Abstract
Carbon dioxide removal technologies are urgently needed to stay within the 2 °C warming limit. This study evaluated Gran Canaria lapilli and a commercially available lava basalt as feedstocks for bio-weathering in flow-through columns inoculated with Aureobasidium pullulans, Suillus variegatus, Bacillus subtilis, and Cupriavidus metallidurans. Weathering rates for biotic lapilli (2.92 ± 0.28 × 10−12 mol m−2 s−1) and lava basalt (2.17 ± 0.15 × 10−12 mol m−2 s−1) are statistically significantly greater than the abiotic controls (lapilli = 1.08 ± 0.01 × 10−12, lava basalt = 0.98 ± 0.02 × 10−12 mol m−2 s−1), with inoculation explaining most of the variance. While lapilli had faster weathering rates and greater phospholipid fatty acid biomasses, lava basalt sequestered more carbon: 1.38 and 0.61 mmol C (biotic and abiotic) compared to 0.56 and −0.25 mmol C for lapilli. Both materials show promise for CDR in bio-weathering systems.
Similar content being viewed by others
Introduction
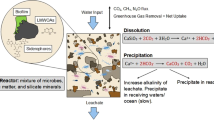
To remain well below the 2 °C warming limit of the Paris agreement requires the development of safe and scalable carbon dioxide removal methods (CDR)1,2,3,4,5. Enhanced silicate weathering, with an estimated climate change mitigation potential of 2–4 Gt CO2 y−1, is one potential CDR6. The buffering of atmospheric CO2 partial pressure through silicate weathering and mineral carbonation has helped balance emissions over geologic timescales and stabilise the climate7,8. The enhancement of natural microbial silicate weathering processes may help significantly mitigate climate change associated with elevated atmospheric CO25,9. Silicate mineral dissolution driven by the reaction with CO2 in the presence of water releases cations (e.g. Ca2+, Mg2+, Na+, and K+) and bicarbonate (HCO3−) into solution, increasing alkalinity and providing storage of CO2, which can be stored in the longer term as calcium and magnesium carbonates upon precipitation in soils or receiving waters10.
Chemical and physical processes driving silicate dissolution can be complemented by biota as they seek to mobilise nutrients held within minerals11. Mycorrhizal fungi form symbiotic relationships with 90% of terrestrial plants, exchanging water and nutrients extracted from organic and mineral substrate in the soil for organic carbon12,13, while saprotrophic fungi can also access nutrients from organic matter14. Mineral dissolution can be supported by microbial processes such as oxidoreduction, acidolysis, chelation/siderophores (complexolysis), and physical disruption15,16,17,18.
Here we sought to compare the weathering rate of a lapilli from Gran Canaria, and its suitability for use as a feedstock for CO2 sequestration in flow-through bio-columns, and compared it to commercially available basalt extrusion rocks. This work builds upon previous research establishing the potential of silicate weathering flow through bio-columns as potential CO2 sequestration tools and the importance of selecting appropriate organic carbon sources19,20. The columns here were inoculated with Aureobasidium Pullulans, Suillus variegatus, Bacillus subtilis, and Cupriavidus metallidurans.
Results
Weathering rate
The weathering rates of all columns decreased through time, with the biotic columns yielding the largest weathering rates throughout the experiment compared to the abiotic columns (Fig. 1A). Based on the linear regression model and analysis of variance (ANOVA) analyses, rock type, inoculation and time accounted for most of the variance in weathering rates (Fig. 1B). Inoculation had a strong statistically significant positive effect on weathering rates. Rock type had a small but significant effect, with lava basalt resulting in lower weathering rates compared to lapilli. Time had a moderate but significant negative effect, with weathering rates decreasing through time. The interactions between time and rock type or inoculation were small and not statistically significant.
The log-transformed time-weighted average weathering rates (Table 1) were all statistically significantly different from each other, except the abiotic columns, based on Tukey’s multiple comparisons test. Inoculation accounted for most of the variance in time-weighted average weathering rates, while rock type accounted for a small but statistically significant variance (Table 1).
Carbon sequestration
The biotic columns produced more inorganic carbon than their corresponding abiotic columns (Fig. 2A). The overall sequestration of carbon followed lava basalt biotic > lapilli biotic ≈ lava basalt abiotic. Weathering of the lapilli columns resulted in the release of solid inorganic carbon, and the net loss in inorganic carbon in the lapilli abiotic columns, i.e., negative TIC. Based on the two-way ANOVA analyses, inoculation accounted for most of the variance in total DIC and nearly half the variance in TIC, while the rock accounted for most of the variance in SIC and TIC. All combinations produced statistically significant different total DIC (Fig. 2A), based on Tukey’s multiple comparisons test. Similarly, all combinations produced statistically significant different TIC, except lapilli biotic versus lava basalt abiotic. The only statistically significant differences in SIC were between the lava basalt abiotic and lapilli biotic or lapilli abiotic columns.
