Abstract
Poly(ester amide) (PEA), which contains ester (–COO–) and amide (–NHCO–) bonds in its molecular chain, is anticipated to exhibit excellent biodegradability, biocompatibility, mechanical strength, and processability through meticulous monomer selection and molecular structure design. As a novel material with significant research value and broad application potential, PEA has garnered increasing attention. This review comprehensively examines recent advancements in bio-based and biodegradable PEA materials, focusing on the effects of bio-based monomers, synthesis methods, and molecular structural parameters-including molecular configuration, sequence architecture, and intermolecular forces on material properties. PEAs derived from α-amino acids, vegetable oils, and plant polysaccharide polyols are highlighted, alongside a detailed comparison of key preparation techniques such as polycondensation, ring-opening polymerization, and enzymatic polymerization. Moreover, the relationships between molecular structures and the thermal, mechanical, and degradation properties of PEAs are thoroughly analyzed. The applications of PEA materials in diverse fields, including drug delivery and tissue engineering in the medical domain, as nanofiltration membranes in separation technologies, and as coatings, are systematically summarized. This review provides valuable insights into the structural design of PEA materials, strategies for regulating biodegradability and functionality, and opportunities for expanding application areas.
Similar content being viewed by others
Introduction
Plastics are essential in daily life but contribute to environmental pollution and accelerate the depletion of non-renewable resources, presenting significant challenges that require urgent attention. Replacing non-degradable polymers with biodegradable alternatives is key to maximize the economic benefits of plastics while minimizing their environmental impact1,2,3,4. Poly(ester amide) (PEA) contains both ester (–COO–) and amide (–NHCO–) bonds within its molecular chain. Ester bonds are readily degradable, while amide bonds are hydrophilic. The nitrogen atoms in amide groups not only promote microbial growth but also form hydrogen bond networks through ester-amide and amide-amide interactions, which enhance the intermolecular forces within PEAs. This property simultaneously confers biodegradability, mechanical strength, and processability to the material. Polyesters such as polylactic acid (PLA), poly(ε-caprolactone) (PCL), and poly(glycolic acid) (PGA) degrade when ester bonds break in microbial or acid-base environments. These materials exhibit good biocompatibility and biodegradability, making them important in applications such as medicine and packaging5,6. However, aliphatic polyester materials generally have weak intermolecular forces, limiting their thermal and mechanical properties. The molecular chain lacks reactive sites for further modification which restricts their application across various fields. In contrast, polyamides, which are linked by amide bonds, form strong hydrogen bond interactions between adjacent molecular chains, resulting in excellent thermal properties, mechanical strength, and corrosion resistance. Consequently, polyamides are widely used in industries such as textiles, automotive, packaging, electronics, and medicine. Although various biobased polyamides have been developed and applied in fields such as hydrogels, most of them lack biodegradability. Fully biodegradable polyamides are relatively rare, with examples including PA4 and poly-γ-glutamic acid (γ-PGA)7,8,9,10,11,12. Through strategic monomer selection and molecular structure design, PEAs can combine the advantages of both polyesters and polyamides, making them a promising polymer material with substantial research potential and application value.
In 1932, Carothers first used ε-aminocaproic acid, diol, and dibasic acid to prepare PEAs13. The material’s flexibility, transparency, and melting point increased with the amino acid content, enabling it to stretch into durable, elastic, and transparent fibers. The exceptional thermal and mechanical properties of PEAs have garnered significant academic and industrial interest. In 1997, a commercial PEA, BAK (trademark of Bayer), was introduced. This thermoplastic, biodegradable plastic can be completely degraded into CO2 and water under composting conditions. It exhibits high flexibility and tensile strength, rendering it applicable to injection molding processes for the production of items such as disposable tableware and fibers14,15. With the continuous advancement in research on PEA materials, their monomer sources, synthetic methods (ranging from polycondensation to ring-opening polymerization), and application areas have been continuously updated and expanded.
Bio-based monomers have attracted widespread attention due to their renewability and environmental friendliness, and have become important raw materials for the synthesis of poly(ester amide) materials. Common bio-based monomers include α-amino acids, vegetable oils, and polysaccharide derivatives. Among them, α-amino acids possess diverse structures, are abundantly available from natural sources, and exhibit excellent cytocompatibility and functional modification capabilities. The advantages of using α-amino acids in the preparation of PEAs include: (i) low toxicity, good cytocompatibility, and biodegradability; (ii) provision of strong amide bonds that enhance the hydrogen bonding network; (iii) the presence of reactive side-chain functional groups that enable further functionalization or chemical conjugation of drugs; and (iv) promotion of favorable cell–polymer interactions16. Natural vegetable oils, such as linseed oil, castor oil, soybean oil17, and palm oil18, are abundantly available, low-cost, environmentally friendly, and biodegradable. These oils primarily consist of triglycerides, containing 94–96% long-chain saturated or unsaturated fatty acids, and are commonly used in melt polycondensation with diacids, anhydrides, and dihydroxy amines to form flexible and heat-resistant PEA materials19. The combination of ester and amide groups with long aliphatic side chains imparts excellent chemical resistance, thermal stability, and flexibility to PEAs20. Reactive sites on the fatty acid chains (such as double bonds, ester bonds, and hydroxyl groups) enable various chemical modifications (e.g., epoxidation, thiol-ene addition). Moreover, the density of hydrogen bonding between amide and ester groups can be tuned to regulate the mechanical properties and thermal stability of PEAs21,22. Polysaccharides such as glucose and isosorbide possess well-defined molecular structures and are rich in hydroxyl groups, making them ideal candidates for constructing hydrophilic polymer backbones. Poly(ester amides) can be synthesized via a variety of methods, including solution polycondensation, interfacial polymerization, melt polycondensation, ring-opening polymerization, and enzymatic catalysis. Solution polycondensation offers mild reaction conditions and relatively low temperatures, making it suitable for the incorporation of α-amino acids and their derivatives. Interfacial polymerization proceeds rapidly under simple conditions, while melt polycondensation, which does not require solvents, is well-suited for large-scale production and is compatible with macromolecular raw materials such as vegetable oils. Table 1 summarizes some of the polycondensation reactions of PEA. Ring-opening polymerization offers excellent reaction rates and structural control, making it ideal for synthesizing high-molecular-weight PEAs and for tuning PEA architectures. Enzymatic catalysis, characterized by its mild, environmentally friendly, and highly selective nature, has emerged as a promising approach in green synthesis. The wide range of biobased monomer sources and various polymerization methods provide significant design flexibility for tailoring the structure and properties of poly(ester amide) (PEA) materials.
The properties of poly(ester amide)s (PEAs) are largely determined by the structural regularity of their molecular chains and the distribution pattern of ester and amide bonds along the backbone. Recent studies have achieved controlled synthesis of PEAs with random, alternating, and block configurations by tuning monomer types, feed ratios, and polymerization pathways. Random structures are typically obtained via one-pot ring-opening polymerization of lactones/lactams or multicomponent melt polycondensation, in which ester and amide bonds are randomly distributed. These structures offer tunable thermal, mechanical, and degradability properties; for instance, increasing the amide content enhances mechanical strength and thermal stability, while higher ester content improves degradability23,24. Block PEAs are synthesized by using preformed polyesters or polyamides as macroinitiators for the ring-opening copolymerization of corresponding lactams or lactones, or by connecting end-functionalized segments. The resulting well-defined block architectures exhibit complementary functions and are commonly employed in the design of amphiphilic copolymers with nanoscale self-assembly capabilities or in materials requiring enhanced mechanical and thermal performance. Alternating PEAs are typically synthesized using intermediates such as diamide-diols or diester-diamines, which ensure the uniform distribution of ester and amide linkages along the polymer chain. This regular chain structure not only facilitates biodegradability but also enhances crystallinity25. However, the complex reaction steps and the need for highly stable intermediates have limited research in this area. In recent years, emerging methods such as solvent-free one-pot synthesis and halogen-induced condensation strategies have provided feasible routes for the efficient synthesis of alternating PEAs, offering simplified processes and improved structural regularity of the resulting polymers14,26,27,28,29. The abundant availability of monomer sources, along with the excellent and tunable properties of PEAs, has made them one of the ideal materials for a wide range of applications, including biomedicine, nanofiltration membranes, and coatings.
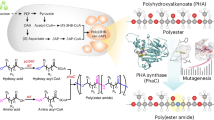
This paper systematically reviews the research progress of bio-based and biodegradable PEAs. The structure and properties of these materials, prepared using α-amino acids, vegetable oils, and polysaccharide polyols as the main monomers, are reviewed. The effects of different preparation methods, including melt polycondensation, solution polycondensation, and ring-opening polymerization, on the structure and properties of PEA materials are discussed. The relationship between structural parameters, such as chain segment sequence structure and molecular chain interactions, and the thermal, mechanical, and degradation properties of PEAs is comprehensively analyzed. Furthermore, the applications of biocompatible PEAs in medical fields, such as drug delivery and tissue engineering, are presented in detail (Fig. 1). This study aims to provide a theoretical reference for the structural design, performance optimization, and application expansion of PEA materials.
A concise overview of poly(ester amide) (PEA), including: Typical biobased sources for PEA synthesis (e.g., α-amino acids, polysaccharides, and plant oils); Major polymerization methods (e.g., polycondensation, ring-opening polymerization, and green synthesis approaches); The structure-property relationships of PEA, focusing on how molecular design influences properties like degradability; Representative applications of PEA in biomedicine, nanofiltration membranes, and coatings.
Synthetic methods for poly(ester amide)s
Poly(ester amide)s based on α-amino acids and their synthesis
α-Amino acids play a crucial role in the development and design of PEA materials. They provide excellent biocompatibility and offer various functionalizable side groups (Fig. 2a)30. Common α-amino acids such as L-glycine, L-alanine (Ala)31, L-isoleucine (Ile)32, L-leucine (Leu)33, L-tyrosine (Tyr)34, L-valine (Val)35, L-methionine (Met)36, and L-phenylalanine (Phe) possess hydrophobic alkyl chains or aromatic rings in their side chains, which are soluble only in organic solvents and are difficult to chemically modify. These are considered non-functional side-chain α-amino acids, which can be incorporated into the PEA backbone to produce Water-insoluble polymers37.On the other hand, amino acids like L-arginine (Arg), L-lysine (Lys), L-histidine (His), L-aspartic acid (Asp), and L-glutamic acid (Glu) typically contain charged functional groups such as carboxyl and amino groups, which can form hydrogen bonds or electrostatic interactions with water molecules. These are classified as functional side-chain α-amino acids. α-Amino acid-based PEAs (AA-PEAs) (Fig. 2b) not only exhibit a chemical structure similar to that of proteins but can also participate in normal physiological metabolism as pseudo-proteins. After simple hydrolysis or enzymatic degradation, the amino acids are released into the circulatory system38.
The common polymerization methods for α-amino acid-based PEA include solution polycondensation and interfacial polymerization. Solution polycondensation typically involves the reaction of α-amino acids with dicarboxylic acids or esters in solvents such as DMF (Fig. 5B). L-Phenylalanine and L-glycine are commonly used to prepare PEA with aromatic or aliphatic backbones. The side groups can influence the material’s hydrophilicity and thermal properties. Han et al.39 prepared PEAs containing Gly, diol, and sebacoyl chloride. These PEAs exhibit lower hydrophilic compared to 4-aminobutyrate-based PEAs without side groups. Phe is often used as a monomer for preparing AA-PEAs. The aromatic side groups significantly enhance the polymer’s thermal stability. Guo et al.40 synthesized a PEA containing unsaturated double bonds in the main chain (UPEA) using Phe, butenediol, and fumaric acid ester as raw materials. The introduction of Phe into the polymer backbone improved the polymer’s thermal stability and degradability by β-chymotrypsin, while the C = C bonds within the polymer skeleton offered reactive sites for subsequent chemical modifications. Ruano et al.41 developed a UPEA-based hydrogel through UV-induced cross-linking of the unsaturated bonds. The resulting structure exhibited a compact morphology with open and interconnected pores, which facilitates ion transport and provides mechanical stability. UPEA-h demonstrated potential as a solid electrolyte for self-powered biomedical implants. Sun et al.42 synthesized an enzyme-reducible α-amino acid-based poly(ester amide)s (SS-PEAs) by conducting a solution polycondensation reaction at low temperature in N,N-dimethylformamide (DMF) using diphenylalanine diester (SS-Phe-2TsOH) and dinitrobenzene hexanedioate (NA) as raw materials. SS-PEAs, whether in membrane or nanoparticle form, exhibited excellent cell compatibility. PEA membranes supported the adhesion and proliferation of fibroblasts, while PEA nanoparticles effectively delivered doxorubicin into cells and rapidly disintegrated under enzymatic conditions (Fig. 3). L-Phe is capable of exerting synergistic effects in combination with other amino acids. Pang et al.43 prepared a series of functional PEAs through solution polymerization of amino acids (L-Phe and DL-2-allyl glycine [AG]) and dicarboxylic acid monomers. The copolymer contains an aromatic ring from L-Phe and a side chain double bond from AG, imparting rigidity and an amorphous structure. The glass transition temperature (Tg) of the PEA was regulated by adjusting the Phe content in the feed. The side chain double bonds in these functional PEA-AG copolymers exhibit high reactivity and offer valuable functionalities, including photochemical reactivity, the potential for conjugation with bioactive agents, and the capacity to design new functional PEA derivatives.
