Abstract
Astronaut nutrition faces supply, logistics, and cost challenges, making space farming a solution. While plants adapt to space microgravity may trigger oxidative stress. Research shows space-grown plants achieve Earth-like growth, but ROS accumulation remains a concern. This study examines ROS buildup in cells and its risks for astronauts, emphasizing effects on homeostasis, disease pathways, gut microbiome, and nutrition. This research provides new insights into oxidative stress in space missions.
Similar content being viewed by others
Introduction
Astronaut nutrition during extended space missions, particularly those aimed at Mars exploration, presents formidable challenges due to intricate logistics and the costs linked to conventional food supply approaches1. In response, cultivating plants within the controlled confines of the International Space Station (ISS) has emerged as a promising remedy2. Nonetheless, this strategy introduces distinct challenges, predominantly revolving around potential cellular repercussions stemming from cosmic radiation-induced oxidative stress and the influences of microgravity on cellular physiology.
Recent investigations within the ISS have yielded promising findings, demonstrating the adaptability of these space-cultivated plants to oxidative stress within microgravity conditions. Consequently, these plants exhibit growth and yield akin to their terrestrial counterparts2,3. However, a central issue remains unresolved: the heightened possibility of reactive oxygen species (ROS) accumulation within plant cells due to prolonged exposure to cosmic radiation and microgravity.
Cosmic radiation, originating from celestial sources beyond Earth, constantly bombards our planet and its atmosphere, albeit with limited direct impact on plant cellular life. While plants have evolved mechanisms to counter radiation, these adaptations are optimized for terrestrial conditions4, where the annual cosmic radiation dose averages approximately 0.39 millisieverts (mSv), largely thanks to the protective ozone layer. In stark contrast, astronauts aboard the ISS face radiation doses ranging from 100 to 200 mSv per year, significantly surpassing terrestrial levels. Moreover, deep space expeditions expose astronauts to potential radiation doses ranging from hundreds to thousands of millisieverts annually, emphasizing the considerable disparity in radiation intensities between the space environment and Earth5,6,7,8,9.
The elevated cosmic radiation in space induces oxidative stress within plant cells, leading to the generation of ROS4. These reactive molecules inflict damage on cellular components, disrupting vital physiological processes10,11. While radiation doses on Earth rarely incite acute toxicity in plants, the cumulative ROS buildup in space-cultivated plants intended for astronaut consumption necessitates a comprehensive evaluation to ascertain potential toxicity at the cellular level.
This concern extends to the suitability of space-grown plants for astronaut sustenance. The significance of ROS-induced oxidative stress in human cellular health, coupled with its occurrence in plant cells, underscores the imperative of scrutinizing potential hazards associated with consuming ROS-enriched foods.
Consuming foods harboring accumulated ROS can have adverse effects on astronaut well-being, influencing various cellular physiological functions and contributing to a spectrum of diseases1,12,13. This accentuates the necessity for a thorough assessment regarding the potential toxicity of ROS-laden foods to astronauts.
To address these concerns, the proposed research advocates for a comprehensive approach to tackle ROS accumulation within space-cultivated plant cells. Through a meticulous and analytical review encompassing bioinformatic studies and past experimental investigations, this study aims to shed light on ROS-related mechanisms within plant cells, evaluate their influence on astronauts’ gut microbiome, nutrition, and overall cellular health, and emphasize the significance of exploring plant breeding strategies that enhance resistance against ROS-induced toxicity. This research delves into the repercussions of ROS accumulation triggered by cosmic radiation exposure in plant cells, concurrently evaluating associated risks linked to astronauts’ consumption.
The unexplored domain of ROS accumulation’s impact on astronaut cellular health and the safety of space-grown plant-based nutrition underscores the exigency of these inquiries. Through the envisioned research framework, our aim is to provide indispensable insights into ROS accumulation within space-cultivated plant cells and its implications for astronauts’ well-being and the sustainable production of food during prolonged space missions. This comprehensive investigation endeavors to provide pivotal insights into ensuring the safety and viability of harnessing space-grown plants as a sustainable nutritional source for astronauts during their extended sojourns in space.
ROS and Oxidative Stress
Oxidative stress is a condition resulting from an imbalance between ROS production and the body’s antioxidant defenses. Oxygen, essential for energy generation, can cause oxidative damage to proteins, lipids, and DNA10. The body’s antioxidants counteract this damage, but an imbalance favoring pro-oxidants leads to oxidative stress14,15. Oxygen’s dual role necessitates a delicate balance10. Atomic oxygen, with two unpaired electrons in distinct orbitals, is highly susceptible to the formation of radicals. When an additional electron is introduced to molecular oxygen, its structure is rejuvenated, resulting in the generation of a diverse array of active oxygen species as shown in Fig. 1.
This image illustrates the intriguing transformation of atomic oxygen into a diverse array of active oxygen species. A Atomic oxygen with two unpaired electrons in separate orbitals, making it highly reactive. B Formation of the superoxide radical (•O2−) through the addition of one electron. C Conversion of superoxide into hydrogen peroxide (H2O2) through dismutation. D Breakdown of hydrogen peroxide into hydroxyl radicals (•OH) via the Fenton reaction. E Further transformation of ROS into singlet oxygen (¹O2) and peroxyl radicals (ROO•). F Interaction of ROS with biomolecules, leading to oxidative stress and cellular damage.
Superoxide Radical (•O2−)
The superoxide radical (•O2 − ) is a chemical species with a single unpaired electron and a net negative charge, characterized by its role as a product of the one-electron reduction of dioxygen16. It can be produced through various chemical reactions (Fig. 1A–C), including the incomplete reduction of molecular oxygen (O2) by the addition of a single electron17 This reaction can be represented using a chemical equation (Eq. 1):
Superoxide radicals can also be generated biologically through enzymatic processes18. For instance, the enzyme superoxide dismutase (SOD) catalyzes the dismutation of two superoxide radicals, converting them into hydrogen peroxide (H2O2) and molecular oxygen (O2) as shown in Eq. 2:
Hydrogen Peroxide (H2O2)
H2O2 is a chemical compound with an oxygen-oxygen single bond between two oxygen atoms16 (Fig. 1D). It is produced in biological processes through ROS and oxidative metabolism. During cellular respiration, ROS, including superoxide radicals (•O2−), can be generated as byproducts of the electron transport chain. These superoxide radicals can subsequently react with each other or other molecules to form H2O2 as shown in Eq. 3:
Peroxides can be produced as byproducts of oxidative metabolism, where molecular oxygen (O2) is involved in various metabolic reactions19. For example, the breakdown of fatty acids can lead to the production of H2O2. Immune cells, such as neutrophils and macrophages, produce peroxides as part of the immune response. These cells generate H2O2 to help destroy invading microorganisms and pathogens as shown in Eq. 4:
They also play a role in antioxidant defense, immune responses, cellular regulation, and lignin degradation. Enzymes like catalase and peroxidase help break down H2O2 into water and oxygen, reducing its harmful effects. Proper regulation of peroxide levels is crucial for maintaining cellular homeostasis and preventing oxidative stress-induced damage17,18,20.
Hydroxyl Radical (•OH)
The hydroxyl radical (•OH) is a highly reactive free radical with an unpaired electron (Fig. 1E, F), causing damage to various biomolecules, including proteins, lipids, and DNA20,21. It is produced through the Fenton reaction, where H2O2 reacts with ferrous iron to produce the hydroxyl radical, hydroxide ions, and ferric iron as shown in Eq. 5:
Despite its damaging effects, hydroxyl radicals also have biological functions, such as acting as signaling molecules in redox signaling pathways19, aiding in immune respons, and contributing to drug and toxins metabolism in the liver18. They are part of the ROS pool generated during oxidative stress. Proper regulation of hydroxyl radicals is crucial for maintaining cellular health and preventing oxidative stress-induced damage18.