A Change in total dissolved inorganic carbon (DIC), solid inorganic carbon (SIC), and total inorganic carbon (TIC), and two-way ANOVA Summary. B Accumulative dissolved inorganic carbon at each sampling time. C Linear model of log10 transformed accumulative DIC and ANOVA summary. D The linear correlation between log10 weathering rate (log10 WR) and dissolved inorganic carbon (DIC), for all data (black line) and the rocks separately with week 1 values excluded (dotted lines).
Dissolved Inorganic Carbon accumulated throughout the experiment, with the biotic columns producing nearly triple the DIC compared to their corresponding abiotic columns throughout the experiment (Fig. 2B). The column order based on DIC production was as follows: lava basalt biotic » lapilli biotic » lava basalt abiotic > lapilli abiotic. Based on the linear model and ANOVA analyses of the accumulative DIC, inoculation had a statistically significant strong positive effect, time also had a statistically significant positive effect, and the accumulative DIC of the lapilli columns was statistically significantly lower than the corresponding lava basalt columns (Fig. 2C). The interaction between time and inoculation produced a small negative but statistically significant effect on accumulative DIC, suggesting the positive effect of inoculation is reduced through time. While the interaction between time and rock produced no statistically significant effect.
Based on Pearson’s correlation calculations, there was a strong linear correlation between the dissolved inorganic carbon and weathering rates for all samples (Fig. 2D). The correlation strengthened when the samples were separated by rock type and week 1 samples were excluded (Fig. 2D).
PLFA and SEM
Two-way ANOVA analysis showed that inoculation accounted for most of the variance in all PLFA derived biomasses (Fig. 3). While rock had a moderate, but not statistically significant, effect on fungi PLFA biomass. Based on Tukey’s multiple comparisons test, each of the bacteria, fungi, and total biomasses was statistically significantly different between the biotic and abiotic columns for both the lava basalt and lapilli. There was no statistically significant difference between the abiotic columns or the biotic columns for all PLFA-derived biomasses. In combination with the SEM (Fig. 4), it appears that sterility of the control columns was maintained.
Extensive colonisation and biofilm formation was visible on both the biotic lapilli and lava basalt rock grains (Fig. 4A–F). Colonisation was not restricted to the organic carbon source. There was no discernible difference in the extent of colonisation and biofilm formation between the two rocks.
Discussion
The weathering rates of the biotic columns were 2–3 times the weathering rates of the corresponding abiotic columns (Fig. 1). Calculating the weathering rates based on the total alkalinity released to the leachate assumes that all reaction products are exported from the columns, therefore the weathering rates calculated here may be underestimated. Cations retained in the columns or bound to the straw would increase the pool of exchangeable cations, which may limit alkalinity production. Total alkalinity, instead of the release of individual cations, was used to calculate weathering rates, as the release of individual cations in similar weathering experiments was non-stoichiometric21. The pH of rock and abiotic (rock + straw) columns were similar, particularly for lava basalt, hence it was deemed appropriate to estimate the contribution of straw to total alkalinity as the difference between the abiotic and rock only columns (Supplementary Fig. 1). Total alkalinity release decreased at each sampling interval, resulting in a decrease in weathering rate throughout the experiment for both the lapilli and lava basalt. SIC production in the lava basalt columns resulted in increased weathering rates when SIC was added to total alkalinity (Table 1).
The inoculated columns yielded statistically significantly faster weathering rates than the corresponding abiotic columns for both the lapilli and lava basalt. The abiotic weathering rates of the lapilli and lava basalt were similar throughout the experiment, however the extent of the enhancement in the weathering rates of the lapilli due to inoculation was greater than for the lava basalt. Inoculation accounted for most of the variance between the abiotic and biotic columns, suggesting that the inoculation of the columns with a mixed culture of B. subtilis, C. metallidurans, A. pullulans, and S. variegatus was responsible for the increased weathering rates of throughout the experiment, and to a greater extent in the lapilli columns. This was further supported by the increased release of cations (Supplementary Fig. 1) in the biotic columns compared to the abiotic columns for both rocks.
Numerous studies assessing microbially driven dissolution of silicate rocks using single cultures have been conducted22,23,24,25,26,27,28,29, while rock bio-weathering results have been mixed in flow-through column experiments. Dunite weathering utilising mixed cultures resulted in a ~100% increase in weathering rates compared to abiotic controls20. Knufia petricola has been reported to accelerate olivine dissolution compared to abiotic controls, through increased solubilisation and binding of Fe23. In contrast, the effect of Pseudomonas reactants and Chaetomium Brasiliense on diopside weathering was weak and solely due to the decrease of pH in the porewaters30. This, along with the larger enhancement in weathering of lapilli compared to lava basalt upon inoculation with a mixed culture, illustrates the need to select and combine appropriate rock feedstocks and microbial inoculums.
The microbes may have supported the rock weathering via oxidoreduction, complexolysis, acidolysis, and physical disruption15,16,17,18. Complexolysis by siderophores, for example, could help reduce cation saturation and inhibit the production of unreactive dissolution products16. The slowest process between transport and interface interactions likely controls dissolution rate31. The transport of the dissolution products away from the reaction sites is likely limited by the initial high dissolution rates of the fine particles or high surface energy sites31.
