Abstract
To address the energy storage needs of wearable electronics, this study developed high-performance, flexible micro-supercapacitors (MSCs) using 2D and 3D patterned fabric-based microelectrodes. The 2D electrodes were created via a screen-printing method with an omnidirectional pre-stretching strategy, while 3D array-structured electrodes were formed through electrostatic actuation. Nano-MnO2 and Na0.77MnO2 were deposited to enhance pseudo-capacitive storage and widen the electrochemical window. The C-C/MnO2-based MSCs exhibited a 21% pseudo-capacitance ratio, achieving an area-specific capacitance of 118.2 mF cm−2 at 5 mV s−1 and an energy density of 39.25 mWh cm−2 at 0.21 mW cm−2. These MSCs maintained 95.05%, 92.04%, and 89.74% of their capacitance under stretched, twisted, and folded conditions, respectively, and showed stable performance across temperatures from −20 °C to 60 °C. Additionally, C-C/Na0.77MnO2-based MSCs extended the electrochemical window to 1.6 V and retained 100.2% capacitance after 6500 cycles. This work offers innovative strategies for advancing portable and wearable electronic devices.
Similar content being viewed by others
Introduction
With the rapid development of flexible electronic devices such as implantable medical monitoring, electronic skin and wearable communication devices, there is an urgent need for lightweight, flexible and biocompatible micro-energy storage devices to supply energy1,2. Therefore, the development of apparel-ready miniature energy storage units is the current hotspot in the research and development of smart textiles3,4,5. The ion transport mechanism of micro-supercapacitors (MSCs) is different from that of traditional stacked supercapacitors, which can integrate microelectrodes, conductive connectors and electrolytes in the same substrate for series/parallel setup and realize the same-plane transport of ions without a diaphragm. The MSCs have the advantages of controllable volume, versatile structure, high charging/discharging efficiency, ease of integration, etc. However, the main problems faced by the development of MSCs lie in the area of the low energy density, poor wearability, and limited industrial applications.
According to the energy density formula of MSCs as \(E=1/2C{V}^{2}\), where C is the capacitance and V is the working voltage, it is known that the capacitance and working window of MSCs needs to be improved simultaneously to enhance the energy density of wearable MSCs. While the flexible electrode materials such as carbonaceous materials6, metal oxides7 and conductive polymers8 currently studied do not have intrinsic stretchability. In order to improve the wearability of MSCs, one of the solutions is to design the non-stretchable electrode materials into special structures, such as wavy9, honeycomb10, spring11, island interconnect12, paper-cut13, etc. Thus the main structure can be protected from deformation by eliminating part of the mechanical stresses in the process of stretching, however, the coexisted defects of poor structural reliability, complicated process and high production cost restricted its scale application. The second solution is to combine the assembled MSCs with the stretchable substrate such as polydimethylsiloxane (PDMS)14, polyurethane (PU)15, silicone rubber (Ecoflex)16, etc. However, the MSCs prepared in this way are prone to shedding and damage during stretching, which limits their existence primarily in the form of pendants, rather than true wear textiles. In contrast, the elastic textile substrate has intrinsic stretchability, wearability comfort, and abundant pore structure that can be used as an electrode carrier and a wearable energy storage integration platform directly17,18,19,20. Porosity exerts a significant influence on the flexibility of electrodes or substrate materials. High porosity provides additional space within the material to accommodate deformation, and interfaces and defects in the porous structure can absorb and disperse stresses to improve the flexibility of the material21,22. To enhance the storage capacitance of flexible textile-based micro-electrodes, the adoption of an omnidirectional pre-stretching strategy is applied, which not only increases the interstitial spaces between fibers, thus augmenting the effective surface area of the electrode material, but also optimizes the pore structure, enabling more thorough contact between the electrode/electrolyte and improving charge transfer efficiency. This strategy concurrently strengthens the adhesion between the electrode and substrate, mitigating the risk of electrode material detachment and damage during stretching processes.
Among the pseudocapacitive materials, transition oxides as MnO2, RuO2, and NiO, etc. have garnered significant attention due to their high theoretical specific capacitance and abundant redox reactions. RuO2 boasts high specific capacitance and excellent conductivity, but its high cost and potential toxicity limit its widespread application. While NiO is cost-effective, it falls behind in terms of specific capacitance and cycling stability. In contrast, MnO2 has emerged as a popular choice for electrode materials due to its high specific capacitance, good cycling stability, and relatively low cost, especially δ-MnO2 with biocompatibility. δ-MnO2 has a layered structure, usually a hierarchical arrangement of manganese-oxygen octahedral units, which allows ions (e.g., H+ or Li+) to migrate rapidly from layer to layer, providing good pseudocapacitive properties. Moreover, the layered structure of δ-MnO2 provides a large specific surface area and better electrochemical cycling stability, which helps to enhance the energy density and power density. However, the pseudocapacitance reaction of pristine δ-MnO2 only occurs at the surface/near-surface regions due to the low intrinsic conductivity, and the limited active sites reduce the utilization rate of active δ-MnO2. Numerous investigations have corroborated that the construction of a three-dimensional array structure offers the potential to simultaneously integrate the loading capacity, electrical conductivity, and electrochemical activity of δ-MnO223,24,25,26. Currently, the main methods to realize the controlled construction of microelectrodes with array structure include laser lithography27,28, 3D printing29, inductively coupled ion etching (ICP)30, etc. However, the generally existed problems as expensive processing equipment, harsh preparation conditions and low efficiency hinder the large-scale application. The preliminary research of our group found that carbon fibers will be polarized and charged under the action of high voltage electric field, and will eventually be vertically implanted on the surface of the substrate after rotation, translation and other complex movements due to the electrostatic driving effect driven by the coulomb force between charges. This method has the advantages of strong structural controllability, high material utilization, high productivity and low energy consumption, which can improve the preparation efficiency and electrochemical performance of MSCs31.
On the other hand, the microstructure and surface charge of the 3D array flexible electrodes need to be optimized to expand their operating voltage and improve the energy density of MSCs. The aqueous electrolyte with a high safety factor has a low theoretical decomposition potential (around 1.23 V) due to its high susceptibility to oxygen (OER)/hydrogen (HER) precipitation on the positive/negative electrode surfaces32. Neutral aqueous electrolytes have a higher hydrogen/oxygen super-potential on the electrode surface due to their lower H+/OH- concentration compared to acidic and alkaline electrolytes. Once the electrode voltage touches the equilibrium potential, the primordial ecological H will be produced by water decomposition as Eq. (1), and subsequently physically adsorbed on the electrode surface as Eq. (2), which in turn will undergo recombined electrochemical/chemical reactions under certain conditions. H2 will be produced when the adsorption energy of the primordial H is lower than its recombination energy. Conversely, the adsorbed H will be embedded in the electrode in a higher adsorption energy state, combining with OH- to produce H2O as Eq. (3)21,33. It is shown that the primordial H tends to be stored in the interlayer spacing of δ-MnO2 with higher adsorption energy, which can be increased by doping metal cations to the interlayer channels of δ-MnO2 with accessorial active sites and capacitive properties34. However, the general atom ratio of embedded cation/Mn is <0.3, and the electrolyte ions and primordial H cannot be effectively embedded in the interlayer channels of δ-MnO2, resulting in a shallow electrochemical reaction and an actual capacitance less than 30% of the theoretical capacitance35,36. Therefore, a high content of cations can be attempted to insert into the interlayer of layered δ-MnO2 to improve the pseudocapacitance utilization and voltage window. In this research, the high content of Na+-doped MnO2 obtained by electrochemical oxidation can be used as the active component of the electrode due to the larger layer spacing, which can accommodate more electrolyte ions, thus enlarging the electrochemical window and further improving the energy density of the MSC.
