Abstract
Invasive breast carcinomas associated with microglandular adenosis (IBC-MGA) represent a rare and poorly characterized form of triple-negative breast cancer (TNBC). We analyzed clinical, pathological, and germline genetic data from 38 patients, including 34 IBC-MGAs and 4 in situ cases. Germline pathogenic or likely pathogenic variants in homologous recombination-deficiency (HRD) genes were found in 42% (16/38) of patients, predominantly in BRCA1 (81%, 13/16). Most tumors were grade 3 invasive ductal or metaplastic carcinomas with limited tumor-infiltrating lymphocytes. No significant clinicopathologic differences were observed between germline HRD-associated and sporadic cases. Paired tumor-normal targeted sequencing revealed frequent TP53 mutations and high HRD scores. These findings underscore the relationship of breast carcinomas associated with MGA with HRD-related germline variants and highlight the potential for targeted therapeutic strategies and the importance of genetic testing in this rare subset of TNBC.
Similar content being viewed by others
Introduction
Microglandular adenosis (MGA) of the breast is a rare and unusual glandular proliferation notable for its lack of a myoepithelial cell layer and is regarded as a non-obligate precursor of triple-negative breast cancer (TNBC)1,2,3,4. Breast carcinomas (BCs) arising in MGA span a histologic spectrum that includes in situ and invasive carcinoma, commonly referred to as breast carcinoma associated with MGA5,6,7.
Invasive carcinoma associated with MGA (IBC-MGA) typically exhibits a triple-negative phenotype and most commonly corresponds to the histologic subtypes of invasive carcinoma of no special type (IDC-NST), acinic cell carcinoma, or matrix-producing metaplastic carcinoma8,9,10,11,12,13,14. We, along with other individual case reports, have observed MGA or IBC-MGA in BRCA1 germline mutation carriers, prompting us to investigate the potential association between IBC-MGA/MGA and BRCA1 germline mutations15,16 and homologous recombination-deficiency (HRD), a process underlying a subset of breast cancers. HRD, due to mutations in canonical HRD genes, such as BRCA1, BRCA2 or PALB2, impairs the error-free repair of DNA double-strand breaks, resulting in genomic instability and tumorigenesis17,18,19. Defining HRD germline predisposition in IBC-MGA would prove invaluable from the therapeutic perspective, given that patients with HRD BCs exhibit benefit from PARP inhibitor (PARPi) therapy, which induce synthetic lethality by blocking single-strand DNA repair pathways20. This study aims to determine the hereditary risk predisposition in patients with IBC-MGA and BC (in situ) associated with MGA (BCis-MGA) through the genetic profiling of these cases.
Results
Study cohort
Our cohort included thirty-eight patients diagnosed with either IBC-MGA (n = 34) or BCis-MGA (n = 4) who had previously undergone germline genetic testing (Table 1 and Supplementary Table S1). The median age at diagnosis across the cohort was 46 years (range: 26−77). The most common clinical presentation was a mammographically detected (34%, 13/38) or palpable mass (34%, 13/38). Magnetic resonance imaging (MRI) revealed mass or non-mass enhancement in nine patients (24%, 9/38). Additionally, three patients (8%) presented with mammographically detected calcifications. A personal history of BC was reported in 24% of patients (9/38), including one patient with synchronous contralateral IDC-NST and one patient with synchronous ipsilateral ductal carcinoma in situ (DCIS). Seven patients had a history of multiple malignancies (18%, 7/38). Non-breast malignancies included tubo-ovarian high-grade serous carcinoma (n = 3), uterine carcinoma (n = 2), melanoma (n = 2), lung cancer (n = 1), colon cancer (n = 1) and meningioma (n = 1; Supplementary Table S1). Notably, a family history of BC was reported in 63% (24/38) of patients with 37% (14/38) of them having a first-degree relative with BC (Table 1 and Supplemental Table S1).
