Absract
The development of wearable devices has increased the need for organic light-emitting diodes (OLEDs) that are soft, stretchable, and can integrate seamlessly with the human body. Traditional intrinsically stretchable OLEDs (is-OLED) often suffer from reduced performance due to orthogonal solvent problem and lamination fabrication process, which can cause defects and delamination. To overcome these challenges, we developed a sequentially coated is-OLED and confirmed the maintenance of the designed morphologies of each layer and a highly stretchable metallic is-cathode. Our is-OLEDs achieved a maximum total luminance of 3151 cd m–2 and a total current efficiency of 5.4 cd A–1. It also demonstrated superior durability, with the ability to stretch up to 70% and maintain 80% luminance after 300 cycles at 40% strain. This advancement suggests a promising future for durable and efficient soft electronic devices.
Similar content being viewed by others
Introduction
Organic light-emitting diodes (OLEDs) are pivotal in current display technology due to their high energy efficiency and vibrant color reproduction1,2,3. The recent shift from rigid devices toward soft electronics has sparked interest in stretchable OLEDs that mimic skin-like properties4,5,6,7,8,9,10,11. Various strategies, including the use of nanocomposites with rigid nanostructures embedded in elastic polymer matrices or strain engineering, have been explored to transform rigid displays into stretchable architectures, though with compromises in device performance, stretchability, or resolution12,13,14. Intrinsically stretchable polymer materials offer a promising alternative, maintaining stretchability even at high device densities15,16,17,18,19,20,21,22,23. While significant progress has been made in enhancing the luminance and stretchability of intrinsically stretchable OLEDs (is-OLEDs), challenges persist in refining manufacturing processes and designing intrinsically stretchable materials.
Previous is-OLED studies have focused on improving the stretchability of inherently rigid organic emissive layer (EML) by blending various materials and molecular structure design15,16,17,18,19,20,21,22,23. Triton X-100 (TX), poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (PEO-PPG-PEO) additives, polyurethane (PU), and polystyrene-block-polybutadiene-block-polystyrene (SBS) elastomers change the microstructure from rigid to stretchable15,16,17,18,19,20. Furthermore, synthesizing emitting molecules incorporating soft substances such as alkyl chains, butadiene, and poly(isoprene) modified the chain dynamics to render them stretchable21,22,23. However, research focusing on other layers and fabrication process remains insufficient.
To fabricate is-OLEDs, solution coating, such as spin-coating, is usually performed. However, in many cases, the lack of solvent orthogonality between layers destroys the designed microstructure of the underlying layer, which significantly reduces the stretchability24,25. Hence, in previous works by others, lamination was used to build up the layers in is-OLEDs. In Zhang et al., emissive layer (EML) and electron transport layer (ETL) surfaces were joined, and in Liu et al., the ETL and cathode surfaces were laminated. In many cases, a lack of solvent orthogonality occurred between the EML, ETL, and cathode layer. The lamination process includes alignment, interfacing, and hot pressing15,16,17,21. During this process, misalignment and pressure-induced defects and voids induce insufficient layer contact, leading to delamination, dark spots, reduced fabrication yield, and decreased performance, especially under repeated stretching11.
For high-performance is-OLEDs, electrode materials such as stretchable Al or Ag films are expected to be most effective because of their inherently high charge carrier density and mobility3. Such materials can be deposited by thermal evaporation, minimizing damage to the underlying organic layers, and are easily patterned. However, metal films inherently lack stretchability26,27,28. Hence, silver nanowires (AgNWs), graphene, and poly(2,3-dihydrothieno-1,4-dioxin)-poly(styrenesulfonate) (PEDOT:PSS) were widely used as stretchable cathodes, which are very limited candidates. AgNWs were embedded in an elastomer matrix to impart stretchability. Hence, they had to be fabricated on a separate substrate, followed by a physical lamination process onto the precoated organic layers16,21. Graphene cannot be directly synthesized on ETL surfaces; hence, its integration also involves a complex transfer process onto cathode surfaces, necessitating a subsequent lamination process16. While the PEDOT:PSS cathode can be directly spin-coated on organic surfaces, this approach risks damaging the underlayer due to the effect of the polar solvent on the ETL and the post-patterning process. As a result, PEDOT:PSS cathodes were also fabricated by a lamination process after the separate fabrication of is-cathodes15. In summary, in addition to their inferior conductivity compared to that of conventional metallic cathodes, these materials inevitably require a manual lamination process during the fabrication of is-OLEDs.
In our previous study, we fabricated an is-OLED using a sequential coating process to achieve high static and cyclic stretchability18. However, there were challenges such as the high roughness of the cathode part, difficulties in cathode patterning, and a lack of analysis on the impact of solution coating on the underlayer. Hence, in this study, we developed a hybrid fabrication process employing sequential solution coating and thermal evaporation. We developed a newly designed morphology-sustainable is-ETL and a highly stretchable metallic is-cathode, previously unexplored in is-OLED research. The is-ETL not only ensures a uniform surface but also sustains the designed morphology of the is-EML, thereby enhancing the electrical and mechanical properties of is-OLEDs. Furthermore, by adjusting the deposition conditions of the conventional brittle Ag metal film, we successfully fabricated a stretchable Ag electrode with high electrical conductivity. As a result, our device achieved a maximum total luminance of 3,151 cd m−2 and a current efficiency of 5.4 cd A−1, maintaining approximately 50% performance at 70% strain and 80% performance after 300 cycles of stretching tests. This work explores new stretchable materials and fabrication processes and discusses potential challenges and advancements in mechanical and electrical properties, paving the way for the promising future of is-OLED technology.
