Abstract
Calcium silicate hydrate (C-S-H) and calcium aluminum silicate hydrate (C-A-S-H) are the primary binding phases in Portland and blended cementitious systems, commonly retaining an amorphous to semi-crystalline structure. The longevity as well as the mechanical performance of such cementitious composites largely rely on the characteristics of C-S-H. On the other hand, ancient Roman concrete contains the crystalline analog of this phase – tobermorite, which is often attributed as the primary source of the extraordinary durability of this cementitious composite. With a goal of understanding the root cause of the enhanced durability of ancient and modern cementitious composites, this study investigated how amorphous and crystalline forms of C-S-H/C-A-S-H phases are affected by decalcification-induced degradation. Two different decalcifying environments were evaluated – using accelerated carbonation and NH4NO3. In-situ Fourier-Transform Infrared (FTIR) spectroscopy was utilized to evaluate the decalcification of these phases for 18 h. The findings indicate that during this period, C-S-H was completely decalcified under accelerated carbonation, as reflected by the decrease in Q2 silicate species in the network and the formation of silicate species with a higher degree of polymerization (Q3 and Q4). Conversely, the crystalline version of these phases (i.e., tobermorite) showed minimal impact from decalcification, with only a 10-15% decrease in Q2 under the same exposure condition. This decalcification observation was corroborated using NH4NO3, yielding similar results. Optimization of low-carbon alternative binders can facilitate favorable conditions for the crystallization of C-S-H and C-A-S-H, thereby fostering the development of a sustainable and durable cementitious composite.
Similar content being viewed by others
Introduction
Calcium silicate hydrate (C-S-H) is the main hydration product of modern portland cement concrete. This phase serves as the primary binding agent that holds the concrete together. As a result of the hydration process, Portland cement produces amorphous C-S-H, which makes up about 60% of the paste by volume1. The increased use of aluminum-rich supplementary cementitious materials (SCMs) in Portland cement or geopolymer concrete production has led to the incorporation of aluminum into the C-S-H structure, forming calcium aluminum silicate hydrate (C-A-S-H)2,3,4. The key properties of concrete, including mechanical performance, durability, and sustainability, are predominantly influenced by C-S-H. Degradation of this phase negatively impacts concrete by reducing its compressive strength5 and modulus of elasticity6, impairing long-term performance with increased shrinkage7, enhancing porosity8, lowering the pH of the pore solution, and elevating the corrosion risk for steel reinforcement9, among other effects.
Al-tobermorite, a crystalline phase of calcium silicate hydrate (C-A-S-H), is a significant hydration product found in ancient Roman concrete structures3,10,11. Roman concrete is an exceptionally durable cement-based material that remains in remarkable condition even after 2000 years of exposure to harsh marine environments3. This phase is considered the crystalline analog for C-S-H, since it is known that the layer structure of the C-S-H phase ideally resembles the layer structure of this crystalline phase10. This mineralogical formation is not usually observed in the conventional Portland cement-based system3. The remarkable longevity of ancient Roman concrete is often credited to the presence of the crystalline Al-tobermorite10.
Among various degradation mechanisms of C-S-H/C-A-S-H, calcium leaching, also known as decalcification, is one of the most common ones. It has been reported to cause significant damage to concrete structures exposed to soft or acidic water, including dams, radioactive waste repositories, and underground constructions, among others12. Decalcification of C-S-H or C-A-S-H is a function of the type of binder, Ca/Si ratio, water-to-binder ratio, porosity, temperature, relative humidity, pH, pore solution composition, type of exposure environment, and their composition, and so on13,14,15,16,17,18. The degradation of portlandite generally occurs before the gradual dissolution of C-S-H, largely because portlandite is more soluble. With the growing use of SCMs in portland cement concrete, the amount of portlandite availability has decreased. As a result, during a chemical attack, C-S-H is more likely to experience decalcification19. The most common ions that promote decalcification are sulfates12,20, carbonates19,21, and chlorides17,22.
Numerous studies have investigated the effects of decalcification on amorphous C-S-H and C-A-S-H phases21,23,24,25. However, to the best of the authors’ knowledge, no research has explored decalcification-induced degradation in crystalline tobermorite or Al-substituted tobermorite. Accordingly, the objective of this study is to monitor the effect of crystallinity and initial pH on the decalcification of C-S-H and C-A-S-H. The decalcification of the amorphous C-S-H and C-A-S-H, and crystalline tobermorite and Al-tobermorite was monitored using the in-situ Fourier-Transform Infrared (FTIR) technique. A custom-made in-situ setup was utilized for this purpose. Previous studies were carried out where in-situ IR was employed to monitor the carbonation kinetics26,27, early age dissolution and geopolymer precipitation28, hydration of C3S29, formation of C-S-H23,30, and so on. The objective of this study is to monitor the effect of the crystallization of C-S-H and C-A-S-H, and the initial pH of the pore solution, on the decalcification due to carbonation of these phases. For these, laboratory-synthesized C-S-H, C-A-S-H, tobermorite, and Al-tobermorite were used under an initial pH of 9 and 13. An initial pH of 13 represents the pore solution pH of the OPC system, and the former one represents the same of the OPC system containing higher dosages of SCMs31. The decalcification kinetics were evaluated primarily due to accelerated carbonation. To further validate the findings from carbonation experiments, NH₄NO₃ was employed as a decalcification agent. Carbonation is inherently a diffusion-controlled process, and the extent of carbonation-induced decalcification can vary based on the degree of carbonation and the precipitation of carbonation products around the material. The rationale for selecting the second decalcification agent lies in the fact that the dissolution process induced by NH4NO3 results in a degradation profile similar to that observed during soft water leaching; however, it occurs with a reduced characteristic time32.
