Abstract
Owing to unique advantages of patternability and high substrate compatibility, screen-printing allows for the fabrication of flexible perovskite solar cells (f-PSCs) with designable device patterns, while the defective and fragile contact at the buried interface seriously restricted the device performance. Herein, a series of siloxane coupling agents (SCAs) with different ending groups i.e., –SH, –NH2, and –CN were incorporated at the SnO2/perovskite interface, which can selectively interact with MA+ and Pb2+ via hydrogen and coordination bonding, respectively. It was revealed that the selection of (3-Cyanopropyl)Triethoxysilane (CN-PTES) can regulate perovskite crystallization with accelerated nucleation and retarded crystal growth, leading to improved crystallinity with released residual lattice strain. Moreover, the incorporated CN-PTES aligned the energy structure of the underlying SnO2 and boosted the interfacial adhesion between perovskite and SnO2, resulting in facilitated electron extraction and enhanced interfacial fracture energy. Consequently, the first screen-printed f-PSCs with improved mechanical resistance were finally obtained.
Similar content being viewed by others
Introduction
Organic-inorganic hybrid lead halide perovskites have attracted worldwide research attention due to their advancing photophysical properties in terms of high absorption coefficient1, tunable band gap2, and long carrier diffusion length3,4, which enabled the achievement of efficient perovskite solar cells (PSCs) with champion power conversion efficiency (PCE) up to 26.9%. Meanwhile, the soft character of the perovskites facilitated the design of flexible photovoltaics5 to extend their application scenario in wearable electronics6,7, smart cars8, photovoltaic-building integration9, and aerospace10,11 because of their light weight, mechanical flexibility, and extremely high energy-to-mass ratio.
Toward mass production of f-PSCs, the manufacturing process should be adaptable with up-scaling methods, and a series of low-cost and high-throughput thin film deposition techniques have been continuously developed, including blade coating12,13, slot-die coating14, meniscus coating15, spray coating16, inkjet printing17 and screen printing18,19. Among them, screen printing exhibits some unique advantages in terms of high substrate compatibility and patternability, which allows for the direct deposition of perovskite films on soft substrates and the design of f-PSCs with diverse patterns. However, a high viscosity of the perovskite precursor ink is generally required for effective screen-printed PSCs, which lead to the difficulty of solvent volatilization and hence defective and fragile contact especially at the buried interface, such as incomplete phase transition, tiny crystals with large amount of grain boundaries, crystal defects or interfacial cracks, leading to restricted efficiency and stability.
Various strategies have been developed to improve the interface quality of printed PSCs, including the engineering of the printing process, precursor solvents, or buried interface. For instance, gas-quenching methods with high-speed gas flow were implemented to blow the as-printed wet films, which accelerated solvent volatilization and contributed to the formation of uniform perovskite films with high crystallinity at the buried interface20,21. Solvent engineering was also employed to modulate perovskite crystallization and interface quality. Particularly, the conventional precursor solvents of N, N-dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) were likely remained within the perovskite films on account of their strong coordination with PbI2. Accordingly, the substitution of the DMSO by n-methylpyrrolidone or n-Butanol, which manifested weaker coordination ability with PbI2, leading to the ease of solvent evaporation and high crystal quality at the buried interface21,22,23. Moreover, interface engineering has been developed to boost interfacial adhesion and regulate perovskite crystallization. For instance, some functional molecules with sticky groups were introduced at the buried interface to enhance the interfacial contact at the fragile ETL/perovskite interface by simultaneously coordinating with upper perovskite and underlying substrate12,24,25. Particularly, hyperbranched polymer with branched three-dimensional molecular structure and Si(OCH3)3 anchor groups, which was easy to self-assemble atop metal oxides and strongly coordinate with the perovskite, were incorporated at the SnO2/perovskite interface to enhance the interfacial fracture energy and hence the mechanical resistance of f-PSCs24,25,26. Moreover, the perovskite crystallization can also be modulated from the buried interface. For example, functional molecules, such as proline hydrochloride27 and entinostat12, were introduced at the buried interface as a versatile medium to boost the perovskite crystallinity with eliminated defect states, and f-PSCs with efficiency approaching 25% were ultimately obtained5,12,27,28. It seems that rational engineering at the buried interface is able to boost the PCE and stability of f-PSCs, while the library of the functional molecules is still limited. Thereby, it is highly designable to diversify the material library and allow for the selection of multifunctional molecules according to the specific requirements of different printing techniques.
Herein, the screen-printing technique was firstly applied to fabricate f-PSCs, and a series of SCAs with a fixed anchoring group and varied ending groups were incorporated at the perovskite/SnO2 interface, which was targeted to strengthen interfacial fracture energy and simultaneously modulate upper perovskite crystallization via their selective interaction with different component of perovskite. As a result, the first screen-printed f-PSC with efficiency of 18.6% was ultimately obtained, and the mechanical stability of the device was simultaneously improved by retaining 90% of the initial efficiency after 6000 bending cycles at a radius of 5 mm.
