjuvant therapy may appear only after long-term follow-up15,16. In the ASPREE trial, the majority of participants were cancer-free at enrollment (81%)10, since the 5-year relative survival rate for breast cancer is 90%17, findings for breast cancer mortality within 3–5 years post-randomization among largely cancer-free individuals at enrollment in the ASPREE trial likely represents a unique subset of highly aggressive tumors.
Using the unique resources from the NHS/NHSII cohorts, with prospectively and repeatedly collected aspirin information both before and after breast cancer diagnosis, extensive data on clinical and participant covariates, and long duration of follow-up after breast cancer diagnosis (up to 34 years), we aimed to evaluate the relations between regular aspirin use (≥2 days/week), dose, and duration of aspirin use and breast cancer survival, taking into consideration variations in follow-up time, and participant, clinical, and tumor molecular features. Using tumor gene expression data, we also investigated the molecular underpinnings of the role of long-term aspirin use before breast cancer diagnosis in tumor prognosis. This analysis includes 57% more breast cancer cases and 68% more breast cancer-specific deaths than our prior paper9.
Results
Participant characteristics
Among the 10,705 eligible participants with stage I, II or III breast cancer, we documented 2825 deaths (26%), of which 1255 (12%) were due to breast cancer. Median duration of follow-up time was 12 years. Compared with nonusers, regular aspirin users after diagnosis were older, and more likely to be diagnosed with vascular diseases (Table 1). Regular aspirin users were also less likely to have been diagnosed with stage III disease or receive chemotherapy (Table 1). Thirty-four percent of regular aspirin users after diagnosis reported the use of baby aspirin. Among women with information on aspirin use before and after diagnosis (n = 9468), 22% women reported regular use both before and after diagnosis, and 20% women initiated aspirin use after diagnosis.
Associations between post-diagnostic aspirin use and long-term breast cancer survival
In multivariable analyses, regular post-diagnosis aspirin use was associated with a 38% lower risk of death from breast cancer and a 28% reduced risk of total mortality compared with nonusers (Table 2). The associations were independent of pre-diagnostic aspirin use. For breast cancer-specific mortality, when stratified by follow-up time, associations appeared stronger with longer time since diagnosis: 5-year HR = 0.74 (95% CI: 0.59–0.92), >5-to-15-year HR = 0.60 (95% CI: 0.49–0.72), and >15-year HR = 0.50 (95% CI: 0.35–0.72). Similar associations were observed for finer categories of aspirin use frequency (Supplementary Table 1). The association remained similar after accounting for other commonly used medications (i.e., beta blockers and statins) (HR = 0.66, 95% CI: 0.58–0.76) and other NSAIDs use (HR = 0.63, 95% CI: 0.55–0.71); when treating death due to all other causes as competing events, the association between regular post-diagnostic aspirin use and breast cancer mortality attenuated but remain significant (HR = 0.72, 95% CI: 0.66, 0.78). Regular use of aspirin before diagnosis was not associated with improved breast cancer survival (HR = 0.93, 95% CI: 0.77–1.12). Compared to women who were not regular aspirin users before and after diagnosis, women who initiated regular aspirin use after breast cancer diagnosis had a 39% decreased risk of death due to breast cancer HR = 0.61, 95% CI: 0.50–0.74).
The associations between aspirin use and breast cancer-specific mortality appeared stronger with a longer total duration of aspirin use as well as with a longer post-diagnostic duration of aspirin use (compared to non-current users, <5 years of aspirin use HR = 0.71 (95% CI: 0.58–0.86); ≥5 years of aspirin use HR = 0.56 (95% CI: 0.46–0.67)) (Table 3). The associations between aspirin and breast cancer mortality were similar across categories of dose. A similar trend was observed for total mortality.
