Abstract
Investigating the association between metabolic syndrome (MetS) and all its components with prediagnostic Parkinson’s disease (PD) may clarify PD pathogenesis and identify candidates for prevention strategies and neuroprotective trials. We conducted a retrospective study of 890 PD patients diagnosed during 2000-2020 and 8,100 matched controls, analyzing electronic health records up to 15 years before PD diagnosis. Prevalence was assessed annually to track changes in association strength over time. PD patients consistently showed lower MetS prevalence (OR = 0.57–0.8). Compared to controls, PD patients had lower triglycerides (OR = 0.44–0.75), BMI (OR = 0.54–0.65), and hyperglycemia (OR = 0.81–0.86) rates, and females showed higher high-density lipoprotein (HDL) (OR = 1.48–1.82), lower low-density lipoprotein/HDL ratio (OR = 0.46–0.64), and reduced hypertension (OR = 0.79–0.85). These findings suggest an inverse association between MetS and PD years before diagnosis. We propose a lipid–blood pressure index incorporating triglycerides, HDL, and hypertension as a potential clinical tool for PD risk stratification.
Similar content being viewed by others
Introduction
Identifying risk factors for Parkinson’s disease (PD) in the prediagnostic period is important for understanding PD pathogenesis, developing preventive drugs, and introducing behavior or lifestyle changes to slow or prevent disease progression.
Several studies have addressed the association between the metabolic syndrome (MetS) and future incident PD1,2,3,4,5. While most identified MetS as a risk factor for the development of PD1,2,3,4, research on its individual components yielded different trends. High levels of triglycerides (TG) and cholesterol and of hypertension were associated with a reduced risk of PD1,4,5,6,7,8,9,10,11,12,13,14,15, whereas the direction of the association of high Body mass index (BMI) and type 2 diabetes with PD was inconsistent across studies3,6,11,15,16,17,18,19,20,21,22. Furthermore, although the neurodegenerative process of PD may begin years before the appearance of motor symptoms23, little is known about the association of MetS and its components with PD before the date of diagnosis (DoD). The aim of the present study was to analyze this association during the 15-year prediagnostic phase of PD.
Results
A total of 1352 patients were diagnosed with PD in 2000–2020 of whom 890, 552 male (62%) and 338 female, met the inclusion criteria. The patients were matched 1:10 with 8100 control subjects, 61% male. There was no between-group difference in the distribution of place of birth (χ2 = 27.48, df = 5, P = 4.6e-05). The available data for each subject spanned from 2 to 15 years, decreasing with time from the DoD. The patients and control subjects had comparable profiles for blood tests and clinic visits, except in the year preceding the DoD, when patients showed an increase in test and visit frequency. Table 1 shows the demographic, clinical, and laboratory characteristics of the patients and controls in the last year before the DoD.
Analysis of the metabolic syndrome and its components
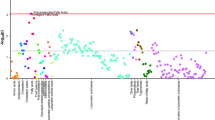
We used the conditional logistic regression model to estimate odds ratio for continuous variables in case-control study, i.e., the conditional odds ratio (COR). As shown in Fig. 1, the prevalence of MetS was lower in patients with PD than controls in each year for both males (COR = 0.732, 95% CI:0.683–0.784, P < 0.001) and females (COR = 0.743, 95% CI:0.676–0.817, P < 0.001), with significant annual differences for the entire 15-year period before the DoD (Supplementary Table S1). Similar findings were reflected in the annual OR values (Fig. 2), which ranged from 0.57 to 0.8 in individuals with MetS and were significant throughout the prediagnostic period.
The graph is a collection of points, each representing the prevalence in a specific year until the DoD (red dashed vertical line). The results are calculated on the basis of data collected in the previous year; for example, “−3” relates to the prevalence calculated during the fourth year (from year 3 to year 4) before the DoD. For controls, the DoD is defined by the DoD of their matched patient (see the Methods section). a Whole cohort. b Stratification by sex. Numbers in the figure indicate the annual count (averaged over 10 datasets) of PD patients and control subjects meeting the criteria for MetS before the DoD.
ORs of blood tests and BMI values are calculated between the fourth and first quartiles, with patients and subjects stratified by age and sex. The lipid-BP index is described in the section on analysis of MetS components. The left column shows the OR values in the first year in which they were significant at 95% confidence, with correspondence to the chart on the right. From this year until the DoD, the OR remained significant (with one exception) or close to significance (P = 0.05; marked with an asterisk). The right column shows the OR values in the last year before the DoD.