Discussion
Screen-printed f-PSCs via SCAs buried interface
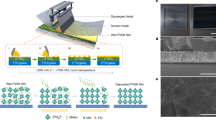
As illustrated in Fig. 1a, the perovskite layer was deposited in ambient air via a fast, cost-effective, and patternable screen-printing technique. Briefly, some perovskite inks were firstly poured into a screen-printing stencil. Under the squeeze of the blade, the perovskite ink was filled into the screen mesh. When the stretched screen mesh was lifted up, the perovskite ink was extracted from the screen mesh and form a wet layer atop soft substrates, which was then transformed into perovskite film via a vacuum-assisted thermal annealing process. As it was revealed that the perovskite is generally crystallized from the top-surface to the buried interface as demonstrated in Fig. 1b29,30, leading to incomplete phase transformation and numerous defect states at the buried interface. It worth to mention that the screen-printing technique enabled the fabrication of devices with various shapes, such as ring, triangle, trapezium, quadrilateral, and so on, as shown in Fig. 1c. In order to improve the quality of as-printed perovskite thin films, a series of SCAs with fixed anchoring group of triethoxysilane and different ending groups in terms of 3-mercaptopropyl (-SH), 3-aminopropyl (-NH2), 3-cyanopropyl (−C ≡ N), which were renamed as SH-PTES, NH2-PTES, CN-PTES, respectively, were applied at the buried SnO2/perovskite interface. The incorporated SCAs are prone to self-assemble atop SnO2 via the formation of Si-O-Sn bond as demonstrated in Fig. 1d, e31,32.
a Schematic illustration of screen-printed f-PSCs. b Crystallization of screen-printed perovskites. c Illustration of chemical interaction of SH-PTES with SnO2 and perovskite at the buried surface. d Illustration of chemical interaction of NH2-PTES and CN-PTES with SnO2 and perovskite buried surface. e Screen-printed perovskite films with different patterns.
Interactions between SCAs and perovskites
The chemical interaction between the SCAs and the upper perovskite was investigated by nuclear magnetic resonance (NMR). The hydrogen NMR (1H NMR) spectra of the SH-PTES, NH2-PTES mixed with PbI2, MAI, respectively, were shown in Fig. 2a, b, the characteristic peaks for hydrogen from ending groups of SH-PTES and NH2-PTES were centered at 1.631 and 1.395 ppm, which were then shifted to 1.652 and 1.241 ppm, respectively, after mixing with PbI2. The large chemical shift when mixing the NH2-PTES and PbI2 indicated a strong interaction between the Pb2+ and the –NH2, while the interaction between the Pb2+ and –SH from SH-PTES was relatively weak. In contrast, a large chemical shift was found when mixing SH-PTES and MAI as seen in Fig. 2a, suggesting a strengthened association between the –SH and the MA+. Moreover, the carbon NMR (13C NMR) spectra in Fig. 2c exhibited that the –CN ending group from CN-PTES is more likely to interact with PbI2 than MAI. The chemical interaction between the perovskite and SCAs was further confirmed by (Fourier transform infrared) FTIR spectroscopy as shown in Supplementary Fig. 1a. Notably, the stretching vibration of -SH in SH-PTES was shifted from 2543.66 to 2511.99 cm−1 when mixed with MAI, which was more pronounced than that mixed with PbI2. Similarly, a larger shift of the stretching vibration of –NH2 from NH2-PTES and –C ≡ N from CN-PTES were observed when mixed with PbI2 compared to MAI as seen in Supplementary Fig. 1b, c. Thereby, the interaction between the ending groups of SCAs and the components of the perovskite were summarized and illustrated in Fig. 2d. As illustrated in Fig. 1c, d, MAI terminated perovskites were likely formed at the buried SnO2/perovskite interface when treated by SH-PTES, while PbI2 terminated perovskites were prone to be constructed at the buried interface by NH2-PTES and CN-PTES.
a 1H NMR spectra of SH-PTES, SH-PTES with PbI2 and SH-PTES with MAI. b 1H NMR spectra of NH2-PTES, NH2-PTES with PbI2 and NH2-PTES with MAI. c 13C NMR spectra of CN-PTES, CN-PTES with PbI2 and CN-PTES with MAI. d Schematic diagram of interaction strength between SCAs and Pb2+ or MA+. e O 1 s XPS spectra of pristine and SCAs-modified SnO2 films.
Furthermore, the interaction between the SCAs and the underlying SnO2 was characterized by X-ray photoelectron spectroscopy (XPS). The presence of Si, S, and N elements in Supplementary Fig. 2a–c indicated the formation of SCAs layer atop SnO2. As shown in Fig. 2e, the O 1 s spectra of pristine SnO2 and SCAs-modified SnO2 films can be fitted with two distinct peaks at 532.23 and 530.97 eV, which were assigned to hydroxyl groups (-OH) and lattice oxygen (O2−), respectively33,34. The lattice oxygen remained constant similar with Sn elements in Supplementary Fig. 2d, while the characteristic peak of -OH groups was moved toward high binding energy area after CN-PTES treatment with a notable increase of the proportion of IO2−/IOH− from 1.786 to 2.083, which indicated that the residual -OH atop SnO2 was partially eliminated by the self-assembled CN-PTES. In contrast, the proportion of IO2−/IOH− were changed to 1.667 and 1.449 atop SnO2 after SH-PTES and NH2-PTES treatments, respectively, which demonstrated that the incorporation of –SH and –NH2 groups atop SnO2 can introduce unfavorable hydroxyl groups because of their hydrogen bonding with moisture from the air.