Chemical, physical, and mineralogical characteristics were likely to also control weathering rates, and the differences between the lapilli and lava basalt. The lapilli and lava basalt produced alkalinity at a similar rate (Supplementary Fig. 2), but the surface area of the lapilli was lower than that of lava basalt (Table 2), resulting in the faster surface area normalised weathering rates reported here. The lapilli and lava basalt were similar in mineral oxide composition (Table 2), which is unlikely to explain the differences in weathering rates. Differences in the relative mineral composition were likely an important control of weathering rates. Minerals such as olivine, pyroxene, and Ca plagioclase, which have high crystallisation temperatures, weather more rapidly at the relatively low standard temperature, compared to quartz, which has a relatively low crystallisation temperature32. The dominant minerals in the lapilli (anorthite 28%, olivine 16%, and diopside 48%) provided a greater total of highly weatherable minerals than the lava basalt (anorthite 11%, nepheline 15%, olivine 7%, diopside 46%, and leucite 14%).
The lava basalt outperformed the lapilli in terms of carbon sequestration (Fig. 2A). The TIC followed lava basalt biotic > lapilli abiotic ≈ lapilli biotic > lava basalt abiotic, with inoculation accounting for most of the variance. The presence of the microbes resulted in statistically significant increases in inorganic carbon (DIC, SIC and TIC) compared to their abiotic and rock only columns (Fig. 2A), and a faster rate of DIC accumulation through time (Fig. 2B). Further, the presence of microbes in the lapilli columns resulted in carbon sequestration, where there was carbon loss in the abiotic columns (i.e. negative SIC) (Fig. 2A). The weathering and carbon sequestration pattern was similar to previous work which found that dunite (forsterite dominated) weathering could be enhanced in similar flow-through experiments, producing increased DIC (127%) and SIC (11%) in the biotic columns compared to abiotic columns20.
Changes in DIC and total alkalinity due to SIC release (lapilli columns) and accumulation (lava basalt columns), resulted in in lower weathering rates for the lapilli and higher weathering rates for the lava (Table 1). Strengthening the relationship between TIC and weathering rate, as the higher SIC corrected weathering rates of the lava basalt resulted in significantly larger TIC, compared to the lapilli. The dissolution of inorganic carbon in the lapilli columns (negative SIC) likely increased DIC (and total alkalinity) in the leachate, resulting in a loss of carbon from the abiotic columns (negative TIC) but not the biotic columns (Fig. 2A).
Inoculation had a different effect on SIC for each rock type. Inorganic carbon release from the lapilli appears reduced due to inoculation with the mixed culture; in comparison, inoculation of the lava basalt resulted in a lower accumulation of SIC. Microbial calcium carbonate dissolution has been hypothesised to occur in soils to reduce salinity and sodicity33, and help weather minerals such as miliolite34, while various B. subtilis strains, for example, have been reported to increase calcium carbonate precipitation via the release of ammonia, production of carbonic anhydrase, and fatty acid metabolism35,36.
The species used in this experiment have reported attachment and weathering mechanisms of silicate minerals. B. subtilis (NCBI 3610) can cause physical and chemical breakdown of plagioclase composing minerals, biotite, and basalt25,28. C. metallidurans (type strain CH34) promotes rock weathering through the reduction of the iron saturation state via processes associated with the formation of biofilms26,27,28. Through the production and secretion of pullulan (a polysaccharide) and other biomolecules such as a uronic acid-based polymer, A. pullulans (DSM 3497) adheres to and weathers quartz37. S. variegatus (DSM 1752) has been reported to promote biotite weathering through the formation of low molecular weight organic acids38,39, and accumulating Fe and heavy metals such as Cu, Cd, Zn, and As potentially solubilised during weathering40,41,42.
The production of CO2 during microbial respiration can lead to further rock weathering through the acidification43. Enhancement of the weathering process beyond those associated with the consumption of the supplied organic carbon by the microbial community and subsequent respiration as CO2 was not determined. While the activity of the biofilm was not determined, the extensive biofilm formation illustrates that the preconditions necessary for many microbial processes were met in the biotic columns. Direct contact with the rock surface, for example, biofilms, is required for many microbial weathering processes such as oxidoreduction and complexolysis15,26,44,45,46. Oxidoreduction and complexolysis involve the transfer of electrons and nutrient extraction via excreted and/or membrane-associated molecules in biofilm regions15,26,44,45,46. Experiments involving 13C labelled organic carbon sources, and isotope analyses, are required, however, to provide stronger estimates of the contribution of microbial respiration to weathering and the total sequestered carbon.