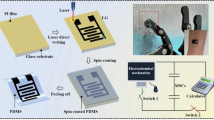
Based on the above analysis, 2D patterned fabric-based stretchable microelectrodes were prepared based on screen-printing method with the omnidirectional pre-stretching strategy, as shown in Fig. 1. Then 3D patterned fabric-based array-structured microelectrodes were constructed by electrostatic actuation. Furthermore, nano-MnO2 and Na0.77MnO2 were deposited by combining electrochemical deposition and oxidization to provide additional pseudocapacitive energy storage and broaden electrochemical window. The influence mechanisms of microelectrode morphology, microstructure and energy storage ratio of pseudo-capacitance under different states were discussed. And the post-optimized MSCs were assembled in series/parallel for power supply of portable devices to analyze their actual wearability. In this study, we observed that the fabricated MSCs exhibit outstanding mechanical flexibility and electrochemical stability. The MSCs maintain their high capacitive performance even under mechanical stresses such as stretching, twisting, and folding. Moreover, the device demonstrates remarkable stability over a wide temperature range, thereby offering the potential for broad applications in the field of wearable electronic devices.
Results and discussion
Morphology and electrochemical properties of 2D C-based MSCs
The mechanical tensile properties of cotton/spandex knitted fabrics are presented in Supplementary Fig. 1. The SEM analysis of the conductive fabric as C with different degrees of omnidirectional pre-stretching from 0% to 50% are shown in Fig. 2a, b and Supplementary Fig. 2. It can be seen that with the increased degrees of omnidirectional pre-stretching of the fabric, the distance between the yarns increased, and a higher amount of CNT-based aqueous pastes able to sufficiently penetrate into yarns/fibers of cotton/spandex fabrics. The conductive C is naturally relaxed to produce a wrinkled shape after natural air-drying as shown in Fig. 2c, d, which are the optical photographs of 30% omnidirectional pre-stretched C before and after relaxation. With the increase of the degree of omnidirectional pre-stretching, the effective area of the microelectrode decreased after relaxation, while the cured CNT-based aqueous slurry tightly stacked and agglomerated together, resulting in a more continuous conductive pathway. Figure 2e shows the SEM image along with corresponding EDS spectra of 30% omnidirectional pre-stretched C, where the thin silver paste layer uniformly covers the surface, forming a continuous conductive network. To quantify the conductivity enhancement, sheet resistance measurements were performed, revealing a dramatic reduction from 870 Ω sq−1 to 42 mΩ sq−1 after silver paste coating. This 20,000-fold decrease in resistance highlights the pivotal role of the silver layer in creating a low-resistance interface, which in turn boosts the efficiency of charge transfer and improves the overall performance of the device. Figure 2f shows the infrared spectra of CNTs before and after acidification, and it can be seen that the characteristic peak of AC-CNTs at 1718.54 cm−1 is more obvious than that of CNTs, which corresponds to C=O stretching vibrational absorption peak. The characteristic peak at 1396.94 cm−1 is attributed to the −COOH absorption peak while the characteristic peaks appearing between 1630.47 cm−1 and 3300 cm−1~3650 cm−1 belong to -OH stretching vibration absorption. The functional groups such as -OH and -COOH were proved to be grafted on the surface of AC-CNTs, which improved the dispersion in polar media. Figure 2g displays the CV curves of C-based MSCs at various scan rates in a dual-electrode system. Due to the inherent electrochemical window limitation of the carbon material, we selected a voltage range of 0–0.9 V for testing. It can be observed that these CV curves exhibit a slight deviation from the rectangular shape, indicating that the C-based MSCs possess highly reversible pseudocapacitive behavior. The sources of pseudocapacitance primarily include the following aspects. (1) The high specific surface area of CNTs and the functional groups on their surface, which can undergo adsorption reactions with ions; (2) The increased number of oxygen-containing functional groups on the surface of acid-treated CNTs, enhancing electrochemical activity; (3) The formation of the silver paste layer, which constructs a conductive network, effectively reducing resistance and thereby improving the pseudocapacitive performance; (4) The pre-stretching treatment of the fabric, which increases the inter-fiber gaps, thereby enhancing the effective surface area of the electrode material; (5) The formation of wrinkle structures after releasing the pre-stretch, further increasing the specific surface area and electrochemical active sites of the electrode. The area-specific capacitances at 5, 10, 20, 50, 100 and 200 mV s−1 are 43.8, 40.6, 35.7, 30.6, 26.1, and 19.6 mF cm−2, respectively. Figure 2h shows the GCD curves of the C-based MSCs at different current densities, and it can be seen that the curves are approximate to isosceles triangles, which indicates the favourate charge/discharge reversibility. And when the current density changes from 0.119 mA cm−2 to 0.278 mA cm−2, the area-specific capacitances of C-based MSCs are 20.3, 20.0, 19.3, 18.6, and 17.6 mF cm−2, respectively. Figure 2i shows the Nyquist plot of C-based MSCs, in which the slope of the straight line is greater than 45° in the low-frequency region, indicating that diffusion and pseudo-capacitive behaviors have occurred. Figure 2i presents the Nyquist plot of C-based MSCs, wherein the slope of the straight line in the low-frequency region exceeds 45°, indicating the occurrence of both diffusive and pseudocapacitive behaviors. The solution resistance (Rs) value is 5.05 Ω, and the charge transfer resistance (Rct) value is 1.30 Ω (Supplementary Table 1), suggesting that the MSCs exhibit low charge transfer resistance and equivalent series resistance. Furthermore, the capacitance retention rates of C-based MSCs after abrasion resistance, water resistance, and washability tests could reach as high as 90.84%, 99.55%, and 99.12%, respectively as illustrated in Supplementary Figs. 3–5, robustly demonstrating the excellent durability and stability of the MSCs in practical applications.
a, b SEM images of 0% and 30% omnidirectional prestretched carbon. c, d Optical photographs of 30% omnidirectional prestretched carbon before and after relaxation. e EDS image of carbon. f Infrared spectra of carbon nanotubes before and after acidification. g, h CV and GCD curves, i Nyquist plot of carbon-based supercapacitor materials.
Morphology and electrochemical properties of 3D C-C-based MSCs
Figure 3a, b show N2 adsorption/desorption curves and the pore size distribution graphs of carbon fibers before and after activation, respectively. The adsorption amount of carbon fiber after activation at low relative pressure increases rapidly, indicating that micropores are formed in the process of activation. And the curve in the medium-pressure region rises slowly due to the transformation of some micro-pores into meso-pores. And the hysteresis loopback phenomenon in carbon fibers after activation ascribed to the capillary condensation effect also proves the existence of mesopores. The pore volume of carbon fiber after activation increases to 0.059 cc g−1 from the original 0.004 cc g−1, and the specific surface area increases to 117.726 m2 g−1 from the original 1.264 m2 g−1. The SEM images of carbon fibers before and after activation are shown in Fig. 3c and Supplementary Fig. 6. Compared with the smooth surface of carbon fibers before activation, the different degrees of etching phenomena lead to grooves, bumps and grooves, etc. They are displayed on the activated surface, which increase the specific surface area of the carbon fibers and are conducive to the subsequent loading of the electrochemical active materials. Figure 3d shows SEM image at low-magnification of C-C with array structure prepared by electrostatic actuation, and it can be seen that the carbon fibers with diameters of 7–10 μm and lengths of 1 mm are basically implanted on the surface of C in an upright state to form 3D vertical array structure. From the SEM image at high-magnification of the corresponding C-C in Fig. 3e, it can be seen that the surface of the activated carbon fiber is rough and has multistage pore structure, which can provide stable self-supporting scaffold for the efficient loading of the subsequent electroactive material.
a, b N2 adsorption/desorption curves and pore size distribution of carbon fiber before and after activation. c SEM of pristine carbon fibers. d, e SEM images of activated carbon fiber (C-C) at low and high magnification. f CV curves of C-C-based MSCs in various voltage windows. g, h CV and CCD curves in 0–1.1 V range. i Nyquist plot of C-C-based supercapacitors.
