Abstract
Although α-synuclein seed amplification assays (α-syn SAA) are promising, its sensitivity may be affected by heterogeneity among patients with Lewy body disease (LBD). We evaluated whether α-syn SAA sensitivity is affected by patient heterogeneity, using 123I-meta-iodobenzylguanidine (MIBG) cardiac scintigraphy in early drug-naïve patients. Thirty-four patients with clinically established or probable Parkinson’s disease (PD) and seven with dementia with Lewy bodies (DLB) or prodromal DLB were included. While 85.2% of patients with abnormal cardiac MIBG were α-syn SAA positive, only 14.3% were positive among those with normal scans. Logistic regression analysis showed that MIBG positivity was the only significant variable associated with α-syn SAA positivity (odds ratio 74.2 [95% confidence interval 6.1–909]). Although α-syn SAA is sensitive for LBD in patients with abnormal MIBG, the sensitivity may be lower in those with normal MIBG. Further studies are necessary to evaluate the association between patient heterogeneity and α-syn SAA sensitivity.
Similar content being viewed by others
Introduction
Pathological hallmarks of idiopathic Parkinson’s disease (PD) and dementia with Lewy bodies (DLB) are the presence of Lewy bodies, which consist of aggregated α-synuclein (α-syn). Recent evidence suggests that aggregated α-syn can be detected in the cerebrospinal fluid (CSF) using real-time quaking-induced conversion (RT-QuIC) assays, which was originally developed for prion disease1 and later used and validated for Lewy body diseases (LBD)2,3,4,5. This assay is now often collectively called the α-syn seed amplification assay (SAA). As previous studies have reported high sensitivity even in preclinical phases of LBD, for disorders such as idiopathic REM sleep behavior disorder (iRBD)6,7 and pure autonomic failure (PAF)6, recently proposed biological classification uses the result of the α-syn SAA to classify whether the patient has LBD8 (or recently proposed terminology of neuronal α-synuclein disease9). However, the result of a recent large study suggested that the sensitivity of the α-syn SAA may be affected by inter-patient heterogeneity such as the presence or absence of olfactory deficits10. Further studies are important to understand the association between the α-syn SAA sensitivity and heterogeneity among patients with LBD.
Recent studies have suggested that there are two different progression pattern of Lewy body pathology11,12,13,14,15. It has been proposed that in the body-first subtype of the disease, pathology initiates in the enteric or peripheral autonomic nervous system, including the cardiac sympathetic nerve, and progresses to the dorsal motor nucleus of the vagus in the medulla before ascending through the brainstem. Conversely, in the brain-first subtype, the pathological process is thought to begin in the unilateral olfactory bulb or amygdala, from where it extends to the substantia nigra and subsequently descends through the brainstem11,12,13,14,15. Although the former is associated with impaired cardiac sympathetic nerve, symmetric symptoms, and increased frequency of prodromal symptoms (iRBD, autonomic impairment, olfactory deficit) and DLB, the latter is associated with preserved cardiac sympathetic nerve, asymmetric symptoms, and decreased frequency of prodromal symptoms and DLB11,12,13,14,15. In the early phase, although further longitudinal studies are needed to support the concept, these subtypes may be identified based on radiological heterogeneity using striatal dopaminergic imaging and 123I-meta-iodobenzylguanidine (MIBG) cardiac scintigraphy12. However, it remained unknown whether α-syn SAA sensitivity is influenced by this radiological heterogeneity.
Therefore, in this study, we evaluated whether α-syn SAA sensitivity is affected by radiological heterogeneity observed using striatal dopaminergic imaging and cardiac MIBG scintigraphy.
Results
Baseline characteristics of the participants
Overall, CSF samples from 105 participants were used for the analysis. Fifty participants received a clinical diagnosis of LBD (PD or DLB). Baseline characteristics did not differ between diagnostic groups (Table 1).
