Abstract
Many drug targets in ongoing Parkinson’s disease (PD) clinical trials have strong genetic links. While genome-wide association studies (GWAS) nominate regions associated with disease, pinpointing causal genes is challenging. Our aim was to prioritize additional druggable genes underlying PD GWAS signals. The polygenic priority score (PoPS) integrates genome-wide information from MAGMA gene-level associations and over 57,000 gene-level features. We applied PoPS to East Asian and European PD GWAS data and prioritized genes based on PoPS, distance to the GWAS signal, and non-synonymous credible set variants. We prioritized 46 genes, including well-established PD genes (SNCA, LRRK2, GBA1, TMEM175, VPS13C), genes with strong literature evidence supporting a mechanistic link to PD (RIT2, BAG3, SCARB2, FYN, DYRK1A, NOD2, CTSB, SV2C, ITPKB), and genes relatively unexplored in PD. Many hold potential for drug repurposing or development. We prioritized high-confidence genes with strong links to PD pathogenesis that may represent our next-best candidates for developing disease-modifying therapeutics.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a neurodegenerative condition with the fastest-growing global prevalence among all neurological disorders, posing significant challenges to healthcare systems and society1. The pathophysiology of PD involves multiple risk factors, including genetics, environmental factors, and aging2. Currently, most therapeutic strategies aim to address dopamine deficiency (e.g., levodopa or dopamine agonists). More advanced therapies, such as deep brain stimulation, focus on modulating neural synchrony in distributed brain networks3. Although these treatments are intended to alleviate symptoms, they do not clearly impact disease progression4. Hence, there is an urgent need to identify specific therapeutic targets that can effectively halt the course of the disease and accelerate the clinical development of disease-modifying therapies.
Recent advancements in drug development for PD have been significantly influenced by over two decades of genetic research. This body of evidence has not only deepened our understanding of the biology underlying PD, but has also led to the identification of numerous drug targets. Some of these genes are currently being evaluated in clinical trials5, including GBA1, LRRK2 and SNCA. Drugs supported by genetic evidence are more than twice as likely to be approved6,7, and human genetic data supported two-thirds of the drugs approved by the FDA from 2013 to 20228,9. Leveraging data from genetic studies, and developing pipelines to prioritize and select the best targets is estimated to significantly impact the development of new drugs10. This is especially true for disease-modifying therapies6,11.
In recent years, various genome-wide association studies (GWAS) have identified over 100 loci associated with PD12,13,14,15,16,17,18. The largest European-ancestry PD GWAS identified 90 independent significant risk signals across 78 genomic regions (37,688 cases, 18,618 first-degree relatives of cases, and 1,417,791 controls)13. A polygenic risk score including 1805 variants explained 16–36% of the heritable risk of PD13. Additionally, 15 population-specific variants were identified through GWAS conducted in individuals of Latino, East Asian, South Asian, and African and African admixed ancestry14,15,16,17. A recent large-scale multi-ancestry meta-analysis of PD GWAS uncovered 12 potentially novel loci and fine-mapped six putative causal variants at six previously identified loci (49,049 cases and 2,452,961 controls from these four ancestral populations)18.
A major limitation of GWAS is that it nominates genomic regions, not the specific genes that confer risk. Typically GWAS hits can be explained by two general mechanisms: (1) a functional coding variant that alters protein function or (2) a non-coding variant that regulates transcription or translation. Often GWAS studies annotate their loci with the closest gene to the lead variant. This is the correct causal gene strikingly often19, although more sophisticated methods have been developed to improve predictive accuracy.
There have been previous gene prioritization efforts using machine learning models that were trained on and applied to non-PD traits (“locus-to-gene” (L2G) model20) or PD specifically (referred to as Yu202421). However, all previous efforts to prioritize PD GWAS genes have done so on a locus-by-locus basis, ignoring information from other significant loci and the rest of the genome. The polygenic priority score (PoPS)19 is a gene prioritization tool that incorporates genome-wide information from MAGMA22 gene-level association tests and more than 57,000 gene-level features (i.e., gene expression, biological pathways, and protein-protein interactions). By intersecting genes with the top PoPS value in their locus with genes nearest to their GWAS signal, they achieved a precision of 79%.
Here we applied PoPS to several PD GWAS summary statistics including European and East Asian ancestries and highlight high-confidence prioritized genes.
Results
Methods overview
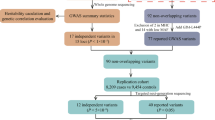
We prioritized PD genes using a meta-analysis of the largest published GWAS in East Asian-16 and European-ancestry13 individuals (44,412 cases, 18,618 proxy cases, and 1,442,642 controls). We identified 120 independent associations with P < 5 × 10–8 (Supplementary Table 1). Across these loci, we prioritized 46 PD genes (Fig. 1, Supplementary Table 2) based on their PoPS scores, distance to the credible set, and presence of non-synonymous variants in the credible set. We further compared our findings with prioritization efforts using the L2G model20 and Yu202421 (Fig. 1).
An overview of the evidence supporting each prioritized gene sorted by gene name. Distance: distance in kilobases between gene and credible set. PoPS: PoPS percentile where 0 represents the smallest genome-wide value and 1 represents the largest. MAGMA: # genes: number of genes in the locus. L2G: Probability of being the causal genes according to the L2G model20. Yu2024: Probability of being the causal genes according to the Yu2024 model21.
Many prioritized genes are also supported by previous literature
Our analysis prioritized several known monogenic or high risk PD genes, including SNCA (OMIM: 168601), LRRK2 (OMIM: 607060), GBA1 (OMIM: 168600), and VPS13C (OMIM: 616840). For some of these, PD clinical trials are already ongoing. LRRK2, thought to cause PD through a gain-of-function mechanism leading to increased LRRK2 kinase activity, is being targeted by kinase inhibitors5. In contrast, other therapies aim to activate glucocerebrosidase (GCase), encoded by GBA1, and increase its activity23,24. Furthermore, there are different approaches of targeting α-synuclein (encoded by SNCA), e.g., by reducing extracellular α-synuclein (PASADENA25 and SPARK26,27 trials) or blocking misfolding28 or aggregation29. VPS13C was relatively far away from its nearby GWAS hit (~150 kb) and had a lower PoPS score (0.111) compared to many other prioritized genes (Fig. 1). Nevertheless, it was the only gene in its locus and is therefore a high-confidence prioritized gene itself. We also prioritized TMEM175, which has been experimentally validated to be the causal gene in its respective GWAS locus30.
