Abstract
This study was aimed to investigate the effects of microgravity on the biochemical and biomechanical change in the lumbar intervertebral disc (IVD) using a modelling approach. The lumbar IVD was considered as an inhomogeneous, porous, mixture consisting of a charged solid phase, an interstitial fluid phase, and a solute phase including charged (e.g., sodium ion, chloride ion) and uncharged (e.g., glucose, oxygen, lactate) solutes. A finite element model of the IVD was established based on the multiphase mixture theory. Our results showed that glucose and oxygen concentrations increased, while lactate concentration decreased under microgravity, compared to those under gravity condition. The metabolic rate of glucose, oxygen and lactate in the nucleus pulposus decreased, the water content increased, while the fluid pressure decreased in the IVD under microgravity compared to those under gravity. The results contribute to a better understanding of the mechanisms of microgravity related biomechanics in the human IVD.
Similar content being viewed by others
Introduction
With the advancements in manned space exploration, astronauts have been facing harsh physical conditions and significant physiological changes in microgravity environments. Studies show that microgravity could lead to back pain and spinal diseases in a large population of astronauts. More than 50% of astronauts report spine pain during space mission, with 86% of which occurred in the lower back1. Though many reasons are related to back pain, such as paravertebral erector spinae atrophy2, loss of spinal muscle strength3, spinal curvature changes4 and IVD degeneration5, etc. But these conclusions above are not necessarily applicable to microgravity condition. Studies have shown that the excessive expansion of lumbar IVD and spinal column elongation under microgravity might be the major causes of the high incidence of back pain in a large number of astronauts under microgravity6,7. Therefore, we paid attention to the changes of biochemical and biomechanical signals within the lumbar IVD under microgravity. The incidence of intervertebral disc (IVD) herniation returned back to the Earth after spaceflight is about 4.3 times higher compared to matched control on Earth, with the highest risk appeared in the first month after returning to the Earth8. The exact causes of lower back pain in microgravity and the high incidence of IVD herniation in astronauts shortly after returning to the Earth are unclear yet.
Numerous studies have investigated the effects of microgravity on the IVD using various methods. Current research indicates that microgravity leads to an increase in disc water content and disc swelling9. This rapid increase in water content within the IVD has been associated with unloading of gravity in dry immersion experiments which simulate the effects of microgravity on Earth10. It has been suggested that changes in biochemical components, such as water content and proteoglycans within the IVD under microgravity may contribute to effects of microgravity on IVD health6,11. For example, increased water content and disc swelling have been shown to reduce spinal mobility under an in vitro simulated microgravity environment12. Animal tail suspension experiments (to simulate microgravity on Earth) have demonstrated a significant reduction in proteoglycan concentration within rabbit IVD. This reduction was accompanied by a decrease in the number of NP cells, exacerbating degenerative changes in the rabbit IVD13. Conversely, other studies have reported that simulated microgravity environments promote the proliferation of rabbit IVD cells14.
Currently, these studies have focused mainly on the water content and proteoglycan change, and the cell density results are controversial among studies. To what extent the water content changes and in which area the water content change locates were largely unknown yet. In addition, how would the mechanical environment and nutritional environment change in the disc were largely unknow either, knowledge of which are very important for advancing the understanding the cell viability and cell activities such as proteoglycan synthesis. Thus, in this study, we aimed to quantify the nutritional and mechanical signals change in the lumbar IVD under microgravity compared to those under gravity using a numerical approach. This study is important for elucidating the impact of microgravity on lumbar IVD health.
Results
The results of model validation
Our model has been validated under various conditions, including the elastic and creep deformation of the tissue under mechanical loading, the swelling behavior of the tissue under osmotic loading, and the transport and metabolism of nutrients in the tissue.
Elastic deformation: Using our model, we validated the elastic deformation of the tissue by simulating the deformation of a rectangular beam under certain mechanical loading. One end (x = 0) of the beam was fixed in all directions and compressive force was applied to the entire bottom face (z = 0). After reaching steady state, the deformation at one of our selected points was 4.16 mm, and the deformation at the same location calculated in the literature by analytic methods was 4.0 mm15.
Creep deformation: Using our model, we validated the creep deformation of the tissue by simulating the deformation of the biphasic material under certain mechanical loading. The upper surface of the tissue was compressed by a constant compressive loading, and the values of the elastic model, diffusion coefficient, and other parameters were consistent with the literature. The displacement of the upper surface of the tissue was to be −0.5 mm from our model after reaching steady state after 90 minutes, which compared well with data from literature16.