Triglycerides
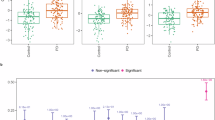
Figure 3a demonstrates that the study group had lower annual average TG levels than controls, with a COR of 0.997 for males (95%, CI:0.996–0.997, P < 0.001) and 0.997 for females (95%, CI:0.996–0.997, P < 0.001). The disparity between the average values became more pronounced towards the DoD and was statistically significant almost throughout the prediagnostic period (Supplementary Fig. S1). Although the whole population showed a decrease in TG levels after age 60 (Supplementary Fig. S2), the slope of the decrease was more substantial in male patients almost throughout the entire period (Fig. 3a). On age stratification, the main differences between patients and controls were observed in the older population first diagnosed at age 67 years or more (Supplementary Fig. S3a). Outcomes were similar when individuals taking medication for dyslipidemia were excluded (Supplementary Fig. S4a). Annual ORs for future incident PD were significant for each of the 6 years prior to diagnosis, ranging from 0.44 to 0.75 (Fig. 2). ORs were significant in males 9 years prior to the DoD, and in females, 4 years prior to the DoD.
The results are calculated based on data collected in the previous year until the DoD (red dashed vertical line); for example, “-3” relates to the mean blood test value calculated during the fourth year (from year 3 to year 4) before the DoD. For controls, the DoD is defined by the DoD of their matched patient with PD (see the Methods section). Numbers of subjects are averaged over 10 datasets of patients and controls with available values. a Mean TG values. b Mean HDL values. c Mean LDL/HDL values. d Mean BMI values. e Prevalence of pharmacologic treatment for hypertension. f Prevalence of pharmacologic treatment for type 2 diabetes.
Cholesterol
The association of HDL with future incident PD differed by sex (Fig. 3b), with a COR of 1.004 for males (95% CI:1.001–1.008, P < 0.05) and 1.015 for females (95% CI:1.011–1.018, P < 0.05). Analysis by year showed that the difference started in the ninth year before the DoD and reached significance from the fifth year (Supplementary Fig. S1). The ORs of future PD supported these findings. Annual ORs were significant from the fifth year onwards in females (1.48-1.82) and in the last 2 years in males (Fig. 2). Upon age stratification, the differences tended to be more evident in the older population first diagnosed at age 67 years or more (Supplementary Fig. S3b)
The PD group had a lower mean LDL/HDL ratio than controls for up to 15 years before the DoD (Fig. 3c), with a COR of 0.852 for males (95% CI :0.814–0.892, P < 0.001) and 0.753 for females (95% CI:0.706–0.803, P < 0.001). The statistical difference started 12 years before the DoD and reached stable significance from the fifth year (Supplementary Fig. S1). This disparity was primarily driven by the female population, in which a significant difference was observed in nearly all 9 years before the DoD. Similar findings were noted on analysis of the OR (Fig. 2). The differences between the age groups tend to be more constant in the younger population (Supplementary Fig. S3c).
The distinction in HDL levels and the LDL/HDL ratio persisted when subjects under pharmacological treatment for dyslipidemia were excluded. There was no between-group difference in the prevalence of statin intake.
BMI
Figure 3d illustrates a decline in average BMI levels in patients compared to controls for 7 years prior to the DoD, with a COR of 0.987 for males (95% CI:0.977– 0.997, P < 0.05) and 0.959 for females (95% CI:0.948–0.969, P < 0.001). The annual difference reached statistical significance in the last 3 years (Supplementary Fig. S1). In females, the decline in BMI commenced 11 years before the DoD and reached significance in the last 4 years. These findings were supported by the annual ORs (Fig. 2). Accordingly, the ratio of overweight/obese patients (ICD-10 code E66) was lower in the PD than in the control group from 9 years before the DoD. Upon age stratification, lower BMI levels in PD were evident among older individuals, while little or no difference, and in some cases, a reversed trend, was observed in the younger group (Supplementary Fig. S3d).
Hypertension
Owing to the unavailability of direct blood pressure data, individuals with hypertension were identified through prescriptions for hypertension medications. Figure 3e illustrates the difference in the prevalence of medication intake in females between the PD and control groups over the years, with a COR of 0.965 for males (95% CI: 0.997–1.025, P > 0.05) and 0.678 for females (95% CI:0.0.625-0.737, P < 0.001) and annual significant differences in the last 8 years (Supplementary Fig. S1). Similar results were achieved for the annual ORs (Fig. 2). Sensitivity analysis of the number of prescriptions (between 1 and 4) yielded similar findings.
The results were supported by analysis of the microalbumin/creatinine ratio, which served as an indicator of hypertension control. Consistently lower mean values were observed in the patients with PD across the entire observational period (Supplementary Fig. S4d).