We identified several genes that were also supported by our literature review. Among these, RIT2, DYRK1A, BAG3, and SCARB2 were particularly promising, with strong evidence highlighting their involvement in PD pathogenesis, although notably, no focussed locus dissection efforts have been performed yet. RIT2 is linked to neuroprotective pathways in dopaminergic neurons31. Reduced RIT2 expression increases neurodegeneration in various preclinical PD models32,33,34,35, while RIT2 overexpression rescues autophagy-lysosome deficits and reduces α-synuclein aggregation32,34. DYRK1A has been linked to several neurodegeneration pathways36,37. DYRK1A appears to regulate α-synuclein inclusion formation38 and directly phosphorylate (and thereby inhibit) parkin39. BAG3 plays a crucial role in macroautophagy and the clearance of alpha-synuclein aggregates40,41. BAG3 overexpression also suppresses neuroinflammation, decreasing the release of pro-inflammatory cytokines and contributing to mitigating neuronal damage in PD42,43,44. SCARB2 is essential for trafficking GCase from the endoplasmic reticulum to lysosomes45. Deficiencies in SCARB2 lead to reduced GCase activity and subsequent α-synuclein accumulation, contributing to neurotoxicity in dopaminergic neurons45,46,47,48,49. Biallelic variants in SCARB2 cause progressive myoclonic epilepsy with or without renal failure.
Furthermore, we prioritized several genes without strong literature evidence but predicted to be the most likely gene responsible underlying the PD GWAS signal with a probability greater than 80% by L2G20 or Yu202421 (SIPA1L2, SH3GL2, TMEM163, MAP4K4, LCORL, CAMK2D, STK39)20,21 (Fig. 1).
Prioritized genes that encode potentially-druggable targets for PD
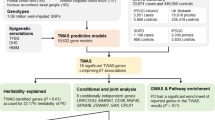
Of the 46 prioritized genes, six (FYN, DYRK1A, NOD2, CTSB, SV2C, ITPKB; Fig. 2) were identified as promising drug targets for PD, each supported by at least eight PD-related publications in our literature review. Their potential as therapeutic targets was established based either on their suitability for repurposing existing drugs to target them in PD (Supplementary Table 3) or their promise for new drug development using the Open Targets platform.
The upper portion of each sub-plot is a LocusZoom plot. Each point represents a different genetic variant, the x-axis represents physical position on the listed chromosome, the left y-axis represents –log10-transformed P value, the right y-axis represents the recombination rate, color represents linkage disequilibrium with the lead variant in the locus (as shown in the legend), and the horizontal dashed line represents the genome-wide significance P value threshold of 5 × 10–8. The lower portion of each figure is a PoPS plot. Genes are denoted as blue bars spanning from their transcription start site to their transcription stop site using the same x-axis as the LocusZoom plot, the y-axis represents the raw PoPS score, the dashed horizontal gray lines represent the top 10% and 1% of PoPS scores genome-wide, and the solid horizontal gray line represents a PoPS score of 0.
FYN inhibitors have been approved to treat various cancers (e.g., chronic myeloid leukemia and acute lymphoblastic leukemia)50,51 and tested in clinical trials for Alzheimer disease52,53,54. FYN plays a significant role in neuroinflammation and protein aggregation55,56,57. Inhibition of FYN has shown promise in reducing these detrimental processes and alleviating L-dopa-induced dyskinesia by regulating the phosphorylation of the NMDA receptor58,59,60.
DYRK1A encodes a dual-specificity kinase with a wide range of functions and interaction partners37. Although no approved drug targeting DYRK1A exists for PD, a number of DYRK1A inhibitors have been investigated in the context of other neurological diseases61,62,63,64. DYRK1A seems to directly phosphorylate a-synuclein and thereby affect aggregate formation and cell death in immortalized hippocampal neurons and brain tissue samples from a MPTP-induced PD mouse model, whereas DYRK1A inhibition suppressed a-synuclein aggregation and reduced dopaminergic neuron apoptosis38,65.
CTSB encodes cathepsin B (catB), a lysosomal enzyme crucial for protein degradation and regulating autophagy. CatB knock-out or inhibition impairs lysosomal functions in dopaminergic neurons, leads to reduced GCase activity66,67, and promotes α-synuclein aggregation66. However, farnesyltransferase inhibitors enhance cathepsin transport and activity, thereby improving lysosomal function and α-synuclein clearance68. These inhibitors, already approved for Hutchinson-Gilford progeria syndrome69, could have potential for repurposing in PD.
Although there is an approved drug targeting NOD2 for osteosarcoma70, it acts as an activator and induces a proinflammatory response. However, data suggest that NOD2 inhibition and an anti-inflammatory response would be desired in PD. In an experimental mouse PD model induced by the neurotoxin 6-hydroxydopamine, NOD2 deficiency was associated with an attenuated inflammatory response and suggested to have protective effects against degeneration of dopaminergic neurons and neuronal death71.
SV2C encodes synaptic vesicle glycoprotein 2 C and is involved in synaptic vesicular function. SV2C expression is enriched in the basal ganglia, particularly in dopaminergic neurons in the substantia nigra72, and is believed to be crucial for dopamine neuron function and homeostasis73,74. In a mouse model, SV2C knock-out resulted in reduced dopamine release in the dorsal striatum, disrupted α-synuclein expression, and mild motor deficits74, suggesting that enhancing SV2C function is beneficial for PD. Clinical trials targeting SV2C are currently ongoing for epilepsy, although it is unclear whether the drug is an activator or inhibitor. Should further preclinical testing find that it is an activator, this drug could potentially be considered for repurposing for PD.