Swelling behavior: Using our model, we validated the swelling behavior of the tissue under osmotic loading by simulating the swelling of a charged and hydrated tissue under osmotic loading. The material parameters were set to the same values in the literature. Our model showed a steady strain of 2.5% at the top surface after 25 minutes under osmotic loading, which was in excellent agreement with the analytical results in the literature under the same condition17.
Transport and metabolism of nutrients in the tissue: Using our model, we validated the transport and metabolism of nutrients in the tissue by simulating a case of cartilage matrix synthesis and spatial variation of matrix products. The difference between the predictions of our model and the analytical solutions from the literature was evaluated by means of a parameter. As the number of elements of the model increases, the value of the difference decreases and eventually stabilizes at 0.1%, which show that excellent agreement was achieved between the analytical solutions and the simulated solutions in our model18.
Glucose concentration and metabolism
Night loading vs Day loading under gravity: After reaching steady state, the glucose concentration averaged in the NP, AF, and CEP regions increased by 1%, 3%, and 1%, the glucose metabolic rate averaged in the NP regions decreased by 4%, and in the AF increased by 3% during night loading compared to those during day loading under gravity (Fig. 1).
Microgravity vs Gravity: After reaching steady state, the glucose concentration averaged in the NP, AF, and CEP regions increased by 2%, 3%, and 1%, respectively, the glucose metabolic rate averaged in the NP, and AF regions decreased by 5% and 1%, under microgravity compared to those under gravity (Fig. 1). The lateral mid-AF regions exhibited the most substantial increase in glucose concentration, contrasting with the minimal increase observed in the NP center (Fig. 5a). Conversely, the most significant decrease in glucose metabolic rate occurred within the NP, with a gradient decline extending towards the inner AF regions. While glucose metabolic rate decreased across most areas of the AF, there was a notable exception in the frontal region, where a substantial increase was observed (Fig. 5e).
Oxygen concentration and metabolism
Night loading vs Day loading under gravity: After reaching steady state, the oxygen concentration averaged in the NP, AF, and CEP regions increased by 4%, 11%, and 2%, the oxygen metabolic rate averaged in the NP regions decreased by 5%, and in the AF increased by 5% during night loading compared to those during day loading under gravity (Fig. 2).
Microgravity vs Gravity: After reaching steady state, the oxygen concentration in the NP, AF, and CEP regions increased by 5%, 6%, and 1%, respectively, and the average oxygen metabolic rate decreased by 5% in the NP region and increased by 1% in the AF region under the microgravity loading compared to those under gravity (Fig. 2). The largest increase in the oxygen concentration is in the inner AF (Fig. 5b). Oxygen metabolic rate decreased in most NP areas, and the decline decreased from NP center to AF. The oxygen metabolic rate in the inner AF increased, while the oxygen metabolic rate in the outermost AF decreased (Fig. 5f).
Lactate concentration and metabolism
Night loading vs Day loading under gravity: After reaching steady state, the lactate concentration averaged in the NP, AF, and CEP regions decreased by 1%, 5%, and 5%, the lactate metabolic rate averaged in the NP regions decreased by 4%, and in the AF increased by 3% during night loading compared to those during day loading under gravity (Fig. 3).
Microgravity vs Gravity: After reaching steady state, the lactate concentration in the NP, AF, and CEP regions decreased by 3%, 5%, and 3%, respectively, and the average lactate metabolic rate in the NP and AF regions decreased by 5%, and 1%, respectively, under the microgravity loading compared to those under gravity (Fig. 3). The least decrease of lactate concentration was in NP region, and the largest decrease was concentrated in the right area of AF, the largest decrease in lactate metabolic rate is NP, the smallest is AF, and it increased greatly in some areas of AF (Fig. 5c and g).
Water content and fluid pressure
Night loading vs Day loading under gravity: After reaching steady state, the average water content in the NP, AF, and CEP regions increased by 1%, 1%, and 5%, the fluid pressure in the NP, AF, and CEP regions decreased by 96%, 103%, and 127% during night loading compared to those during day loading under gravity (Fig. 4).
Microgravity vs Gravity: After reaching steady state, the average water content in the NP, AF, and CEP regions increased by 2%, 2%, and 3%, respectively under microgravity boundary compared to gravity conditions (Figs. 4 and 5d). The fluid pressure in the NP, AF, and CEP regions decreased by 53%, 53%, and 65% under microgravity compared to those under gravity (Fig. 4). The change in the fluid pressure and water content in the IVD was quite uniform (Fig. 5h).
Spatial distributions were shown at mid-axial plane (z = 0, t = 65 h) for: a glucose concentration, b oxygen concentration, c lactate concentration, d water content, e glucose metabolic rate, f oxygen metabolic rate, g lactate metabolic rate, and h fluid pressure. Changes of biochemical and biomechanical signals within the lumbar IVD under microgravity relative to the gravity conditions were calculated as (microgravity-gravity)/gravity.