Methods
Preparation of is-EML and is-HTL
For preparation of the is-EML solution, TX (Sigma‒Aldrich) was first dissolved in toluene at a concentration of 2.5 mg ml–1. Then, SY (PDY-132, Sigma‒Aldrich) was blended in the TX-dissolved toluene at a concentration of 5 mg ml–1. The blended solution was stirred at 300 rpm for 12 h at 60 °C. After stirring, the is-EML was spin-coated on the is-HTL composed of a 1:1 solution of PEDOT:PSS (Al 4083, Heraeus Clevios) and isopropyl alcohol (IPA) with 5 wt% TX.
Preparation of is-ETLs and Upper ETL
For the is-ETL and upper ETL, a PFN-Br:PEIE:TX solution and a d-PEIE solution were prepared. For the d-PEIE solution, PEIE (Sigma‒Aldrich, 37 wt%) and Cs2CO3 (Sigma‒Aldrich) were codissolved in 2-methoxyethanol (for which the weight ratio of PEIE to Cs2CO3 was 10:1) at a 4 wt% concentration. After sonication of the d-PEIE solution via ultrasonication, the d-PEIE solution was stirred at 80 °C for 12 h. For the PFN-Br:PEIE:TX solution, PEIE and TX were dissolved in methanol at a concentration of 4 mg ml−1. Then, 2 mg of PFN-Br (Ossila) was dissolved in 0.5 ml of PEIE and 0.25 ml of a TX-blended solution (weight ratio of PFN-Br:PEIE:TX of 2:2:1) or 0.5 ml of a TX-blended solution (weight ratio of PFN-Br:PEIE:TX of 2:2:2).
Mechanical characterization of is-EMLs, is-ETLs, and is-cathode
For the stretchable substrate, a PDMS substrate was cured on a polyethylene terephthalate (PET) substrate. The PDMS (Sylgard 184, Dow Corning) was prepared by mixing the base and agent at a 10:1 weight ratio and spin-coating the mixture on the PET substrate at 500 rpm for 30 s. The spin-coated PDMS was cured at 120 °C for 12 h. Then, the cured PDMS was peeled off from the PET substrate and used as a stretchable substrate.
For mechanical characterization of the is-EMLs, COS analysis was performed for various solvent treatments. On the cured PDMS substrate, oxygen plasma was applied at 140 W for 90 s, and the is-HTL was spin-coated at 500 rpm for 60 s and 1000 rpm for 10 s. PDMS with the is-HTL was annealed on a hot plate at 120 °C for 15 min. Then, the sample was transferred to a glove box filled with nitrogen gas. The is-EML was spin-coated on the is-HTL at 1500 rpm for 30 s and annealed at 90 °C for 10 min. Then, MeOH, IPA, and 2-methoxyethanol were spin-coated on the is-EML at 4000 rpm for 30 s and annealed at 100 °C for 10 min. In the case of the is-ETL, the is-ETL was spin-coated at 5000 rpm for 30 s on the plasma-treated PDMS substrate and annealed on the hot plate at 90 °C for 10 min in the glove box. The is-EMLs and is-ETLs on the PDMS substrate were loaded on a homemade stretching jig. Strain was applied in increments of 10% from 0 to 100%.
Fabrication of the ITO anode based rigid OLEDs
The slide glass substrate (Paul Marienfeld GmbH & Co. KG, Germany) was cut to 2.5 cm × 2.5 cm and sequentially washed in the sonication baths of acetone, IPA, and deionized water for 5 min each. On precleaned glass, ITO (150 nm thick) was deposited using direct current (DC) magnetron sputtering and annealed at 300 °C for 30 min inside a glove box filled with nitrogen gas. On the ITO-coated glass, oxygen plasma was applied at 140 W for 90 s. On the plasma-treated ITO glass, an is-HTL solution was spin-coated at 2000 rpm for 60 s after being filtered through a 1 μm polytetrafluoroethylene (PTFE) syringe filter and annealed at 120 °C for 20 min. The sample was then transferred into a glove box filled with nitrogen gas. On the is-HTL, the is-EML was spin-coated at 1500 rpm for 30 s and annealed at 100 °C for 10 min. Then, the PFN-Br, PEIE, and TX-blended solution was spin-coated on the is-EML at 5000 rpm for 30 s and annealed at 100 °C for 10 min. Finally, the Al cathode was thermally evaporated on the is-ETL with a 150 nm thickness.
Fabrication of electron only device
For electron only device fabrication, 150 nm Ag anode was deposited on the pre-cleaned glass substrate using thermal evaporator. On the Ag anode, 0.5 wt% d-PEIE solution was spin coated at 5000 rpm for 30 s and annealed at 100 °C for 10 min. It was then transferred into a glove box filled with nitrogen gas. On the d-PEIE, is-EML was spin-coated at 1500 rpm for 30 s and annealed at 100 °C for 10 min. Then, PFN-Br, PFNBr:PEIE, PFNBr:PEIE:TX blend solution was spin-coated on the is-EML at 5000 rpm for 30 s and annealed at 100 °C for 10 min. Finally, Al cathode was thermally evaporated on the is-ETL with 150 nm thickness.
Preparation of FOTS on glass substrate
A precleaned glass substrate was first treated by O2 plasma at 140 W for 90 s. Then, the glass substrate was vapor annealed in a desiccator with trichloro(1H,1H,2H,2H-perfluorooctyl)silane (FOTS, Sigma‒Aldrich) solution for 30 min and annealed at 150 °C for 30 min.
Fabrication of is-OLEDs
For the is-anode, 0.5 ml of a AgNW (with a diameter of 30 nm and a length of 30 μm; Novarials) solution (1 mg ml–1 concentration) was spray-coated on the FOTS-treated glass substrate using a patterned metal mask. On the AgNWs, an aerogel solution with 4 wt% ethanol was spin-coated at 1000 rpm for 30 s and annealed at 100°C for 10 min. Then, a PDMS solution in tetrahydrofuran (THF) solvent (base and agent ratio of 10:1 at 20 mg ml–1) was spin-coated on the aerogel surface at 1000 rpm for 30 s. Additionally, PDMS (base and agent ratio of 10:1) was spin-coated at 300 rpm for 30 s and cured at 120°C for 12 h. After the curing process, the AgNW-embedded PDMS was released from the glass substrate.