The inverse associations between aspirin use and breast cancer mortality did not differ statistically by age of breast cancer diagnosis, BMI, smoking status, stage, or hormone receptor status (Table 4), although associations were attenuated with higher stage and with breast cancers diagnosed at older ages. Regular post-diagnosis aspirin use was associated with a 49% reduced risk of death from breast cancer for stage I survivors and a 22% lower risk of breast cancer mortality for stage III survivors. For women whose breast cancer was diagnosed ≥70, regular aspirin use after diagnosis was inversely, though not statistically associated with decreased breast cancer mortality (HR = 0.84, 95% CI: 0.59–1.20). The associations between aspirin and breast cancer survival were comparable in COX2-positive (HR = 0.71, 95% CI: 0.49–1.04) and in COX2-negative (HR = 0.42, 95% CI: 0.29–0.59) tumors (p-interaction = 0.09).
Mean age of breast cancer diagnosis was 60 years old and 81% of breast cancer survivors had age at diagnosis <70. Initiating aspirin use before (HR = 0.63, 95% CI: 0.51–0.77) or after (HR = 0.59, 95% CI: 0.46–0.77) diagnosis at a younger age (i.e., <60 years old) was associated with reduced breast cancer mortality (Supplementary Table 2). Although associations attenuated slightly, initiating aspirin use before (HR = 0.74, 95% CI: 0.56–0.97) or after (HR = 0.58, 95% CI: 0.46–0.74) diagnosis at ≥60 was also associated with reduced breast cancer mortality.
Long-term pre-diagnostic aspirin use and breast tumor gene expression
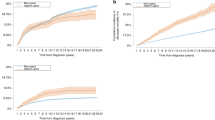
Pre-diagnostic long-term aspirin use was associated with downregulation of gene expression pathways implicated in cell cycle (mitotic spindle, G2M checkpoint), proliferation (estrogen response, PI3K-AKT mTOR signaling, E2F targets), DNA repair, and cellular stress (unfolded protein response) in primary tumors in the NHS (FDR ≤ 0.05) (Fig. 1a). A gene expression signature derived from the aspirin-associated pathways in the NHS was predictive of better survival in METABRIC: those with high (tertile 3) vs. low (tertile 1) aspirin gene expression score had 32% decreased risk of recurrence (Fig. 1b).
a Breast tumor gene expression pathways associated with pre-diagnostic long-term aspirin use in the NHS. Redline denotes FDR significance (FDR ≤ 0.05), blue line denotes nominal significance (p ≤ 0.05). b Aspirin gene expression signature derived from the aspirin-associated pathways from the NHS and recurrence free survival in METABRIC.
Discussion
In this large prospective analysis of breast cancer survivors with up to 34 years of follow-up, regular use of aspirin after breast cancer diagnosis was associated with reduced risk of breast cancer-specific and total mortality. The inverse associations were more evident with a longer duration of aspirin use and were stronger with a longer time since diagnosis, supporting a possible long-term benefit of aspirin use on cancer prognosis. Associations between aspirin use and breast cancer survival were attenuated for women with higher stage and with breast cancer diagnosis at older ages. It is worth noting that the majority of participants in our analysis were early stage (89% stage I and II), hormone receptor-positive breast cancers, and 81% had the age diagnosis <70 years. Our study provides a novel finding that long-term aspirin use before diagnosis was associated with the down-regulation of the cell cycle, proliferation, and cellular stress pathways in primary breast tumors, suggesting long-term aspirin use before diagnosis may have sub-clinical effects on breast tumor prognosis by possibly altering the tumor microenvironment towards a less proliferative type.