Prolonged bed rest vs microgravity
Compared to day loading under gravity environment, the trend of change were similar between prolonged bed rest (PBR) and microgravity conditions in the following signals: the compressive and shear forces on the lumbar IVD, water content, fluid pressure, concentrations of glucose, oxygen, and lactate in the lumbar IVD, and the metabolic rates of glucose, oxygen, and lactate in the NP regions (Tables 1 and 2). But the magnitudes of these changes were larger in the PBR condition compared to those in the microgravity one. Compared to day loading under gravity, the trend of change were different between PBR and microgravity conditions in the following signals: the metabolic rates of glucose, oxygen, and lactate in the AF region.
Discussions
The effects of microgravity on nutrient transport, metabolic rate, water content, and mechanical signals change within the lumbar IVD were studied using a finite element model based on the multiphase mixture theory. We found that the concentrations of glucose and oxygen increased, while the concentration of lactate decreased; the metabolic rate of glucose, oxygen and lactate in the NP region decreased; the water content increased, while the fluid pressure decreased in most areas of lumbar IVD under microgravity compared to those under gravity. The decreased fluid pressure throughout the tissue are consequences of the lumbar IVD swelling due to osmotic loading under microgravity. To the best of our knowledge, this study is the first quantitative investigation to evaluate the effect of microgravity on nutrient transport and metabolic rate, as well as mechanical signals within the human lumbar IVD.
Our results report increased tissue hydration in the lumbar IVD under microgravity. On Earth, the swelling pressure and intrinsic elastic forces of the lumbar IVD balance the mechanical loading exerted on the lumbar IVD by the muscle, ligament, body weight, etc. Microgravity reduces the compressive loading exerted on the lumbar IVD19, leading to an imbalance in the forces in the lumbar IVD, thus the lumbar IVD undergoes water absorption and swelling. This is consistent with findings in the literature, on changes in the water content, of the lumbar IVD under microgravity or simulated microgravity10,20,21. For example, Yoshihiro et al. found that the water content of the discs increased significantly after bed rest using magnetization transfer magnetic resonance imaging (MT-MRI)21. Treffel et al. found a significant increase in lumbar IVD water content (+17%) in all subjects after the dry immersion22, which was consistent with the increase trend of water content within the lumbar IVD in our results, and the increase range was greater than those in our results. It was found that the height within the lumbar IVD increased significantly in dry immersion condition, and it was speculated that the increase of water content within the lumbar IVD was the possible mechanism of the height increase23,24.
The rates of glucose and oxygen metabolism in the NP decreased under microgravity compared to gravity conditions, whereas the concentrations of glucose and oxygen increased and the concentration of lactate decreased, indicating that glucose metabolism within the lumbar IVD slowdown under microgravity. These results are consistent with findings in the literature investigating the effects of microgravity on human cellular metabolism. For example, Li et al. cultured endothelial cells on the SJ-10 returnable scientific satellite and monitored the glucose and lactate concentrations in the cell culture supernatant. They found that the glucose concentration in the culture medium increased and the lactate concentration decreased in the microgravity environment, indicating a inhibition of glucose metabolism25. Chakraborty et al. found a significant inhibitory effect of the microgravity environment on the energy metabolism of human endothelial cells using metabolomics analysis26. Hughes-Fulford studied the growth of osteoblasts aboard the STS-56 space shuttle and reached similar conclusions27. Ahlers et al. found that glucose metabolism in rats was impeded under simulated microgravity through rat suspension experiments28.
Both glucose concentration and oxygen concentration were significantly increased within the lumbar IVD under microgravity compared to gravity conditions. This is because the rate of glucose and oxygen consumption is reduced under microgravity, whereas in our model, nutrients are supplied continuously from the tissue boundaries, which results in an increase in the concentration of nutrients within the tissue. Sélard et al. simulated the transport and metabolism of oxygen, glucose, and lactate within the lumbar IVD using a finite element model, and they found that nutrients such as glucose are accumulated in the tissue when the rate of glucose metabolism decreases29. This finding is consistent with our observation of increased glucose concentrations in lumbar IVD under microgravity. In our results, fluid pressures were significantly reduced in the lumbar IVD under microgravity. Due to the unloading in the microgravity, the compressive loading on the lumbar IVD decreased, which decreased the fluid pressure in the lumbar IVD. In addition, the lumbar IVD swells in microgravity due to the imbalances in the osmotic pressure and the lowered external loads, resulting in the increase of disc water content which leads to the decrease of osmotic pressure inside the lumbar IVD and eventually the drop of the fluid pressure.