ITPKB encodes a kinase involved in calcium homeostasis75. While it is often possible to inhibit kinases, inhibiting ITPKB is predicted to be detrimental in PD since it has been associated with increased α-synuclein aggregation and impaired autophagy76. However, previous work has shown that MICU3 (a brain-specific mitochondrial calcium uniporter) operates downstream of ITPKB and inhibiting MICU3 was able to rescue increased ITPKB activity77. MICU3, has the highest PoPS score in one of our GWAS loci, but was not the nearest gene and was therefore not prioritized (Supplementary Fig. 1).
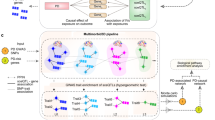
Finally, we prioritized several genes with limited existing literature or functional studies pertaining to PD. Despite this, many of these genes have promising potential as drug targets for PD, e.g., by repurposing existing drugs approved for other conditions (Supplementary Table 3). For example, inhibitors of exportin 1 (encoded by XPO1), a nuclear export protein crucial for maintaining cellular homeostasis, have been developed and approved for different cancer conditions78. PIK3CA encodes a lipid kinase essential for cell proliferation and survival, and has an approved inhibitor for breast cancer79. We identified five additional genes (EP300, MAP4K4, CAMK2D, NCOR1, and WDR43) that may encode druggable proteins (Fig. 3). Among those, MAP4K4 and CAMK2D were also prioritized by the L2G20 and Yu202421 models (Fig. 1). Further, EP300 is not only the nearest gene with the top local PoPS score, but there is also a non-synonymous variant (rs20551) in the credible set in high LD (R2 = 87%) with the lead variant (rs9611522).
Predicted tractability of the 38 prioritized genes that are not already targets of approved or investigational drugs. Data was extracted from the Open Targets platform using GraphQL API queries (https://platform.opentargets.org/) (see Methods). Various forms of evidence that suggest that a target may be tractable are shown on the x-axis, sorted from highest-quality to lowest. Structure with Ligand: a Protein Data Bank co-crystal structure exists for the target and a small molecule. High-Quality Ligand: the target is bound by a ligand that (1) has a property forecast index ≤7, (2) binds ≤2 distinct protein domains and motifs identified by SMART (Simple Modular Architecture Research Tool), and (3) is derived from ≥2 distinct chemical scaffolds. High-Quality Pocket: the target has a DrugEBIlity score ≥0.7. Med-Quality Pocket: the target has a DrugEBIlity score 0–0.7. Druggable Family: the target was reported to be a member of the druggable genome in Finan et al. 90. Light green cells indicate that a given gene is supported by a given form of evidence, while dark green cells indicate an absence of such evidence. For more information on ongoing targeted drug trials for a selection of genes, see Supplementary Table 3.
Discussion
The field of targeted therapies driven by genetic discoveries for PD is rapidly evolving, with new therapies entering trials and ongoing studies bearing hope for more effective treatments. The goal of our study was to nominate high-confidence genes that bear promising potential as novel drug targets for PD. Notably, the list of prioritized genes is speculative and extensive follow-up investigations are required to determine their exact role in the pathogenesis of PD and thus, their potential as druggable targets for PD. Following established monogenic genes with ongoing trials already, e.g., GBA1, LRRK2, and SNCA, our prioritized genes may have the next-strongest links to PD and may represent our next-best opportunities for disease-modifying drug targets.
We prioritized 46 genes that are likely to be driving their signal in their respective GWAS loci and are predicted to play a potentially important role in PD. In addition to well-established PD genes, including SNCA, LRRK2, GBA1, TMEM175 and VPS13C, we identified six genes (FYN, DYRK1A, NOD2, CTSB, SV2C, and ITPKB) as promising targets for drug repurposing or novel therapeutic development. Our literature review found that each of these genes had a plausible mechanistic link to PD pathogenesis, e.g., due to their involvement in pathways linked to neuroinflammation, autophagy, and neuronal degeneration. Another strategy for some of these genes is to evaluate how already existing drugs for other conditions may be repurposed for PD. However, extensive further preclinical work and validation experiments are required to determine if and how these genes can be targeted and how to translate this into potential drug development. We hope these results stimulate interest in initiating drug development programs focused on these targets, all of which represent promising candidates for future PD therapies.
Furthermore, our approach identified genes that are relatively unexplored in PD. However, our target tractability assessment indicated that some of these genes may be druggable targets, e.g., XPO1, PIK3CA, EP300, MAP4K4, CAMK2D, NCOR1, and WDR43. Moreover, XPO1 and PIK3CA inhibitors, currently approved for the treatment of various cancer types, hold potential for repurposing as therapeutic agents for PD78,79. Lastly, we identified genes such as RIT2, BAG3, and SCARB2, with strong literature evidence supporting their involvement in neurodegenerative pathways linked to PD. Together, we hope this first piece of evidence will stimulate further investigations and advocate for studies exploring the potential effects of overexpression and knockdown of these genes in cell and animal models for PD to better understand their potential mechanistic role. Such extensive preclinical work is crucial to facilitating and advancing drug development programs by pharmaceutical companies, and represents a critical step toward establishing these genes as viable therapeutic targets for PD.
Several previous gene prioritization efforts have aimed to identify the causal genes in PD GWAS loci. The L2G model20 is an XGBoost model trained on data from 445 curated gold-standard GWAS loci from 194 traits20. L2G20 integrates fine-mapping with functional genomics data, gene distance, and in silico functional predictions. The model has been applied to a large number of GWAS from a variety of traits, including the largest European-ancestry PD GWAS13. L2G identified three genes with probability >80% of being a causal PD gene that were not prioritized by our methods: KCNS3, MBNL2, and ASXL321. However, there is only one genome-wide significant variant in the KCNS3 locus (gnomAD non-Finnish European MAF = 9.7%) and all other variant P values are greater than 1 × 10–5, suggesting this may be a spurious association. MBNL2 and ASXL3 were both the nearest gene in their loci, but did not have the top PoPS value.