In a meta-analysis of randomized trials of daily aspirin use originally designed for vascular events prevention (n = 23,535), a significantly lower risk of cancer death was observed during the trial period (<9 years), and the beneficial effect of aspirin use was only evident with ≥5 years of treatment and ≥5 years of follow-up5. In post-trial analysis with long-term follow-up, daily aspirin use reduced the 20-year risk of death due to all solid cancers, and the association was stronger for patients with a longer duration of treatment (≥5 years)5. Similarly, our results suggest that the potential survival benefit of aspirin may be more evident with a longer duration of aspirin use and a longer length of follow-up. In a meta-analysis of cohort and case-control studies and electronic medical record data (n = 149,860), post-diagnostic aspirin use was significantly associated with lower breast cancer mortality (HR = 0.79, 95% CI: 0.64, 0.98)18. The associations were stronger for ER-positive breast cancer and in patients with lower stage18. Nevertheless, in the recent ABC trial12 and the ASPREE trial10, daily aspirin use was not associated with reduced breast cancer death or iDFS10,11,12.
In the recently completed ABC phase III randomized trial of 3020 breast cancer survivors aged 18–70 with a primary invasive HER2-negative cancer, 300 mg daily aspirin did not reduce iDFS with a median follow-up of 33.8 months vs. placebo13. All breast cancer survivors in the ABC trial were stage II and III at diagnosis, 89% were hormone receptor-positive, and 87.5% were diagnosed within 5 years of enrollment12. In the NHS with over 34 years of follow-up, we observed an association between longer duration of post-diagnostic aspirin use, longer follow-up, and increased breast cancer survival. It is possible the follow-up time and duration of aspirin use in ABC was insufficient to examine the long-term effect. There is increasing awareness that early dissemination of clinical trials may miss the late benefit of adjuvant therapies for breast cancer14. For example, in the randomized, phase III Suppression of Ovarian Function Trial with a 5-year scheduled treatment, the initial analysis (median follow-up 67 months) revealed no benefit of adding ovarian suppression to tamoxifen for disease-free recurrence16. However, after a median follow-up of 8 years, a survival benefit of the treatment was observed15. If the beneficial effects of aspirin emerge late, survival outcomes that occur earlier in the trial may largely reflect events not affected by aspirin, and analysis with short follow-up time may reveal no benefit of aspirin use. The Add-Aspirin trial, a large ongoing multi-center clinical study, that randomized 3662 breast cancer patients for aspirin use with longer treatment duration and follow-up time, may shed light on the use of longer-term aspirin as a secondary preventive agent for breast cancer recurrence/mortality.
In the ASPREE trial, which enrolled 19,114 older individuals without major chronic diseases, 100 mg daily aspirin use did not reduce breast cancer mortality over a median of 4.7 years10. It is plausible that starting aspirin at older ages may be too late to modulate the trajectory of tumor development19. In the U.S., 69% of new invasive breast cancers were diagnosed at age <7020. In the NHS cohorts, 81% of breast cancer patients were <70 at diagnosis. Our study was underpowered to study disease-free women, age ≥70, who initiated regular aspirin use after age 70 and died of breast cancer within 5 years. Moreover, since only 251 participants were diagnosed with breast cancer, the ASPREE trial had very limited power to evaluate mortality after breast cancer diagnosis with just 4.7 years of follow-up. Methodological studies also reveal that early treatment effects in a clinical trial may fluctuate considerably over time because of the small number of events21.
Several biological mechanisms have been hypothesized to underpin the influence of aspirin on cancer prognosis, including its anti-coagulation and anti-inflammation properties. Pro-coagulation factors may protect cancer cells from immune elimination and support the establishment of downstream secondary sites22. Higher platelet counts have been associated with poor prognosis of breast cancer23,24,25. Future research is needed to examine whether the relations between aspirin use and breast cancer survivorship differ by coagulation factors. Aspirin may also inhibit COX2-mediated inflammation. In both the current analysis and our prior report26, the survival benefit of aspirin did not differ by tumor COX2 expression26, suggesting that pathways other than COX2-mediated anti-inflammation processes may explain the associations26. Our gene expression analysis in primary breast tumors from the NHS revealed downregulation of cell cycle, proliferation, and cellular stress pathways associated with long-term pre-diagnostic aspirin use, this may suggest that aspirin use before breast cancer diagnosis has sub-clinical effects on breast tumor prognosis by altering primary tumor microenvironment towards a less proliferative molecular type and support a potential beneficial role of aspirin use at the early phases of carcinogenesis.