PLFA alone was used to estimate total microbial biomass because peaks 14:0, 15:0, 16:0, and 17:0 occur in all microorganisms and should sufficiently capture combined fungal and bacterial biomasses47. The biotic columns yielded statistically significantly larger PLFA biomasses than the abiotic and rock only columns (Fig. 3). Although the mineral compositions were similar, the larger PLFA biomasses in the lapilli compared to the lava suggests that the mineral composition of the lapilli better supported the microbes utilised here despite phosphate release being lower in the lapilli columns (Supplementary Fig. 3). No microbes were visible in the SEM images of the abiotic columns (Fig. 4), combined with the low PLFA biomasses suggests sterility of the abiotic columns was maintained throughout the experiment.
PLFA in combination with the SEM imaging was able to demonstrate a difference in biomass and surface colonisation between the biotic and abiotic columns (Figs. 4 and 5), which combined with the statistical importance of inoculation to weathering rates and carbon sequestration (Figs. 2 and 3), suggests the use of mixed cultures in flow-through columns to weather silicate rocks and capture CO2 is a viable CDR strategy.
Here we investigated the effect of combined bacterial and fungal inoculation on the weathering and carbon sequestration potential of two rocks, a Gran Canaria lapilli and the commercial lava basalt, in simple flow-through bio-columns. The Gran Canaria lapilli have been reported to support extensive microbial communities48. Inoculation resulted in increased weathering rates and carbon sequestration, with the inoculated lava basalt sequestering more carbon despite having lower PLFA-derived biomasses. Weathering of the Gran Canaria lapilli resulted in carbon sequestration, primarily as DIC; however, the lava basalt sequestered ~2 times the DIC and TIC. Both rocks demonstrated potential as feedstocks for microbially enhanced weathering in flow-through columns and their potential as a CDR strategy.
Methods
Experimental setup
The experiment consisted of six different column combinations, totalling 17 columns. There were three replicates for each combination, except the lapilli rock only columns for which there was two replicates due to insufficient rock mass for a third replicate (Fig. 5). The flow-through columns consisted of 100 g of rock (lapilli or lava basalt) and 2.5 g of straw (Fig. 5). The components were well mixed prior to addition to the flow-through columns. The flow-through columns consisted of 2 magenta boxes (Merck, Sweden) stacked on each other; the upper box had a 9 mm hole drilled in each corner and one in the centre with a 20-μm nylon mesh filter laid over the holes, sealed with a lid. The flow-through columns were then autoclaved (121 °C for 20 min), and allowed to cool to room temperature, prior to inoculation with 5 mL of each of the pre-grown fungi and bacteria (20 mL total). The abiotic columns containing straw also received 20 mL of sterile nutrient broth (nutrient concentrations available in Supplementary Table 1), to compensate for additional alkalinity provided by the media during inoculation. The rock (no straw) columns did not receive nutrient broth, so the weathering of the rocks alone could be monitored.
Each flow-through column received 50 mL of autoclaved distilled water (pH 5.0) using a volumetric flask, Monday to Friday, for 6 weeks. The water flow-through (leachate) in the lower magenta box was collected at the beginning of each week for analysis. At the completion of the 6 weeks of watering, the solid mixture was homogenised in each column and samples collected into sterile 50 mL DNA/RNA-free centrifuge tubes and stored at −20 °C for PLFA and solid carbon analyses.
Rock selection
Two rock types were selected, both formed via basaltic lava extrusions. The rocks (Table 2), the Gran Canaria lapilli (lapilli; sample reference GC-Bandama-Lap-3)48 and the commercially available basalt lavagestein (lava basalt) (Eifelfold lava Union, Germany), were manually crushed and sieved to 1–2 mm. The 1730–1736 Lanzarote eruption was the longest recorded basaltic fissure eruption in the history of the Canaries, changing the agricultural productivity of Lanzarote and resulting in the adaptation of using pyroclasts to enhance topsoil48. The ability of the lapilli to retain moisture inside the vesicles and maintain a hydrous micro-climate has been postulated to support abundant communities of microbes, increasing the transport of nutrients from the volcanic matter to plants48. Lapilli soil amendments were utilised throughout the Canarie Islands, the lapilli investigated here was from a disused quarry on Gran Canaria. The comparison to the lava basalt was useful due to the similarity to lapilli in formation and composition.
Fusion Inductively Coupled Plasma, Mass Spectroscopy (Thermo ICAP 6500 ICP, ActLabs 4B2 Lithium Metaborate/Tetraborate Fusion ICP-MS protocol), and loss on ignition (450 °C for 4 h) were used to determine the oxide and trace element composition (Actlabs, Ontario, Canada), after autoclavation (reference materials and recovery percentages—Supplementary Material). The negative LOI value of the lapilli may have arisen from the oxidation of Fe2+ to Fe3+, resulting in a mass increase greater than the loss due to volatiles49, however, this was not examined here. The trace element composition was provided in the Supplementary Table 2. The Brunauer-Emmett-Teller specific surface area (BET) of the materials was determined using a Micromeritics ASAP 2020 surface area analyser (Norcross, GA, USA) by recording nitrogen adsorption and desorption isotherms at −195 °C. Before analysis, samples underwent pre-treatment: they were heated to 100 °C under a dynamic vacuum (1 × 10−4 Pa) using a Micromeritics SmartVacPrep sample preparation unit. Equilibrium adsorption data points were collected when the pressure change dropped below 0.01% within a 10 s interval, with a minimum delay of 100 s. The XRD spectra were obtained using a D8 ADVANCE Powder Bruker diffractometer using Cu anode (0.15406 nm), Bragg−Brentano geometry in the 2θ range from 10 to 80°, and a step size of 0.017°.