On germline genetic testing, 42% (16/38) of patients were found to carry a germline pathogenic or likely pathogenic variant (P/LPV) in an HRD gene. Most of them affected BRCA1 (81%, 13/16), including recurrent P/LPVs, such as p.Gln1756fs in four patients and p.Ser267Lysfs in two patients. Besides BRCA1, other P/LPVs affected BRCA2 (13%, 2/16) and PALB2 (6%, 1/16) (Supplementary Table S1). Two patients (5%) carried a germline alteration in HRD genes classified as a variant of unknown significance (VUS), including mutations in BRCA1 p.Thr1691 (Case 16) and BARD1 p.Ile764Thr (Case 38).
Clinicopathologic characteristics
Histologically, most BC in this study were IBC-MGA (89%, 34/38); four cases (11%; 4/38) corresponded to BCis-MGA (Table 1). The median untreated invasive tumor size was 15 mm (range 2 to 55 mm). The cohort was enriched for matrix-producing metaplastic carcinomas, which constituted almost a third of the cases in the study (32%, 11/34, Fig. 1a). Most, however, were IDC-NST (59%, 20/34), with two tumors showing acinic cell features (6%, 2/34; Fig. 1b). One tumor was a spindle cell metaplastic carcinoma (3%, 1/34). All IBC-MGA were ER-negative and HER2-negative (Supplementary Table S1), and the majority showed Nottingham histologic grade 3 (82%, 28/34; Fig. 1c). Lymphovascular invasion was present in 18% (7/38) of cases, and 21% had lymph node metastases (7/33). The majority of tumors (85%, 29/34) demonstrated <50% stromal tumor-infiltrating lymphocytes most of which exhibited <20% stromal TILs (82%, 28/34).
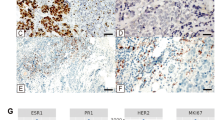
A Case 2, a 46 year-old BRCA1 c.181 T > G carrier with matrix-producing metaplastic carcinoma associated with atypical microglandular adenosis (B). Case 35, a 32 year-old with invasive carcinoma showing acinic cell features and associated atypical microglandular adenosis. Prominent granules in acinic cell-like areas merge with invasive carcinoma (C) Case 6, a 47 year-old BRCA1 c.3607 C > T carrier with invasive ductal carcinoma of no special type and adjacent breast carcinoma (in situ) associated with MGA (BCis-MGA) and atypical microglandular adenosis (2X) Areas of frank invasion transition from BCis-MGA (D) Oncoplot illustrating the germline pathogenic/likely pathogenic variants in patients along with their associated clinicopathologic characteristics. Stacked bar plots showing comparison of clinicopathologic features between HRD germline carriers and sporadic tumors, including tumor infiltrating lymphocytes (TILs; E), % of response (pCR) to neoadjuvant chemotherapy (F), and histologic type (G). HRD homologous recombination deficiency, VUS variant of unknown significance, IDC-NST invasive ductal carcinoma of no special type, BCis-MGA breast carcinoma (in situ) associated with microglandular adenosis, TILs tumor infiltrating lymphocytes, pCR pathologic complete response.
Most patients underwent mastectomy (55%, 21/38), while 45% (16/38) had breast conserving surgery. Eleven patients with IBC-MGA (32%, 11/34) received neoadjuvant chemotherapy (NACT), and pathologic complete response (pCR) was observed in 18% (2/11) of them (Table 1).
Most IBC-MGA received adjuvant treatment, including radiotherapy (68%, 21/31) and chemotherapy (77%, 24/31). Six patients with IBC-MGA experienced disease recurrence, including one local recurrence (3%, 1/31) and five distant metastases (16%, 5/31).
One of four patients with BCis-MGA received radiotherapy and none received chemotherapy. No patients with BCis-MGA (n = 4) experienced disease recurrence (Supplementary Table S1).
Clinicopathologic features of BC-MGA in patients with germline HRD alterations and sporadic cases
Given the distinct clinicopathologic features associated with HRD in BCs and the high prevalence of germline HRD variants in BCs associated with MGA, we conducted a secondary analysis to compare cases with germline HRD alterations (gHRD, n = 16) to sporadic cases (n = 22; Fig. 1d; Supplementary Table S2). Patients with gHRD and had a comparable median age at presentation to sporadic cases (45 years (range: 26−67) vs. 46.5 years (range 32-77) (P-value = 0.9).