In order to select the suitable operating voltage that C-C-based MSCs can achieve, CV tests were performed at a scanning rate of 50 mV s−1 by subjecting C-C-based MSCs to four different voltage windows as 0–0.9 V, 0–1.0 V, 0–1.1 V, and 0–1.2 V, as shown in Fig. 3f. The C-C-based MSCs can be charged/discharged normally, and the shape of the CV curves maintains well under the voltage window of 0–1.1 V, and the CV curves are polarized due to the oxygen precipitation when the voltage window is enlarged from 1.1 V to 1.2 V. Although the C-C three-dimensional structure contributes to the enhanced utilization of the material and could potentially optimize its overall performance, it does not fundamentally alter the inherent electrochemical attributes of the carbon material. Consequently, there remains a certain limitation on its working voltage. The voltage window of 3D C-C-based MSCs (1.1 V) is higher than that of 2D C-based MSCs (0.9 V), which is attributed to the unique advantages of the 3D structure. The 3D structure offers a larger specific surface area, facilitating electrolyte infiltration and ion adsorption, thus enhancing capacitive performance. Furthermore, the carbon fiber microstructure within the 3D structure can more effectively dissipate stress, preventing structural failure under high voltage, thereby allowing for a higher operating voltage21,33. Figure 3g shows the CV curves of C-C-based MSCs with different scanning rates at 0–1.1 V, in which no obvious redox peak appeared indicates the constant charging/discharging rate. And the corresponding area-specific capacitance values of C-C-based MSCs were 64.3, 58.4, 50.6, 44.1, 36.7, and 28.5 mF cm−2, respectively at scanning rates at 5, 10, 20, 50, 100 and 200 mV s−1. Figure 3h shows the GCD curves of C-C-based MSCs under the voltage window of 0–1.1 V at different current densities, in which the near-triangle shape of charging/discharging curves indicates the rapid reversible charging/discharging behaviors. And the area-specific capacitance can still be maintained at 89.14% with the current density changing from 0.198 mA cm−2 to 0.357 mA cm−2, suggesting that the C-C-based MSCs have a favorable pseudo-capacitive reversibility and charging/discharging multiplicity performance. The energy density of 21.19 mWh cm−2 and the power density of 0.11 mW cm−2 are obtained at the current density of 0.198 mA cm−2. Figure 3i shows the Nyquist plots of C-C-based MSCs, in which a linear straight line-like behavior occurs at low-frequency region and a semicircular situation results at high-frequency region indicate the satisfactory frequency response characteristic. And the Rs and Rct of the C-C-based MSCs are 6.14 Ω and 0.84 Ω from the built-in enlarged view of the high-frequency region (Supplementary Table 1).
Morphology, structure and electrochemical properties of C-C/MnO2-based MSCs
Figure 4a–c exhibit the SEM images of the C-C/MnO2-based microelectrodes at different magnifications. As shown in Fig. 4b, the surface of most carbon fibers is uniformly coated with an ultra-thin MnO2 nanofilm, which is beneficial for achieving high-speed ion and electron transport, as well as efficient pseudocapacitive reactions. However, as depicted in Fig. 4c, a small portion of the carbon fibers exhibit uneven agglomerate morphology due to the deposition of MnO2. From the EDS spectra of C-C/MnO2-based microelectrodes in Fig. 4d–g and Supplementary Fig. 7, it can be seen that the silver paste forms a continuous conductive layer beneath the carbon fibers and MnO2, while the elements of O and Mn are uniformly distributed in regions corresponding to the carbon fiber array structure. The Ag signal in EDS is attenuated due to surface coverage by the overlying materials, as explained in Supplementary Fig. 7.
Figure 5a displays the CV curves of the C-C/MnO2 composite-based MSCs within a voltage range of 0–1.1 V. The maintenance of the voltage window at 1.1 V can be rationalized as follows. (1) Within the composite electrode, an electrochemical potential equilibrium might have been established between the carbon material and MnO2, with the equilibrium potential aligning with that of the pure carbon electrode. (2) The content of MnO2 in the composite electrode might not have reached the critical proportion necessary to significantly modulate the electrochemical window. It can be seen that the curves present a similar symmetrical shape with obvious redox peaks, indicating that the C-C/MnO2-based MSCs have a highly reversible pseudocapacitive property. The corresponding area-specific capacitance values of C-C/MnO2-based MSCs at 0–1.1 V are 118.2, 96.6, 67.7, 48.8, 35.7, and 26.3 mF cm−2 at scanning rates ranging from 5 to 200 mV s−1. The oxidation peak and reduction peak observed in the CV curves of C-C/MnO2-based MSCs correspond to the oxidation and reduction reactions of MnO2, respectively. As the voltage increases, Mn ions within MnO2 lose electrons and undergo oxidation, leading to the formation of the oxidation peak. Conversely, when the voltage decreases, the oxidized Mn ions regain electrons, resulting in the formation of the reduction peak. The specific reaction equation for its Mn2+ during redox reaction is Eq. (4). The redox peaks in the CV curves of C, C-C and C-C/MnO2 based MSCs are presented in Supplementary Fig. 8. Compared with those of C-C-based MSCs and C/MnO2-based MSCs as shown in Supplementary Figs. 9–12, the area-specific capacitance is increased by 83.8% and 15.8% at 5 mV s−1, respectively, indicating that the constructed 3D array structure and electrodeposited MnO2 can effectively improve the electrochemical properties. The shift of redox peaks in the CV curves of C-C/MnO2-based MSCs at different scan rates as depicted in Fig. 5a is primarily associated with the following factors. (1) Enhanced polarization effect. As the scan rate increases from 5 to 200 mV s−1, an imbalance occurs between the charge transfer rate at the electrode surface and the ion diffusion rate, leading to an enhanced polarization effect. (2) Differences in reaction kinetics. The redox reaction of MnO2 (Mn2+↔MnO2) involves two processes as surface adsorption and bulk diffusion. At low scan rate as 5 mV s−1, ions have sufficient time to diffuse into the interior of the material, and the contribution of bulk diffusion is significant. In contrast, the reaction is constrained by rapid surface charge transfer at high scan rates, leading to peak position shifts. (3) Microstructure of the electrode material. The carbon fiber substrate with a 3D array structure (Fig. 3d, e) may exhibit non-uniform local electric field distribution during high-speed scanning. This non-uniformity can affect the uniform reaction of the MnO2 nanofilm, further exacerbating the variations in peak positions. Figure 5b shows the GCD curves of C-C/MnO2-based MSCs at 0–1.1 V. The obvious peaks appearing in both charging and discharging processes fully indicate the pseudo-capacitive behavior. The area-specific capacitance and coulombic efficiency at 1.984 mA cm−2 are 41.44 mF cm−2 and 82.66%, respectively. Correspondingly, the energy density and power density are 19.96 mW cm−2 and 0.97 mWh cm−2, respectively. However, the curves show a significant decrease in Coulombic efficiency at low current densities. The main reasons are as follows. The electrochemical redox reaction cannot be completely discharged at low current density, and the charging/discharging time decreases with the increase of current density, the electrochemical process is mainly affected by the bilayer, so the coulombic efficiency becomes larger. Additionally, the impedance of the electrolyte and electrode materials has a more significant effect on the overall performance at low current density. As shown in Table 1, the area-specific capacitance of MSCs prepared herein is superior to that of previously reported