Bacteria and fungi
The fungi selected were A. pullulans (DSM 3497) and S. variegatus (DSM 1752) (Leibniz Institute, Germany). The bacteria selected were B. subtilis (NCIB 3610), and C. metallidurans (type strain CH34) (Leibniz Institute, Germany). All fungi and bacteria are risk group 1, under the Technical Rules for Biological Agents50.
Culturing
Freeze dried cultures were rehydrated and propagated following the suppliers’ instructions. New cultures were prepared via transferring approximately a hundredth volume of the culture to sterile fresh media23,51. All reagents were purchased from Merc (Sweden).
Malt extract broth, containing 2% (w/v) malt extract, 0.1% (w/v) casein-digested peptone, and 2% (w/v) D-(+)-glucose dissolved in MilliQ water (18.2 mΩ) water, was used to grow A. pullulans and S. Variegatus at room temperature. S. Variegatus cultures were disaggregated gently in a sterile kitchen blender prior to inoculation of the flow-through columns. B. subtilis and C. metallidurans (Leibniz Institute, Germany) were grown at 30 °C in nutrient broth (DSMZ media 1), containing 0.5% (w/v) Bacto Peptone, 0.3% (w/v) NaCl, and 0.3% (w/v) meat extract dissolved in MilliQ water. All cultures were grown on shaker plates at 30 rpm in the darkness.
Carbon source
Wheat straw (Welkoop, The Netherlands) was used to provide the necessary carbon and some of the essential nutrients to support microbial growth in the flow-through columns. Upon drying and grinding of the straw, total carbon and nitrogen were determined using an elemental analyser (FlashSmart, Thermo Fisher Scientific, USA) alongside acetanilide standards (Carlo Erba Instruments, Italy). The primary elemental composition was determined via HR-ICP-MS (Thermo Element 2, Thermo Fisher Scientific, USA), upon digestion via a modified salicylic acid/sulphuric acid digestion method (Table 3)52, alongside single element and multi-element standards (Inorganic Ventures, New Jersey, USA).
Analyses
The leachate for each week, collected at the beginning of the subsequent week, was filtered to 0.25 μm using polyethersulfone filters (Fisherbrand, USA) prior to analysis for pH, alkalinity, conductivity, dissolved organic and inorganic carbon (DOC and DIC) (Sievers M9, General Electric, USA), cations (Na+, K+, Mg2+, Ca2+, and NH4+) and anions (NO3−, SO42−, PO43−, Cl−, and F−) (Metrohm ion chromatography system 883 basic IC Plus, Switzerland). Manual end-point titration, of 40 mL of leachate to pH 4.2 with 0.02 mol L−1 HCl while stirring, was used to measure total alkalinity (Piston Burette E 274, Metrohm, Switzerland). Gravimetric dilutions (20:1, 10:1, 5:1) with MilliQ water for weeks 1 to 3, were performed for dissolved carbon and ion analyses, due to the potential for the high DOC to exceed the quantification range and potential for damage to the ion chromatography componentry. Samples that were not analysed within 48 h were stored at −20 °C.
Solid organic and inorganic carbon (SOC and SIC) analyses via loss on ignition and elemental analysis (Costech Instruments ECS 4010, USA) were performed on the homogenised flow-through column rock/straw contents after 6 weeks of watering, and on the unreacted materials. Samples from each column were oven dried at 105 °C for 24 h, before grinding at 650 rpm for 10 min in a ball mill (Retsch PM 100 CM). Subsamples of 10 g were weighed into muffled ceramic crucibles, and combusted at 550 °C for 3 h, before reweighing. Solid organic carbon (SOC) was calculated as the loss of mass upon combustion and converted to the weight percentage of carbon in the straw (Table 3). Subsamples (70 mg) of the combusted material were weighed into tin capsules for SIC analysis via combustion in an elemental analyser, alongside acetanilide standards (71.09% carbon), EDTA, and high organic carbon sediment quality controls.
A modified glutaraldehyde, ethanol gradient and bis(trimethylsilyl)amine (HMDS) method was used for bacterial and fungal cell fixation, as previously described20. Samples were placed on stubs with carbon tape and coated with palladium and gold for Scanning Electron Microscopy (SEM) imaging using a Zeiss Supra 35 VP (Carl Zeiss SMT, Oberkochen, Germany) equipped with a field emission gun.