Defense Mechanisms Against Oxidative Damage
The immobile nature of plants has driven the development of an intricate antioxidant defense system, characterized by a network of numerous enzymatic components, essential for overcoming various stress conditions. This defense system in plants comprises a variety of enzymes primarily involved in either preventing the Haber-Weiss reaction or facilitating the Foyer–Halliwell–Asada pathway, which reduces H2O2 and utilizes the reducing potential of NADPH22. These enzymes work in synergy as part of the antioxidant defense system. Plants have evolved sophisticated antioxidant mechanisms to neutralize ROS and protect themselves from oxidative damage. Notable enzymatic antioxidants include SOD, which converts superoxide radicals into H2O2 and oxygen; Catalase (CAT), which decomposes H2O2 into water and oxygen; Ascorbate Peroxidase (APX), which utilizes ascorbate to reduce H2O2 to water; and Glutathione Peroxidase (GPX), which reduces H2O2 and organic hydroperoxides using glutathione23,24,25. Additionally, non-enzymatic antioxidants such as Ascorbic Acid (Vitamin C), Glutathione (GSH), Carotenoids, and Tocopherols (Vitamin E) contribute to scavenging ROS and regenerating other antioxidants. Moreover, ROS-scavenging pathways, including the Ascorbate-Glutathione Cycle and Glutathione-Peroxidase Cycle, play a role in detoxifying H2O2. Stress signaling and gene regulation are also crucial, with ROS acting as signaling molecules that activate transcription factors, and plant hormones like abscisic acid (ABA), salicylic acid (SA), and jasmonic acid (JA) modulating antioxidant defenses22,26.
In food systems, ROS can initiate lipid peroxidation, leading to the formation of lipid hydroperoxides and secondary oxidation products like aldehydes and ketones. These oxidation products can negatively impact the sensory qualities of food, such as flavor, color, and texture. Additionally, ROS can react with proteins, causing protein oxidation, which may result in the loss of essential amino acids, decreased protein solubility, and the formation of protein aggregates. The presence of antioxidants, both natural and synthetic, can mitigate the effects of ROS by scavenging free radicals and chelating metal ions, thereby protecting food quality and extending shelf life27. In general, both astronauts and individuals on Earth are susceptible to cellular and tissue damage induced by ROS. However, astronauts are subjected to approximately 100 times more ionizing radiation compared to the general population, leading to an abnormally high level of ROS production in their cells. Consequently, the formulation of a nutritionally balanced diet for astronauts, enriched with antioxidants and other essential nutrients, is a critical priority for any space agency28.
Understanding Mechanisms and Sites of Active Oxygen Production
ROS, also known as active oxygen, are produced in various cellular compartments like chloroplasts, mitochondria, cell membrane, peroxisomes, apoplast, endoplasmic reticulum, and cell wall. Space conditions like cosmic radiation and microgravity increase ROS production, causing oxidative stress (Fig. 2).
Peroxisomes as a Source of Active Oxygen Production
Peroxisomes are membrane-bound organelles in eukaryotic cells that play a crucial role in metabolic processes, including the breakdown of fatty acids and detoxification of harmful molecules. They can also contribute to the production of ROS, also known as active oxygen29. One-way peroxisomes generate ROS is through the metabolism of fatty acids, a process known as beta-oxidation. This process breaks down fatty acids into smaller units, producing acetyl-CoA and transferring electrons to molecular oxygen (O2) via the electron transport chain. This leads to the generation of superoxide radicals (•O2 − ), a type of ROS. The overall reaction can be summarized as shown in Eq. 6:
The enzyme responsible for the breakdown of fatty acids in peroxisomes is called fatty acyl-CoA oxidase (FAO), which initiates the beta-oxidation process. The transfer of electrons to molecular oxygen and the subsequent formation of superoxide radicals involves various enzymatic steps within the electron transport chain in peroxisomes30. Peroxisomes also contain antioxidant defense mechanisms to help manage and regulate ROS levels. Catalase, an enzyme abundant in peroxisomes, catalyzes the breakdown of H2O2 into water and oxygen, serving as a protective mechanism. Peroxisomes are also pivotal in essential metabolic processes, including the breakdown of long-chain fatty acids, detoxification of harmful compounds, and the synthesis of certain phospholipids and bile acids29,31.
Chloroplast as a Source of Active Oxygen Production
Chloroplasts are organelles in plant cells that play a crucial role in photosynthesis, converting light energy into chemical energy in the form of glucose. They also produce ROS, also known as active oxygen, as a natural byproduct of the photosynthetic electron transport chain32. During photosynthesis, chlorophyll molecules absorb light energy, leading to the transfer of electrons through a series of protein complexes. In some cases, electrons can prematurely interact with molecular oxygen, forming superoxide radicals (•O2 − ). The overall process can be summarized as shown in Eq. 7:
The production of ROS in chloroplasts is an inherent part of photosynthesis, but plants have evolved mechanisms to balance ROS production and detoxification. Antioxidant enzymes like SOD and ascorbate peroxidase play critical roles in neutralizing ROS and preventing oxidative damage to chloroplast components. Chloroplasts’ functions extend beyond energy production, impacting oxygen release, carbon fixation, and plant responses to their environment. Overall, chloroplasts play a vital role in photosynthesis and the overall health of plants32,33.
Apoplast as a Source of Active Oxygen Production
The apoplast, the extracellular space outside plant cell membranes, is a crucial part of plant physiology and can also be a source of ROS, or active oxygen34. ROS production in the apoplast is linked to various physiological processes, including cell wall remodeling, defense responses, and interactions with the external environment. The production process involves enzymes like NADPH oxidases, peroxidases, and polyphenol oxidases, which generate ROS through reactions like the reduction of molecular oxygen (O2). The overall process can be summarized as shown in Eq. 8:
ROS production in the apoplast is essential for plant defense and signaling mechanisms. Excessive ROS can be damaging, but plants have developed intricate systems to regulate ROS levels and harness their potential benefits. ROS are crucial for plant immune responses to pathogen attacks, strengthening cell walls, and regulating physiological processes like gene expression, growth, and development. They also contribute to wound healing and sealing damaged plant tissues34,35. In interactions between plant roots and soil microorganisms, ROS are produced, influencing nutrient uptake and symbiotic relationships. In summary, the apoplast plays a significant role in plant physiology, defense responses, cell wall reinforcement, signaling, wound healing, and root-soil interactions. Proper regulation of apoplastic ROS levels is essential for maintaining plant health, defense, and interactions with the environment.
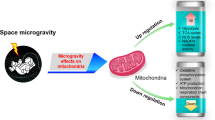
Mitochondria as a Source of Active Oxygen Production
Mitochondria, double-membraned organelles in eukaryotic cells, are crucial for energy production through oxidative phosphorylation. They are primarily known for their role in ATP synthesis but can also produce ROS, also known as active oxygen. ROS production within mitochondria occurs as a byproduct of the electron transport chain (ETC), a series of protein complexes embedded in the mitochondrial inner membrane36. During oxidative phosphorylation, electrons are transferred through the ETC, and a small percentage of these electrons can leak prematurely, leading to the reduction of molecular oxygen to superoxide radicals (•O2 − ). The overall process can be summarized as shown in Eq. 9:
The exact sites of ROS production within the ETC can vary, and multiple enzymatic reactions are involved. Complex I and complex III of the ETC are major sites of ROS production, with enzyme coenzyme Q also involved37.
Mitochondrial ROS production is a natural consequence of oxidative phosphorylation and electron transport. Cells have evolved antioxidant defense mechanisms to regulate ROS levels, with enzymes like manganese superoxide dismutase (MnSOD) and glutathione peroxidase helping neutralize ROS and prevent oxidative stress. Low levels of mitochondrial ROS can act as signaling molecules, influencing cellular responses such as gene expression and adaptation to stress36,37.
Endoplasmic Reticulum as a Source of Active Oxygen Production
The endoplasmic reticulum (ER) is a crucial organelle involved in protein synthesis, lipid metabolism, and calcium storage36. It can also produce ROS, also known as active oxygen, which is linked to cellular conditions like ER stress and alterations in calcium homeostasis. During ER stress, unfolded or misfolded proteins accumulate in the ER lumen, leading to the activation of the unfolded protein response (UPR). The UPR can induce ROS generation through various mechanisms, including the activity of enzymes like NADPH oxidases. The production of ROS in the ER can be summarized as shown in Eq. 10:
The chemical reactions leading to ROS generation in the ER can vary based on the specific context and enzymes involved. NADPH oxidases are key enzymes responsible for ROS production during ER stress, which transfer electrons from NADPH to molecular oxygen, leading to the formation of superoxide radicals (•O2 − ). The generation of ROS in the ER has specific functional implications, with moderate levels serving as signaling molecules, influencing gene expression, cell survival, and adaptation to stress. ROS can also influence calcium homeostasis in the ER, impacting cellular signaling and protein folding36,38. In summary, the ER is primarily involved in protein synthesis and lipid metabolism but can also produce ROS in response to cellular stress. Proper ER function and ROS regulation are essential for cellular health and adaptation to changing environmental conditions.
