Abstract
The effect of artificial-ageing on corrosion behaviour of aluminium alloy (AA)2024-T3 was investigated. The natural ageing which takes place during the aircraft's lifespan was simulated with isothermal heat-treatments at 190 °C. Electrochemical impedance spectroscopy measurements were performed in different heat-treated specimens to examine the prevailing corrosion mechanisms. Additionally, pre-corroded tensile specimens from different heat-treatment conditions were mechanically tested to assess the corrosion-induced degradation. Different forms of corrosion were revealed in the investigated ageing tempers; intense localized attack was noticed in the initial (T3) and under-aged (UA) tempers. UA condition exhibited the highest susceptibility to corrosion propagation followed by T3, according to the charge transfer resistance RCT and degradation rate of tensile elongation at fracture Af ( ≈ 0.118) and ( ≈ 0.103), respectively. Corrosion-induced degradation rates for the peak-aged (PA) and over-aged (OA) tempers ( ≈ 0.042 and 0.054, respectively) were almost one third of UA, attributed to volume fraction and size of the precipitated second-phase particles.
Similar content being viewed by others
Introduction
Aluminium alloy (AA)2024 (based on the Al-Cu-Mg system) has traditionally been one of the most utilised aeronautical alloys due to its balance of physical and mechanical properties. The microstructure of AA2024, typically used in the naturally aged condition (Τ3), consists of the Al-based solid solution matrix, as well as several constituent-type intermetallic particles (IMs) and dispersoids. Upon extended natural ageing and artificial ageing, S-phase (Al2CuMg) and its precursors S΄ and S΄΄, are the most common precipitated intermetallic particles in the microstructure of AA20241,2. Dispersoid particles such as Al6(Cu, Fe, Mn), Al7Cu2Fe and (Al, Cu)6Mn form in response to the low solubility of transition metal alloying elements, and do not respond to any thermal treatment. S-phase precursors (i.e., S΄ and S”) are considered as the dominant hardening phases3,4. Shearing of the precipitates by dislocations is not possible upon extended artificial ageing, i.e., in the over-aged (OA) condition, where coarsening and growth of precipitates takes place5. Additionally, the precipitates lose their coherency with the matrix and their surrounding strain fields decline, thus, leading to lower mechanical strength of the alloy. The size, shape and volume fraction of precipitates is evolving with appropriate heat-treatment and this kinetics affect the alloy’s mechanical behaviour6.
Among different approaches used in laboratory scale for the simulation of natural ageing of Al-alloys, artificial ageing heat-treatments are utilised to accelerate microstructural transformations7,8,9 and assess their effect on material’s properties10,11,12,13,14,15. A drastic effect of artificial ageing holding time and isothermal temperature on yield stress and elongation at fracture of AA2024-T3 was observed in prior work11. Additionally, a significant effect on hardness is referred by Astika12 and Ringer et al.16, showing that it is controlled by the type, size, and distribution of precipitates. Wang et al.13 found that both strength and ductility properties are influenced by the precipitation of S΄΄ and S’ phases and the subsequent decrease in dislocation density. In recent years, many researchers have studied the strengthening mechanisms of Al alloys during solution and ageing treatments17,18.
Several studies19,20 have shown that ageing may influence the corrosion behaviour of aluminium alloys due to occurrence of galvanic corrosion phenomena between the intermetallic phases—i.e., θ- and S-type in case of AA2024—and the matrix or the adjacent Cu-depleted particles. According to21, the precipitated phases continuously distributed along grain boundaries lead to intergranular corrosion (IGC) which is easily evolved into exfoliation corrosion (EXCO). EXCO is considered as a particular form of intergranular corrosion attack that occurs on the corroded surfaces of aircraft structures comprising of elongated grains22,23. The ASTM G34 standard24 defines EXCO as corrosion that proceeds laterally along planes parallel to the surface and forms voluminous corrosion products which induce internal stresses and force the metal apart. Susceptibility of AA2024 in IGC after artificial ageing heat-treatments (i.e., solution treating and re-ageing) was examined by Wang et al.25; S-phase precipitation on dislocation cell walls accelerated anodic dissolution of the cell interiors and led to aggregated pitting corrosion in over-re-aged samples. Higher Cu content in the matrix of the altered surface layer (ASL) of AA2024 resulted in less pitting corrosion susceptibility of the ASL when compared to the underlying substrate26. The role of microstructure on the tensile ductility decrease of pre-corroded specimens of AA2024 was also investigated in Alexopoulos et al.27; however, only one case of short time corrosion exposure was studied, i.e., 2 h EXCO exposure, since no significant pitting corrosion was evident at this time and the ductility decrease was ascribed to hydrogen embrittlement phenomenon. The corrosion-induced tensile ductility decrease at short exposure time (2 h EXCO) was correlated with the precipitation of S-phase particles, showing that the specimens at peak-aged (S΄΄and S΄ precipitates) and over-aged (S precipitates) conditions exhibited the lowest corrosion-induced decrease, while the highest ductility decrease (∼ 26 %) was noticed for the T3 condition where limited Guinier–Preston (GPB) zones on grain boundaries were present. Zhang and Frankel28 also studied the pitting and intergranular corrosion behaviour of various tempers of AA2024. Artificial ageing was found to have a strong effect on polarization curves and localized corrosion morphology of AA2024. Even though several studies dealing with the effect of artificial ageing on corrosion susceptibility of AA2024 started several years ago28,29,30,31,32, the crucial mechanisms of corrosion in different ageing tempers remain still unclear. Investigation of the corrosion behaviour on ageing aircrafts is of major importance since several studies33,34,35 on structural parts of old aircrafts (at the end of their service life, i.e., above 20 years) have shown that although the tensile mechanical properties (i.e., yield stress, elongation at fracture etc.) were not significantly influenced, high susceptibility to corrosion attack as well as intergranular corrosion fracture mechanism were observed on fractured components.
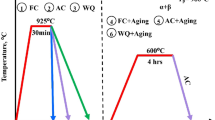
In the present work, the effect of artificial ageing on corrosion attack and its propagation on AA2024-T3 regarding tensile mechanical properties degradation was investigated. Since several aged aircrafts with AA2024 in their fuselage were found to be high susceptible to corrosion attack33,34,35, the investigation of corrosion mechanism on aged specimens of AA2024 is of major importance. Rectangular (for electrochemical and microstructural analysis) and tensile specimens of AA2024-T3 were artificially aged for different times, to simulate Under-Aged (UA), Peak-Aged (PA) and Over-Aged (OA) conditions. The artificial ageing time and temperature which corresponds to the above-mentioned different ageing tempers were selected from a relevant article of the authors27. Afterwards, exposure of the artificially aged specimens to exfoliation corrosion environment for different exposure times was performed to investigate the rate of corrosion-induced ductility degradation in different ageing tempers.
Results
Macroscopic evaluation of corroded specimens
Exposure of AA2024 specimens to the investigated corrosive environment (EXCO) resulted in deterioration of the specimens’ surface due to breakdown of the passive oxide layer19,20, and the subsequent nucleation of corrosion-induced surface pits which are shown with red arrows in Figs. 1–4 for the different artificial ageing conditions. For the case of T3 condition (refer to Fig. 1), pitting formation on the corroded surfaces remained rather limited for short exposure times and up to 2 h, e.g., Fig. 1a,b. With increasing exposure time to EXCO solution, an increase in pitting density was evident. Localized corrosion in the form of randomly dispersed pits (black signs shown with red arrows) was evident for all exposure times while exfoliated layers noticed after 24 hours (refers to Fig. 1c). Additionally, salt crystals with a white coloration were evident above pits in high exposure times, as it is shown with blue arrows in Fig. 1c.