A three-step phospholipid fatty acid (PLFA) protocol (extraction, lipid fractionation, and alkaline methanolysis) was used to assess microbial biomass via GCMS using a splitless method, as previously described in detail20. All vials and glass pipettes were muffled at 500 °C for 3 h before use. Solid sample from each column after 6 weeks, was freeze-dried prior to extraction. Fungal and Bacterial Acid Methyl Ester (FAME and BAME) mixes were used to identify the peaks (Supplementary Table 3), and a nonadecanoate internal standard was used to calibrate the peaks.
Weathering rate
Total alkalinity (Ct) of the abiotic and biotic columns was calculated by subtracting the difference between the abiotic and rock-only columns from each column, similarly to our previous work20. Weathering rate, as total alkalinity release rate (R), was calculated in mol m2 s−1, using Eq. 1. Where Q = the outflux volume (L) from the column per time step t (s), Ct = total alkalinity (mol L−1) at time t, m = rock mass in the column (g), A = specific surface area of the rock (m2 g−1), and τ (s) = residence time of the solution occupying the material pore space21.
Residence time (τ) was calculated using Eq. 2. Where Vc = column volume occupied with rock (m3), Vo = outflow volume (m3), mc = initial mass of the starting material (g), p = material specific density (g m−3), for time t (s)21.
The weathering rates were calculated based on total alkalinity produced, average weathering rates for the whole flow-through column21.
Time-weighted average weathering rates were estimated to account for the effect of SIC accumulation or loss in the columns on weathering rates. Total alkalinity, here, equalled the sum of the total alkalinity (mol) produced throughout the experiment for each column, divided by the total leachate volume (L). Time-weighted average weathering rates, where SIC increased or decreased, were estimated by adding SIC to DIC, which was subsequently used to calculate the total alkalinity using the seacarb package (flag 9, temperature = 20 °C and salinity = 0).
Data corrections and statistical analyses
Weathering rates, DIC, SIC, TIC, and PLFA were corrected for the contribution of straw to total alkalinity, DIC, SIC, and PLFA by subtracting the difference between the abiotic and rock-only columns from the biotic and abiotic columns. The SIC present in the unreacted starting material was also subtracted from the SIC values. TIC was the sum of the corrected DIC and SIC.
All statistical analyses were performed using PRISM 10.1.2 (GraphPad Software, USA). Graphs and tables report standard deviations for all replicates. For the calculation of cumulative averages, the values for each individual column for the full experiment were summed before being averaged with their replicates, and the standard deviation was calculated.
All data were assessed via Spearman’s test for heteroscedasticity, and the D’Agostino-Pearson omnibus and Shapiro-Wilk normality tests53. To meet homoscedastic and normality assumption,s log transformations were performed prior to statistical analysis, where applicable. Linear models and ANOVA analyses54 were used to determine the effect (magnitude and direction) of rock type, inoculation, and time on weathering rates and DIC. Two-way ANOVA54 was also used to calculate the contribution of rock and inoculation to the variance in TIC, SIC, total DIC, and PLFA biomasses. The significance of the difference between the means of each combination was determined via Tukey’s test55. The relationship between DIC and weathering rate (log transformed) was computed via Pearson correlation.
Data availability
All data analysed during this study are included in this published article [and its supplementary information files]. The datasets used and analysed during this study are available in the supplementary material as an excel file.
References
Rockström, J. et al. A roadmap for rapid decarbonization. Science 355, 1269–1271 (2017).
UNFCC. (United Nations, 2015).
Gasser, T., Guivarch, C., Tachiiri, K., Jones, C. D. & Ciais, P. Negative emissions physically needed to keep global warming below 2 °C. Nat. Commun. 6, 7958 (2015).
Fuss, S. et al. Negative emissions—part 2: costs, potentials and side effects. Environ. Res. Lett. 13, 063002 (2018).
Vicca, S. et al. Is the climate change mitigation effect of enhanced silicate weathering governed by biological processes? Glob. Change Biol. 28, 711–726 (2021).
Smith, S. M. et al. The state of carbon dioxide removal - 1st edn. (The State of Carbon Dioxide Removal, 2023).
Walker, J. C. G., Hays, P. B. & Kasting, J. F. A negative feedback mechanism for the long-term stabilization of Earth’s surface temperature. J. Geophys. Res. Oceans 86, 9776–9782 (1981).
Berner, R. A., Lasaga, A. C. & Garrels, R. M. The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years. Am. J. Sci. 283, 641–683 (1983).
Beerling, D. J. et al. Potential for large-scale CO2 removal via enhanced rock weathering with croplands. Nature 583, 242–248 (2020).
Hartmann, J. et al. Enhanced chemical weathering as a geoengineering strategy to reduce atmospheric carbon dioxide, supply nutrients, and mitigate ocean acidification. Rev. Geophys. 51, 113–149 (2013).
Finlay, R. D. et al. Reviews and syntheses: Biological weathering and its consequences at different spatial levels – from nanoscale to global scale. Biogeosciences 17, 1507–1533 (2020).
Brundrett, M. C. & Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. N. Phytol. 220, 1108–1115 (2018).