Diagnostic performance of α-syn SAA for differentiating LBD versus other diseases
The positive rates of α-syn SAA for PD and DLB were 52.4% and 87.5%, respectively, which were significantly higher than those in other diagnostic groups (Fig. 1). Example SAA curves and interpretations are provided in Supplementary Fig. 2. The diagnostic performances of α-syn SAA in several situations are summarized in Table 2. While the specificity of α-syn SAA for the diagnosis of LBD was high (91%) and the sensitivity for DLB was also high (88%), the sensitivity for PD was 52%, which was lower than expected.
Radiological heterogeneity among participants with LBD and association between α-syn SAA positivity and cardiac MIBG abnormality
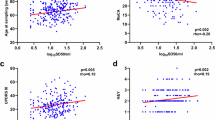
Of the 50 patients with a clinical diagnosis of LBD, 45 had available results for both DAT SPECT and MIBG cardiac scintigraphy (the patients with missing results were excluded). Furthermore, we excluded four patients with a diagnosis of PD not fulfilling the MDS-PD clinically established or probable criteria. The remaining 41 patients were included in further analysis. Thirty-four patients were in the PD group and seven were in the DLB group. None were suspected to have familial forms and genetic testing was not conducted. Twenty-seven were evaluated within two years from disease onset. The SAA positivity was numerically lower in those evaluated within 2 years from disease onset but the difference was nonsignificant (56% vs 71%, p = 0.50). The indices of striatal DAT and cardiac MIBG of each patient are plotted in Fig. 2 in reference to a previous study reporting radiological heterogeneity12. When displayed in a scatter plot, a wide distribution for both DAT and MIBG measures was observed. Correlation between DAT Z-SBR and MIBG delayed H/M ratio was weak (r = 0.12) and nonsignificant (p = 0.45). While normal cardiac MIBG was observed in 41% (14/34) of patients with PD, all patients with DLB showed abnormal cardiac MIBG (Fig. 2A). Not only those meeting the probable criteria but also 15% of those meeting clinically established MDS-PD criteria16 showed normal cardiac MIBG results (Fig. 2B).
A–C Striatal DAT Z-SBR and cardiac MIBG delayed H/M ratio of each patient. Vertical and horizontal dot lines represent predetermined cut-off lines. A Although normal cardiac MIBG was observed in 41% (14/34) of patients with PD, all patients with DLB showed decreased cardiac MIBG H/M ratio irrespective of DAT measure. B Plots are limited to patients with PD. Not only those meeting the probable PD criteria but also 15% of those meeting clinically established MDS-PD criteria showed normal cardiac MIBG results. C Plots of all patients with LBD and their α-syn SAA positivity. D The difference in α-syn SAA positivity between MIBG normal and abnormal groups was significant for both analyses limited to patients with PD (85.0% vs 14.3%, p < 0.001) and patients with clinically established PD (91.7% vs 0%, p < 0.01).
LBD patients with or without MIBG abnormality were similar in age (73.6 ± 10.0 vs. 74.4 ± 6.6), sex (female 48.1% vs 35.7%), and disease duration (1 [1–2.0] vs 1 [1–2.5] years) (Table 3). Although other baseline characteristics were also similar except for the frequency of DLB and geriatric depression scale (Table 3), the frequency of α-syn SAA positivity was significantly higher in patients with MIBG abnormality (85.2% vs 14.3%, p < 0.001) (Fig. 2C). This difference remained significant when limiting the analysis to patients with PD or to patients with clinically-established PD (Fig. 2D).
When patients were grouped by α-syn SAA positivity, there were no significant differences in baseline characteristics such as age, sex, disease duration, UPDRS part 3 score, and DAT Z-SBR, and disease type among the groups (Supplementary Table 1). However, the prevalence of MIBG abnormalities was significantly higher in patients with a positive α-syn SAA. Furthermore, all MIBG measures, including the early heart-to-mediastinum (H/M) ratio, delayed H/M ratio, and washout rate, were significantly altered and abnormal in this group (Supplementary Table 1). Logistic regression analysis showed that α-syn SAA positivity was significantly associated with MIBG positivity (odds ratio 74.2 [95% confidence interval = 6.1–909]), but not with age, sex, disease duration, and PD or DLB.