The effect of corrosion seems to be more intense in AA2024-T3 + 2 h@190°C specimens, simulating under-aged (UA) condition, regarding surface deterioration. Notable differences in the surfaces of pre-corroded specimens were noticed with increasing exposure time; for short exposure times pitting corrosion (shown in red arrows) remained limited, e.g., Fig. 2a, while slight increase in pitting density as well as in the surface roughness and accumulation of salt crystals (shown in blue arrows) was evident on the surface of specimens corroded for 2 h, e.g., Fig. 2b. Severe localized corrosion was noticed for the AA2024-T3 + 2 h@190°C specimens after 24 h exposure time, which evolved to exfoliation corrosion as it shown in specific sites (highlighted with green arrows) of the surface in Fig. 2c. Corrosion attack in this temper seems to propagate intergranularly.
Regarding specimens of AA2024-T3 + 8 h@190°C simulating peak-aged condition of AA2024-T3 (refer to Fig. 3), and AA2024-T3 + 63 h@190°C simulating over-aged condition (refer to Fig. 4), no significant differences in surface deterioration were noticed with increasing exposure time to EXCO solution and especially after 2 h exposure time. Pitting corrosion remained limited for the very short exposure times (i.e., 0.5 h) as was the case for T3 and AA2024-T3 + 2 h@190°C specimens. Nevertheless, increased volume fraction of pitting was noticed for higher exposure times, e.g., Figs. 3b and 4b. Further corrosion exposure (up to 24 h) did not affect the surface of pre-corroded specimens significantly when compared to 2 h. Corrosion morphology—characterized by uniformly/smoothly dispersed pits throughout exposed surface (no severe localized corrosion around specific areas)—was almost the same for the specimens exposed to EXCO solution for 2 (Figs. 3b, 4b), and 24 h (Figs. 3c, 4c) for both ageing conditions, respectively. The whole surface of the specimens was exfoliated after long exposure times, instead of specific sites in the case of under-aged specimens. This can be attributed to the higher density of precipitates which are more uniformly distributed throughout specimen’s surfaces as was also shown in previous studies regarding this material36,37,38,39. Increase in the volume fraction of precipitates with increasing ageing time was also observed in38, where cluster of Cu and Mg elements in solid solution state (before ageing) was increased from 0.02 vol.% to 0.88 vol.% and 1.21 vol.% for 8 h and 24 h at 190 °C, respectively. Furthermore, after 8 h artificial ageing (peak-aged) the volume fraction of GPB zones was ~0.86 vol.%, while after 24 h (over-aged) it was decreased to 0.17 vol.% GPB zones and simultaneously the volume fraction of S’ phase increased to 1.02 vol.%38. The extended ageing times, along with the induced increase in second-phase precipitates’ volume fraction, leads to increased pitting rates, thus making the alloy more susceptible to corrosion attack36,37. Nevertheless, second-phase precipitates up to 3 nm width value are considered as tolerable from a corrosion perspective according to Ralston et al.37. More uniform surface deterioration at higher ageing times (e.g., peak- and over-aged) was also noticed in a previous paper of the authors27.
It seems that not only the corrosion propagation mechanism but also the macroscopic corrosion morphology, such as width of pits as well as depth of cracks, affects the tensile properties as was also shown in previous publications of the authors40,41.
Electrochemical impedance measurements
Electrochemical impedance spectroscopy (EIS) results for the different artificial ageing conditions of AA2024-T3, as a function of immersion time in 3.5 wt. % NaCl solution, are shown in the form of Bode diagrams in Fig. 5a to Fig. 5d, respectively. Figure 5a presents the results for the as-received T3 temper where two-time constants are evident at low (10-2-10-1) and medium (100-103) frequencies regime after above 1 h of immersion. In contrast, at the initial period of immersion (e.g., 5 min represented by black curve) the relaxation process at higher frequencies being responsible for the response from the passive film can also be evidenced. Time constants in medium frequencies are related to charge transfer processes coupled to the double layer capacitance, while the time constant at low frequencies is likely to be associated to a diffusion-control. Slight fluctuations of the curves in 1 h and 3 h at low frequencies regime can be caused by non-stationarities of the system at this period resulted from the metastable pitting formation. After 6 h of exposure, the pits seem to become more stable leading to much less scattering of the low-frequency impedance values. The same behaviour was noticed for AA2024-T3 + 2 h@190 °C specimens; nevertheless, stable pits formed slightly faster than in T3 temper, as can be seen in Fig. 5b. Regarding AA2024-T3 + 8 h@190°C and AA2024-T3 + 63 h@190 °C specimens, formation of stable pits seems to become faster with increasing artificial ageing time.
Electrochemical impedance spectroscopy results of AA2024-T3 specimens measured in 3.5 wt. % NaCl solution are provided in the form of Bode figures for the as-received condition in (a), for 2 h @ 190 °C (UA) in (b), for 8 h @ 190 °C (PA) in (c) and for 63 h @ 190 °C (OA) in (d). The left figure presents the phase angle as a function of frequency and the right figure presents the impedance modulus as a function of frequency. The different coloured curves represent the various exposure times to the electrolyte after which each measurement was performed.
Artificial ageing induced microstructural changes, such as increased volume fraction of second phase precipitates38, leads to variations in corrosion susceptibility36,37 due to the different electrochemical corrosion potential of each phase and the Al-matrix or adjacent phases and depleted zones39. Differences in corrosion potential between the different intermetallic compounds not only enhance galvanic corrosion but also affect the corrosion mechanism of the alloy42,43. Regarding AA2024, in which S phase precipitates are the main strengthening phases, the corrosion mechanism is described by the dissolution of Al2CuMg (Ecorr = -883 mVsce at 0.1 M NaCl) at the initial stages of corrosion attack due to the selective dissolution of the high active elements of Al and Mg. Thus, a Cu-rich layer with a porous structure is remained. Dissolution of Al2CuMg was found to be manifested by dealloying and this accounts for its high corrosion rate as well as retainment of its shape during corrosion attack44. The dealloying kinetics were found to be accelerated at high temperature in NaCl solution45. Cu enrichment of the intermetallics surface results to an increase in corrosion potential (shift to more noble values), which can be higher than the respective of the Al- matrix (Ecorr = -602 mVsce at 0.1 M NaCl), thus reversing the galvanic relationship between Al2CuMg precipitate and the matrix, leading to trenching around phases.
Impedance modulus of specimens in T3 condition slightly increase for short exposure times and up to 3 h at low frequencies regime (10-2-10-1) followed by a gradual decreasing trend for higher exposure times, e.g., Fig. 5a. Essential decrease was noticed between 6 and 12 h of exposure, while further corrosion exposure did not found to reduce impedance modulus essentially since by that time stable pits are formed and the corrosion process is controlled by the diffusion limited cathodic oxygen reduction as well as reduction of protons at more negative potentials. Formation of voluminous corrosion products after long exposure times seal the pits and impede transport to/from the active areas. As for the AA2024-T3 + 2 h@190°C specimens (resembling UA temper) shown in Fig. 5b, a similar behaviour was observed. The main difference is that the stabilization of the pits occurred after shorter time. The measurement performed after 3 h demonstrated a clear drop of low frequency impedance value and absence of data scattering related to the metastable pits. Regarding AA2024-T3 + 8 h@190°C specimens (refer to Fig. 5c), the impedance modulus at low frequencies almost remained unaffected by corrosion for short exposure times and up to 3 h. It is also important that the disappearance of the time constant related with the oxide film takes significantly longer in this case. Additionally, the impedance modulus at low frequencies was higher in this condition even after the highest exposure time (e.g., 48 h) in comparison to the other systems. In AA2024-T3 + 63 h@190°C specimens fluctuations were noticed, and only slight decrease of the impedance modulus was observed for the time range between 6–48 h of exposure.