PBR has been widely used to simulate the microgravity environment8,30,31. Studies have shown similar changes in lumbar IVD water content and morphology in both PBR and microgravity conditions32,33. The similar trend of change between PBR and microgravity conditions in the biomechanical signals (i.e., compressive and shear forces on the lumbar IVD, water content, fluid pressure, concentrations of glucose, oxygen, and lactate in the lumbar IVD, and the metabolic rates of glucose, oxygen, and lactate in the NP regions) is mainly due to the similar mechanical unloadings on the lumbar IVD in the axial direction between PBR and microgravity, leading to similar decreases in compressive and shear forces on the lumbar IVD in both conditions. However, the different mechanical loadings in the sagittal direction on the lumbar IVD between PBR and microgravity led to higher anterior-posterior shear forces on the lumbar IVD under PBR than those under microgravity34. That the magnitudes of these changes were larger in the PBR condition than those in the microgravity one is mainly caused by the difference in forces in the lumbar spinal muscles and ligaments. Our results showed that the total lumbar spinal muscle force decreased by 78% and the total ligament force decreased by 59% under PBR compared to those under microgravity (data not shown), this led to lower compressive forces on the lumbar IVD under PBR than those under microgravity. The large decrease in the compressive forces on the lumbar IVD caused an imbalance in the osmotic pressure and fluid pressure within the lumbar IVD, thus the lumbar IVD underwent water absorption and swelling, leading to higher water content and lower fluid pressure in the lumbar IVD under PBR condition. This further lead to lower metabolic rates of glucose and oxygen, which consequently lead to the accumulation of glucose and oxygen and less production of lactate in the lumbar IVD under the PBR condition.
The differences between day and night loading were caused by the differences in the mechanical loading on the lumbar IVD. Night loading led to lower compressive forces on the lumbar IVD than the day loading, which caused an imbalance in the osmotic pressure and fluid pressure within the lumbar IVD, thus the lumbar IVD underwent water absorption and swelling, leading to higher water content and lower fluid pressure in the lumbar IVD under night loading. This further lead to lower metabolic rates of glucose and oxygen, which consequently lead to the accumulation of glucose and oxygen and less production of lactate in the lumbar IVD under the PBR condition. In addition, the nutrient concentration and metabolic rate were also affected by the alternative day and night loading sequence, in our multiphasic mixture theory based model, the loading history affects current state of the nutrient concentrations and metabolic rates, which caused the opposite trend in the NP and AF regions. Our results under constant day and night loading showed that nutrient concentrations and metabolic rates in the NP and AF regions showed consistent trend of change (data not shown).
This study has several limitations. First, due to the lack of nutritional data in microgravity environments, the same nutrient boundary condition and the parameters of metabolic rates within the lumbar IVD was applied to the lumbar IVD under both gravity (obtained from experimental measurements) and microgravity conditions. These assumptions may have an impact on our results. These parameters will be refined when more data becomes available in the future studies. In the loading boundary setting of the model, a constant load from the standing posture was applied during the day (for 16 hours) for the gravity condition, and another constant load from lying supine was applied during the night (for 8 hours). This simplification may not accurately represent the different loading magnitudes experienced during various daily activities. Our previous study indicates that the compressive loading on the lumbar IVD during various daily activities, such as sitting slouched, sitting straight, standing flexed, and lifting, is comparable to or exceeds that during standing35. Therefore, loading boundary based on standing posture during the daytime may alter the magnitude of differences in the biochemical and mechanical signals between microgravity and gravity conditions, but not the trend of this change between them. And in the gravity condition, the compressive forces on the lumbar IVD change because of various activities such as sitting, standing and standing flexed during day loading. Some studies have reported that dynamic loading was beneficial to the metabolism within IVD than static loading36. Therefore, the nutrient metabolism within the IVD under gravity in this study might be lower than those under the actual condition, which means that the difference of nutrient metabolism within IVD between gravity and microgravity in this study may be lower than the actual condition. Further research is needed to validate these findings experimentally and to explore the development of targeted protective measures for the lumbar IVD under microgravity.
In summary, the effects of microgravity on nutrient transport and metabolic rates, water content, and mechanical signals within the lumbar IVD was quantitatively studied with a finite element approach based on the multiphase mixture theory. Our results suggest that microgravity decreases the nutrient metabolism in the lumbar IVD, increases the water content, and decreases the fluid pressure within lumbar IVD. These results are helpful for understanding how the microgravity affects biochemical and biomechanical signals within the lumbar IVD, which is important for understanding the possible mechanisms by which microgravity affects human lumbar IVD health. The knowledge gained from this study may also provide helpful insight for studying patients with long-term bed rest in clinic.