The figure illustrates the structure and function of a disulfide-containing poly(ester amide) (SS-PEA) polymer designed for responsive drug delivery and cell culture applications. The polymer backbone contains disulfide bonds, enabling degradation under reductive intracellular conditions, while ester and amide bonds allow for degradation.
Amino acids with functional side groups, such as L-lysine and L-arginine, can enhance hydrophilicity and introduce reactive sites. Liu et al.44 synthesized arginine-based PEA (Arg-PEA) by chemically bonding bis-L-arginine p-toluenesulfonate (Arg-2-S) with dicarboxylic acid dinitrobenzenesulfonate (NF) in a solution environment. Subsequently, a hydrogel was prepared under UV irradiation through both chemical reaction and electrostatic interaction between Arg-PEA and methacrylic anhydride-modified hyaluronic acid (HA-MA). This hydrogel enables in situ gelation and is suitable for irregularly shaped wounds. By varying the Arg-PEA content, the internal morphology, mechanical properties, and biodegradability of the hydrogel can be finely tuned. At higher Arg-PEA concentrations, the hydrogel exhibits larger pore sizes, faster degradation rates, and lower compressive modulus and brittleness. Due to the abundant ester and amide bonds in Arg-PEA, it is highly susceptible to hydrolysis, enabling sustained release of L-arginine at the wound site, thereby achieving dual anti-inflammatory and antioxidant effects.
Incorporating α-amino acids with functional side chains into PEAs represents a promising strategy for creating engineered tissue scaffolds capable of delivering drugs or facilitating cell signaling26. A simple method is using Lys for introducing functional side chain amino groups. Deng et al.45 adjusted the feed ratio of a Lys derivative, ε-(benzyloxycarbonyl)-L-lysine N-carboxylate hydride (Z-LysNCA), and L-Phe to synthesize a copolymer (PEA-Lys-NH2) composed of L-Phe, diol diester, and sebacic acid (SA). The resulting structure contained pendant functional amino groups, and higher Lys content significantly enhanced hydrolysis and enzymatic degradation rates due to increased hydrophilicity. Similarly, Ghaffar et al.46 synthesized block PEAs from Leu and Lys, demonstrating excellent biocompatibility and biodegradability under enzyme-mediated conditions. Although solution polycondensation is an attractive method for PEA synthesis, the polymer must be rigorously purified after the reaction to remove toxic solvents and by-products. Additionally, this method requires relatively long reaction times and high-purity monomers to achieve stoichiometric balance for the preparation of high molecular weight PEA30,47.
Compared to solution polycondensation, interfacial polycondensation typically involves dissolving the monomers in two immiscible solvents (an aqueous phase and an organic phase). Upon diffusion and contact at the interface, the monomers rapidly react. Since the reactive monomers are continuously supplied at the interface, overall stoichiometric balance is not required. The reaction proceeds rapidly and irreversibly at moderate temperatures (0–50 °C) (Fig. 5C). Fonseca et al.48 prepared PEA via interfacial polycondensation by slowly adding a dichloromethane solution of L-lactate-based diacyl chloride into an aqueous solution containing bis(α-amino acid) diamine synthesized from glycine and diethylene glycol, along with Na₂CO₃. The resulting PEA was obtained as a precipitate. Asp has a negatively charged functional carboxylate side group, which has been widely used to provide side chain carboxylate groups. Soleimani et al.49 synthesized an amphiphilic, photoresponsive Asp-PEA via interfacial polymerization. L-Aspartic acid served as a comonomer forming the hydrophobic backbone, with its carboxylic side chains acting as reactive sites for ester linkage to the anticancer drug paclitaxel (PTX). Poly(ethylene oxide) was conjugated via amide bonds to confer amphiphilicity. Micelles were prepared from aqueous solution using a nanoprecipitation method. Upon entering the body, hydrolysis of the ester bonds released the drug. Under UV irradiation, partial cleavage of o-nitrophenyl esters in the hydrophobic PEA chain occurred, leading to partial disintegration of the micelles and increased exposure of ester bonds to water, thereby accelerating the hydrolytic release of PTX. Li et al.50 used L-Gly and azelaoyl dichloride as raw materials to prepare allylated PEAs through interfacial polymerization and linked alginate via a mercapto-alkenyl click reaction (Fig. 4). Due to the adsorption inhibition of the hydrophobic PEA skeleton and the disruptive effect of the alginate side groups on the ordered arrangement of freezable water molecules, the copolymers were able to inhibit 55% ice recrystallization at 10 mg/mL. This finding offers important guidance for the design and synthesis of polymeric cryoprotectants.
α-Amino acids are thermally unstable and prone to decarboxylation or decomposition under molten conditions, making it difficult to control the polymerization process. As a result, there are relatively few reports on the synthesis of α-amino acid-based PEA via melt polycondensation (Fig. 5A). Ye et al.51 synthesized a biodegradable copolymer P(LA-co-Asp) via simple melt polycondensation of L-aspartic acid (L-Asp) and a polylactic acid (PLA) prepolymer. The resulting copolymer featured a PEA backbone with PLA side chains and exhibited a higher glass transition temperature (Tg) compared to PLA homopolymer. The PEA component was suitable for drug delivery applications. Lu et al.52 synthesized PEA by melt polycondensation of glutamic acid, lactic acid, and glycolic acid under solvent-free conditions. The resulting copolymer was amorphous and characterized by low melt viscosity and a low melting temperature. Asín et al.53 prepared PEA by melt polycondensation of glycine with various diols and dicarboxylic acids. The properties of the resulting products were influenced by the rigidity of the diols. Among the PEAs synthesized, those containing aromatic units exhibited the highest glass transition temperatures. Kadri et al.54 synthesized poly(ester amide)s using ε-caprolactone (CL) and L-phenylalanine (L-Phe) as raw materials via melt polycondensation. The copolymer exhibited excellent thermal stability and glass transition temperature (Tg), which increased to 47 °C with the increase in amino acid content. The PEA demonstrated high cell viability and chemical stability under hydrolytic and oxidative conditions, opening possibilities for the development of materials suitable for biomedical applications, particularly as drug delivery systems.
The α-amino acid-based PEA has been extensively studied and modified into pH and enzyme-responsive nano-assemblies and hydrogels for applications such as drug delivery and wound treatment. Future research should focus on expanding its biomedical applications, such as conjugating fluorescent substances to develop biological probes capable of selectively targeting cancer cells. However, challenges such as limited thermal stability and low polymerization activity have constrained the synthesis of α-amino acid-based PEAs to solution polycondensation methods. This limitation leads to low molecular weight polymers suitable primarily for biomedical applications with moderate thermal and mechanical requirements. Additionally, the potential toxicity of solvents used during polymer synthesis warrants careful consideration. Therefore, solvent-free methods are imperative for preparing α-amino acid PEAs, enhancing molecular weight and performance to expand their application range.
Poly(ester amide)s based on vegetable oils and their synthesis
Plant oils such as linseed oil, castor oil, and palm oil are rich in unsaturated fatty acids and serve as important bio-based monomer sources for PEA synthesis. Their derivatives can form PEA materials through melt polycondensation with dicarboxylic acids, diols, and amine-based monomers (Fig. 6).
He et al.55 synthesized PEAs via melt polycondensation using vegetable oil-derived 21-carbon dicarboxylic acid and hydroxyl-containing straight-chain diamines. By varying the ratio of dicarboxylic acid to diamine, the chain length and cross-linking density were adjusted, resulting in materials with tunable thermal and mechanical properties. The rich hydrogen bonding from esters, amides, and carboxyl groups imparts strong cohesion and interfacial adhesion to the PEA, making it processable through heating-induced rearrangement of hydroxyl ester bonds in the cross-linking network. Flaxseed oil is one of the most widely used vegetable oils. Flaxseed oil-based PEA resin exhibits significantly enhanced hardness, rapid drying, and superior water vapor resistance compared with ordinary alkyd coatings. Alam et al.56 prepared room-temperature-curable PEA coatings by copolymerizing styrene-co-maleic anhydride with linseed oil fatty amide and ethylenediaminetetraacetic acid. These composites demonstrated excellent mechanical properties and corrosion resistance, maintaining stable performance at temperatures up to 150 °C. Castor oil, a renewable polyol derived from castor seeds, serves as a valuable precursor for preparing PEAs via melt polycondensation. Cheng et al.57 synthesized elastic and plastic PEAs by reacting sebacic acid, 4-aminophenyl disulfide (APD), and a castor oil derivative, and PA1010 salt (Fig. 7). The dynamic covalent bonds formed by APD (via disulfide exchange reactions) enable repeated reprocessing of the material without compromising its network structure or mechanical properties. Such innovations highlight the potential of vegetable oil-based PEAs for broader applications. Further enhancing material properties, Vikash et al.58 prepared polyurethane-modified PEA films using castor oil and isocyanate as raw materials. The high hydroxyl content in castor oil ensures a high cross-linking density, thereby improving thermal stability and flame retardancy at room temperature. The choice of comonomer in vegetable oil-based PEAs plays a critical role in determining material performance and offers opportunities for fine-tuning specific properties. Natarajan et al.59 synthesized a bio-based, plant oil-derived PEA for biomedical applications by reacting epoxidized soybean oil, diethylenetriamine (DETA), and dicarboxylic acids of varying chain lengths at 150 °C under catalyst-free conditions. The degradation rate and modulus of the resulting copolymers could be tuned by adjusting the chain length of the dicarboxylic acids.
Poly(ester amide)s based on polysaccharides and Their Synthesis
Polysaccharides are widely distributed in both plants and animals, making them abundant, inexpensive, and easily accessible. They can be extracted from biomass or obtained through biotransformation. Common monomers include monosaccharides (such as glucose and L-arabinose) and polysaccharides (such as glycogen and maltose). The stereochemical diversity of these molecules provides structural tunability, and the abundant hydroxyl groups along the molecular chains impart hydrophilicity and offer sites for post-modifications like methylation. These characteristics make polysaccharides ideal raw materials for the flexible segments of PEA27,60.
Polysaccharide-derived PEAs exhibit excellent hydrophilicity, making hydrolysis a central focus of research efforts. Pinilla et al.61 synthesized PEAs containing L-arabinose and 5-amino-1-O-[(pentachlorophenoxy)glutaryl]pentanol to investigate the factors influencing PEA hydrolysis. Their findings revealed that both crystallinity and hydrophilicity play critical roles in PEA degradation, with hydrophilicity positively correlating with the degradation rate. Additionally, altering the chain length of dicarboxylic acid monomers modulates the hydrophilicity/hydrophobicity of the copolymers, which in turn allows for controlled degradation of PEA. Gomurashvili et al.62 esterified isosorbide with α-amino acids in the presence of p-toluenesulfonic acid, and isolated the resulting bis(p-toluenesulfonate ammonium) ester, which was then reacted with dicarboxylic acids of different chain lengths to synthesize PEA. The enzymatic degradation behavior was evaluated using chymotrypsin or lipase. PEAs derived from long-chain dicarboxylic acids exhibited higher hydrophobicity and a reduced susceptibility to chymotrypsin-catalyzed hydrolysis. Similar trends were observed in the preparation of biodegradable PEAs using isosorbide60.