To gain a better insight into corrosion mechanisms, EIS response was modelled using equivalent electrical circuits, as shown in Fig. 6. The R1 element in circuit model represents the resistance of electrolyte. The pair R2//Q1 stands for the polarization resistance, i.e., the resistance of electron flow through double layer, in parallel with capacitance of double layer. A constant phase element (CPE—that was denoted with Q in the present work—was used instead of an ideal capacitor to take into consideration the heterogeneity of the surface, as already mentioned in literature, e.g. refs. 46, and47. W element refers to Warburg impedance which is ascribed to the response at low frequencies being controlled by diffusion processes. It is worth mentioning that for short exposure times in PA condition (AA2024-T3 + 8 h@190°C) another equivalent circuit model showed good fitting. The model included an extra constant phase element (R//Q) that corresponds to oxide film resistance in parallel with conductive pathways associated with defective sites created by intermetallic particles (IMCs). Nevertheless, the simple electrical circuit model that better simulates most of the investigated cases used in this research work to simulate corrosion mechanism in AA2024-T3 specimens exposed to different ageing heat-treatments.
The charge transfer resistance RCT (R2 in the present model configuration) values with increasing immersion time are depicted in Fig. 7. Charge transfer resistance was found to decrease even from the early stages of corrosion for AA2024-T3 + 2 h@190°C specimens (UA) while this was not the case for the other three investigated conditions. The lower values of resistance noticed for AA2024-T3 + 2 h@190°C specimens throughout immersion time range indicate fast charge transfer on aluminium surface. A notable increase of RCT was noticed for the first hours of exposure in T3 temper, followed by a significant sudden drop to lower values that almost reached the respective of AA2024-T3 + 2 h@190°C specimens after 6 h of immersion. However, for higher exposure times the charge transfer resistance was almost stabilized for both ageing conditions. Regarding, AA2024-T3 + 8 h@190°C (simulating PA) and AA2024-T3 + 63 h@190°C (simulating OA) specimens an increasing trend was evident in the first hours of immersion, followed by a slight decreasing trend after 3 h. No significant alterations in the charge transfer resistance were evident for higher immersion times while the higher values of RCT revealed that electron flow through double layer is slower in more aged specimens.
NaCl solution for AA2024-T3 exposed to different artificial ageing conditions. The values of RCT derived from the R2 of the equivalent electrical circuit model that better fitted the EIS results. The values with the lowest error (< 10 %) were selected from at least three specimens. Each different curve in the figure simulates the different ageing conditions named as under-aged, peak-aged and over-aged.
Mechanical evaluation of pre-corroded specimens
Typical tensile flow curves of the pre-corroded to an EXCO solution specimens of AA2024-T3 in different ageing conditions are presented in Fig. 8. The nominal stress calculation was based on the nominal cross-section of the tensile specimens; namely, (width x thickness) = (12.5 mm x 3.2 mm), respectively. Prior exposure of AA2024-T3 to the EXCO solution resulted in significant modifications to the overall tensile mechanical properties of the alloy. However, the corrosion-induced modifications were different for each artificial ageing condition of the alloy. The effect of corrosion was more severe in T3 and under-aged (AA2024-T3 + 2 h@190°C) conditions, refer to Fig. 8a and b, respectively. A significant decrease of the axial nominal strain was noticed after only 0.5 h of corrosion exposure in T3 condition, shown in Fig. 8a. With increasing corrosion exposure time, the axial nominal strain was further decreased; almost half value of the initial property value was noticed after 24 h corrosion exposure time. On the contrary, the axial nominal stress was less decreased (lower degradation between corrosion times) even after the highest exposure time, i.e., 24 h, in T3 condition.
The σ-ε flow curves for different exposure times in a as-received, b under-aged, c peak-aged and d over-aged conditions are presented. They were evaluated from the tension tests. The nominal stress calculation was based on the nominal cross-section of the tensile specimens, namely width x thickness = 12.5 mm x 3.2 mm. Each different coloured curve corresponds to different pre-exposure time of specimens to EXCO solution.
Several differences in the overall tensile mechanical behaviour were noticed for the pre-corroded AA2024-T3 + 2 h@190°C specimens (Fig. 8b). Axial nominal strain was not notably decreased for the very short exposure times, i.e., 0.5 h where hydrogen embrittlement is the dominant degradation mechanism41,48, while after 2 h of corrosion exposure, where pitting corrosion was evident on the specimen’s surface as shown in Fig. 2b, a sudden drop of both the axial nominal strain and the axial nominal stress was noticed. The significant stress drop noticed in this alloy is attributed to the decrease in the effective thickness due to micro-cracking propagation, as referred by Alexopoulos and Papanikos49. Further increase of corrosion exposure time was found to decrease the tensile mechanical properties of AA2024-T3 + 2 h@190°C specimens significantly.
Regarding AA2024-T3 + 8 h@190°C (PA) and AA2024-T3 + 63 h@190°C (OA) specimens—refer to Fig. 8c and Fig. 8d, respectively—both the axial nominal strain and the axial nominal stress showed a decreasing trend with corrosion propagation but in lower rate when compared to T3 and AA2024-T3 + 2 h@190°C (UA) specimens. The slight decrease of stress even after the highest exposure time (i.e., 24 h) revealed that micro-cracks propagate in lower rates in higher artificial ageing times, since they are blocked by the precipitations formed after ageing.
The limited decrease of the axial nominal stress in T3 condition can be attributed to the intergranular corrosion attack (IGC) and propagation as also referred in50; on the contrary, the artificially aged specimens have probably exhibited a mixed mode of intergranular and transgranular corrosion attack due to increased volume fraction of second-phase precipitates even inside grains.
The effect of ageing conditions on corrosion propagation
Figure 9a summarizes the conventional yield stress Rp0.2% (based on the nominal cross-section of the specimens) results for the pre-corroded—for different exposure times to EXCO solution—specimens in different ageing conditions. Marked in diagram are the regions of under-, peak- and over-aged conditions as a function of ageing time with different background colours. Each curve in the diagram represents the propagation of Rp0.2% with increasing ageing time for the different pre-exposed specimens. It is obvious that with increasing artificial ageing time, Rp0.2% tends to increase up to its maximum value in the peak-aged condition; further increase of ageing time (OA condition) leads to Rp0.2% decrease due to the growth and coarsening of the precipitates5,51. Regarding the effect of artificial ageing time on corrosion-induced degradation of Rp0.2%, it was evident that the highest decrease due to corrosion exposure took place for AA2024-T3 + 2 h@190°C; approximately 23% degradation was observed after 24 h of exposure. The corrosion-induced degradation of Rp0.2% is more severe in AA2024-T3 + 2 h@190 °C specimens, where the highest difference in the property values between the different exposure times was noticed.
In figure 9a the conventional yield stress Rp0.2% is provided, while figure 9b shows the respective tensile elongation at fracture Af results. The mechanical properties are presented as average values of at least three different specimens for each case, as a function of artificial ageing time. The standard deviation of the measurements is also included. The different coloured background corresponds to the regions of different ageing conditions while the different coloured curves correspond to different corrosion exposure times.
On the contrary, the less corrosion-induced decrease of conventional yield stress between the different corrosion exposure times was noticed in T3 as well as in AA2024-T3 + 63 h@190°C specimens. Nevertheless, significant degradation of Rp0.2% due to corrosion exposure was also noticed in T3 temper but the respective degradation tends to be eliminated with increasing exposure time. For instance, the non-artificially aged (T3 condition) and non-corroded material (shown in black squares and black solid line) exhibited ~15% decrease of the conventional yield stress after only 0.5 h corrosion exposure time (decrease from 387 MPa to 330 MPa)27. Further increase of corrosion exposure time did not decrease the Rp0.2% significantly, since after the highest exposure time (e.g., 24 h) the conventional yield stress was ~320 MPa (decreased by almost 18 % from the initial value and only 3 % from the first corrosion exposure time). As the logarithmic scale was used to express the artificial ageing time in hours, it is mathematically impossible to illustrate the reference condition T3 without any artificial ageing time (zero hours); to cope with this problem a very small value of 0.015 h was used in all figures to address the reference specimens without any kind of artificial ageing heat-treatment.