Methods
Theory
We aimed to quantitatively investigate the effects of microgravity on the biochemical and biomechanical signals within the lumbar IVD using a numerical method. Based on the multiphase mixture theory, the lumbar IVD was considered as an inhomogeneous, porous, mixture consisting of a charged solid phase, an interstitial fluid phase, and a solute phase with multiple species, including charged (e.g., sodium ion, chloride ion) and uncharged (e.g., glucose, oxygen, and lactate) solutes. The governing equations were cast in terms of displacement of solid matrix and electrochemical potentials of fluid and solutes37,38. Specifically, the equation of mass balance for the nutrients were given as
where α denotes the solid, liquid, ionic and molecular phases; \({c}^{\alpha }\) is the concentration (mole per tissue volume) of component α, and \({{\boldsymbol{v}}}^{\alpha }\) denotes the velocity of component α. and \({Q}^{\alpha }\) (mole per tissue volume per time) is the rate of component α production (or consumption), The specific expression for \({Q}^{\alpha }\) is as follows:
The molar flux of the nutrients was written as follows:
ρcell is the cell density (per tissue volume), where \({V}_{\max }^{\text{'}}\) (were set to be 5.27 nmol/million cells-hour for NP cells and 3.64 nmol/million cells-hr for AF cells) is the maximum consumption rate of oxygen39, \({k}_{m}^{{O}_{2}}\) (were set to be 3.4 μM for NP cells and 12.3 μM for AF cells) is the Michaelis-Menten constant for oxygen39, \({k}_{m}^{l}\) (was set to be 0.19 mM for NP and AF cells) is the Michaelis-Menten constant for lactate40. HA is the convection coefficient (hindrance factor) of solute α, JW is the volume flux of water relative to the solid phase, ϕw is the water volume fraction (also known as water content), Da is the diffusivity of solute α, εa is the modified electrochemical potential for solute α.
According to the theoretical equation proposed by Zhu et al. for calculating the rate of change of lumbar IVD tissue cell density40,41, the conservation equation for cell density is as follows:
where ζ is essentially the rate of cell density change, ζ is calculated from experimental measurements obtained in the literature. In addition, the momentum balance equation for the mixture is as follows:
Where \({{\boldsymbol{\sigma }}}^{s}\) is the stress of the solid phases, p is the pressure of fluid phase, I is the identity tensor.
Finite element method
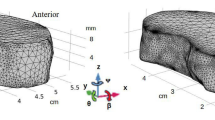
We developed a 3D finite element model based on the theoretical framework. The geometry of the disc was generated based on a L2-L3 human disc40,42,43. Considering that the stiffness of the vertebral body is much larger than that of the disc, we modeled vertebral body as a rigid body. due to the geometric symmetric along the mid-sagittal and mid-axial planes in the lumbar IVD, in order to reduce computational costs, we modeled only the upper right quarter of the lumbar IVD. The mesh consisted of 7872 sextic order, hexahedral lagrange elements. The finite element model of the lumbar IVD was developed with COMSOL software (COMSOL 6.0, COMSOL, Inc., MA) based on the finite element method developed by Sun et al.17.
Material properties
The thickness of the lumbar IVD is set to be 10 mm based on a healthy human lumbar IVD40,42. The values of the Lame constants (λ and μ) in nucleus pulposus (NP) were λ = 0.391 MPa and μ = 0.009 MPa; for annulus fibrosus (AF), λ was linearly increased from 0.391 to 1.009 MPa and μ linearly increased from 0.009 to 0.291 MPa from the innermost AF region to the outermost AF regions based on experimental results, respectively44,45,46. The vertebra was modeled as a single-phase solid with linear elastic mechanical properties of λ = 86.5 MPa, and μ = 57.7 MPa based on experimental results47. The water content (volume fraction) at the healthy state was 0.85 in the NP, and linearly decreased from 0.85 to 0.7 from the innermost AF region to the outermost AF regions from healthy human lumbar IVD40,48,49. The initial values of cell density in NP and AF were 4000 cells/mm3 and 9000 cells/mm3 from healthy human lumbar IVDs, respectively50.
Model validation
This multiphasic mixture theory based finite element model has been previously validated for various biomechanical and biochemical behaviors of the human lumbar IVD against experimental data from literature, including the elastic and creep deformation of the lumbar IVD under mechanical loading, the swelling behavior of the human lumbar IVD under osmotic loading, and the transport and metabolism of oxygen, glucose and lactate in the lumbar IVD51. Briefly, the elastic deformation of the solid phase under mechanical loading compared well with data from literature15. The creep deformation which represents the mechanical coupling between the solid-liquid phase under compressive loading was simulated with our model and compared well with data in literature16. The swelling behavior of the lumbar IVD which represents the mechano-electrochemical couplings among charged solid, fluid, and solute phases under osmotic loading was simulated and compared well with data from literature17. The transport and metabolism of nutrients within the lumbar IVD was also simulated and compared well with data in the literature18.