In addition to isosorbide, Okada et al.63 investigated the synthesis of a series of PEAs composed of D-sorbitol, alanine, glycine, and p-nitrophenyl fatty acid esters with methylene chain lengths ranging from 4 to 10, and examined the influence of molecular structure on their biodegradability. During enzymatic degradation, PEAs containing shorter methylene chains were more readily degraded by porcine pancreatic lipase. Moreover, most of the poly(ester amide)s were degraded faster by papain compared to their corresponding polyesters lacking amide linkages. Inmaculada et al.64 prepared a series of stereo-structured aromatic PEAs via polycondensation of amino-tri-O-methyl-xylitol with aromatic diacids, including terephthalic acid, isophthalic acid, and thiophene-2,5-dicarboxylic acid. These hydrophilic, amorphous PEAs primarily undergo hydrolysis through the cleavage of ester bonds. Their high hydrolysis rates result in final compounds that retain their amide structures.
Preparation of PEA by ring-opening polymerization
Cyclic monomers, including L-LA, ε-caprolactone (CLO), ε-caprolactam (CLA), morpholine-2,5-dione, and their derivatives, are primarily used in the preparation of PEAs through ring-opening polymerization. These monomers undergo polymerization initiated by low-molecular-weight reactive molecules, forming corresponding PEAs. Common catalytic systems for ring-opening polymerization (ROP) include alkali metals (such as sodium and magnesium), metal salts (such as Sn(Oct)₂), and organic bases (such as DBU). The ROP reaction conditions are mild, and the stereochemistry of the polymer can be controlled. The ring-opening copolymerization (ROP) of CLA and CLO is a common method for the preparation of PEA (Fig. 8).
Using CLO and CLA as raw materials and sodium caprolactam as the catalyst, Mateva et al.65 synthesized PEAs with ester/amide ratios ranging from 75/25 to 10/90 at 160 °C. The tensile properties of the copolymers were directly proportional to the amide content, with higher amide content promoting crystallization. Compared with conventional sodium metal, magnesium bromide caprolactam exhibits lower sensitivity to polar impurities, resulting in higher molecular weight quality and purity in the PEAs prepared with it as the initiator. Brozek et al.66 used caprolactam magnesium bromide as a catalyst for the ROP of CLO and CLA, producing high-molecular-weight PEAs with CLO content ranging from 0% to 100% and melting points between 50°C and 211°C, depending on the CLO unit content. Deshayes et al.67,68,69 synthesized P(CLO-co-CLA) using hypophosphorous acid as a catalyst at 250 °C. By altering the monomer addition sequence of CLO and CLA, they prepared PEA with both random and block structures. In the former, the two cyclic monomers were added simultaneously, while in the latter, the cyclic monomers were added sequentially.
Poly(lactic acid) (PLA) is a typical biodegradable polyester, and the corresponding Poly(ester amide)s can be synthesized through the ring-opening polymerization of lactide. Basterretxea et al.70 investigated the catalytic effects of two different organic catalysts (Brønsted acids and bases) on the copolymerization of lactide (L-LA) and ε-caprolactam. The resulting polymers exhibited three distinct microstructures: block, random, and alternating, depending on the catalyst type and feeding ratio. Under Brønsted base catalysis, high concentrations of L-LA produced random copolymers, while high concentrations of ε-caprolactam favored block copolymer formation. In contrast, Brønsted acid catalysis resulted in completely reversed structural trends, with high L-LA concentrations favoring block structures and high ε-caprolactam concentrations yielding random copolymers. Hong et al.71,72 utilized mechanochemical ball-milling method to initiate the ring-opening polymerization of lactide with amino-terminated PA11, thereby synthesizing a PLA-b-PA11-b-PLA triblock PEA. They further prepared (PLA-b-PA11)n multiblock copolymers via diisocyanate coupling. The resulting multiblock copolymers exhibited high toughness, yield stress, and modulus comparable to PLA, along with thermoplastic processability. When used as compatibilizers in PLA/PA11 blends, the block PEA significantly enhanced interfacial adhesion, attributed to the “interpenetration” effect at the interface (Fig. 9).
An alternative strategy for ring-opening polymerization (ROP) involves the homopolymerization of cyclic monomers that simultaneously contain ester and amide linkages in their molecular structure. Common monomers in this category include morpholine-2,5-diones (MDs), 2-azoline, and their derivatives. Poly(morpholine-2,5-dione)s, also known as polydepsipeptides, are alternating copolymers of α-amino acids and α-hydroxy acids. The ROP of MD analogs is typically carried out by direct polymerization with stannous octanoate (Sn(Oct)2) as the catalyst and alcohols or water as initiators, under vacuum at temperatures of 110–160 °C. In 1975, Goodman et al.73 obtained PEAs through the ring-opening polymerization of morpholine-2,5-dione with stannous octoate as catalyst and alcohol or water as initiator; the reaction conforms to the first-order kinetics. The narrow molecular weight distribution and high monomer conversion rate are advantages of Sn(Oct)2 as a catalyst, but stannous octoate has certain toxicity, the reaction is difficult to control at high temperatures, and side reactions are difficult to prevent. To address these issues, organocatalysts have emerged as excellent alternatives to Sn(Oct)2. Lian et al.74 investigated methionine-derived morpholine-2,5-dione organocatalyzed ROP using 1,8-diazabicyclo[5.4.0]undec-7-ene/thiourea (DBU/TU) binary organocatalysts to overcome the problem of uncontrollability of the reaction catalyzed by metal catalysts (Tu eliminates the interaction of MD nitrogen protons with DBU and prevents uncontrolled ROP). The PEA product has a high molecular weight (8.1–28.2 kg/mol) and a narrow molecular weight distribution (1.07–1.12). The thioether group hanging from the polymer molecular chain can be used as a “click” reaction site to introduce functionalized groups (Fig. 10).
Organocatalytic polymerization process, where methionine-containing monomers are polymerized using an organocatalyst to form the PEA backbone, and the postpolymerization modification of the PEA, where the methionine side chains are chemically modified to introduce functional groups, enabling further tailoring of the material properties.
Green synthetic methods such as enzymatic catalysis
Enzymatic catalysis is an efficient method for preparing PEAs. This process involves the synthesis of polymers through a biosynthetic pathway catalyzed by enzymes, which operate under mild reaction conditions. Enzymes are highly enantioselective, generate few side reactions, and are derived from renewable resources. Additionally, they are environmentally friendly, and non-toxic. One of the key advantages of enzymatic catalysis is that it requires less stringent post-processing compared to traditional catalysts, making it especially suitable for biomedical applications.75. Sharma et al.76 investigated the Saccharomyces cerevisiae lipase B (N435) catalyzed polycondensation reaction of dimethyl adipate, diaminosiloxane, and 1,8-octanediol to obtain a PEA containing organosilicon units with an Mn of 6000–11,000 g/mol and polydispersity of 1.5–2.2. Meimoun et al.77 added straight-chain fatty diamines dissolved in dichloromethane to a mixture of acetic acid, triethylamine, and ethyl chloroformate; sodium borohydride was added dropwise and stirred to produce fatty diol diamine monomers with different chain lengths. Then, they were condensed with diethyl adipate under N435 catalysis to obtain PEA. The number average molecular weight of the product was 1500–7200 g/mol, the dispersion was 1.4–1.8, and the yield was 56–84%. Change in chain length of the fatty diol can adjust the Tg of the polymer.
In addition to the common methods previously discussed, PEAs can also be prepared using other techniques, such as solid–liquid polycondensation78 and microwave-assisted methods79. In situ or ex situ polymerization for the preparation of hyperbranched PEAs has been developed80,81. For example, Li et al.82,83 synthesized hyperbranched PEAs by reacting dicarboxylic acids with polyhydroxy sec-amides (Fig. 11). The sec-amides reacted with one of the carboxyl groups of the dicarboxylic acids to form an ABx intermediate, which underwent thermal condensation in the absence of a catalyst, resulting in aliphatic and semi-aromatic hyperbranched PEAs with multiple hydroxyl groups. The abundant hydroxyl groups at the polymer terminals can serve as reactive sites for subsequent modification.
The cost of the polycondensation reaction is low, and the preparation of PEA in the molten state is simple and efficient, making it promising for industrial production. However, solution polycondensation requires a long reaction time, and the molecular weight achieved only meets the minimal requirements for biomedical applications with low molecular weight demands. Ring-opening polymerization of lactams and lactones combines the advantages and disadvantages of both polyamide and polyester materials. There is significant scope for performance optimization, with structural adjustments possible through catalyst selection and feeding strategies. Alkali metal catalytic systems for preparing PEAs offer high yields but generate metal residues, which are undesirable for biomedical applications. Organic catalysis requires a long reaction time, and the molecular weight and its distribution are difficult to control. Additionally, the ester/amide ratio in the copolymer often deviates significantly from the theoretical composition. An ideal catalytic system for controlling the ring-opening copolymerization of PEA has yet to be developed, and the material properties still require further optimization. Future research should prioritize the exploration of novel cyclic monomers and selective green catalysts to enable the efficient, versatile, and functionalized design of PEA materials.
Regulation of poly(ester amide)s structure and properties
Poly(ester amide)s are promising materials owing to their modifiable structures and tunable properties. The properties of PEAs strongly dependent on the structure of the molecular chains (random, alternating, or block), the type of monomers or prepolymers employed, and the monomer feed ratio and sequence. Adjusting the chemical composition and microstructure of the polymers can tailor their material properties, such as thermal, mechanical, and biodegradation behaviors (Fig. 12).
Structural design and modulation of Poly(ester amide)s
As one of the most promising classes of materials, poly(ester amide)s (PEAs) feature a readily modifiable structure and tunable properties. The performance of PEAs is highly dependent on the molecular architecture—whether random, alternating, or block—as well as the types of monomers or prepolymers used and the monomer addition sequence. By tailoring the chemical composition and microstructure of the polymer, key properties such as thermal behavior, mechanical performance, and degradability can be systematically regulated. Nakayama et al.84 used n-butyllithium to catalyze the ring-opening copolymerization of butyrolactam (PRN) and CLO, by varying the molar ratio of PRN to CLO, which modulated the amide-to-ester bond ratio. The melting point of the copolymers increased proportionally with amide content, ranging from 60 °C to 265 °C. Microbial degradation tests revealed that amide-rich copolymers degraded rapidly, while CLO-rich PEAs exhibited rapid degradation during enzymatic hydrolysis with lipase. Wang et al.85 carried out the one-pot melt polycondensation of 1,4-butanediol, 1,10-decanediamine (DD), 1,10-decanedioic acid, and itaconic acid to prepare random linear PEAs. Increasing the DD content in the feed elevated the proportion of amide groups in the copolymer chains, resulting in higher Tg, tensile strength, and thermal decomposition temperatures, while elongation at break decreased. Amide groups contribute to the mechanical properties of PEAs, while ester bonds enhance toughness and degradation behavior. PEAs with DD contents of 30% and 50% exhibit higher tensile strength and excellent degradation properties. Using melt polycondensation, Ding et al.86 copolymerized polycaprolactam prepolymers with PLA prepolymers to prepare PEAs. The introduction of polycaprolactam segments affected PLA chain movement, increasing its glass transition temperature, melting point, and enhancing the thermal stability of the copolymers.
In block-structured PEAs, polyester or polyamide is incorporated as segments within the molecular chain, allowing each segment to retain its inherent characteristics. For example, PEG serves as a hydrophilic segment, forming an amphiphilic block copolymer with a hydrophobic α-amino acid-based PEA. This copolymer spontaneously assembles into nanoscale micelles with a core/shell structure in water, suitable for drug delivery. Block PEAs can be synthesized through the ring-opening polymerization of pre-synthesized polyester or polyamide as macromolecular initiators or via end-group linking reactions. Chen et al.87 prepared a PLLA-b-PA4 block PEA by the “click” reaction between thiolated PLLA and alkenylated PA4. They also studied the electrospinning fiber properties of PEAs; they found that the PLLA block remained amorphous when it was shorter than the PA4 block; when the PLLA block was long, each block formed independent crystalline regions, but crystallization was mutually constrained; the melting points of both blocks were lower than their respective homopolymer melting points. PLLA-rich copolymer electrospun fibers exhibited high hydrophobicity, smooth surfaces, and ultrafine, bead-free morphologies. The average fiber diameter was influenced by the PLLA chain length. As the chain length increases, entanglement causes the diameter to increase from 620 ± 61 nm to 850 ± 60 nm. Hashimoto et al.88 synthesized block Poly(ester amide)s PEG-b-PA4 by employing a PEG-based macromolecular initiator for the anionic ring-opening polymerization of butyrolactam. PEG enhanced the flexibility of PA4 but impeded the degradation of the copolymer. Hideaki et al.89 achieved efficient condensation of amino-terminated PBS and carboxyl-terminated PA4 in solution to synthesize block-structured PEA with a molecular weight of Mn=6.6 kDa. They investigated the amphiphilicity and self-assembly behavior of the resulting immiscible block copolymers.