The respective results for elongation at fracture Af are presented in Fig. 9b. As expected, the essential yield stress increase in AA2024-T3 + 8 h@190°C specimens was followed by a ductility decrease where elongation at fracture Af reached to a minimum value. A different corrosion-induced degradation mechanism was noticed for the elongation at fracture in different artificial ageing conditions, when compared to the conventional yield stress. As for the case of Rp0.2%, the specimens of AA2024-T3 + 2 h@190°C exhibited the highest Af degradation due to corrosion exposure, e.g., -51 % after 24 h of exposure. Additionally, significant Af decrease was evident for the T3 condition, where higher degradation between the different corrosion exposure times was noticed for the short corrosion exposure times and up to 4 h. In this case, the less effect of corrosion exposure was evident in AA2024-T3 + 8 h@190°C condition followed by AA2024-T3 + 63 h@190°C, with the highest decrease of Af to be -21 % and -26 % after 24 h of exposure, respectively.
Fractographic analysis
The tensile test results revealed that corrosion-induced mechanical properties decrease (i.e., Rp0.2% and Af) is artificial-ageing sensitive; hence, the corrosion-induced degradation mechanism depends on artificial-ageing condition of the alloy. In order to investigate the possible phenomena related to the behaviour noticed in tensile tests, pre-corroded fractured specimens from under-, peak- and over-aged conditions were examined with the aid of stereoscope (refer to Figs. 10–13) and scanning electron microscope (SEM) (refer to Figs. 14–16). Stereoscopical analysis revealed an increasing surface deterioration, by means of pitting density and size, with increasing EXCO time in T3 as well as in AA2024-T3 + 2 h@190°C conditions, shown in Figs. 10 and 11, respectively. Furthermore, a decrease in ductility with increasing exposure time was evident in these conditions from the small side-surfaces, where the presence of necking was eliminated. On the contrary, regarding AA2024-T3 + 8 h@190°C (PA) (Fig. 12) and AA2024-T3 + 63 h@190°C (OA) (Fig. 13) specimens no essential difference in the surface corrosion morphology was observed with increasing corrosion exposure time, while these specimens seemed to maintain their ductility even after long exposure times where the necking phenomenon was still evident.
The surfaces of pre-corroded tensile specimens after tension testing are presented in macroscopic scale. The different exposure times to exfoliation corrosion solution are depicted in different images, i.e., the 0.5 h is provided in (a), the 2 h in (b), the 4 h in (c) and the 24 h in (d), respectively. Upper images show the upper (big) flat surfaces of tensile specimens, and the bottom images show the small side-surfaces (thickness).
The surfaces of pre-corroded tensile specimens after tension testing are presented in macroscopic scale. The different exposure times to exfoliation corrosion solution are depicted in different images, i.e., the 0.5 h is provided in (a), the 2 h in (b), the 4 h in (c) and the 24 h in (d), respectively. Upper images show the upper (big) flat surfaces of tensile specimens, and the bottom images show the small side-surfaces (thickness).
The surfaces of pre-corroded tensile specimens after tension testing are presented in macroscopic scale. The different exposure times to exfoliation corrosion solution are depicted in different images, i.e., the 0.5 h is provided in (a), the 2 h in (b), the 4 h in (c) and the 24 h in (d), respectively. Upper images show the upper (big) flat surfaces of tensile specimens, and the bottom images show the small side-surfaces (thickness).
The surfaces of pre-corroded tensile specimens after tension testing are presented in macroscopic scale. The different exposure times to exfoliation corrosion solution are depicted in different images, i.e., the 0.5 h is provided in (a), the 2 h in (b), the 4 h in (c) and the 24 h in (d), respectively. Upper images show the upper (big) flat surfaces of tensile specimens, and the bottom images show the small side-surfaces (thickness).
Figure 14 shows SEM pictures from the pre-corroded AA2024-T3 + 2 h@190°C specimens (simulating under-aged condition) after tensile testing. The right column of the figure provides a general view of the fractured specimens for 0.5, 4, 24 h of corrosion exposure (in different rows) where a ductility-based fracture mechanism is evident from the 45° inclination of the fracture surface. It was noticed that pitting density in the large, exposed surfaces (L/LT direction) of the specimens was increased with increasing exposure time, leading to exfoliation after 24 h. Regarding fracture surface shown in the left column of the figure, it is evident that the specimen exposed to corrosion for 0.5 h was characterized by several dimples throughout the fractured surface interrupted by some smooth surfaces near the edge of the specimen’s fractured surface. With increasing exposure time, the smooth surfaces were expanded to the specimen’s thickness along with formation of micro-cracks up to a maximum depth shown for 4 and 24 h. Additionally, fracture resembling cup and cone geometry was noticed after 24 h of corrosion exposure.
Figure 15 presents the SEM images of the fractured AA2024-T3 + 8 h@190°C specimens. Right column provides the general view of the fractured specimens corroded for 0.5, 4, 24 h (in different rows) where a ductility-based fracture mechanism is still evident from the 45° inclination of the fracture surface up to the highest exposure time. An increase in pitting density on the large, exposed surfaces (L/LT direction) was evident with increasing exposure time. The fractured surfaces of the AA2024-T3 + 8 h@190°C specimens were characterized by dimples for all the investigated exposure times. Additionally, the existence of some smooth areas next to the exposed surface was evident; nevertheless, these areas expand to a less extend into the specimen’s thickness than in the case of AA2024-T3 + 2 h@190°C specimens. Furthermore, there was no notable crack propagation along the thickness of the specimens—as was the case for AA2024-T3 + 2 h@190°C specimens—that could reduce the specimens’ effective thickness significantly. This observation is in accordance with the tensile test results, where the yield stress was not essentially reduced by corrosion propagation for the AA2024-T3 + 8 h@190 °C specimens. Regarding AA2024-T3 + 63 h@190°C specimens shown in Fig. 16, the fractured surfaces were characterized by large smooth surfaces with the dimples to be concentrated to the middle thickness. There were no notable differences between the different exposure times, meaning that fracture mechanism was not essentially altered by corrosion propagation. Cracking formation was obvious, but the cracks did not propagate deep into the mid-thickness of the material sheet.
