Abstract
Breast cancer (BC) displays age-related histopathologic and transcriptomic heterogeneity. Whether BC in elderly patients differs genetically from that of younger individuals remains unclear. We re-analyzed sequencing data from 1918 BCs previously subjected to an FDA-cleared paired tumor-normal targeted sequencing assay across elderly (≥65 years), middle-aged (>45 and <65 years) and young (≥45 years) patients. BCs in elderly individuals exhibited fewer germline but were numerically enriched in somatic homologous recombination deficiency (HRD)/DNA damage response (DDR) genetic alterations. Primary ER+/HER2- BC in elderly patients showed shifts in the spectrum of actionable PI3K/AKT alterations, whereas metastatic cases were enriched in FAT1 and RB1 mutations and fewer ESR1 mutations, suggesting age-dependent therapeutic resistance mechanisms. Metastatic ER+/HER2- lobular BCs were enriched in actionable ERBB2 mutations. Resistance-associated alterations were more prevalent in metastatic vs primary BC in elderly patients. Our findings reveal distinct actionable genetic features in elderly patients, highlighting the importance of genomic profiling and treatment personalization in this population.
Similar content being viewed by others
Introduction
Breast cancer (BC) is vastly heterogeneous in its histopathologic and genomic features, clinical outcomes and responses to therapy1,2,3. There is growing evidence to suggest that the biology, pathogenesis and genetic landscape of BC might vary according to age4. Compared to BCs in elderly individuals, BCs in younger patients have been found to be enriched for the basal-like molecular subtype5,6. Moreover, responses to chemotherapy markedly vary by age group, as reported in randomized clinical trials7.
For instance, patients with early-stage hormone receptor-positive/HER2-negative (HR+/HER2-) BC younger than 50 years old seem to derive greater benefit from the addition of adjuvant chemotherapy to endocrine therapy compared to their older counterparts, based on TAILORx and RxPONDER trial results8,9.
Whether the repertoire of genetic alterations affecting cancer genes in BC differs between elderly and younger patients remains to be fully elucidated. Previous work indicates that BCs occurring in older patients harbor a higher number of copy number alterations (CNAs), including 18p and 6q27 losses, a higher tumor mutational burden (TMB), and are enriched for KMT2D and FOXA1 somatic mutations, compared to younger patients10. Even though the frequency of BRCA1 and BRCA2 germline mutations in triple-negative BCs in patients over 65 years old is not negligible11, it is lower than that observed in BCs occurring in younger individuals12, that are enriched for genetic alterations affecting cancer predisposition genes13.
Here, we sought to determine the spectrum of genetic alterations in cancer-related genes and mutational signatures in BC in elderly individuals (≥65), compared to middle-aged (>45 and <65) and young (≤45) patients. Age thresholds of 45 and 65 years reflect established epidemiologic and policy frameworks14,15,16 and have been previously associated with distinct tumor biology17,18. Furthermore, we aimed to define the genomic drivers of progression in elderly patients through a comparison of the genetic alterations affecting metastatic and primary BC samples in the elderly population. To this aim, we performed a re-analysis of a large series of BC previously subjected to targeted sequencing using an FDA-authorized multigene sequencing assay, and investigated their mutational signatures utilizing methods applicable to formalin-fixed paraffin-embedded (FFPE) samples profiled with targeted capture sequencing.
Results
Clinicopathologic characteristics of breast cancer differ according to age
We reanalyzed targeted sequencing data from 1918 BC samples, including 918 primary and 1000 metastatic BC samples, previously subjected to the Memorial Sloan Kettering Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT)19, a paired tumor-normal targeted sequencing assay including up to 468 cancer genes. In this cohort, 318 patients were classified as elderly (≥ 65 years), 1009 as middle-aged (>45 and <65 years) and 591 as young (≤45 years).
The distribution across histologic subtypes was found to be different in primary BCs in elderly individuals compared to young patients (P < 0.05), with a higher proportion of invasive lobular carcinoma (ILC) in the elderly group (39/220, 18%), compared to the young subgroup (21/234, 9%) as shown in previous studies20. These findings are depicted in Supplementary Fig. S1, which displays the clinicopathologic characteristics according to age. Differences were also detected in the histologic grade, with BCs in elderly patients being of lower grade than those in young individuals (P < 0.001; Supplementary Fig. S1a). In primary BCs, the distribution of estrogen receptor (ER)/HER2 status was found to be distinct in the elderly population compared to middle-aged (P < 0.05) and young (P < 0.05) patients, with a higher prevalence of ER+/HER2- primary BCs in elderly patients (81%) than in the young group (73%), and a lower proportion of HER2+ cases in elderly individuals (6%) compared to young (14%) and middle-aged (9%) patients; (Supplementary Fig. S1a). Elderly patients with primary BC presented with a lower TNM stage at diagnosis compared to middle-aged (P < 0.01) and young patients (P < 0.001; Supplementary Fig. S1a), possibly reflecting earlier detection in this age group. Post-neoadjuvant therapy primary BC samples were less frequent in elderly (3.2%) vs young patients (10.7%; P < 0.01) and similar to middle-aged patients (5.4%; P > 0.05).
In metastatic BCs, we observed differences in histologic subtype between elderly and young patients (P < 0.001), with an enrichment in ILC in the elderly group (28% vs 10%; Supplementary Fig. 1b). No statistically significant differences in histologic grade/differentiation or ER/HER2 status were observed in the metastatic cohort (Supplementary Fig. 1b). We also found that elderly patients with metastatic BC more often presented with higher TNM stage at diagnosis (P < 0.01, vs middle-aged; P < 0.001, vs young; Supplementary Fig. 1b), possibly reflecting selection bias for advanced cases undergoing biopsy. In line with these findings, we observed a higher proportion of de novo metastatic disease in elderly (34.7%) vs middle-aged (21.7%; P < 0.01) and young patients (16.2%; P < 0.001), which may explain, at least in part, the TNM stage differences. Moreover, metastatic BC samples from elderly patients (72%) were more frequently collected prior to systemic therapy for metastatic disease compared to those from young patients (58%; P < 0.05), and at a comparable rate to those from middle-aged patients (66%; P > 0.05). Among pre-treated cases, samples from elderly patients were obtained after fewer lines of therapy compared to young patients (median 2, range 1–7 vs median 3 range, 1–4 lines; P < 0.01), with no differences compared to middle-aged patients (median 3, range 1–12; P > 0.05).