Alternating structures are typically synthesized by reacting intermediates, such as diamide diols, diester diamines, and diamide diacids, with diacids and diols. Owing to their excellent biocompatibility, α-amino acids are often utilized in the synthesis of diester diamine and diamide diacid intermediates. However, amino acids are prone to self-polymerization during polymerization. To prepare intermediates, they usually undergo a protection-deprotection process before reacting with diacids or diols. The steps are complex, and the intermediates exhibit poor thermal stability90,91. Nguyen et al.92 utilized various diacids and ethanolamines to first synthesize diamide diols, which were then reacted with diacids to produce alternating PEAs. The copolymer molecular weight ranges from 4400 mol/g to 12,000 mol/g and the properties were modulated by varying the methylene chain length of the diacids, resulting in melting points ranging from 118.7 °C to 150 °C and demonstrating good thermal stability. Jang et al.93 prepared diamide diols by reacting glycolide with diamines of different methylene chain length and then prepared alternately structured PEAs by reacting them with diacids under solution polymerization conditions; they found that this preparation process required a long reaction time for the materials to have the desired mechanical properties.
To address the challenges of multistep polymerization, high monomer purity requirements, and organic solvent usage in the synthesis of alternating PEAs, Liu et al.28 proposed a solvent-free, one-pot strategy using water as a regulator. This method leverages the difference in the equilibrium constants of amidation and esterification reactions (Fig. 13). By introducing a controlled amount of water and nitrogen gas at the early stage of polymerization, esterification is suppressed, allowing the preferential amidation of adipic acid and amino alcohol to form di-amide diol intermediates. Subsequently, polyesterification is initiated by vacuum-driven water removal. The resulting alternating PEA (Tm = 102 °C, ΔHm = 30 J g−1) exhibits a well-ordered arrangement of ester and amide bonds in the molecular chain, demonstrating significantly superior properties compared to the corresponding random PEA (Tm = 72 °C, ΔHm < 1 J g−1). Additionally, Zeng et al.29 developed alternating PEAs without relying on intermediates. This was achieved by first synthesizing an AB-type PEA prepolymer via the amidation of 6-bromohexanoyl chloride and 6-aminocaproic acid, followed by repeated nucleophilic reactions. A comparison of the thermal properties revealed that the alternating PEA exhibited a higher melting point than the random PEA of the same composition. Moreover, the alternating PEA demonstrated a more regular microstructure with a narrower melting peak. These PEAs also displayed a critical dissolution temperature in DMSO, transitioning between opaque and transparent states at low and high temperatures, respectively.
Performance modulation of Poly(ester amide)s
Thermal properties
PEA molecules contain repeating ester and amide groups in their main chains, forming ester-amide and amide-amide hydrogen bonds that strengthen intermolecular interactions and enhance thermal properties. PEAs synthesized by the one-pot method typically exhibit random structures. Adjusting the feed ratio of monomers, such as diamine and diacid, alters the ester-to-amide ratio in the copolymer, which directly impacts thermal properties such as Tm and Tg. Feng et al.94 synthesized PEAs from 1,3-diamino-2-propanol (DP) and dimethyl 2,5-furan dicarboxylate (DMFDCA) using solution polycondensation in a microreactor. Increasing the DMFDCA/DP ratio elevates the ester bond content in the backbone, reducing hydrogen bond density and yielding PEAs with tunable Tg values (144.2–114.7 °C) and high thermal stability, The thermal decomposition temperature reached 330 °C. Similarly, Ding et al.95 synthesized carboxyl-terminated prepolymers through melt polycondensation of amide diol (ADM) and diacid, followed by copolymerization with polyether diamine (PD). They found that decreasing ADM content lowered amide bond density, while PD side groups disrupted crystallinity, which reducing Tg values. In addition to varying the feed ratio, the odd–even number of methylene units, the rigidity of copolymerized monomers and steric hindrance significantly influence the thermal properties of PEAs. Soleimani et al.96 used melt polycondensation and interfacial polymerization to synthesize PEAs with diols of varying methylene chain lengths, dicarboxylic acids, and α-amino acids (Ala and Phe). PEAs with rigid units (e.g., short-chained diols or cyclohexyl/aromatic structures) exhibited higher Tg and viscosities, while those with flexible, long-chained diols were semicrystalline. Jang et al.93 synthesized poly(ester amide)s (PEAs) via polycondensation of diamino diols derived from glycolide, containing varying numbers of methylene units, with aliphatic dicarboxylic acids of different chain lengths. The introduction of amide bonds enhanced the thermal decomposition temperature of the materials, thereby broadening the processing window of PGA. The study investigated the effect of methylene unit number on the thermal properties of the materials and found that PEAs with an odd number of methylene units exhibited lower melting temperatures compared to those with an even number. This phenomenon was attributed to differences in crystal structure and the orientation of dipole moments. The steric configuration of copolymerized monomers impacts the molecular chain’s regularity and crystalline properties. Tsuji et al.97,98 investigated the crystalline, thermal, and degradation properties of enantiomeric random copolymers P(LLA-LAL) and P(DLA-DAL) and found that the alanine content affected the properties of the copolymers with different crystalline configurations to different extents. The strength of hydrogen bonding between amide bonds of the same configuration is higher than that between opposing configurations, resulting in polymers with high Tm.
Block-structured PEAs exhibit distinct morphological and thermal behaviors compared to their corresponding random copolymers. They can be utilized as thermoplastic elastomers, compatibilizers for blends, and interfacial modifiers. Typically, random PEAs are miscible in the amorphous state, displaying a single glass transition temperature (Tg) and melting temperature (Tm). In contrast, the individual blocks of block PEAs are mutually incompatible, undergoing phase separation and crystallizing independently in the molten state47. Deshayes et al.67,68,69 compared the effects of block versus random architectures on the thermal properties of P(CLO-co-CLA) copolymers. In block PEAs, increasing the CLO content resulted in only a slight decrease in the melting point of the CLA block—from 198.4 °C to 191.1 °C. In contrast, random copolymers with similar compositions exhibited a significant reduction in melting point, from 203.1 °C to 92.4 °C, due to their disordered microstructure. Using native polymerization, Gu et al.99 synthesized triblock PEAs by first preparing a PA6 prepolymer through the ring-opening polymerization of caprolactam (CPL) and then using tetraphenyltin as a catalyst to initiate the ring-opening polymerization of caprolactone (CL) (Fig. 14). They investigated the relationship between the CPL/CL feed ratio and the resulting copolymer properties. At high CPL content, PA6 dominated the composition, and the PCL chains were too short to crystallize. Instead, the PCL chains integrated as defects within the PA6 crystal lattice. The copolymer exhibited a single melting peak, with a melting point 10 °C lower than that of pure PA6. Conversely, at low CPL content, the increased PCL ratio allowed the two components to crystallize independently, producing distinct melting points. Some PCL chains also formed hydrogen bonds within the PA6 crystalline regions, creating a eutectic structure that reduced the Tg of the PA6 segments.
Mechanical properties
PEAs composed of rigid amide segments and flexible ester segments offer adjustable mechanical properties due to the distinct roles of each phase. Amide segments, as hard phases, enhance physical cross-linking, dissipate energy, and increase the strength and modulus of the material under tensile stress. In contrast, ester segments, as soft phases, enhance flexibility and ductility. The mechanical properties of the copolymers can be tailored by varying the molecular weights and contents of the hard and soft segments. Gao et al.100 introduced 1,12-dodecanediamine into PET under melt conditions to synthesize PEAs. The incorporation of amide bonds led to hydrogen bonding, which enhanced intermolecular interactions, resulting in increased crystallinity and improved mechanical properties. PEA films prepared via extrusion and biaxial stretching exhibited lower water and oxygen permeability compared to pure PET polyester films. Lipsa et al.101 used 1,4-diaminobutane and CLO to form a bisamide diol prepolymer, which was then melt-polycondensed with 1,4-butanediol and dimethyl adipate for PEA preparation. By changing the ratio of bisamide diol to 1,4-butanediol, a polymer with amide content ranging from 10 mol% to 85 mol% was obtained. As the amide content increased, the elastic modulus increased from 70 MPa to 524 MPa, fracture stress increased from 8 MPa to 28 MPa, and fracture strain decreased from 820% to 370%. Periodicals et al.102 prepared block-structured PEAs using azo-capped PA4 to initiate the free radical polymerization of vinyl acetate with different molar ratios and investigated the relationship between the composition of the blocks and their properties, which were composition dependent. Tensile strength increased with PA4 segment content, whereas the elongation at break increased with poly(vinyl acetate) segment content. Using the melt polycondensation method, Liu et al.103 synthesized PEAs using polybutylene glycol (PTMG) as the soft segment and PA6 as the hard segment (Fig. 15). The inclusion of PTMG transformed the brittle fracture behavior of PA6 into ductile fracture. With increasing PTMG content, the elongation at break improved significantly. PEA with 55% PTMG content demonstrated the highest tensile strength (9.2 ± 1.1 MPa) and elongation at break (569% ± 100%).
Degradation performance
Amide bonds within the molecular chains of PEAs exhibit moderate hydrophilicity, which increases the susceptibility of ester bonds to enzymatic attack, ultimately leading to molecular chain scission. This property underscores the potential of PEAs as promising candidates for biodegradable materials. Comprehensive studies have been conducted to explore the cellular interactions and biodegradation mechanisms of PEAs.The degradation rate of PEAs accelerates under extreme pH conditions (both acidic and alkaline) and elevated temperatures104. The hydrolysis of PEA occurs mainly in the amorphous region, and thus the degree of crystallinity is crucial for the degradation of PEAs. To increase the biodegradation rate of PEAs, enzymes are usually added to a buffer solution, and enzymes involved in the degradation of PEAs include esterases, protein hydrolases (proteases), and lipases5.
Nakayama et al.105 synthesized γ-aminobutyric acid (GABA)-based PEAs via solution polymerization and evaluated their degradability in seawater. The study revealed that aliphatic PEAs with short-chained diol units degraded faster than those containing dicarboxylic acid units. This finding indicates that biodegradation primarily occurs through the cleavage of ester bonds between GABA and diol units rather than through the amide bonds between GABA and dicarboxylic acid. Furthermore, the degradation rate was found to be influenced by both hydrophilicity and crystallinity, increasing with higher ester content in the molecular chain and decreasing with higher crystallinity. Lee et al.106 synthesized PEAs with amide content varying from 10 mol% to 30 mol% using adipic acid, 1,4-butanediol, and 1,4-butanediamine as monomers and investigated the biodegradation process; the hydrophilicity of the copolymer improved with increasing amide content, thus increasing the degradation rate. IR spectroscopy results showed that the area ratios of all the amide characteristic bands increased during hydrolysis, and the area ratios of the amide–ester hydrogen bonding bands decreased, indicating that degradation occurred mainly in the ester region and that degradation of the ester unit led to the decomposition of the amide–ester hydrogen bonds. Abid et al.107 studied the acid hydrolysis rate of sulfonated furan PEAs at 37 °C. The results demonstrated that an increase in the amide content within the copolymer backbone enhanced hydrophilicity, which significantly accelerated the hydrolysis rate.
The rigidity and chemical isomerization differences of copolymer monomers are important factors affecting the degradation rate. Al-Tayyem et al.108 reported the synthesis and hydrolytic degradation characteristics of AA-PEAs derived from isosorbide and α-amino acids. The side groups of α-amino acids played a critical role in the degradation process. Polymers with hydrophobic side chains or rigid dicarboxylic acid groups exhibited decreased degradation rates due to the polymer chain stacking, which hindered the penetration of degradation media into the polymer matrix. Fan et al.109 investigated the reaction of phenylalanine with varying enantiomeric compositions (L/D ratios) to synthesize AA-PEAs. The L/D ratio did not affect the polymer yield or molecular weight. However, PEAs based on L-amino acids exhibited enhanced enzyme specificity, and the enzymatic degradation of PEAs derived from L-Phe was significantly superior to that of PEAs based on D-Phe.