A similar but PD-specific XGBoost model is the Yu202421 model. They trained their model on the largest European-ancestry PD GWAS13, using only seven well-established PD-associated genes as positive labels: GBA1, LRRK2, SNCA, GCH1, MAPT, TMEM175, and VPS13C. They used predictive features derived from gene expression and splicing in PD-relevant tissues such as brain, dopaminergic neuron subpopulations, and microglia. Yu2024 identified 15 top-ranked genes with probability >80% of being a causal PD gene that were not prioritized by our methods. Of these, 6 were in complex loci containing >10 genes that are intrinsically challenging to resolve: ZBTB4 (93 genes), SETD1A (32 genes), RPS6KL1 (18 genes), KPNA1 (13 genes), KCTD7 (12 genes), and HIP1R (12 genes). Additionally, Yu2024 prioritized KCNS3 (like L2G), MBNL2 (like L2G), CYLD (we prioritized NOD2), INPP5F (we prioritized BAG3), GCH1, GRN, IGSF9B, MAPT, and SCAF11.
The main advantage of the Yu202421 model over the L2G model20 model is its PD-centered design. However, predictions made by the Yu202421 model appear to be poorly calibrated, possibly due to the small number of positive labels in its training set. For example, the largest European-ancestry PD GWAS13 identified a GWAS hit in strong linkage disequilibrium (LD, r2 = 97.9%) with a non-synonymous variant in the FCGR2A gene, strongly suggesting that this is the causal gene in the locus. Although conditional analyses revealed that this was the only independent signal in the locus, the Yu202421 model predicted that 19 genes in this locus had >75% probability of being the causal gene, albeit with FCGR2A still ranking first.
All these previous efforts used a locus-by-locus approach, disregarding data from other GWAS loci and the remainder of the genome. In comparison, PoPS incorporates genome-wide information. The initial PoPS publication19 shows that PoPS outperformed other similarity-based and locus-based gene prioritization methods across numerous complex traits. By combining PoPS with nearest gene and non-synonymous credible set variant information, we prioritized a list of high-confidence genes as promising candidates for follow-up functional studies and potential drug development programs.
Our study had several limitations. We were unable to assess genes on chromosome X because PoPS gene features are restricted to autosomes. We failed to prioritize GCH1, a well-established PD risk gene13,80 implicated in dopamine synthesis in nigrostriatal cells. Although GCH1 had the top PoPS value in its locus, the PIP-weighted center of the credible set was slightly closer to the WDHD1 gene. Proximity-based gene prioritization criteria may be less reliable in loci like GCH1, that have a broad, large LD block GWAS peak covering multiple genes. In addition, there are well-documented examples where the causal gene lies further than 300 kb from the credible set that will be missed by proximity-based criteria81. Further, our method of using a combination of PoPS and the nearest gene restricted us to only nominate one gene per locus, even though some loci may have more than one causal gene.
Finally, using GWAS data only from European and East Asian- ancestry could potentially restrict the generalizability of our findings across diverse genetic populations. The limited representation of these populations in GWAS highlights the need for larger studies in underrepresented groups to ensure equitable insights into PD genetics. Initiatives like the Global Parkinson’s Genetics Program (GP2) have been pivotal in addressing this gap, dedicating considerable efforts to advancing the study of underrepresented populations and fostering global collaborations in PD research.
Gene prioritization efforts play a crucial role in nominating the underlying causal genes within GWAS risk loci, enabling the identification of potential novel drug targets. These efforts are essential for translating genetic discoveries into actionable therapies and have paved the way for personalized medicine approaches. Using PoPS and other methods, we prioritized a high-confidence list of 46 genes predicted to play an important role in PD pathogenesis and representing potential therapeutic targets. Prioritizing known and well-established PD genes, already targeted in clinical drug trials, strengthens the robustness and reliability of our approach. Newly prioritized genes may represent our next-best candidates for disease-modifying therapeutics. We hope our findings stimulate further investigations and preclinical work to facilitate drug development programs and potentially establish these genes as viable therapeutic targets for PD.
Methods and materials
Ethics statement
This study was conducted in accordance with the ethical standards of the institutional and national research committees. Details on Institutional Review Board approvals of the individual studies included in the presented work are provided in the original publications13,16.
GWAS summary statistics
We analyzed two published GWAS datasets. First, an East Asian-ancestry (EAS) meta-analysis of 6,724 cases and 24,851 controls (effective sample size [Neff] = 15,886)16. Second, a European-ancestry (EUR) meta-analysis of 37,688 cases, 18,618 proxy cases (first degree relative with PD), and 1,417,791 controls (Neff = 127,626)13. We performed a fixed-effects meta-analysis of these two datasets using METAL82 to generate a combined EAS + EUR dataset.
Reference panels
We used all available data from the Haplotype Reference Consortium (HRC) release 1.1, which consists of 54,330 phased haplotypes with 36,678,860 variants, to construct three linkage disequilibrium (LD) reference panels: an EAS panel (N = 538), an EUR panel (N = 16,860), and an EAS + EUR panel that included both EAS and EUR individuals in the same proportions as the GWAS summary statistics—11% EAS and 89% EUR (NEUR = 4,322, NEAS = 538). We merged the EUR and EAS panels using PLINK 1.9. We did not directly generate LD matrices; rather, we generated binary PLINK files (.bed, bim, and fam) which were used as inputs for COJO and MAGMA (see below).
Variant quality control
We removed EUR GWAS variants with: (1) a reported allele frequency that differed from the EUR reference panel frequency by >0.1 (42 variants removed) and (2) a reported allele frequency that differed from the EUR reference panel frequency by >12-fold (an additional 462 variants removed). No variants failed these same quality control checks for the EAS GWAS and EAS + EUR meta-analysis. After quality control, the number of variants remaining was 5,347,472 for EAS, 6,584,031 for EUR, and 7,593,632 for EAS + EUR.