Hyperglycemia
Hyperglycemia was inversely associated with the future incidence of PD in both sexes, with a COR of 0.895 for males (CI: 0.834–0.96, P < 0.01), and 0.797 for females (95% CI:0.0.721–0.882, P < 0.001). The annual difference reached significance at 3 years prior to the DoD, with ORs ranging from 0.81 to 0.86 (Fig. 2). There was also an inverse association of the components determining a diagnosis of hyperglycemia, i.e., medication intake and blood test values. The rate of purchase of diabetes medications was lower in patients with PD (Fig. 3f). Mean HbA1c values were lower in patients than controls for almost the entire 15-year duration (Supplementary Figs. S4b, S4c), more evident in females (COR = 0.753, 95% CI: 0.679–0.834, P < 0.001) than males (COR = 0.906, 95% CI: 0.857–0.958, P < 0.001). The trend in HbA1c levels was more prominent starting from the eighth year before the DoD. There was a noticeable between-group difference among males for 8 years before the DoD, and among females, for 13 years before the DoD, largely driven by the females under pharmacological treatment (not shown). The decrease in HbA1c values with age was likely related to the increase in consumption of diabetes medications (Fig. 3f). Indeed, HbA1c values remained relatively steady (not shown) during the prediagnostic period in both patients and controls who did not take diabetes medications, but decreased with age in patients and controls took diabetes medications.
Suggested new lipid-blood pressure index
Analysis of the prevalence of each component of the MetS over the years revealed that those with the greatest impact were dyslipidemia, based on TG and HDL values, and hypertension (Supplementary Fig. S5). The impact of obesity changed over time, with the prevalence increasing from ~10% to almost ~50%. The impact of hyperglycemia was modest.
Therefore, we propose a novel PD predictive tool, termed the lipid-blood pressure (lipid-BP) index, based on the markers that had the highest prevalence among the patients with MetS and demonstrated a more prominent association with PD. The lipid-BP index identifies individuals with TG levels in the fourth quartile, HDL levels in the first quartile, and high hypertension. The difference in annual ORs of future incident PD between these individuals and those with low TG levels, high HDL levels (fourth quartile), and no hypertension was significant already from the fifth year prior to the DoD onward, ranging from 0.41 to 0.58 (Fig. 2). The lipid-BP index demonstrates the lowest OR values of all markers shown in Fig. 2 for the overall population and by sex for enough years before the DoD, which makes this index a practical, easy-to-apply method.
Discussion
This comprehensive retrospective study of 890 patients with PD and 8100 matched controls showed that incident MetS and its components during the prediagnostic phase of PD are associated with a lower risk of future incident PD, with a strong relationship to sex.
Previous studies investigating the association between MetS and future diagnosis of PD have yielded conflicting findings. A prospective trial found that newly diagnosed or worsening MetS was a risk factor for PD, whereas an improvement in MetS parameters or no diagnosis of MetS lowered the risk of PD development1. That study looked at the dynamics of MetS and had a shorter timespan than ours. The results were supported by two nationwide studies2,4. The meta-analysis of Souza et al.3 led to similar conclusions. Conversely, another prospective study suggested that patients with MetS had a lower incidence of PD, with up to a 50% reduction in relative risk5. Accordingly, our research showed a significantly lower PD incidence among subjects with MetS over 15 years before the DoD, with annual ORs ranging from 0.57 to 0.8.
Evaluating MetS based on the NCEP ATP III criteria24 was imperative, as the rate of diagnosis of MetS (ICD-10 E88) among individuals in our database was substantially lower than the rate indicated by their medical records, suggesting that MetS was present before the physician’s diagnosis. To gain a comprehensive picture, we examined all components (criteria) of MetS. Previous studies also highlighted associations between lipidome profile and PD25,26,27,28. We sought to determine whether these associations also manifest during the prediagnostic phase.
In terms of TG alone, the results in the literature were clearer. Both prospective and retrospective studies of various lipid profile and cardiovascular factors in this context reported a direct association of high levels of TG with a lower risk of PD5,8,10,11,15,28. The present study, which investigated the 15-year period before PD diagnosis, supported these findings. Moreover, the results remained valid when individuals receiving pharmacological treatment were excluded.
Similarly, most prospective and case-control studies point to an association of lower HDL levels, hypercholesterolemia, higher total cholesterol levels, higher LDL/HDL ratio, and higher LDL levels with a lower future risk of PD PD6,7,8,9,15. There are some exceptions, however, including a recent nationwide, population-based, cohort study from South Korea wherein patients with a lower HDL level had a higher incidence of PD29. In the present study, female patients in the PD group had significantly higher HDL levels than female control subjects and a lower LDL/HDL ratio.