We next sought to determine the repertoire of somatic genetic alterations and mutational signatures in primary and metastatic BC in elderly patients, compared to middle-aged and young patients. To account for the molecular heterogeneity of BCs based on ER/HER2 status and histologic subtype21,22,23, we conducted the subset reanalysis of genetic alterations and mutational signatures of the ER+/HER2- BC cohort, which provided a sufficiently large sample size to draw robust conclusions (n = 1501). We separately analyzed primary (n = 739) and metastatic (n = 762) ER+/HER2- BCs from elderly, middle-aged and young patients, and subsequently according to histologic subtype. Analyses of the ER-/HER2- and HER2+ cohorts were not performed given the limited number of elderly patients in these groups, that would preclude meaningful conclusions to be drawn.
Enrichment in PIK3CA and CDH1 mutations and lower genomic instability in primary ER+/HER2- breast cancer in elderly patients
We conducted a comparative analysis in primary ER+/HER2- BC by age group. No statistically significant differences were observed in the histologic subtype distribution in elderly patients compared to the other two age groups among primary ER+/HER2- BCs (Fig. 1a and Supplementary Table S1), with most cases being invasive ductal carcinoma of no special type (IDC-NST) followed by ILC in elderly (74% and 20%), middle-aged (74% and 19%) and young (82% and 12%) individuals; (Fig. 1a and Supplementary Table S1).
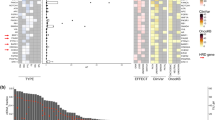
a Histologic subtype and histologic grade of primary ER+/HER2- breast cancer (BC) in elderly (n = 179), middle-aged (n = 390) and young (n = 170) patients. b Recurrent somatic genetic alterations in primary ER+/HER2- BC in elderly, middle-age, and young patients. Cases are shown in columns and genes in rows. Histological subtype, grade are shown in phenobars (top). Genetic alterations are color-coded according to the legend. Forest plots showing odds ratio (OR) of cancer genes altered at statistically significantly different rates in elderly individuals compared to young patients (c), and compared to middle-aged patients (d). Tumor mutational burden (TMB; e) and fraction of genome altered (FGA; f) of primary ER+/HER2- BC in elderly, middle-age, and young patients. g Dominant mutational signatures in primary ER+/HER2- BC in elderly, middle-age, and young patients. n.s., non-significant; *P < 0 .05, **P < 0.01, ***P < 0.001; Mann-Whitney U test and Fisher’s exact test. IDC-NST invasive ductal carcinoma of no special type, HRD homologous recombination deficiency, ILC invasive lobular carcinoma, mixed D-L mixed ductal-lobular.
In primary ER+/HER2- BCs from elderly (n = 179), middle-aged (n = 390) and young (n = 170) patients PIK3CA (52%, 43% and 35%, respectively) was the most frequently mutated gene across all groups, followed by CDH1 (22%), MAP3K1 (16%), TP53 and GATA3 (15%, each) in elderly patients (Fig. 1b). We observed a shift in the spectrum of PI3K/AKT/mTOR pathway genetic alterations according to age. AKT1 mutations were less frequent in elderly individuals (1%) compared to middle-aged (7%: P < 0.01) and young (6%: P < 0.01), while PIK3CA mutations were (52%) were more frequent in elderly vs young patients (35%; P < 0.01; Fig. 1b–d). Notably, AKT1 mutations were mutually exclusive (CoMet P = 0.031) with PIK3CA mutations in the whole cohort (Fig. 1b). In addition, mutations in CDH1 (22% vs 10%; P < 0.01) and TBX3 (8% vs 3%; P < 0.05), both characteristic of ILC24,25,26, were enriched in elderly compared to young and middle-aged patients, respectively (Fig. 1b–d). This likely reflects the numerically higher proportion of ILC cases in elderly patients (20%) compared to young patients (12%; Fig. 1a). Other genes more frequently altered in primary ER+/HER2- BC in elderly patients were the chromatin remodelers KMT2C (3% vs 0%; P < 0.05) compared to young patients, and KMT2B (3% vs 0.3%; P < 0.05) compared to middle-aged patients (Fig. 1b–d). Conversely, TP53 mutations were less frequent in elderly patients, compared to young (24%; P < 0.05) and middle-aged individuals (24%; P < 0.05; Fig. 1b–d), in line with their lower histologic grade (Fig. 1a and Supplementary Table S1).
The tumor mutation burden (TMB) of ER+/HER2- primary BC was comparable across age groups (Fig. 1e). No differences in the frequency of specific CNAs were observed as depicted in Supplementary Fig. S2a, that displays comparisons of CNAs across age groups. Nonetheless, the fraction of genome altered (FGA) was significantly lower in BCs in elderly (median, 0.12; range, 0–0.72) compared to young patients (median, 0.17; range, 0–0.85; P < 0.05; Fig. 1f). These findings indicate lower genome-wide genomic instability in elderly patients, in line with their lower frequency of rate of TP53 mutations, a key driver of genetic instability in BC.
To assess age related differences in mutational processes, we inferred mutational signatures using Signature Multivariate Analysis (SigMA)27, a bioinformatics tool optimized for targeted capture sequencing data from FFPE samples, in cases with ≥5 somatic single base substitutions (SBS), a threshold previously validated for MSK-IMPACT data by our group28,29. This resulted in a dataset of 79, 170 and 71 ER+/HER2- primary BCs from elderly, middle-age and young patients, respectively. BCs in elderly individuals showed a numerically higher frequency of aging/clock-like signature (49%) and numerically lower APOBEC signature (23%), compared to middle-aged (aging, 45%; APOBEC, 32%; P > 0.05) and young (aging, 41%; APOBEC, 38%; P > 0.05) patients (Fig. 1g). The lack of statistical significance observed could be due to the limited number of cases with ≥5 SBS per group (Fig. 1g).
Enrichment of ILC-associated alterations in elderly patients and age-related shifts in resistance alterations in metastatic ER+/HER2- breast cancer
Our analyses in the metastatic ER+/HER2- cohort revealed the histologic subtype distribution to be different between elderly and young BC patients (P < 0.001) with a higher frequency of ILC and mixed ductal-lobular cases in elderly (31% and 10%), compared to middle-aged (22% and 5%) and young (12% and 3%) individuals (Fig. 2a and Supplementary Table S1). No differences in histologic grade/differentiation were observed (Fig.2a and Supplementary Table S1), In addition, the metastatic site distribution differed according to age. Elderly individuals had fewer metastases to the liver and ovary (P < 0.05, each) and more chest wall metastases (P < 0.01) sequenced, compared to young patients. Chest wall metastases were also more frequent in elderly vs middle-aged patients (P < 0.01; Supplementary Fig. S3a). Of note, prior exposure to CDK4/6 or mTOR inhibitors was similar across age groups, suggesting that these treatments are unlikely to confound the rates of resistance associated genetic alterations.