These findings provide a framework for tailoring the thermal, mechanical, and degradation properties of PEA materials. Factors such as the rigidity of the polyester–amide monomer and the ratio of ester to amide bonds significantly influence the final material properties. The ester groups confer degradability and molecular chain flexibility, while the amide groups enhance intermolecular interactions through non-covalent bonding, contributing to improved thermal stability and energy dissipation during mechanical stretching. Consequently, the material exhibits high modulus and strength. Bulky and rigid copolymerization monomers, either in the main chain or as side groups, can introduce significant steric hindrance. This resistance restricts the mobility of molecular chains, thereby reducing flexibility. PEAs tend to exhibit an amorphous form with high thermal stability and viscosity. The chemical isomerization of PEA monomers primarily impacts biodegradation. PEAs synthesized from L-amino acids degrade more rapidly due to the enzyme specificity of proteases. The large design space and diverse molecular structures of PEAs provide considerable potential for modulating material properties to meet practical application requirements.
Application of poly(ester amide)s
The demand for biodegradable and biocompatible materials has increased significantly in biomedical fields, particularly in applications such as drug delivery, tissue scaffold engineering, and gene delivery vector preparation. PEAs offer significant potential in these areas due to their ability to degrade under various enzymatic and non-enzymatic conditions. Moreover, their monomers can be derived from a range of non-toxic metabolic intermediates, making them highly versatile and safe for biomedical use. Various forms of PEA-based materials, including particles, microspheres, hydrogels, three-dimensional scaffolds, electrospun fibers, and fibrous membranes, have been developed. These materials exhibit excellent biocompatibility and tunable degradation rates, which are critical for applications such as sustained drug release, cell growth support, and tissue regeneration. Their adaptability and customizable properties make PEAs a promising candidate for advancing biomedical technologies.
Drug delivery
Drug delivery systems aim to enhance target specificity, reduce systemic toxicity, and protect drugs from biochemical degradation. The incorporation of amino acids into nanodrug delivery systems offers additional therapeutic benefits by modulating signaling pathways linked to cellular metabolism, thereby improving efficacy110,111. AA-PEAs with excellent processability, biocompatibility, and biodegradability, making them ideal candidates for drug delivery carriers. Chudecka–Głaz et al.112 synthesized hydrophobic polymer segments by reacting tyrosine derivatives with diacids. These segments were copolymerized with PEG to produce a block PEA with both hydrophilic and hydrophobic domains, capable of drug encapsulation. The drug release rate was controlled by the molecular weight of PEG, with lower molecular weights leading to faster release. Xin et al.113 synthesized Phe-PEA with good drug-carrying properties using Phe, dibasic acid, and diol as raw materials and used nanoprecipitation to prepare hydrophobic doxorubicin (DTX) carriers Phe-PEA nanoparticles for sustained drug release. Phe-PEA nanoparticles have smaller particle sizes and higher DTX loading, which can effectively inhibit HSCLC tumor growth, block tumor metastasis, and increase apoptosis. Zhang et al.114 prepared hydrogels for transdermal drug delivery of insulin by crosslinking arginine-based PEA (Arg-PEA) with polyvinyl alcohol dipropylene amide under light irradiation. Arg-PEA not only carries a positive charge, enabling the adsorption of protein drugs and transdermal peptides, but also crosslinks with the polymer to form a hydrogel scaffold. The hydrogel exhibits excellent mechanical properties, is suitable for cell growth without causing skin irritation, and can continuously release insulin and transdermal peptides to effectively regulate blood glucose levels. Khuddus et al.115 reported amide side-chain-functionalized polyesters based on biogenic side groups, such as L-aspartic acid, diols, and aromatic or aliphatic compounds (Fig. 16). Their study explored the influence of copolymerization monomers on the thermal properties of PEA. Furthermore, they grafted hydrophilic PEG onto the side chains to produce amphiphilic PEA-PEG, which self-assembled in aqueous media into spherical nanoparticles with an average size of 140 ± 10 nm. These nanoparticles demonstrated superior encapsulation efficiency and sustained release capabilities for various anticancer drugs.
The process of solvent-free melt polycondensation, where L-aspartic acid and a polyol are polymerized under heat to form the L-aspartyl PEA, occurs without the need for a solvent, making the process more environmentally friendly and efficient. The figure highlights the L-aspartyl PEA is used to encapsulate anticancer drugs and release them within cancer cells, improving cellular uptake and controlling the drug release profile.
Tissue engineering
Tissue engineering focuses on tissue regeneration and restoring organ function by implanting cells or tissues cultivated in vitro, or by stimulating cell growth within an implantable matrix47. Three-dimensional scaffolds or electrospun fibers prepared with biocompatible PEA materials exhibit low cytotoxicity and inflammatory properties. These properties contribute to cell adhesion, proliferation, growth, and differentiation, making them suitable for the recovery of traumatized organs or tissues. Stone et al.116 incorporated reduced graphene oxide into PEA-chitosan fiber scaffolds to improve their electrical conductivity, mitigate the low porosity of electrostatic spinning through ultrasonication, and effectively promote cell penetration and tissue maturation; the PEA scaffolds showed excellent performance in supporting cardiac differentiation. He et al.117 developed PEA-based hybrid hydrogels using arginine-based unsaturated poly(ester amide)s (Arg-UPEA) and glycidyl methacrylate chitosan, cross-linked under UV light. These hydrogels effectively regulated arginase activity and arginine metabolism in traumatized macrophages, promoting wound healing. Zhou et al.118 synthesized photo-cross-linked bioactive hydrogels using Arg-UPEA and HA-MA as raw materials. Arg-UPEA, efficiently loaded into the hydrogels through chemical bonding and charge interactions, can provide high NO production and arginase activity, promoting calcium deposition and cellular osteogenesis.
Gene transfer
Gene therapy aims to transfer genetic material, functional genes, or DNA/RNA fragments into specific cells to prevent or treat a disease119. PEA has low cytotoxicity and biocompatibility, simplifying DNA delivery and intracellular unpacking, thus improving transfection120. Dai et al.121 synthesized a cationic L-Arg-based PEA with high plasmid DNA binding capacity. The transfection efficiency in smooth muscle cells was comparable to that of the commercial reagent Superfect, but the cytotoxicity was significantly lower. This PEA facilitated DNA delivery via a temperature-sensitive endocytosis mechanism, which provided an efficient and biocompatible pathway for gene delivery. Using Arg-based PEA, You et al.122 developed a series of structurally tunable Arg-PEA molecules and combined them with nanoparticles through ionic electrostatic interactions (Fig. 17). Small structural modifications, such as variations in CH2 unit length, effectively tuned the physicochemical properties, including hydrophobicity, nanoparticle size, and zeta potential. These adjustments enabled precise regulation of transfection efficiency and gene silencing. Arg-PEA/CpG nanoparticle complexes demonstrated controlled immune response modulation to CpG and optimized release rate for various nucleic acids, making them promising candidates for gene delivery applications.
Nanofiltration membranes
Poly(ester amide)s (PEA) selective layers synthesized via interfacial polymerization between the hydroxyl and amino groups of hydrophilic monomers and tricarbonyl chloride (TMC) are rich in ester and amide bonds. These layers exhibit high cross-linking densities and narrow pore size distribution123. Thin-film composite nanofiltration (TFC) membranes, comprising a selective layer, support layer, and non-woven fabric, are fabricated through techniques such as interfacial polymerization and layer-by-layer assembly. These membranes are widely applied in water treatment and separation processes. However, traditional polyamide selective layers are prone to chlorine degradation and membrane fouling, leading to reduced performance and lifespan in practical applications. Jayarani et al.124 investigated the chlorine resistance of various TFC membranes and demonstrated that ester bonds remain unaffected by chlorine corrosion. This finding suggests that incorporating ester-containing monomers in aqueous solutions during membrane fabrication can yield PEA-based nanofiltration (NF) membranes with enhanced chlorine resistance. Furthermore, unreacted hydroxyl groups on the surface of PEAs contribute to improved antifouling properties.The separation performance of PEA membranes can be modulated by altering the type of hydroxyl-containing monomer or adjusting the ester/amide ratio in the polymer structure125. Such tunability enables the optimization of membrane characteristics for specific applications, addressing key challenges in the water treatment industry.
Cheng et al.126 prepared a PEA nanofiltration membrane using bis(triphenylpropane) and TMC on a polyether sulfone ultrafiltration membrane (Fig. 18). The Poly(ester amide)s layer has a negative charge and hydrophilicity due to the carboxyl and hydroxyl groups on its surfaces. The LNF membrane exhibits a rejection rate of over 96% for various dyes and 30% and 10% for Na2SO4 and NaCl, respectively, demonstrating good separation of dye/salt mixtures. Rezania et al.127 further advanced PEA membranes by designing a carboxylaromatic diamine diol (CDA) monomer. The hydrophilic carboxyl and hydroxyl groups on the CDA surface contributed to high permeability (5.3 LMH·bar−1) and superior antifouling properties. The membrane achieved salt rejection rates of 91% for Na2SO4, 70% for NaCl, and 17% for CaCl2, demonstrating its versatile separation capabilities. In subsequent research, a structurally similar monomer, sulfonated aromatic diamide-diol (SDA), was synthesized to enhance NF membrane performance128. Membranes prepared with SDA alone achieved high permeability (6.2 LMH bar−1) and significant Na2SO4 retention (86%). Incorporating a small proportion of piperazine (PIP) was incorporated into the aqueous phase during fabrication, the resulting membranes exhibited enhanced salt rejection (97% for Na2SO4) at a slightly reduced permeability (5.0 LMH bar−1). These findings highlight the tunability of PEA membranes for tailored separation performance, making them promising for industrial dye separation and water purification applications.
Common nanofiltration membranes. Nanofiltration membranes often face a trade-off between selectivity and permeability, and dual-high performance membranes are rarely reported. Cai et al.129 A high-performance PEA nanofiltration membrane was developed with a polyamide-polyester composite layer and a polydopamine (PDA) surface layer. The interfacial polymerization process was facilitated by a mixture of piperazine and triethanolamine (TEOA) in the aqueous phase and a TMC solution in the organic phase on a PSF support layer. TEOA incorporation increased the free volume fraction and chain spacing, enhancing the migration of permeants. Additionally, the abundant hydroxyl groups improved hydrophilicity, selectivity, and water permeability. The PDA surface layer further enhanced antifouling properties, significantly extending the membrane’s service life. The TFC/PDA membrane achieved a water permeability of 21.3 LMH bar−1 and an Na2SO4 rejection rate of 97.6%. It also demonstrated excellent pollutant removal, achieving rejection rates of up to 98% for rhodamine B and basic blue B. These features underline its potential for applications in water purification and dye separation processes.
Coatings
Vegetable oil–based PEA is an ideal alternative to petroleum-based coatings due to its excellent biodegradability. However, the tight network of a cross-linked PEA as coating results in poor mechanical properties (e.g. brittleness). Therefore, through a rational molecular design, a variety of non-covalent bonds, such as hydrogen bonds, are introduced into PEA cross-linked networks, and the strong covalent network maintains the structural integrity of PEAs. Intermolecular interactions act as sacrificial bonds to effectively dissipate energy to further enhance the rigidity and ductility of polymer materials. Gao et al.130 catalyzed the conversion of Sapium sebiferum oil into 9,10-dihydroxyoctadecanoic acid (C18-OH) and prepared thermoset PEA by melt polycondensation with 1,6-hexanediamine (Fig. 19); their performance benefited from the synergistic effect of reversible hierarchical hydrogen bonding between the molecular chains and flexible long-chain hydroxyl ester bonding; both bonds acted as a sacrificial bonds to dissipate energy, and the PEA exhibited excellent mechanical strength (55.2 ± 3.5 MPa), toughness (130.1 ± 18.8 MJ/m3), adhesion (shear strength of aluminum plate up to 10.29 ± 0.21 Mpa), and reprocessability. The introduction of nanoparticles into PEAs is another effective method to enhance PEA, and the introduction of nanoparticles improves the adhesion and mechanical strength of a coating through PEA–nanoparticle interactions. Bakshi et al.131 reported a polypyrrole (PPy)-coated polysorbate cerium dioxide (PSCeO2) nanoparticle-filled N,N-bis(2-hydroxyethyl)tung oil–based Poly(ester amide)s (UTOPESA-PPy-PSCeO2) coating. The nanoparticles doped with PEA filled the intermolecular gaps, the hydroxyl groups of the PEA pre-polymer were fully cross-linked with toluene-2,4-toluene-2,4-diisocyanate and PPy-PSCeO2, the dense structure effectively prevented the exchange and deposition of corrosive ions, and the coating exhibited good corrosion resistance, was flame retardant and hydrophobic (contact angle of 95°), and can be used safely at 200 °C.