Discussion
In this study, we confirmed that the RT-QuIC assay we used as α-syn SAA is specific for LBD. Furthermore, our results suggest that the α-syn SAA sensitivity may be affected by inter-patient radiological heterogeneity, namely the presence or absence of cardiac MIBG abnormalities (Fig. 3).
α-syn SAA positivity was high in patients with MIBG abnormal LBD. From previous reports, these patients should have Lewy bodies at least in the cardiac sympathetic system and substantia nigra in PD. However, α-syn SAA positivity was low in patients with MIBG normal PD. Although neuropathological data is unavailable at this point, from previous reports, most of the patients with non-young-onset and non-familial PD should have Lewy bodies at least in the substantia nigra. (The elements of the figure were created by the author using Adobe Illustrator and Microsoft PowerPoint).
While our study showed high specificity (91%) of α-syn SAA for LBD in line with previous reports, the sensitivity was lower (58%) than that reported in previous studies5,6,7,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31. This difference could be due to a number of factors including differences in sample handling, assay characteristics, or clinical characteristics of the participants. As a previous study on a different PD cohort using the same α-syn RT-QuIC assay showed a higher sensitivity (unpublished) and a recent large study suggested that the positivity of α-syn SAA may be affected by heterogeneity among participants10, we evaluated whether α-syn SAA sensitivity was affected by heterogeneity among participants in our cohort.
MIBG cardiac scintigraphy abnormalities reflect the degeneration of postganglionic cardiac sympathetic nerve terminal32,33,34. Overall, cardiac MIBG uptake is reduced in patients with clinical diagnosis of PD and DLB and the sensitivity is reported to range between 64.5–100% for PD35 and 82.4–100% for DLB36. In the PD group, up to 40% of patients may show normal cardiac MIBG at the initial scan and a decreased cardiac MIBG at the follow-up37,38. This change between the initial scan and the follow-up might be associated with different clinical characteristics37. In an autopsy validation study conducted at our institution, although the MIBG H/M ratio strongly correlated with residual cardiac sympathetic fibers, the sensitivity for the LBD pathology was 80%33. Those with normal cardiac MIBG and LBD pathology at autopsy (false-negative) showed preserved cardiac sympathetic fibers even at autopsy; thus, suggesting a different progression pattern33. Recent reports proposed that the MIBG cardiac scintigraphy is useful for differentiating subtypes of disease progression associated with clinical characteristics11,12,13,14,15, the presence of which was also suggested13,14 using autopsy data39. In this study, because the MIBG cardiac scintigraphy results were available in most participants and were normal in one-third of the patients with LBD, we decided to evaluate whether α-syn SAA sensitivity was associated with cardiac MIBG abnormality. Although α-syn SAA sensitivity in patients with abnormal cardiac MIBG was high (85%) and similar to that observed in previous reports, the sensitivity was as low as 14% in patients with normal cardiac MIBG. The difference was significant and logistic regression analysis confirmed that the association was not confounded by other factors. These results suggest that α-syn SAA sensitivity is affected by the presence of cardiac MIBG abnormality, and that the high rate of MIBG normality in our cohort contributed to the lower sensitivity compared to previous studies.
The difference in pathological distribution and load could be contributing to the difference in α-syn SAA sensitivity (Supplementary Fig. 3). In previous autopsy validation studies, while α-syn SAA was 100% positive in individuals with widespread cortical distribution of Lewy bodies, the sensitivity was lower in those with pathology limited to the amygdala or brainstem31,40. As the brain-first LBD is considered to start in amygdala and spread by descending into the brainstem, it is associated with normal cardiac MIBG11,12,13,14,15. In this study, patients with normal cardiac MIBG may have had Lewy body pathology relatively limited to the amygdala or brainstem, and thus showed negative α-syn SAA results. Alternatively, body-first LBD is associated with abnormal cardiac MIBG results and the pathology may also persist within the brainstem. Faster clinical progression and accelerated dementia14,15 suggest that the pathology may have already spread to the neocortex by the time motor symptoms manifest. Consequently, this subtype often exhibits positive α-syn SAA results (Supplementary Fig. 3). Another possibility may include different α-syn strains (Supplementary Fig. 4) or inhibitory matrix in the CSF of LBD with normal cardiac MIBG41. Although recently proposed biological classifications rely on the assumption that α-syn SAA sensitivity is very high from the prodromal phase8,9 based on previous studies in iRBD6,7 and PAF6, these prodromal symptoms may be characteristic only for LBD with abnormal cardiac MIBG11,12,13,14,15. Following these results, future studies may be necessary to evaluate whether high α-syn SAA sensitivity can be replicated in the early or prodromal phase of LBD with normal cardiac MIBG.