Results
Decalcification due to carbonation
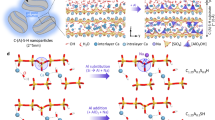
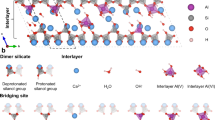
C-S-H is an amorphous or semi-crystalline phase with a structure that shares some similarities with the tetrahedral-octahedral-tetrahedral (T-O-T) framework of smectite, where the tetrahedral SiO₂ chains (analogous to planes in smectites) are linked to a “pseudo-octahedral” CaO sheet, where calcium atoms are seven-fold coordinated33,34. During carbonation, when CO₂ dissolves in water, it forms bicarbonate ions (HCO3-). Calcium in the interlayer of the silicate chains is removed, reacts with bicarbonate ions, and precipitates as calcium carbonate. This decalcification process leads to the condensation (cross-linking) of silicate chains, resulting in the development of more polymerized and longer silicate sites21. Incorporating aluminum allows it to substitute at the bridging or pairing silicate sites, depending on the Ca/Si ratio and the alkalinity available to balance the negative charge in C-A-S-H.
The FTIR spectra for silicate compounds usually exhibit the primary absorption between 800 and 1200 cm−1, which corresponds to the asymmetrical stretching vibration (ν3) of the Si–O bond with various degrees of polymerization (Qn where n = 0, 1, 2, 3, 4)19,35. Figure 1 shows that after 18 h of accelerated carbonation, the Q2 polymerization at 960 cm−1 in C-S-H (Fig. 1a) significantly decreased, while higher polymerization at 1050 cm−1 (Q3) increased with time. For both pH environments, degradation of the Q2 site was prominent and development of the Q3 peak was observed. It is important to note here that initially, at 0 min, there was no peak observed for Q3 polymerization. The C-S-H is primarily comprised of Q2 silicate chain, which agrees with the literature19. When Al was incorporated into the network, the positions of Q2 and Q3 merged in the C-A-S-H sample (Fig. 1c, d), where both Q2 and Q3 were initially present. With time, the Q3 peak became more prominent and resulted in a broad peak. It is important to note that while the starting pH was controlled, it is expected that during the carbonation, the pH is reduced due to the carbonic acid formation, which primarily causes the decalcification.
The tobermorite sample primarily exhibited an asymmetric Si-O stretching vibration at 965 cm⁻¹, with additional shoulders at 948 cm⁻¹ and 930 cm⁻¹. A minor shoulder at 1028 cm⁻¹ was also observed, attributed to Q³ polymerization of the silicate tetrahedron, present as an impurity in the starting material. Similar spectral features were noted in the Al-tobermorite sample. When the crystallized phases, tobermorite (Fig. 2a, b) and Al-tobermorite (Fig. 2c, d), were exposed to accelerated carbonation, the degradation rate was found to be very slow under both pH 9 and pH 13 conditions. The Q² peak showed minimal reduction, and the formation of the Q³ peak at 1050 cm⁻¹ was not prominent, indicating the stability of these crystalline phases during carbonation.
Figure 3 depicts the degradation rate of Q2 polymerization caused by decalcification under carbonation across gel phases. Initially, the C-S-H samples contained 65–67% Q2, but over time, decalcification led to a decline in Q2 levels, which dropped to approximately 30% after 18 h. At pH 9, C-S-H exhibited the highest degradation rate among all samples, with significant decalcification occurring within 300 min. Previous studies have highlighted that in low-pH environments, C-S-H primarily consists of Q3 polymerization, observed at higher wavenumbers36. In contrast, at pH 13, the degradation rate was slower, resembling the stability of C-S-H in a conventional cementitious composite, where it remains stable in high-pH pore solutions. For C-A-S-H (Fig. 3b), the initial Q2 content was approximately 55%, indicating the coexistence of both Q2 and Q3 structures within the C-A-S-H gel. Following accelerated carbonation, the Q2 content decreased to around 42–44%. The relatively low conversion rate is attributed to the overlapping peaks of Q2 and Q3. Regardless of the pH, the degradation rate for C-A-S-H remained consistent.
Figure 4 depicts the degradation rate of Q2 polymerization caused by decalcification under carbonation across crystalline phases. The Tobermorite sample had around 73–75% Q2 initially (Fig. 4a). Over time, the decalcification rate was very low. For pH 9, the line of slope remained almost straight with a final Q2 of 70%. For pH 13, the final Q2 was around 67%. The slopes of these lines are one to two orders of magnitude lower than that of the C-S-H and C-A-S-H samples. For the Al-tobermorite sample, pH 13 (Fig. 4b) also showed higher degradation with time compared to the pH 9 sample. The initial quantity of Q2 was around 74–77%. After 18 h, it became 63% for the starting pH of 9 and around 56% for the starting pH of 13.
Both crystalline phases of calcium silicate hydrate exhibited a very low degradation rate, highlighting their greater stability against carbonation-induced degradation compared to gel phases, regardless of aluminum content. When comparing pH 9 and pH 13, the lower pH condition provided better stability under similar conditions. Previous studies have also noted that tobermorite stabilizes more effectively at pH 10 or below10, which explains the enhanced stability of crystalline phases at lower pH levels. The overall drop in Q2% ranged from 5% to 15% (Fig. 4a, b).
In 11 Å tobermorite cross-linking between aluminosilicate chains has been observed in low-Ca (Ca/Si < 1) cements and at elevated temperatures, resulting in disordered analogs of ‘double chain’ calcium silicate minerals37. When considering the effect of aluminum, crystalline Al-tobermorite showed a higher degradation rate compared to its non-aluminum counterpart under their respective starting pH conditions. This could be due to the incorporation of Al in the bridging sites, which balances the negative charge of the silicate chain38, thereby facilitating calcium leaching from the interlayer. In other words, the presence of Al in C-S-H with a lower Ca/Si ratio makes it more prone to decalcification caused by carbonation.