Regarding hypertension, results of various prospective and case-control studies are conflicting, with some reporting a significant inverse association with PD6,8,11. and others reporting a positive or no association12,30. In the present study, the prevalence of hypertension in the female patients with PD was lower than in the controls throughout the 15-year study period. Furthermore, analysis of the microalbumin/creatinine ratio, which served as an indirect measure of hypertension control, yielded similar findings. However, because hypertension was defined by the purchase of hypertension medication, we could not ascertain whether the observed inverse association was influenced by medication consumption, as suggested by previous research13. Additionally, further investigation is necessary to establish causality.
Findings for BMI, obesity, and type 2 diabetes in the literature are inconsistent as well. These parameters were found to be inversely6,8,17,19,21,22 or directly14,31,32 associated with PD, or not associated at all18. For example, one large-scale investigation of over two million patients with type 2 diabetes and over six million reference subjects found significantly elevated rates of PD following the development of type 2 diabetes (hazard ratio 1.32%)31. By contrast, earlier, mainly case-control studies, found that PD rates were lower following type 2 diabetes6,8,21. In the present study, BMI and type 2 diabetes were inversely associated with the future occurrence of PD. On analysis of the association between intake of medication for type 2 diabetes and HbA1c levels, results were similar.
Overall, the factors with the greatest difference from controls and the greatest impact for extended periods before the DoD were TG level, HDL level, and hypertension. On this basis, we propose a risk assessment tool, termed the lipid-BP index. The ORs for future development of PD were ~0.5 for individuals with TG level in Q4, HDL level in Q1, and hypertension compared to individuals with TG level in Q1, HDL level in Q4, and no hypertension.
Sex differences have long been recognized in PD. The prevalence of the disease is higher in males33. Female patients are particularly vulnerable to the development of non-motor fluctuations and dyskinesias34,35 and have higher rates of depression, fatigue, pain, and urologic dysfunction36,37. Male patients have greater cognitive and behavioral impairments and progress faster to dementia38. The cause of these differences is unknown but may involve sex-related differences in the nigrostriatal circuitry arising from genetic, developmental, and hormonal factors39. In the present study, stratification by sex revealed differences in average values of the metabolic biomarkers, prevalence of pharmacological treatment, and associations with PD. The association of PD with TG was more evident in males, and with HDL, HDL/LDL ratio, hypertension, and type 2 diabetes, in females, both in magnitude and the time before the DoD, particularly for HDL. These findings are in line with previous research11.
To explain the mechanisms underlying the lower prevalence of MetS and its components already in the prediagnostic phase of PD, some researchers have pointed to the reduced sympathetic activity in PD. This leads to a decrease in the release of cortisol and catecholamines from the hypothalamic-pituitary-adrenal axis8, affecting carbohydrate, TG, and cholesterol metabolism. The sympathetic nervous system also controls blood pressure. Hence, individuals with increased sympathetic activity, which causes hypertension, have a lower incidence of PD. Catecholamines also stimulate noradrenaline release, which stimulates dopaminergic receptors and hypothetically has a neuroprotective effect on dopaminergic neurons40,41. The lower incidence of MetS might explain the reduced weight, which is common in PD and might be related to reduced energy intake reflected in the lower glucose and TG levels. In addition, lipid metabolism is suspected to play an important role in PD pathogenesis, reflected, for example, in the complex interplay between α-synuclein, found in Lewy bodies in PD, fatty acids, and TG42,43. Some studies suggested that these pathways may be amenable to potential intervention with neuroprotective substances, such as omega-3 polyunsaturated fatty acids that act on lipid metabolism44. Our findings support this direction and may further hint at possible interventions that might capitalize on the protective effects of certain metabolic pathways in preventing PD.
Another key factor that might impact the metabolic profile of PD patients is the gut microbiome. There is a growing amount of evidence for gut microbiome dysbiosis in PD45. It is well known that the intestinal microbiome is associated with obesity, type II diabetes, and lipid metabolism46. Microbial-derived lipids can be directly sensed by the host to modulate innate and adaptive immune pathways and to regulate metabolic pathways, all of which can influence the progression of chronic inflammation and metabolic syndrome47. Microbiome species also play an important role in biotransformation, detoxification, and digestion of dietary lipids, and the resulting downstream products can affect local tissues and systemic immunity and metabolism in the host48.
To our knowledge, this retrospective study is the first to comprehensively examine the association between MetS and all its components with PD within a single investigation. A unique aspect of our research is the longitudinal analysis of very comprehensive EHR data. This made it possible for us to map timelines and observe changes in the magnitude of the association for each component up to 15 years before the DoD, providing deeper insights into the prediagnostic phase. Our method and framework were meticulously designed to mitigate selection bias among patients with PD and controls and to address confounding variables such as age, sex, and medication use. Rigorous criteria were applied to ascertain the reliability of the PD diagnosis. The determination of disease onset was not based solely on diagnosis, enhancing the robustness of our findings. Additionally, the diverse ethnicities represented in Israel’s demographics added to the study’s strength. The integration of metabolic biomarkers with pharmacological treatments, coupled with sex stratification, yielded a comprehensive overview of each metabolic biomarker. This approach led to the proposal of a practical new tool, the lipid-BP index, to aid physicians in assessing future PD risk more effectively and earlier.