Although two patients with IBC-MGA in the gHRD group achieved pCR compared to none in the sporadic group, this finding did not achieve statistical significance (40%, 2/5 vs. 0%, 0/6; P-value = 0.4). No other clinicopathologic factors were found to be distinct between the two cohorts (Fig. 1e–g; Supplementary Table S2), although interpretation is limited due to the small cohort size.
Somatic genetic alterations and HRD features of IBC-MGA
Panel sequencing targeting up to 505 genes (MSK-IMPACT) was performed on six IBC-MGA cases, including four primary tumors (two matrix-producing metaplastic carcinomas, one spindle cell metaplastic carcinoma, and one IDC-NST and two metastatic tumors (one matrix-producing metaplastic carcinoma and one IDC-NST). Among these, 2 of 6 cases were associated with P/LPV germline alterations: Case 5, a metastatic matrix-producing metaplastic carcinoma, was a BRCA1 germline mutation carrier, and Case 37, a metastatic IDC-NST, was from a PALB2 germline mutation carrier. All other tumors subjected to panel sequencing lacked P/LP germline alterations in HRD genes, including Case 38, a primary metaplastic spindle cell carcinoma.
The median tumor mutational burden and fraction of genome altered were 5.8 mut/Mb (range, 1.8−10) and 41% (range, 1−99), respectively. Whole-genome duplication was observed in 2/6 (33%) cases (Fig. 2a). Five of six BCs (83%, 5/6) harbored pathogenic TP53 mutations. Recurrent alterations were also observed in genes involved in chromatin remodeling (67%, 4/6), particularly KMT2A (50%, 3/6) and KMT2C (33%, 2/6) (Fig. 2a). Two BCs (33%, 2/6) harbored alterations in either PTEN and/or CASP8. No somatic alterations affecting HRD genes were observed.
Heatmap depicting individual cases are displayed in columns, while genes are organized in rows. Phenobars in the right panel indicate the invasive carcinoma subtype, the sample type sequenced, identified genetic alterations and IMPACT-HRD scores (A). IMPACT-HRD scores across cases (B), including number of telomeric imbalances, nTAI (C), large scale state transitions, LST(D) and genome wide loss of heterozygosity, LOH (E). TMB tumor mutational burden, FGA fraction of genome altered, HRD homologous recombination deficiency, WGD whole genome duplication, IBC-MGA invasive breast carcinoma associated with microglandular adenosis, LOH loss of heterozygosity, P/LPV pathogenic/likely pathogenic variant, VUS variant of unknown significance.
Given the observed prevalence of germline HRD alterations in IBC-MGAs, we next investigated whether these tumors exhibited genomic features associated with HRD defects. We utilized an IMPACT-HRD score21, which incorporates genome wide LOH, nTAI, and LST, with a threshold of ≥50 considered indicative of the HRD phenotype (see Methods). In the five IBC-MGA cases analyzed (100%, 5/5; Fig. 2b–e) all exhibited high HRD scores (≥50, see Methods), including Case 5, which harbored a germline BRCA1 mutation and somatic LOH.
Although this analysis was conducted on a limited number of cases, these findings align with the high prevalence of germline HRD alterations in this cohort and further support a key role for HRD in the pathogenesis of IBC-MGA.
Discussion
Through germline profiling, this study identified a novel association between IBC-MGA and germline alterations in HRD-associated genes, found in 42% of cases. In line with these findings, all IBC-MGA cases with available paired tumor/normal targeted sequencing data exhibited HRD genomic features and were classified as HRD. This association has significant therapeutic implications, as a subset of patients with IBC-MGA may benefit from PARPi. These findings highlight the importance of germline genetic testing in IBC-MGA patients to guide treatment decisions.
Although all IBC-MGA cases exhibited HRD genomic features, only one case harbored a pathogenic germline BRCA1 P/LPV with biallelic LOH. No somatic alterations in other core HRD genes were identified in the remaining cases. The underlying genomic basis for HRD was not elucidated in these cases. It is plausible that IBC-MGA may harbor genetic or epigenetic alterations in HRD pathway genes that are not detectable by targeted sequencing, such as complex structural rearrangements or epigenetic alterations.