The released AgNW-embedded PDMS substrate was reattached to a glass substrate with the AgNW electrode facing up. Before coating with the is-HTL, oxygen plasma was applied at 140 W for 90 s. Then, an is-HTL solution was spin-coated at 500 rpm for 60 s and 1000 rpm for 10 s after being filtered through a 1 μm PTFE syringe filter and annealed at 120 °C for 15 min. The is-HTL-coated sample was transferred to a N2-filled glove box. In the glove box, the is-EML was spin-coated at 1500 rpm for 30 s and annealed at 90 °C for 5 min. On the is-EML, is-ETLs was sequentially coated. An is-ETL solution (PFN-Br:PEIE:TX) was spin-coated at 5000 rpm for 30 s and annealed at 90 °C for 5 min. Then, a Ag cathode was evaporated on the is-ETL at 1 Å s–1 up to 60 nm thickness. After the evaporation process, 4 wt% d-PEIE solution was spin-coated on the Ag surface at 1000 rpm for 30 s and annealed at 90 °C for 5 min. Finally, the is-OLED was released from the glass substrate. The 3 × 2 array device fabrication process was almost the same as the is-OLED fabrication process. Only the anode and cathode patterns and glass size were different from those for the is-OLEDs.
Other characterization methods
The surface morphology of each film was characterized using AFM (NX-10, Park Systems) and field-emission scanning electron microscopy (FE-SEM, IT-500HR, JEOL). The PL spectra of the devices were measured using a spectrofluorometer (FP- 8550, JASCO Co.). The microstructures were characterized by TEM (JEM-ARM200F “NEO ARM,” JEOL) and energy-dispersive X-ray spectrometry (EDS, JED-2300T (Dual), JEOL). The luminance–voltage–current density (L–V–J) characteristics and EL spectra were measured using a 2400 Keithley Sourcemeter and a CS-200 (CS-2000) Konica-Minolta Chromameter. The optical transmittance of the devices was measured using an ultraviolet–visible (UV–VIS) spectrophotometer (V-650, JASCO Co.).
Results
Designs of intrinsically stretchable OLED
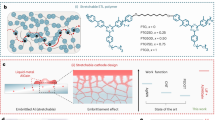
Figure 1a shows a schematic image of the is-OLEDs fabricated in this work. Figure 1b summarizes our sequential coating processes from the is-anode to the upper ETL. All the detailed process for fabricating is-OLEDs is illustrated in Supplementary Fig. 1. As shown in Fig. 1c, for the is-OLEDs, an intrinsically stretchable hole transport layer (is-HTL) is formulated from PEDOT:PSS and the nonionic surfactant TX, and an intrinsically stretchable EML (is-EML) is composed of a commercial EML, Super Yellow (SY) and TX18. TX affects the conformation and microstructure of PEDOT:PSS and SY, resulting in highly stretchable is-HTL and is-EML18.
a Schematic of an is-OLED structure from the is-anode to the upper ETL. b is-OLED fabrication process with sequential coating. c Device structure and components of an is-OLED. d Schematics of the is-EML morphology change after ETL solvent coating. Based on the various analysis results, by adding Triton X into the ETL solvent, the SY chains maintain an extended morphology after the is-ETL coating process, resulting in high stretchability. e Schematics of the is-cathode morphology and electron conducting mechanism under stretching conditions.
As illustrated in Fig. 1c, an intrinsically stretchable ETL (is-ETL) is spin-coated on the is-EML. Firstly, PFN-Br, distinguished by its high lowest unoccupied molecular orbital (LUMO) level of 2.7 eV and low highest occupied molecular orbital (HOMO) level of 5.6 eV, was selected as the is-ETL (Fig. 1c). It offers significant advantages for electron injection and hole blocking in OLED structures15,29. Furthermore, the addition of PEIE contributes to a notable increase in the stretchability of the material, reaching approximately 70% strain (Supplementary Fig. 2). In addition, we aimed to enhance the stretchability of the is-OLED by incorporating TX in ETL solution. Since TX, a nonionic surfactant, is soluble in most organic solvents, we anticipated potential changes in the characteristics of the EML when TX present on the SY surface is washed away. To preserve the morphology of the is-EML, TX was also introduced into the ETL. This addition ensures the maintenance of the designed morphology from the HTL to the ETL during the continuous coating process. Incorporating TX into the PFN-Br and PEIE matrix has been demonstrated to improve both the mechanical and electrical properties of the is-OLED, resulting in enhanced overall device performance.
Following the is-ETL coating process, an is-metal cathode is developed using evaporation, as shown in Fig. 1e. To render metal cathode stretchable, modifying the interaction with the underlayer and adjusting the deposition conditions are crucial. Since our cathodes are positioned on top of the devices rather than directly above the elastomer substrate, options for the deposition surface are more constrained than those in previously reported stretchable metal studies30,31,32,33. The deposition surface must have (1) a smooth surface, (2) high compatibility (adhesion) with Ag atoms, and (3) appropriate energy levels for electron injection. Considering these conditions, we successfully fabricated a stretchable Ag electrode with high electrical conductivity by fine-tuning the deposition conditions of the traditionally brittle silver metal film. The precisely adjusted evaporation conditions for the stretchable metal cathode resulted in only microcracks on the surface under stretching conditions, enabling efficient electron movement between the microcracks (Fig. 1e).