Discussion
To justify the observations from the fractographic analysis, a mechanical model was exploited to calculate the “effective thickness” of the specimens exposed for different times to EXCO solution. The “effective thickness” concept was introduced in a previous publication of the authors41 and refers to the cross-section (and hence to the thickness of the specimen) that remains unaffected from corrosion exposure, as the surface pits and micro-cracks essentially decrease the loading capability of the cross-section. The model was introduced in the previous article and is referred as iso-modulus of elasticity based on the concept that the modulus of elasticity of the unaffected “non-corroded” material remains the same with the reference material. To this end, it utilizes the decrease of the tensile modulus of elasticity E of pre-corroded specimens to address the effective thickness calculation problem. It is well known that corrosion-induced degradation of AA2024 is attributed to the synergistic effect of micro-cracking formation (and subsequent propagation) and hydrogen embrittlement phenomenon, e.g.,41,52,53,54. Pitting/micro-cracks are considered as preferential pathways for the diffusion of hydrogen (produced during redox reaction) from the corroded surface into the inner layers of the material, causing hydrogen embrittlement on the aluminium matrix just below the pit/crack edge. Nevertheless, mechanical properties degradation on pre-corroded specimens is also found even in cases where no significant cracking formation is noticed, i.e., in short corrosion exposure times. In41 a synergistic effect of micro-cracking and hydrogen embrittlement was found on AA2024, with the total decrease in tensile ductility to be attributed to two different mechanisms: the first one consisted of the ¼ reduction of total elongation at fracture due to hydrogen embrittlement, while the rest ¾ was attributed to micro-crack formation and propagation. The synergistic mechanisms were experimentally derived from the plots of tensile ductility over depth of attack (cracks) and hydrogen embrittlement calculated to the case of zero depth of cracking. In case that hydrogen embrittlement phenomenon is not taken into consideration (as it is difficult to quantify), then the change in the slope of the nominal stress-strain tensile curves in the elastic region is attributed to surface-cracking phenomena. Micro-cracking leads to reduction of the effective thickness of the specimen, according to Alexopoulos et al.41. It is thought that the true value of E of the specimen’s effective thickness is independent of corrosion exposure time as it is supposed to be unaffected by corrosion. However, for the assumptions of the proposed model the modulus of elasticity depends solely on the effective thickness of specimens and not to other phenomena which may influence the material’s property, such as loss of surface-elongated grains with increased work-hardening capability. Thus, the decrease in effective thickness calculated by the following equation:
The results derived from the above-mentioned mechanical model are shown in Fig. 17, where it is obvious that the specimens in AA2024-T3 + 2 h@190 °C (UA) exhibited the higher decrease of the effective thickness while lower degradation was noticed for specimens in T3 and AA2024-T3 + 8 h@190 °C (PA) conditions. There was no significant difference of the effective thickness of AA2024-T3 + 2 h@190 °C and AA2024-T3 + 63 h@190 °C (OA) specimens up to 0.5 h corrosion exposure time, while after 2 h of exposure specimens of AA2024-T3 + 63 h@190°C exhibited slightly higher decrease of the effective thickness, probably due to easier bypassing of precipitates from the dislocations coming from micro-cracks. Nevertheless, for long exposure times and after 4 h corrosion exposure time the effective thickness of AA2024-T3 + 63 h@190 °C specimens reached a plateau while for the case of AA2024-T3 + 2 h@190 °C specimens’ effective thickness was continuously reduced.
The specimens’ thickness carrying the mechanical load, i.e. “effective thickness”, was calculated using the “iso-modulus of elasticity” model and is presented as a function of increasing exposure time to EXCO solution. The average values of modulus of elasticity after different corrosion exposure times were calculated for each ageing condition and was used as an input for the model. The standard deviation of the values was also calculated and included in figure as error bars.
Figure 18 summarises the respective results of conventional yield stress and elongation at fracture as in Fig. 9 shown in a different way, i.e., as a function of corrosion exposure time instead of artificial ageing time, in order to highlight the corrosion-induced degradation in different ageing conditions of AA2024-T3. Marked in diagram are the regions of short exposure times (grey background) and long exposure times (yellow background) as were arbitrarily selected from the authors in the regions where the slope of the degradation curve was changed. It is evident from Fig. 18a that the AA2024-T3 + 2 h@190°C (UA) specimens exhibited the highest corrosion-induced degradation, with the Rp0.2% to be significantly decreased even after the early stages of corrosion exposure time (i.e., 0.5 h); the property was continuously decreasing up to 24 h of corrosion exposure reaching to lower values than the respective in T3 condition. For the case of T3 condition the respective degradation was lower for short exposure times while for long corrosion exposure times (> 4 h) the conventional yield stress was stabilized probably because the micro-cracks reached their maximum depth as was noticed in Alexopoulos et al.55. and Charalampidou et al.56. for the pre-corroded specimens of AA2024 with 0.4 mm and 1.6 mm nominal thickness, respectively. Regarding AA2024-T3 + 8 h@190°C (PA) and AA2024-T3 + 63 h@190°C (OA) specimens, a notable decrease of Rp0.2% due to corrosion exposure was evident for up to 4 h. Nevertheless, the respective degradation percentage of Rp0.2% with increasing corrosion exposure time was significantly lower when compared against AA2024-T3 + 2 h@190°C and T3 specimens. Additionally, as was the case for T3 temper, the Rp0.2% tend to be stabilized in long corrosion exposure times.
Figure (a) shows the conventional yield stress Rp0.2% and figure (b) the elongation at fracture Af values for each ageing temper and at different exposure time to EXCO solution. Each point in the curves represents the average value of the respective property which was extracted from at least three specimens of AA2024-T3 of each artificially aged condition and in different exposure time to EXCO. The standard deviation of the values was also calculated and included in the figure as error bars. The equation of the fitting curve is also included in the figure.
As for the case of elongation at fracture Af the highest corrosion-induced degradation was noticed in AA2024-T3 + 2 h@190°C specimens followed by T3 (refer to Fig. 18b. Significant degradation of the property was evident even from the short exposure times (i.e., 0.5 h), where no essential surface pitting was evident (refer to Figs. 1–4), that is attributed to hydrogen embrittlement phenomenon48,57. Elongation at fracture was found to continuously decrease up to the highest corrosion exposure time (i.e., 24 h) for both, AA2024-T3 + 2 h@190°C and T3 conditions, while for AA2024-T3 + 8 h@190°C and AA2024-T3 + 63 h@190°C specimens the Af seemed not to be notably affected by corrosion for the whole exposure time range. Less but not least, it should be referred that the curve fitting used in Fig. 18,—that better simulates the corrosion-induced degradation of the mechanical properties—was the exponential linear fitting and its mathematical type was included in the figures. Nevertheless, for the convenience of the readers, logarithmic diagrams were also used to present the corrosion-induced degradation rate factor of the mechanical properties that was calculated by the slope of degradation curves as shown in Fig. 19b. It is obvious from Fig. 19 that the slope of degradation curves is higher in AA2024-T3 + 2 h@190°C condition for both, investigated mechanical properties (Rp0.2% and Af) followed by T3 temper. The calculated slope values are summarized in Table 1 where it was noticed that the AA2024-T3 + 2 h@190°C specimens exhibited the highest Af degradation rate factor (i.e., −0.11804), while the AA2024-T3 + 8 h@190°C specimens exhibited the lowest Af degradation rate factor of approximately − 0.042.
The average values of the conventional yield stress Rp0.2% shown in (a) and elongation at fracture Af shown in figure (b) as calculated in Fig. 18 are presented in logarithmic scale. The standard deviation of the values was also calculated and included in the figure as error bars. The equation of the fitting curve is also included in the figure.
Thus, it seems that even if the increase in precipitates in long ageing times accelerates electrochemical kinetics45 and enhances corrosion susceptibility, precipitates formed in PA condition (AA2024-T3 + 8 h@190°C) impede corrosion propagation since they block micro-cracks movement and therefore decrease the degradation rate of the mechanical properties of the alloy. However, it should be mentioned that the present results may overestimate the alloy’s degradation, regarding both the mechanical properties and corrosion mechanism, since accelerated experimental methods are used for the simulation of the technological problem. For instance, in the work of Prudhomme et al.58, where a comparison of the mechanical properties of AA2024 from a decommissioned A320 aircraft (wing panels) with an artificially aged AA2024-T351 was performed, 55 h at 150 °C were found to correspond to 10,000 h exposure to 80 °C during service life of an aircraft. Nevertheless, the obtained results contribute to the understanding of degradation kinetics influenced by the heat-treatments and can also be used as a mean for comparison of different ageing conditions.