Despite a range of histologic phenotypes including a notable enrichment in metaplastic carcinomas, IBC-MGA appears to have lower overall levels of tumor-infiltrating lymphocytes (TILs), with only 5% of cases showing >50% TILs22, compared to triple-negative IDC-NST. Additionally, the pathologic complete response (pCR) rate was slightly lower at 18%, compared to 28% observed in conventional TNBC23. Both examples of pCR occurred in patients with P/LPV variants in HRD genes. Further studies with larger sample sizes are recommended to verify this observation. Interestingly, all four patients with BCis-MGA remained disease-free at the time of the study. This supports the notion that in the absence of frankly invasive carcinoma with stromal desmoplasia and inflammation, BCis-MGA may behave akin to ER-negative ductal carcinoma in situ, as previously reported24. Nonetheless, the disease recurrence observed in a subset of patients with IBC-MGA (16%) underscores the importance of potential targeted therapy. Given the high rate of germline alterations in HRD genes and HRD genomic features identified in these breast cancers, PARPi may offer potential benefit.
Paired tumor-normal targeted sequencing in six cases, including a gHRD case with a BRCA1 inactivating mutation, revealed highly recurrent TP53 somatic mutations in IBC-MGA, akin to both MGA and those reported in conventional TNBC and mammary acinic cell carcinoma (AciCC)12,13. Mammary AciCC is a BC that shares significant morphologic and genomic overlap with IBC-MGA, and indeed 6% of IBC-MGA in our study showed acinic cell features. Recent studies have shown that mammary AciCC is a distinct entity from its salivary gland counterpart, as it lacks the characteristic t(4;9) (q13;q31) genomic rearrangement and NR4A3 overexpression, both of which are hallmark features of salivary gland AciCC25,26. Interestingly, P/LPV BRCA1 germline variants have been identified in patients with AciCC. These observations support the notion that mammary AciCC and IBC-MGA or BCis-MGA with acinic cell features are very closely related lesions, and may in fact represent a spectrum of the the same entity.
This study has limitations, such as the small cohort size, due to the rarity of IBC-MGA, in which <100 of these tumors are described in the literature. Additionally, MSK-IMPACT was conducted in only six cases and the MSK-IMPACT panel includes only 505 cancer genes, which targets a predefined set of genes, restricting the identification of a broader group of genetic alterations.
In summary, we identified an association between BC-MGA and germline pathogenic/likely pathogenic variants in HRD-associated genes, particularly in BRCA1. While IBC-MGA in germline and sporadic contexts shares similar clinical features, these findings may have important implications for future treatment strategies and personalized medicine approaches.
Methods
Study cohort and data collection
The cohort consisted of 38 patients, including 34 patients with IBC-MGA and 4 patients with BCis-MGA over a 24 year period. This study was approved by the Institutional Review Board at Memorial Sloan Kettering Cancer Center (MSK). Clinicopathologic data for all cases were collected, including genetic testing data, patient demographics (age and family history), tumor size, treatment regimens, and outcomes. Individuals with likely pathogenic or pathogenic mutations in cancer susceptibility genes (CAGs) were curated by a board-certified medical geneticist (PT) following the criteria established by the American College of Medical Genetics and Genomics27.
Histopathologic assessment
Histopathologic assessment of each tumor was evaluated by at least two study breast pathologists (CS and HYW). The assessment of histologic subtype was conducted following the World Health Organization classification criteria1. Estrogen receptor (ER) and human epidermal growth factor 2 (HER2) were obtained from pathology reports and previously assessed according to the American Society of Clinical Oncology/College of American Pathologists recommendations28. The extent of tumor-infiltrating lymphocytes (TILs) was determined in all invasive tumors following the criteria set forth by the International TILs Working Group22.
Statistical analysis
Statistical analyses were conducted using R version 1.2. Fisher’s exact test was used for categorical variables, and continuous variables were compared using the student’s t-test. All tests were two-sided, with a P-value of <0.05 considered statistically significant.