Boundary conditions
The loading conditions on the lumbar IVD were quantitatively calculated with a lumbar musculoskeletal model containing the swelling characteristics of the lumbar IVD developed in our previous study19,35. Using this model, the compressive loading on the lumbar IVD at relaxed floating posture under microgravity was 0.082 MPa (averaged over the five lumbar discs: L1L2, L2L3, L3L4, L4L5, L5S1), this value was applied as the loading boundary on the lumbar IVD under microgravity. The compressive loading on the lumbar IVD was calculated as 0.237 MPa at relaxed standing and 0.022 MPa at lying under gravity environment. Diurnal compressive loading was applied on lumbar IVD under gravity with 0.237 MPa during the day for 16 hours and 0.022 MPa at night for 8 hours respectively. The loading protocol under gravity consists of three above 24-hour diurnal loading cycles. The initial state (marked as ‘0 h’) represents the unloaded state before the loading was initiated. After three cycles of loading, the biochemical and biomechanical signals within the lumbar IVD reached a steady state. The loadings were applied to the top surface of the vertebra (z = 9.5 mm).
On the top surface of the vertebra (z = 9.5 mm), the displacements were zero in the horizontal directions. There was no relative motion at the disc-vertebra interface (z = 4.5 mm). The AF and vertebra periphery were set to be traction free, the periphery of the lumbar IVD was allowed to move freely in the horizontal direction. The mid-sagittal plane (x = 0) and the mid-axial plane (z = 0) were assumed to be symmetric. The interface between AF and vertebra was assumed to be impermeable, while the interface between NP and vertebra was set to be permeable as well as at the AF periphery40.
The boundary conditions for nutrients were set as follows: oxygen, glucose and lactate concentrations at the AF periphery and cartilage endplate(CEP)-vertebra interface were 6.4 kPa (The oxygen solubility was set to 1.0268 × 10−6 mol/kPa)52, 5.6 mM and 1.0 mM, respectively. The changes in the average concentrations and metabolic rates of glucose, oxygen and lactate, water content, and mechanical signals over time in the NP, AF and CEP regions under microgravity and gravity were quantitatively analyzed, respectively. The changes in the spatial distribution of biochemical and biomechanical signals within the mid-axial plane of the lumbar IVD (z = 0) in microgravity (relative to gravity) were reported in microgravity at the 65th hour. The 65th hour is a time point in the loading cycle three where the day loading was applied, it was used as an example to show the differences in spatial distribution of biochemical and biomechanical signals between microgravity and gravity conditions.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Kerstman, E. L., Scheuring, R. A., Barnes, M. G., DeKorse, T. B. & Saile, L. G. Space adaptation back pain: a retrospective study. Aviat., space, Environ. Med. 83, 2–7 (2012).
Berry, D. B. et al. The effect of high-intensity resistance exercise on lumbar musculature in patients with low back pain: a preliminary study. BMC Musculoskelet. Disord. 20, 290 (2019).
Althobaiti, S., Deane, J. A. & Falla, D. Responsiveness of hand-held dynamometry for measuring changes in trunk muscle strength in people with chronic low back pain. BMC Musculoskelet. Disord. 26, 1–11 (2025).
Swain, C. T. V., Pan, F., Owen, P. J., Schmidt, H. & Belavy, D. L. No consensus on causality of spine postures or physical exposure and low back pain: A systematic review of systematic reviews. J. Biomech. 102, 109312 (2020).
Freemont, A. J. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology 48, 5–10 (2009).
Sayson, J. V., Lotz, J., Parazynski, S. & Hargens, A. R. J. A. A. Back pain in space and post-flight spine injury: Mechanisms and countermeasure development. Acta Astronautica. 86, 24–38 (2013).
Bailey, J. F. et al. Biomechanical changes in the lumbar spine following spaceflight and factors associated with postspaceflight disc herniation. Spine. J. 22, 197–206 (2021).
Johnston, S. L., Campbell, M. R., Scheuring, R. & Feiveson, A. H. Risk of herniated nucleus pulposus among US astronauts. Aviat., space, Environ. Med. 81, 566–574 (2010).
Belavy, D. L. Comment on Matsumura et al.: changes in water content of intervertebral discs and paravertebral muscles before and after bed rest. J. Orthop. Sci. 19, 693–694 (2014).
Treffel, L. et al. DI-5-Cuffs: Lumbar Intervertebral Disc Proteoglycan and Water Content Changes in Humans after Five Days of Dry Immersion to Simulate Microgravity. Int. J. Mol. Sci. 21, 3748 (2020).