Rosling, A. Trees, mycorrhiza and minerals –field relevance of in vitro experiments. Geomicrobiol. J. 26, 389–401 (2009).
Tunlid, A., Floudas, D., Op De Beeck, M., Wang, T. & Persson, P. Decomposition of soil organic matter by ectomycorrhizal fungi: Mechanisms and consequences for organic nitrogen uptake and soil carbon stabilization. Front. Forests Glob. Change 5, 934409 (2022).
Uroz, S., Calvaruso, C., Turpault, M.-P. & Frey-Klett, P. Mineral weathering by bacteria: ecology, actors and mechanisms. Trends Microbiol. 17, 378–387 (2009).
Kirtzel, J. et al. Organic acids, siderophores, enzymes and mechanical pressure for black slate bioweathering with the basidiomycete Schizophyllum commune. Environ. Microbiol. 22, 1535–1546 (2020).
Hoffland, E. et al. The role of fungi in weathering. Front. Ecol. Environ. 2, 258–264 (2004).
Adebayo, A. A., Harris, R. F. & Gardner, W. R. Turgor pressure of fungal mycelia. Trans. Br. Mycol. Soc. 57, 145–151 (1971).
Calogiuri, T. et al. Design and construction of an experimental setup to enhance mineral weathering through the activity of soil organisms. JoVE, e65563 (2023).
Corbett, T. D. W. et al. Organic carbon source controlled microbial olivine dissolution in small-scale flow-through bioreactors, for CO2 removal. npj Mater. Degrad. 8, 34 (2024).
Amann, T., Hartmann, J., Hellmann, R., Pedrosa, E. T. & Malik, A. Enhanced weathering potentials—the role of in situ CO2 and grain size distribution. Front. Clim. 4, 929268 (2022).
Gerrits, R. et al. High-resolution imaging of fungal biofilm-induced olivine weathering. Chem. Geol. 559, 119902 (2021).
Gerrits, R. et al. How the rock-inhabiting fungus K. petricola A95 enhances olivine dissolution through attachment. Geochim. Cosmochim. Acta 282, 76–97 (2020).
Wang, W., Sun, J., Dong, C. & Lian, B. Biotite weathering by Aspergillus niger and its potential utilisation. J. Soils Sediment. 16, 1901–1910 (2016).
Song, W., Ogawa, N., Oguchi, C. T., Hatta, T. & Matsukura, Y. Effect of Bacillus subtilis on granite weathering: a laboratory experiment. CATENA 70, 275–281 (2007).
Olsson-Francis, K., VAN Houdt, R., Mergeay, M., Leys, N. & Cockell, C. S. Microarray analysis of a microbe-mineral interaction. Geobiology 8, 446–456 (2010).
Byloos, B. et al. The impact of space flight on survival and interaction of cupriavidus metallidurans CH34 with basalt, a volcanic moon analog rock. Front Microbiol. 8, 671 (2017).
Cockell, C. S. et al. Space station biomining experiment demonstrates rare earth element extraction in microgravity and Mars gravity. Nat. Commun. 11, 5523 (2020).
Niron, H., Vienne, A., Frings, P., Poetra, R. & Vicca, S. Exploring the synergy of enhanced weathering and Bacillus subtilis: A promising strategy for sustainable agriculture. Glob. Change Biol. 30, e17511 (2024).
Pokrovsky, O. S., Shirokova, L. S., Zabelina, S. A., Jordan, G. & Bénézeth, P. Weak impact of microorganisms on Ca, Mg-bearing silicate weathering. npj Mater. Degrad. 5, 51 (2021).
Brantley, S. L. In Kinetics of Water-Rock Interaction (ed. James D. Kubicki Susan L. Brantley, Art F. White) Ch. 5, 151–210 (Springer, 2007).
Sparks, D. L., Singh, B. & Siebecker, M. G. In Environmental Soil Chemistry. 3rd edn. (eds Donald L. Sparks, Balwant Singh, & Matthew G. Siebecker) 39–104 (Academic Press, 2023).
Tamilselvi, s., Thiyagarajan, C. & Uthandi, S. Calcite dissolution by Bacillus subtilis SSRI02:An invitro study for the reclaimation saline sodic soils. Indian J. Geo-Mar. Sci. 47 (2018).
Subrahmanyam, G., Vaghela, R., Bhatt, N. P. & Archana, G. Carbonate-dissolving bacteria from ‘miliolite’, a bioclastic limestone, from Gopnath, Gujarat, Western India. Microbes Environ. 27, 334–337 (2012).
Han, Z. et al. Mechanism of biomineralization induced by bacillus subtilis J2 and characteristics of the biominerals. Minerals 9, 218 (2019).
Barabesi, C. et al. Bacillus subtilis gene cluster involved in calcium carbonate biomineralization. J. Bacteriol. 189, 228–235 (2007).
Pouliot, J. M., Walton, I., Nolen-Parkhouse, M., Abu-Lail, L. I. & Camesano, T. A. Adhesion of aureobasidium pullulans is controlled by uronic acid based polymers and pullulan. Biomacromolecules 6, 1122–1131 (2005).