Designed morphology sustainable electron transport layer
For the is-EML, TX mixed with SY-conjugated polymers (Fig. 1c) alters the morphology of the polymers from coiled to extended, resulting in high stretchability and electrical properties18. This morphological change is evident through the redshifted absorbance (Abs), blueshifted photoluminescence (PL), and changes in the atomic force microscopy (AFM) surface phase (Fig. 2a, Supplementary Figs. 4, 5). Here, we coated various solvents on is-EMLs to investigate the effect of dissolution of is-EMLs in the solvent on the changes in the conformation and morphology of the is-EMLs (Fig. 2a). Regardless of the TX concentration in the SY and ETL materials, applying a solvent coating on the is-EML to coat the is-ETL reverts the Abs, PL, and phase changes to those of pristine SY (Supplementary Figs. 3, 4, 5).
a UV‒vis absorption spectra of is-EMLs with various TX blending ratios, and solvent coating effect on the is-EMLs. b UV-vis absorption spectra of is-EMLs before and after TX-blended solvent coating (TX1 = 1 mg ml–1, TX2 = 2 mg ml–2, TX4 = 4 mg ml–1). c Photoluminescence spectra of is-EMLs before and after TX-blended solvent coating. d COS of is-EMLs before and after TX-blended solvent coating. e COS of is-ETLs with various component ratios when coated on PDMS or is-EMLs. f UPS intensity of is-ETL and Ag films with various TX ratios. g Current density of electron-only devices with various ETL component ratios. h Energy-level alignment diagram for the conventional OLED device with an is-ETL. i V-L curve, (j) J-V curve, and (k) εc-L curve of the conventional OLED device with an is-ETL on a glass substrate.
To confirm the effect of TX incorporation in ETL solution, we measured the Abs and PL after spin coating the TX blended solvent on the is-EML. Figure 2b, c shows the redshifted Abs and blueshifted PL peaks after coating, akin to the behavior of the is-EML. Moreover, increasing the TX concentration better sustain the is-EML Abs and PL peaks up to 4 mg ml–1. Figure 2d shows the effect of TX on the COS of the is-EML (Supplementary Figs. 3, 10). Mixing SY with TX increases the COS. Applying the solvent coating alone decreases the COS to 40%, whereas the solvent containing TX yields a COS of 60% at 1 mg ml–1, 80% at 2 mg ml–1, and 90% at 4 mg ml–1. These findings suggest that the TX-blended solvent maintains the is-EML morphology, ensuring high stretchability. Therefore, coating of an ETL solution with TX is crucial for preserving the extended morphology of the is-EML.
Figure 2e shows the mechanical characteristics of the ternary blends of PFN-Br, PEIE, and TX. TX increases the COS of the is-ETL by increasing the free volume within the material and decreasing the crystallinity of the conjugated polymer (Supplementary Fig. 2)18,29. Hence, the ternary blend of the is-ETL with a ratio of 2:2:2 inherently exhibits high stretchability and is capable of withstanding up to 100% COS. When this is-ETL is applied over the is-EML, the COS increases as the ratio of TX increases, matching the COS of the is-EML itself. The introduction of TX into the PFN-Br:PEIE ETL has dual effects: providing high stretchability of the is-ETL and sustaining the designed morphology of the is-EML. This stretchability alignment between the is-ETL and is-EML is a significant achievement, ensuring that the overall device can maintain its structural integrity and functional performance even under considerable mechanical strain. Furthermore, the ternary blended is-ETL shows a uniform surface morphology, as shown in Supplementary Fig. 11.
Figure 2f shows the electrical properties of the is-ETLs. Ultraviolet photoelectron spectroscopy (UPS) measurements, based on the TX blending ratio, reveal that the work function of 4.8 eV for the pristine Ag film decreases to 3.3 eV following coating with a PFN-Br:PEIE (2:2) solution. Additional blending of the ETL with TX results in work functions of 3.25 eV for a 2:2:1 ratio and 3.35 eV for a 2:2:2 ratio, indicating similar electron injection characteristics (Supplementary Fig. 13, Supplementary Table 1). Triton X is an electrical insulator, but we estimate that the dipole of the nonionic surfactant TX lowers the work functions of PEIE and PFN-Br.
As shown in Fig. 2g, we fabricated electron-only devices using the is-ETLs, the detailed structure of which is presented in Supplementary Fig. 14. Compared to PFN-Br, the PFN-Br:PEIE ETL exhibits a higher current density. Furthermore, as the TX blending ratio increases, the electron current density correspondingly increases. This finding implies that the TX-blended is-ETL improves the electron transport properties by maintaining the extended morphology of the is-EML. Then, we fabricated devices with a conventional OLED structure using the is-ETLs (Fig. 2h). Figure 2i demonstrates the improved performance obtained with the TX-blended is-ETLs compared to that obtained with the 2:2 ratio, and the increase in the efficiency is shown in Fig. 2k. Hence, the TX-blended is-ETLs not only exhibit inherently high mechanical and electrical properties but also help sustain the designed morphology of the is-EML, thereby enhancing its mechanical and electrical characteristics. Although the 2:2:1 ratio results in a slightly better performance, we chose a 2:2:2 ratio of the is-ETL for the is-OLED fabrication to enhance the is-EML stretchability.
Intrinsically stretchable Ag metal cathode
To render the Ag cathode stretchable, we first selected the underlying layer. The structure and properties of the deposited metal film are significantly influenced by the underlying layer. Our is-ETL (PFN-Br:PEIE:TX), serving as the underlayer for the Ag cathode, exhibits low surface roughness and suitable energy levels for electron transport (Supplementary Fig. 11 and Fig. 2f). Moreover, the amine groups present in PEIE are compatible with Ag atoms through coordination bonds, acting as seed layers for the formation of ultrathin Ag films26. Therefore, the is-ETL surface is suitable for forming a stretchable metal cathode.