Effect of SCAs on perovskite crystallization
To explore the effect of SCAs on perovskite crystallization, in situ UV–Vis spectra measurements were carried out. As presented in Fig. 3a, the perovskite crystallization was mainly composed of three stages. First, the absorption intensity of the as-printed films in the range of 300– 400 nm was increased and the peak position was slightly red-shifted as seen in Supplementary fig. 3a, which was attributed to the growth of iodide plumbates (i.e., PbI3−, PbI42−) within the film as the rapid volatilization of residual solvent. Secondly, several characteristic peaks at 460, 510, 570, 650, 730 nm, which might be assigned to low-dimensional perovskites with n = 1, 2, 3, 4, ≥5, respectively35, were appeared and then gradually disappeared as shown in Fig. 3b, indicating the formation of low-dimensional perovskites and their transformation to 3D perovskites. Thirdly, the absorption intensity started to decrease and leveled off with stabilized spectra as seen in Supplementary Fig. 3b. The initial high absorption intensity was susceptibly originated from the confinement effect of small perovskite crystallites and stabilized as their growth into large ones36. These three stages were clearly distinguished for both pristine and SCAs-assisted perovskite crystallization, and their dwell times (τ) are related to the activation energy for each stage. As summarized in Fig. 3a, τ = 5.5, 1 s at the first two stages, respectively, were observed for the perovskite crystallization on pristine SnO2. In contrast, the dwell time was changed to τ = 4, 3 s and τ = 4.5, 3 s upon SH-PTES and CN-PTES modification, respectively, while the τ = 4.5, 1.5 s were obtained after NH2-PTES treatment. Thereby, the perovskite nucleation was accelerated, and phase transformation was retarded when the perovskites were deposited atop SCAs-modified SnO2. In situ PL spectra in Supplementary Figs. 4 and 5 confirmed the conclusions from the in situ absorption spectra. Clearly, the SH-PTES and CN-PTES are more pronounced on affecting the crystallization dynamics because of the strong interaction between the terminated groups of –SH, –C ≡ N, and the perovskite components of MA+, Pb2+, respectively, while the effect of NH2-PTES is less significant due to the relatively weak interaction between –NH2 and the perovskite.
Scanning electron microscope (SEM) images of the perovskite films and their corresponding grain size are shown in Fig. 3c. It is interesting to notice that the grain sizes were enlarged when applying SCAs with strong association with PbI2, i.e., NH2-PTES, CN-PTES. For instance, the average grain size of CN-PTES-modified perovskites was increased from 525 nm to 750 nm. In contrast, the grain size was decreased when SCAs with strong interaction with MAI was applied, such as SH-PTES. Grazing incident wide-angle X-ray scattering (GIWAXS) spectra were performed to evaluate the crystallinity of screen-printed perovskite films with different buried interfaces. As shown in Supplementary fig. 6, both the pristine and SCAs-modified perovskite demonstrated a diffraction pattern at q ≈ 10 nm–1, which was assigned to the (100) facet of MAPbI3. Notably, narrow and bright scattering rings were noticed after CN-PTES treatment, indicating improved crystallinity. Moreover, depth-dependent resolved GIWAXS was measured to analyze the residual strain of perovskite lattices with or without the aid of SCAs. As seen in Supplementary Fig. 7, the diffraction peaks at q ≈ 10 nm−1 of pristine perovskites and SH-PTES, NH2-PTES treated perovskites were shifted to lower diffraction angles as probing angles increased from 0.1 to 0.6°, which demonstrated that the crystal plane distance was increased in the perovskite film, suggesting the presence of residual tensile strain within the perovskite lattice. Correspondingly, the diffraction peak retained constant in CN-PTES-modified perovskites, which exhibited constant crystal plane distance, testifying the residual strain was released with the assistance of CN-PTES. Based on the equation: \(G=\frac{z{\sigma }^{2}h}{E^{\prime} }\) (\(G\) is the driving force for mechanical failure, \(z\) is a nondimensional scaling factor, \(\sigma\) is the residual stress, \(h\) is the thickness of the perovskite layer, and \(E^{\prime}\) is the biaxial modulus), the release of residual stress can reduce the \(G\) on the perovskites, which can improve its mechanical resistance37. Furthermore, a series of double-peaks at q ≈ 10.12, 10.2 nm−1, which represented asymmetric tetragonal I4/mcm phase and symmetric cubic Pm-3m phase, respectively, were observed in pristine and SH-PTES-, NH2-PTES-modified perovskites at various probing angles38,39,40. By contrast, only a single peak at q ≈ 10.2 nm−1 was appeared in the CN-PTES-treated perovskites, indicating the presence of pure cubic phase. It has been revealed that the appearance of the cubic phase within the perovskite films could effectively enhance the photovoltaic performance of PSCs, while the tetragonal phase accelerated the performance degradation of PSCs40,41,42.