a Histologic subtype and histologic differentiation of metastatic ER+/HER2- breast cancer (BC) in elderly (n = 81), middle-aged (n = 408) and young (n = 273) patients. b Recurrent somatic genetic alterations in metastatic ER+/HER2- BC in elderly, middle-age, and young patients. Cases are shown in columns and genes in rows. Histological subtype and histologic differentiation are shown in phenobars (top). Genetic alterations are color-coded according to the legend. Forest plots showing odds ratio (OR) of cancer genes altered at statistically significantly different rates in elderly individuals compared to young patients (c), and compared to middle-aged patients (d). Tumor mutational burden (TMB; e) and fraction of genome altered (FGA; f) of metastatic ER+/HER2- BC in elderly, middle-age, and young patients. g Dominant mutational signatures in metastatic ER+/HER2- BC in elderly, middle-age, and young patients. n.s. non-significant; *P < 0 .05, **P < 0.01, ***P < 0.001; Mann-Whitney U test and Fisher’s exact test. IDC-NST invasive ductal carcinoma of no special type, HRD homologous recombination deficiency, ILC invasive lobular carcinoma, mixed D-L mixed ductal-lobular.
Analysis of metastatic ER+/HER2- BC in elderly (n = 81), middle-aged (n = 408) and young individuals (n = 273) revealed a higher frequency of mutations in CDH1 in elderly (27%), compared to young patients (8%; P < 0.001; Fig. 2b, c). Moreover, in elderly patients, mutations in ILC associated genes were more frequent, including ERBB2 (15%) and the transcription factors NCOR1 (15%), RUNX1 (6%) and FOXA1 (10%), compared to young (ERBB2, 4%, P < 0.01; NCOR1, 3%, P < 0.001; RUNX1, 2%, P < 0.01) and middle-aged patients (ERBB2, 7%, P < 0.05; NCOR1, 4%, P < 0.01; FOXA1, 4%, P < 0.05; Fig. 2b–d). Accordingly, GATA3 mutations, reported to have a lower prevalence in ILC24 were less frequent in elderly vs young patients (12% vs 24%; P < 0.05; Fig. 2b, c), These findings likely reflect the enrichment of ILC observed in elderly compared to young individuals (Fig. 2a).
Metastatic ER+/HER2- BCs in elderly patients had a higher frequency of alterations linked to resistance to CDK4/6 inhibitors30,31, such as in FAT1 (11%) compared to young patients (4%; P < 0.05), and in RB1 (8%) compared to young (4%; P < 0.05) and middle-aged (3%; P < 0.05) patients (Fig. 2b–d). Notably, ESR1 mutations (10% vs 20%; P < 0.05), associated with resistance to endocrine therapy32, were less common in elderly compared to young patients (Fig. 2b–d). Given that ESR1 mutations have been reported to vary according to metastatic site33, we further examined their distribution in liver metastasis, which were less frequent in elderly patients. None of the ER+/HER2- liver metastasis from elderly individuals (0%; 0/14) harbored ESR1 mutations compared to 35% (30/85; P < 0.05) in middle-aged, and 28% (31/110; P < 0.01) in young patients (Supplementary Fig. S3b), suggesting that the differences observed cannot be explained solely by metastatic site distribution. These findings suggest age-related differences in therapeutic resistance mechanisms.
The TMB of metastatic ER+/HER2- BCs was higher in elderly (median 3.9, range 0–41) than in young patients (median, 3.9; range, 0–56; P < 0.05; Fig. 2e), but high TMB ( ≥ 10 mutations/Mb), an actionable biomarker for anti-PD1 immunotherapy benefit34, was uncommon (Fig. 2e). FGA was lower in elderly patients (median 18%, range 0–100%) than in young individuals (median 24%, range 0–100%; P < 0.05; Fig. 2f), with no differences in the frequency of specific CNAs (Supplementary Fig. S2b).
Mutational signatures were inferred in 56, 261 and 165 metastatic ER+/HER2- BC samples with ≥ 5 somatic mutations in elderly, middle-aged and young patients, respectively. APOBEC mutational signature, linked to endocrine resistance29,35,36, was the most prevalent in all groups (elderly, 38%; middle-aged, 41%; young, 35%), with no differences according to age (Fig. 2g).
Age related differences in PI3K/AKT/mTOR and MAPK/ERK pathways in invasive ductal carcinoma of no special type
We evaluated somatic genetic alterations in primary and metastatic ER+/HER2- BC by histologic subtype, conducting a separate analysis for IDC-NST and for ILC.
PIK3CA was the most frequently mutated gene in primary ER+/HER2- IDC-NST across all age groups (elderly, 47%; middle-aged, 41%; and young, 30%; Fig. 3a), which a higher mutational frequency in elderly than in young individuals (47% vs 30%; P < 0.01). Conversely, AKT1 mutations were less frequent in elderly compared to middle-aged patients (2% vs 6%; P < 0.05; Fig. 3a–c). MAP3K1 mutations were more prevalent in elderly vs young patients (17% vs 5%; P < 0.01), while MAP2K4 mutations were less common than in middle-aged individuals (2% vs 5%; P < 0.05; Fig. 3a–c). These findings highlight age-related differences in targetable genetic alterations in PI3K/AKT/mTOR and MAPK/ERK pathways. No differences in TMB or FGA were observed according to age (Supplementary Fig. S4a, b). Although not statistically significant, we observed a higher proportion of cases with a dominant aging signature in elderly patients (49%), compared to middle-aged (45%) and young (41%) patients (Fig. 3d).
Recurrent somatic genetic alterations in primary ER+/HER2- invasive ductal carcinoma of no special type (IDC-NST; a) and in metastatic lobular breast cancer (ILC; e) in elderly (primary IDC-NST, n = 133; metastatic ILC, n = 25), middle-aged (primary IDC-NST, n = 287; metastatic ILC, n = 90), and young (primary IDC-NST, n = 139; metastatic ILC, n = 32) patients. Cases are shown in columns and genes in rows. Genetic alterations are color coded according to legend. Forest plots depicting odds ratio (OR) of cancer genes altered at statistically significantly different rates between young and elderly (b, f) and middle-aged and elderly patients (c, g). Dominant mutational signatures in primary IDC-NST (d) and metastatic ILC (h) ER+/HER2- BC in elderly, middle-age, and young patients. (i) Lollipop plot of ERBB2 mutations in metastatic ER+/HER2- ILC in elderly, middle-aged, and young patients. ERBB2 domains and mutations are color coded according to the legend. n.s., non-significant; *P < 0 .05, **P < 0.01, ***P < 0.001; Mann-Whitney U test and Fisher’s exact test. IDC-NST invasive ductal carcinoma of no special type, HRD homologous recombination deficiency, ILC invasive lobular carcinoma, mixed D-L mixed ductal-lobular.