Decalcification in presence of NH4NO3
Figure 5 illustrates the impact of NH₄NO₃, used as a decalcification agent, on the Q² polymerization of binder phases. An NH₄NO₃ concentration of 18 mol/L was selected, as this is below the saturation limit under laboratory conditions39. Figure 5a shows the changes in silicate polymerization within the C-S-H sample. After 18 h of exposure to NH4NO3 solution, the Q² peak intensity decreased slightly, and the formation of a Q³ peak at 1045 cm⁻¹ was observed. For the C-A-S-H sample, complete decalcification of the Q² peak did not occur. Instead, the co-existence of Q² and Q³ peaks was observed following decalcification. The Q² peak shifted slightly to the right, merging with the Q³ peak. The overall reductions in Q2 peaks for C-S-H and C-A-S-H were 25.6% and 18.9%, respectively.
In contrast, the crystalline phases, such as tobermorite and Al-tobermorite, showed no significant effect on the primary Q² peak, and no Q³ peak formation was observed (Fig. 5c, d). These results align with those obtained from carbonation degradation, where carbonation, being a diffusion-based process, exhibits similar effects. Here, NH₄NO₃ was in direct contact with the sample, further supporting the consistency of results across different decalcification methods.
Figure 6 illustrates the degradation rate of Q² under the influence of NH₄NO₃. The data indicate that the crystalline phases, tobermorite and Al-tobermorite, remained unchanged throughout the observed time frame. In contrast, the gel phases exhibited degradation of the Q² structure. For the C-S-H sample, the Q² content decreased from 83% to approximately 65%. In the C-A-S-H sample, where Q² and Q³ peaks overlapped, the degradation of the Q² peak was less pronounced, with its quantity reducing from 58% to 47% over the same period. This trend is consistent with the decalcification behavior observed due to carbonation, as shown in Fig. 3 and Fig. 4.
Decalcification due to chemical attack
To evaluate the decalcification of calcium-silicate-hydrate (C-S-H) under sulfate attack, a sodium sulfate solution was employed at the concentration specified in ASTM C1012 as a decalcification agent. All other parameters were maintained consistent with prior studies. In situ infrared spectroscopy (IR) was conducted, and the findings are illustrated in Fig. 7. The Q2 polymerization of the silicate structure exhibited minimal alteration throughout the duration of the study. Although the emergence of the Q3 peak at 1080 cm−1 was noted, its significance was negligible. The extent of Q2 degradation remained insufficient to indicate substantial alteration. Therefore, it can be concluded that the specified concentration of sodium sulfate did not possess adequate strength to compromise the integrity of the C-S-H structure within the tested duration. Figure 8 and Fig. 9 illustrate the effects of sulfuric acid and hydrochloric acid, both at a pH of 6, on the C-S-H structure. In both instances, the Q2 peak exhibited a broadening. However, no evidence of higher polymerized silicate sites was observed in either case. This absence may be attributed to the dissolution of some silica. Consequently, the equation employed to calculate C-S-H degradation, represented as Q2*100/(Q2 + Q3), becomes inapplicable in this context. Therefore, as depicted in Figs. 8b and 9b, there is no observable degradation of Q2 in relation to the total Q2 and Q3 content.
Observations from 29Si NMR
²⁹Si NMR was conducted alongside in-situ IR to characterize the carbonated material compared to the starting material, providing detailed, site-specific insights into the local chemical environment of silicon atoms, including their coordination states, connectivity, and structural organization. In the 29Si NMR spectra, tetrahedral coordination is represented by Q1, Q2b, Q2p, Q3, and Q4, which indicate the chemical shift (ppm) of a silicon atom bonded to n bridging oxygens. Q1 corresponds to a chain-end tetrahedron. In the case of Q2, two of the three tetrahedra share O−O edges with the central Ca−O region of the C-S-H layer, forming what is commonly referred to as “paired” tetrahedra (Q2p). The third tetrahedron, which shares an oxygen atom at the pyramidal apex of a calcium polyhedron, bridges the paired tetrahedra and is therefore called “bridging” (Q2b)34. Q3 represents a tetrahedron surrounded by three silica tetrahedra, where two are Q2 and the third is another Q3, linking two silicate chains in the interlayer space33,40. Finally, Q4 corresponds to the polymerized 3D network of silicate tetrahedra41.
Figure 10 represents 29Si NMR spectra of the C-S-H sample before and after carbonation. Before carbonation, the C-S-H sample predominantly exhibited a Q2p peak at -86 ppm, with a shoulder at -95 ppm corresponding to Q3, accounting for approximately 89.5% of the area under the Q2p peak (Table 1). After carbonation, the Q2 peak disappeared, while prominent Q3 and Q4 peaks emerged, signifying the degradation of the original C-S-H structure and the formation of a highly polymerized three-dimensional silica gel network, regardless of the starting pH. Under carbonation with a starting pH of 13, the Q3 peak at -89.3 ppm decreased in intensity compared to that of pH 9 and overlapped with the adjacent Q3 peak at -99 ppm.
Figure 11 represents the 29Si NMR spectra of the tobermorite sample before and after carbonation exposure. Tobermorite, a hydrothermally formed crystalline calcium silicate hydrate phase, displayed sharp peaks in the 29Si NMR spectrum. The primary peak, located at -85 ppm, corresponds to the pairing sites of silicate tetrahedra (Q2p), while a secondary peak around -83 ppm, attributed to the bridging sites of silicate tetrahedra (Q2b), overlapped with the Q2p peak. As shown in Table 2, the bridging site had a higher area percentage (40.4%) compared to the pairing site (24.4%). Additionally, Q1 sites and approximately 25% Q3 sites were identified. It is worth noting that the Q3% appears slightly higher in the NMR (Fig. 10) plot compared to the in-situ IR plot (Fig. 4). This discrepancy arises because, following in-situ IR monitoring, the samples were preserved and shipped for the NMR experiment, which took approximately 2–3 weeks. During this period, the samples may have undergone slight carbonation from exposure to the atmosphere.