This study has several important strengths. The long duration of follow-up enabled us to evaluate the associations between regular aspirin use and breast cancer deaths over up to 34 years, which may be particularly relevant for breast cancer with good long-term survival rates27. Aspirin use was ascertained both before and after breast cancer diagnosis, allowing us to disentangle post-diagnostic aspirin use from pre-diagnostic aspirin use. To our knowledge, our study was the first to examine pre-diagnostic long-term aspirin use and breast tumor gene expression. Limitations include that the study was observational and aspirin use was not randomized. To minimize confounding by indication28,29, we employed propensity score methods to take into consideration population differences between regular and non-regular aspirin users. We also adjusted for an extensive list of breast cancer prognostic factors including nSES, and many dietary and lifestyle factors in the multivariant Cox regression. Our results were comparable after adjusting for participant, clinical, and tumor characteristics associated with breast cancer survival. However, we cannot rule out the possibility that aspirin use may be reflective of other breast cancer prognostic factors associated with prolonged survival that were not included in the regression models. Aspirin use in the NHS was based on self-reported questionnaires, however, as health professionals, it is likely that women’s responses reflected their actual use. The vast majority of breast cancers in the NHS were hormone receptor-positive and stage I and II at diagnosis, and the relationship between aspirin use and breast cancer survival was attenuated for women with higher stage and with breast cancer diagnosis at an older age. Thus, our findings may not be applicable to breast cancer survivors with aggressive forms of the disease or to individuals who initiated aspirin at older ages (≥70 years old) and died shortly after diagnosis. By updating the aspirin exposure every 2 years, we accounted for a participant’s aspirin exposure in the prior 2 years and minimized potential misclassification of the exposure. However, we cannot update changes in adherence within the 2-year interval. Participants in the NHS cohorts are predominately White, female nurses with a high socioeconomic status. Future studies assessing the use of long-term aspirin and breast cancer survival in more diverse populations are warranted. Finally, side effects of aspirin use, such as gastrointestinal tract bleeding will need to be considered.
To conclude, in this large prospective analysis of breast cancer survivors, regular use of aspirin after diagnosis was associated with reduced risk of breast cancer-specific and total mortality over 34 years of follow-up. The inverse associations were more evident with a longer duration of aspirin use and longer follow-up after diagnosis, supporting a potential latent benefit of aspirin use on cancer prognosis. Long-term aspirin use before diagnosis was associated with down-regulation of the cell cycle, proliferation, and cellular stress pathways in primary breast tumors, suggesting potential sub-clinical effects of aspirin on breast tumor prognosis by possibly altering the primary tumor microenvironment towards a less proliferative type. Clinical trials with longer intervention and follow-up after a cancer diagnosis may be useful.
Methods
Study population
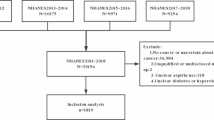
This study includes participants from the NHS and NHSII, two prospective cohorts of female registered nurses in the U.S., described previously30,31. In brief, the NHS was established in 1976 when 121,701 female, married registered nurses aged 30–55 years returned an initial questionnaire; and the NHSII was established in 1989 when 116,429 female registered nurses aged 25–42 years completed an initial questionnaire. Participants have been followed via biennial mailed questionnaire30. Diagnosis of breast cancer was reported by women on biennial questionnaires, and participants gave written permission for study physicians to review their medical records. Ninety-nine percent of self-reported breast cancers for which medical records were obtained have been confirmed by study physicians (blinded to exposure information)9. Eligible participants included women with confirmed invasive breast cancer in 1980–2019 for NHS and 1989–2019 for NHSII. We excluded participants if they had in situ, stage IV tumors, missing information on stage, or reported any cancer (other than nonmelanoma skin) before breast cancer diagnosis or before baseline aspirin assessment or had missing aspirin information on the first post-diagnostic questionnaire. This study included 10,705 participants who were diagnosed with stage I, II, or III breast cancer. The study protocol was approved by the institutional review boards (IRBs) of the Brigham and Women’s Hospital, Harvard T.H. Chan School of Public Health, and participating registries (1999P011117/BWH). The IRBs allowed participants’ completion of questionnaires to be considered as implied consent. The study was performed in accordance with the Declaration of Helsinki.