Comparisons of metastatic ER+/HER2- IDC-NST according to age were conducted and the findings depicted in Supplementary Fig. S5. In this subset, PIK3CA, TP53, and GATA3 were the most frequently mutated genes and ESR1 was frequently altered in all age groups (Supplementary Fig. S5a). We observed a higher frequency of FAT1 alterations in elderly (16%) compared to middle-aged (4%, P < 0.01) and young patients (4%, P < 0.01; Supplementary Fig. 5a). Elderly patients had lower FGA as compared to young patients (P < 0.05), with no differences were observed in TMB or mutational signatures (Supplementary Fig. S5b–d).
Enrichment of ERBB2 mutations in metastatic ER+/HER2- lobular breast cancer in elderly patients
Comparisons of primary ER+/HER2- ILC according to age were conducted and are shown in Supplementary Fig. S6. In primary ER+/HER2- ILC, as expected, CDH1 and PIK3CA were the most frequently altered genes across all age groups. Other frequent alterations affected ILC enriched genes, such as TBX3 and PTEN (Supplementary Fig. S6a). No significant differences were observed in mutational frequency, TMB, FGA and mutational signatures were identified between elderly and younger patients (Supplementary Fig. S6b–d).
Analysis of metastatic ER+/HER2- ILC in elderly (n = 25), middle-aged (n = 90) and young (n = 32) individuals showed CDH1 as the most frequently altered gene in all groups (68%, 86% and 63%, respectively), with PIK3CA, TP53 and ESR1 also frequently mutated (Fig. 3e). ILC-related genes, such as TBX3 (12%, 11% and 22%), NCOR1 (20%, 8% and 3%) and FOXA1 (12%, 8% and 9%) were frequently altered in all three groups (Fig. 3e). Notably, elderly patients had a significantly higher frequency of ERBB2 activating mutations (44%) compared to middle-aged (8%; P < 0.01) and young patients (9%; P < 0.001; Fig. 3e–g). No significant differences were observed in TMB, FGA or mutational signatures (Fig. 3h and Supplementary Fig. S4c, d).
Seventy-two percent of ERBB2 mutations identified across all age groups were oncogenic hotspot mutations (72%, 18/25 Fig. 3e). In elderly patients, most affected the L755 (33%, 4/12) and S310 (25%, 3/12) hotspot loci, both causing dysregulation of HER2 kinase activity37,38,39,40,41 and classified as clinically actionable (OncoKB actionability level 3A and an ESCAT level IIb42). In contrast, only two out of six ERBB2 mutations in young patients were oncogenic, which corresponded to Y772-A775 Exon 20 duplications43,44, that have OncoKB 3A and ESCAT IIb actionability levels38,42.
APOBEC mutagenesis and resistance alterations in progression of ER+/HER2- breast cancer in the elderly
To identify genetic alterations involved in BC progression, we compared primary (n = 179) and metastatic (n = 81) ER+/HER2- BCs in elderly patients. Metastatic cases were enriched for genetic alterations linked to endocrine resistance, such as ESR1 (10% vs 3%; P < 0.05) and ERBB2 (15% vs 2%; P < 0.001) and CDK4/6 inhibitor resistance, such as FAT1 (11% vs 2%, P < 0.01) and RB1 (9% vs 2%, P < 0.05; Fig. 4a). This was in line with an enrichment of dominant aging signature and a numerically higher frequency of APOBEC-dominant cases in metastatic vs primary ER+/HER2- BC in the elderly cohort (Fig. 4b), consistent with the association of APOBEC mutagenesis with endocrine resistance29,35,36. In agreement with these findings, evaluation of the individual mutational signature exposures revealed a higher APOBEC mutagenesis contribution in metastatic compared to primary cases (P < 0.05), and a trend toward lower aging signature exposure in metastases vs primary cases (Fig. 4c, d). Both TMB (median, 5.6; range 0–24) and FGA (median, 19%; range 0–78%) in metastatic ER+/HER2- BC of elderly patients were higher than in primary BC samples (TMB, median 3, range 0–19, P < 0.001; FGA, median 12%, range 0–72%, P < 0.01; Fig 4e, f). These findings suggest that APOBEC mutagenesis, linked to high TMB and genomic instability, might play key roles in ER+/HER2- BC progression of in the elderly population.
a Forest plots showing odds ratio (OR) of cancer genes altered at statistically significantly different rates between metastatic (n = 81) and primary (n = 179) ER+/HER2- breast cancer (BC) samples in elderly individuals. b Dominant mutational signatures in primary and metastatic ER+/HER2- BC in elderly patients. Boxplots depicting mutational signature exposures of aging (c) and APOBEC (d) signatures in primary and metastatic ER+/HER2- BC in elderly patients. Tumor mutation burden (TMB; e) and fraction of genome altered (FGA; f) or primary and metastatic ER+/HER2- BC. n.s., non-significant; *P < 0 .05, **P < 0.01, ***P < 0.001; Mann-Whitney U test and Fisher’s exact test.
Germline and somatic homologous recombination deficiency and DNA damage response in breast cancer in the elderly patients
Lastly, we assessed the frequency of pathogenic germline genetic alterations affecting homologous recombination-deficiency (HRD) and DNA damage response (DDR) genes, including BRCA1, BRCA2, ATM, CHEK2, PALB2, RAD51B, BARD1, BRIP1, RAD51C and RAD51D in the whole cohort, regardless of ER/HER2 status, comparing elderly patients to younger individuals. We observed that BCs in elderly patients harbored a numerically lower frequency of germline genetic alterations in HRD/DDR genes compared to BCs in young patients (3.9% vs 13.2%; P > 0.05; Fig. 5a). Conversely, BC in elderly patients had a numerically higher frequency of somatic alterations in HRD/DDR genes (4.7%) compared to middle-aged patients (3.1%; P > 0.05) and to young patients (2.9%; P > 0.05; Fig. 5a). The gene most frequently affected by pathogenic somatic alterations in the elderly was BRCA2 (2.2%), while ATM was the most frequent somatically altered HRD/DDR gene in middle-aged (0.9%) and young patients (1.2%; Fig. 5a). Notably, our analyses revealed that, although not statistically significant, a lower proportion of genetic alterations in HRD/DDR genes were bi-allelic in elderly patients (36.8%), compared to middle-aged (53.7%) and young (57.9%) patients (Fig. 5b). When restricting this analysis to somatic HRD/DDR alterations, the proportion of bi-allelic inactivation remained lower in elderly (35.3%) vs middle-aged patients (54.8%) but was comparable to the one in young patients (38.9%), as shown in Supplementary Table S2, which summarizes the germline and somatic HRD/DDR alterations by age group. Notably, most germline HRD/DDR alterations in young patients (75%) and 50% of those in middle-aged individuals were bi-allelic. Only two elderly patients in our cohort had germline alterations in an assessed HRD/DDR gene, one had a bi-allelic BRCA2 mutation, and the other a mono-allelic BARD1 mutation (Supplementary Table S2). These findings suggest that albeit at a higher frequency, a subset of somatic alterations in HRD/DDR genes in elderly patients might not constitute drivers of the genomic instability in these cancers.