Isolating independent association signals
In order to disentangle statistically-independent genetic signals in the EAS + EUR dataset, we first clumped variants using PLINK v1.983 (P < 5 × 10–8, r2 < 0.1, window size = 3 Mbp) and our EAS + EUR reference panel, expanded the boundaries of each clump by 500 kb on either side, and merged overlapping boundaries. Within each resulting region, we ran COJO84 and removed hits with joint P > 5 × 10–8. If multiple independent hits in a region were found, we used COJO to isolate each signal by performing leave-one-hit-out conditional analysis. For each isolated signal, we computed credible sets (CSs) using the finemap.abf function in the coloc R package85,86. Finally, we defined loci as ±300 kb around each credible set.
MAGMA and PoPS
We performed gene-based association tests using MAGMA22 (“SNP-wise mean model”) and all variants with MAF > 1%. We analyzed the EAS- and EUR-based GWAS separately using the corresponding ancestry-specific reference panel and MAFs. We mapped variants to protein-coding genes using Genome Reference Consortium Human Build 37 (GRCh37) gene start and end positions from GENCODE v4487. We removed genes that had fewer than three variants mapped to them. For each gene, we meta-analyzed the resulting ancestry-specific MAGMA z-scores weighted by the square root of sample size82. Using the ancestry-specific MAGMA results as input, we performed PoPS19 using all 57,543 gene-based features as predictors. These features were not available for chrX so we restricted our analysis to autosomal genes. The resulting ancestry-specific PoPS values were then also meta-analyzed weighted by the square root of sample size. We only used the meta-analyzed MAGMA and PoPS values for gene prioritization.
Gene prioritization criteria
Following the original PoPS publication, we prioritized genes that met both of the following criteria: (1) had the top PoPS value in a given locus and (2) were the nearest gene to the corresponding GWAS signal based on the posterior inclusion probability (PIP)-weighted average position of credible set variants (the smallest set of variants expected to contain the causal variant with 95% probability). We also prioritized genes that had PIP>50% for non-synonymous credible set variants affecting the gene. We used non-synonymous variants from the “baseline-LF 2.2.UKB model” (80,693 variants)88. If a locus contained multiple prioritized genes, we only included the gene that was prioritized due to non-synonymous credible set variants.
Comparison with previous PD gene prioritization efforts
We compared our prioritized genes with those highlighted in two previous studies: L2G20 and Yu202421. Both studies trained XGBoost models to predict the probability that a given gene is causal in a given GWAS locus, and have been applied to the EUR PD GWAS data. We downloaded results for the L2G study20 from the Open Targets Genetics website (https://genetics.opentargets.org/Study/GCST009325/). We extracted results for the Yu202421 study from Supplementary Table 2. Although there only appears to be one GWAS hit near the FCGR2A gene in the EUR dataset13, Yu202421 causal probabilities in this locus sum to over 2500%. We therefore subsetted to genes that Yu2024 designated as being in the top three per locus.
Drug repurposing and tractability
We determined whether our prioritized genes were targeted by approved or investigational drugs using GraphQL API queries of the Open Targets platform89, which in turn queries the EMBL-EBI ChEMBL database. For genes that were not targeted by approved or investigational drugs, we performed additional Open Targets API queries to extract evidence of drug tractability—the probability of identifying a small molecule drug that is able to bind and modulate a given target. We considered genes to encode proteins that may be druggable if they have been co-crystallized with a small molecule.
Literature review
We performed a PubMed search (https://pubmed.ncbi.nlm.nih.gov/) for each prioritized gene using the standardized search term: “GENE_NAME[Title/Abstract] AND parkinson[Title/Abstract]”. We excluded known monogenic or high risk genes: LRRK2 (OMIM: 607060), GBA1 (OMIM: 168600), SNCA (OMIM: 168601), and VPS13C (OMIM: 616840). We also excluded TMEM175, which has been experimentally confirmed as the causal gene in its locus. For each search that returned at least eight publications, we performed a more detailed literature review. We excluded genes with fewer than eight publications from further analyses. We screened the abstracts for evidence of a potential role of each gene in the pathogenesis of PD and information regarding the potential mechanism of action, thereby nominating it as a potential drug target. If required, an additional full-text review was performed.
Data availability
Data and code used in preparation of the manuscript are linked in the manuscript. ChEMBL Database: https://www.ebi.ac.uk/chembl/. HRC reference release 1.1: https://ega-archive.org/datasets/EGAD00001002729. Gencode release 44: https://www.gencodegenes.org/human/release_44.html. OpenTargets platform: https://platform-docs.opentargets.org/. Details for accessing the EUR13 and EAS16 GWAS datasets can be found their original publications.
Code availability
Custom code used in the presented study is stored at https://github.com/kheilbron/cojo_pipe and https://github.com/kheilbron/brett. COJO: https://yanglab.westlake.edu.cn/software/gcta/#COJO. MAGMA: https://cncr.nl/research/magma/. PLINK 1.9: https://www.cog-genomics.org/plink/. PoPS: https://github.com/FinucaneLab/pops.
References
GBD 2016 Parkinson’s Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 17, 939–953 (2018).
Periñán, M. T. et al. Effect modification between genes and environment and Parkinson’s disease risk. Ann. Neurol. 92, 715–724 (2022).
Neumann, W.-J., Horn, A. & Kühn, A. A. Insights and opportunities for deep brain stimulation as a brain circuit intervention. Trends Neurosci. 46, 472–487 (2023).
Davidson, B., Milosevic, L., Kondrataviciute, L., Kalia, L. V. & Kalia, S. K. Neuroscience fundamentals relevant to neuromodulation: Neurobiology of deep brain stimulation in Parkinson’s disease. Neurotherapeutics 21, e00348 (2024).
McFarthing, K. et al. Parkinson’s disease drug therapies in the clinical trial pipeline: 2023 update. J. Parkinsons. Dis. 13, 427–439 (2023).