After carbonation at pH 9, the intensity of the pairing site decreased, although 12% of the Q2 sites retained their structure even under 99% carbonation. Decalcification led to increased polymerization of the silicate chain, resulting in the appearance of Q3 and Q4 peaks. Following carbonation at pH 13, the pairing site peak further diminished, with 8.8% of the Q2 sites still maintaining their structure, albeit with a broader peak. This suggests that the crystallinity of tobermorite was impacted by decalcification under highly alkaline conditions.
C-S-H primarily exhibits an amorphous morphology, appearing as lath-like or foil-like deposits at the nanoscale under transmission electron microscopy (TEM) before exposure19. Following accelerated carbonation, initiated at a pH of 13, small globular structures with dense peripheries were observed. These globules are indicative of polymerized silica gel in Fig. 12a, b characteristic morphology consistent with findings reported in the literature19.
Tobermorite was synthesized hydrothermally, demonstrating a predominantly acicular shape with sharp edges before exposure10. Data from in-situ FTIR (Fig. 2) and 29Si NMR analyses (Fig. 11) revealed that tobermorite exhibited higher resistance to degradation caused by decalcification. This suggests that carbonation effects on these nanostructures were minimal. TEM observations further supported these findings; the primary needle-like morphology was preserved post-carbonation. However, slight rounding of the needle edges was observed (Fig. 12d). In Fig. 12c, a gel-like phase formation is evident near the needle ends. This spherical gel-like structure is likely attributable to the formation of amorphous calcium carbonate. Thus, after 18 h of accelerated carbonation, while degradation of this crystalline tobermorite phase due to carbonation had just begun, the C-S-H gel phase had already undergone significant degradation
Products of carbonation
The gel phases, C-S-H and C-A-S-H, undergo decalcification under different pH conditions in different ways. As a result, the formation of different polymorphs of calcium carbonate is unpredictable and dependent on the solution compositions and reaction mechanisms25. Initially, amorphous calcium carbonate forms, which converts to vaterite or more thermodynamically stable calcite. This conversion is a function of HCO3− saturation, pH, RH%, and temperature42. Upon accelerated carbonation, well-formed rhombohedral calcite crystals were prominently visible in the gel phases across all pH levels, as illustrated in Fig. 10. In C-S-H samples at pH 13, metastable vaterite was observed alongside calcite. At pH 9, calcite crystals in C-S-H exhibited sharp edges, whereas those in C-A-S-H samples at both pH 9 (Fig. 13c) and pH 13 (Fig. 13d) appeared with rounded edges. Notably, at pH 13, petal-shaped vaterite crystals were prominently observed alongside calcite, as evident in Fig. 13d. The stabilization of vaterite at a higher pH is in line with the literature43.
After carbonation, tobermorite crystals retained their original shape (Fig. 14a), indicating that not all crystals were affected after 18 h of exposure to 99% CO₂. Figure 11b shows vaterite precipitation at pH 13. The co-existence of Al-tobermorite crystals and calcium carbonate polymorphs is evident in Fig. 14c. Additionally, calcite crystals were identified in both tobermorite and Al-tobermorite samples after carbonation, regardless of pH.
X-ray diffraction (XRD) was used to identify the carbonation products in the gel and crystalline phases of calcium silicate hydrate (C-S-H), both with and without Al. Since the carbonation products were similar across the gel and crystalline phases, the XRD spectra for C-S-H and tobermorite are presented in Fig. S.1. Before carbonation, the C-S-H sample was largely amorphous or poorly crystalline, showing only a minor peak at 29.6° (two theta). After carbonation, various polymorphs of calcium carbonate formed, with calcite being the dominant phase at pH 9. At pH 13, metastable phases such as vaterite and aragonite were also observed alongside calcite44. For the crystalline tobermorite phase, distinct peaks corresponding to tobermorite crystals were evident. Even after accelerated carbonation for 18 h, the tobermorite peaks remained visible, indicating that some of the tobermorite crystals were unaltered. These results align with observations from in-situ IR and 29Si NMR analyses. As carbonation products, calcite was predominant under both pH conditions, while vaterite was additionally detected in the samples with a starting pH of 13.
Discussion
This study introduces an innovative approach to monitor the carbonation-induced degradation of calcium silicate hydrate phases utilizing the in-situ IR technique. Two methods of decalcification were employed: one involving accelerated carbonation and the other using NH4NO3. In the first method, decalcification occurs through a gaseous agent, with its effectiveness depending on its diffusivity in various media. In the second method, the decalcification agent is a solid dissolved in water, allowing direct contact with the material. The effect of two different pH environments (pH 9 and 13) on the decalcification kinetics of gel and crystalline phases of calcium silicate hydrate with or without Al content was studied.
The carbonation behavior of C-S-H is intrinsically linked to the characteristics of the C-S-H matrix, wherein the leaching of Ca ions during hydration, the adsorption of Ca ions by CO32− ions, and the subsequent deposition of carbonates on the matrix surface establish a dynamic equilibrium. Literature showed that this equilibrium involves the interplay between alterations in the matrix’s electronegativity and carbonate deposition45. The initial stage of decalcification involves the dissolution of Ca ions, which is primarily governed by the free energy requirements of the process. Compared to crystalline phases, the amorphous C-S-H gel phase exhibits a lower free energy for Ca dissolution, making it more susceptible to decalcification46. Furthermore, the free energy for calcium dissolution decreases with increasing Ca/Si ratios, indicating that C-S-H with higher Ca/Si ratios is more prone to decalcification46,47.