Next-generation sequencing and HRD score analysis
We investigated somatic genetic alterations in subset of cases (n = 6) that had been previously subjected to the FDA-approved paired tumor-normal targeted sequencing using the MSK Integrated Mutation Profiling of Actionable Targets (MSK-IMPACT) assay in the clinical setting29. Targeted sequencing data of these cases was retrieved from cBioPortal30 and retrospectively analyzed. Loss of heterozygosity of the wild type allele (LOH) of affected germline HRD genes was assessed using FACETS31. We inferred previously established measures of HRD phenotype using an algorithm optimized for MSK-IMPACT32. In brief, the IMPACT-HRD score is computed as an unweighted sum of the genome-wide loss of heterozygosity (LOH), the number of telomeric imbalances (nTAI) and large-scale transitions (LST). The IMPACT-HRD scores were strongly correlated and concordant with Myriad’s Genomic Instability Scores in a cohort of germline BRCA1/2 wild-type ovarian cancer patients who received Myriad testing as well as MSK-IMPACT profiling as part of routine clinical care (data not shown)21. Here, we used a conservative IMPACT-HRD score threshold of 50 to determine a tumor to be HRD-positive. Of note, this is significantly greater than Myriad’s FDA-approved threshold of 42 for ovarian cancer patients and an exploratory threshold of 33 for breast cancer patients32,33.
Data availability
Genetic alterations identified by MSK-IMPACT will be available on cBioPortal upon publication.
References
International Agency for Research on Cancer. WHO Classification of Tumours-Breast Tumours 5th edn. https://www.iarc.who.int/news-events/who-classification-of-tumours-5th-edition-volume-2-breast-tumours/ (2019).
Pareja, F. et al. Triple-negative breast cancer: the importance of molecular and histologic subtyping, and recognition of low-grade variants. NPJ Breast Cancer 2, 16036 (2016).
Pareja, F. et al. Unraveling complexity and leveraging opportunities in uncommon breast cancer subtypes. NPJ Breast Cancer 11, 6 (2025).
Thomas, A., Reis-Filho, J. S., Geyer, C. E. & Wen, H. Y. Rare subtypes of triple negative breast cancer: Current understanding and future directions. NPJ Breast Cancer 9, 55 (2023).
Koenig, C., Dadmanesh, F., Bratthauer, G. L. & Tavassoli, F. A. Carcinoma arising in microglandular adenosis: an immunohistochemical analysis of 20 intraepithelial and invasive neoplasms. Int. J. Surg. Pathol. 8, 303–315 (2000).
James, B. A., Cranor, M. L. & Rosen, P. P. Carcinoma of the breast arising in microglandular adenosis. Am. J. Clin. Pathol. 100, 507–513 (1993).
Zhong, F. et al. Carcinoma arising in microglandular adenosis of the breast: triple negative phenotype with variable morphology. Int J. Clin. Exp. Pathol. 7, 6149–6156 (2014).
Tsang, J. Y. & Tse, G. M. Microglandular adenosis: a prime suspect in triple-negative breast cancer development. J. Pathol. 239, 129–132 (2016).
Salarieh, A. & Sneige, N. Breast carcinoma arising in microglandular adenosis: a review of the literature. Arch. Pathol. Lab Med 131, 1397–1399 (2007).
Khalifeh, I. M. et al. Clinical, histopathologic, and immunohistochemical features of microglandular adenosis and transition into in situ and invasive carcinoma. Am. J. Surg. Pathol. 32, 544–552 (2008).
Guerini-Rocco, E. et al. Microglandular adenosis associated with triple-negative breast cancer is a neoplastic lesion of triple-negative phenotype harbouring TP53 somatic mutations. J. Pathol. 238, 677–688 (2016).
Wen, Y. H., Weigelt, B. & Reis-Filho, J. S. Microglandular adenosis: a non-obligate precursor of triple-negative breast cancer?. Histol. Histopathol. 28, 1099–1108 (2013).
Geyer, F. C. et al. Genetic analysis of microglandular adenosis and acinic cell carcinomas of the breast provides evidence for the existence of a low-grade triple-negative breast neoplasia family. Mod. Pathol. 30, 69–84 (2017).