Frequency of germline and somatic genetic alterations in homologous recombination deficiency (HRD)/DNA damage response (DDR) genes in elderly, middle-aged and young patients (a), and proportion of mono- and bi-allelic inactivation in HRD/DDR-altered cases according to age (b). Analysis includes all breast cancer samples regardless of sample type or ER/HER2 status. n.s. non-significant; Fisher’s exact test.
To further evaluate genomic instability, we assessed microsatellite instability (MSI) using MSIsensor scores derived from MSK-IMPACT data, available for 184 elderly, 743 middle-aged and 461 young patients. MSI-high status was observed in 1.1%, 0.8% and 2.2% of BC in elderly, middle-aged and young individuals, respectively. No statistically significant differences were observed across age groups (Supplementary Table S3).
Discussion
Our re-analysis of curated MSK-IMPACT targeted sequencing data of primary and metastatic BC samples22 in elderly patients compared to younger individuals showed an overall similar genetic make-up between the groups but also revealed important differences.
Metastatic ER+/HER2- BCs from elderly patients, compared to young patients were found to harbor a lower frequency of ESR1 mutations, that are associated with resistance to aromatase inhibitors32, and represent an actionable alteration with Elacestrant45,46. Conversely, metastatic ER+/HER2- BCs in elderly patients, compared to young patients, had a higher frequency in genetic alterations in RB1 and FAT1, associated to resistance to CDK4/6 inhibitors in ER + BC30. These findings indicate that mechanisms of therapeutic resistance may exhibit differential activity according to age. Moreover, the emergence of resistance associated alterations, such as RB1 and FAT1 mutations may reflect the combined effect of a longer cumulative duration of endocrine and other therapies, and changes in tumor biology associated with age, such as the accumulation of somatic mutations over time47, DNA methylation drift48, and diminished immunosurveillance49, which could help shape the evolution of BC in elderly patients.
Intratumor heterogeneity may also shape the genomic landscape of BC in elderly patients. While ESR1 mutations have been reported to be enriched in liver metastases33, the lower ESR1 mutation rate we observed in metastatic ER+/HER2- BC in elderly patients persisted even in liver lesions, suggesting that metastatic site distribution alone does not fully explain the differences observed. Future studies using paired primary-metastatic and multi-site samples, as well as single cell, spatial or liquid biopsy approaches, will help resolve the BC heterogeneity in this population.
The spectrum of genetic alterations in the PI3K/AKT/mTOR and MAPK pathways appears to vary according to age group. A lower frequency of AKT1 mutations and a higher prevalence of PIK3CA mutations were observed in primary ER+/HER2- BC in elderly patients compared to younger patients. Furthermore, a subset analysis of cases according to histologic type revealed that primary ER+/HER2- IDC-NST in elderly patients, had a higher frequency of MAPK3K1 mutations, and a lower frequency of AKT1 and MAP2K4 mutations, than in younger patients. These findings may reflect age-related differences in molecular subtype distribution, as previously reported18, with PIK3CA mutations enriched in Luminal A and AKT1 mutations in Luminal B tumors50. These differences were not seen in the metastatic BC studied here, possibly due to therapy induced changes that obscure age related or subtype specific mutational patterns.
Metastatic ER+/HER2- ILC affecting elderly patients, compared to middle-age and young patients, were found to harbor a higher frequency of ERBB2 activating mutations. While not directly assessed in this study, these mutations are known to confer resistance to trastuzumab, pertuzumab and first generation anti-HER2 tyrosine kinase inhibitors (TKIs), such as lapatinib37,51,52. These findings suggest that elderly patients could be candidates to an alternative treatment with HER2 irreversible inhibitors such as neratinib, newer generation TKIs, such as tucatinib, antibody drug conjugates, that show activity in patients with ERBB2-mutant tumors37,51,52,53. We observed differences in the mechanism of ERBB2 dysregulation according to age. While metastatic ER+/HER2- ILC in elderly patients were found to harbor predominantly ERBB2 extracellular domain or Exon 18 activation mutations, these are not found in young patients, whose tumors harbored Exon 20 insertion mutations, that are associated with lower activity or resistance to neratinib and other HER2-TKIs43. Further studies are warranted to define the basis and biological implications of the different modalities of ERBB2 mutations in this context.
Our comparison of ER+/HER2- metastatic and primary BC samples from elderly patients revealed important differences, such as higher rates of alterations in genes associated to resistance to CDK4/6 inhibitors (FAT1 and RB1) and to endocrine therapy (ESR1 and ERBB2). These findings suggest that the genomic landscape of metastatic ER+/HER2- BC in elderly patients is shaped in great part by therapeutic pressure. Notably, our analyses revealed greater exposure to APOBEC mutational signatures along with high TMB and FGA in metastatic vs primary BC in the elderly subgroup. These findings suggest that APOBEC mutagenesis, that induces hypermutation and genomic instability, and that might play roles in resistance to endocrine therapy, might be a major driver of progression in elderly patients.
In agreement with previous work13, we observed a lower frequency of germline genetic alterations in HRD/DDR genes in elderly patients compared to young patients. Interestingly, we also observed a numerically higher frequency of somatic alterations in this group of genes in elderly patients; these were mostly mono-allelic, however, in contrast to the predominantly bi-allelic events identified in younger patients. These findings suggest a lower likelihood of functional HRD in elderly patients, potentially reflecting age-associated mutagenesis with an increase in likely passenger alterations in these cases, rather than true selection of HRD tumor clones. Although we did not assess epigenetic alterations as a second hit, and a formal HRD score could not be applied given that these analyses were based on a targeted sequencing panel, both limitations of our study, the lack of enrichment in HRD mutational signatures in BCs from elderly individuals, supports this interpretation. Nonetheless, a subset of somatic HRD/DDR alterations in BCs from elderly individuals may still have therapeutic relevance, which warrants further investigation. Although there is conflicting information on the actionability of somatic HRD/DDR genetic alterations other than BRCA1/254, somatic mutations in BRCA2, that we observed in the elderly population, have an OncoKB actionability level 3A, and an ESCAT level IIB for PARP inhibitors, based on the results of the TBCRC-048 clinical trial assessing olaparib in patients with metastatic BC with HRD mutations54. This highlights the importance of comprehensive functional and allelic characterization of HRD/DDR alterations in the elderly population.