carbon-based MSCs or C/MnO2-based MSCs. Supplementary Fig. 13 shows the Nyquist plots of C-C/MnO2-based MSCs at open-circuit voltage, in which Rs is 7.0 Ω and Rct is 2.5 Ω. As can be seen from the Ragon plots in Fig. 5c, the electrochemical performance of C-C/MnO2-based MSCs is superior to that of previously reported carbon-based or C/MnO2-based MSCs29,37,38,39,40,41,42. Figure 5d shows the log(ip) and log(v) curves of C-C/MnO2-based MSCs, which were charged and discharged with b-values of 0.423 and 0.572, respectively. b-value of charging less than 0.5 indicates the chemical reaction of the MSC and b-value of discharging in the range of 0.5–1 indicates the presence of pseudocapacitive behavior. The contribution of pseudo-capacitance in C-C/MnO2-based MSCs can be calculated to be 47.3% from colored shaded area in the CV curves at a scan rate of 50 mV s−1 as shown in Fig. 5e. And the corresponding pseudocapacitance contributions of C-C/MnO2-based MSCs at other scan rates from 5 to 100 mV s−1 are displayed in Fig. 5f43. Figure 5g shows the CV curves of C-C/MnO2-based MSCs in the original, stretched by 30%, twisted and folded states, respectively. By employing a pre-stretching strategy, the pore structure and flexibility of the electrode were successfully optimized, enhancing charge transfer efficiency and strengthening the adhesion between the electrode and the substrate. The CV curves at various states nearly coincide at a scan rate of 50 mV s−1, with 95.05%, 92.04%, and 89.74% of the area-specific capacitance retained compared to the initial state when subjected to 30% stretching, twisting, and folding, respectively. This indicates the exceptional electrochemical stability exhibited by the C-C/MnO2-based MSCs. The preservation of voltage peaks across these curves further confirms that the charge storage capability of this supercapacitor remains largely unaffected under deformation conditions. Figure 5h shows the CV curves of C-C/MnO2-based MSCs at 50 mV s−1 from −20 °C to 60 °C. It can be seen that the specific capacitance retention relative to the room temperature (20 °C) at −20 °C, 0 °C, 40 °C, and 60 °C are 90.29%, 98.54%, 99.50% and 95.28%, respectively. The electrochemical stability of C-C/MnO2-based MSCs for 3000 charging/discharging cycles was carried out at a current density of 1.190 mA cm−2 as shown in Fig. 5i. The capacitance retention is 85.9% and the coulombic efficiency is maintained at 78.6% after 3000 cycles. As shown in the inset of Fig. 5i, there is no significant difference in the charge/discharge curves before and after 3000 cycles, which indicates that the battery has excellent electrochemical stability. The excellent capacitive performance is mainly attributed to the efficient loading of straticulate MnO2 and high utilization of pseudocapacitive reaction. The microelectrode with three-dimensional array structure increases the specific surface area of the active material exposed to the electrolyte, while reducing the electrode impedance and promoting the reaction kinetics of ions/electrons. In Fig. 5i, we observed a sudden increase in the capacitance of the C-C/MnO2-based MSCs, which can be primarily attributed to the following two factors. (1) With the progression of multiple charge-discharge cycles, the MnO2 material undergoes a gradual activation process, leading to the exposure of additional active sites. (2) During the charging/discharging process, the structural adjustment helps to increase the specific surface area of the electrode, promoting the adsorption of electrolyte ions on the surface of the electrode, which is a potential reason for the capacitance elevation.
a CV curves, b GCD curves, c Ragone plots, d log(ip)-log(v) curves. e Pseudocapacitive contribution at 50 mV s−1. f Pseudocapacitive contributions at different scan rates. g CV curves under original, stretched by 30%, twisted, and folded states. h CV curves at different temperatures from −20 °C to 60 °C. i Cycling stability at 1.190 mA cm−2.
Morphology, structure and electrochemical properties of C-C/Na0.77MnO2-based MSCs
Figure 6a, b presents the SEM images of the C-C/Na0.77MnO2-based microelectrodes at various magnifications. It can be observed that the carbon fiber surface is uniformly coated with a nanoscale flower-like Na0.77MnO2 structure. This not only effectively reduces the charge transfer resistance but also provides abundant adsorption sites, which is favorable for the ion intercalation process44. The N2 adsorption/desorption curves and pore size distribution for the C-C/Na0.77MnO2-based microelectrode are presented in Supplementary Fig. 14. The physical phases and crystal structures of C-C, C-C/MnO2, and C-C/Na0.77MnO2-based microelectrodes were analyzed by XRD tests as shown in Fig. 6c. The XRD patterns of the C-C, C-C/MnO2, and C-C/Na0.77MnO2-based microelectrodes all exhibit diffraction peaks attributed to the (111), (200), (220), and (311) planes of Ag (JCPDS No. 87-0597), indicating that the silver paste layer has been coated onto the microelectrodes and functions as a crucial conductive layer. The diffraction peaks appeared at 36.81° and 65.70° in the XRD pattern of C-C/MnO2-based microelectrode correspond to the (006) and (119) diffraction of δ-MnO2 (JCPDS No. 18-0802). The XRD patterns of C-C/Na0.77MnO2-based microelectrodes with diffraction peaks at 31.95°, 37.03°, 48.93°, 61.98°, 62.44°, and 77.10° corresponded to the crystal surface of Na0.77MnO2 (JCPDS No.27-0752) at (004), (111), (114), (116), (130), and (028), respectively. The new diffraction peaks in the 27–30° range may indicate the presence of Na2O or other oxymanganese compounds in the C-C/Na0.77MnO2-based microelectrode. As can be seen from EDS spectra of C-C/Na0.77MnO2-based microelectrode in Fig. 6d–g, the elements of O, Mn and Na are uniformly distributed in the positions where the carbon fibers appear, and the distribution of each element in Supplementary Fig. 15 further intuitively indicate the loading of Na0.77MnO2 on the carbon fibers.
Figure 6h shows the full XPS spectra of C-C/MnO2 and C-C/Na0.77MnO2-based microelectrodes, and it can be seen that there are mainly three elements as C, O, and Mn in the C-C/MnO2 spectrum, and four elements as C, O, Mn, and Na in the C-C/Na0.77MnO2 spectrum. Figure 6i shows the C 1 s spectra of C-C/MnO2 and C-C/Na0.77MnO2-based microelectrodes. C-C/MnO2-based microelectrode has three obvious characteristic peaks at 284.82 eV, 286.40 eV and 288.68 eV corresponding to C=C/C-C, C-O and C=O, respectively. While two more distinct peaks existed at 284.59 eV and 286.83 eV for C-C/Na0.77MnO2-based microelectrode correspond to the presence of C=C/C-C and C=O. As shown in Fig. 6j, two pronounced fitted peaks at 531.64 eV and 532.88 eV of C-C/MnO2 are attributed to the presence of Mn-O-H and C=O/C-O. And two fitted peaks at 531.70 eV and 536.23 eV of C-C/Na0.77MnO2 are ascribed to the presence of Mn-O-H and O=O, where the doped O is beneficial to capacity enhancement. As can be seen from Mn 2p spectra in Fig. 6k, two distinct characteristic peaks at 642.03 eV and 653.71 eV of C-C/MnO2 and 642.09 eV and 653.64 eV of C-C/Na0.77MnO2 are attributed to the presence of Mn 2p3/2 and Mn 2p1/2, respectively. The calculated spin potential energy differences of the two peaks are 11.68 eV and 11.55 eV, respectively, indicating the presence of Mn4+ in both microelectrodes. Figure 6l shows the nuclear spectrum Mn 3 s of C-C/Na0.77MnO2-based microelectrode with an energy separation of 5.69 eV, indicating that the oxidation state of Mn is significantly improved after electrochemical oxidation. Na 1s of electrochemically oxidized C-C/Na0.77MnO2 is shown in Fig. 6m, confirming the successful intercalation of Na+ into MnO2. The Na/Mn atomic ratio in the C-C/Na0.77MnO2-based microelectrode measured by inductively coupled plasma is 0.77, which fully reflects the high Na+ content.