Factors Contributing to ROS Generation in Space
Microgravity induces ROS production through multiple interconnected mechanisms. One primary factor is mitochondrial dysfunction, where alterations in mitochondrial dynamics under microgravity conditions lead to electron leakage in the electron transport chain, increasing ROS generation. Additionally, microgravity affects antioxidant defense systems, potentially downregulating key antioxidant enzymes, thereby reducing the cell’s ability to neutralize ROS. Another critical aspect is mechanotransduction and cellular signaling, as the absence of gravitational force disrupts mechanical stress responses, altering pathways that regulate oxidative stress. Collectively, these factors contribute to a significant increase in ROS accumulation, impacting plant growth and cellular homeostasis in space environments39,40,41,42.
The induction factors of ROS in plants grown in space can be attributed to various factors and environmental conditions unique to the spaceflight environment. These factors can contribute to the production of ROS, which are highly reactive molecules containing oxygen that can cause cellular damage and oxidative stress. Here are some key sources and explanations for the induction of ROS in plants grown in space (Fig. 3):
This image provides insights into the induction factors of ROS in plants cultivated in space. The unique conditions of the spaceflight environment lead to the production of ROS, highly reactive oxygen-containing molecules known to cause cellular damage and oxidative stress. The illustration delves into the various factors and environmental conditions unique to space, shedding light on how they contribute to the generation of ROS in plants.
Microgravity Effects
Plants grown in microgravity experience altered mechanical and gravitational forces. This change in physical stress can disrupt cellular processes and affect the balance of ROS production and scavenging43. The absence of normal gravity-related processes may lead to increased ROS production. Microgravity disrupts normal physiological processes in plants, leading to increased production of ROS and oxidative stress. This inefficiency in the electron transport chain in mitochondria causes electrons to leak and react with oxygen, forming ROS, which accumulate and damage cellular components like proteins, lipids, and DNA44. Additionally, microgravity impacts the orientation and growth direction of plant roots and shoots, causing disoriented growth patterns and changes in cell structures. To study these effects, clinostat devices simulate microgravity by rotating plants to negate gravity’s influence, allowing researchers to understand how plants perceive and adapt to microgravity. CRISPR/Cas9 genome-editing techniques are also employed to investigate and enhance plant adaptation to space environments by targeting specific genes involved in stress responses and growth regulation. The combined use of clinostat devices and CRISPR/Cas9 techniques provides valuable insights into plant adaptation mechanisms, crucial for successful space farming and long-term space missions. The TOAST database can be a valuable tool for researchers to investigate the effects of microgravity on plants by analyzing gene expression patterns and responses to spaceflight-related stresses39,40,42,45.
Radiation Exposure
Space environments expose plants to elevated levels of ionizing radiation, including cosmic rays and solar radiation, which can generate ROS and cause cellular damage46. Astronauts aboard the ISS experience 100–200 mSv annually, far exceeding Earth’s 0.39 mSv, with deep-space missions potentially reaching several thousand mSv per year47. Plants face varying levels of low- and high-LET radiation, with high-LET radiation (e.g., heavy ions) causing more severe damage due to higher ionization density. Chronic exposure can lead to genomic instability, reduced growth, and altered physiological responses, while recent studies highlight the role of DNA repair mechanisms and antioxidant defenses in mitigating radiation stress. Research is now focused on developing radiation-tolerant crops and shielding strategies to support sustainable agriculture in space exploration48,49.
Altered Atmospheric Conditions
Spaceflight environments have different atmospheric compositions and pressures compared to Earth. These changes can affect plant metabolism and photosynthesis, potentially leading to the accumulation of ROS due to imbalances in energy production and utilization. Spaceflight conditions, including altered oxygen levels and elevated CO2, have a significant impact on plant metabolism, leading to redox imbalances and increased ROS production. In the microgravity environment of space, the distribution of gases, including oxygen, is affected, which can result in hypoxic conditions (low oxygen levels) within the plant growth environment. Hypoxia disrupts the normal functioning of the electron transport chain in mitochondria, causing inefficient respiration and increased production of ROS. Under these conditions, electrons leak and react with oxygen to form ROS, leading to oxidative stress and potential damage to cellular components such as proteins, lipids, and DNA50. Additionally, spaceflight environments often have elevated levels of CO2 to support human respiration. While CO2 is essential for photosynthesis, excessive levels can disrupt the balance of photosynthetic processes, leading to the overproduction of reducing equivalents (NADPH) and ATP, which can overwhelm the plant’s metabolic capacity51. This imbalance in the redox state of the cell causes an overproduction of ROS during photosynthesis and other metabolic processes, further contributing to oxidative stress. Plants have evolved antioxidant defense systems, including enzymatic and non-enzymatic antioxidants, to mitigate the effects of ROS. However, the unique conditions of spaceflight can overwhelm these defense systems, leading to chronic oxidative stress. Furthermore, spaceflight conditions can trigger changes in gene expression related to stress responses, upregulating genes encoding antioxidant enzymes and other protective proteins to counteract increased ROS production51. In addition to growth and performance, CO2 concentration also affects the nutritional content of plants intended for space missions to supplement astronauts’ diets50. Plants in space face unique challenges due to higher levels of cosmic radiation and the absence of gravity52. Cosmic radiation, including high-energy particles from solar particle events (SPE) and galactic cosmic rays (GCR), can penetrate plant tissues, causing oxidative stress and damage to cellular components like DNA, proteins, and lipids, ultimately affecting plant growth and health. Additionally, the absence of gravity leads to hypoxic conditions, disrupting normal physiological processes such as respiration, photosynthesis, and nutrient uptake, which results in increased ROS production and oxidative stress. These conditions also impact the orientation and growth direction of plant roots and shoots50.
Oxygen Availability
The availability of oxygen in space is different from that on Earth. Altered oxygen levels can impact ROS production during respiration and other cellular processes53,54,55,56,57. Spaceflight is an environmentally stressful condition for biological organisms because there is a lower level of gravity, less oxygen availability, and greater cosmic radiation as compared to on Earth58. The microgravity environment and the closed systems used for growing plants can lead to hypoxic conditions. This can affect the normal physiological processes of plants. In hypoxic conditions, the efficiency of the electron transport chain in mitochondria is compromised, leading to an increase in ROS production. This can disrupt the balance between ROS production and scavenging, resulting in oxidative stress59 and affects plant growth. In Arabidopsis thaliana, alcohol dehydrogenase (ADH) is differently affected by redox modifications when under spaceflight stress due to transcriptome variation in hypoxic conditions60. There are unique responses in the novel environment of spaceflight with heat shock factors and heat shock protein genes response from Arabidopsis thaliana cell cultures in spaceflight. The root apex in Arabidopsis thaliana is impacted due to root zone hypoxia. Root zone hypoxia also occurs in Brassica rapa due to the lack of oxygen in the spaceflight environment61.
Nutrient Imbalances
Spaceflight conditions can disrupt nutrient uptake and distribution in plants. Nutrient imbalances, such as altered levels of minerals and trace elements, can affect enzymatic activities involved in ROS metabolism62,63. Microgravity, radiation, and other space-specific conditions can disrupt nutrient balance, leading to altered levels of minerals and trace elements. These imbalances can affect enzymatic activities involved in ROS metabolism64. For example, microgravity conditions can lead to imbalances in certain nutrients in Arabidopsis thaliana. These imbalances may negatively affect plant growth and nutritional quality during long-duration space missions. Possible mechanisms for such effects include reduced transpiration, altered expression of channels or transporters, and direct effects on nutrient uptake65. Also studies with the Micro-Tom dwarf tomato variety highlighted the negative effect of microgravity on nutrient acquisition and plant growth, including the overall yield66. However, rethinking traditional earth-based cultivation methods, researchers propose that space conditions can be exploited to enhance plant growth and resilience, ultimately supporting efficient food production for long-term space missions and extraterrestrial colonization. Researchers consider traditional soil-based systems ineffective. They propose adaptable hydroponic systems, leveraging water dispensation and removal in a substrate-free environment. These innovative systems incorporate misting of nutrient solutions, hydrogels, and specific root module geometries to optimize nutrient delivery67. Efficient delivery of water and nutrients is crucial for plant growth in space, so hydroponic and aeroponic systems are used. These systems require precise control to ensure proper nutrient uptake68.