Future studies should investigate the functional relevance of somatic HRD/DDR alterations in elderly patients, including the role of epigenetic silencing as second hit in mono-allelic cases, as well as the assessment of HRD genomic scars using whole-genome sequencing (WGS) data. WGS could also uncover the identification of large structural variants not detectable by targeted sequencing and allow the better characterization of APOBEC mutagenesis. In addition, given the association of APOBEC mutagenesis with resistance to endocrine therapies and targeted therapies29, and actionability of HRD/DDR alterations55,56, evaluation of these processes in BCs from elderly patients in clinical trials may inform their role as predictive biomarkers in this population.
Our study has limitations, such as the lack of gene expression data for BC subtype identification. In addition, our analysis was limited to 468 cancer genes present in the MSK-IMPACT panel. Moreover, the numbers of elderly patients were small in the ER-/HER2- and HER2+ cohorts, which precluded the analyses in those groups. Differences in the number of treatment lines across age groups in the metastatic cohort represent an additional limitation. Moreover, we observed age related differences in the proportion of primary BC samples collected post-neoadjuvant therapy, introducing further heterogeneity into the cohort. Despite these limitations, our analyses revealed important differences in the genomic landscape of BC in elderly patients compared to younger individuals, such as distinct rates of germline and somatic HRD/DDR alterations. We also observed differences in the spectrum of PI3K/ATK/mTOR and MAPK/ERK pathway actionable alterations in ER+/HER2- BC, and a higher frequency of actionable ERBB2 hotspot mutation in metastatic ER+/HER2- ILC in elderly individuals.
These findings support routine genomic profiling of BC in elderly patients for the identification of actionable genetic alterations and to guide treatment personalization.
Methods
Subject and samples
This study was approved by an MSK Institutional Review Board (IRB) and as part of the study initially published by Razavi et al.22, for which informed consent was provided. Targeted massively parallel sequencing data from 1918 BCs (primary, n = 918; metastatic, n = 1000) previously analyzed using the FDA-authorized MSK-IMPACT assay were retrieved from the study by Ravazi et al.22. Among 1918 samples, 82 were primary-metastasis pairs, (41 pairs), while 1836 were unpaired. Of the 1000 metastatic samples, 986 (98.6%) were distant metastases and 14 (1.4%) were local recurrences.
Patients were classified according to age at BC diagnosis as elderly (≥ 65 years), middle-age (between >45 and <65 years) and young (≤45 years)10,57. BCs were classified into subtypes according to ER expression defined by immunohistochemistry (IHC) and HER2 expression by IHC and/or fluorescence in situ hybridization (FISH), as previously described22 and following the American Society of Clinical Oncology (ASCO) and College of American Pathology (CAP) guidelines58,59.
Targeted massively parallel sequencing analysis
SBSs, insertions/deletions (Indels) and gene-specific CNAs of up to 468 cancer-related genes identified by clinical FDA-authorized MSK-IMPACT60,61 sequencing were retrieved from the study by Razavi et al.22. Mutational hotspots were detected and annotated using a previously described algorithm39,40 from Razavi et al.22. TMB was calculated as total number of mutations per megabase. The raw MSK-IMPACT sequencing data (i.e., BAM files) were reprocessed using our validated bioinformatics pipeline, as previously described19,62,63, for the inference of genome-wide copy number gains and losses, loss of heterozygosity (LOH) of genes targeted by somatic mutations and mutational signatures. Mutational signatures were defined using the SigMA tool27 in all cases with at least five SBSs, as previously described64. CNAs were detected using the FACETS algorithm65, as previously described. FGA, calculated as the fraction of the genome that is not diploid divided by total genome, was retrieved from Razavi et al.22. Gene mutational frequencies were represented as elderly, middle-age and young, respectively. Germline variants for BRCA1, BRCA2, ATM, CHEK2, PALB2, RAD51B, RAD51C, RAD51D, BRIP1 and BARD1 were retrieved under a prospective IRB-approved protocol66,67,68. Variants with >1% frequency in the Genome Aggregation Database69 were discarded. Remaining variants were classified based on the American College of Medical Genetics and Genomics guidelines70. Germline analysis included exon deletions and duplications. Splice-site variants targeting intronic positions up to 1–3 bp from exon-intron junctions were considered. MSI status was assessed using MSIsensor71 scores and categorized as MSI-high (score ≥ 10), MSI-indeterminate (score ≥ 3 and <10) or MSI-stable (score < 3)72,73.
Statistical analysis
Statistical analysis was carried out using R v3.1.2. All tests were two-tailed. Fisher’s exact test was performed for comparison of categorical variables. Chi-square tests were applied for larger contingency tables when expected cell counts were sufficient. Mann-Whitney U test was performed for continuous variables. Mutual exclusivity was calculated using CoMEt74 in the maftools package75, as previously described76. Statistical tests were performed to compare BC in elderly vs in middle-aged individuals, BC in elderly vs in young individuals, and metastatic vs primary BC in elderly patients, stratified by ER/HER2 status and/or histologic subtype. P < 0.05 (unadjusted) were considered statistically significant. Microsatellite instable cases were excluded from TMB comparative analysis.
Data availability
The MSK-IMPACT sequencing data supporting the findings of this study are publicly available in cBioPortal at the following accession: https://identifiers.org/cbioportal:breast_msk_2018.
References
Martelotto, L. G., Ng, C. K., Piscuoglio, S., Weigelt, B. & Reis-Filho, J. S. Breast cancer intra-tumor heterogeneity. Breast Cancer Res. 16, 210 (2014).
Weigelt, B., Geyer, F. C. & Reis-Filho, J. S. Histological types of breast cancer: how special are they?. Mol. Oncol. 4, 192–208 (2010).
Pareja, F. & Reis-Filho, J. S. Triple-negative breast cancers—a panoply of cancer types. Nat. Rev. Clin. Oncol. 15, 347–348 (2018).
Feng, Y. et al. Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 5, 77–106 (2018).
Wang, M. X., Ren, J. T., Tang, L. Y. & Ren, Z. F. Molecular features in young vs elderly breast cancer patients and the impacts on survival disparities by age at diagnosis. Cancer Med. https://doi.org/10.1002/cam4.1544 (2018).
Jenkins, E. O. et al. Age-specific changes in intrinsic breast cancer subtypes: a focus on older women. Oncologist 19, 1076–1083 (2014).
Piccart, M. J. et al. Gene expression signatures for tailoring adjuvant chemotherapy of luminal breast cancer: stronger evidence, greater trust. Ann. Oncol. 32, 1077–1082 (2021).