Wallander, H. & Wickman, T. Biotite and microcline as potassium sources in ectomycorrhizal and non-mycorrhizal Pinus sylvestris seedlings. Mycorrhiza 9, 25–32 (1999).
Rosenstock, N. P., van Hees, P. A. W., Fransson, P. M. A., Finlay, R. D. & Rosling, A. Biological enhancement of mineral weathering by Pinus sylvestris seedlings – effects of plants, ectomycorrhizal fungi, and elevated CO2. Biogeosciences 16, 3637–3649 (2019).
Szubstarska, J., Jarzyńska, G. & Falandysz, J. Trace elements in variegated Bolete (Suillus variegatus) fungi. Chem. Pap. 66, 1026–1031 (2012).
Kalač, P. In Mineral Composition and Radioactivity of Edible Mushrooms (ed. Pavel Kalač) 75–298 (Academic Press, 2019).
Blaudez, D. et al. Differential responses of ectomycorrhizal fungi to heavy metals in vitro. Mycol. Res. 104, 1366–1371 (2000).
Lian, B., Chen, Y., Zhu, L. & Yang, R. Effect of microbial weathering on carbonate rocks. Earth Sci. Front. 15, 90–99 (2008).
Hernandez, M. E., Kappler, A. & Newman, D. K. Phenazines and other redox-active antibiotics promote microbial mineral reduction. Appl. Environ. Microbiol. 70, 921–928 (2004).
Leyval, C. & Berthelin, J. Interactions between Laccaria laccata, Agrobacterium radiobacter and beech roots: influence on P, K, Mg, and Fe mobilization from minerals and plant growth. Plant Soil 117, 103–110 (1989).
Newman, D. K. How bacteria respire minerals. Science 292, 1312–1313 (2001).
Joergensen, R. G. Phospholipid fatty acids in soil—drawbacks and future prospects. Biol. Fertil. Soils 58, 1–6 (2022).
Troll, V. R. et al. Volcanic particles in agriculture and gardening. Geol. Today 33, 148–154 (2017).
Vandenberghe, R. E., de Resende, V. G., da Costa, G. M. & De Grave, E. Study of loss-on-ignition anomalies found in ashes from combustion of iron-rich coal. Fuel 89, 2405–2410 (2010).
ABAS. 5-252 (Federal Institute for Occupational Safety and Health, 2010).
Noack-Schönmann, S. et al. Genetic transformation of Knufia petricola A95 - a model organism for biofilm-material interactions. AMB Express 4, 80 (2014).
Novozamsky, I., van Eck, R., Houba, V. J. G. & van der Lee, J. J. Solubilization of plant tissue with nitric acid-hydrofluoric acid-hydrogen peroxide in a closed-system microwave digestor. Commun. Soil Sci. Plant Anal. 27, 867–875 (1996).
Ghasemi, A. & Zahediasl, S. Normality tests for statistical analysis: a guide for non-statisticians. Int. J. Endocrinol. Metab. 10, 486–489 (2012).
Christensen, R. In International Encyclopedia of the Social & Behavioral Sciences (eds. Neil J. Smelser & Paul B. Baltes) 473–480 (Pergamon, 2001).
Tukey, J. W. The philosophy of multiple comparisons. Stat. Sci. 6, 100–116 (1991).
Acknowledgements
We would like to thank Christoffer Bergval (IEG, Uppsala University) for his assistance with analytical instrumentation and Veera Nogerius (Department of Ecology and Genetics, Uppsala University) for her support in setting up the experiments and culturing. This research was supported by the European Commission: H2020 EIC pathfinder project Super Bio-Accelerated Mineral weathering: a new climate risk hedging column technology’—‘BAM’ (grant agreement number 964545). This research is a contribution to ROTTnROCK, a research project funded by the European Research Council under the European Union's Horizon Europe Programme (ERC synergy grant number: ERC-2023-SyG 101118491).
Funding
Open access funding provided by Uppsala University.
Author information
Authors and Affiliations
Contributions
T.C.: experiment design, setup, and analyses, data processing, and manuscript writing. E.O. and T.U.: experiment operation, setup, and analyses, data processing, and manuscript editing. T.J.: experimental analyses. A.S.: experiment design and analyses, and manuscript editing. S.V. and M.H.: funding, experiment design, and manuscript editing. T.B. and O.T.: experiment analyses, manuscript editing. R.P.: experimental analysis and design. J.H., I.A.H., and S.E.V.: funding and experiment design. H.N. funding, experiment design, and manuscript editing. M.V.T.: experiment design. A.N. funding, experiment design, analyses, and manuscript editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Corbett, T., Odelius, E., Uebel, T. et al. Microbial dissolution of Gran Canaria lapilli in small-scale flow through columns: carbon dioxide removal potential. npj Mater Degrad 9, 60 (2025). https://doi.org/10.1038/s41529-025-00611-9
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41529-025-00611-9