Devices performance of f-PSCs
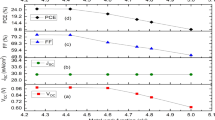
To investigate the impact of SCAs modification on photovoltaic performance, a series of f-PSCs with a shared device structure of PEN/ITO/SnO2/(w or w/o SCAs) /MAPbI3/Spiro-OMe TAD/MoO3/Ag were fabricated, and their typical J–V curves were plotted in Fig. 4a. Particularly, the device based on pristine perovskite exhibited a PCE of 17.3% with an open circuit voltage (VOC) of 1.10 V, a JSC of 21.4 mA cm−2, and a fill factor (FF) of 73.7%. Upon buried interface modification, the efficiencies of the devices with NH2-PTES and CN-PTES treatment were increased, while the efficiency of the SH-PTES-modified device was slightly decreased. Among them, the CN-PTES modified device yielded a champion PCE of 18.6% with a VOC of 1.09 V, a JSC of 23.3 mA cm−2, and a FF of 73.1%, which is the highest value for screen-printed flexible PSCs up to date. The performance improvement was mainly originated from the increase of VOC and JSC, and the increase of EQE at the entire wavelength range as seen in Fig. 4b indicated facile charge extraction after the interface optimization. For long-term storage stability, the PCE of the control device was rapidly decayed to 46.5% of the initial value, while the CN-PTES modified PSCs maintained 90.7% of its initial efficiency after shelf storage of 1000 hours (Fig. 4c). Figure 4d summarized the photovoltaic metrics and their statistic deviation from 20 individual devices, and the measured short circuit density (JSC) from the J–V characterization were double confirmed by integrating the external quantum efficiency (EQE) and solar spectrum as shown in Fig. 4b.
a J–V curves of the champion flexible devices with or without SCAs. Inset is a photo of the flexible device. b External quantum efficiency (EQE) spectra and integrated current of the f-PSCs with or without SCAs modification. c Long-term storage stability measurement of devices. d PCE, VOC, JSC, and FF statistics of 20 devices based on pristine SnO2 and SCAs-modified SnO2.
Photophysical properties of perovskite films and flexible devices
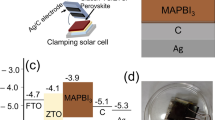
In order to understand the reason for performance improvement, the optoelectronic properties of the perovskites were systematically investigated. The energy level structures of pristine and SCAs-modified SnO2 films were first evaluated by ultraviolet photoelectron spectroscopy (UPS). As seen in Fig. 5a, the valence band maximum energy (EVBM) of SnO2, SH-PTES-SnO2, NH2-PTES-SnO2, and CN-PTES-SnO2 films were calculated to be −7.67, −7.86, −7.51 and −7.61 eV, respectively. It was noted that the absorption and bandgap of the underlying SnO2 and the upper perovskites remained constant after the buried interface modification as shown in Supplementary Figs. 8, 9, and 10, and the conduction band minimum energy (ECBM) were estimated to be −4.22, −4.51, −4.06 and −4.16 eV, respectively. As plotted in Fig. 5b, the CN-PTES-modified SnO2 exhibited a cascade energy level alignment with perovskite, which was beneficial to interfacial electron extraction. As shown in Fig. 5c, the steady-state photoluminescence (PL) of the perovskite was effectively quenched after NH2-PTES and CN-PTES modification, suggesting favored charge transfer from the perovskite to the ETL, which was confirmed by the reduced charge-carrier lifetime extracted from time-resolved PL (TRPL) in Fig. 5d. In contrast, the PL and TRPL exhibited an inverse tendency after SH-PET modification, which was ascribed to the unfavored energy structure as seen in Fig. 5b and low crystallinity as measured in Fig. 3b, c. The dark J–V of the pristine and SCAs-modified f-PSCs was shown in Fig. 5e, and the CN-PTES-modified devices exhibited the lowest dark-state current density, indicating suppressed nonradiative recombination of charge carriers. As shown in Fig. 5f, the ideality factors n of solar cells with or without modifications were calculated by the light-intensity-dependent VOC. According to the formula: \({V}_{oc}=(nkT)/q{\rm{l}}{\rm{n}}({{\rm{P}}}_{\mathrm{light}})\) (\(k\) is Boltzmann constant, \(T\) is room temperature and \(q\) is the elementary charge)43, the ideality factors were estimated to be 2.16, 2.55, 1.89, and 1.47 for pristine, SH-PTES, NH2-PTES, and CN-PTES modified devices, respectively. The smallest ideality factor of CN-PTES modified device indicated suppressed trap-assisted charge recombination. To quantitatively estimate the trap density (\({N}_{{trap}}\)) within the device, electron-only devices (ITO/SnO2/(w or w/o SCAs)/perovskite/PC61BM/Ag) were fabricated. As calculated from the equation: \({N}_{{trap}}=2{\varepsilon }_{r}{\varepsilon }_{0}{V}_{{TFL}}/e{L}^{2}\) (\({\varepsilon }_{r}\) is the relative permittivity, \({\varepsilon }_{0}\) is the vacuum permittivity, \(e\) is the electron charge, \(L\) is the perovskite film thickness, and \({V}_{{TFL}}\) is the limiting voltage full of traps)44, the \({N}_{{trap}}\) of CN-PTES-modified devices was decreased from 2.48 × 1016 to 1.96 × 1016 cm−3 as summarized in Fig. 5g, Supplementary fig. 11. The built-in potential was analyzed by the Mott–Schottky equation: \({C}^{-2}=2\left({V}_{{bi}}-V\right)/\left({A}^{2}q{\varepsilon }_{r}{\varepsilon }_{0}{N}_{d}\right)\), where \({V}_{{bi}}\) is built-in potential, \({N}_{d}\) is charge density45. As noticed in Fig. 5h, the Vbi was improved from 0.77 to 0.80 and 0.82 V after the treatment with NH2-PTES and