Conclusion
Monomer selection and molecular structure design enable the production of PEAs with biodegradability and biocompatibility comparable to polyester, alongside excellent thermal and mechanical properties similar to polyamide. These PEAs represent a promising class of degradable materials with diverse bio-based monomer sources, including vegetable oils, α-amino acids, and polysaccharides. As human metabolites, α-amino acids serve as structural units in PEAs, not only imparting desirable thermal and mechanical properties but also facilitating the chemical coupling of drug functional groups, making them suitable as drug carriers. Furthermore, the side chains of PEAs can be modified to adjust their hydrophobicity, drug release rates, and other properties. PEA resins derived from the fatty acids of vegetable oil extracts exhibit strong intermolecular bonding performance due to the abundance of amide bonds, resulting in superior mechanical strength, heat resistance, and chemical resistance compared to traditional polyester resins. PEAs can be synthesized through various methods, including polycondensation, ring-opening polymerization, and enzymatic polymerization. Factors such as copolymerization monomers, synthesis techniques, reaction conditions, and dosing methods significantly influence the structure and properties of PEAs. The structural design of PEAs offers a wide range of possibilities. Their physical and chemical properties can be tuned through structural modifications, and the arrangement of ester and amide bonds in the main chain can be categorized into three types: random, alternating, and block. Random PEAs are typically prepared via a multicomponent one-pot method, which is simple and uses a wide range of raw materials. The thermal and mechanical properties of these PEAs can be regulated by altering the molar ratio of esters to amides in the feedstock. Alternating PEAs, prepared using intermediates such as diamide diols and monomers like diacids and hydroxy acids, exhibit a regular distribution of ester and amide bonds, resulting in uniform structures with improved crystallinity and thermal properties compared to random PEAs of similar composition. Block PEAs incorporate polyester and polyamide segments in their molecular chains, allowing for material functionalization through the introduction of distinct blocks. The flexibility of the main chain, as well as the crystallinity and viscosity of PEA materials, are influenced by the rigidity of comonomers and the length of methylene chains. Due to their excellent biodegradability, biocompatibility, diverse monomer sources, versatile preparation methods, and flexible and adjustable structure-property relationships, PEA materials have been widely used in biomedicine, nanofiltration membranes, and coatings.
Although research on PEAs has been fruitful and PEAs have a wide range of applications in various fields, some gaps in their research and issues in their development have been noted. The following scientific issues and technological challenges need to be consider to further advance the development of these materials:
-
(1)
The multicomponent polycondensation reaction for PEA synthesis involves diverse active functional groups, including hydroxyl, carboxyl, amino, and ester groups. These functional groups participate in various key processes, such as esterification and amidation, along with side reactions like ester exchange and ester-acyl exchange. The complexity of this reaction system poses challenges for the efficient preparation of high-molecular-weight PEAs, and the performance of the product fluctuates greatly.
-
(2)
The relationship between the structure and performance of PEAs requires further investigation. Current studies tend to focus on regular factors such as the monomer feeding ratio, main chain length, and stiffness, as they relate to thermal, mechanical, and degradation properties. However, the role of side chains in PEAs deserves more attention, given their significant influence on material hydrophilicity, hydrophobicity, stability, and dynamic responsiveness to environmental changes. To fully leverage the unique advantages and characteristics of PEA materials, it is essential to systematically elucidate their conformational relationships. This approach will facilitate the optimization of material design and properties, enabling tailored applications across diverse practical scenarios.
-
(3)
Compared to other monomers, α-amino acids display superior biocompatibility, effectively minimizing immune responses and toxic side effects. The unique side chain functional groups allow for specific stimulatory responses of carriers or carried drugs, laying a foundation for responsive drug delivery systems. Further modifications of these side chains are requisite to enhance the function, release rate, and drug delivery efficiency of AA-PEAs. Additionally, reducing cytotoxicity should be given priority, and clinical trials should be conducted to validate their safety and efficacy. These endeavors will create a path for the development of more efficient and personalized treatment strategies customized to individual patient requirements.
In conclusion, PEA materials are developing toward multifunctionalization and diversification and occupy an important position in daily life, environmental protection, and medical fields. In the future, it is still necessary to continue expanding, updating, and optimizing the monomer sources and synthesis methods of PEA, building a more systematic structure–property relationship, following the principles of green chemistry, promoting the development of new biodegradable materials, and assisting the “dual-carbon” strategy.
Data availability
No datasets were generated or analyzed during the current study.
References
Wang, G.-X., Huang, D., Ji, J.-H., Völker, C. & Wurm, F. R. Seawater-degradable polymers—fighting the marine plastic pollution. Adv. Sci. 8, 2001121 (2021).
Haider, T. P., Völker, C., Kramm, J., Landfester, K. & Wurm, F. R. Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angew. Chem. Int. Ed. 58, 50–62 (2019).
Nair, L. S. & Laurencin, C. T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 32, 762–798 (2007).
Agostinho, B., Silvestre, A. J. D., Coutinho, J. A. P. & Sousa, A. F. Synthetic (bio)degradable polymers—when does recycling fail?. Green. Chem. 25, 13–31 (2023).
Ranganathan, P., Chen, C.-W., Rwei, S.-P., Lee, Y.-H. & Ramaraj, S. K. Methods of synthesis, characterization and biomedical applications of biodegradable poly(ester amide)s—a review. Polym. Degrad. Stab. 181, 109323 (2020).
Zhang, Q. et al. Bio-based polyesters: Recent progress and future prospects. Prog. Polym. Sci. 120, 101430 (2021).
Yamano, N., Kawasaki, N., Ida, S. & Nakayama, A. Biodegradation of polyamide 4 in seawater. Polym. Degrad. Stab. 166, 230–236 (2019).
Yamano, N., Kawasaki, N., Ida, S., Nakayama, Y. & Nakayama, A. Biodegradation of polyamide 4 in vivo. Polym. Degrad. Stab. 137, 281–288 (2017).
Hocker, S. J. A. et al. Graphene oxide reduces the hydrolytic degradation in polyamide-11. Polymer 126, 248–258 (2017).
Hejazi, S. et al. Chitosan/poly-γ-glutamic acid crosslinked hydrogels: characterization and application as bio-glues. Int. J. Biol. Macromol. 277, 133653 (2024).
Hejazi, S. et al. Physicochemical characterization of chitosan/poly-γ-glutamic acid glass-like materials. Int. J. Mol. Sci. 24, 12495 (2023).
Winnacker, M. & Rieger, B. Biobased polyamides: recent advances in basic and applied research. Macromol. Rapid Commun. 37, 1391–1413 (2016).
Carothers, W. H. & Hill, J. W. Studies of polymerization and ring formation. Xiii. Polyamides and mixed polyester—polyamides. J. Am. Chem. Soc. 54, 1566–1569 (1932).
Winnacker, M. & Rieger, B. Poly(ester amide)s: recent insights into synthesis, stability and biomedical applications. Polym. Chem. 7, 7039–7046 (2016).
Mecking, S. Nature or petrochemistry?—Biologically degradable materials. Angew. Chem. Int. Ed. 43, 1078–1085 (2004).
Ansari, V. et al. Additive manufacturing of α-amino acid based poly(ester amide)s for biomedical applications. Biomacromolecules 23, 1083–1100 (2022).
Irfan, M., Bhat, S. I. & Ahmad, S. Waterborne reduced graphene oxide dispersed bio-polyesteramide nanocomposites: an approach towards eco-friendly anticorrosive coatings. N. J. Chem. 43, 4706–4720 (2019).
More, A. P. & Mhaske, S. T. Anticorrosive coating of polyesteramide resin by functionalized ZnO- Al2O3-Fly ash composite and functionalized multiwalled carbon nanotubes. Prog. Org. Coat. 99, 240–250 (2016).
Zhu, G., Liu, C. & Zhang, C. Plant oil-based polymers. Phys. Sci. Rev. 8, 895–936 (2023).
Yoshinaka, Y. & Miller, S. A. Bio-oil derived polyesteramides as water-degradable replacements for polyethylene. Green. Chem. 27, 4152–4164 (2025).
Karak, N. In Vegetable Oil-Based Polymers (ed. Karak, N.) 126–145 (Woodhead Publishing, 2012). https://doi.org/10.1533/9780857097149.126.
Alam, M., Akram, D., Sharmin, E., Zafar, F. & Ahmad, S. Vegetable oil based eco-friendly coating materials: a review article. Arab. J. Chem. 7, 469–479 (2014).
Park, S. B. et al. Development of marine-degradable poly(ester amide)s with strong, up-scalable, and up-cyclable performance. Adv. Mater. 37, 2417266 (2025).
Kluge, M. et al. A facile method to synthesize semicrystalline poly(ester amide)s from 2,5-furandicarboxylic acid, 1,10-decanediol, and crystallizable amido diols. ACS Sustain. Chem. Eng. 8, 10812–10821 (2020).
Cheng, K. et al. Preparation and characterization of quasi alternating polyester amides based on ε-caprolactone, 1,6-hexanediamine, and adipic acid. ACS Appl. Polym. Mater. 7, 2007–2016 (2025).
Murase, S. K. & Puiggalí, J. Chapter 8 - Poly(ester amide)s: recent developments on synthesis and applications. In Natural and Synthetic Biomedical Polymers (eds Kumbar, S. G., Laurencin, C. T. & Deng, M.) 145–166 (Elsevier, 2014).
Han, S. & Wu, J. Recent advances of poly(ester amide)s-based biomaterials. Biomacromolecules 23, 1892–1919 (2022).
Liu, T. et al. Sequence-controlled synthesis of alternating poly(ester amide)s using water as control agent in one-pot. Chin. J. Polym. Sci. 42, 1015–1020 (2024).
Zeng, F.-R. et al. Copolymers of ε-caprolactone and ε-caprolactam via polyesterification: towards sequence-controlled poly(ester amide)s. Polym. Chem. 11, 1211–1219 (2020).
Sun, H. et al. α-Amino acid containing degradable polymers as functional biomaterials: rational design, synthetic pathway, and biomedical applications. Biomacromolecules 12, 1937–1955 (2011).
Bonillo Martínez, A. D., Galán, I. C. R. & Bellver, M. V. M. Application of a biodegradable polyesteramide derived from l-alanine as novel excipient for controlled release matrix tablets. AAPS PharmSciTech 18, 3286–3295 (2017).
Dirauf, M. et al. Block copolymers composed of petox and polyesteramides based on glycolic acid, l-Valine, and l-Isoleucine. Macromolecules 53, 3580–3590 (2020).
Lamas, M. L. et al. Towards the development of electrospun mats from poly(ε-caprolactone)/poly(ester amide)s miscible blends. Polymer 150, 343–359 (2018).
Kim, B.-H., Lee, C.-W. & Gong, M.-S. Preparation of polyesteramides based on aliphatic amine-containing phenol derivatives via interfacial polymerization. Macromol. Res. 11, 328–333 (2003).
Deng, M., Wu, J., Reinhart-King, C. A. & Chu, C.-C. Biodegradable functional poly(ester amide)s with pendant hydroxyl functional groups: synthesis, characterization, fabrication and in vitro cellular response. Acta Biomater.7, 1504–1515 (2011).
Karimi, P., Rizkalla, A. S. & Mequanint, K. Versatile biodegradable poly(ester amide)s derived from α-amino acids for vascular tissue engineering. Materials 3, 2346–2368 (2010).
Kantaria, T. et al. Biodegradable nanoparticles made of amino-acid-based ester polymers: preparation, characterization, and in vitro biocompatibility study. Appl. Sci. 6, 444 (2016).
Xie, R., Li, J., Zhao, M. & Wu, F. Recent advances in the development of poly(ester amide)s-based carriers for drug delivery. Saudi Pharm. J. 32, 102123 (2024).