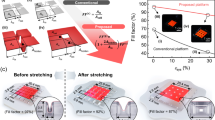
The deposition conditions were optimized to achieve both good optoelectrical properties and stretchability. The evaporation rate and thickness were adjusted during the thermal evaporation process (Ag20 to Ag100, where AgXX refers to a device with an XX nm deposition based on the quartz crystal microbalance). As the deposition proceeded, Ag cathodes exhibited three distinct stages according to the Volmer–Weber growth mechanism: (1) Stage I : discrete islands (Ag20), (2) Stage II : partially connected film (Ag40 to Ag80), (3) Stage III : continuous film (Ag100) (Fig. 3a and Supplementary Fig. 15).
a Schematics of is-cathode surface morphology from thin Ag to thick Ag film with various Ag deposition parameter. b Sheet resistance, stretchability of is-cathode, and Luminance of is-OLED at various is-cathode deposition parameters. c Optimal Ag deposition parameters for the is-cathode. Deposition rate and deposition condition are controlled by quartz crystal microbalance. d Resistance change of Ag cathode film with various Ag deposition parameter from Ag30 to Ag70 during the static stretching tests. e Resistance change of the is-cathode deposited by Ag60 during cyclic stretching tests at 20% and 40% strain. f TEM cross-sectional image of the is-OLED. g Charge conducting mechanism of the is-cathode under stretched condition. h SEM surface image of the is-cathode under stretched condition up to 60% strain.
Discrete island morphologies in Stage I exhibit poor conductivity. On the other hand, continuous film morphologies in Stage III make the cathode brittle34. Therefore, we focused on characterizing Ag cathodes in Stage II. As shown in scanning electron microscopy (SEM) and transmission electron microscopy (TEM) cross-section images in Supplementary Fig. 16, Ag grains start to coalescence to enhance connectivity between islands rather than grow in the thickness direction. As the deposition progressed from Ag40 to Ag60, the grains became increasingly densely connected, and the thickness reached approximately 35 nm. Greater connectivity ensures high bulk conductivity but can also make the film brittle. Furthermore, a high evaporation rate leads to continuous films by suppressing Volmer–Weber growth, while a lower rate enhances stretchability due to the interlocking interface between the metal and substrate and the optimal grain size32,35.
To verify our strategy, we fabricated stretchable cathodes under various deposition conditions with the same structure as the is-OLED shown in Fig. 1a and characterized their optoelectrical properties, focusing on maximum luminance (Fig. 3b, c) and cathode conductivity under static and cyclic stretching conditions (Fig. 3d, e). The conductivity rapidly decreases below Ag40 due to the poor connectivity between Ag grains (Fig. 3b, d and Supplementary Fig. 15). Ag cathodes above Ag60 have high conductivity and show a superior luminance of over 3200 cd m–2 when used as cathodes in is-OLEDs but cannot endure strains above 60% due to the brittle nature of the dense, continuous Ag grains (Fig. 3b, d, Supplementary Fig. 15, and Supplementary Fig. 17). Ag cathodes with an evaporation rate above 1 Å s–1 also exhibited a significant decrease in luminance under 40% and 60% stretching (Fig. 3c and Supplementary Fig. 18).
Ag60 cathode with an evaporation rate of 1 Å s–1 exhibits a low sheet resistance of 6 Ω sq–1 and a maximum luminance of more than 2300 cd m–2, maintaining a resistance change of R R0−1 < 10 under 70% strain, simultaneously ensuring high electrical conductivity and mechanical stretchability (Fig. 3b, c, d, and Supplementary Fig. 18). Additionally, the material maintains its conductivity during 200 cycles of 40% strain stretching (Fig. 3e). Therefore, we selected Ag60 deposited at a rate of 1 Å s–1 as our optimal stretchable cathode.
We investigated the relationship between the microstructures and electrical properties of Ag cathodes using SEM, TEM, and time-of-flight secondary ion mass spectrometry (TOF-SIMS). Partially connected morphologies of Ag grains are observed, as shown in Fig. 3f, g. Due to the slow deposition process, an intermixed region of Ag and organic layers is observed in the TOF-SIMS and TEM cross-section images (Fig. 3f and Supplementary Figs. 19–21). No observable channel cracks appear on the film in the optical microscopy (OM) images until 60% strain (Supplementary Fig. 22). Instead, microcracks develop throughout the film during the stretching process. Under stretching, strain is released by widening of preexisting cracks while conducting paths are preserved through connected grains (Fig. 3d, g, h). The cracks reversibly close after strain release; thus, the Ag cathode also shows high electrical and mechanical stability under cyclic stretching (Fig. 3e, h and Supplementary Fig. 25).
Our stretchable Ag cathode’s performance is based on two unique structural features of is-OLEDs. First, the multilayers beneath the cathode (from the is-HTL to the is-ETL) provide a gradient in the Young’s modulus between the soft elastomer substrate and the stiff metal cathodes. This enhances the cathode’s stretchability compared to structures without multilayer configurations (Supplementary Fig. 26). Second, the partially interconnected Ag morphologies allow additional layers to be overcoated on the cathodes. We overcoated cesium carbonate-doped PEIE (d-PEIE) (upper ETL) on an Ag cathode, which possesses superior electron injection properties36.
The upper ETL increases the interfacial contact area between the cathode and the ETLs, effectively enhancing electron injection (Supplementary Figs. 27a). Additionally, the upper ETL acts as a matrix for the Ag cathode, providing extra adhesion and thereby improving mechanical stability (Supplementary Fig. 27b). If d-PEIE is placed between is-ETL and is-cathode, it could hinder electron transport due to its electrically insulating nature and disrupt the intended Ag nucleation and growth processes by altering the surface properties. However, when applied as an overcoating layer above the cathode, it primarily influences the lowering of the energy barrier for electron injection through porous channels within Ag films. Therefore, the d-PEIE overcoating layer contributes to the high luminance and improved stretchability of the is-OLEDs (Supplementary Fig. 27b–e).