CN-PTES, respectively, and the increased built-in electric field can accelerate carrier transport within the device. Electrochemical impedance spectroscopy (EIS) measurements were performed on the photovoltaic devices. The Nyquist plots of pristine and SCAs-modified devices were fitted by an equivalent circuit diagram, as seen in Fig. 5i, and the semicircle in the high-frequency region corresponded to carrier recombination impedance (Rrec)27,46. Lower Rrec (296.1 Ω) of the device modified by SH-PTES, and higher Rrec (504.8 Ω, 857.8 Ω) of the device treated by NH2-PTES, CN-PTES, respectively, than that (343.4 Ω) of the pristine device indicated that the nonradiative recombination at the ETL/ PVK interface was largely suppressed after CN-PTES and NH2-PTES treatment. To sum up, the above results indicated that the incorporation of SCAs such as NH2-PTES and CN-PTES, which can strongly associate with PbI2 at the buried interface, can effectively align the energy structure of the underlying SnO2 and modulate the crystallization of the upper perovskite, resulting in improved device performance. On the other hand, the introduction of SCAs at the buried interface, which is selectively interacted with MAI such as SH-PTES, is detrimental to the device performance.
a UPS spectra of secondary electron cut-off and valence bands of SnO2 film and SCAs-SnO2 film. b Energy level diagram of SnO2/perovskite and SnO2/SCAs/perovskite. c Steady-state PL and d TRPL decay spectra of perovskite films with or without SCAs modification. e Dark J–V curves of PSCs. f VOC as a function of light intensities of PSCs. g Statistical diagram of trap density of the electron-only device. h Mott–Schottky plots. i Nyquist plots (inset is an equivalent circuit for data fitting).
Mechanical stability of flexible PSCs
To investigate the influence of SCAs modification on mechanical resistance of the f-PSCs, the devices were cyclically bent at a radius of 5 mm. As shown in Fig. 6a, only 69.7% of the initial PCE was retained after 6000 bending cycles for the control device. In contrast, more than 90% of the initial PCE was maintained for the CN-PTES-modified f-PSCs. To unexplored the reason for the improved mechanical stability, the Young’s modulus of perovskites was calculated from nanoindentation measurements. As presented in Fig. 6b, the CN-PTES-modified perovskite exhibited Young’s modulus of 29.68 GPa, which was lower than the control one (35.7 GPa), suggesting improved bendability. Furthermore, load–displacement measurements of double cantilever beam (DCB) specimens without or with SCAs modification were performed to evaluate interfacial fracture energy of the perovskite/ETL interface in the f-PSCs. As seen in Fig. 6c, larger critical loads, PC, were measured when the interface were treated by NH2-PTES and CN-PTES, versus small ones obtained from the pristine and SH-PTES-modified specimens, indicating enhanced fracture energy as aided by NH2-PTES and CN-PTES. Optical microscope images of the delaminated SnO2 top surface and perovskite buried interface after the tensile test were presented in Fig. 6d and Supplementary Figs. 12 and 13. Particularly, the SnO2 top surface was very smooth and the grain size of the buried perovskite can be clearly distinguished from the pristine device. In contrast, the buried perovskites from the CN-PTES modified device were partially destroyed, and some of them were resident at the SnO2 surface after the tensile test, which suggested that the interfacial adhesion was significantly enhanced by adding the SCAs monolayer, and the same phenomenon was observed in SEM images (Supplementary Fig. 14). Thus, interfacial delamination can be effectively prevented by incorporating CN-PTES at the buried interface, which was beneficial for the mechanical stability of the f-PSCs.
a Normalized PCE of f-PSCs as a function of bending cycles at a radius of 5 mm. Inset is a photo of the bending test. Error bars represent the standard deviations from the statistic results of three individual devices. b Elastic modulus of perovskite films deposited on SnO2 and SCAs-modified SnO2 films. c Tensile load–displacement response curves of the perovskites with or without SCAs modification. d Schematic illustration of tensile test of DCB specimens without or with CN-PTES modification.
In summary, a series of SCAs with various terminal groups, i.e., SH-, NH2- and CN-, were introduced at the buried interface of screen-printed f-PSCs, which can strongly anchor with underlying SnO2 and interact with upper perovskite via selectively coordinating with MA+ or Pb2+. It was revealed that the incorporation of CN-PTES, which can strongly associate with PbI2 at the buried interface, can effectively regulate upper perovskite crystallization with accelerated nucleation and retarded crystal growth, leading to improved crystallinity with suppressed defect states and released residual lattice strain. Moreover, the incorporated CN-PTES can align the energy structure of the underlying SnO2 and boost the interfacial adhesion between the perovskite and the SnO2, resulting in facilitated electron extraction and enhanced interfacial fracture energy. As a result, a champion PCE of 18.6% was obtained as aided by the CN-PTES, which is so far the highest value among the screen-printed f-PSCs. Also, the mechanical resistance of flexible PSCs was simultaneously improved, and above 90% of the efficiency was maintained after 6000 cyclic bending at a radius of 5 mm.