Our study has limitations inherent to its retrospective design, including the possibility of reverse causality due to prodromal PD symptoms influencing metabolic features, such as weight loss. Nevertheless, our study offers valuable insights from a real-world population, encompassing all patients with PD within a specific region and spanning up to 15 years before the DoD. Second, since each subject was diagnosed at a different point within the timeframe of the study, the number of subjects in the dataset decreased as we drew further away from the DoD. Third, to mitigate the likelihood of variations in blood tests taken among individuals, we used an annual time frame within which all the results were averaged. Fourth, the reliance on pharmacological treatment alone to define hypertension raises concerns about causal inference.
In conclusion, this is one of the largest cohort studies of PD, leveraging real-world data to explore factors preceding and correlating with PD diagnosis. Our findings demonstrate an association between MetS and its components with a lower PD incidence over an extensive prediagnostic period, with sex playing a significant role. Larger-scale studies are warranted to corroborate these findings and elucidate the causal nature of the relationship between MetS and each of its components with PD and suggest a mechanistic explanation of our results. We recommend that a prospective study be performed to follow patients with and without MetS for future PD incidence, and to validate the lipid-BP index and explore mechanistic pathways in the interrelation between PD and MetS.
Methods
Participants
The study group consisted of patients with PD attending the Movement Disorders Clinic of Rabin Medical Center who were members of Clalit Health Services (CHS), the largest healthcare organization in Israel. The Movement Disorders Clinic at Rabin Medical Center is a specialist outpatient clinic affiliated with CHS. It provides care for PD patients but is not part of an inpatient hospital setting. Patients who were diagnosed by a physician in the community or during hospitalization (ICD-10 code G20) between 1 January 2000 and 30 April 2020 and had visited the clinic at least three times (either before or after the DoD) were considered for inclusion. The study was approved by the local Helsinki Committee of Rabin Medical Center. Informed consent was not required.
The DoD was defined as the earliest date on which the patient was either diagnosed with PD, first visited the Movement Disorders Clinic, or first purchased medication prescribed for PD.
The control group consisted of subjects of CHS without PD, matched for sex, age, and residence (10:1) to each patient on the date of patient diagnosis. This means that a control subject had the same (artificial) DoD as their matched patient, and test results for both subjects were collected with respect to the same age. Patients diagnosed with Lewy body dementia, multisystem atrophy, progressive supranuclear palsy, drug-induced parkinsonism, or vascular parkinsonism after diagnosis of PD were excluded from the study. The inclusion/exclusion of patients with a subsequent diagnosis of essential tremor or vascular parkinsonism was determined after review of their medical history by a medical expert.
The diagnosis of MetS was based on the criteria of the National Cholesterol Educational Program Adult Treatment Panel III (NCEP ATP III, 2005 revision)24, (Supplementary Table S2). Since waist circumference was not routinely available, BMI was used as the criterion defining obesity. The definition of prediabetes or diabetes was based on the current “Standards of Care in Diabetes”49.
Data collection
The following data were extracted for all subjects from the EHRs: sex, date of birth, country of birth, BMI, visits to physicians, ICD-10 diagnoses, blood test values, and medications purchased. Blood tests included levels of TG, high-density lipoprotein (HDL), low-density lipoprotein (LDL)/HDL, microalbumin/creatinine, glucose, and HbA1c. Although the LDL/HDL ratio is not part of the NECP ATP III criteria for MetS, it was analyzed here to further explore the role of cholesterol50. Microalbumin/creatinine was included based on findings of a strong association of microalbuminuria with elevated blood pressure51,52. and HbA1c was included because it is considered a very robust diagnostic test for type II diabetes mellitus (T2DM) and is not affected by the specific nutrition close to the test date53.
Statistical analysis
To analyze the longitudinal data, several raw data preprocessing steps were performed.
-
1.
Outlier removal. Extreme outliers were removed from the blood test analysis based on Tukey’s outer fences54. (See Supplementary Material). BMI values of less than 12 or more than 42 were excluded from the analysis55.
-
2.
Temporal analysis. For each patient and each blood test, the time before the DoD was discretized to one-year windows, except for 13–15 years before the DoD, which was discretized to a 2-year window because of the scarcity of data for this period. For controls, these windows were based on the DoD of their matched patient. No imputation was conducted for missing blood test values; the analyses were based solely on actual measurements. For subjects with multiple measurements within the same year, the results were averaged for that year.