Fully intrinsically stretchable OLED
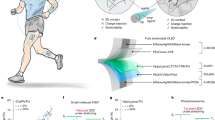
After detailed characterization of the is-ETL and is-cathode, is-OLEDs were fabricated. The fabrication involved sequential coating of the is-ETL, is-cathode, and an upper ETL over the is-EML and is-HTL based on AgNW-embedded polydimethylsiloxane (PDMS). The structural design and energy-level alignment of these devices are illustrated in Fig. 4a, b. Figure 4c shows the characteristic current density–voltage–luminance (J-V-L) curves for the is-OLEDs. These devices display a turn-on voltage of 6 V, achieving a maximum brightness of approximately 2340 cd m–2 at 9 V, for a measurement taken from the AgNW side. The maximum current efficiency recorded for this side is approximately 4.2 cd A–1.
a Schematics of the is-OLED structure and thickness. b Energy-level alignment diagram of the is-OLED. c J-V-L curve of the is-OLEDs. d Relative L change (L ∙ L0−1) of the is-OLEDs during static stretching tests when operated at 8 V. Error bars indicate mean ± standard deviation. e L ∙ L0−1 changes of the is-OLEDs during 40% strain cyclic stretching tests. f Radar plot comparing the electroluminescence (EL) performance, stretchability, and fabrication complexity of is-OLEDs produced using the sequential coating or lamination method. The fabrication complexity corresponds to the number of transfer and lamination processes during the is-OLED fabrication process. g Optical images of the is-OLEDs with an original emission area of 3.0 × 3.0 mm2 operated under various strains. h Optical photographs of 3×2 is-OLED array devices operated under various strains and deformations.
On the opposite side, where a semitransparent Ag cathode film is used, the performance is approximately 40% of that observed on the AgNW side. This side shows a luminance of 811 cd m–2 and a current efficiency of 1.2 cd A–1. The diminished performance on this Ag side is primarily due to its lower transmittance and reflectance compared to those on the AgNW side. When considering the total performance of the device, combining both the anode and cathode sides, the overall luminance reaches an impressive 3151 cd m–2, with a corresponding current efficiency of 5.4 cd A–1. This indicates the high overall performance of the is-OLEDs, demonstrating the effectiveness of the sequential coating process and the synergy between the is-ETL and is-cathode in enhancing the device efficiency and brightness.
Furthermore, to analyze the overall impact of thermal treatment temperature on device performance, PL intensity of is-EML and L-V-J curves of the is-OLED were measured according to the thermal treatment temperature after coating the is-ETL and upper ETL, as shown in Supplementary Fig. 28. High-temperature thermal annealing degraded the is-EML and induce substrate expansion, negatively affecting the device, while excessively low-temperature thermal treatment adversely affect multilayer film formation during sequential coating process37. Hence, we set the annealing temperature to 90 °C for device fabrication, resulting in the highest performance is-OLED.
The stretchability of the is-OLEDs was tested to evaluate their durability and adaptability under mechanical stress. These tests involved both static and cyclic stretching tests, with the outcomes presented in Fig. 4d, focusing on the relative luminance change in response to various levels of strain at 8 V. At 0% strain, the average luminance across five samples was recorded as 791 cd m–2. As the strain increases, the devices demonstrate remarkable resilience. Up to a 20% strain at 8 V, there is a negligible reduction in performance, indicating a high level of stretchability. However, as the strain is further increased, the luminance gradually decreases. At 40% strain, the devices maintain approximately 75% of their original luminance. This value decreases to approximately 55% at 60% strain and further decreases to approximately 45% at 70% strain. These results are comparable to those achieved by devices using stretchable electrodes such as AgNWs and PEDOT:PSS15,16,17,18. This performance underlines the significant potential of metal-based stretchable cathodes in future applications.
Supplementary Fig. 29 illustrates the performance distribution of the fabricated is-OLEDs. As shown in Supplementary Fig. 29a, among the ten devices produced, eight out of the ten samples fall within the error range compared to the luminance values presented in Fig. 4c. Supplementary Fig. 29b shows all the samples used to calculate the average in Fig. 4d. Most samples exhibit deviations within 10% of the mean value and follow a similar trend. It demonstrated the high repeatability of our is-OLED fabrication process.
Figure 4e illustrates the changes in the performance of the is-OLEDs under cyclic stretching conditions at 40% strain. A special jig for the cyclic test is shown in Supplementary Fig. 30. In the early stages of the cyclic stretching test, specifically up to 50 cycles, the performance of the devices decreases, and the luminance decreases to approximately 85% of its original value. However, when the test is extended to 300 cycles, the luminance of the devices remarkably stabilizes, maintaining approximately 80% of the initial value. This indicates a significantly greater cyclic stretchability than that reported in previous studies using lamination processes, which exhibited a performance below 80% after 100 cycles at 15% and 40% strain15,17. Notably, our devices maintain stable performance for up to 300 cycles, surpassing the 200 cycles achieved in similar studies using sequential coating18.
After the cyclic test, the top surface of the is-OLEDs was observed via SEM (Supplementary Fig. 31). This analysis reveals the formation of microcracks on the surfaces of the is-OLEDs due to stretching. However, microcracks and crack closure are vital characteristics, as they allow for continued flow and injection of charge across the affected areas, ensuring sustained device functionality. Furthermore, Supplementary Fig. 32 shows the cross-sectional image of the is-OLEDs before and after 200 cyclic stretching at 40% strain. SEM and TEM images confirm that all layers in the is-OLEDs maintain uniform film morphology and good adhesion during mechanical stretching. This resilience of the is-OLEDs under cyclic stretching, particularly their ability to maintain a high level of performance after 300 cycles at 40% strain, is an significant improvement over previous devices15,16,17,18. These results demonstrate the superior mechanical and electrical properties of the is-ETL and is-metal cathode compared to those achieved with conventional stretchable electrodes15,16,17,18.