Methods
Materials
All materials used in the experiments were purchased and used without further purification, which include: formamidinium iodide (FAI, GreatCell Solar, 99.9%); formamidinium bromide (FABr, GreatCell Solar, 99.9%); lead iodide (Advanced Election Technology Co., Ltd, China, 99.999%); chlorobenzene (CB, Sigma-Aldrich, 99.7%); isopropanol (IPA, Sigma-Aldrich, 99.5%); acetonitrile (ACN, Sigma-Aldrich, 99.8%); (3-Mercaptopropyl)Triethoxysilane (SH-PTES, Adamas-beta, 98.0%); (3-Aminopropyl)Triethoxysilane (NH2-PTES, TCI, 98.0%); (3-Cyanopropyl)Triethoxysilane (CN-PTES, Adamas-beta, 98.0%); Spiro-OMe TAD (Advanced Election Technology Co., Ltd, China, 99.5%); bis(trifluoromethane)sulfonimide lithium salt (Li-TFSI, Sigma-Aldrich, 99.95%); 4-tert-butylpyridine (tBP, 99.9%, Sigma-Aldrich); methylamine alcohol solution (Sigma-Aldrich, 33 wt.% in absolute ethanol); acetic acid (Sigma-Aldrich, anhydrous, 99%); absolute ethyl alcohol (abs. EtOH, WUXI CITY YASHENG CHEMICAL Co., Ltd, China, 99%); diethyl ether (Et2O, Yonghua Chemical Co., Ltd, China, 99.5%); tin oxide nanoparticles (Xi’an Yuri Solar Co., Ltd, China, 12% in H2O colloidal dispersion). Polyethylene naphthalate (PEN)/ITO was purchased from Advanced Election Technology Co., Ltd. The solvent of MAAc was synthesized by ourselves. Particularly, 27.6 ml acetic acid and 90 ml methylamine alcohol solution were mixed in a 500 mL round-bottomed and stirred in an ice bath (0 °C) for 2 h, which was evaporated by rotation for 1.5 h at 55 °C and the liquid product was stored in a refrigerator for 12 h to crystallize. The white product was purified by washing 3–5 times with diethyl ether and then dissolved again with ethanol solution. The final product was formed by a rotary evaporator for 1.5 h at 55 °C.
Device fabrication
Flexible PEN/ITO substrates were ultrasonic cleaned with ethanol for 5 min, then dried with wiping cloths and treated with plasma for 30 s. SnO2 films were deposited on the cleaned PEN/ITO substrates by spin-coating at 4000 rpm for 30 s, and annealed at 120 °C for 40 min in ambient air, which were then treated by plasma for 30 s after cooling to room temperature. For SCAs modification, 60 μL solutions of SCAs (1 μL/mL SH-PTES, NH2-PTES, and CN-PTES) were deposited on SnO2 by spin coating at 3000 rpm for 30 s. After spin coating, SCAs films were annealed at 100 °C for 10 min. Then, PbI2 (253.4 mg), FAI (94.5 mg), and FABr (2.1 mg) were mixed in 1 mL MAAc by stirring and heating at 100 °C for 20 h in a nitrogen-filled glovebox. The perovskite ink was transferred onto the pristine and SCAs-modification SnO2 films by screen-printing technique in 20 ~ 80% RH, with a printing speed of 20–80 mm s−1, a printing gap of 0.8–1.2 mm, a printing pressure of 0.2 ~ 0.5 Pa and a mesh count of 300–500, then annealed at 115 °C for 5 min to form perovskite thin films as aided by hair dryer. Afterward, 1 mL chlorobenzene solution with 72.3 mg of Spiro-OMe TAD, 29 μL of tBP, and 17.5 μL of Li-TFSI/ACN (520 mg/mL dissolved in ACN) were stirred for 2 h to form the Spiro-OMe TAD precursor solution at room temperature. It was coated at 3000 rpm 30 s. Finally, 100 nm Ag were plated on HTLs through vacuum evaporation at a pressure of 5.0 × 10−4 Pa.
Device measurements
The photovoltaic performance of flexible devices was tested by the Enlitech (Taiwan) SS-F5-3A system with a Keithley 2400 source in a nitrogen glovebox (AM 1.5 G, 100 mW cm−2 calibrated by the NIST-certified monocrystalline Si solar cell). The active area of the flexible was 0.1 cm2, which can ensure the accuracy of JSC measured from J–V scans. J–V curves were scanned by reverse (from 1.2 V to −0.2 V) with the step of 0.02 V and delay time of 1 ms during the measurement. The external quantum efficiency (EQE) measurements were made by an EQE test system (a model of QTest high-finity 5 from Crown-tech Co.). VOC dependence on light intensity were measured with light intensity ranges from 0 to 100 mW/cm2. Dark J–V curves were determined by the SS-F5-3A simulator with a Keithley 2400 source. For long-term storage stability measurements, the flexible devices were stored in a N2 glovebox and measured intermittently for 1000 h, and mechanical stability were tested by a self-built automatic bending equipment.