-
3.
BMI calculation. Height and weight measurements were used to calculate BMI. If height and weight were not consistently measured, BMI was imputed on the basis of previous BMI results (see Supplementary Material for data imputation method for BMI).
-
4.
Cohort data balancing. To avoid over-representation of the control group because of the imbalance in size between the groups, 10 balanced datasets were created, each including the data of all the patients with PD and one of their matched controls. All results presented here were averaged over these 10 balanced datasets. This method was preferred to repetitive random sampling because it ensured alignment of sex and age between the patients and controls and used all the controls.
-
5.
Trend identification. Trends in blood test results were measured over the control group and stratified for age and sex.
-
6.
Decision on MetS. The presence of MetS was assessed annually according to the EHRs.
-
7.
Medications purchased calculation. Medication was calculated on the basis of the percentage of individuals who purchased at least one prescription out of the total number of individuals in each year. Sensitivity analysis was conducted for varying numbers of prescriptions (n = 1, 2, 3, or 4) to examine whether drug intake impacted the results.
For group (PD and controls) comparison:
-
1.
Odds ratios were estimated using conditional logistic regression, adjusted for age and sex, over the entire period before the DoD for the 1:10 matched case-control design.
-
2.
To explore the annual association between PD and MetS and its components, the following analyses were performed:
-
Chi-squared test for independence was used to compare the prevalence of MetS and hypertension between controls and patients.
-
Welch’s two-sided t-test (designed for unequal variances) was used to analyze blood tests and BMI results. To avoid the impact of pharmacological treatment on the test results, the test was repeated after individuals under treatment were excluded.
-
The false discovery rate (FDR) correction was used to account for multiple comparisons56.
To calculate the annual ORs for future incident PD while accounting for variations in concentrations due to age and sex, blood test and BMI values of the control subjects were divided into quartiles for each subgroup of sex and age decade (40–50, 50–60, etc.) (Supplementary Table S3). For example, for males between 70 and 80 years of age, Q1 TG level is defined as 84 mg/dL, and Q3 as 154 mg/dL. Based on these quartiles, each blood test result for each individual (patients/controls) was defined as high (above 75%), medium (25%–75%), or low (below 25%) by the individual’s sex and age. Thereafter, ORs of blood tests and BMI values were calculated for each year between the fourth and first quartiles of patients and controls, stratified by age group and sex. The OR presentation was supported by P values and 95% confidence intervals (CI), computed using Fisher’s exact test, to accommodate the different sample sizes of the blood tests through the years.
Data availability
Data was extracted from Clalit Health Services (CHS) using Clalit’s data-sharing platform powered by MDClone (https://www.mdclone.com), ensuring anonymity. Permission to use this dataset for the current analysis was granted by CHS HMO. The dataset for our study, along with the data dictionary, and full documentation, are securely held by CHS HMO. Due to ethical restrictions, data for the current study cannot be shared.
Code availability
The underlying code for this study is not publicly available but may be made available to qualified researchers on a reasonable request from the corresponding author.
Change history
10 June 2025
In this article the affiliation details for Author Boaz Lerner were incorrectly given as '1Department of Industrial Engineering & Management, Ben-Gurion University of the Negev, Beer Sheva, Israel and 4Present address: Neurological Institute, Division of Movement Disorders, Tel-Aviv Souraski Medical Center, Tel Aviv, Israel.' but should have been '1Department of Industrial Engineering & Management, Ben-Gurion University of the Negev, Beer Sheva, Israel'.
References
Park, S. H. et al. Association of dynamic changes in metabolic syndrome status with the risk of parkinson’s disease: a nationwide cohort study. J. Parkinson’s Dis. 11, 1751–1759 (2021).
Roh, J., Lee, S. & Yoon, J. Metabolic syndrome and Parkinson’s disease incidence: a nationwide study using propensity score matching. Metab. Syndr. Relat. Disord.19, 1–7 (2021).
Souza, A. P.dS. et al. Effect of metabolic syndrome on Parkinson’s disease: a systematic review. Clinics 76, e3379 (2021).
Nam, G. E. et al. Metabolic syndrome and risk of Parkinson disease: a nationwide cohort study. PLoS Med. 15, e1002640 (2018).
Sääksjärvi, K., Knekt, P., Männistö, S., Lyytinen, J. & Heliövaara, M. Prospective study on the components of metabolic syndrome and the incidence of Parkinson’s disease. Parkinsonism Relat. D21, 1148–1155 (2015).
Miyake, Y. et al. Case–control study of risk of Parkinson’s disease in relation to hypertension, hypercholesterolemia, and diabetes in Japan. J. Neurol. Sci. 293, 82–86 (2010).