In addition, different plant species and their genetic makeup play a significant role in how they respond to the space environment. Some plants may be more resilient to nutrient imbalances, while others may require genetic modifications to thrive in space. For example, Arabidopsis thaliana can experience delayed flowering and changes in gene expression under spaceflight conditions. These changes include up-regulation of genes involved in temperature response, wounding, and protein stabilization, and down-regulation of genes related to circadian rhythm, gibberellins, and mRNA processes. Also, wheat grown in weightlessness experienced various developmental and biochemical anomalies. Specifically, the leaves of the wheat were hyperhydric, senesced precociously, and accumulated aromatic and branched-chain amino acids typical of tissues experiencing oxidative stress69. These factors highlight the complexity of growing plants in space and the need for continued research to develop effective solutions for nutrient management. By understanding these challenges, scientists aim to improve crop production in space, which is essential for long-term space missions and potential colonization of other planets.
Stress and Microbial Interactions
It is well established that both abiotic and biotic stress can induce ROS production as part of defense mechanisms. In intensified environments such as microgravity conditions, altered microbial interactions—such as shifts in beneficial plant-associated bacteria—may disrupt plant health and further contribute to ROS accumulation. Additionally, certain pathogenic microbes may exhibit altered virulence in microgravity, further exacerbating plant stress and ROS generation. For example, studies have shown that gamma radiation exposure leads to microbial dysbiosis in the rhizosphere of rice plants, reducing bacterial diversity and negatively affecting plant health and phosphorus uptake. Similarly, cosmic radiation in space may have comparable effects, potentially altering microbial communities associated with space-grown plants and influencing ROS dynamics70. Understanding how space-induced microbial shifts influence ROS dynamics in plants is crucial for designing sustainable plant growth systems in extraterrestrial environments.
Beyond plant health, consuming space-grown plants with elevated ROS levels could also negatively impact gut microbiota balance in astronauts. Given that ROS plays a key role in shaping microbial communities, excessive dietary ROS intake may contribute to gut microbiome dysbiosis, further compounding the physiological challenges associated with long-term space missions11,71.
Mechanical Stress
Launch, landing, and other aspects of space travel subject plants to mechanical stress. Mechanical forces and vibrations can disrupt cellular structures and trigger ROS production72.
Altered Gene Expression
Gene expression patterns in plants can change in response to spaceflight conditions. Studies have shown that microgravity and cosmic radiation influence the expression of key ROS-related genes, including those involved in antioxidant defense (e.g., SOD, CAT, GPX) and ROS-producing pathways (e.g., RbohD, RbohF)73,74. These changes can lead to an imbalance between ROS production and detoxification, resulting in ROS accumulation.
Additionally, epigenetic modifications such as DNA methylation and histone modifications have been observed in space-grown plants, affecting stress response pathways and ROS homeostasis3,75. Differential methylation in ROS-related genes could modulate their expression levels, impacting oxidative stress regulation. Understanding these molecular changes is crucial for developing strategies to enhance plant resilience in space environments.
Limited Antioxidant Defense
Spaceflight conditions can impact the availability of antioxidants that help neutralize ROS. Reduced antioxidant capacity may lead to ROS buildup due to multiple factors76,77, including altered gene expression, downregulation of key antioxidant enzymes, and disruption of mechanotransduction pathways39,40,41,42. Studies have shown that microgravity and cosmic radiation can reduce the activity of critical antioxidant enzymes, such as superoxide dismutase (SOD)21, catalase, and glutathione peroxidase, thereby impairing the cell’s ability to detoxify ROS36,37. Additionally, mitochondria and peroxisomes—both key organelles involved in ROS metabolism—contain antioxidant mechanisms that may become dysregulated in space, further exacerbating oxidative stress30.
Prolonged exposure to ROS without sufficient antioxidant protection could disrupt the body’s equilibrium of antioxidant defenses, impair nutrient absorption, and increase susceptibility to oxidative damage78,79,80,81,82. This imbalance highlights the need for targeted countermeasures, such as dietary antioxidant supplementation or genetic modifications in space-grown plants, to support cellular resilience against oxidative stress in space environments61,62.
Challenges in Water Management
Proper water distribution in microgravity can be challenging. Water stress or imbalances can influence ROS production in plant cells.
It’s important to note that while these factors can induce ROS in plants grown in space, plants have also evolved various defense mechanisms to manage and mitigate oxidative stress. These include the activation of antioxidant enzymes, upregulation of stress-responsive genes, and adjustments in metabolic pathways. Understanding the complex interplay between ROS induction factors and plant responses is crucial for successful plant growth in space and optimizing space agriculture83,84.
Diseases Linked to ROS in Astronauts
The intricate balance between ROS production and cellular defense mechanisms is crucial for maintaining optimal health. Imbalances that tip the scales toward excessive ROS production can lead to detrimental effects on cellular components, contributing to various human diseases. These multifaceted connections between ROS and disease pathology highlight the significance of understanding ROS dynamics in the context of human health.
Moreover, a potential yet understudied source of ROS exposure for astronauts comes from space-grown plants, which are cultivated as a sustainable food source during long-term missions. Studies have demonstrated that these plants accumulate significantly higher levels of ROS due to the combined effects of microgravity and cosmic radiation. If consumed, such ROS-enriched plant-derived foods could contribute to oxidative stress in astronauts, potentially increasing the risk of ROS-associated diseases, including cardiovascular, neurological, and immune-related disorders2,3. To better assess these risks, we review current knowledge on ROS-related disease mechanisms and their potential relevance to space travelers. (Fig. 4).
This image explores the consequences of disrupted ROS balance in biological systems. ROS, natural byproducts of cellular processes, are essential for functions like apoptosis and immunity. However, when ROS production surpasses the body’s defense mechanisms, it leads to oxidative stress. This imbalance damages vital cellular components and is linked to various human diseases. Understanding these dynamics is crucial for unraveling disease mechanisms and potential treatments.
Cancer and ROS
The link between ROS and cancer has garnered substantial attention. Cancer cells often exhibit heightened levels of ROS due to factors such as oxidative burst triggered by infiltrating macrophages and increased ROS production in tumor vasculature. These conditions foster an environment of oxidative stress that can lead to DNA damage. ROS-mediated DNA damage can result in the inactivation of tumor suppressor genes and the activation of oncogenes, facilitating the transformation of normal cells into cancerous counterparts. The intricate interplay between ROS, DNA mutations, and the activation of proto-oncogenes underscores the role of oxidative stress in driving carcinogenesis85,86,87.
Cardiovascular Diseases and ROS
ROS play a pivotal role in the landscape of cardiovascular diseases. Vascular cells express NADPH oxidase enzymes that generate ROS, contributing to various vascular disorders. Dysregulation of NADPH oxidase expression is implicated in conditions such as hypertension. Elevated superoxide radicals reduce nitric oxide availability, impairing vasodilation. Additionally, ROS-induced proliferation and hypertrophy of vascular smooth muscle cells contribute to increased vascular resistance and blood pressure78,79,88.
Neurological Disorders and ROS
ROS are intricately involved in neurological diseases, with implications spanning from neuroprotection to neurodegeneration. Proper levels of ROS are essential for neuronal function, but excessive ROS production can contribute to neurodegenerative diseases like Alzheimer’s. The NADPH oxidase enzyme expressed in microglia cells generates ROS, and the accumulation of ROS-producing amyloid exacerbates neuronal damage, leading to cognitive decline80.
Respiratory Ailments and ROS
Respiratory disorders, including asthma and chronic obstructive pulmonary disease (COPD), exhibit associations with oxidative stress and ROS. Inflammation in lung tissue is exacerbated by ROS-induced activation of inflammatory signaling pathways and transcription factors. The intricate interplay between ROS and inflammatory responses contributes to the progression of these diseases81,82.
Sensory Impairments and ROS
Age-related sensory disorders such as cataracts, retinal degeneration, and hearing loss are intricately linked to ROS production and oxidative stress. ROS contribute to cellular damage and dysfunction in ocular and auditory tissues, accelerating the progression of age-related sensory impairments89.
Infertility and ROS
ROS also exert a significant influence on fertility, particularly in males. ROS can be generated internally by leukocytes and immature spermatozoa or externally due to factors like alcohol and tobacco consumption. Infections and inflammation stimulate leukocytes to produce ROS, which can have detrimental effects on sperm motility, morphology, and viability. The presence of high levels of ROS in semen can contribute to male infertility90.
As research advances, new connections between ROS and diseases continue to emerge. The complex interplay between ROS, cellular responses, and disease progression highlights the need for innovative therapeutic strategies that restore redox balance. In space, an additional concern is the consumption of ROS-enriched plants, which may contribute to oxidative stress in astronauts. Further research is needed to determine how dietary ROS affects cellular homeostasis and long-term astronaut health.