This study had several limitations. First, diagnoses were clinical, and neuropathological confirmation was unavailable, thus, some of the patients with normal MIBG results may later develop other Parkinsonian disorders. However, diagnoses were made by board-certified neurologists, and analysis regarding radiological heterogeneity was limited to those meeting clinically established or probable PD criteria (MDS criteria), the diagnostic accuracy of which is reported to be 92.5% even for probable PD42. Second, all participants were Japanese and genetic testing to rule out genetic forms of LBD was not conducted. Third, we only used our α-syn RT-QuIC assay and a single cut-off to determine α-syn SAA positivity. Using different assays and cut-offs may yield different sensitivities and may show higher sensitivity, especially in the MIBG normal group. Future studies using different α-syn SAA assays in our MIBG normal group samples would be important to further understand the difference in diagnostic characteristics between different assays. Fourth, not all clinical information was blinded to the tester. However, since we used objective criteria to determine SAA positivity and the tester was blinded to the final clinical diagnosis and the results of cardiac MIBG scintigraphy, the main findings of the study are likely not biased by this point. Fifth, although we focused on DAT SPECT and MIBG cardiac scintigraphy results that were available for most of the participants in the biobank, data regarding other important factors associated with heterogeneity among patients with LBD such as the presence of RBD and olfactory function were insufficient to conduct a formal analysis in the present study. We are collecting these data for further studies.
In conclusion, although α-syn SAA is specific for LBD and is also sensitive in patients with MIBG abnormality, the sensitivity may be lower in those with normal cardiac MIBG. Further studies are warranted to evaluate the association between patient heterogeneity and α-syn SAA sensitivity.
Materials and methods
Participants
Patients who underwent lumbar puncture for CSF biomarker measurement at the Tokyo Metropolitan Institute for Geriatrics and Gerontology between December 2021 and June 2023 were recruited for the Tokyo Medical Biobank. Most patients were recruited during the initial diagnostic workup and were in the early course of the disease as well as drug-naïve. Those who consented to biobanking with sufficient remaining CSF samples were included in this study. This study was performed in accordance with the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of the Tokyo Metropolitan Institute for Geriatrics and Gerontology (R23-031). The remaining CSF, serum, and plasma samples were stored as part of the Tokyo Medical Biobank for future study. The clinical diagnosis of each patient was based on the latest information obtained on April 1, 2024. Patients with a diagnosis of PD were considered the PD group and evaluated using the MDS clinical diagnostic criteria16. Patients with DLB or prodromal DLB were evaluated using previous criteria43,44 and considered the DLB group. Patients with multiple system atrophy (MSA), corticobasal syndrome and progressive supranuclear palsy syndrome, and Alzheimer’s disease (AD) were separated into the MSA, other Parkinsonian disease, and AD groups. CSF biomarker measurement methods and cut-offs for the AD diagnosis were as previously described45,46,47. All patients with other diagnoses including non-AD mild cognitive impairment or dementia, primary progressive aphasia, and amyotrophic lateral sclerosis were enrolled in the Other group. The LBD groups include the patients in the PD and DLB groups.