Huang, X. et al. Statins, plasma cholesterol, and risk of Parkinson’s disease: a prospective study. Mov. Disord. 30, 552–559 (2015).
Scigliano, G. et al. Reduced risk factors for vascular disorders in Parkinson disease patients: a case-control study. Stroke 37, 1184–1188 (2006).
Huang, X., Abbott, R. D., Petrovitch, H., Mailman, R. B. & Ross, G. W. Low LDL cholesterol and increased risk of Parkinson’s disease: prospective results from Honolulu-Asia Aging Study. Mov. Disord. 23, 1013–1018 (2008).
Fu, X. et al. A systematic review and meta-analysis of serum cholesterol and triglyceride levels in patients with Parkinson’s disease. Lipids Health Dis.19, 97 (2020).
Vikdahl, M., Bäckman, L., Johansson, I., Forsgren, L. & Håglin, L. Cardiovascular risk factors and the risk of Parkinson’s disease. Eur. J. Clin. Nutr. 69, 729–733 (2015).
Kizza, J. et al. Cardiovascular risk factors and Parkinson’s disease in 500,000 Chinese adults. Ann. Clin. Transl. Neurol.6, 624–632 (2019).
Lee, Y. et al. Antihypertensive agents and risk of Parkinson’s disease: a Nationwide cohort study. PLoS ONE 9, e98961 (2014).
Han, K., Kim, B., Lee, S. H. & Kim, M. K. A nationwide cohort study on diabetes severity and risk of Parkinson disease. npj Parkinsons Dis. 9, 1–8 (2023).
Fang, F. et al. Lipids, apolipoproteins, and the risk of Parkinson disease: a prospective cohort study and a Mendelian randomization analysis. Circ. Res. 125, 643–652 (2019).
Sääksjärvi, K. et al. Reduced risk of Parkinson’s disease associated with lower body mass index and heavy leisure-time physical activity. Eur. J. Epidemiol. 29, 285–292 (2014).
Jeong, S. et al. Body mass index, diabetes, and the risk of Parkinson’s disease. Mov. Disord. 35, 236–244 (2020).
Roos, E. et al. Body mass index, sitting time, and risk of Parkinson disease. Neurology 90, e1413–e1417 (2018).
Noyce, A. J. et al. Estimating the causal influence of body mass index on risk of Parkinson disease: a Mendelian randomisation study. PLoS Med. 14, e1002314 (2017).
Schernhammer, E., Hansen, J., Rugbjerg, K., Wermuth, L. & Ritz, B. Diabetes and the risk of developing Parkinson’s disease in Denmark. Diabetes Care 34, 1102–1108 (2011).
D’Amelio, M. et al. Diabetes preceding Parkinson’s disease onset. A case–control study. Parkinsonism Relat. D. 15, 660–664 (2009).
Domenighetti, C. et al. Association of body mass index and Parkinson's disease. Neurology 103, e209620 (2024).
Hawkes, C. H. The prodromal phase of sporadic Parkinson’s disease: does it exist and if so how long is it?. Mov. Disord. 23, 1799–1807 (2008).
Grundy, S. M. et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 112, 2735–2752 (2005).
Avisar, H. et al. Lipidomics prediction of Parkinson's disease severity: a machine-learning analysis. J. Parkinson’s Dis. 11, 1141–1155 (2021).
Avisar, H. et al. Lipid level alteration in human and cellular models of alpha synuclein mutations. npj Parkinson’s Dis. 8, 52 (2022).
Saedi, S. et al. Serum lipid profile of Parkinson’s disease patients: a study from the Northwest of Iran. Casp. J. Intern. Med. 12, 155–161 (2021).
Wei, Q. et al. Reduced serum levels of triglyceride, very low density lipoprotein cholesterol and apolipoprotein B in Parkinson’s disease patients. PLoS ONE 8, e75743 (2013).
Park, J. et al. Association of high-density lipoprotein cholesterol variability and the risk of developing Parkinson disease. Neurology 96, E1391–E1401 (2021).
Hou, L. et al. Hypertension and diagnosis of Parkinson’s disease: a meta-analysis of cohort studies. Front. Neurol. 9, 162 (2018).
Pablo-Fernandez, E. D., Goldacre, R., Pakpoor, J., Noyce, A. J. & Warner, T. T. Association between diabetes and subsequent Parkinson disease: a record-linkage cohort study. Neurology 91, e139–e142 (2018).
Rhee, S. Y. et al. Association between glycemic status and the risk of Parkinson disease: a nationwide population-based study. Diabetes Care 43, 2169–2175 (2020).
Elbaz, A. et al. Risk tables for Parkinsonism and Parkinson’s disease. J. Clin. Epidemiol. 55, 25–31 (2002).