Figure 4f presents a radar plot comparing the performance and fabrication complexity of is-OLEDs made using traditional lamination processes and our sequentially coated is-OLEDs (Supplementary Table 2). While the luminance and efficiency are similar or slightly lower than those achieved with lamination methods, our is-OLEDs exhibit higher static stretchability and notably superior cyclic stretchability. In terms of fabrication complexity, our approach shows the most advantageous characteristics, indicative of the reduced need for lamination and transfer processes during the fabrication process. The sequentially coated is-OLEDs, made using the designed morphology-sustainable is-ETL and is-metal cathode, simplify the traditionally complex processes associated with lamination methods, achieving high levels of cyclic stretchability.
Figure 4g depicts the light emitted from an is-OLED when it is stretched. The image shows that the device emits light consistently across its entire surface area both in a neutral state (0% strain) and under significant stretching (up to 70% strain). Further expanding on the capabilities of these devices, Fig. 4h shows a 3 × 2 array device fabricated using the is-OLEDs subjected to stretching. The detailed structure of this array is illustrated in Supplementary Fig. 33. This array device shows stable light emission up to 50% strain. Furthermore, the array device also demonstrates resilience to various other forms of deformation, including bending, folding, and crumpling. This ability to maintain stable light emission even under deformation conditions is a testament to the mechanical robustness and versatility of these devices.
In Supplementary Fig. 34, we assessed the operational lifespan of the is-OLEDs, revealing a short duration of about 1 min. Additionally, their efficiency still appears relatively low compared to conventional OLEDs. Although improvements to the is-ETL and is-cathode in this study led to a modest increase in lifespan and efficiency, there were limitations in achieving dramatic enhancements in these aspects. We attribute these challenges to two primary factors. Firstly, the absence of encapsulation layers exposes the devices to oxygen and moisture, contributing to their diminished operational stability18. Secondly, the use of AgNW embedding PDMS as the anode substrate introduces issues such as the one-dimensional morphology of AgNWs, a low work function, and protrusions. These factors contribute to a reduced contact area, compromised hole injection, and increased leakage current, consequently impacting both the lifespan and efficiency of the devices24. Therefore, ongoing advancements in elastomer substrates and the adoption of a uniform, high hole injection film-forming anode could facilitate the development of highly stable and efficient is-OLEDs.
Discussion
In summary, this study has made significant strides in the field of is-OLEDs by innovating and developing new stretchable component materials, such as designed morphology-sustainable is-ETLs and is-Ag metal cathodes. These advancements have been crucial in enhancing the overall performance of is-OLEDs. The meticulously adjusted formulation of PFN-Br, PEIE, and TX blended into the is-ETL has been instrumental in enhancing both the mechanical and electrical properties of is-OLEDs. The blending of PFN-Br and PEIE has demonstrated the ability to form a stretchable matrix simultaneously exhibiting high electron injection characteristics. Additionally, the incorporation of TX into this blend has been effective in sustaining the morphology of the underlying is-EML, leading to simultaneous improvements in both the mechanical and electrical properties of the is-EML and is-ETL. The is-cathode, engineered under controlled deposition conditions, has achieved significant advancements in terms of morphology control and adhesion with the is-ETL. This has led to the creation of a metal cathode that not only is highly stretchable but also maintains excellent electrical conductivity, which is a critical attribute for effective is-OLED operation.
Furthermore, this research represents a departure from more complex lamination techniques through rational material engineering and optimized device construction. This approach significantly contributes to simplifying the fabrication process, making it more accessible for the development of next-generation stretchable optoelectronic devices. While acknowledging that the current performance of these is-OLEDs still lags behind that of conventional OLEDs, this study underscores the immense potential for future enhancements. In particular, the prospects of improving the performance through fine-tuning aspects such as the Ag stretchable cathode as well as the thickness and combination of the ETL are promising. Looking ahead, the continued development and utilization of stretchable metallic cathodes are expected to lead to significant performance improvements in is-OLEDs, opening up new possibilities and applications in the realm of flexible and wearable electronics.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Koo, J. H. et al. Flexible and stretchable smart display: Materials, fabrication, device design, and system integration. Adv. Funct. Mater. 28, 1801834 (2018).
Chen, H. W. et al. Liquid crystal display and organic light-emitting diode display: present status and future perspectives. Light. Sci. Appl. 7, 17168 (2018).
Wang, S. M. et al. Towards high-power-efficiency solution-processed OLEDs: Material and device perspectives. Mater. Sci. Eng. R. Rep 140, 100547 (2020).
Someya, T., Bao, Z. N. & Malliaras, G. G. The rise of plastic bioelectronics. Nature 540, 379–385 (2016).
Sekitani, T. et al. Stretchable active-matrix organic light-emitting diode display using printable elastic conductors. Nat. Mater. 8, 494–499 (2009).
Singh, M., Haverinen, H. M., Dhagat, P. & Jabbour, G. E. Inkjet printing-process and its applications. Adv. Mater. 22, 673–685 (2010).
Yook, K. S. & Lee, J. Y. Small molecule host materials for solution processed phosphorescent organic light-emitting diodes. Adv. Mater. 26, 4218–4233 (2014).
Chiba, T., Pu, Y. J. & Kido, J. Solution-processable electron injection materials for organic light-emitting devices. J. Mater. Chem. C. 3, 11567–11576 (2015).
Liang, J. J. et al. Elastomeric polymer light-emitting devices and displays. Nat. Photonics 7, 817–824 (2013).
Gao, H., Chen, S., Liang, J. & Pei, Q. Elastomeric light emitting polymer enhanced by interpenetrating networks. ACS Appl. Mater. Inter. 8, 32504–32511 (2016).
Yin, H. et al. Structures and materials in stretchable electroluminescent devices. Adv. Mater. 34, 2106184 (2021).