CSF sample collection
CSF samples were collected and stored as previously reported with minor modifications. Briefly, CSF samples were obtained using a standard lumbar puncture procedure and the first tube was sent for cell counting and routine biochemical testing. Subsequent CSF samples were directly collected in polypropylene low-binding tubes and centrifuged at 800 × g for 10 min at 4 °C. Lastly, 0.5 mL of each sample was aliquoted into 1.5 mL tubes (Proteosave SS 1.5 mL, Sumitomo Bakelite Co., Ltd., Tokyo, Japan) and stored at −80 °C.
α-syn SAA (RT-QuIC)
The α-syn recombinant protein was expressed and purified as previously described3. The RT-QuIC reaction mix consisted of 50 mM PIPES (pH 7.0), 170 mM NaCl, 10 µM thioflavin-T (ThT), 0.1 mM ethylenediaminetetraacetic acid tetrasodium salt hydrate (EDTA), 0.015‰ sodium dodecyl sulfate (SDS), and 0.1 mg/mL human recombinant α-syn protein. Each well of a black 96-well plate with a clear bottom (Nunc 96 well; Sigma-Aldrich, St. Louis, MO, USA) contained 100 μL with two 0.5 mm zirconium/silica beads. Reactions were seeded with 20 μL of undiluted CSF to a final reaction volume of 100 μL. The plates were sealed with a plate sealer film and incubated in a BMG OPTIMA Fluo STAR plate reader at 53 °C for 120 h with intermittent shaking cycles: double orbital with 1 min shake (400 rpm), 15 min rest. ThT fluorescence measurements (450 nm excitation and 480 nm emission) were taken every 15 min. Each sample was run in triplicate, allowing two negative control samples (reactions seeded with prion disease brain homogenate), one positive control (reaction seeded with LBD brain homogenate), an unseeded reaction, and 20 CSF samples to be tested on one plate. The α-syn amyloid formation was monitored for 72 h. The specificity of this assay was confirmed using samples from disease controls such as Creutzfeldt–Jakob disease (Supplementary Fig. 1). Age, sex, initial clinical diagnosis, and date of CSF obtainment were provided, and other detailed clinical information including the final clinical diagnosis and the results of cardiac MIBG scintigraphy were blinded to the tester. A positive reaction is indicated when the ThT fluorescence value exceeds 20,000 arbitrary units in two or more wells within 200 cycles, as per our established protocol. In instances where only a single well of the sample exhibits an elevated value, we take the precautionary measure of retesting the sample. Subsequently, a positive reaction is confirmed if more than one well demonstrates a ThT fluorescence value of 20,000 arbitrary units or greater.
Radiological assessment
Dopamine transporter (DAT) single photon emission computed tomography (SPECT) images were acquired and evaluated as previously described48,49. Briefly, 185 MBq of 123I-ioflupane (DaT SCAN®, Nihon Medi-Physics, Tokyo, Japan) was administered and images were acquired after 3 h. The specific binding ratios (SBR) of striatal DAT binding were semi-quantitatively calculated using the DAT VIEW software (Nihon Medi-Physics) with the Southampton method50 after phantom calibration. The z-score of the average striatal DAT SBR (Z-SBR) for age- and sex-matched Japanese participants51 was calculated and a cut-off was set at -2.
123I-MIBG cardiac scintigraphy images were acquired and assessed as previously described using smart MIBG (PDRadiopharma Inc., Tokyo, Japan)33,49. Briefly, 111 MBq of 123I-MIBG (PDRadiopharma Inc.) was administered, whereas early and delayed images were obtained after 15–30 min and 3–4 h, respectively. The heart-to-mediastinum (H/M) ratios underwent standardized conversion to a value comparable to that of a medium-energy-type collimator52, and washout rates were calculated from early and delayed images33. The cut-off for the delayed H/M ratio was set at 2.2.
Abnormalities of DAT SPECT and MIBG cardiac scintigraphy were identified by an expert nuclear medicine physician (M. Kameyama) with reference to these quantitative indices.