Han, S.-I., Kim, B.-S., Kang, S.-W., Shirai, H. & Im, S. S. Cellular interactions and degradation of aliphatic poly(ester amide)s derived from glycine and/or 4-amino butyric acid. Biomaterials 24, 3453–3462 (2003).
Guo, K., Chu, C. C., Chkhaidze, E. & Katsarava, R. Synthesis and characterization of novel biodegradable unsaturated poly(ester amide)s. J. Polym. Sci. A: Polym. Chem. 43, 1463–1477 (2005).
Ruano, G. et al. Biohydrogel from unsaturated polyesteramide: synthesis, properties and utilization as electrolytic medium for electrochemical supercapacitors. Polym. Test. 82, 106300 (2020).
Sun, H. et al. Enzymatically and reductively degradable α-amino acid-based poly(ester amide)s: synthesis, cell compatibility, and intracellular anticancer drug delivery. Biomacromolecules 16, 597–605 (2015).
Pang, X. & Chu, C.-C. Synthesis, characterization and biodegradation of functionalized amino acid-based poly(ester amide)s. Biomaterials 31, 3745–3754 (2010).
Liu, T. et al. l-Arginine based polyester amide/hyaluronic acid hybrid hydrogel with dual anti-inflammation and antioxidant functions for accelerated wound healing. Chin. Chem. Lett. 33, 1880–1884 (2022).
Deng, M., Wu, J., Reinhart-King, C. A. & Chu, C.-C. Synthesis and characterization of biodegradable poly(ester amide)s with pendant amine functional groups and in vitro cellular response. Biomacromolecules 10, 3037–3047 (2009).
Ghaffar, A. et al. Monitoring the in vitro enzyme-mediated degradation of degradable poly(ester amide) for controlled drug delivery by LC-ToF-MS. Biomacromolecules 12, 3243–3251 (2011).
Fonseca, A. C., Gil, M. H. & Simões, P. N. Biodegradable poly(ester amide)s—a remarkable opportunity for the biomedical area: Review on the synthesis, characterization and applications. Prog. Polym. Sci. 39, 1291–1311 (2014).
Fonseca, A. C., Serra, A. C., Coelho, J. F. J., Gil, M. H. & Simões, P. N. Novel poly(ester amide)s from glycine and L-lactic acid by an easy and cost-effective synthesis. Polym. Int. 62, 736–743 (2013).
Soleimani, A., Borecki, A. & Gillies, E. R. Photodegradable poly(ester amide)s for indirect light-triggered release of paclitaxel. Polym. Chem. 5, 7062–7071 (2014).
Li, Z., Zhu, K., Ren, L. & Yuan, X. Synthesis of trehalose-grafted poly(ester amide) with potential ice recrystallization inhibition activity. Polymer 296, 126819 (2024).
Ye, R.-R., Wang, Z.-Y., Wang, Q.-F., Yang, K. & Luo, S.-H. Synthesis of biodegradable material poly(lactic acid-co-aspartic acid) via direct melt polycondensation and its characterization. J. Appl. Polym. Sci. 121, 3662–3668 (2011).
Lu, D., Ren, Z., Zhou, T., Wang, S. & Lei, Z. Synthesis and characterization of amphiphilic biodegradable poly(glutamic acid-co-lactic acid-co-glycolic acid) by direct polycondensation. J. Appl. Polym. Sci. 107, 3638–3643 (2008).
Asín, L., Armelin, E., Montané, J., Rodríguez-Galán, A. & Puiggalí, J. Sequential poly(ester amide)s based on glycine, diols, and dicarboxylic acids: thermal polyesterification versus interfacial polyamidation. Characterization of polymers containing stiff units. J. Polym. Sci. A: Polym. Chem. 39, 4283–4293 (2001).
Kadri, L. et al. Highly efficient one-pot synthesis of polyesteramides from ɛ-caprolactone and l-phenylalanine with high cell-viability and chemical stability. Polymer 319, 127981 (2025).
He, J., Ding, Y., Jiang, F. & Wang, Z. Poly(ester amide)s derived from low-value plant oil as reusable low-temperature tolerant adhesives. Eur. Polym. J. 198, 112387 (2023).
Alam, M. & Alandis, N. M. Development of ambient cured polyesteramide coatings from linseed oil: a sustainable resource. J. Polym. Environ. 19, 391–397 (2011).
Chen, J.-H., Yuan, W.-Q., Li, Y.-D., Weng, Y.-X. & Zeng, J.-B. Malleable and sustainable poly(ester amide) networks synthesized via melt condensation polymerization. ACS Sustain. Chem. Eng. 7, 15147–15153 (2019).
Ganvit, V. M., Patel, V., Patel, M., Dharva, A. & Sharma, R. K. Synthesis, physicochemical and thermal properties of urethane-modified polyesteramide films using mahua and castor oil as sustainable resources. J. Appl. Polym. Sci. 141, e54872 (2024).
Natarajan, J. et al. Poly(ester amide)s from soybean oil for modulated release and bone regeneration. ACS Appl. Mater. Interfaces 8, 25170–25184 (2016).
Galbis, J. A., García-Martín, M., de, G., de Paz, M. V. & Galbis, E. Synthetic polymers from sugar-based monomers. Chem. Rev. 116, 1600–1636 (2016).
Molina Pinilla, I., Bueno Martínez, M., Zamora Mata, F. & Galbis, J. A. Carbohydrate-based copolymers. synthesis and characterization of copoly(ester amide)s containing l-arabinose units. Macromolecules 35, 2977–2984 (2002).
Gomurashvili, Z., Kricheldorf, H. R. & Katsarava, R. amino acid based bioanalogous polymers. synthesis and study of new poly(ester amide)s composed of hydrophobic α-amino acids and dianhydrohexitoles. J. Macromol. Sci. A 37, 215–227 (2000).
Okada, M., Yamada, M., Yokoe, M. & Aoi, K. Biodegradable polymers based on renewable resources. V. Synthesis and biodegradation behavior of poly(ester amide)s composed of 1,4:3,6-dianhydro-D-glucitol, α-amino acid, and aliphatic dicarboxylic acid units. J. Appl. Polym. Sci. 81, 2721–2734 (2001).
Pinilla, I. M., Martínez, M. B. & Galbis, J. A. Synthesis and hydrolytic degradation of stereoregular aromatic poly(ester amide)s derived from D-xylose. J. Polym. Sci. Part A: Polym. Chem. 48, 4711–4720 (2010).
Toncheva, N. & Mateva, R. Kinetic investigation and regularities during the synthesis of Poly(ε-caprolactam-co-ε-caprolactone) and Poly(ε-caprolactam-co-δ-valerolactone) biodegradable copolymers. Polym. Bull. 60, 27–36 (2008).
Chromcová, D., Baslerová, L., Roda, J. & Brožek, J. Polymerization of lactams. 99 Preparation of polyesteramides by the anionic copolymerization of ε-caprolactam and ε-caprolactone. Eur. Polym. J. 44, 1733–1742 (2008).
Michell, R. M. et al. Effect of sequence distribution on the morphology, crystallization, melting, and biodegradation of poly(ε-caprolactone-co-ε-caprolactam) copolymers. Macromolecules 42, 6671–6681 (2009).
Bernášková, A., Chromcová, D., Brožek, J. & Roda, J. Polymerization of lactams, 95 Preparation of polyesteramides by the anionic polymerization of ε-caprolactam in the presence of poly(ε-caprolactone). Polymer 45, 2141–2148 (2004).
Deshayes, G. et al. Novel polyesteramide-based di- and triblock copolymers: From thermo-mechanical properties to hydrolytic degradation. Eur. Polym. J. 47, 98–110 (2011).
Basterretxea, A. et al. Synthesis and characterization of poly (ε-caprolactam-co-lactide) polyesteramides using Brønsted acid or Brønsted base organocatalyst. Eur. Polym. J. 95, 650–659 (2017).
Hong, S.-J. et al. Semicrystalline-glassy multiblock copolymers via mechanochemical synthesis toward tough poly(lactide). ACS Sustain. Chem. Eng. 10, 14523–14538 (2022).
Hong, S.-J., Kim, G. R., Kim, N.-K., Shin, J. & Kim, Y.-W. Poly(amide11)-Incorporated block copolymers as compatibilizers to toughen a poly(lactide)/polyamide 11 blend. ACS Appl. Polym. Mater. 6, 1224–1235 (2024).
Carothers, W. H., Dorough, G. L. & van Natta, F. J. Studies of polymerization and ring formation. X. The reversible polymerization of six-membered cyclic esters. ACS Publications https://pubs.acs.org/doi/pdf/10.1021/ja01341a046 (2002).
Lian, J., Li, M., Wang, S., Tao, Y. & Wang, X. Organocatalytic polymerization of morpholine-2,5-diones toward methionine-containing poly(ester amide)s: preparation and facile functionalization. Macromolecules 53, 10830–10836 (2020).
Douka, A., Vouyiouka, S., Papaspyridi, L.-M. & Papaspyrides, C. D. A review on enzymatic polymerization to produce polycondensation polymers: the case of aliphatic polyesters, polyamides and polyesteramides. Prog. Polym. Sci. 79, 1–25 (2018).
Sharma, B. et al. Enzymatic synthesis and solid-state properties of aliphatic polyesteramides with polydimethylsiloxane blocks. Macromolecules 40, 7919–7927 (2007).
Meimoun, J. et al. Lipase-catalysed polycondensation of levulinic acid derived diol-diamide monomers: access to new poly(ester-co-amide)s. Polym. Chem. 11, 7506–7514 (2020).
Çakır, S., Eriksson, M., Martinelle, M. & Koning, C. E. Multiblock copolymers of polyamide 6 and diepoxy propylene adipate obtained by solid state polymerization. Eur. Polym. J. 79, 13–22 (2016).
Borriello, A., Nicolais, L., Fang, X., Huang, S. J. & Scola, D. A. Synthesis of poly(amide-ester)s by microwave methods. J. Appl. Polym. Sci. 103, 1952–1958 (2007).
Morell, M. et al. New epoxy thermosets modified with hyperbranched poly(ester-amide) of different molecular weight. Eur. Polym. J. 46, 1498–1509 (2010).
Kelland, M. A. Tuning the thermoresponsive properties of hyperbranched poly(ester amide)s based on diisopropanolamine and cyclic dicarboxylic anhydrides. J. Appl. Polym. Sci. 121, 2282–2290 (2011).
Li, X. et al. Synthesis and characterization of hyperbranched poly(ester-amide)s from commercially available dicarboxylic acids and multihydroxyl primary amines. Macromolecules 39, 7889–7899 (2006).
Lin, Y., Dong, Z. & Li, Y. One-pot synthesis and characterization of hyperbranched poly(ester-amide)s from commercially available dicarboxylic acids and multihydroxyl secondary amines. J. Polym. Sci. A: Polym. Chem. 46, 5077–5092 (2008).
Nakayama, A., Yamano, N., Kawasaki, N. & Nakayama, Y. Synthesis and biodegradation of poly(2-pyrrolidone-co-ε-caprolactone)s. Polym. Degrad. Stab. 98, 1882–1888 (2013).
Wang, R. et al. One-pot synthesis of biodegradable and linear poly(ester amide)s based on renewable resources. J. Appl. Polym. Sci. 133 (2016).
Zhao, X., Ding, Z., Zhang, Y., Wang, Y. & Peng, S. Preparation and crystallization kinetics of polyesteramide based on poly(L-lactic acid). e-Polym. 18, 97–104 (2018).
Chen, T., Zhong, G.-C., Zhang, Y.-T., Zhao, L.-M. & Qiu, Y.-J. Bio-based and biodegradable electrospun fibers composed of poly(L-lactide) and polyamide 4. Chin. J. Polym. Sci. 38, 53–62 (2020).
Hashimoto, K., Sudo, M., Sugimura, T. & Inagaki, Y. Synthesis of novel block copolymers containing polyamide4 segments and control of their biodegradability. J. Appl. Polym. Sci. 92, 3492–3498 (2004).
Ono, H., Minamikawa, H., Nemoto, K. & Yoshida, M. Self-assembly and amphiphilic behavior of poly(ester)-block-poly(amide) diblock copolymer based on biodegradable poly(butylene succinate) and poly(2-pyrrolidone). Eur. Polym. J. 163, 110961 (2022).