Energy storage mechanism and application of C-C/Na0.77MnO2-based MSCs
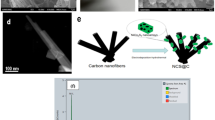
The interconnected ultrathin nanosheets are present on C-C/Na0.77MnO2 as shown in the TEM image (Fig. 7a), which can provide a large amount of specific surface area for ion adsorption/desorption. The well-resolved lattice stripes with an intrafacial spacing of 0.66 nm are observed in Fig. 7b corresponding to (002) crystallographic plane of Na0.77MnO2, which is larger than that of MnO2 as 0.55 nm30 due to the high content of Na+ insertion. The insertion of Na+ significantly enlarges the lattice spacing of MnO2, which facilitates the orderly transport and sufficient adsorption of electrolyte ions. Additionally, the expanded lattice spacing effectively inhibits the occurrence of OER/HER, thus enhancing the operating voltage. Furthermore, the insertion of Na+ also mitigates the volume expansion of MnO2 during the charging and discharging process, improving the structural stability of the electrode and subsequently prolonging the cycle life34,45. The prepared C-C/Na0.77MnO2 nanosheets are also proved to be polycrystalline in structure as shown by the closed circles in the SEAD diagram (inserted in Fig. 7b). In order to select the suitable voltage range that C-C/Na0.77MnO2-based MSCs can achieve, the CV tests were performed by subjecting the C-C/Na0.77MnO2-based MSCs to different voltage windows at a scanning rate of 50 mV s−1 as shown in Fig. 7c. The CV curves are all nonstandard rectangular shapes and maintain well in the range of 0–1.6 V, while when the voltage window expands from 1.6 V to 1.7 V, the curves become polarized and oxygen evolution occurs. Therefore, 0–1.6 V was chosen as a suitable operating voltage for C-C/Na0.77MnO2-based MSCs, which was increased by 0.5 V compared to that of C-C/MnO2-based MSCs. This phenomenon can be attributed to the unique structural characteristics of Na0.77MnO2. The intercalation of Na+ ions leads to an increase in the interlayer spacing of MnO2, which effectively suppresses the oxygen evolution reaction, thereby broadening the operational voltage window. The specific reaction equation for its Na+ during the redox reaction is Eq. (5). Figure 7d shows CV curves of C-C/Na0.77MnO2-based MSCs at 0–1.6 V, and the corresponding area-specific capacitances are 8.21, 5.12, 3.42, 2.22, 1.35, 0.63, 0.41, and 0.34 mF cm−2 at scanning rates ranging from 10 to 1000 mV s−1. Figure 7e shows the GCD curves of C-C/Na0.77MnO2 based MSCs at different current densities from 0 V to 1.6 V. At the high current density of 0.238 mA cm−2, the area-specific capacitance and coulombic efficiency are 2.37 mF cm−2 and 77.78%, corresponding to energy density and power density of 1.90 mW cm−2 and 0.15 mWh cm−2. The lower Coulombic efficiency observed in the C-C/Na0.77MnO2-based MSCs is mainly attributed to the structural changes in the electrode materials and the increase in charge transfer resistance. Structural changes such as lattice expansion or contraction may occur in Na0.77MnO2 electrodes during charging/discharging at high current densities, affecting the efficiency of charge storage and release. Moreover, as can be seen in Figs. 5b and 7e, the GCD curves of both C-C/MnO2-based MSCs and C-C/Na0.77MnO2-based MSCs show a significant IR decrease. This is primarily attributed to the internal resistance of the electrode materials, the resistance of the electrolyte, and the contact resistance between the electrode and the current collector46,47. The Nyquist plots and equivalent circuit diagram of the C-C/Na0.77MnO2-based MSCs are shown in Fig. 7f, and it can be seen that Rs is 67.27 Ω, and Rct is 38.83 Ω (Supplementary Table 1). The slope b can be obtained from the log(ip) and log(v) curves of C-C/Na0.77MnO2-based MSCs as shown in Fig. 7g. The b values of 0.330 and 0.397 for the charging and discharging exhibit mainly bilayer behavior. As shown in Supplementary Fig. 16, the shaded area indicates that the pseudo-capacitance contributes 6.8% in C-C/Na0.77MnO2-based MSCs at a scan rate of 50 mV s−1. As shown in Fig. 7h, the corresponding pseudo-capacitance contributions of C-C/Na0.77MnO2-based MSCs at scan rates of 10, 25, 50, 100, and 200 mV s−1 are 4%, 5%, 7%, 10%, and 16%, respectively. The electrochemical stability of C-C/Na0.77MnO2-based MSCs for 6500 charging/discharging cycles at a current density of 0.159 mA cm−2 is shown in Fig. 7i. The area-specific capacitance retention is as high as 100.2% after 6500 cycles and the coulombic efficiency is calculated to be 85.9% at the 6500th cycle. In the figure, we distinctly observe significant capacitance fluctuations in the C-C/Na0.77MnO2-based MSCs. This phenomenon can be primarily attributed to the following two key factors. (1) During the charge-discharge cycles, Na+ ions in Na0.77MnO2 undergo processes of intercalation and deintercalation. This process may lead to volumetric expansion and contraction of the electrode material, subsequently causing fluctuations in capacitance. (2) Compared to C-C/MnO2, the introduction of Na+ into the C-C/Na0.77MnO2 composite electrode material may impact the structural stability of the electrode. The incorporation of Na+ could result in dynamic changes to the electrode structure, thereby inducing unstable fluctuations in the capacitance performance. Moreover, there is no obvious difference between the charge/discharge curves with stable and approximate triangles before and after 6500 cycles as inserted in Fig. 7i, revealing the satisfactory electrochemical stability. Compared to the electrochemical properties of C-C/MnO2-based MSCs, the ideal working voltage and cycling stability of C-C/Na0.77MnO2-based MSCs are mainly attributed to the following aspects. (1) High content of inserted Na+ is proved to expand the lattice spacing of MnO2 as shown in TEM images (Fig. 7a, b), which can ensure the ordered electron transfer rate and sufficient ion adsorption to prevent the occurring of oxygen (OER)/hydrogen (HER) precipitation, thus expanding the working voltage. While as for the normal δ-MnO2, the electrolyte ions and primordial H cannot be effectively embedded in the interlayer channels, resulting in a shallow electrochemical reaction. (2) High content of inserted Na+ can slow down the volume expansion of MnO2 during charging/discharging cycles, thereby reducing the stress concentration between materials and structural damage. (3) High content of doped Na+ can regulate the chemical environment of MnO2 as reducing the surface stripping and solvation phenomenon to improve the chemical stability.
a TEM image at low magnification with inset at high magnification. b Selected area electron diffraction (SEAD) plot. c CV curves at various voltages. d CV curves at 0–1.6 V. e GCD curves at 0–1.6 V. f Nyquist plots with inset of equivalent circuit diagram. g log(ip)-log(v) curves. h Pseudocapacitive contribution at different scan rates. i Cyclic stability of C-C/Na0.77MnO2-based MSCs.
Furthermore, the C-C/Na0.77MnO2 microelectrode exhibits exceptional electrochemical performance in LiCl/PVA electrolyte, with its electrochemical mechanisms primarily encompassing the following aspects. (1) Ion transport and intercalation. The crystal structure of Na0.77MnO2 boasts high ion channel openness and large interlayer spacing, favorable for the intercalation and deintercalation of Li+ ions. During charging and discharging, Li+ ions migrate to the electrode surface through the LiCl/PVA electrolyte and intercalate into the interlayer structure of Na0.77MnO2, enabling energy storage and release. This process not only enhances the specific capacitance performance of the electrode but also improves its cycling stability. (2) Redox reaction. The intercalated Li+ ions undergo redox reactions with oxygen atoms in MnO2, forming LixMnO2 compounds accompanied by electron transfer (Eq. (6)). This reaction process provides additional charge storage capacity for the electrode, further boosting its electrochemical performance. (3) Expansion of electrochemical window. High content of doped Na+ expands the lattice spacing of MnO2, ensuring an ordered electron transfer rate and sufficient ion adsorption, which prevents the occurrence of oxygen (OER)/hydrogen (HER) evolution, thereby extending the working voltage. At the same time, it can regulate the chemical environment of MnO2 as reducing the surface stripping and solvation phenomenon to improve the chemical stability.