Minikel, E. V., Painter, J. L., Dong, C. C. & Nelson, M. R. Refining the impact of genetic evidence on clinical success. Nature https://doi.org/10.1038/s41586-024-07316-0 (2024).
King, E. A., Davis, J. W. & Degner, J. F. Are drug targets with genetic support twice as likely to be approved? Revised estimates of the impact of genetic support for drug mechanisms on the probability of drug approval. PLoS Genet 15, e1008489 (2019).
Ochoa, D. et al. Human genetics evidence supports two-thirds of the 2021 FDA-approved drugs. Nat. Rev. Drug Discov. 21, 551 (2022).
Rusina, P. V. et al. Genetic support for FDA-approved drugs over the past decade. Nat. Rev. Drug Discov. 22, 864 (2023).
Nelson, M. R. et al. The support of human genetic evidence for approved drug indications. Nat. Genet. 47, 856–860 (2015).
Cully, M. Genetic information adds supporting weight. Nat. Rev. Drug Discov. 14, 525–525 (2015).
Khani, M. et al. Towards a Global View of Parkinson’s Disease Genetics. Ann. Neurol. 95, 831–842 (2024).
Nalls, M. A. et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 18, 1091–1102 (2019).
Loesch, D. P. et al. Characterizing the genetic architecture of Parkinson’s disease in Latinos. Ann. Neurol. 90, 353–365 (2021).
Foo, J. N. et al. Genome-wide association study of Parkinson’s disease in East Asians. Hum. Mol. Genet. 26, 226–232 (2017).
Foo, J. N. et al. Identification of Risk Loci for Parkinson Disease in Asians and Comparison of Risk Between Asians and Europeans: A Genome-Wide Association Study. JAMA Neurol. 77, 746–754 (2020).
Rizig, M. et al. Identification of genetic risk loci and causal insights associated with Parkinson’s disease in African and African admixed populations: a genome-wide association study. Lancet Neurol. 22, 1015–1025 (2023).
Kim, J. J. et al. Multi-ancestry genome-wide association meta-analysis of Parkinson’s disease. Nat. Genet. 56, 27–36 (2024).
Weeks, E. M. et al. Leveraging polygenic enrichments of gene features to predict genes underlying complex traits and diseases. Nat. Genet. 55, 1267–1276 (2023).
Mountjoy, E. et al. An open approach to systematically prioritize causal variants and genes at all published human GWAS trait-associated loci. Nat. Genet. 53, 1527–1533 (2021).
Yu, E. et al. Machine learning nominates the inositol pathway and novel genes in Parkinson’s disease. Brain 147, 887–899 (2024).
de Leeuw, C. A., Mooij, J. M., Heskes, T. & Posthuma, D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput. Biol. 11, e1004219 (2015).
Giladi, N. et al. Safety and efficacy of venglustat in GBA1-associated Parkinson’s disease: an international, multicentre, double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 22, 661–671 (2023).
den Heijer, J. M. et al. A phase 1B trial in GBA1-associated parkinson’s disease of BIA-28-6156, a glucocerebrosidase activator. Mov. Disord. 38, 1197–1208 (2023).
Pagano, G. et al. A phase II study to evaluate the safety and efficacy of prasinezumab in early Parkinson’s disease (PASADENA): Rationale, design, and baseline data. Front. Neurol. 12, 705407 (2021).
Brys, M. et al. Randomized phase I clinical trial of anti-α-synuclein antibody BIIB054. Mov. Disord. 34, 1154–1163 (2019).
Lang, A. E. et al. Trial of cinpanemab in early Parkinson’s disease. N. Engl. J. Med. 387, 408–420 (2022).
Price, D. L. et al. The small molecule alpha-synuclein misfolding inhibitor, NPT200-11, produces multiple benefits in an animal model of Parkinson’s disease. Sci. Rep. 8, 16165 (2018).
Pagan, F. L. et al. Nilotinib effects on safety, tolerability, and potential biomarkers in Parkinson disease: A phase 2 randomized clinical trial. JAMA Neurol. 77, 309–317 (2020).
Jinn, S. et al. Functionalization of the TMEM175 p.M393T variant as a risk factor for Parkinson disease. Hum. Mol. Genet. 28, 3244–3254 (2019).
Uenaka, T. et al. In silico drug screening by using genome-wide association study data repurposed dabrafenib, an anti-melanoma drug, for Parkinson’s disease. Hum. Mol. Genet. 27, 3974–3985 (2018).
Obergasteiger, J. et al. The small GTPase Rit2 modulates LRRK2 kinase activity, is required for lysosomal function and protects against alpha-synuclein neuropathology. NPJ Parkinsons Dis. 9, 44 (2023).
Kearney, P. J. et al. Silencing Parkinson’s risk allele Rit2 sex-specifically compromises motor function and dopamine neuron viability. NPJ Parkinsons Dis. 10, 41 (2024).
Olsen, A. L. & Feany, M. B. Parkinson’s disease risk genes act in glia to control neuronal α-synuclein toxicity. Neurobiol. Dis. 159, 105482 (2021).
Wang, Q. et al. Molecular profiling of human substantia nigra identifies diverse neuron types associated with vulnerability in Parkinson’s disease. Sci. Adv. 10, eadi8287 (2024).
Demuro, S., Di Martino, R. M. C., Ortega, J. A. & Cavalli, A. GSK-3β, FYN, and DYRK1A: Master regulators in neurodegenerative pathways. Int. J. Mol. Sci. 22, 9098 (2021).
Arbones, M. L., Thomazeau, A., Nakano-Kobayashi, A., Hagiwara, M. & Delabar, J. M. DYRK1A and cognition: A lifelong relationship. Pharmacol. Ther. 194, 199–221 (2019).
Kim, E. J. et al. Dyrk1A phosphorylates alpha-synuclein and enhances intracellular inclusion formation. J. Biol. Chem. 281, 33250–33257 (2006).
Im, E. & Chung, K. C. Dyrk1A phosphorylates parkin at Ser-131 and negatively regulates its ubiquitin E3 ligase activity. J. Neurochem. 134, 756–768 (2015).