Characterizations
Nuclear magnetic resonance measurements were performed on a JOEL NMR spectrometer (JNMECZ400S, 400 MHz, Japan). Fourier transform infrared spectra were measured by a Thermo Fisher IS50 with a reflectance accessory. X-ray photoelectron spectroscopy and ultraviolet photoelectron spectroscopy were received from Thermo ESCALAB 250XI. In situ absorption spectra and in situ photoluminescence spectra were tested by ISAS-HI001 system (Nanjing Ouyi Optoelectronics Technology). Scanning electron microscop images were taken by JEOL’s JSM-7800F. Grazing incident wide-angle X-ray scattering was obtained at the beamline BL02U2 of the Shanghai Synchrotron Radiation Facility (SSRF). Absorption spectra were acquired by Shimadzu UV-1780 UV–vis spectrophotometer. Steady-state photoluminescence spectra were scanned by a fluorescence spectrophotometer (F-7100, HITACHI), and time-resolved photoluminescence data were measured using a TCSPC (excited with a 550 nm laser, emitted with the wavelength of 790 nm). Built-in potential and Nyquist plots were performed at the electrochemical workstation (CHI600E, Shanghai Chenhua Instrument Co., Ltd.) and the values of impedance were calculated by the software named ZSimpWin. Young’s Modulus was tested by means of Hysitron TI 950. Mechanical testing: “Sandwich” double cantilever beam specimens were loaded in a universal testing machine (HY-0350) with the structure: PEN/ITO/SnO2/(w or w/o SCAs)/perovskite/UV curing adhesive/ITO/PEN, with a rate of 5 mm min−1 until SnO2 and perovskite films were separated under load. Optical microscope images were taken by a light microscope (SHENZHEN AOSVI OPTICAL INSTRUMENT CO., LTD.).
Data availability
Data are provided by contacting the corresponding authors.
References
De Wolf, S. et al. Organometallic halide perovskites: sharp optical absorption edge and its relation to photovoltaic performance. J. Phys. Chem. Lett. 5, 1035–1039 (2014).
Eperon, G. E. et al. Formamidinium lead trihalide: a broadly tunable perovskite for efficient planar heterojunction solar cells. Energy Environ. Sci. 7, 982–988 (2014).
Xing, G. et al. Long-range balanced electron and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 342, 341–344 (2013).
Stranks, S. D. et al. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 342, 344–347 (2013).
Ren, N. et al. 25% - Efficiency flexible perovskite solar cells via controllable growth of SnO2. iEnergy 3, 39–45 (2024).
Lee, G. et al. Ultra-flexible perovskite solar cells with crumpling durability: toward a wearable power source. Energy Environ. Sci. 12, 3182–3191 (2019).
Hu, X. et al. Wearable power source: a newfangled feasibility for perovskite photovoltaics. ACS Energy Lett. 4, 1065–1072 (2019).
Kaltenbrunner, M. et al. Flexible high power-per-weight perovskite solar cells with chromium oxide–metal contacts for improved stability in air. Nat. Mater. 14, 1032–1039 (2015).
Batmunkh, M. et al. Recent advances in perovskite-based building-integrated photovoltaics. Adv. Mater. 32, 2000631 (2020).
Tu, Y. et al. Perovskite solar cells for space applications: progress and challenges. Adv. Mater. 33, 2006545 (2021).
Hoang, M. T. et al. Are metal halide perovskite solar cells ready for space applications?. J. Phys. Chem. Lett. 13, 2908–2920 (2022).
Xu, W. et al. Multifunctional entinostat enhances the mechanical robustness and efficiency of flexible perovskite solar cells and minimodules. Nat. Photon. 18, 379–387 (2024).
Xue, T. et al. Self-healing ion-conducting elastomer towards record efficient flexible perovskite solar cells with excellent recoverable mechanical stability. Energy Environ. Sci. 17, 2621–2630 (2024).
Beynon, D. et al. All-printed roll-to-roll perovskite photovoltaics enabled by solution-processed carbon electrode. Adv. Mater. 35, e2208561 (2023).
Fan, B. et al. A bionic interface to suppress the coffee-ring effect for reliable and flexible perovskite modules with a near-90% yield rate. Adv. Mater. 34, e2201840 (2022).
Park, M. et al. Scalable production of high performance flexible perovskite solar cells via film-growth-megasonic-spray-coating system. Int. J. Pr. Eng. Man-GT 10, 1223–1234 (2022).
Gao, B. & Meng, J. Flexible CH3NH3PbI3 perovskite solar cells with high stability based on all inkjet printing. Sol. Energy 230, 598–604 (2021).
Chen, C. et al. Perovskite solar cells based on screen-printed thin films. Nature 612, 266–271 (2022).
Chen, C. et al. Fully screen-printed perovskite solar cells with 17% efficiency via tailoring confined perovskite crystallization within mesoporous layer. Adv. Energy Mater. 13, 2302654 (2023).
Wen, J. et al. Steric engineering enables efficient and photostable wide-bandgap perovskites for all-perovskite tandem solar cells. Adv. Mater. 34, 2110356 (2022).