The optimized C-C/MnO2-based MSCs were connected in series and parallelly for diversified applications. Figure 8a, b show the CV and GCD profiles of 2–4 C-C/MnO2-based MSCs in series/parallel, and electrochemical tests show that the integrated MSCs in series or parallel have stable electrochemical performance and negligible attenuation. In addition, both the CV and GCD profiles of series-connected MSCs show a gradual and linear increase in the operating voltage, while the current density in the CV profile of parallel-connected MSCs steadily increases under the same voltage. The discharge times of 2, 3 and 4 MSCs in parallel are almost double, triple and quadruple those of single MSCs at the same discharge current density. As shown in Fig. 8c, three C-C/MnO2-based MSCs connected in series can continuously power a small light bulb, and the brightness still does not significantly decay when the small light bulb is lit for 120 s, as can be seen from the photographs taken under dark conditions. This state lasted for up to 10 min until the LEDs gradually dimmed. It can also supply power to the hourly clock and the photographs of the first 35 min of power are shown in Fig. 8d. At the same time, 3 series-connected MSCs can be worn on the wrist to power a smartwatch as displayed in Fig. 8e. In addition, three C-C/MnO2-based MSCs can also be connected in series on the lab coat to power an hourly clock as shown in Fig. 8f, which fully demonstrates the practical wearability of the fabricated MSCs herein.
a CV curves for 2–4 MSCs in series and parallel. b GCD curves for 2–4 MSCs in series and parallel. c Lighting a small bulb with 3 MSCs in series for the first 120 s. d Powering an hourly clock with 3 MSCs in series for the first 35 min. e Powering a smartwatch with 3 MSCs in series. f Powering an hourly clock with 3 MSCs in series worn on a lab coat.
Reaction equations
The key electrochemical reactions involved in this work are listed below:
-
1.
Water decomposition and hydrogen adsorption:
$${{\boldsymbol{H}}}_{{\bf{2}}}{\boldsymbol{O}}+{\boldsymbol{e}}\to {\boldsymbol{H}}+{{\boldsymbol{OH}}}^{-}$$(1)$${\boldsymbol{C}}+{\boldsymbol{H}}\to {{\boldsymbol{CH}}}_{{\boldsymbol{ad}}}$$(2)$${\bf{2}}{{\boldsymbol{CH}}}_{{\boldsymbol{ad}}}+{\boldsymbol{OH}}\to {\boldsymbol{C}}\left({\boldsymbol{e}}\right)+{{\boldsymbol{H}}}_{{\bf{2}}}{\boldsymbol{O}}$$(3) -
2.
Redox reaction of MnO2:
$${{\boldsymbol{Mn}}}^{{\bf{2}}+}+{{\bf{2}}{\boldsymbol{H}}}_{{\bf{2}}}{\boldsymbol{O}}\rightleftarrows {\boldsymbol{Mn}}{{\boldsymbol{O}}}_{{\bf{2}}}+{\bf{2}}{{\boldsymbol{H}}}^{+}+{\bf{2}}{{\boldsymbol{e}}}^{-}$$(4) -
3.
Sodium intercalation into MnO2:
$${\boldsymbol{Mn}}{{\boldsymbol{O}}}_{{\bf{2}}}+{\bf{0.77}}{\boldsymbol{N}}{{\boldsymbol{a}}}^{+}+{\bf{0.77}}{{\boldsymbol{e}}}^{-}\rightleftarrows {\boldsymbol{N}}{{\boldsymbol{a}}}_{{\bf{0.77}}}{\boldsymbol{Mn}}{{\boldsymbol{O}}}_{{\bf{2}}}$$(5) -
4.
Lithium interaction with Na0.77MnO2:
Methods
Materials
NaOH, KOH, (C3H5NO)n and C2H7NO2 were purchased from Tianjin Damao Chemical Reagent Co. Ltd. The refining agent and penetrating agent JFC were homemade. C2H6O was purchased from Tianjin Yongda Chemical Reagent Co. Ltd. CNT and carbon fiber were purchased from Cangzhou Zhongli New Material Science and Technology Co. Ltd. H2SO4 and HNO3 were purchased from Beijing Chemical Industry Co. Ltd. Aqueous CNT slurry (CNT content in solids of 5 wt%) was purchased from Suzhou Carbonfund Graphene Science and Technology Co. Ltd. C3H6O was purchased from Tianjin Komeo Chemical Reagent Co. C4H14MnO8, Na2SO4 and PVA were purchased from Sinopharm Group Chemical Reagent Co. LiCl was purchased from Shanghai McLean Biochemistry Technology Co. The above reagents are all analytical purity.
Preparation of 2D C-based MSCs with different degrees of omnidirectional pre-stretching
Cotton/spandex fabrics were immersed in a solution of 10 g L−1 NaOH, 1 g L−1 high-efficiency refiner and 3 g L−1 penetrant JFC with a bath ratio of 30:1, heated at 80 °C for 60 min, washed and then immersed in an aqueous solution with a C2H6O concentration of 30% for 30 min, washed, dried and set aside. 1.8 g of CNTs was put in a mixed acidic solution of H2SO4 and HNO3 with a volume ratio of 3:1, and sonicated at 40 °C for 4 h. Then centrifuged, filtered, dried and ground to get acidified carbon nanotubes defined as AC-CNTs. Cotton/spandex fabrics were fixed after having been pre-stretched by 30%, and aqueous AC-CNTs-based inks with mass ratios of 2% was printed on their surfaces for air-drying. The silver paste was printed again for 8 times, pre-baked in an oven at 80 °C for 5 min, and baked at 120 °C for 10 min to obtain microelectrodes with an effective area of 2.52 cm2 (defined as the geometric area of the entire device exposed to the electrolyte). Then the prepared PVA/LiCl gel electrolyte was uniformly coated on the substrate to obtain C-based MSCs. During the all-directional pre-stretching process, we employed a range of multi-stage stretching degrees from 0% to 50%. These specific stretching ratios were precisely calculated using the following formula as \({\boldsymbol{tensileratio}}=\frac{{{\boldsymbol{L}}}_{{\boldsymbol{stretched}}}-{{\boldsymbol{L}}}_{{\boldsymbol{original}}}}{{{\boldsymbol{L}}}_{{\boldsymbol{original}}}}{\boldsymbol{\times }}{\boldsymbol{100}} \%\).
Preparation of 3D C-C-based MSCs
The 1 mm carbon fiber was soaked in a solution of C3H6O and C2H6O in concentration of 30% for 30 min and 60 min, respectively, then washed, filtered and dried. 2.1 g of KOH was dissolved in 2 mL of deionized water, 48 mL of C2H6O was added and stirred for 1 h. 1.05 g of carbon fibers were soaked in the solution for 30 min, then filtered and dried, and then heated up to 900 °C at a rate of 2 °C min−1 under nitrogen atmosphere at a constant temperature of 3 h to get activation. And then the appropriate amount of carbonized/activated carbon fiber was added to 200 mL of deionized water, and 4 mL of polyacrylamide solution with a mass concentration of 1 g L−1 was added followed by stirring and ultrasonication to obtain completely dispersed carbon fiber.