Cao, Y.-L. et al. A role of BAG3 in regulating SNCA/α-synuclein clearance via selective macroautophagy. Neurobiol. Aging 60, 104–115 (2017).
Seidel, K. et al. The HSPB8-BAG3 chaperone complex is upregulated in astrocytes in the human brain affected by protein aggregation diseases. Neuropathol. Appl. Neurobiol. 38, 39–53 (2012).
Sheehan, P. W. et al. An astrocyte BMAL1-BAG3 axis protects against alpha-synuclein and tau pathology. Neuron 111, 2383–2398.e7 (2023).
Lu, S.-Z. et al. Suppression of astrocytic autophagy by αB-crystallin contributes to α-synuclein inclusion formation. Transl. Neurodegener. 8, 3 (2019).
Ying, Z.-M. et al. BAG3 promotes autophagy and suppresses NLRP3 inflammasome activation in Parkinson’s disease. Ann. Transl. Med 10, 1218 (2022).
Keo, A. et al. Transcriptomic signatures of brain regional vulnerability to Parkinson’s disease. Commun. Biol. 3, 101 (2020).
Thomas, R., Moloney, E. B., Macbain, Z. K., Hallett, P. J. & Isacson, O. Fibroblasts from idiopathic Parkinson’s disease exhibit deficiency of lysosomal glucocerebrosidase activity associated with reduced levels of the trafficking receptor LIMP2. Mol. Brain 14, 16 (2021).
Pérez-Roca, L. et al. Glucocerebrosidase regulators SCARB2 and TFEB are up-regulated in Lewy body disease brain. Neurosci. Lett. 706, 164–168 (2019).
Zunke, F. et al. Characterization of the complex formed by β-glucocerebrosidase and the lysosomal integral membrane protein type-2. Proc. Natl. Acad. Sci. USA 113, 3791–3796 (2016).
Ambrosi, G. et al. Ambroxol-induced rescue of defective glucocerebrosidase is associated with increased LIMP-2 and saposin C levels in GBA1 mutant Parkinson’s disease cells. Neurobiol. Dis. 82, 235–242 (2015).
Kohal, R. et al. Fyn, Blk, and Lyn kinase inhibitors: A mini-review on medicinal attributes, research progress, and future insights. Bioorg. Med. Chem. Lett. 102, 129674 (2024).
Peng, S. & Fu, Y. FYN: emerging biological roles and potential therapeutic targets in cancer. J. Transl. Med. 21, 84 (2023).
Ramos, R. & Vale, N. Dual drug repurposing: The example of saracatinib. Int. J. Mol. Sci. 25, 4565 (2024).
Nygaard, H. B. et al. A phase Ib multiple ascending dose study of the safety, tolerability, and central nervous system availability of AZD0530 (saracatinib) in Alzheimer’s disease. Alzheimers Res. Ther. 7, 35 (2015).
van Dyck, C. H. et al. Effect of AZD0530 on cerebral metabolic decline in Alzheimer disease: A randomized clinical trial. JAMA Neurol. 76, 1219–1229 (2019).
Panicker, N. et al. Fyn kinase regulates microglial neuroinflammatory responses in cell culture and animal models of Parkinson’s disease. J. Neurosci. 35, 10058–10077 (2015).
Panicker, N. et al. Fyn kinase regulates misfolded α-synuclein uptake and NLRP3 inflammasome activation in microglia. J. Exp. Med. 216, 1411–1430 (2019).
Low, C. Y. B. et al. Isoform-specific upregulation of FynT kinase expression is associated with tauopathy and glial activation in Alzheimer’s disease and Lewy body dementias. Brain Pathol. 31, 253–266 (2021).
Sanz-Blasco, S. et al. The kinase Fyn as a novel intermediate in L-DOPA-induced dyskinesia in Parkinson’s disease. Mol. Neurobiol. 55, 5125–5136 (2018).
Saminathan, H. et al. Fyn kinase mediates pro-inflammatory response in a mouse model of endotoxemia: Relevance to translational research. Eur. J. Pharmacol. 881, 173259 (2020).
Saminathan, H. et al. Fyn kinase-mediated PKCδ Y311 phosphorylation induces dopaminergic degeneration in cell culture and animal models: Implications for the identification of a new pharmacological target for Parkinson’s disease. Front. Pharmacol. 12, 631375 (2021).
Pathak, A. et al. DYRK1A kinase inhibition with emphasis on neurodegeneration: A comprehensive evolution story-cum-perspective. Eur. J. Med. Chem. 158, 559–592 (2018).
Nguyen, T. L., Fruit, C., Hérault, Y., Meijer, L. & Besson, T. Dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) inhibitors: a survey of recent patent literature. Expert Opin. Ther. Pat. 27, 1183–1199 (2017).
Feki, A. & Hibaoui, Y. DYRK1A protein, a promising therapeutic target to improve cognitive deficits in down syndrome. Brain Sci. 8, 187 (2018).
Jarhad, D. B., Mashelkar, K. K., Kim, H.-R., Noh, M. & Jeong, L. S. Dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) inhibitors as potential therapeutics. J. Med. Chem. 61, 9791–9810 (2018).
Yong, Y. et al. Dyrk1a phosphorylation of α-synuclein mediating apoptosis of dopaminergic neurons in Parkinson’s disease. Parkinsons Dis. 2023, 8848642 (2023).
Jones-Tabah, J. et al. The Parkinson’s disease risk gene cathepsin B promotes fibrillar alpha-synuclein clearance, lysosomal function and glucocerebrosidase activity in dopaminergic neurons. Res. Sq. https://doi.org/10.21203/rs.3.rs-3979098/v1 (2024).
Kim, M. J., Jeong, H. & Krainc, D. Lysosomal ceramides regulate cathepsin B-mediated processing of saposin C and glucocerebrosidase activity. Hum. Mol. Genet. 31, 2424–2437 (2022).
Drobny, A. et al. Reciprocal effects of alpha-synuclein aggregation and lysosomal homeostasis in synucleinopathy models. Transl. Neurodegener. 12, 31 (2023).