Huang, C. et al. Meniscus-modulated blade coating enables high-quality α-phase formamidinium lead triiodide crystals and efficient perovskite minimodules. Joule 8, 1–15 (2024).
Wang, Z. et al. An embedding 2D/3D heterostructure enables high-performance FA-alloyed flexible perovskite solar cells with efficiency over 20%. Adv. Sci. 8, e2101856 (2021).
Zheng, X. et al. Solvent engineering for scalable fabrication of perovskite/silicon tandem solar cells in air. Nat. Commun 15, 4907 (2024).
Li, Z. et al. Hyperbranched polymer functionalized flexible perovskite solar cells with mechanical robustness and reduced lead leakage. Nat. Commun. 14, 6451 (2023).
Dai, Z. et al. Interfacial toughening with self-assembled monolayers enhances perovskite solar cell reliability. Science 372, 618–622 (2021).
Dai, Z. et al. Dual-interface-reinforced flexible perovskite solar cells for enhanced performance and mechanical reliability. Adv. Mater. 34, 2205301 (2022).
Xu, R. et al. Optimizing the buried interface in flexible perovskite solar cells to achieve over 24% efficiency and long-term stability. Adv. Mater. 36, e2308039 (2023).
Zhang, M. et al. Vertically oriented perovskites with minimized intrinsic boundaries for efficient photovoltaics. J. Phys. Chem. Lett. 15, 5150–5158 (2024).
Chen, S. et al. Stabilizing perovskite-substrate interfaces for high-performance perovskite modules. Science 373, 902–907 (2021).
Pu, D. et al. Enhancing efficiency and intrinsic stability of large-area blade-coated wide-bandgap perovskite solar cells through strain release. Adv. Funct. Mater. 34, 2314349 (2024).
Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 96, 1533–1554 (1996).
Hu, M. et al. Structure and morphology of self-assembled 3-mercaptopropyltrimethoxysilane layers on silicon oxide. Appl. Surf. Sci. 181, 307–316 (2001).
Jung, E. H. et al. Bifunctional surface engineering on SnO2 reduces energy loss in perovskite solar cells. ACS Energy Lett. 5, 2796–2801 (2020).
Sun, Q. et al. SnO2 surface modification and perovskite buried interface passivation by 2,5-furandicarboxylic acid for flexible perovskite solar cells. Adv. Funct. Mater. 34, 2404686 (2024).
Blancon, J. C. et al. Scaling law for excitons in 2D perovskite quantum wells. Nat. Mater. 9, 2254 (2018).
Nah, Y. et al. Narrowing the phase distribution of quasi-2D perovskites for stable deep-blue electroluminescence. Adv. Sci. 9, 2201807 (2022).
Zhang, H. & Park, N. G. Strain control to stabilize perovskite solar cells. Angew. Chem. Int. Ed. 61, e202212268 (2022).
Fransson, E. et al. Revealing the free energy landscape of halide perovskites: metastability and transition characters in CsPbBr3 and MAPbI3. Chem. Mater. 35, 8229–8238 (2023).
Whitfield, P. S. et al. Structures, phase transitions and tricritical behavior of the hybrid perovskite methyl ammonium lead iodide. Sci. Rep. 6, 35685 (2016).
Leonard, A. A. et al. Light-induced transient lattice dynamics and metastable phase transition in CH3NH3PbI3 nanocrystals. ACS Nano 17, 5306–5315 (2023).
Li, M. et al. Acceleration of radiative recombination for efficient perovskite LEDs. Nature 630, 631–635 (2024).
Qin, W. et al. Suppressing non-radiative recombination in metal halide perovskite solar cells by synergistic effect of ferroelasticity. Nat. Commun. 14, 256 (2023).
Zheng, K. et al. Improved phase stability of CsPbI2Br perovskite by released microstrain toward highly efficient and stable solar cells. InfoMat 3, 1431–1444 (2021).
Cao, Y. et al. Designed additive to regulated crystallization for high performance perovskite solar cell. Angew. Chem. Int. Ed. 63, e202404401 (2024).
Meng, Y. et al. Pre-buried ETL with bottom-up strategy toward flexible perovskite solar cells with efficiency over 23%. Adv. Funct. Mater. 33, 2214788 (2023).
Yi, Z. et al. Dual-interface engineering induced by silane coupling agents with different functional groups constructing high-performance flexible perovskite solar cells. Chem. Eng. J. 469, 143790 (2023).
Acknowledgements
This work was financially supported by the Natural Science Foundation of China (22425903, U24A20568, 61705102, 62288102, 22409091, 22409090 and 62205142), the National Key R&D Program of China (2023YFB4204500, 2020YFA07099003), the Jiangsu Provincial Departments of Science and Technology (BK20241875, BE2022023, BK20220010, and BZ2023060), the Excellent Doctoral Dissertation Cultivation Program of Nanjing Tech University (2023-02).
Author information
Authors and Affiliations
Contributions
M.D. wrote the main manuscript text, and J.Y., T.L. and J.W. prepared figures. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Duan, M., Yang, J., Li, T. et al. Mechanically stable screen-printed flexible perovskite solar cells via selective self-assembled siloxane coupling agents. npj Flex Electron 9, 30 (2025). https://doi.org/10.1038/s41528-025-00407-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41528-025-00407-6