Statistical methods
Statistical analyses were conducted using GraphPad Prism version 9 (GraphPad Software, San Diego, CA, USA), MedCalc Statistical Software version 20.218 (MedCalc Software Ltd., Ostend, Belgium; https://www.medcalc.org; 2023), or R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria) and a graphical interface EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan)53. Missing data were handled using a pairwise deletion approach. Categorical variables are expressed as percentages and differences between groups were evaluated using Fisher’s exact test. Pairwise comparisons were performed using Holm’s method. Normally distributed continuous variables are expressed as mean ± standard deviation and differences between groups were tested using the Student’s t-test. Continuous variables without a normal distribution are expressed as median (interquartile range) and differences between groups were tested using the Mann–Whitney U test. Correlation was evaluated using Spearman’s method. Logistic regression analysis was performed with α-syn SAA positivity as the dependent variable and age, sex, disease duration, PD or DLB, and MIBG positivity as independent variables. P < 0.05 were considered statistically significant.
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Atarashi, R. et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat. Med. 17, 175–178 (2011).
Fairfoul, G. et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann. Clin. Transl. Neurol. 3, 812–818 (2016).
Sano, K. et al. Prion-Like Seeding of Misfolded alpha-Synuclein in the Brains of Dementia with Lewy Body Patients in RT-QUIC. Mol. Neurobiol. 55, 3916–3930 (2018).
Nakagaki, T., Nishida, N. & Satoh, K. Development of alpha-synuclein real-time quaking-induced conversion as a diagnostic method for alpha-synucleinopathies. Front Aging Neurosci. 13, 703984 (2021).
Grossauer, A. et al. alpha-synuclein seed amplification assays in the diagnosis of synucleinopathies using cerebrospinal fluid-A systematic review and meta-analysis. Mov. Disord. Clin. Pract. 10, 737–747 (2023).
Rossi, M. et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 140, 49–62 (2020).
Iranzo, A. et al. Detection of alpha-synuclein in CSF by RT-QuIC in patients with isolated rapid-eye-movement sleep behaviour disorder: a longitudinal observational study. Lancet Neurol. 20, 203–212 (2021).
Hoglinger, G. U. et al. A biological classification of Parkinson’s disease: the SynNeurGe research diagnostic criteria. Lancet Neurol. 23, 191–204 (2024).
Simuni, T. et al. A biological definition of neuronal alpha-synuclein disease: towards an integrated staging system for research. Lancet Neurol. 23, 178–190 (2024).
Siderowf, A. et al. Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using alpha-synuclein seed amplification: a cross-sectional study. Lancet Neurol. 22, 407–417 (2023).
Borghammer, P. & Van Den Berge, N. Brain-first versus gut-first Parkinson’s disease: a hypothesis. J. Parkinsons Dis. 9, S281–S295 (2019).
Horsager, J. et al. Brain-first versus body-first Parkinson’s disease: a multimodal imaging case-control study. Brain 143, 3077–3088 (2020).
Borghammer, P. et al. Neuropathological evidence of body-first vs. brain-first Lewy body disease. Neurobiol. Dis. 161, 105557 (2021).
Borghammer, P. et al. A postmortem study suggests a revision of the dual-hit hypothesis of Parkinson’s disease. NPJ Parkinsons Dis. 8, 166 (2022).
Borghammer, P. The brain-first vs. body-first model of Parkinson’s disease with comparison to alternative models. J. Neural Transm. (Vienna) 130, 737–753 (2023).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Shahnawaz, M. et al. Development of a biochemical diagnosis of Parkinson disease by detection of alpha-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol. 74, 163–172 (2017).
Groveman, B. R. et al. Rapid and ultra-sensitive quantitation of disease-associated alpha-synuclein seeds in brain and cerebrospinal fluid by alphaSyn RT-QuIC. Acta Neuropathol. Commun. 6, 7 (2018).
Bongianni, M. et al. alpha-Synuclein RT-QuIC assay in cerebrospinal fluid of patients with dementia with Lewy bodies. Ann. Clin. Transl. Neurol. 6, 2120–2126 (2019).
Manne, S. et al. Ultrasensitive detection of aggregated alpha-synuclein in glial cells, human cerebrospinal fluid, and brain tissue using the RT-QuIC assay: new high-throughput neuroimmune biomarker assay for Parkinsonian disorders. J. Neuroimmune Pharm. 14, 423–435 (2019).