The above C-based microelectrode as prepared was used as the substrate, and carbon fibers were implanted by electrostatic actuation, in which the process parameters were as follows. The electrostatic voltage is defined as 25 kV, electrostatic field spacing is 6 cm, and implant-time is 25 s. A gel electrolyte composed of 0.09 M PVA and 0.2 M anhydrous LiCl was dissolved in 40 mL of deionized water, followed by stirring at 85 °C for 2 h. Upon cooling to room temperature, a clear and transparent PVA/LiCl gel electrolyte was obtained. The implanted fabrics were pre-baked at 80 °C for 5 min, and baked at 120 °C for 10 min, and assembled with the PVA/LiCl gel electrolyte to obtain 3D C-C-based MSCs.
Preparation of C-C/MnO2-based MSCs
In a three-electrode system, MnO2 was deposited on C-C microelectrodes as prepared using 0.01 mol L−1 C4H14MnO8 and 0.02 mol L−1 C2H7NO2 as electrodeposition solution, C-C as the working electrode, platinum sheet as the counter electrode, and Ag/AgCl as the reference electrode by constant current deposition method. The current density was set as 0.4 mA cm−2, and the time was 600 s to prepare C-C/MnO2 microelectrode. Then the prepared PVA/LiCl gel electrolyte was uniformly coated on the microelectrode to obtain C-C/MnO2-based MSCs.
Preparation of C-C/Na0.77MnO2-based MSCs
In the three-electrode system, the C-C microelectrode as the working electrode, the Pt sheet as the counter electrode, and Ag/AgCl as the reference electrode with 0.1 mol L−1 Na2SO4 and 0.1 mol L−1 C4H14MnO8 as the electrodeposition solution, and constant voltage deposition was carried out for 20 min under the voltage parameter of −1.8 V and the condition of 70 °C. Then, 3.0 M Na2SO4 as the electrodeposition solution, and Na+ doping was carried out using electrochemical oxidation at a scan rate of 100 mV s−1, a voltage window of 0–1.3 V, and 200 cycles. The prepared PVA/LiCl gel electrolyte was uniformly coated on the microelectrode to obtain C-C/Na0.77MnO2-based MSCs. The estimated masses of the active substances in all types of devices involved in this study are presented in Supplementary Table 2.
Characterization
The apparent morphology of the samples was analyzed using a field emission scanning electron microscope (FESEM, S-4800, Hitachi, Japan). The characteristic peaks of functional groups of the samples were analyzed using a Fourier infrared spectrometer (FT-IR, Thermo Scientific Nicolet iS20, Shanghai, Linley Instruments Co.). Specific surface area, pore volume and pore size distribution of the samples were tested using a nitrogen adsorption desorption analyzer (BET, Mike ASAP2460, Connaught Instruments, USA). The crystal structure of the samples was characterized using an X-ray diffractometer (XRD, D8 Advance, Bruker, Germany). The elemental composition, chemical state and molecular structure of the samples was obtained using X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250XI, Thermo Fisher Scientific, USA). Inductively coupled plasma mass spectrometry was used to determine the relative amount of each element in the samples (ICP-MS, Agilent 7800, Agilent, USA). The electrochemical properties of the microdevices were evaluated using an electrochemical workstation (CHI 660E, Shanghai Chenhua Instrument Co., Ltd.). The formulas for calculating capacitance, energy density, and power density based on the CV and GCD methods are presented in Supplementary Eqs. (3)–(6).
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files.
References
Feng, X. et al. Recent advances in micro-supercapacitors for AC line-filtering performance: from fundamental models to emerging applications. EScience 1, 124–140 (2021).
Zhang, P., Wang, F., Yu, M., Zhuang, X. & Feng, X. Two-dimensional materials for miniaturized energy storage devices: from individual devices to smart integrated systems. Chem. Soc. Rev. 47, 7426–7451 (2018).
Huang, P. et al. On-chip and freestanding elastic carbon films for micro-supercapacitors. Sci 351, 691–695 (2016).
Kyeremateng, N. A., Brousse, T. & Pech, D. Microsupercapacitors as miniaturized energy-storage components for on-chip electronics. Nat. Nanotechnol. 12, 7–15 (2017).
Wang, D. et al. Chemical formation of soft metal electrodes for flexible and wearable electronics. Chem. Soc. Rev. 47, 4611–4641 (2018).
Chen, X., Paul, R. & Dai, L. Carbon-based supercapacitors for efficient energy storage. Natl Sci. Rev. 4, 453–489 (2017).
Mustaqeem, M. et al. Rational design of metal oxide based electrode materials for high performance supercapacitors-a review. J. Energy Storage 55, 105419 (2022).
Wang, Y., Ding, Y., Guo, X. & Yu, G. Conductive polymers for stretchable supercapacitors. Nano Res. 12, 1978–1987 (2019).
Xu, P. et al. Carbon nanotube fiber based stretchable wire‐shaped supercapacitors. Adv. Energy Mater. 4, 1300759 (2014).
Pu, J., Wang, X., Xu, R. & Komvopoulos, K. Highly stretchable microsupercapacitor arrays with honeycomb structures for integrated wearable electronic systems. ACS Nano 10, 9306–9315 (2016).
Lu, Z., Foroughi, J., Wang, C., Long, H. & Wallace, G. G. Superelastic hybrid CNT/graphene fibers for wearable energy storage. Adv. Energy Mater. 8, 1702047 (2018).
Kim, D., Shin, G., Kang, Y. J., Kim, W. & Ha, J. S. Fabrication of a stretchable solid-state micro-supercapacitor array. ACS Nano 7, 7975–7982 (2013).
Xu, R. et al. Kirigami-inspired, highly stretchable micro-supercapacitor patches fabricated by laser conversion and cutting. Microsyst. Nanoeng. 4, 1–10 (2018).
Hong, S. Y. et al. High-density, stretchable, all-solid-state microsupercapacitor arrays. ACS Nano 8, 8844–8855 (2014).
Yue, Y. et al. Highly self-healable 3D microsupercapacitor with MXene–graphene composite aerogel. ACS Nano 12, 4224–4232 (2018).
Lim, Y. et al. Biaxially stretchable, integrated array of high performance microsupercapacitors. ACS Nano 8, 11639–11650 (2014).
Cong, Z. et al. Stretchable coplanar self-charging power textile with resist-dyeing triboelectric nanogenerators and microsupercapacitors. ACS Nano 14, 5590–5599 (2020).
Zhang, H., Qiao, Y. & Lu, Z. Fully printed ultraflexible supercapacitor supported by a single-textile substrate. ACS Appl. Mater. Inter. 8, 32317–32323 (2016).
Xie, X. et al. Stencil-printed electrodes without current collectors and inactive additives on textiles for in-plane microsupercapacitors. J. Mater. Chem. A. 9, 25042–25050 (2021).
Lv, J. et al. Transforming commercial regenerated cellulose yarns into multifunctional wearable electronic textiles. J. Mater. Chem. C. 8, 1309–1318 (2020).
Li, X. et al. Wearable solid-state supercapacitors operating at high working voltage with a flexible nanocomposite electrode. ACS Appl. Mater. Inter. 8, 25905–25914 (2016).
Xiaoping, L. et al. Design and construction of 1D/2D/3D fabric-based wearable micro-supercapacitors. J. Power Sources 560, 232712 (2023).
Gao, P. et al. The critical role of point defects in improving the specific capacitance of δ-MnO2 nanosheets. Nat. Commun. 8, 14559 (2017).
Wang, Y., Zhang, Y.-Z., Dubbink, D. & ten Elshof, J. E. Inkjet printing of δ-MnO2 nanosheets for flexible solid-state micro-supercapacitor. Nano Energy 49, 481–488 (2018).
Abbas, S. et al. Zn doping induces rich oxygen vacancies in δ-MnO2 flower-like nanostructures for impressive energy density coin cell supercapacitor. J. Energy Storage 87, 111455 (2024).