In this study, the gel phases exhibited a higher rate of decalcification compared to the crystalline phases, regardless of the decalcification medium, which is in line with the literature. Under accelerated carbonation, C-S-H underwent roughly 37% loss of its original Q2 structure, resulting in a more polymerized Q3 state, and a reduced starting pH led to an increased rate of degradation (Fig. 3a). The formation of vaterite, along with calcite, was also observed from SEM (Fig. 13) and XRD (Fig. S.1). Literature also showed that vaterite formation delays carbonation19. Alternatively, it can be stated that a higher pH environment promotes the formation of vaterite48, thereby reducing further carbonation-induced degradation. Additionally, the literature indicates that the extent of decalcification of C-S-H continuously rises as the pH in the system decreases during sulfate attack24. When Al was incorporated in this study, the initial FTIR spectrum indicated the co-existence of Q2 and Q3 in the starting C-A-S-H. After 18 h, Q2 degradation was limited to 13%. It is important to consider that the starting C-A-S-H already contained some Q3. The Q2 and Q3 peaks were overlapped, resulting in a broader peak within the 900–1200 cm⁻¹ wavenumber range (Fig. 1c, d). This overlapping of Q2 and Q3 peaks made it challenging to accurately monitor Q2 degradation in C-A-S-H through in-situ IR. Notably, the reduction of Q2% of C-A-S-H was similar across both tested starting pH levels.
For the crystalline phases, such as tobermorite and Al-tobermorite, the reduction of Q2% was significantly lower (Fig. 4) compared to the gel phases (Fig. 3). Literature also showed that in the case of crystalline tobermorite, the lowest free energy for Ca dissolution is located at the coordinate (0, 0, 0) with z-axis symmetry, as the layered structural arrangement is oriented parallel to the z-axis. This structural symmetry inhibits calcium dissolution, thereby conferring greater resistance to decalcification in tobermorite compared to amorphous C-S-H46. This finding aligns with the results of this study. When comparing the crystalline phases with and without aluminum content, Al-tobermorite exhibited a higher rate of decalcification than tobermorite (Fig. 4). This suggests that the presence of aluminum in the silicate chain network facilitates the dissolution of calcium from the interlayer, enabling subsequent reactions with carbonate ions. Literature showed that the bridging quad-coordination aluminum in the C-A-S-H gel phase is thermodynamically metastable, which is why it can be easily dealuminated49,50. The water-assisted bond dissociation energy of Si, that of Al-O, is significantly higher than Si-O, highlighting the breaking of Al-O bonds is more likely to occur under carbonation or another chemical attack20,49. Both crystalline phases experienced greater degradation at higher pH levels (starting pH 13), likely because higher pH conditions are not favorable for the formation and stabilization of these phases10. Under NH4NO3 exposure, the crystalline phases exhibited minimal decalcification relative to the gel phases, consistent with the trend observed during accelerated carbonation (Fig. 6). It is important to note that the Q2 degradation rate was quantified using raw data without background correction to minimize human error. If the background were corrected, the calculated degradation rate would be higher.
NMR spectra of the C-S-H sample revealed that carbonation completely destroyed the Q2 structure, leading to the formation of more polymerized Q3 and Q4 phases, regardless of the initial pH (Fig. 10). In contrast, the crystalline tobermorite sample retained approximately 8–12% of its Q2 sites after 18 h of accelerated carbonation (Table 2). This highlights the stability of the crystalline phase against degradation due to decalcification.
Carbonation reaction products were further analyzed using XRD and SEM. The primary carbonation products were identified as calcite and vaterite. Notably, prominent vaterite formation was observed in samples with a higher starting pH (pH 13). For the crystalline phases, sharp acicular tobermorite crystals remained prominent after 18 h of accelerated carbonation, accompanied by the formation of vaterite and calcite crystals (Fig. 14).
The findings of this study suggest that crystalline calcium silicate hydrate phases, i.e., tobermorite and Al-tobermorite, offer significant advantages in resisting degradation caused by decalcification compared to the gel phases. These findings provide new insights into why the presence of tobermorite/Al-tobermorite as a binding phase may contribute to the superior durability of ancient Roman concrete in harsh marine environments (decalcifying environment), compared to modern portland cement, where C–S–H gel is the primary binding phase. However, the findings also suggest that having Al could be detrimental to the stability of crystalline tobermorite. Nowadays, SCMs are commonly used to replace a portion of ordinary portland cement (OPC) that contains a significant amount of aluminum. Careful consideration should be given when selecting SCMs, as a higher aluminum content can negatively impact durability performance.
The primary limitation of this study is that, under certain chemical attacks, the decalcification of C-S-H or C-A-S-H occurs without the subsequent formation of higher polymerized silicate species. In some cases, silica gel might get dissolved. In such scenarios, the quantification method outlined in this study might not provide the most accurate representation of the process.
Methods
The synthesis of calcium silicate hydrate (C-S-H), calcium aluminum silicate hydrate (C-A-S-H), tobermorite, and Al-tobermorite was similar to our previous study10. The chemicals, including Calcium nitrate (Ca(NO3)2.4H2O), sodium silicate (Na2SiO3.9H2O), calcium hydroxide (Ca(OH)2), fumed silica (SiO2), aluminum oxide (Al2O3), aluminum nitrate (Al(NO3)3.9H2O), and sodium hydroxide (NaOH) were used as received from VWR and used without further modifications. To synthesize C-S-H and C-A-S-H, a mixture of calcium nitrate, sodium silicate, aluminum nitrate, and water (adjusted to a pH of 11.5 with NaOH) was placed in a High-Density Polyethylene (HDPE) plastic bottle with a water-to-solid ratio of 5:1. All the chemicals were combined with water and stirred using a mechanical stirrer at 600 rpm for 48 h at 50 °C on a hot plate to ensure the reaction was complete. Following the reaction period, the C-S-H or C-A-S-H gel was filtered in an environment purged with nitrogen gas to prevent carbonation. The filtered gel was washed three times with water to eliminate any unreacted ions and then placed in an oven at 50 °C for 24 h while being purged with nitrogen gas to dry. After drying, chunks of C-S-H or C-A-S-H were produced, and to obtain a uniform powder, these chunks were manually ground in a mortar and pestle in a nitrogen gas-controlled environment. The synthesis steps are shown in Fig. 15. The final Ca/Si ratio of the synthesized C-S-H was 0.93; for C-A-S-H, the aluminum-to-silicon ratio was 0.14, as measured using XRF.