Shahnawaz, M. et al. Discriminating alpha-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature 578, 273–277 (2020).
Singer, W. et al. Alpha-synuclein oligomers and neurofilament light chain in spinal fluid differentiate multiple system atrophy from lewy body synucleinopathies. Ann. Neurol. 88, 503–512 (2020).
Bargar, C. et al. Streamlined alpha-synuclein RT-QuIC assay for various biospecimens in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol. Commun. 9, 62 (2021).
Brockmann, K. et al. Association between CSF alpha-synuclein seeding activity and genetic status in Parkinson’s disease and dementia with Lewy bodies. Acta Neuropathol. Commun. 9, 175 (2021).
Mammana, A. et al. RT-QuIC detection of pathological alpha-synuclein in skin punches of patients with Lewy body disease. Mov. Disord. 36, 2173–2177 (2021).
Orru, C. D. et al. A rapid alpha-synuclein seed assay of Parkinson’s disease CSF panel shows high diagnostic accuracy. Ann. Clin. Transl. Neurol. 8, 374–384 (2021).
Perra, D. et al. Alpha-synuclein seeds in olfactory mucosa and cerebrospinal fluid of patients with dementia with Lewy bodies. Brain Commun. 3, fcab045 (2021).
Rossi, M. et al. Diagnostic value of the CSF alpha-synuclein real-time quaking-induced conversion assay at the prodromal MCI stage of dementia with Lewy bodies. Neurology 97, e930–e940 (2021).
Russo, M. J. et al. High diagnostic performance of independent alpha-synuclein seed amplification assays for detection of early Parkinson’s disease. Acta Neuropathol. Commun. 9, 179 (2021).
Sokratian, A. et al. Heterogeneity in alpha-synuclein fibril activity correlates to disease phenotypes in Lewy body dementia. Acta Neuropathol. 141, 547–564 (2021).
Hall, S. et al. Performance of alphaSynuclein RT-QuIC in relation to neuropathological staging of Lewy body disease. Acta Neuropathol. Commun. 10, 90 (2022).
Orimo, S. et al. Cardiac sympathetic denervation precedes neuronal loss in the sympathetic ganglia in Lewy body disease. Acta Neuropathol. 109, 583–588 (2005).
Matsubara, T. et al. Autopsy validation of the diagnostic accuracy of (123)I-metaiodobenzylguanidine myocardial scintigraphy for Lewy body disease. Neurology 98, e1648–e1659 (2022).
Amino, T. et al. Profound cardiac sympathetic denervation occurs in Parkinson disease. Brain Pathol. 15, 29–34 (2005).
Orimo, S., Suzuki, M., Inaba, A. & Mizusawa, H. 123I-MIBG myocardial scintigraphy for differentiating Parkinson’s disease from other neurodegenerative parkinsonism: a systematic review and meta-analysis. Parkinsonism Relat. Disord. 18, 494–500 (2012).
Nihashi, T., Ito, K. & Terasawa, T. Diagnostic accuracy of DAT-SPECT and MIBG scintigraphy for dementia with Lewy bodies: an updated systematic review and Bayesian latent class model meta-analysis. Eur. J. Nucl. Med Mol. Imaging 47, 1984–1997 (2020).
Tsujikawa, K. et al. Chronological changes of 123I-MIBG myocardial scintigraphy and clinical features of Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 86, 945–951 (2015).
Ryu, D. W. et al. Initial versus follow-up sequential myocardial 123I-MIBG scintigraphy to discriminate Parkinson disease from atypical Parkinsonian syndromes. Clin. Nucl. Med 44, 282–288 (2019).
Tanei, Z. I. et al. Lewy pathology of the esophagus correlates with the progression of Lewy body disease: a Japanese cohort study of autopsy cases. Acta Neuropathol. 141, 25–37 (2021).
Samudra, N. et al. Clinicopathological correlation of cerebrospinal fluid alpha-synuclein seed amplification assay in a behavioral neurology autopsy cohort. Alzheimers Dement https://doi.org/10.1002/alz.13799 (2024).