Impact of ROS on Astronaut Gut Microbiota
The effect of the accumulation of ROS on the intestinal microbiota population has gained increasing attention in recent research. ROS are known to play a crucial role in maintaining gut homeostasis by modulating various physiological processes and immune responses. However, when ROS levels become dysregulated and accumulate, it can lead to significant alterations in the composition and function of the intestinal microbiota, which in turn can have implications for overall gut health and various disease states. Several studies have explored the intricate relationship between ROS accumulation and changes in the intestinal microbiota population71,91,92,93 (Fig. 5).
This insightful image delves into the impact of ROS accumulation on the human gut microbiota. Recent research has highlighted the crucial role of ROS in maintaining gut homeostasis by regulating physiological processes and immune responses. However, when ROS levels become dysregulated and accumulate, they can significantly alter the composition and function of the intestinal microbiota. This disruption has profound implications for overall gut health and is linked to various disease states. The illustration explores the intricate relationship between ROS accumulation and changes in the gut microbiota, emphasizing the delicate balance necessary for a healthy gut environment.
Shifts in Microbial Composition
Excessive ROS levels in the gut can lead to shifts in the relative abundance of different microbial taxa. These shifts may include an increase in potentially harmful bacteria and a decrease in beneficial commensal microbes. Such imbalances in microbial composition have been associated with conditions like inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and other gastrointestinal disorders71.
Microbial Dysbiosis
ROS accumulation can disrupt the delicate balance between microbial species in the gut, leading to a state of microbial dysbiosis. Dysbiosis is characterized by alterations in the diversity and richness of the gut microbiota, potentially paving the way for the overgrowth of opportunistic pathogens and reduction in beneficial microbes. This dysbiosis can further exacerbate inflammation and contribute to the progression of gut-related disorders94.
Impaire d Intestinal Barrier Function
ROS-induced damage to the gut epithelial barrier can compromise its integrity, leading to increased permeability (leaky gut). A leaky gut allows harmful microbial components to translocate into the bloodstream, triggering immune responses and inflammation. This chronic low-grade inflammation can perpetuate a cycle of ROS accumulation and further dysbiosis, contributing to the pathogenesis of various gastrointestinal diseases95.
Impact on Metabolites and Microbial Metabolism
ROS accumulation can also influence the production of microbial metabolites, which play a key role in maintaining gut health. Disruption of microbial metabolism due to ROS can lead to alterations in the production of short-chain fatty acids (SCFAs), neurotransmitters, and other metabolites that have important regulatory functions in the gut-brain axis, immune system, and overall physiological processes71.
Role in Gut-Brain Axis
Accumulation of ROS in the gut may impact the gut-brain axis, which is the bidirectional communication network between the gut and the brain. Changes in the gut microbiota composition and function can affect neurotransmitter production and signaling, potentially contributing to mood disorders and cognitive dysfunction96.
In summary, while no direct studies have examined the impact of ROS accumulation from space-grown plants on astronaut gut microbiota, previous research suggests that excessive ROS in the gut can lead to microbial dysbiosis and population shifts. Additionally, studies have shown that spaceflight conditions, including microgravity and cosmic radiation, already alter the gut microbiota composition of astronauts.
Given these findings, it is plausible that consuming ROS-enriched foods could further contribute to microbiome imbalances, though this remains to be experimentally confirmed. Further research is needed to clarify these interactions, assess potential health risks, and develop targeted strategies—such as antioxidant supplementation or dietary modifications—to help maintain gut microbial balance during long-term space missions.
ROS Status in Space-Grown Plants
Growing plants in space, a crucial aspect of sustaining astronauts during extended missions, presents its own array of challenges. One prominent concern revolves around the potential buildup of ROS within these space-cultivated plants. These ROS, encompassing superoxide anions and hydrogen peroxide, represent highly reactive molecules that may accumulate due to shifts in gravity, exposure to radiation, and limited air circulation aboard spacecraft or space stations. This accumulation could hold significant ramifications for both the nutritional quality of the produced food and its absorption within the astronauts’ digestive systems2,12,97.
NASA’s research and spaceflight experiments provide valuable insights into ROS accumulation in space-grown plants. Mingqi Zhou et al. 3 conducted a bioinformatics analysis comparing Arabidopsis seeds grown on Earth and aboard the International Space Station (ISS). Their findings revealed that 46% of differentially methylated cytosine-DEG sets in leaves were associated with ROS-related genes, demonstrating a significant increase in ROS activity under microgravity conditions.
Similarly, Manabu Sugimoto et al. on Mizuna plants cultivated in space and on Earth showed that 20 out of 32 ROS oxidative markers were upregulated more than two-fold in space-grown plants. Notably, two key ROS-producing genes (NADPH oxidase genes: RbohD and RbohF) exhibited a 3-fold and 5.1-fold increase in expression, respectively. While ROS-scavenging enzymes, such as thioredoxin and glutaredoxin, were also upregulated, their increase was comparatively lower2.
These studies confirm that ROS levels in space-grown plants rise significantly due to gene expression changes. While plants appear to adapt by reprogramming their defense systems, it remains uncertain whether this adaptation is sufficient to prevent ROS accumulation in edible tissues. The key concern is that ROS accumulation in plant tissues intended for astronaut consumption could pose a health risk, potentially affecting gut microbiota and cellular homeostasis2,3,98.
Moreover, while previous studies primarily attribute ROS accumulation to microgravity, an overlooked factor is cosmic radiation exposure. Upon further analysis of Zhou et al.’s findings, similarities were observed between oxidative stress caused by high light intensity and UV radiation on Earth and oxidative stress in space-grown plants. This strongly suggests that cosmic radiation, rather than microgravity alone, plays a dominant role in ROS accumulation in space agriculture2,3.
The most concerning aspect is the potential magnitude of ROS accumulation in plant tissues, which some studies suggest could be up to five times higher than in Earth-grown plants. While ROS-scavenging enzymes also increase, their upregulation (approximately two-fold) is insufficient to counterbalance the three- to five-fold rise in ROS production markers, indicating that a substantial portion of ROS may remain in the plant2,3.
Astronauts aboard the ISS receive an annual radiation dose of 100 to 200 millisieverts (mSv), far exceeding the 0.39 mSv typically experienced on Earth. For deep-space missions, such as Mars exploration, these doses could rise to several hundred to several thousand mSv per year, further emphasizing the need for radiation-resistant crop cultivation strategies47.
The repercussions of ROS accumulation in space-grown plants are twofold. Primarily, the oxidative stress stemming from heightened ROS levels might lead to the degradation of vital nutrients like vitamins, minerals, and antioxidants. Such deterioration would result in a diminished nutritional value of the harvested crops. Moreover, the ROS-induced oxidation of lipids and other compounds could alter the taste, aroma, and overall sensory perception of the food, making it less appealing to astronauts. Additionally, the breakdown of proteins triggered by ROS could impact the texture and shelf life of the space-cultivated produce, potentially reducing its storage viability12,89.
Beyond the implications for food quality, the accumulation of ROS could also affect nutrient absorption within the astronauts’ digestive tracts. Oxidative stress has the potential to disrupt the regular operations of the gastrointestinal system, thereby influencing the processes of digestion and nutrient uptake. Furthermore, compromised intestinal barriers due to ROS-related damage might lead to heightened permeability and inflammation. These consequences, in turn, could hinder nutrient absorption and compromise the overall health of the gut. Additionally, prolonged exposure to ROS without sufficient antioxidant protection could disrupt the body’s equilibrium of antioxidant defenses, potentially impeding its ability to counteract oxidative stress and maintain optimal nutrient absorption78,79,80,81,82.
The buildup of ROS in the digestive system could profoundly impact the composition, diversity, and function of the intestinal microbiota. This complex interplay between ROS and the gut microbiota carries significant implications for gastrointestinal health, inflammation, and the development of various gut-related diseases91,94,99.
Addressing these challenges necessitates comprehensive strategies. Optimizing the cultivation environments, refining nutrient delivery systems, and introducing antioxidant supplementation all stand as essential steps to mitigate ROS accumulation. Furthermore, endeavors to boost the antioxidant content of space-grown plants or engineer antioxidant pathways could provide potential solutions to counteract the effects of ROS. Through continuous research and innovative techniques, space agencies and scientists are actively working to ensure that space-grown food retains its nutritional value and remains capable of supporting astronauts’ well-being amid the demanding conditions of extended space missions.
Addressing Concerns Regarding Astronauts’ Health and Food Safety
The intricate relationship between space-grown plants, ROS, and the nourishment of astronauts in space gives rise to several fundamental questions that underline the complexity of this issue. These inquiries encompass both profound concerns and potential solutions that directly impact the nutritional well-being of space travelers.