Summarizing, it was noticed that specimens in T3 as well as at early stages of artificial ageing, e.g., under-aged (UA) condition, exhibited intense localized corrosion attack and high corrosion propagation rates. Nucleation and growth of metastable S-phase precipitates enhances corrosion susceptibility due to differences in the electrochemical potential between the second-phase precipitates and Al-matrix, thus leading to localized corrosion attack. On the contrary, less intense corrosion damage was noticed in peak-aged (PA) and over-aged (OA) conditions, with more uniform distribution of pits on the exposed surfaces. Specimens in PA and OA condition were less affected by corrosion propagation among the investigated conditions, in terms of electrochemical kinetics and tensile elongation at fracture Af degradation rate ( ≈ 0.042) and ( ≈ 0.054), respectively, due to the fine dispersion of precipitates. The following results are concluded in terms of higher to minimum corrosion-induced degradation rate for the different investigated heat treatment conditions of AA2024. These degradation rates were attributed to the volume fraction and size of the precipitated second-phase particles:
under-aged condition > initial (T3) condition > over-aged condition > peak-aged condition.
Methods
Materials
The material used in the current research work was the aeronautical aluminium alloy 2024 in T3 condition, which was received in 3.2 mm nominal thickness. The chemical composition of AA2024 according to the sheet manufacturer is a mixture of the following elements as percentage by weight; 4.35% Cu, 1.50% Mg, 0.64% Mn, 0.50% Si, 0.50% Fe, 0.25% Zn, 0.15% Ti, 0.10% Cr, 92.01% Al. T3 condition includes solution heat-treatment at 495 °C, quenching in water at 0 °C, and natural age in room temperature (25 °C) to a substantially stable condition. Tensile as well as small rectangular metallographic specimens were machined from the longitudinal (L) rolling direction of the sheet in accordance with ASTM E8 specification59. The specimens were cut from the aluminium sheets by exploiting a waterjet machine in order to avoid any temperature increase on the material that will influence natural ageing and the precipitates formation. Afterwards, the specimens were cleaned with alcohol and were ground with different SiC papers to meet the standard guidelines for tensile specimens’ preparation. The geometrical dimensions of the tensile specimens were 12.5 mm x 3.2 mm at the reduced cross section, with a total length of 190 mm, while the respective dimensions for the small rectangular metallographic specimens were 10 mm x 20 mm x 3.2 mm.
Preparation
Exfoliation corrosion (EXCO) solution was used for the exposure of tensile specimens, in order to investigate the corrosion-induced degradation of tensile mechanical properties in different artificially aged specimens. It consists of ~ 4.0 M sodium-chloride (NaCl), 0.5 M potassium-nitrate (KNO3) and 0.1 M nitric acid (HNO3). Solution preparation started with the addition of 234 g sodium chloride (reagent grade NaCl with 99.0 % purity), 50 g of potassium nitrate (reagent grade KNO3 with 99 % purity) and an additional 6.3 ml of 70 % concentrated nitric acid (HNO3) to 1 Liter of distilled water, in accordance with ASTM G34 standard24. Solution volume was calculated per exposure area of the specimens; 20 ml/cm2 was selected for all specimens, in accordance with ASTM G34 standard. The selected area of exposure was 55 ± 0.01 mm lengthwise and 12.5 ± 0.01 mm widthwise within the reduced cross-section of tensile specimens to ensure fracture within gauge length. Additionally, masking of the side-surfaces (L/ST) was performed in order to eliminate the effect of side-surfaces micro-cracking in ductility (elongation at fracture) decrease, where according to Charalampidou et al.56 the side-surfaces attack has a contribution percentage to the total ductility decrease of > 60 %, at short exposure times to EXCO solution. Furthermore, this kind of exposure better simulates the exfoliation corrosion in 2xxx and 7xxx aeronautical alloys24.
For the investigation of corrosion initiation and propagation mechanisms on different ageing conditions of AA2024-T3, electrochemical impedance spectroscopy (EIS) measurements were performed in naturally aerated 3.5 wt. % NaCl solution according to ASTM G44 standard60. Solution preparation included the addition of 35 gr of NaCl to 1 Liter of distilled water in room temperature. The 3.5 wt. % NaCl solution was used for these tests since the in-service obtained corrosion damage correlates well to the one caused by this solution61. Additionally, this solution is milder than EXCO and leads to smoother transition between the different electrochemical kinetics, making them more easily defined.
Prior to EXCO solution exposure, the side-surfaces of tensile specimens (L/ST) as well as the upper surfaces of the small rectangular specimens (L/LT, surfaces to be exposed) were polished up according to ASTM E8 specification59. Further preparation of the tensile specimens included shielding of specimens’ surfaces so that only specific areas of interest were exposed. The specimens were masked with appropriate insulating PVC tape (with excellent adherence) to avoid crevice corrosion beneath the masking tape. Afterwards, the specimens’ surfaces (both tensile and rectangular) were cleaned with alcohol according to ASTM G1 specification62.
Natural ageing of AA2024-T3 on aircraft structures was simulated by performing isothermal artificial ageing heat-treatment at 190 °C in the as-received condition, i.e., T3. The ageing temperature of 190 °C was selected as an intermediate heat-treatment temperature for artificial ageing of AA2024-T3 (range varies from 170 °C to 210 °C) according to the study of Alexopoulos et al.11, where it was shown that essentially no prolonged ageing times, i.e., below 10 h, to attain the peak-aged (PA) condition. Yield stress continuously increased with ageing time up to 8 h. This increase in yield stress is characterized as under-aged (UA) region. A plateau in yield stress was noticed from 6 h up to 12 h ageing time, which is typical of PA condition. Finally, by further increasing the artificial ageing time to 63 h at 190 °C an essential decrease in yield stress was observed due to coarsening of the precipitates, so that this heat-treatment condition is considered to belong to the over-aged (OA) region. To this end, the following three (3) different artificial ageing times were selected for the present study, namely 2, 8, and 63 h corresponding to the three different investigated (UA, PA, and OA) conditions, respectively. The specimens of AA2024-T3 were prior isothermally artificially aged in an electric oven Elvem T101 (2600 W) with ± 0.1 °C temperature control at 190 °C and subsequently exposed to corrosion exposure (refer to Table 2), to investigate the corrosion mechanism in different artificial ageing conditions. After corrosion exposure, the specimens were removed from the containers and cleaned with acetone before performing the tensile tests.
Electrochemical measurements
The corrosion behaviour of AA2024-T3 in different ageing conditions was assessed by electrochemical impedance spectroscopy (EIS) tests, which were carried out using a PalmSens® potentiostat. The experiments were carried out in naturally aerated 3.5 wt. % NaCl solution at room temperature (25 ± 1) °C. A typical three electrodes cell system that was composed of a platinum electrode as counter electrode, a silver chloride electrode Ag/AgCl as reference electrode and the small rectangular specimens of the investigated aluminium alloy as working electrodes was used. A surface area of 0.5 cm2 of the working electrode was exposed to the electrolyte. The voltage perturbation range used in the EIS measurements was 10 mV (rms), and the acquisition rate was 10 points per decade in a frequency range from 105 Hz down to 0.01 Hz. The measurements were repeated at fixed intervals of 0 (after 5 min immersion), 1, 3, 6, 12, 24, and 48 h of immersion time. Each test was performed at least three times to evaluate the reproducibility of the results. The obtained data was fitted employing a PStrace5® analysis software.