Yasuoka, H. et al. Effects of reloading after simulated microgravity on proteoglycan metabolism in the nucleus pulposus and anulus fibrosus of the lumbar intervertebral disc: an experimental study using a rat tail suspension model. Spine 32, 734–740 (2007).
Laws, C. J., Berg-Johansen, B., Hargens, A. R. & Lotz, J. C. The effect of simulated microgravity on lumbar spine biomechanics: an in vitro study. Eur. Spine J. 25, 2889–97 (2016).
Wu, D., Zhou, X., Zheng, C., He, Y. & Liu, Y. The effects of simulated +Gz and microgravity on intervertebral disc degeneration in rabbits. Sci. Rep. 9, 16608 (2019).
Liu, Y., Zhu, S. & Li, G. Construction of tissue engineered intervertebral disc under microgravity culture. Orthoped. J. China. 21, 291–295 (2013).
Land, S. et al. Verification of cardiac mechanics software: benchmark problems and solutions for testing active and passive material behaviour. Proc. R. Soc. A Mathem. Phys. 471, 20150641 (2015).
Mow, V. C., Kuei, S. C., Lai, W. M. & Armstrong, C. G. Biphasic Creep and Stress Relaxation of Articular Cartilage in Compression: Theory and Experiments. J. Biomech. Eng. 102, 73–84 (1980).
Sun, D., Gu, W., Guo, X. E., Lai, W. M. & Mow, V. C. A mixed finite element formulation of triphasic mechano-electrochemical theory for charged, hydrated biological soft tissues. Int. J. Numer. Methods Eng. 45, 1375–1402 (1999).
Dimicco, M. A. & Sah, R. L. Dependence of Cartilage Matrix Composition on Biosynthesis, Diffusion, and Reaction. Biomech. Modeling Mechanobiol. 50, 57–73 (2003).
Wu, B. et al. Effect of microgravity on mechanical loadings in lumbar spine at various postures: a numerical study. npj Microgravity 9, 16 (2023).
Zhang, S., Wang, K., Zhu, R., Jiang, C. & Niu, W. Penguin Suit and Fetal Position Finite Element Model to Prevent Low Back Pain in Spaceflight. Aerosp. Med. Hum. Perform. 92, 312–318 (2021). 5.
Matsumura, Y. et al. Changes in water content of intervertebral discs and paravertebral muscles before and after bed rest. J. Orthop. Sci. 14, 45–50 (2009).
Treffel, L. et al. Intervertebral disc swelling demonstrated by 3D and water content magnetic resonance analyses after a 3-day dry immersion simulating microgravity. Front. Physiol. 7, 605 (2016).
Robin, A. et al. Spinal changes after 5-day dry immersion as shown by magnetic resonance imaging (DI-5-CUFFS). Am. J. Physiol.-Regulatory, Integr. Comp. Physiol. 323, R310–R318 (2022).
Green, D. A. & Scott, J. P. R. Spinal Health during Unloading and Reloading Associated with Spaceflight. Front. Physiol. 8,1126 (2018).
Li, N. et al. Microgravity-induced alterations of inflammation-related mechanotransduction in endothelial cells on board SJ-10 satellite. Front. Physiol. 9, 1025 (2018).
Chakraborty, N. et al. Gene-metabolite profile integration to understand the cause of spaceflight induced immunodeficiency. npj Microgravity. 4, 4 (2018).
Hughes-Fulford, M. L. Effects of microgravity on osteoblast growth activation. Exp Cell Res. 224,103–9 (1995).
Ahlers, I. et al. Suspension hypokinesia in rats - effects of the time of day on metabolic and hormonal changes. Physiological Res. 42, 397–402 (1993).
Sélard, E., Shirazi-Adl, A. & Urban, J. P. G. Finite Element Study of Nutrient Diffusion in the Human Intervertebral Disc. Spine 28, 1945–1953 (2003).
Belavy, D. L., Adams, M., Brisby, H., Cagnie, B. & Wilke, H. J. Disc herniations in astronauts: What causes them, and what does it tell us about herniation on earth?. Eur. Spine J. 25, 144–154 (2016).
Sayson, J. V. & Hargens, A. R. Pathophysiology of Low Back Pain during Exposure to Microgravity. Aviat. Space Environ. Med. 79, 365–373 (2008).
Kordi, M. et al. Loss and re-adaptation of lumbar intervertebral disc water signal intensity after prolonged bedrest. J. Musculoskelet. Neuronal Interact. 15, 294 (2015).
Harrison, M. F. et al. Preflight, in-flight, and postflight imaging of the cervical and lumbar spine in astronauts. Aerosp. Med. Hum. Perform. 89, 32–40 (2018).