Just, M. K. et al. Alpha-synuclein strain variability in body-first and brain-first synucleinopathies. Front Aging Neurosci. 14, 907293 (2022).
Virameteekul, S., Revesz, T., Jaunmuktane, Z., Warner, T. T. & De Pablo-Fernandez, E. Clinical diagnostic accuracy of Parkinson’s disease: where do we stand? Mov. Disord. 38, 558–566 (2023).
McKeith, I. G. et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 89, 88–100 (2017).
McKeith, I. G. et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology 94, 743–755 (2020).
Kurihara, M. et al. CSF P-Tau181 and other biomarkers in patients with neuronal intranuclear inclusion disease. Neurology 100, e1009–e1019 (2023).
Kurihara, M. et al. Neuropathological changes associated with aberrant cerebrospinal fluid p-tau181 and Abeta42 in Alzheimer’s disease and other neurodegenerative diseases. Acta Neuropathol. Commun. 12, 48 (2024).
Kurihara, M. et al. Relationship Between Cerebrospinal Fluid Alzheimer’s Disease Biomarker Values Measured via Lumipulse Assays and Conventional ELISA: Single-Center Experience and Systematic Review. J. Alzheimers. Dis. 99, 1077–1092 (2024).
Goto, R. et al. Correlations between cerebrospinal fluid homovanillic acid and dopamine transporter SPECT in degenerative parkinsonian syndromes. J. Neural Transm. (Vienna) 130, 513–520 (2023).
Shimasaki, R. et al. Associations of cerebrospinal fluid monoamine metabolites with striatal dopamine transporter binding and 123I-meta-iodobenzylguanidine cardiac scintigraphy in Parkinson's disease: Multivariate analyses. Parkinsonism Relat. Disord. 128, 107129 (2024).
Tossici-Bolt, L. et al. [(123)I]FP-CIT ENC-DAT normal database: the impact of the reconstruction and quantification methods. EJNMMI Phys. 4, 8 (2017).
Matsuda, H. et al. Japanese multicenter database of healthy controls for [(123)I]FP-CIT SPECT. Eur. J. Nucl. Med Mol. Imaging 45, 1405–1416 (2018).
Nakajima, K. et al. Multicenter cross-calibration of I-123 metaiodobenzylguanidine heart-to-mediastinum ratios to overcome camera-collimator variations. J. Nucl. Cardiol. 21, 970–978 (2014).
Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 48, 452–458 (2013).
Acknowledgements
The authors thank the participants of the Tokyo Medical Biobank for donating their clinical information and biosamples. We also thank all members of the Department of Neurology, Healthy Aging Innovation Center (HAIC), and the Integrated Research Initiative for Living Well with Dementia (IRIDE) of the Tokyo Metropolitan Institute for Geriatrics and Gerontology (TMIG) for their assistance. This study was supported by KAKENHI from the Japan Society for the Promotion of Science (JSPS) to M. Kurihara (JP23K14789) and K.S. (JP21K07417), and Translational Research Grant from the Tokyo Metropolitan Institute for Geriatrics and Gerontology to M. Kurihara.
Author information
Authors and Affiliations
Contributions
Conceptualization, formal analysis, visualization, original draft: M. Kurihara; Data Curation: M. Kurihara, R.S., K.H., and K.O.; Funding Acquisition: M.K. and K.S.; Investigation: K.S. (RT-QuIC) and M.Kameyama (Radiological assessments); Methodology: K.S.; Resources: R.S., K.H., K.O., K.T., R.I., M.H., Y.N., M. Kameyama, and A.I.; Supervision: A.I.; Review & Editing: K.S, R.S., K.H, and M. Kameyama. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kurihara, M., Satoh, K., Shimasaki, R. et al. α-synuclein seed amplification assay sensitivity may be associated with cardiac MIBG abnormality among patients with Lewy body disease. npj Parkinsons Dis. 10, 190 (2024). https://doi.org/10.1038/s41531-024-00806-y
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41531-024-00806-y