A central question revolves around the potential toxicity of ROS-infused sustenance. The markedly elevated ROS levels found in crops cultivated in space, compared to those grown on Earth, raise the question of whether consuming such food could lead to harm. The assessment hinges on whether harm arises from a single instance of consuming ROS-enriched plant-based food or if the risk accumulates over multiple servings or extended consumption. Addressing this dilemma requires meticulous experiments involving mice fed with space-cultivated plants, offering insights into potential health outcomes.
Another perplexing issue relates to the alteration of the intestinal metagenome resulting from the consumption of ROS-laden plants. While the space environment itself negatively affects the metagenome, emerging evidence suggests that ROS might negatively affect beneficial bacterial populations. The intricate interplay between cosmic conditions and ROS as factors influencing the intestinal bacterial ecosystem necessitates thorough investigation, with profound implications for astronaut health.
One prospective avenue to counteract ROS accumulation due to cosmic radiation lies in plant breeding. Developing genetically modified plants adapted to space conditions, equipped with resistance to ROS buildup, holds promise. However, the timing of these breeding initiatives and their alignment with upcoming Mars missions add complexity that requires careful evaluation.
To address these multifaceted concerns, a proposed comprehensive strategy involves a three-pronged data collection approach, encompassing space-grown plants, their Earth counterparts, and those cultivated under simulated microgravity and cosmic radiation conditions. This approach utilizes well-defined and comprehensive datasets to fuel bioinformatics analyses, enabling nuanced insights and meaningful interpretations, and even studies in quantum biology and quantum bioinformatics100 in space, in the absence of Earth’s gravity and magnetic field, are essential. In light of the complexities of ROS accumulation, establishing a specialized field of study dedicated to oxidative stress in plant analysis is recommended. This field could encompass various aspects, including the biological pathways of oxidative stress and ROS regulation, exploration of breeding techniques for ROS-resistant plants, investigation of the nutritional implications of ROS-enriched plants on mammalian physiology, and an anticipated exploration of their effects on the composition of beneficial bacteria and the intricate landscape of the intestinal metagenome.
Equally significant is the degree of attention bestowed upon these intricate challenges by prominent institutions like NASA and its affiliated space contractors. Amid the overarching pursuit of sustenance safety and the perpetuation of food sources, understanding the scope of research and contemplation directed toward addressing ROS accumulation in space-grown plants assumes paramount importance.
Conclusion
The cultivation and consumption of salads derived from plants grown in space has sparked a significant concern, prompting a thorough investigation into potential ramifications. Building upon the earlier discourse, the prospect of plants acquiring toxic attributes looms large, bearing the potential for enduring physiological consequences and the onset of debilitating ailments within the astronaut cohort. This apprehension gains further significance when considering the financial implications entailed in supplying sustenance to spacefarers. The substantial cost of ferrying each kilogram of consumable provisions to the space station is estimated at approximately $10,000. This financial outlay experiences a marked escalation, reaching approximately $150,000 per kilogram for lunar missions, and surging to an astounding $1 million per kilogram for voyages destined for Mars. Notably, while provisioning food supplies for missions to the International Space Station or lunar sojourns spanning around 3 days appears viable, the endeavor becomes markedly formidable during extended interplanetary expeditions such as the 9-month journey to Mars. During this protracted odyssey, a substantial food stock becomes imperative, notwithstanding the attendant financial and logistical intricacies. Consequently, a compelling rationale emerges for the in-situ cultivation of sustenance, specifically plant-based, within the spatial confines.
However, the tantalizing prospect of extraterrestrial horticulture is beset by a paramount challenge - the specter of plant-borne toxicity in the space environment. This predicament assumes heightened significance considering imminent space exploration endeavors. Notably, NASA’s ambitious Artemis project is poised to reclaim lunar exploration by 2024, while Elon Musk’s visionary aspirations envision human habitation on Mars by 2030. Given these imminent undertakings, the resolution of potential plant toxicity becomes an exigent priority. Failure to adequately address this challenge could cast a shadow over these pioneering initiatives, potentially impeding their progress and realization. In light of these imperatives, concerted efforts and multidisciplinary collaboration are warranted to decipher and mitigate the underlying mechanisms driving plant toxicity in space. Such endeavors hold the key to establishing a sustainable and secure food production system, bolstering the success of upcoming space missions, and advancing the frontiers of human exploration in the cosmos.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Pandith, J. A., Neekhra, S., Ahmad, S. & Sheikh, R. A. Recent developments in space food for exploration missions: a review. Life Sci. Space Res. 36, 123–134 (2023).
Sugimoto, M. et al. Genome-wide expression analysis of reactive oxygen species gene network in mizuna plants grown in long-term spaceflight. BMC Plant Biol. 14, 4 (2014).
Zhou, M., Sng, N. J., LeFrois, C. E., Paul, A.-L. & Ferl, R. J. Epigenomics in an extraterrestrial environment: organ-specific alteration of DNA methylation and gene expression elicited by spaceflight in Arabidopsis Thaliana. BMC Genomics 20, 205 (2019).
De Micco, V., Arena, C., Pignalosa, D. & Durante, M. Effects of sparsely and densely ionizing radiation on plants. Radiat. Environ. Biophys. 50, 1–19 (2011).
Reitz, G. Characteristic of the radiation field in low earth orbit and in deep space. Z. Für Med. Phys. 18, 233–243 (2008).
Atri, D. & Melott, A. L. Cosmic rays and terrestrial life: a brief review. Astropart. Phys. 53, 186–190 (2014).
Naito, M. et al. Radiation dose and its protection in the moon from galactic cosmic rays and solar energetic particles: at the lunar surface and in a Lava Tube. J. Radiol. Prot. 40, 947 (2020).
Krukowski, K. et al. The impact of deep space radiation on cognitive performance: from biological sex to biomarkers to countermeasures. Sci. Adv. 7, eabg6702 (2021).
Almeida Filho, D., Federico, C. A., da Silva, F. C. A. & Souza-Santos, D. Evaluation of Neutron Doses in Brazilian Aircrew by the Monte Carlo Method. Radiat. Meas. 166, 106959 (2023).
Hajam, Y. A. et al. Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells 11, 552 (2022).
Khan, M. et al. The Key Roles of ROS and RNS as a Signaling Molecule in Plant-Microbe Interactions. Antioxid. Basel Switz. 12, 268 (2023).
Choe, E. & Min, D. B. Chemistry and reactions of reactive oxygen species in foods. Crit. Rev. Food Sci. Nutr. 46, 1–22 (2006).
Serra, A. J., Pinto, J. R., Prokić, M. D., Arsa, G. & Vasconsuelo, A. Oxidative stress in muscle diseases: current and future therapy 2019. Oxid. Med. Cell. Longev. 2020, 6030417 (2020).
García-Caparrós, P. et al. Oxidative stress and antioxidant metabolism under adverse environmental conditions: a review. Bot. Rev. 87, 421–466 (2021).
Hussain, S. et al. Oxidative Stress and Antioxidant Defense in Plants Under Drought Conditions. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Hasanuzzaman, M., Hakeem, K. R., Nahar, K., Alharby, H. F., Eds.; Springer International Publishing: Cham, 2019; pp 207–219. https://doi.org/10.1007/978-3-030-06118-0_9.
Hasanuzzaman, M. et al. Regulation of Reactive Oxygen Species and Antioxidant Defense in Plants under Salinity. Int. J. Mol. Sci. 22, 9326 (2021).
Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxid. Basel Switz. 9, 852 (2020).
Jakubczyk, K. et al. Reactive oxygen species - sources, functions, oxidative damage. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 48, 124–127 (2020).
Sies, H. et al. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 23, 499–515 (2022).
Checa, J. & Aran, J. M. Reactive oxygen species: drivers of physiological and pathological processes. J. Inflamm. Res. 13, 1057–1073 (2020).
Das, K. & Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2 (2014).
Rajput, V. D. et al. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 10, 267 (2021).
Veljović Jovanović, S., Kukavica, B., Vidović, M., Morina, F. & Menckhoff, L. Class III Peroxidases: Functions, Localization and Redox Regulation of Isoenzymes. In Antioxidants and Antioxidant Enzymes in Higher Plants; Gupta, D. K., Palma, J. M., Corpas, F. J., Eds.; Springer International Publishing: Cham, 2018; pp 269–300. https://doi.org/10.1007/978-3-319-75088-0_13.
Nianiou-Obeidat, I. et al. Plant Glutathione Transferase-Mediated Stress Tolerance: Functions and Biotechnological Applications. Plant Cell Rep. 36, 791–805 (2017).