Assessment of aspirin use
Aspirin use was first assessed in 1980 in NHS and in 1989 in NHSII and every 2 years thereafter. Specifically, we collected information on frequency (number of days/week) and dose (number of tablets/week) and estimated total (pre- and post-diagnostic) and post-diagnostic duration (in years) of standard-dose (325 mg) aspirin use. Beginning in 1994 for NHS, to reflect secular trends in the consumption of low-dose baby aspirin (81 mg), participants were asked to convert the intake of 4 baby (81 mg) aspirin to 1 standard aspirin tablet. Since 2000 (NHS) and 2001 (NHSII), we collected information on the use of baby and standard-dose aspirin separately. Regular aspirin use was defined as using aspirin (standard- or low-dose) ≥2 days per week; nonusers were those who reported no use or use of aspirin <2 days per week. When calculating tablets per week, we converted the dose of baby aspirin to regular aspirin (divided by 4). We defined the age of regular aspirin initiation as the age of the participant’s first report of regular aspirin use (≥2 days/week). Post-diagnostic aspirin use was treated as a time-varying exposure updated every 2 years after diagnosis.
Assessment of death
Deaths were documented by reports from next of kin or the U.S. Postal Service or by searching the National Death Index32. Specific causes of death were ascertained by study physicians (blinded to exposure information) through medical records review or review of the death certificate.
Tumor gene expression
In NHS/NHSII, we profiled transcriptome-wide gene expression in tumor and tumor-adjacent normal tissue using microarray chips for formalin-fixed paraffin-embedded tumor tissues33. A detailed protocol has been published previously34,35,36,37. The NHS/NHSII included 359 women who were diagnosed with stage I, II, or III invasive breast cancer and reported aspirin use prior to diagnosis. In the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC), transcriptional profiling was performed on the Illumina HT-12 v3 platform38,39. All clinical and gene expression data was deposited at the European Genome–Phenome Archive under accession number EGAS0000000008338,39. A total of 953 stage I, II or III invasive cases were included in the METABRIC analysis.
Assessment of covariates
We obtained demographic characteristics, reproductive history, smoking status, physical activity, medical history, and medication use from biennial questionnaires. Body mass index (BMI) was calculated using height reported at baseline and weight reported biennially. A composite score of neighborhood socioeconomic status (nSES) was calculated based on census tract level of information from participants’ geocoded addresses, which included information on median income, median home value, percent white, percent in poverty, percent with a college degree, percent families with interest or dividends, percent occupied housing, and percent families headed by single female40,41. Tumor estrogen receptor (ER), progesterone receptor (PR), and HER2 status were determined with immunohistochemistry33. COX2 expression was assessed by dual-staining tumor microarrays with monoclonal antibodies produced by Cayman and Thermo Fisher Scientific42. Breast cancer treatment information was obtained from self-reported supplementary questionnaires.