Schwartz, C. J. et al. Microglandular adenosis is an advanced precursor breast lesion with evidence of molecular progression to matrix-producing metaplastic carcinoma. Hum. Pathol. 85, 65–71 (2019).
Sabaté, J. M. et al. Microglandular adenosis of the breast in a BRCA1 mutation carrier: radiological features. Eur. Radio. 12, 1479–1482 (2002).
Ricci, C. et al. Ductal invasive carcinoma arising within atypical microglandular adenosis in a patient with BRCA-1 mutation: a case report. Hum. Pathol. Case Rep. 8, 41–45 (2017).
Lord, C. J. & Ashworth, A. The DNA damage response and cancer therapy. Nature 481, 287–294 (2012).
Turner, N., Tutt, A. & Ashworth, A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat. Rev. Cancer 4, 814–819 (2004).
Riaz, N. et al. Pan-cancer analysis of bi-allelic alterations in homologous recombination DNA repair genes. Nat. Commun. 8, 857 (2017).
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005).
Muldoon, D. M. et al. Pan-cancer analysis identifies signatures of HRD-positivity in germline BRCA1/2 mutated non-BRCA associated cancers. Cancer Res. 84, 1757 (2024).
Salgado, R. et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an international TILs working group 2014. Ann. Oncol. 26, 259–271 (2015).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384, 164–172 (2014).
Rosen, P. P. Microglandular adenosis. A benign lesion simulating invasive mammary carcinoma. Am. J. Surg. Pathol. 7, 137–144 (1983).
Richardson, E. T. et al. NR4A3 Expression is consistently absent in acinic cell carcinomas of the breast: a potential nosologic shift. Mod. Pathol. 36, 100144 (2023).
Qin, F. et al. Breast carcinomas resembling acinic cell carcinoma: comprehensive analysis of 14 cases and review of the literature. Am. J. Surg. Pathol. 49, 448–457 (2025).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical genetics and genomics and the association for molecular pathology. Genet Med 17, 405–424 (2015).
Wolff, A. C. et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 31, 3997–4013 (2013).
Cheng, D. T. et al. Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J. Mol. Diagn. 17, 251–264 (2015).
Gao, J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 6, pl1 (2013).
Shen, R. & Seshan, V. E. FACETS: allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic Acids Res. 44, e131 (2016).
Telli, M. L. et al. Homologous recombination deficiency (HRD) score predicts response to platinum-containing neoadjuvant chemotherapy in patients with triple-negative breast cancer. Clin. Cancer Res. 22, 3764–3773 (2016).
Lenz, L. et al. Identifying homologous recombination deficiency in breast cancer: genomic instability score distributions differ among breast cancer subtypes. Breast Cancer Res. Treat. 202, 191–201 (2023).
Acknowledgements
This work was funded in part by the Marie-Josée and Henry R. Kravis Center for Molecular Oncology and the National Cancer Institute Cancer Center Core Grant No. P30-CA008748. FP is funded in part by an NIH P50 CA247749-01 grant and an FDA 1U01FD007909-01A1 grant. BW is funded in part by Breast Cancer Research Foundation and NIH P50 CA247749-01 grants.
Author information
Authors and Affiliations
Contributions
C.J.S. and H.W. conceived the work. Conceptualization and methodology: All authors. Supervision: H.W. and F.P. Data curation and formal analysis: C.J.S., I.G., M.R., P.T., A.G., D.R., D.M., C.B., F.P. Writing—original draft: C.J.S. Writing—review and editing: All authors.
Corresponding authors
Ethics declarations
Competing interests
F.P. reports membership on the Scientific Advisory Board of MultiplexDx and has received consulting fees and served on advisory boards for AstraZeneca. BW reports research funding by Repare Therapeutics and SAGA Diagnostics paid to the institution, and employment of an immediate family member at AstraZeneca. All remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schwartz, C.J., Genco, I., Repetto, M. et al. Breast carcinomas associated with microglandular adenosis are linked to germline alterations in homologous recombination-deficiency genes. npj Breast Cancer 11, 76 (2025). https://doi.org/10.1038/s41523-025-00794-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-025-00794-z