Tensile mechanical tests
The tensile properties of the specimens were evaluated after testing in an INSTRON 8801 Testing Machine. The software was programmed to apply a tensile strain to the specimen—under crosshead displacement control—at a rate of 0.7 mm/min. The relevant specimen dimensions (i.e., thickness t0, width b0 and gauge length L0) were measured and programmed into software. Thereafter, specimens were individually installed into the machine’s grips, ensuring good alignment with the loading axis. An extensometer of 50 mm initial gauge length was installed onto specimens and zeroed before applying a small preload to the specimen. The test was then allowed to commence, with termination set to occur after complete fracture of the specimens. After termination of the tests, the relevant specimen dimensions were measured again and tabulated. The raw data from each test was then analysed using a specially programmed Excel spreadsheet and edited with the aid of Microcal Origin© software. For the reproducibility of the results, at least three tensile tests have been carried out per each test series. Evaluated properties were the conventional yield stress Rp0.2% (0.2 % proof stress), ultimate tensile strength Rm and elongation at fracture Af.
Data availability
The datasets used and/or analysed during the current study available from the corresponding authors on reasonable request.
References
Buchheit, R. G., Grant, R. P., Hlava, P. F., Mckenzie, B. & Zender, G. L. Local dissolution phenomena associated with S phase (Al2CuMg) particles in aluminum alloy 2024-T3. J. Electrochem. Soc. 144, 2621–2628 (1997).
Boag, A. et al. How complex is the microstructure of AA2024-T3? Corros. Sci. 51, 1565–1568 (2009).
Ringer, S. P., Hono, K., Polmear, I. J. & Sakurai, T. Nucleation of precipitates in aged Al-Cu-Mg-(Ag) alloys with high Cu:Mg ratios. Acta Mater. 44, 1883–1898 (1996).
Ringer, S. P., Caraher, S. K. & Polmear, I. J. Response to comments on cluster hardening in an aged Al-Cu-Mg alloy. Scr. Mater. 39, 1559–1567 (1998).
Martin, J. W. Precipitation Hardening 2nd edn, Vol. 240 (Butterworth-Heinemann Ltd, 1998).
Starke, E. A. & Staley, J. T. Application of modern aluminum alloys to aircraft. Prog. Aerosp. Sci. 32, 131–172 (1996).
Moy, C. K. S., Weiss, M., Xia, J. & Sha, G. Influence of heat treatment on the microstructure, texture and formability of 2024 aluminium alloy. Mater. Sci. Eng. A 552, 48–60 (2012).
Davis, J. R. Aluminum and Aluminum Alloys (ASM InternationalASM International, Ohaio, USA, 1993).
Radutoiu, N., Alexis, J., Lacroix, L., Abrudeanu, M. & Petit, J.-A. Study of the influence of the artificial ageing temperature on the AA2024 alloy microstructure. Key Eng. Mater. 550, 115–125 (2013).
Yang, R. et al. Multistage-aging process effect on formation of GP zones and mechanical properties in Al−Zn−Mg−Cu alloy. Trans. Nonferrous Met. Soc. China 26, 1183–1190 (2016).
Alexopoulos, N. D., Velonaki, Z., Stergiou, C. I. & Kourkoulis, S. K. Effect of ageing on precipitation kinetics, tensile and work hardening behavior of Al-Cu-Mg (2024) alloy. Mater. Sci. Eng. A 700, 457–467 (2017).
Astika, I. M. Hardness improvement of aluminum alloy 2024 Τ3 after artificial aging treatment. IOP Conf. Ser. 539, 012004 (2019).
Wang, Z., Chen, M., Jiang, H., Li, H. & Li, S. Effect of artificial ageing on strength and ductility of an Al-Cu-Mg-Mn alloy subjected to solutionizing and room-temperature rolling. Mater. Charact. 165, 110383 (2020).
Xu, X., Deng, Y., Chi, S. & Guo, X. Effect of interrupted ageing treatment on the mechanical properties and intergranular corrosion behavior of Al-Mg-Si alloys. J. Mater. Res. Technol. 9, 230–241 (2020).
Pakravan, K., Monazzah, A. H. & Farahmand, S. Ageing condition of tensile specimens: fracture behavior of notched Αl2024 sheet under tensile loading. Mater. Res. Express 7, 056522 (2020).
Ringer, S. P., Marceau, R. K. W., Sha, G., Ferragut, R. & Dupasquier, A. Solute clustering in Al–Cu–Mg alloys during the early stages of elevated temperature ageing. Acta Mater. 58, 4923–4939 (2010).
Araghchi, M., Mansouri, H., Vafaei, R. & Guo, Y. Optimization of the mechanical properties and residual stresses in 2024 aluminum alloy through heat treatment. J. Mater. Eng. Perform. 27, 3234–3238 (2018).
Liang, M. C., Chen, L., Zhao, G. Q. & Guo, Y. Y. Effects of solution treatment on the microstructure and mechanical properties of naturally aged EN AW 2024 Al alloy sheet. J. Alloy. Compd. 824, 153943 (2020).
Lacroix, L., Ressier, L., Blanc, C. & Mankowski, G. Statistical study of the corrosion behavior of Al2CuMg intermetallics in AA2024-T351 by SKPFM. J. Electrochem. Soc. (JES) 155, 8–15 (2008).
Soltis, J. Passivity breakdown, pit initiation and propagation of pits in metallic materials—review. Corros. Sci. 90, 5–22 (2015).
Sun, S., Fang, Y., Zhang, L., Li, C. & Hu, S. Effects of aging treatment and peripheral coarse grain on the exfoliation corrosion behaviour of 2024 aluminium alloy using SR-CT. J. Mater. Res. Technol. 9, 3219–3229 (2020).
Posada, M. et al. Exfoliation and related microstructures in 2024 aluminum body skins on aging aircrafts. Mater. Charact. 38, 259–272 (1997).
Robinson, M. J. & Jackson, N. C. The influence of grain structure and intergranular corrosion rate on exfoliation and stress corrosion cracking of high strength Al–Cu–Mg alloys. Corros. Sci. 41, 1013–1028 (1999).
ASTM International. ASTM G34, Standard Test Method for Exfoliation Corrosion Susceptibility in 2XXX and 7XXX Series Aluminum Alloys (EXCO Test) https://standards.globalspec.com/std/3828310/astm-g34-01-2007.
Wang, Z. et al. The intergranular corrosion susceptibility of 2024 Al alloy during re–ageing after solution treating and cold–rolling. Corros. Sci. 114, 156–168 (2017).
Wang, S., Yang, F. & Frankel, G. S. Effect of altered surface layer on localized corrosion of aluminum alloy 2024. J. Electrochem. Soc. 164, 317–323 (2017).
Alexopoulos, N. D., Velonaki, Z., Stergiou, C. I. & Kourkoulis, S. K. The effect of artificial ageing heat treatments on the corrosion-induced hydrogen embrittlement of 2024 (Al–Cu) aluminium alloy. Corros. Sci. 102, 413–424 (2016).
Zhang, W. & Frankel, G. S. Transitions between pitting and intergranular corrosion in AA2024. Electrochem. Acta 48, 1193–1210 (2003).
Larignon, C., Alexis, J., Andrieu, E., Odemer, G. & Blanc, C. The contribution of hydrogen to the corrosion of 2024 aluminum alloy exposed to thermal and environmental cycling in chloride media. Corros. Sci. 69, 211–220 (2013).
Marlaud, T., Malki, B., Deschamps, A. & Baroux, B. Electrochemical aspects of exfoliation corrosion of aluminium alloys: the effects of heat treatment. Corros. Sci. 53, 1394–1400 (2011).
Ghosh, K. S., Hilal, M. D. & Bose, S. Corrosion behaviour of 2024 Al-Cu-Mg alloy of various tempers. Trans. Nonferrous Met. Soc. China 23, 3215–3227 (2013).
Korb, L., Olson, D. In Corrosion 9th ed. 13 (ASM International, OH, USA, 1992).
Schmidt, H. J., Schmidt-Brandecker, B., Trey, H. In Third Joint FAA/DoD/NASA Conference on Aging Aircraft Albuquerque. 20–23 (Albuquerque, New Mexico1999).