Sparano, J. A. et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N. Engl. J. Med. 379, 111–121 (2018).
Kalinsky, K. et al. 21-Gene assay to inform chemotherapy benefit in node-positive breast cancer. N. Engl. J. Med. 385, 2336–2347 (2021).
Azim, H. A. Jr., Nguyen, B., Brohee, S., Zoppoli, G. & Sotiriou, C. Genomic aberrations in young and elderly breast cancer patients. BMC Med. 13, 266 (2015).
Boddicker, N. J. et al. Risk of late-onset breast cancer in genetically predisposed women. J. Clin. Oncol. JCO2100531, https://doi.org/10.1200/JCO.21.00531 (2021).
Young, S. R. et al. The prevalence of BRCA1 mutations among young women with triple-negative breast cancer. BMC Cancer 9, 86 (2009).
Apostolou, P. & Fostira, F. Hereditary breast cancer: the era of new susceptibility genes. Biomed. Res. Int. 2013, 747318 (2013).
Jung, A. W. et al. Multi-cancer risk stratification based on national health data: a retrospective modelling and validation study. Lancet Digit. Health 6, e396–e406 (2024).
Tittmann, J. et al. Breast cancer stage and molecular subtype distribution: real-world insights from a regional oncological center in Hungary. Discov. Oncol. 15, 240 (2024).
Institute of Medicine (US) Committee to Design a Strategy for Quality Review and Assurance in Medicare. Medicare: A Strategy for Quality Assurance. Washington (DC) (Lohr K. N.) (National Academies Press (US), 1990).
Anders, C. K. et al. Age-specific differences in oncogenic pathway deregulation seen in human breast tumors. PLoS ONE 3, e1373 (2008).
Anders, C. K. et al. Breast carcinomas arising at a young age: unique biology or a surrogate for aggressive intrinsic subtypes?. J. Clin. Oncol. 29, e18–e20 (2011).
Cheng, D. T. et al. Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J. Mol. Diagn. 17, 251–264 (2015).
Van Baelen, K. et al. Current and future diagnostic and treatment strategies for patients with invasive lobular breast cancer. Ann. Oncol. 33, 769–785 (2022).
Pareja, F., Weigelt, B. & Reis-Filho, J. S. Problematic breast tumors reassessed in light of novel molecular data. Mod. Pathol. 34, 38–47 (2021).
Razavi, P. et al. The genomic landscape of endocrine-resistant advanced breast cancers. Cancer Cell 34, 427–438.e426 (2018).
Pareja, F. et al. The genomic landscape of metastatic histologic special types of invasive breast cancer. NPJ Breast Cancer 6, 53 (2020).
Ciriello, G. et al. Comprehensive molecular portraits of invasive lobular breast cancer. Cell 163, 506–519 (2015).
Desmedt, C. et al. Genomic characterization of primary invasive lobular breast cancer. J. Clin. Oncol. 34, 1872–1881 (2016).
Corso, G., Veronesi, P., Sacchini, V. & Galimberti, V. Prognosis and outcome in CDH1-mutant lobular breast cancer. Eur. J. Cancer Prev. 27, 237–238 (2018).
Gulhan, D. C., Lee, J. J., Melloni, G. E. M., Cortes-Ciriano, I. & Park, P. J. Detecting the mutational signature of homologous recombination deficiency in clinical samples. Nat. Genet. 51, 912–919 (2019).
Selenica, P. et al. APOBEC mutagenesis, kataegis, chromothripsis in EGFR-mutant osimertinib-resistant lung adenocarcinomas. Ann. Oncol. 33, 1284–1295 (2022).
Gupta, A. et al. APOBEC3 mutagenesis drives therapy resistance in breast cancer. Nat. Genet. https://doi.org/10.1038/s41588-025-02187-1 (2025).
Condorelli, R. et al. Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann. Oncol. 29, 640–645 (2018).
Li, Z. et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the hippo pathway. Cancer Cell 34, 893–905 e898 (2018).
Oesterreich, S. & Davidson, N. E. The search for ESR1 mutations in breast cancer. Nat. Genet. 45, 1415–1416 (2013).
Rinaldi, J. et al. The genomic landscape of metastatic breast cancer: insights from 11,000 tumors. PLoS One 15, e0231999 (2020).
Marcus, L. et al. FDA approval summary: pembrolizumab for the treatment of tumor mutational burden-high solid tumors. Clin. Cancer Res. 27, 4685–4689 (2021).
Bertucci, F. et al. Genomic characterization of metastatic breast cancers. Nature 569, 560–564 (2019).
Law, E. K. et al. The DNA cytosine deaminase APOBEC3B promotes tamoxifen resistance in ER-positive breast cancer. Sci. Adv. 2, e1601737 (2016).
Bose, R. et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 3, 224–237 (2013).
Chakravarty, D. et al. OncoKB: a precision oncology knowledge base. JCO Precis. Oncol. 2017, https://doi.org/10.1200/PO.17.00011 (2017).
Chang, M. T. et al. Identifying recurrent mutations in cancer reveals widespread lineage diversity and mutational specificity. Nat. Biotechnol. 34, 155–163 (2016).
Chang, M. T. et al. Accelerating discovery of functional mutant alleles in cancer. Cancer Discov. 8, 174–183 (2018).
Robichaux, J. P. et al. Pan-cancer landscape and analysis of ERBB2 mutations identifies poziotinib as a clinically active inhibitor and enhancer of T-DM1 activity. Cancer Cell 36, 444–457 e447 (2019).
Crimini, E. et al. Precision medicine in breast cancer: from clinical trials to clinical practice. Cancer Treat. Rev. 98, 102223 (2021).
Robichaux, J. P. et al. Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat. Med. 24, 638–646 (2018).
Tan, A. C. et al. Clinical and genomic features of HER2 exon 20 Insertion mutations and characterization of HER2 expression by immunohistochemistry in East Asian non-small-cell lung cancer. JCO Precis Oncol. 6, e2200278 (2022).
Bidard, F. C. et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized phase III EMERALD trial. J. Clin. Oncol. 40, 3246–3256 (2022).
Boscolo Bielo, L. et al. Genomic and clinical landscape of metastatic hormone receptors-positive breast cancers carrying ESR1 alterations. ESMO Open 9, 103731 (2024).
Martincorena, I. Somatic mutation and clonal expansions in human tissues. Genome Med 11, 35 (2019).
Horvath, S. Erratum to: DNA methylation age of human tissues and cell types. Genome Biol. 16, 96 (2015).
Pawelec, G. Immunosenescence and cancer. Biogerontology 18, 717–721 (2017).