Bela, K. et al. Plant Glutathione Peroxidases: Emerging Role of the Antioxidant Enzymes in Plant Development and Stress Responses. J. Plant Physiol. 176, 192–201 (2015).
Hasanuzzaman, M. et al. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxid. Basel Switz. 9, 681 (2020).
Chemistry and Reactions of Reactive Oxygen Species in Foods: Critical Reviews in Food Science and Nutrition: Vol 46, No https://www.tandfonline.com/doi/abs/10.1080/10408390500455474 (accessed 2025-02-28).
Gómez, X. et al. Key Points for the Development of Antioxidant Cocktails to Prevent Cellular Stress and Damage Caused by Reactive Oxygen Species (ROS) during Manned Space Missions. NPJ Microgravity 7, 35 (2021).
Sachdev, S., Ansari, S. A. & Ansari, M. I. Peroxisomes and ROS Under Stress Conditions. In Reactive Oxygen Species in Plants: The Right Balance; Sachdev, S., Ansari, S. A., Ansari, M. I., Eds.; Springer Nature: Singapore, 2023; pp 107–123. https://doi.org/10.1007/978-981-19-9884-3_7.
Frontiers | Plant Peroxisomes: A Factory of Reactive Species. https://www.frontiersin.org/articles/10.3389/fpls.2020.00853/full (accessed 2023-08-08).
Corpas, F. J., del Río, L. A. & Palma, J. M. Impact of Nitric Oxide (NO) on the ROS Metabolism of Peroxisomes. Plants 8, 37 (2019).
Foyer, C. H. & Hanke, G. ROS Production and Signalling in Chloroplasts: Cornerstones and Evolving Concepts. Plant J. 111, 642–661 (2022).
Mitra, S. et al. Impact of ROS Generated by Chemical, Physical, and Plasma Techniques on Cancer Attenuation. Cancers 11, 1030 (2019).
Reactive oxygen species and organellar signaling | Journal of Experimental Botany | Oxford Academic. https://academic.oup.com/jxb/article/72/16/5807/6278282 (accessed 2023-08-08).
Parke, D. V. & Sapota, A. Chemical Toxicity and Reactive Oxygen Species. Int. J. Occup. Med. Environ. Health 9, 331–340 (1996).
Hernansanz-Agustín, P. & Enríquez, J. A. Generation of Reactive Oxygen Species by Mitochondria. Antioxidants 10, 415 (2021).
Tapeinos, C. & Pandit, A. Physical, Chemical, and Biological Structures Based on ROS-Sensitive Moieties That Are Able to Respond to Oxidative Microenvironments. Adv. Mater. 28, 5553–5585 (2016).
Jeanmaire, D. et al. Chemical Specificity in REDOX-Responsive Materials: The Diverse Effects of Different Reactive Oxygen Species (ROS) on Polysulfide Nanoparticles. Polym. Chem. 5, 1393–1404 (2014).
Tan, S., Pei, W., Huang, H., Zhou, G. & Hu, W. Additive Effects of Simulated Microgravity and Ionizing Radiation in Cell Death, Induction of ROS and Expression of RAC2 in Human Bronchial Epithelial Cells. NPJ Microgravity 6, 34 (2020).
Nguyen, H. P., Tran, P. H., Kim, K.-S. & Yang, S.-G. The Effects of Real and Simulated Microgravity on Cellular Mitochondrial Function. NPJ Microgravity 7, 44 (2021).
Choi, W.-G., Barker, R. J., Kim, S.-H., Swanson, S. J. & Gilroy, S. Variation in the Transcriptome of Different Ecotypes of Arabidopsis Thaliana Reveals Signatures of Oxidative Stress in Plant Responses to Spaceflight. Am. J. Bot. 106, 123–136 (2019).
Singh, R., Rajput, M. & Singh, R. P. Simulated Microgravity Triggers DNA Damage and Mitochondria-Mediated Apoptosis through ROS Generation in Human Promyelocytic Leukemic Cells. Mitochondrion 61, 114–124 (2021).
Xu, P., Chen, H., Jin, J. & Cai, W. Single-base resolution methylome analysis shows epigenetic changes in Arabidopsis seedlings exposed to microgravity spaceflight conditions on board the SJ-10 recoverable satellite. Npj Microgravity 4, 1–11 (2018).
Kiss, J. Z., Wolverton, C., Wyatt, S. E., Hasenstein, K. H. & van Loon, J. J. W. A. Comparison of Microgravity Analogs to Spaceflight in Studies of Plant Growth and Development. Front. Plant Sci. 2019, 10. https://doi.org/10.3389/fpls.2019.01577.
Barker, R., Lombardino, J., Rasmussen, K. & Gilroy, S. Test of Arabidopsis Space Transcriptome: A Discovery Environment to Explore Multiple Plant Biology Spaceflight Experiments. Front. Plant Sci. 11, 147 (2020).
Arena, C., De Micco, V., Macaeva, E. & Quintens, R. Space radiation effects on plant and mammalian cells. Acta Astronaut 104, 419–431 (2014).
Peracchi, S. et al. Modelling of the silicon-on-insulator microdosimeter response within the International Space Station for Astronauts’ radiation protection. Radiat. Meas. 128, 106182 (2019).
Barker, R. et al. Rad-Bio-App: A Discovery Environment for Biologists to Explore Spaceflight-Related Radiation Exposures. Npj Microgravity 7, 1–9 (2021).
Maffei, M. E. et al. The Physiology of Plants in the Context of Space Exploration. Commun. Biol. 7, 1–12 (2024).
Farooq, M. et al. Investigating Plant Responses to Microgravity and Adaptations in Gravisensitive Environments. Environ. Sci. Eur. 36, 28 (2024).
Plants in Space: Novel Physiological Challenges and Adaptation Mechanisms | SpringerLink. https://link.springer.com/chapter/10.1007/124_2021_53#DOI (accessed 2025-02-26).
Hydroponics for plant cultivation in space – a white paper - ScienceDirect. https://www.sciencedirect.com/science/article/pii/S221455242400066X (accessed 2025-02-26).
Giannetto, A. et al. Effects of oxygen availability on oxidative stress biomarkers in the mediterranean mussel mytilus galloprovincialis. Mar. Biotechnol. 19, 614–626 (2017).
Welker, A. F., Moreira, D. C., Campos, ÉG. & Hermes-Lima, M. Role of redox metabolism for adaptation of aquatic animals to drastic changes in oxygen availability. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 165, 384–404 (2013).
Lombardino, J. et al. Genomic characterization of potential plant growth-promoting features of sphingomonas strains isolated from the International space station. Microbiol. Spectr. 10, e01994–21 (2022).
Barker, R. et al. Meta-analysis of the space flight and microgravity response of the arabidopsis plant transcriptome. Npj Microgravity 9, 1–15 (2023).
Rutter, L. et al. A New Era for Space Life Science: International Standards for Space Omics Processing. Patterns 2020, 1. https://doi.org/10.1016/j.patter.2020.100148.
Manian, V., Gangapuram, H., Orozco, J., Janwa, H. & Agrinsoni, C. Network analysis of local gene regulators in Arabidopsis Thaliana under spaceflight stress. Computers 10, 18 (2021).
Sasidharan, R., Schippers, J. H. M. & Schmidt, R. R. Redox and low-oxygen stress: signal integration and interplay. Plant Physiol. 186, 66–78 (2021).
Loreti, E., Valeri, M. C., Novi, G. & Perata, P. Gene regulation and survival under hypoxia requires starch availability and metabolism. Plant Physiol. 176, 1286–1298 (2018).
Evidence of Root Zone Hypoxia in Brassica rapa L. Grown in Microgravity | International Journal of Plant Sciences: Vol 162, No https://www.journals.uchicago.edu/doi/10.1086/319585 (accessed 2025-02-26).
Chakraborty, K. et al. Ionic basis of salt tolerance in plants: nutrient homeostasis and oxidative stress tolerance. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp 325–362. https://doi.org/10.1007/978-981-10-9044-8_14.
Nogueirol, R. C., Monteiro, F. A., Gratão, P. L., de Alcântara da Silva, B. K. & Azevedo, R. A. Cadmium application in tomato: nutritional imbalance and oxidative stress. Water Air. Soil Pollut. 227, 210 (2016).
Hessel, V. et al. Eustress in Space: Opportunities for Plant Stressors Beyond the Earth Ecosystem. Front. Astron. Space Sci. 2022, 9. https://doi.org/10.3389/fspas.2022.841211.