Pure 11 Å tobermorite was produced through hydrothermal synthesis from the combination of calcium hydroxide and fumed silica, with or without the inclusion of aluminum oxide, within a 50 mL autoclave. For this, deionized water was utilized at a pH of 7 in the hydrothermal synthesis of tobermorite. A water-to-solid ratio of 10:1 was employed, maintaining a Ca/Si ratio of 0.90 for both tobermorite and Al-tobermorite, with Al/(Al + Si) = 0.10 for Al-tobermorite. The synthesis took place at a temperature of 180 °C over a period of 72 h. The resulting tobermorite/ Al-tobermorite was then filtered, dried in an oven at 50 °C, and ground into a powder using the same method as for C-S-H or C-A-S-H powder.
Figure S.2 shows the X-ray diffraction patterns, and Fig. S.3 shows Scanning Electron Microscopy (SEM) images of the initial materials. Both C-S-H and C-A-S-H exhibited an amorphous gel-like structure before exposure. In contrast, crystalline tobermorite and Al-tobermorite displayed well-defined, acicular-shaped crystals10,19. No noticeable differences in shape or appearance were observed with or without aluminum, consistent with previous studies10.
To perform the in-situ Fourier-Transform Infrared (FTIR), first, the background spectrum was collected using a 2 μL water drop in a CO2 atmosphere in a customized setup (Fig. 16). A solid-to-liquid ratio of 1:250 was used for this experiment. Such a high liquid ratio was used to ensure that the reaction would not be limited due to the lack of moisture availability. At first, the powder sample was placed on the ATR tip, and then water with different pH (pH 9 and pH 13) was put on top of the powder sample. NaOH pellets were used to increase the pH of the water.
For the accelerated carbonation exposure, the purging of CO2 gas commenced immediately following the placement of the paste sample onto the ATR tip. A total of 280 spectra were acquired, with a time interval of 3.75 min between each spectrum. The purging of 99% CO2 gas was sustained, with a resolution set at 4 cm⁻¹, involving 32 scans for each sample. The signal-to-noise ratio was maintained at a level below 3:1.
For the NH4NO3 exposure condition, 18 mol/L of NH4NO3 solution was used. In-situ measurements of FTIR were collected over 18 h of accelerated carbonation to monitor the carbonation-induced polymerization of calcium silicate hydrate with and without Al.
Over the 18 h, a total of 280 spectra were collected and analyzed. To determine the degradation rate of Q², the absorbance peak intensities from the raw data were collected across 280 spectra at wavenumber associated with Q2 and Q3, as indicated in Figs. 3, 4, and 6. The relative percentage of Q², in relation to both Q² and Q³, was monitored over time.
It is evident from the results obtained from Fig. 17 that three consecutive tests conducted on the same material produced analogous outcomes, with a coefficient of variance of 10%, which is statistically within the acceptable range. This finding demonstrates the repeatability of the test method across different samples.
After 18 h of accelerated carbonation, the samples were collected for further characterization. To minimize additional atmospheric carbonation at this stage, the samples were stored in sealed vials. All the 29Si solid-state NMR experiments were collected at the SCS NMR Facility of the University of Illinois at Urbana-Champaign, using a Bruker NEO spectrometer operating at B0 = 11.7 T (νL(29Si) = 99.30 MHz). A 6 mm Phoenix HXY magic-angle spinning (MAS) probe was used for all the 29Si NMR experiments under a spinning speed of 7 kHz at room temperature. All samples were ground into fine powders and packed into 6 mm o.d. standard zirconia rotors inside a nitrogen-filled glove bag. Compound A4 was ground with 30 mg KBr. The experiment for A4 was conducted with a spinning speed of 5 kHz. The 29Si pulse width used was 3.5 µs, corresponding to a p/4 pulse length. SPINAL-64 1H decoupling (~23 kHz) was applied during acquisition. A recycle delay was set to be 30 s. 2048 transients were used for all the samples. The 29Si NMR chemical shifts were referenced to tetramethylsilane (TMS) (d = 0 ppm). The powder obtained after in-situ IR was vacuum-dried and used in this experiment.
X-ray diffraction (XRD) patterns of the powdered sample were acquired utilizing a Cu-Kα source. The diffraction patterns were recorded within the angular range of 5° to 50°, employing a step size of 0.03° (2θ) per second.
The morphology of the carbonated products was assessed utilizing the Hitachi S4800 II Field Emission Scanning Electron Microscope (FE-SEM). The instrument was operated in high-vacuum mode, with an accelerated voltage of 30 kV and a working distance of approximately 11 mm. For this analysis, samples obtained from the in-situ FTIR were employed. Prior to imaging, the samples were coated with a Gold (Au)-Platinum (Pt) layer to enhance the quality of the scanning electron microscopy (SEM) images.
For transmission electron microscopy (TEM) images, the samples obtained after in-situ IR were placed in isopropanol and ultrasonicated for dispersion. A few drops of the solution were then placed on lacey carbon 300 mesh, gold grid (Ted Pella Inc., USA) for imaging. Hitachi H-9500 was used at 300 kV.