Turova, P. et al. The Breast Cancer Classifier refines molecular breast cancer classification to delineate the HER2-low subtype. NPJ Breast Cancer 11, 19 (2025).
Giordano, S. H. et al. Systemic therapy for advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO guideline update. J. Clin. Oncol. JCO2200519, https://doi.org/10.1200/JCO.22.00519 (2022).
Hyman, D. M. et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554, 189–194 (2018).
Ferraro, E., Drago, J. Z. & Modi, S. Implementing antibody-drug conjugates (ADCs) in HER2-positive breast cancer: state of the art and future directions. Breast Cancer Res. 23, 84 (2021).
Tung, N. M. et al. TBCRC 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J. Clin. Oncol. 38, 4274–4282 (2020).
Henry, N. L. et al. Biomarkers for systemic therapy in metastatic breast cancer: ASCO guideline update. J. Clin. Oncol. 40, 3205–3221 (2022).
Batalini, F. et al. Homologous recombination deficiency landscape of breast cancers and real-world effectiveness of poly ADP-ribose polymerase inhibitors in patients with somatic BRCA1/2, germline PALB2, or homologous recombination deficiency signature. JCO Precis. Oncol. 7, e2300091 (2023).
Anders, C. K., Johnson, R., Litton, J., Phillips, M. & Bleyer, A. Breast cancer before age 40 years. Semin Oncol. 36, 237–249 (2009).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: ASCO-College of American Pathologists Guideline Update. J. Clin. Oncol. 41, 3867–3872 (2023).
Allison, K. H. et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J. Clin. Oncol. 38, 1346–1366 (2020).
Chin, C. L. et al. A human expression system based on HEK293 for the stable production of recombinant erythropoietin. Sci. Rep. 9, 16768 (2019).
Penson, A. et al. Development of genome-derived tumor type prediction to inform clinical cancer care. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2019.3985 (2019).
Pareja, F. et al. Loss-of-function mutations in ATP6AP1 and ATP6AP2 in granular cell tumors. Nat. Commun. 9, 3533 (2018).
Weigelt, B. et al. The landscape of somatic genetic alterations in breast cancers from ATM germline mutation carriers. J. Natl. Cancer Inst. 110, 1030–1034 (2018).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Shen, R. & Seshan, V. E. FACETS: allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic Acids Res. 44, e131 (2016).
Terraf, P. et al. Comprehensive assessment of germline pathogenic variant detection in tumor-only sequencing. Ann. Oncol. 33, 426–433 (2022).
Cheng, D. T. et al. Comprehensive detection of germline variants by MSK-IMPACT, a clinical diagnostic platform for solid tumor molecular oncology and concurrent cancer predisposition testing. BMC Med Genom. 10, 33 (2017).
Mandelker, D. et al. Mutation detection in patients with advanced cancer by universal sequencing of cancer-related genes in tumor and normal DNA vs guideline-based germline testing. JAMA 318, 825–835 (2017).
Karczewski, K. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 (2020).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 17, 405–424 (2015).
Niu, B. et al. MSIsensor: microsatellite instability detection using paired tumor-normal sequence data. Bioinformatics 30, 1015–1016 (2014).
Middha, S. et al. Reliable pan-cancer microsatellite instability assessment by using targeted next-generation sequencing data. JCO Precis. Oncol. 2017, https://doi.org/10.1200/PO.17.00084 (2017).
Yang, S. R. et al. Microsatellite instability and mismatch repair deficiency define a distinct subset of lung cancers characterized by smoking exposure, high tumor mutational burden, and recurrent somatic MLH1 inactivation. J. Thorac. Oncol. 19, 409–424 (2024).
Leiserson, M. D., Wu, H. T., Vandin, F. & Raphael, B. J. CoMEt: a statistical approach to identify combinations of mutually exclusive alterations in cancer. Genome Biol. 16, 160 (2015).
Mayakonda, A., Lin, D. C., Assenov, Y., Plass, C. & Koeffler, H. P. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 28, 1747–1756 (2018).
da Silva, E. M. et al. TERT promoter hotspot mutations and gene amplification in metaplastic breast cancer. NPJ Breast Cancer 7, 43 (2021).
Acknowledgements
Research reported in this publication was supported in part by a Cancer Center Support Grant of the NIH/NCI (Grant No. P30CA008748). A.M. is supported by the European Society for Medical Oncology (ESMO) José Baselga Fellowship for Clinician Scientists sponsored by AstraZeneca. S.C. and B.W. are supported in part by the Breast Cancer Research Foundation. S.C., B.W., and F.P. are supported in part by an NIH/NCI MSK SPORE on Genomic Instability in Breast Cancer P50CA247749 01 grant. F.P. is funded in part by an FDA 1U01FD007909-01A1 grant.
Author information
Authors and Affiliations
Contributions
J.S.R.F., B.W., and F.P. conceived and designed the study. P.S., H.D., M.R., T.B., A.M.G., C.J.S., F.D., A.M., L.F. accrued and analyzed the data. J.S.R.F., B.W. and F.P. contributed to the interpretation of the results. P.S., H.D., M.R. and F.P. drafted the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
A.M. reported consulting or advisory role for Menarini/Stemline and AstraZeneca, honoraria as speaker’s bureau from Roche and Eli Lilly, received travel accommodation from Menarini/Stemline and Daiichi Sankyo, outside the submitted work. S.C. has received institutional grant/funding from Daiichi-Sankyo, AstraZeneca, and Lilly, Share options Totus Medicines, and consultation/Ad board/Honoraria from AstraZeneca, Lilly, Casdin Capital, Nuvalent, Blueprint, and SAGA Diagnostics. J.S.R.-F. is an employee of AstraZeneca and owns AstraZeneca stocks. Prior Conflicts of Interest in the last 2 yeas include the receipt of personal fees for the following activities: Board Membership at Grupo Oncoclinicas, consultant for Goldman Sachs Merchant Banking, consultant for Bain Capital, consultant for and SAB member of Paige.ai, consultant for and SAB member of Repare Therapeutics, Consultant of SAGA Diagnostics, Consultant of Personalis, and consultant at MultiplexDx. B.W. reports research grants from REPARE Therapeutics and SAGA Diagnostics paid to the institution, and employment of an immediate family member at AstraZeneca. F.P. reports membership on advisory boards for AstraZeneca and MultiplexDx, as well as receipt of consultancy fees from AstraZeneca. All other authors have nothing to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Selenica, P., Dopeso, H., Repetto, M. et al. Genomic landscape of breast cancer in elderly patients. npj Breast Cancer 11, 70 (2025). https://doi.org/10.1038/s41523-025-00781-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41523-025-00781-4