Polinski, E. et al. 2-D clinorotation alters the uptake of some nutrients in Arabidopsis Thaliana. J. Plant Physiol. 212, 54–57 (2017).
Growth, yield and reproduction of dwarf tomato grown under simulated microgravity conditions: Plant Biosystems - An International Journal Dealing with all Aspects of Plant Biology: Vol 141, No https://www.tandfonline.com/doi/abs/10.1080/11263500601153735 (accessed 2025-02-26).
Hasenstein, K. H. & Miklave, N. M. Hydroponics for Plant Cultivation in Space – a White Paper. Life Sci. Space Res. 43, 13–21 (2024).
Morrow, R. C. et al. Matrix-Based Porous Tube Water and Nutrient Delivery System; SAE International, 1992. https://doi.org/10.4271/921390.
Carman, J. G., Hole, P., Salisbury, F. B. & Bingham, G. E. Developmental, nutritional and hormonal anomalies of weightlessness-grown wheat. Life Sci. Space Res. 6, 59–68 (2015).
Dysbiosis of the rhizosphere microbiome caused by γ-irradiation alters the composition of root exudates and reduces phosphorus uptake by rice in flooded soils | Plant and Soil. https://link.springer.com/article/10.1007/s11104-022-05726-5?utm_source=chatgpt.com (accessed 2025-02-26).
Singh, V., Ahlawat, S., Mohan, H., Gill, S. S. & Sharma, K. K. Balancing reactive oxygen species generation by rebooting gut microbiota. J. Appl. Microbiol. 132, 4112–4129 (2022).
Porter, T. K. et al. A theory of mechanical stress-induced H2O2 signaling waveforms in planta. J. Math. Biol. 86, 11 (2022).
Hasanuzzaman, M. et al. Regulation of ROS metabolism in plants under environmental stress: a review of recent experimental evidence. Int. J. Mol. Sci. 21, 8695 (2020).
Van Ruyskensvelde, V., Van Breusegem, F. & Van Der Kelen, K. Post-transcriptional regulation of the oxidative stress response in plants. Free Radic. Biol. Med. 122, 181–192 (2018).
Epigenomic Regulators Elongator Complex Subunit 2 and Methyltransferase 1 Differentially Condition the Spaceflight Response in Arabidopsis - PubMed. https://pubmed.ncbi.nlm.nih.gov/34589093/ (accessed 2025-02-26).
Huchzermeyer, B., Menghani, E., Khardia, P. & Shilu, A. Metabolic pathway of natural antioxidants, antioxidant enzymes and ROS providence. Antioxid. Basel Switz. 11, 761 (2022).
Zia-Ur-Rehman, M. et al. Nanoparticles assisted regulation of oxidative stress and antioxidant enzyme system in plants under salt stress: a review. Chemosphere 314, 137649 (2023).
Murphy, E. & Liu, J. C. Mitochondrial calcium and reactive oxygen species in cardiovascular disease. Cardiovasc. Res. 119, 1105–1116 (2023).
Dubois-Deruy, E., Peugnet, V., Turkieh, A. & Pinet, F. Oxidative stress in cardiovascular diseases. Antioxidants 9, 864 (2020).
Cheng, Y. et al. Ferroptosis mediated by lipid reactive oxygen species: a possible causal link of neuroinflammation to neurological disorders. Oxid. Med. Cell. Longev. 2021, e5005136 (2021).
Maynard, S. et al. Relationships between human vitality and mitochondrial respiratory parameters, reactive oxygen species production and dNTP levels in peripheral blood mononuclear cells. Aging 5, 850–864 (2013).
Gao, X. et al. Cryptococcal Hsf3 controls intramitochondrial ROS homeostasis by regulating the respiratory process. Nat. Commun. 13, 5407 (2022).
Velikanov, G. A., Sibgatullin, T. A., Belova, L. P. & Ionenko, I. F. Membrane water permeability of maize root cells under two levels of oxidative stress. Protoplasma 252, 1263–1273 (2015).
Li, J. et al. Water extract from herpetospermum pedunculosum attenuates oxidative stress and ferroptosis induced by acetaminophen via regulating Nrf2 and NF-κB pathways. J. Ethnopharmacol. 305, 116069 (2023).
Moloney, J. N. & Cotter, T. G. ROS Signalling in the Biology of Cancer. Semin. Cell Dev. Biol. 80, 50–64 (2018).
Pelicano, H., Carney, D. & Huang, P. ROS Stress in Cancer Cells and Therapeutic Implications. Drug Resist. Updat. 7, 97–110 (2004).
Ziyaei, K., Mokhtari, M., Hashemi, M., Rezaei, K. & Abdi, F. Association between Exposure to Water Sources Contaminated with Polycyclic Aromatic Hydrocarbons and Cancer Risk: A Systematic Review. Sci. Total Environ. 924, 171261 (2024).
Xu, T. et al. Oxidative Stress in Cell Death and Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2019, e9030563 (2019).
Juan, C. A., Pérez de la Lastra, J. M., Plou, F. J. & Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 22, 4642 (2021).
Villaverde, A. I. S. B., Netherton, J. & Baker, M. A. From Past to Present: The Link Between Reactive Oxygen Species in Sperm and Male Infertility. Antioxidants 8, 616 (2019).
Yardeni, T. et al. Host Mitochondria Influence Gut Microbiome Diversity: A Role for ROS. Sci. Signal. 12, eaaw3159 (2019).
Chen, Q. et al. Natural Exosome-like Nanovesicles from Edible Tea Flowers Suppress Metastatic Breast Cancer via ROS Generation and Microbiota Modulation. Acta Pharm. Sin. B 12, 907–923 (2022).
Xie, J. et al. Protective Effect of Quercetin on Streptozotocin-Induced Diabetic Peripheral Neuropathy Rats through Modulating Gut Microbiota and Reactive Oxygen Species Level. Biomed. Pharmacother. 127, 110147 (2020).
Kakade, A., Salama, E.-S., Pengya, F., Liu, P. & Li, X. Long-Term Exposure of High Concentration Heavy Metals Induced Toxicity, Fatality, and Gut Microbial Dysbiosis in Common Carp, Cyprinus Carpio. Environ. Pollut. 266, 115293 (2020).
Wang, H., Zhai, N., Chen, Y., Fu, C. & Huang, K. OTA induces intestinal epithelial barrier dysfunction and tight junction disruption in IPEC-J2 cells through ROS/Ca2+-mediated MLCK activation. Environ. Pollut. 242, 106–112 (2018).
Ikeda, Y., Saigo, N. & Nagasaki, Y. Direct evidence for the involvement of intestinal reactive oxygen species in the progress of depression via the gut-brain axis. Biomaterials 295, 122053 (2023).
Cooper, M., Douglas, G. & Perchonok, M. Developing the NASA food system for long-duration missions. J. Food Sci. 76, R40–R48 (2011).
Manzano, A., Carnero-Diaz, E., Herranz, R. & Medina, F. J. Recent transcriptomic studies to elucidate the plant adaptive response to spaceflight and to simulated space environments. iScience 25, 104687 (2022).
Khodadad, C. L. M. et al. Microbiological and nutritional analysis of lettuce crops grown on the international space station. Front. Plant Sci. 11, 199 (2020).
Mokhtari, M., Khoshbakht, S., Ziyaei, K., Akbari, M. E. & Moravveji, S. S. New classifications for quantum bioinformatics: Q-bioinformatics, QCt-bioinformatics, QCg-bioinformatics, and QCr-bioinformatics. Brief. Bioinform. 25, bbae074 (2024).
Acknowledgements
We would like to express our gratitude for the support and advice we received during this research from The NASA GeneLab Plant Analysis Working Groups (AWGs), and no other substantial contributions were received from non-authors. Several graphical icons used in this manuscript were adapted under Creative Commons licenses (e.g., CC0 1.0, CC BY 3.0, CC BY-NC 4.0) or equivalent public domain resources. All image credits, licenses, and source URLs are detailed in Supplementary File 1. This study received no funding.
Author information
Authors and Affiliations
Contributions
M.M. contributed significantly to the study's conceptualization, methodology, and original draft writing, and also participated in the manuscript's revision and editing. S.R. and R.B. provided guidance and supervision in the conceptualization of the study. B.B. and K.Z. contributed to the review and editing of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
All authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mokhtari, M., Reinsch, S.S., Barcenilla, B.B. et al. Space-driven ROS in cells: a hidden danger to astronaut health and food safety. npj Microgravity 11, 52 (2025). https://doi.org/10.1038/s41526-025-00492-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41526-025-00492-x