Can, T. et al. Optimizing the electrons/ions diffusion kinetics in δ-MnO2 for realizing an ultra-high rate-capability supercapacitor. Chem. Eng. J. 471, 144784 (2023).
Gnerlich, M., Ben-Yoav, H., Culver, J. N., Ketchum, D. R. & Ghodssi, R. Selective deposition of nanostructured ruthenium oxide using Tobacco mosaic virus for micro-supercapacitors in solid Nafion electrolyte. J. Power Sources 293, 649–656 (2015).
Kim, K.-W. et al. Deformable micro-supercapacitor fabricated via laser ablation patterning of Graphene/liquid metal. npj Flex. Electron. 8, 18 (2024).
Liu, L. et al. 3D printing of high-performance micro-supercapacitors with patterned exfoliated graphene/carbon nanotube/silver nanowire electrodes. Sci. China Technol. Sci. 64, 1065–1073 (2021).
Wang, X., Yin, Y., Hao, C. & You, Z. A high-performance three-dimensional micro supercapacitor based on ripple-like ruthenium oxide-carbon nanotube composite films. Carbon 82, 436–445 (2015).
Li, X. et al. Three-dimensional stretchable fabric-based electrode for supercapacitors prepared by electrostatic flocking. Chem. Eng. J. 390, 124442 (2020).
Yu, M., Lu, Y., Zheng, H. & Lu, X. New insights into the operating voltage of aqueous supercapacitors. Chem. Eur. J. 24, 3639–3649 (2018).
Zhao, L. et al. Honeycomb porous MnO2 nanofibers assembled from radially grown nanosheets for aqueous supercapacitors with high working voltage and energy density. Nano Energy 4, 39–48 (2014).
Li, X. et al. Different metal cation-doped MnO2/carbon cloth for wide voltage energy storage. J. Colloid Interf. Sci. 649, 731–740 (2023).
Shan, X. et al. Framework doping of Ni enhances pseudocapacitive Na-ion storage of (Ni) MnO2 layered birnessite. Chem. Mater. 31, 8774–8786 (2019).
Shan, X. et al. Structural water and disordered structure promote aqueous sodium-ion energy storage in sodium-birnessite. Nat. Commun. 10, 4975 (2019).
Eustache, E. et al. High areal energy 3D-interdigitated micro‐supercapacitors in aqueous and ionic liquid electrolytes. Adv. Mater. Technol. 2, 1700126 (2017).
Zheng, X. et al. Highly stretchable CNT/MnO2 nanosheets fiber supercapacitors with high energy density. J. Mater. Sci. 55, 8251–8263 (2020).
Liu, N. et al. Cable‐type supercapacitors of three‐dimensional cotton thread based multi‐grade nanostructures for wearable energy storage. Adv. Mater. 25, 4925–4931 (2013).
Wang, J. et al. Polymorphous supercapacitors constructed from flexible three-dimensional carbon network/polyaniline/MnO2 composite textiles. ACS Appl. Mater. Inter. 10, 10851–10859 (2018).
Wang, Y. et al. Silicon-based 3D all-solid-state micro-supercapacitor with superior performance. ACS Appl. Mater. Inter. 12, 43864–43875 (2020).
Du, J. et al. High-performance pseudocapacitive micro-supercapacitors with three-dimensional current collector of vertical ITO nanowire arrays. J. Mater. Chem. A. 7, 6220–6227 (2019).
Liu, J. et al. Advanced energy storage devices: basic principles, analytical methods, and rational materials design. Adv. Sci. 5, 1700322 (2018).
Gao, P. et al. Understanding the synergistic effects and structural evolution of Co(OH)2 and Co3O4 toward boosting electrochemical charge storage. Adv. Funct. Mater. 32, 2108644 (2022).
Jabeen, N. et al. High‐performance 2.6 V aqueous asymmetric supercapacitors based on in situ formed Na0.5MnO2 nanosheet assembled nanowall arrays. Adv. Mater. 29, 1700804 (2017).
Yazar, S., Arvas, B., Arvas, M. B., Yolacan, C. & Sahin, Y. A biocompatible and flexible supercapacitor for wearable electronic devices. J. Energy Storage 74, 109400 (2023).
Gürkan, K., Karaman, H. & Ballikaya, S. Optimization of high-performance flexible thermoelectric generator from material synthesis to simulation and device application. Energ. Convers. Manag. 291, 117335 (2023).
Wang, Y. et al. Fixture-free omnidirectional prestretching fabrication and integration of crumpled in-plane micro-supercapacitors. Sci. Adv. 8, eabn8338 (2022).
Ren, J. et al. Twisting carbon nanotube fibers for both wire‐shaped micro‐supercapacitor and micro‐battery. Adv. Mater. 25, 1155–1159 (2013).
Kim, C. & Moon, J. H. 3D bicontinuous structure of a pseudocapacitive ultrathin shell/carbon core: a novel electrode for thin-film supercapacitors with high areal energy density. ACS Sustain. Chem. Eng. 8, 14711–14717 (2020).
Sajedi-Moghaddam, A., Gholami, M. & Naseri, N. Inkjet printing of MnO2 nanoflowers on surface-modified A4 paper for flexible all-solid-state microsupercapacitors. ACS Appl. Mater. Inter. 15, 3894–3903 (2023).
Xu, R., Wang, Z., Gao, L., Wang, S. & Zhao, J. Effective design of MnO2 nanoparticles embedded in laser-induced graphene as shape-controllable electrodes for flexible planar microsupercapacitors. Appl. Surf. Sci. 571, 151385 (2022).
Boruah, B. D., Maji, A. & Misra, A. Flexible array of microsupercapacitor for additive energy storage performance over a large area. ACS Appl. Mater. Inter. 10, 15864–15872 (2018).
Patil, S. J., Park, J. S., Kim, Y. B. & Lee, D. W. A quasi 2D flexible micro‐supercapacitor based on MnO2//NiCo2O4 as a miniaturized energy‐storage device. Energy Technol. 6, 1380–1391 (2018).
Hu, H., Pei, Z., Fan, H. & Ye, C. 3D interdigital Au/MnO2/Au stacked hybrid electrodes for on‐chip microsupercapacitors. Small 12, 3059–3069 (2016).
Tao, S. et al. Important effect of Pt modification at the collector/active material interface of flexible micro-supercapacitors. Appl. Surf. Sci. 456, 410–418 (2018).
Wang, N., Liu, J., Zhao, Y., Hu, M. & Shan, G. High-performance asymmetric micro-supercapacitors based on electrodeposited MnO2 and N-doped graphene. Nanotechnology 30, 235403 (2019).
Acknowledgements
This research was supported by Scientific Research Project for Higher Education Institution in Hebei Province (No. BJK2023085), Natural Science Foundation Project of Hebei Province (E2024208048), National Natural Science Foundation of China (Grant No. 22005083) and Introduction of talents Research Funds for the Hebei University of Science and Technology (No. PYA2018012).
Author information
Authors and Affiliations
Contributions
Xiaoping Lin: Conceptualization, data curation, writing-Original draft preparation. Shangbo Li: Visualization, investigation, methodology. Xiaoyan Li: Writing-reviewing & editing, supervision. Xuming Huang: Funding & editing. Luhua Jia: Supervision, reviewing & editing. Wei Zhang: Reviewing & editing. Zaisheng Cai: Reviewing & editing. Gunel Imanova: Reviewing & editing. Sridhar Komarneni: Supervision, reviewing & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, X., Li, S., Li, X. et al. 3D patterned fabric-based wearable micro-supercapacitor operating at high voltage by electrostatic actuation. npj Flex Electron 9, 60 (2025). https://doi.org/10.1038/s41528-025-00435-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41528-025-00435-2