Statistical analysis
We used multivariable Cox proportional hazards regression to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the prospective associations between post-diagnostic aspirin use, updated every 2 years, and breast cancer-specific and all-cause mortality. We used the time since diagnosis as the time scale to account for left truncation due to variations between participants in the timing of post-diagnostic assessment43,44. The Anderson–Gill data structure was used to handle time-varying exposure and covariates, updated 2 years. We started follow-up from the return date of the first post-diagnostic questionnaire. To avoid short-term changes in medication use during active treatment, we defined the first post-diagnostic aspirin use as aspirin reported on the first questionnaire collected ≥12 months after diagnosis45. Participants were followed until death or the end of follow-up (June 1, 2019), whichever came first. In each model, we adjusted for time-invariant covariates of age at diagnosis, calendar year of diagnosis, stage, treatment, pre-diagnostic BMI, oral contraceptive use, menopausal hormone use, parity, menopausal status, and ER status; and time-varying covariates updated every two years of change in BMI from pre- to post-diagnosis, post-diagnostic physical, post-diagnostic smoking, post-diagnostic nSES. We stratified all models by cohort and follow-up period. The proportional hazards assumption was tested by assessing the interaction term between aspirin exposure and time within each 2-year follow-up interval. No violation of the proportional hazards assumption was noted. To minimize population differences between regular and non-regular aspirin users, we computed a propensity score by estimating the probability of regular use of aspirin based on age, BMI, physical activity, nSES, and history of angina, myocardial infarction, stroke, or rheumatoid arthritis. As a sensitivity analysis, we further adjusted for the use of beta-blockers, statins and other non-steroidal anti-inflammatory drug (NSAIDs). To better approach the parameters of the trials, we modeled the relation between post-diagnostic regular aspirin use and mortality stratified by time since diagnosis and assessed whether total duration (pre- and post-diagnostic) and post-diagnostic duration of aspirin use were associated with mortality. We performed stratified analyses by participant, clinical, and tumor characteristics. As a sensitivity analysis, we used Fine and Gray sub-distribution hazards to account for competing risks when modeling breast cancer-specific mortality, with competing events defined as death due to all other causes.
Using a competitive gene set testing—which compared genes from one pathway to all other genes from the genome—we explored functional enrichment of pathways associated with pre-diagnostic long-term aspirin use (≥2 days/week for at least 10 years) compared to women who were not long-term regular aspirin users prior to diagnosis46. We chose the 50 cancer “hallmark” gene-sets from the Molecular Signature Database (http://www.broadinstitute.org/gsea/msigdb/)47. We identified individual genes contributing to significantly enriched pathways (FDR ≤ 0.05)48 and created a gene expression signature from the aspirin-associated pathways, calculated as the weighted sum of the relative levels of each gene by their coefficient with aspirin use.
Data availability
Further information including the procedures to obtain and access data from the Nurses’ Health Studies and Health Professionals’ Follow-up Study is described at https://www.nurseshealthstudy.org/researchers (contact email: nhsaccess@channing.harvard.edu) and https://sites.sph.harvard.edu/hpfs/for-collaborators/.
Code availability
The code used in this analysis is available upon request.
References
Henry, W. S. et al. Aspirin suppresses growth in PI3K-mutant breast cancer by activating AMPK and inhibiting mTORC1 signaling. Cancer Res. 77, 790–801 (2017).
Alfonso, L. F. et al. Aspirin inhibits camptothecin-induced p21CIP1 levels and potentiates apoptosis in human breast cancer cells. Int. J. Oncol. 34, 597–608 (2009).
Saha, S. et al. Aspirin suppresses the acquisition of chemoresistance in breast cancer by disrupting an NFkappaB-IL6 signaling axis responsible for the generation of cancer stem cells. Cancer Res. 76, 2000–2012 (2016).
Bhattacharya, A. et al. SMAR1 repression by pluripotency factors and consequent chemoresistance in breast cancer stem-like cells is reversed by aspirin. Sci. Signal. 13, eaay6077 (2020).
Rothwell, P. M. et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377, 31–41 (2011).
Peto, R. et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br. Med. J. (Clin. Res. Ed. 296, 313–316 (1988). .
Farrell, B. et al. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J. Neurol. Neurosurg. Psychiatry 54, 1044–1054 (1991).
Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk The Medical Research Council’s general practice research framework. Lancet 351, 233–241 (1998)..
Holmes, M. D. et al. Aspirin intake and survival after breast cancer. J. Clin. Oncol. 28, 1467–1472 (2010).
McNeil, J. J. et al. Effect of aspirin on all-cause mortality in the healthy elderly. N. Engl. J. Med. 379, 1519–1528 (2018).