Data availability
All data will be available upon request.
References
Raki, L., Beaudoin, J., Alizadeh, R., Makar, J. & Sato, T. Cement and Concrete Nanoscience and Nanotechnology. Materials 3, 918–942 (2010).
Borno, I. B., Tahsin, A. & Ashraf, W. Utilization of industrial waste sodium sulfate for calcined clay-based sustainable binder. Constr. Build Mater. 440, 137073 (2024).
Ashraf, W. et al. Mimicking the cementation mechanism of ancient Roman seawater concrete using calcined clays. Appl Clay Sci. 230, 106696 (2022).
Chen, X., Sutrisno, A. & Struble, L. J. Effects of calcium on setting mechanism of metakaolin-based geopolymer. J. Am. Ceram. Soc. 101, 957–968 (2018).
Jebli, M. et al. Leaching effect on mechanical properties of cement-aggregate interface. Cem. Concr. Compos. 87, 10–19 (2018).
Constantinides, G. & Ulm, F.-J. The effect of two types of CSH on the elasticity of cement-based materials: Results from nanoindentation and micromechanical modeling. Cem. Concr. Res 34, 67–80 (2004).
Chen, J. J., Thomas, J. J. & Jennings, H. M. Decalcification shrinkage of cement paste. Cem. Concr. Res 36, 801–809 (2006).
Ashraf, W. Carbonation of cement-based materials: Challenges and opportunities. Constr. Build Mater. 120, 558–570 (2016).
Tang, Y.-J., Zuo, X.-B., Yin, G.-J., Davoudi, H. & Li, X.-N. Influence of calcium leaching on chloride diffusivity in cement-based materials. Constr. Build Mater. 174, 310–319 (2018).
Borno, I. B., Haque, M. I. & Ashraf, W. Crystallization of C-S-H and C-A-S-H in artificial seawater at ambient temperature. Cem. Concr. Res. 173, 107292 (2023).
Rispoli, C. et al. Unveiling the secrets of Roman craftsmanship: mortars from Piscina Mirabilis (Campi Flegrei, Italy). Archaeol. Anthropol. Sci. 12, 8 (2020).
Liu, X., Feng, P., Yu, X. & Huang, J. Decalcification of calcium silicate hydrate (C-S-H) under aggressive solution attack. Constr. Build Mater. 342, 127988 (2022).
Kamali, S., Moranville, M. & Leclercq, S. Material and environmental parameter effects on the leaching of cement pastes: Experiments and modelling. Cem. Concr. Res. 38, 575–585 (2008).
Harris, A. W., Manning, M. C., Tearle, W. M. & Tweed, C. J. Testing of models of the dissolution of cements—leaching of synthetic CSH gels. Cem. Concr. Res. 32, 731–746 (2002).
de Larrard, T., Benboudjema, F., Colliat, J.-B., Torrenti, J.-M. & Deleruyelle, F. Concrete calcium leaching at variable temperature: Experimental data and numerical model inverse identification. Comput Mater. Sci. 49, 35–45 (2010).
Liu, X. et al. Effects of pH on the nano/micro structure of calcium silicate hydrate (CSH) under sulfate attack. Cem. Concr. Res. 140, 106306 (2021).
Glasser, F. P., Pedersen, J., Goldthorpe, K. & Atkins, M. Solubility reactions of cement components with NaCl solutions: I. Ca (OH) 2 and CSH. Adv. Cem. Res. 17, 57–64 (2005).
Kamali, S., Gérard, B. & Moranville, M. Modelling the leaching kinetics of cement-based materials––influence of materials and environment. Cem. Concr. Compos. 25, 451–458 (2003).
Haque, M. I., Borno, I. B., Khan, R. I. & Ashraf, W. Reducing carbonation degradation and enhancing elastic properties of calcium silicate hydrates using biomimetic molecules. Cem. Concr. Compos. 136, 104888 (2023).
Hou, D. et al. Structural deterioration of calcium aluminosilicate hydrate by sulfate attack: Atomic processes of Al-Si chain breaking. Constr. Build Mater. 401, 132867 (2023).
Sevelsted, T. F. & Skibsted, J. Carbonation of C–S–H and C–A–S–H samples studied by 13 C, 27 Al and 29 Si MAS NMR spectroscopy. Cem. Concr. Res 71, 56–65 (2015).
Hill, J., Harris, A. W., Manning, M., Chambers, A. & Swanton, S. W. The effect of sodium chloride on the dissolution of calcium silicate hydrate gels. Waste Manag. 26, 758–768 (2006).
Liu, L., et al. Influence of decalcification on structural and mechanical properties of synthetic calcium silicate hydrate (C-S-H). Cem. Concr. Res. 123, 105793 (2019).
Liu, X. et al. Effects of pH on the nano/micro structure of calcium silicate hydrate (C-S-H) under sulfate attack. Cem. Concr. Res. 140, 106306 (2021).
Uno, T. et al. Understanding the Carbonation Phenomenon of C–S–H through layer structure changes and water exchange. J. Phys. Chem. C. 128, 11802–11816 (2024).
Haque, M. I., Borno, I. B. & Ashraf, W. Enhancing CO 2 Sequestration in Beta Dicalcium Silicate (β-C 2 S) with Polyacrylic acid addition and improving performance of carbonated composites. ACS Sustain Chem. Eng. 13, 744–753 (2025).
Nishat, F. M., Borno, I. B., Tahsin, A. & Ashraf, W. New insights into the interaction between seawater and CO2-activated calcium silicate composites. Cem. Concr. Compos 157, 105929 (2025).
Król, M., Rożek, P., Chlebda, D. & Mozgawa, W. ATR/FT-IR studies of zeolite formation during alkali-activation of metakaolin. Solid State Sci. 94, 114–119 (2019).