Mullard, A. The FDA approves a first farnesyltransferase inhibitor. Nat. Rev. Drug Discov. 20, 8 (2021).
Frampton, J. E. Mifamurtide: a review of its use in the treatment of osteosarcoma. Paediatr. Drugs 12, 141–153 (2010).
Cheng, L. et al. NOD2 promotes dopaminergic degeneration regulated by NADPH oxidase 2 in 6-hydroxydopamine model of Parkinson’s disease. J. Neuroinflamm. 15, 243 (2018).
Dardou, D. et al. Distribution of SV2C mRNA and protein expression in the mouse brain with a particular emphasis on the basal ganglia system. Brain Res. 1367, 130–145 (2011).
Bucher, M. L. et al. Synaptic vesicle glycoprotein 2C enhances vesicular storage of dopamine and counters dopaminergic toxicity. Eur. J. Neurosci. 59, 2483–2501 (2024).
Dunn, A. R. et al. Synaptic vesicle glycoprotein 2C (SV2C) modulates dopamine release and is disrupted in Parkinson disease. Proc. Natl. Acad. Sci. USA 114, E2253–E2262 (2017).
Kovacs, G., Reimer, L. & Jensen, P. H. Endoplasmic reticulum-based calcium dysfunctions in synucleinopathies. Front. Neurol. 12, 742625 (2021).
Di Leva, F. et al. Increased levels of the Parkinson's disease-associated gene ITPKB correlate with higher expression levels of à-synuclein, independent of mutation status. Int. J. Mol. Sci. 24, 1984 (2023).
Apicco, D. J. et al. The Parkinson's disease-associated gene ITPKB protects against α-synuclein aggregation by regulating ER-to-mitochondria calcium release. Proc. Natl. Acad. Sci. USA. 118, e2006476118 (2021).
Azmi, A. S., Uddin, M. H. & Mohammad, R. M. The nuclear export protein XPO1 - from biology to targeted therapy. Nat. Rev. Clin. Oncol. 18, 152–169 (2021).
André, F. et al. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 380, 1929–1940 (2019).
Chang, D. et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat. Genet. 49, 1511–1516 (2017).
Claussnitzer, M. et al. FTO obesity variant circuitry and adipocyte browning in humans. N. Engl. J. Med. 373, 895–907 (2015).
Willer, C. J., Li, Y. & Abecasis, G. R. METAL: Fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010).
Chang, C. C. et al. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 4, 7 (2015).
Yang, J. et al. Conditional and joint multiple-SNP analysis of GWAS summary statistics identifies additional variants influencing complex traits. Nat. Genet. 44, 369–375 (2012).
Giambartolomei, C. et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet 10, e1004383 (2014).
Wallace, C. Eliciting priors and relaxing the single causal variant assumption in colocalisation analyses. PLoS Genet 16, e1008720 (2020).
Frankish, A. et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 47, D766–D773 (2019).
Weissbrod, O. et al. Functionally informed fine-mapping and polygenic localization of complex trait heritability. Nat. Genet. 52, 1355–1363 (2020).
Koscielny, G. et al. Open Targets: a platform for therapeutic target identification and validation. Nucleic Acids Res. 45, D985–D994 (2017).
Finan, C. et al. The druggable genome and support for target identification and validation in drug development. Sci. Transl. Med. 9, eaag1166 (2017).
Acknowledgements
This project was supported by the Global Parkinson’s Genetics Program (GP2). GP2 is funded by the Aligning Science Across Parkinson’s (ASAP) initiative and implemented by The Michael J. Fox Foundation for Parkinson’s Research (https://gp2.org). For a complete list of GP2 members see https://gp2.org. We would like to thank the research participants and employees of 23andMe, Inc. for making this work possible. We thank SURF (www.surf.nl) for the support in using the Snellius National Supercomputer. This work utilized the computational resources of the NIH HPC Biowulf cluster (https://hpc.nih.gov). This article has been posted on the medRxiv preprint server. C.C.C. was supported by a Vanier Canada Graduate Scholarship from the Canadian Institutes of Health Research. J.K. and S.R. were supported by the German Center for Mental Health (DZPG). AB, JK, and SR were supported by the European Union’s Horizon program (101057454, “PsychSTRATA”). A.B. and S.R. were supported by The German Research Foundation (402170461, grant “TRR265”). DP and MS were supported by The Netherlands Organization for Scientific Research (NWO Gravitation: BRAINSCAPES: A Roadmap from Neurogenetics to Neurobiology—Grant No. 024.004.012). D.P. was supported by The European Research Council (Advanced Grant No ERC-2018-AdG GWAS2FUNC 834057). N.B. and D.P. were supported by the European Union’s Horizon program (964874, “REALMENT”). K.H. was supported by a Humboldt Research Fellowship from the Alexander von Humboldt Foundation. G.P., S.A., D.P., S.R., and the research reported in this publication were supported by the National Institute Of Mental Health of the National Institutes of Health under Award Number R01MH124873. The content is the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was supported in part by the Intramural Research Program of the National Institute on Aging (NIA), and the Center for Alzheimer’s and Related Dementias (CARD), within the Intramural Research Program of the NIA and the National Institute of Neurological Disorders and Stroke.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
S.R., C.B., and K.H. were responsible for study conceptualization, study execution, and leading the analyses. L.M.L. and C.C.C. were involved in the data analyses and interpretation. They were also responsible for the literature review. J.K., A.B., S.A., G.P., M.S., N.B., and D.P. supported the literature review and data interpretation. L.M.L. and C.C.C. wrote the initial draft of the manuscript. All co-authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
K.H. is a former employee of 23andMe, Inc. and a current employee of Bayer AG. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lange, L.M., Cerquera-Cleves, C., Schipper, M. et al. Prioritizing Parkinson’s disease risk genes in genome-wide association loci. npj Parkinsons Dis. 11, 77 (2025). https://doi.org/10.1038/s41531-025-00933-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-025-00933-0