McNeil, J. J. et al. Effect of aspirin on cancer incidence and mortality in older adults. J. Natl Cancer Inst. 113, 258–265 (2021).
Chen, W. Y. et al. A randomized phase III, double-blinded, placebo-controlled trial of aspirin as adjuvant therapy for breast cancer: the Aspirin after Breast Cancer (ABC) Trial. J. Clin. Oncol. 40, 360922–360922 (2022).
Chen, W. Y. et al. Aspirin vs. placebo as adjuvant therapy for breast cancer: the alliance A011502 randomized trial. JAMA 331, 1714–1721 (2024).
Lohmann, A. E. et al. The futility of futility analyses in adjuvant trials in hormone receptor-positive breast cancer. J. Natl Cancer Inst. 114, 924–929 (2022).
Francis, P. A. et al. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N. Engl. J. Med. 379, 122–137 (2018).
Francis, P. A. et al. Adjuvant ovarian suppression in premenopausal breast cancer. N. Engl. J. Med. 372, 436–446 (2015).
Siegel, R. L. et al. Cancer statistics, 2022. CA Cancer J. Clin. 72, 7–33 (2022).
Baker, A. & Kartsonaki, C. Aspirin use and survival among patients with breast cancer: a systematic review and meta-analysis. Oncologist 29, e1-e14 (2024).
Chan, A. T. Aspirin and the USPSTF—what about cancer?. JAMA Oncol. 8, 1392–1394 (2022).
Giaquinto, A. N. et al. Breast cancer statistics, 2022. CA Cancer J. Clin. 72, 524–541 (2022).
Lesaffre, E. et al. Statistical controversies in clinical research: futility analyses in oncology-lessons on potential pitfalls from a randomized controlled trial. Ann. Oncol. 28, 1419–1426 (2017).
Gay, L. J. & Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 11, 123–134 (2011).
Liu, S. et al. Elevated platelet count predicts poor prognosis in breast cancer patients with supraclavicular lymph node metastasis. Cancer Manag. Res. 12, 6069–6075 (2020).
Lal, I., Dittus, K. & Holmes, C. E. Platelets, coagulation and fibrinolysis in breast cancer progression. Breast Cancer Res. 15, 207 (2013).
Dirix, L. Y. et al. Coagulation/fibrinolysis and circulating tumor cells in patients with advanced breast cancer. Breast Cancer Res. Treat. 192, 583–591 (2022).
Holmes, M. D. et al. COX-2 expression predicts worse breast cancer prognosis and does not modify the association with aspirin. Breast Cancer Res. Treat. 130, 657–662 (2011).
Weiss, A. et al. Validation study of the American Joint Committee on Cancer Eighth Edition Prognostic Stage compared with the anatomic stage in breast cancer. JAMA Oncol. 4, 203–209 (2018).
Brookhart, M. A. et al. Propensity score methods for confounding control in nonexperimental research. Circ. Cardiovasc. Qual. Outcomes 6, 604–611 (2013).
Sturmer, T. et al. Propensity scores for confounder adjustment when assessing the effects of medical interventions using nonexperimental study designs. J. Intern. Med. 275, 570–580 (2014).
Eliassen, A. H. et al. Adult weight change and risk of postmenopausal breast cancer. JAMA 296, 193–201 (2006).
Eliassen, A. H. et al. Use of aspirin, other nonsteroidal anti-inflammatory drugs, and acetaminophen and risk of breast cancer among premenopausal women in the Nurses’ Health Study II. Arch. Intern. Med. 169, 115–121 (2009).
Stampfer, M. J. et al. Test of the National Death Index. Am. J. Epidemiol. 119, 837–839 (1984).
Tamimi, R. M. et al. Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res. 10, R67 (2008).
Wang, J. et al. Alcohol consumption and breast tumor gene expression. Breast Cancer Res. 19, 108 (2017).