Higl, J., Hinder, D., Rathgeber, C., Ramming, B. & Lindén, M. Detailed in situ ATR-FTIR spectroscopy study of the early stages of C-S-H formation during hydration of monoclinic C3S. Cem. Concr. Res. 142, 106367 (2021).
Borno, I. B., Ashraf, W., Khan, R. I. & Tahsin, A. Multi-technique Approaches to Evaluate the Pozzolanic Properties of Alkali-Rich Calcined Kaolinite and Bentonite Clay Blends. Adv. Civ. Eng. Mater. 11, 20210156 (2022).
Vollpracht, A., Lothenbach, B., Snellings, R. & Haufe, J. The pore solution of blended cements: a review. Mater. Struct. 49, 3341–3367 (2016).
Wang, L., Jin, M., Zhou, S., Tang, S. & Lu, X. Investigation of microstructure of C-S-H and micro-mechanics of cement pastes under NH4NO3 dissolution by 29Si MAS NMR and microhardness. Measurement 185, 110019 (2021).
Faucon, P. et al. Aluminum Incorporation in Calcium Silicate Hydrates (C−S−H) Depending on Their Ca/Si Ratio. J. Phys. Chem. B 103, 7796–7802 (1999).
Richardson, I. G. The calcium silicate hydrates. Cem. Concr. Res. 38, 137–158 (2008).
Borno, I. B., Nair, N. & Ashraf, W. Alkali thermal fusion: A prospective route to enhance the reactivity of low-grade clay and utilize as supplementary cementitious material (SCM). Cem. Concr. Compos. 147, 105417 (2024).
García-Lodeiro, I., Fernández-Jiménez, A., Blanco, M. T. & Palomo, A. FTIR study of the sol–gel synthesis of cementitious gels: C–S–H and N–A–S–H. J. Sol Gel Sci. Technol. 45, 63–72 (2008).
Yan, Y. et al. Kinetics of Al uptake in synthetic calcium silicate hydrate (C-S-H). Cem. Concr. Res. 172, 107250 (2023).
Wei, S. et al. The correlation between Al incorporation and alkali fixation by calcium aluminosilicate hydrate: Observations from hydrated C3S blended with and without metakaolin. Cem. Concr. Res. 172, 107249 (2023).
Sigtryggsson, C., Hamnér, K. & Kirchmann, H. Laboratory studies on dissolution of nitrogen fertilizers by humidity and precipitation. Agric. Environ. Lett. 5, https://doi.org/10.1002/ael2.20016 (2020).
Andersen, M. D., Jakobsen, H. J. & Skibsted, J. Incorporation of Aluminum in the Calcium Silicate Hydrate (C−S−H) of Hydrated Portland Cements: A High-Field 27 Al and 29 Si MAS NMR Investigation. Inorg. Chem. 42, 2280–2287 (2003).
Ashraf, W. & Olek, J. Carbonation behavior of hydraulic and non-hydraulic calcium silicates: potential of utilizing low-lime calcium silicates in cement-based materials. J. Mater. Sci. 51, 6173–6191 (2016).
Kogo, M., Umegaki, T. & Kojima, Y. Effect of pH on formation of single-phase vaterite. J. Cryst. Growth 517, 35–38 (2019).
Sheng Han, Y., Hadiko, G., Fuji, M. & Takahashi, M. Crystallization and transformation of vaterite at controlled pH. J. Cryst. Growth 289, 269–274 (2006).
Haque, M. I., Borno, I. B. & Ashraf, W. Enhancing the properties of carbonation cured gamma dicalcium silicates (γ-C2S) using biomimetic molecules. J. Sustain. Cem. Based Mater. 13, 690–705 (2024).
Zheng, H. et al. Molecular-scale insights into the leaching of Ca ions and carbonate deposition behavior in CSH under carbonation. J. Build. Eng. 98, 111358 (2024).
Tao, Y., Gao, Y., Sun, Y., Pellenq, R. J.-M. & Poon, C. S. CSH decalcification in seawater: The view from the nanoscale. Cem. Concr. Res. 175, 107385 (2024).
Hong, S.-Y. & Glasser, F. P. Alkali sorption by CSH and CASH gels: Part II. Role of alumina. Cem. Concr. Res. 32, 1101–1111 (2002).
Song, X., Zhang, L., Cao, Y., Zhu, J. & Luo, X. Effect of pH and temperatures on the fast precipitation vaterite particle size and polymorph stability without additives by steamed ammonia liquid waste. Powder Technol. 374, 263–273 (2020).
Ye, H., Chen, Z. & Huang, L. Mechanism of sulfate attack on alkali-activated slag: The role of activator composition. Cem. Concr. Res 125, 105868 (2019).
Richardson, I. G. Tobermorite/jennite-and tobermorite/calcium hydroxide-based models for the structure of CSH: applicability to hardened pastes of tricalcium silicate, β-dicalcium silicate, Portland cement, and blends of Portland cement with blast-furnace slag, metakaolin, or silica fume. Cem. Concr. Res. 34, 1733–1777 (2004).
Acknowledgements
This work was conducted with funding support from the US Defense Advanced Research Projects Agency (W911NF2010308) at the University of Texas at Arlington. All opinions, findings, conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the sponsor.
Author information
Authors and Affiliations
Contributions
Ishrat Baki Borno: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. Muhammad Intesarul Haque: Data curation, Formal analysis, Investigation, Writing – review & editing. Warda Ashraf: Conceptualization, Formal analysis, Investigation, Funding acquisition, Supervision, Writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Borno, I.B., Haque, M.I. & Ashraf, W. Decalcification kinetics of calcium silicate hydrates: role of Al content, crystallinity, and initial pH. npj Mater Degrad 9, 121 (2025). https://doi.org/10.1038/s41529-025-00669-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41529-025-00669-5