Salimon, S. R., Salimon, A. I. & Korsunsky, A. M. The evolution of electrochemical, microstructural, and mechanical properties of aluminium alloy 2024-T4 (D16AT) during fatigue cycling. Proc. Inst. Mech. Eng. Part G J. Aerosp. Eng. 224, 339–353 (2010).
Brunet, M. et al. Comparison of long-term natural aging to artificial aging in Duralumin. MATEC Web Conf., EDP Sci. 326, 04007 (2020).
Ralston, K. D., Birbilis, N., Weyland, M. & Hutchinson, C. R. The effect of precipitate size on the yield strength-pitting corrosion correlation in Al–Cu–Mg alloys. Acta Mater. 58, 5941–5948 (2010).
Ralston, K. D. et al. Role of nanostructure in pitting of Al–Cu–Mg alloys. Electrochem. Acta 55, 7834–7842 (2010).
Cao, Y. et al. Quantitative study of dimensional stability mechanism and microstructure evolution during precipitation process of 2024Al alloy. J. Mater. Sci. Technol. 90, 85–94 (2021).
Birbilis, N. & Buchheit, G. Electrochemical characteristics of intermetallic phases in aluminum alloys. J. Electrochem. Soc. 152, B140–B151 (2005).
Alexopoulos, N. D., Siskou, N., Charalampidou, Ch, M. & Kourkoulis, S. K. Simulation of the corrosion-induced damage on aluminum alloy 2024 specimens with equivalent surface notches. Frat. ed. Integrità Strutt. 50, 342–353 (2019).
Alexopoulos, N. D., Charalampidou, C., Skarvelis, P. & Kourkoulis, S. K. Synergy of corrosion-induced micro-cracking and hydrogen embrittlement on the structural integrity of aluminium alloy (Al-Cu-Mg) 2024. Corros. Sci. 121, 32–42 (2017).
Li, J., Dang, J. A summary of corrosion properties of Al-rich solid solution and secondary phase particles in Al alloys. Metals 7, 84 (2017).
JI, Y. et al. Review of micro-scale and atomic-scale corrosion mechanisms of second phases in aluminum alloys. Trans. Nonferrous Met. Soc. China 31, 3205–3227 (2021).
Buchheit, R. G. et al. The electrochemistry of intermetallic particles and localized corrosion in Al alloys. JOM J. Miner. Met. Mater. Soc. 53, 29–33 (2001).
Li, J., Hurley, B. & Buchheit, R. Effect of temperature on the localized corrosion of aa2024-t3 and the electrochemistry of intermetallic compounds during exposure to a dilute NaCl solution. Corrosion 72, 1281–1291 (2016).
Schmutz, P. & Frankel, G. S. Characterization of AA2024-T3 by scanning Kelvin probe force microscopy. J. Electrochem. Soc. 145, 2285–2295 (1998).
Blanc, C., Lavelle, B. & Mankowski, G. The role of precipitates enriched with copper on the susceptibility to pitting corrosion of the 2024 aluminium alloy. Corros. Sci. 39, 495–510 (1997).
Kamoutsi, H. Corrosion induced hydrogen embrittlement in high strength Al alloys. Corros. Sci. 48, 1209–1224 (2004).
Alexopoulos, N. D. & Papanikos, P. Experimental and theoretical studies of corrosion induced mechanical properties degradation of aircraft 2024 aluminum alloy. Mater. Sci. Eng. A 498, 248–257 (2008).
Charalampidou, C. M., Pretorius, C. C. E., Mostert, R. J. & Alexopoulos, N. D. Effect of solution aggressiveness on the crack growth resistance and cracking mechanism of AA2024-T3. Corrosion 77, 1029–1040 (2021).
Polmear, I. Metallurgy of the light alloys. In: Light Alloys, Metallurgy and Materials Science (eds. Polmear, I., StJohn, D., Nie, J.-F., Qian, M.) 544 (Elsevier Science, 1995).
Kermanidis, Al. Th, Petroyiannis, P. V. & Pantelakis, Sp. G. Fatigue and damage tolerance behaviour of corroded 2024 T351 aircraft aluminum alloy. Theor. Appl. Fract. Mech. 43, 121–132 (2005).
Sankaran, K. K., Perez, R. & Jata, K. V. Effects of pitting corrosion on the fatigue behavior of aluminum alloy 7075-T6: modeling and experimental studies. Mater. Sci. Eng. A 297, 223–229 (2001).
Bray, G. H., Bucci, R. J., Colvin, E. L., Kulak, M. Effects of prior corrosion on the S/N fatigue performance of aluminum alloys 2024-T3 and 2524-T3. In: Effects of Environment on the Initiation of Crack Growth ASTM STP 1298 (eds. Sluys, W. A. V. D., Robert, S., Piascik, Robert Zawierucha, R.) 89 (ASTM International, 1997).
Alexopoulos, N. D., Dalakouras, C. J., Skarvelis, P. & Kourkoulis, S. K. Accelerated corrosion exposure in ultra thin sheets of 2024 aircraft aluminium alloy for GLARE applications. Corros. Sci. 55, 289–300 (2012).
Charalampidou, C., Dietzel, W., Zheludkevich, M., Kourkoulis, S. K. & Alexopoulos, N. D. Corrosion-induced mechanical properties degradation of Al-Cu-Li (2198-T351) aluminium alloy and the role of side-surface cracks. Corros. Sci. 183, 109330 (2021).
Pantelakis, Sp. G., Setsika, D., Chamos, A. & Zervaki, A. Corrosion damage evolution of the aircraft aluminum alloy 2024 T3. Int. J. Struct. Integr. 7, 25–46 (2016).
Prudhomme, M. et al. Effect of actual and accelerated ageing on microstructure evolution and mechanical properties of a 2024-T351 aluminium alloy. Int. J. Fatigue 107, 60–71 (2018).
ASTM International. ASTM E8M, Standard Test Methods for Tension Testing of Metallic Materials. https://www.instron.com (2009).
ASTM International. ASTM G44, Standard Practice for Exposure of Metals and Alloys by Alternate Immersion in Neutral 3.5% Sodium Chloride Solution. https://www.astm.org/g0044-21.html (2005).
Guérin, M., Andrieu, E., Odemer, G., Alexis, J. & Blanc, C. Effect of varying conditions of exposure to an aggressive medium on the corrosion behavior of the 2050 Al–Cu–Li alloy. Corros. Sci. 85, 455–470 (2014).
ASTM International. ASTM G.1, Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. https://www.astm.org/g0001-90r99e01.html (1999).
Acknowledgements
This research work was financed by the Hellenic Foundation for Research and Innovation (H.F.R.I.), under the 2nd Call for H.F.R.I. Research Projects to support Faculty Members and Researchers (Proposal ID: 03385, MIS Acronym: CorLi, Title: Corrosion susceptibility, degradation and protection of advanced Al-Li aluminium alloys).
Author information
Authors and Affiliations
Contributions
N.D.A.: Conceptualization, Methodology, Writing—review and editing, Funding acquisition. C.-M.C.: Investigation, Methodology, Experimental work, Data curation, Writing—original draft. N.S.: Experimental work. D.G.: Experimental work. M. Z.: Writing—review and editing. S.K.K.: Writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Charalampidou, C.M., Siskou, N., Georgoulis, D. et al. Corrosion of aluminium alloy AA2024-Τ3 specimens subjected to different artificial ageing heat treatments. npj Mater Degrad 8, 93 (2024). https://doi.org/10.1038/s41529-024-00503-4
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41529-024-00503-4
This article is cited by
-
Long-term corrosion performance of superhydrophobic vs. superhydrophilic laser-modified AA2024 surfaces under ambient storage
npj Materials Degradation (2025)