Heng, Y. J. et al. Molecular mechanisms linking high body mass index to breast cancer etiology in post-menopausal breast tumor and tumor-adjacent tissues. Breast Cancer Res. Treat. 173, 667–677 (2019).
Kensler, K. H. et al. PAM50 molecular intrinsic subtypes in the Nurses’ Health Study Cohorts. Cancer Epidemiol. Biomarkers Prev. 28, 798–806 (2019).
Peng, C. et al. Prediagnostic 25-hydroxyvitamin D concentrations in relation to tumor molecular alterations and risk of breast cancer recurrence. Cancer Epidemiol. Biomark. Prev. 29, 1253–1263 (2020).
Curtis, C. et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486, 346–352 (2012).
Prat, A. et al. Molecular features and survival outcomes of the intrinsic subtypes within HER2-positive breast cancer. J. Natl Cancer Inst. 106, dju152 (2014).
DeVille, N. V. et al. Neighborhood socioeconomic status and mortality in the nurses’ health study (NHS) and the nurses’ health study II (NHSII). Environ. Epidemiol. 7, e235 (2022).
Kim, C. S. et al. Long-term aircraft noise exposure and risk of hypertension in the Nurses’ Health Studies. Environ. Res. 207, 112195 (2022).
McGee, E. E. et al. Erythrocyte membrane fatty acids and breast cancer risk by tumor tissue expression of immuno-inflammatory markers and fatty acid synthase: a nested case-control study. Breast Cancer Res. 22, 78 (2020).
Song, M. et al. Fiber intake and survival after colorectal cancer diagnosis. JAMA Oncol. 4, 71–79 (2018).
Cain, K. C. et al. Bias due to left truncation and left censoring in longitudinal studies of developmental and disease processes. Am. J. Epidemiol. 173, 1078–1084 (2011).
Wang, T. et al. Diabetes risk reduction diet and survival after breast cancer diagnosis. Cancer Res. 81, 4155–4162 (2021).
Wu, D. & Smyth, G. K. Camera: a competitive gene set test accounting for inter-gene correlation. Nucleic Acids Res. 40, e133 (2012).
Liberzon, A. et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 1, 417–425 (2015).
Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B (Methodol.) 58, 267–288 (1996).
Acknowledgements
This work was supported by the National Institutes of Health/National Cancer Institute (UM1 CA186107, P01 CA87969, R01 CA49449, U01 CA176726, R01 CA67262, R01 CA50385, T32 CA009001, U19 CA148065, R01 CA166666) and National Institute on Aging (K01AG080030). The authors would like to acknowledge the contribution to this study from central cancer registries supported through the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and/or the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program. Central registries may also be supported by state agencies, universities, and cancer centers. Participating central cancer registries include the following: Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Indiana, Iowa, Kentucky, Louisiana, Massachusetts, Maine, Maryland, Michigan, Mississippi, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Puerto Rico, Rhode Island, Seattle SEER Registry, South Carolina, Tennessee, Texas, Utah, Virginia, West Virginia, Wyoming. The authors assume full responsibility for analyses and interpretation of these data.
Author information
Authors and Affiliations
Contributions
C.P. contributed to data curation, formal analysis, investigation, methodology, manuscript writing, and manuscript review. P.J.S. contributed to resources and manuscript review. M.D.H., W.Y.C., W.C.W., and M.J.S. contributed to data curation, resources, and manuscript review. T.T.W., K.D.B., P.A.L., Y.J.H., and B.A.R. contributed to conceptualization, investigation, methodology, and manuscript review. A.H.E. contributed to conceptualization, funding acquisition, investigation, methodology, project administration, resources, supervision, manuscript writing, and manuscript review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Peng, C., Wang, T., Holmes, M.D. et al. Regular aspirin use, breast tumor characteristics and long-term breast cancer survival. npj Breast Cancer 11, 62 (2025). https://doi.org/10.1038/s41523-025-00775-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41523-025-00775-2