Aslankoohi, N. & Mequanint, K. Poly(ester amide)–bioactive glass hybrid biomaterials for bone regeneration and biomolecule delivery. ACS Appl. Bio Mater. 3, 3621–3630 (2020).
Ahamad, T., Kumar, V., Parveen, S. & Nishat, N. In vitro antibacterial and antifungal assay of poly-(ethylene oxamide-N,N′-diacetate) and its polymer–metal complexes. Appl. Organomet. Chem. 21, 1013–1021 (2007).
Nguyen, T. H. N., Balligand, F., Bormann, A., Bennevault, V. & Guégan, P. Synthesis of new biobased linear poly(ester amide)s. Eur. Polym. J. 121, 109314 (2019).
Jang, Y.-J., Sangroniz, L. & Hillmyer, M. A. Ductile gas barrier poly(ester–amide)s derived from glycolide. Polym. Chem. 13, 3882–3891 (2022).
Feng, Y. et al. Controllable synthesis of furan-based poly(ester amide)s and its study on adhesive and aggregation-induced emission properties. ACS Appl. Polym. Mater. https://doi.org/10.1021/acsapm.4c00160 (2024).
Ding, Y. et al. Sustainable ultra-strong polyesteramide elastomers with rapid degradation and high resilience. Eur. Polym. J. 210, 112901 (2024).
Soleimani, A., Drappel, S., Carlini, R., Goredema, A. & Gillies, E. R. Structure–property relationships for a series of poly(ester amide)s containing amino acids. Ind. Eng. Chem. Res. 53, 1452–1460 (2014).
Tsuji, H. et al. Stereocomplex crystallization, homocrystallization, and polymorphism of enantiomeric copolyesteramides poly(lactic acid-co-alanine)s from the melt. Polym. Crystallization 3, e10094 (2020).
Tsuji, H. et al. Thermal properties and degradation of enantiomeric copolyesteramides poly(lactic acid-co-alanine)s. Polym. Degrad. Stab. 171, 109047 (2020).
Gu, Y., Liu, J., Gao, W., Liu, M. & Yi, C. One-pot in situ bulk polymerization of triblock copolymer PCL-b-PA6-b-PCL based on PA6 by using tetraphenyl tin. Polymer 300, 126968 (2024).
Gao, H., Cao, W., He, J. & Bai, Y. Highly transparent biaxially oriented poly(ester amide) film with improved gas barrier properties and good mechanical strength. Eur. Polym. J. 156, 110620 (2021).
Lips, P. A. M., Broos, R., van Heeringen, M. J. M., Dijkstra, P. J. & Feijen, J. Synthesis and characterization of poly(ester amide)s containing crystallizable amide segments. Polymer 46, 7823–7833 (2005).
Kawasaki, N., Yamano, N. & Nakayama, A. Polyamide4-block-poly(vinyl acetate) via a polyamide4 azo macromolecular initiator: thermal and mechanical behavior, biodegradation, and morphology. J. Appl. Polym. Sci. 132 (2015).
Liu, Y. et al. Flexible preparation of PA6-based thermoplastic elastomer filaments with enhanced elasticity, melt spinnability and transparency enabled by high-molecular-weight soft segments. Polym. Chem. 14, 4352–4361 (2023).
Ferré, T., Franco, L., Rodrı́guez-Galán, A. & Puiggalı́, J. Poly(ester amide)s derived from 1,4-butanediol, adipic acid and 6-aminohexanoic acid. Part II: composition changes and fillers. Polymer 44, 6139–6152 (2003).
Nakayama, Y. et al. Synthesis, properties, and biodegradation of sequential poly(ester amide)s containing γ-aminobutyric acid. Int. J. Mol. Sci. 21, 3674 (2020).
Yeol Lee, S., Park, J. W., Yoo, Y. T. & Im, S. S. Hydrolytic degradation behaviour and microstructural changes of poly(ester-co-amide)s. Polym. Degrad. Stab. 78, 63–71 (2002).
Abid, M., Mhiri, S., Bougarech, A., Triki, R. & Abid, S. Preparation, characterization and degradation study of novel sulfonated furanic poly(ester-amide)s. Designed Monomers Polym. 23, 16–24 (2020).
Al-Tayyem, B. H. & Sweileh, B. A. Synthesis, characterization and hydrolytic degradation of novel biodegradable poly(ester amide)s derived from Isosorbide and α-amino acids. J. Polym. Res. 27, 120 (2020).
Fan, Y., Kobayashi, M. & Kise, H. Synthesis and biodegradation of poly(ester amide)s containing amino acid residues: The effect of the stereoisomeric composition of L- and D-phenylalanines on the enzymatic degradation of the polymers. J. Polym. Sci. A: Polym. Chem. 40, 385–392 (2002).
Li, C. et al. A metabolic reprogramming amino acid polymer as an immunosurveillance activator and leukemia targeting drug carrier for T-cell acute lymphoblastic leukemia. Adv. Sci. 9, 2104134 (2022).
Feng, Y., Lu, J., Behl, M. & Lendlein, A. Progress in depsipeptide-based biomaterials. Macromol. Biosci. 10, 1008–1021 (2010).
Chudecka-Głaz, A., Szczeblińska, J., Cymbaluk-Płoska, A., Kohn, J. & El Fray, M. New poly(ester-amide) copolymers modified with polyether (PEAE) for anticancer drug encapsulation. J. Microencapsul. 33, 702–711 (2016).
Chen, X. et al. Significant suppression of non-small-cell lung cancer by hydrophobic poly(ester amide) nanoparticles with high docetaxel loading. Front. Pharmacol. 9 (2018).
Zhang, S. et al. Poly(ester amide)-based hybrid hydrogels for efficient transdermal insulin delivery. J. Mater. Chem. B 6, 6723–6730 (2018).
Khuddus, M. & Jayakannan, M. Melt polycondensation strategy for amide-functionalized l-aspartic acid amphiphilic polyester nano-assemblies and enzyme-responsive drug delivery in cancer cells. Biomacromolecules 24, 2643–2660 (2023).
Stone, H., Lin, S. & Mequanint, K. Preparation and characterization of electrospun rGO-poly(ester amide) conductive scaffolds. Mater. Sci. Eng. C 98, 324–332 (2019).
He, M., Potuck, A., Zhang, Y. & Chu, C.-C. Arginine-based polyester amide/polysaccharide hydrogels and their biological response. Acta Biomater.10, 2482–2494 (2014).
Zhou, Y. et al. Arginine based poly (ester amide)/ hyaluronic acid hybrid hydrogels for bone tissue Engineering. Carbohydr. Polym. 230, 115640 (2020).
Robbins, P. D. & Ghivizzani, S. C. Viral vectors for gene therapy. Pharmacol. Therapeutics 80, 35–47 (1998).
Chen, C.-K. et al. Biodegradable polymers for gene-delivery applications. IJN 15, 2131–2150 (2020).
Yamanouchi, D. et al. Biodegradable arginine-based poly(ester-amide)s as non-viral gene delivery reagents. Biomaterials 29, 3269–3277 (2008).
You, X. et al. Arginine-based poly(ester amide) nanoparticle platform: from structure–property relationship to nucleic acid delivery. Acta Biomater.74, 180–191 (2018).
Wu, X. et al. A critical review on polyamide and polyesteramide nanofiltration membranes: Emerging monomeric structures and interfacial polymerization strategies. Desalination 577, 117379 (2024).
Jayarani, M. M., Rajmohanan, P. R., Kulkarni, S. S. & Kharul, U. K. Synthesis of model diamide, diester and esteramide adducts and studies on their chlorine tolerance. Desalination 130, 1–16 (2000).
Zhang, Z., Fan, K., Liu, Y. & Xia, S. A review on polyester and polyester-amide thin film composite nanofiltration membranes: synthesis, characteristics and applications. Sci. Total Environ. 858, 159922 (2023).
Cheng, J. et al. A novel polyester-amide loose composite nanofiltration membrane for effective dye/salt separation: the effect of long molecule on the interfacial polymerization. J. Membr. Sci. 657, 120675 (2022).
Rezania, J., Vatanpour, V., Shockravi, A. & Ehsani, M. Preparation of novel carboxylated thin-film composite polyamide-polyester nanofiltration membranes with enhanced antifouling property and water flux. React. Funct. Polym. 131, 123–133 (2018).
Rezania, H.(Jafar)., Vatanpour, V., Shockravi, A. & Ehsani, M. Study of synergetic effect andcomparison of novel sulfonated and carboxylated bulky diamine-diol and piperazine in preparation ofnegative charge NF membrane. Sep. Purif. Technol. 222, 284–296 (2019).
Cai, W., Wang, M., Yang, G. Q. & Li, J. High-performance nanofiltration membranes with a polyamide-polyester composite layer and a polydopamine surface layer for desalination and dye pollutant removal. Polymer 268, 125720 (2023).
Gao, H., Wu, X., Fan, J. & Wang, Z. Mechanically strong and tough plant oil-based poly(ester amide) vitrimers with closed-loop recyclability. ACS Sustain. Chem. Eng. 12, 2668–2677 (2024).
Bakshi, M. I. & Ahmad, S. In-situ synthesis of synergistically active ceria doped polypyrrole oleo-polyesteramide hybrid nanocomposite coatings: Corrosion protection and flame retardancy behaviour. Prog. Org. Coat. 147, 105778 (2020).
Ali Mohamed, A., Salhi, S., Abid, S., El Gharbi, R. & Fradet, A. Random and quasi-alternating polyesteramides deriving from ε-caprolactone and β-alanine. Eur. Polym. J. 53, 160–170 (2014).
Young, E. L. & McDonald, A. G. Preparation and characterization of biobased lignin-co-polyester/amide thermoplastics. Molecules 26, 2437 (2021).
Papadopoulos, L. et al. Unlocking the potential of furan-based poly(ester amide)s: an investigation of crystallization, molecular dynamics and degradation kinetics of novel poly(ester amide)s based on renewable poly(propylene furanoate). Polym. Chem. 12, 5518–5534 (2021).
Cao, K. et al. Fabrication and in situ cross-linking of carboxylic-acid-functionalized poly(ester amide) scaffolds for tissue engineering. ACS Appl. Polym. Mater. 1, 2360–2369 (2019).
Wu, J., Mutschler, M. A. & Chu, C.-C. Synthesis and characterization of ionic charged water soluble arginine-based poly(ester amide). J. Mater. Sci: Mater. Med. 22, 469–479 (2011).
Kiros, S., Lin, S., Xing, M. & Mequanint, K. Embryonic mesenchymal multipotent cell differentiation on electrospun biodegradable poly(ester amide) scaffolds for model vascular tissue fabrication. Ann. Biomed. Eng. 48, 980–991 (2020).
Gloria, A. et al. The influence of poly(ester amide) on the structural and functional features of 3D additive manufactured poly(ε-caprolactone) scaffolds. Mater. Sci. Eng.: C. 98, 994–1004 (2019).
Tian, J., Song, B., Gao, S., Van der Bruggen, B. & Zhang, R. Omnifarious performance promotion of the TFC NF membrane prepared with hyperbranched polyester intervened interfacial polymerization. J. Membr. Sci. 642, 119984 (2022).
Chen, P. et al. Preparation, characterization and acid/alkali stabilities of aromatic polyesteramide-based composite membranes for water desalination. Sep. Purif. Technol. 269, 118739 (2021).
Acknowledgements
The work is funded by the National Natural Science Foundation of China (51273060), and Hubei Province Technology Innovation Plan Key Research and Development Project (2023BAB110), and Green Industry Technology Leading Program of Hubei University of Technology-Independent Exploration Plan (XJKY2022004). Hubei Provincial Science and Technology Innovation Talent Program (2024DJC027), Green Industrial Science and Technology Leading Project of Hubei University of Technology (XJ2024000301).
Author information
Authors and Affiliations
Contributions
Xipo Zhao*: Writing—review editing, Conceptualization. Fan Mo: Writing—Original Draft, Writing—Reviewing & Editing, Investigation Caibo Zhao: Formal analysis, Investigation. Shunmei Li: Investigation. Min Wang: Formal analysis. Shixing Dong: Methodology. Ling Zhou: Writing—review editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, X., Mo, F., Zhao, C. et al. Preparation, structure–property relationships, and applications of novel bio-based and biodegradable polyesteramide materials. npj Mater Degrad 9, 71 (2025). https://doi.org/10.1038/s41529-025-00612-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41529-025-00612-8