Hargens, A. R. & Vico, L. Long-Duration Bed Rest as an Analog to Microgravity. J. Appl. Physiol. 120, 00935.02015 (2016).
Qin, B. et al. Effect of lumbar muscle atrophy on the mechanical loading change on lumbar intervertebral discs. J. Biomech. 139, 111120–111120, https://doi.org/10.1016/j.jbiomech.2022.111120 (2022).
Wang, D. L., Jiang, S. D. & Dai, L. Y. Biologic response of the intervertebral disc to static and dynamic compression in vitro. Spine (Phila Pa 1976). 32, 2521–2528 (2007)
Ateshian, G. A. On the theory of reactive mixtures for modeling biological growth. Biomech. Model. Mechanobiol. 6, 423-445 (2007).
Gu, W., Lai, W. M. & Mow, V. C. A Mixture Theory for Charged-Hydrated Soft Tissues Containing Multi-electrolytes: Passive Transport and Swelling Behaviors. J. Biomech. Eng. 120, 169–180 (1998).
Huang, C.-Y. & Gu, W. Y. Effects of mechanical compression on metabolism and distribution of oxygen and lactate in intervertebral disc. J. Biomech. 41, 1184–1196 (2008).
Zhu, Q., Jackson, A. R. & Gu, W. Cell viability in intervertebral disc under various nutritional and dynamic loading conditions: 3d finite element analysis. J. Biomech. 45, 2769–2777 (2012).
Lin, J., Lee, S.-M., Lee, H.-J. & Koo, Y.-M. Modeling of typical microbial cell growth in batch culture. Biotechnol. Bioprocess Eng. 5, 382–385 (2000).
Jackson, A. R., Huang, C. Y. & Gu, W. Effect of endplate calcification and mechanical deformation on the distribution of glucose in intervertebral disc: a 3D finite element study. Computer Methods Biomech. Biomed. Eng. 14, 195–204 (2011).
Gu, W., Zhu, Q., Gao, X. & Brown, M. D. Simulation of the Progression of Intervertebral Disc Degeneration Due to Decreased Nutritional Supply. Spine 39, 1411–1417, https://doi.org/10.1097/brs.0000000000000560 (2014).
Périé, D., Korda, D. & Iatridis, J. C. Confined compression experiments on bovine nucleus pulposus and annulus fibrosus: sensitivity of the experiment in the determination of compressive modulus and hydraulic permeability. J. Biomechan. 38, 2164–2171 (2005).
Périé, D. et al. Assessment of compressive modulus, hydraulic permeability and matrix content of trypsin-treated nucleus pulposus using quantitative MRI. J. Biomechan. 39, 1392–1400 (2006).
Iatridis, J. C. et al. Degeneration affects the anisotropic and nonlinear behaviors of human anulus fibrosus in compression. J. Biomech. 31, 535–544 (1998).
Goldstein, S. A. The mechanical properties of trabecular bone: dependence on anatomic location and function. J. Biomech. 20, 1055–1061 (1987).
Magnier, C., Boiron, O., Wendling-Mansuy, S., Chabrand, P. & Deplano, V. Nutrient distribution and metabolism in the intervertebral disc in the unloaded state: a parametric study. J. Biomech. 42, 100–108 (2009).
Antoniou, J. et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J. Clin. Investig. 98, 996–1003 (1996).
Maroudas, A., Stockwell, R., Nachemson, A. & Urban, J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J. Anat. 120, 113 (1975).
Gao, X. & Gu, W. Verification of a numerical model for simulating intervertebral disc pathophysiology. Proceedings of the ASME 2020 Verification and Validation Symposium. V001T09A001 (2020).
Soukane, D. M. Analysis of Nonlinear Coupled Diffusion of Oxygen and Lactic Acid in Intervertebral Discs. J. Biomech. Eng. 127, 1121 (2005).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (12372306, 82151311, and 11902154). The Funders had no role in study design, data collection, data analyses, interpretation, or writing of this manuscript.
Author information
Authors and Affiliations
Contributions
B.Q.: Writing—original draft, Visualization, Validation, Software, Formal analysis, Data curation. X.G.: Methodology. J.W.: Writing—review and editing, Software, Investigation, Formal analysis, Data curation. L.Z.: Writing—review and editing, Formal analysis. Z.Q.: Writing—review and editing, Methodology. Q.Z.: Writing—review and editing, Writing—original draft, Validation, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Qin, B., Gao, X., Wu, J. et al. Numerical study on the effect of microgravity on biomechanics in human lumbar intervertebral discs. npj Microgravity 11, 40 (2025). https://doi.org/10.1038/s41526-025-00483-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41526-025-00483-y