Choi, D. K. et al. Highly efficient, heat dissipating, stretchable organic light-emitting diodes based on a MoO3/Au/MoO3 electrode with encapsulation. Nat. Commun. 12, 2864 (2021).
Nam, M. et al. Highly reliable and stretchable OLEDs based on facile patterning method: toward stretchable organic optoelectronic devices. Npj Flex. Electron. 8, 17 (2024).
Yao, L. Q. et al. High-efficiency stretchable organic light-emitting diodes based on ultra-flexible printed embedded metal composite electrodes. Infomat 5, e12410 (2023).
Zhang, Z. et al. High-brightness all-polymer stretchable LED with charge-trapping dilution. Nature 603, 624–630 (2022).
Zhou, H. et al. Graphene-based intrinsically stretchable 2D-contact electrodes for highly efficient organic light-emitting diodes. Adv. Mater. 34, e2203040 (2022).
Liu, Y. et al. A self-assembled 3D penetrating nanonetwork for high-performance intrinsically stretchable polymer light-emitting diodes. Adv. Mater. 34, e2201844 (2022).
Kim, J. H. & Park, J. W. Intrinsically stretchable organic light-emitting diodes. Sci. Adv. 7, eabd9715 (2021).
Oh, J. H. & Park, J. W. Intrinsically stretchable phosphorescent light-emitting materials for stretchable displays. ACS Appl. Mater. Inter. 15, 33784–33796 (2023).
Jeon, K. H. & Park, J. W. Light-emitting polymer blended with elastomers for stretchable polymer light-emitting diodes. Macromolecules 55, 8311 (2022).
Liu, W. et al. High-efficiency stretchable light-emitting polymers from thermally activated delayed fluorescence. Nat. Mater. 22, 737–745 (2023).
Jao, C. C. et al. Novel stretchable light-emitting diodes based on conjugated-rod block elastic-coil copolymers. Polym. Int. 70, 426–431 (2021).
Li, X. C. et al. Intrinsically stretchable electroluminescent elastomers with self-confinement effect for highly efficient non-blended stretchable OLEDs. Angew. Chem.-Int. Ed. 62, e202213749 (2023).
Zhou, H. et al. Intrinsically stretchable low-dimensional conductors for wearable organic light-emitting diodes. Device 1, 100060 (2023).
Han, S. J. et al. Achieving low-voltage operation of intrinsically-stretchable organic light-emitting diodes. Adv. Funct. Mater. 33, 2211150 (2023).
Kang, H. et al. Polymer-metal hybrid transparent electrodes for flexible electronics. Nat. Commun. 6, 6503 (2015).
Bi, Y. G. et al. Ultrathin metal films as the transparent electrode in ITO-free organic optoelectronic devices. Adv. Opt. Mater. 7, 1800778 (2019).
Park, S. I. et al. Theoretical and experimental studies of bending of inorganic electronic materials on plastic substrates. Adv. Funct. Mater. 18, 2673–2684 (2008).
Ohisa, S. et al. Conjugated polyelectrolyte blend with polyethyleneimine ethoxylated for thickness-insensitive electron injection layers in organic light-emitting devices. ACS Appl. Mater. Inter. 10, 17318–17326 (2018).
Jiang, Z. et al. A 1.3-micrometre-thick elastic conductor for seamless on-skin and implantable sensors. Nat. Electron. 5, 784–793 (2022).
Zhu, T. et al. Highly stable and strain-insensitive metal film conductors via manipulating strain distribution. Mater. Horiz. 10, 5920–5930 (2023).
Han, S. et al. Enhanced stretchability of metal/interlayer/metal hybrid electrode. Nanoscale 13, 4543–4550 (2021).
Kim, S. W. et al. Omnidirectionally stretchable metal films with preformed radial nanocracks for soft electronics. ACS Appl. Nano. Mater. 3, 7192–7200 (2020).
Won, D. et al. Transparent electronics for wearable electronics application. Chem. Rev. 123, 9982–10078 (2023).
Kim, M. H. et al. Mechanically robust stretchable semiconductor metallization for skin-inspired organic transistors. Sci. Adv. 8, eade2988 (2022).
Kim, J. H. & Park, J. W. Designing an electron-transport layer for highly efficient, reliable, and solution-processed organic light-emitting diodes. J. Mater. Chem. C. 5, 3097–3106 (2017).
Zhao, F. W., Wang, C. R. & Zhan, X. W. Morphology control in organic solar cells. Adv. Energy. Mater. 8, 1703147 (2018).
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) Grant funded by the Korea Government (MSIT) (RS-2023-00302611) and the Technology Innovation Program Development Program (“20022479”, “Development of deuterium oxide localization and deuterium benzene synthesis technology to improve OLED lifetime by 25%“) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).
Author information
Authors and Affiliations
Contributions
J.-H. O., K.-H. J. and J.-W. P. designed the project. J.-H. O. and K.-H. J. carried out the experiments and analysis. J.-H. O., K.-H. J. and J.-W. P. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oh, JH., Jeon, KH. & Park, JW. Intrinsically stretchable OLEDs with a designed morphology-sustainable layer and stretchable metal cathode. npj Flex Electron 8, 43 (2024). https://doi.org/10.1038/s41528-024-00332-0
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41528-024-00332-0
This article is cited by
-
Enabling efficient electron injection in stretchable OLED
Nature Materials (2025)
-
Rise of intrinsically stretchable electroluminescent materials: toward free-form displays
npj Flexible Electronics (2025)
-
Future trends of display technology: micro-LEDs toward transparent, free-form, and near-eye displays
Light: Science & Applications (2025)
-
Interconnected and Layered Morphological Structure of Polycarbazole-Vanadium Pentoxide Nanocomposites for the Application of OLED as ETL Material
Journal of Inorganic and Organometallic Polymers and Materials (2025)