Donzuso, G. et al. Gender differences in non-motor fluctuations in Parkinson’s disease. J. Neural Transm. 130, 1249–1257 (2023).
Iwaki, H. et al. Differences in the presentation and progression of Parkinson’s disease by sex. Mov. Disord. 36, 106–117 (2021).
Nicoletti, A. et al. Gender effect on non-motor symptoms in Parkinson’s disease: a men more at risk?. Parkinsonism Relat. D. 35, 69–74 (2017).
Gao, L., Yang, Y., Cai, L. & Xiong, Y. Gender differences in pain subtypes among patients with Parkinson’s disease. J. Integr. Neurosci. 21, 120 (2022).
Cholerton, B. et al. Sex differences in progression to mild cognitive impairment and dementia in Parkinson’s disease. Parkinsonism Relat. D. 50, 29–36 (2018).
Patel, R. & Kompoliti, K. Sex and gender differences in Parkinson’s disease. Neurol. Clin. 41, 371–379 (2023).
O′Neill, E. & Harkin, A. Targeting the noradrenergic system for anti-inflammatory and neuroprotective effects: implications for Parkinson’s disease. Neural Regen. Res. 13, 1332–1337 (2018).
Jovanovic, P. et al. Sustained chemogenetic activation of locus coeruleus norepinephrine neurons promotes dopaminergic neuron survival in synucleinopathy. PLoS ONE 17, e0263074 (2022).
Fanning, S. et al. Lipidomic analysis of α-synuclein neurotoxicity identifies stearoyl coa desaturase as a target for parkinson treatment. Mol. Cell 73, 1001–1014.e8 (2019).
Cole, N. B. et al. Lipid droplet binding and oligomerization properties of the Parkinson’s disease protein α-synuclein. J. Biol. Chem. 277, 6344–6352 (2002).
Gómez-Soler, M. et al. Triglyceride form of docosahexaenoic acid mediates neuroprotection in experimental Parkinsonism. Front. Neurosci. Switz. 12, 604 (2018).
Wang, Q., Luo, Y., Ray Chaudhuri, K., Reynolds, R. & Tan, E. The role of gut dysbiosis in Parkinson’s disease: mechanistic insights and therapeutic options. Brain J. Neurol. 144, 2571–2593 (2021).
Ghaisas, S., Maher, J. & Kanthasamy, A. Gut microbiome in health and disease: linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharm. Ther. 158, 52–62 (2016).
Yoon, H., Shaw, J. L., Haigis, M. C. & Greka, A. Lipid metabolism in sickness and in health: emerging regulators of lipotoxicity. Mol. Cell 81, 3708–3730 (2021).
Brown, E. M., Clardy, J. & Xavier, R. J. Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe 31, 173–186 (2023).
2. Diagnosis and classification of diabetes: standards of care in diabetes—2024. Diabetes Care 47, S20–S42 (2024).
Fernandez, M. L. & Webb, D. The LDL to HDL cholesterol ratio as a valuable tool to evaluate coronary heart disease risk. J. Am. Coll. Nutr. 27, 1–5 (2008).
Palatini, P. Microalbuminuria in hypertension. Curr. Hypertens. Rep. 5, 208–214 (2003).
Poudel, B., Yadav, B. K., Nepal, A. K., Jha, B. & Raut, K. B. Prevalence and association of microalbuminuria in essential hypertensive patients. N. Am. J. Med. Sci. 4, 331–335 (2012).
Care, D. 2. Classification and diagnosis of diabetes: standards of care in. Diabetes Care 46, S19 (2023).
Tukey, J. W. in Exploratory Data Analysis (Reading, 1977).
Goodloe, R., Farber-Eger, E., Boston, J., Crawford, D. C. & Bush, W. S. Reducing clinical noise for body mass index measures due to unit and transcription errors in the electronic health record. AMIA Jt Summits Transl. Sci. Proc. 2017, 102–111 (2017).
Benjamini, Y. & Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Statist. 29, 1165–1188 (2001).
Acknowledgements
We would like to express our sincere gratitude to Mrs. Shiri Kushnir, manager of the data research department at Rabin Medical Center, Research Authority, for her valuable support in data extraction. Hila Avisar was supported by the Ben-Gurion University High-tech, Bio-tech, Chem-tech–STEM Fellowship, and is currently supported by the Aufzien Family Center for the Prevention and Treatment of Parkinson's Disease at Tel Aviv University.
Author information
Authors and Affiliations
Contributions
Conception and design of the study: H.A., U.G., R.D., B.L. Acquisition and analysis of data: H.A., R.D., B.L. Drafting a significant portion of the manuscript H.A. U.G., R.D., B.L. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Avisar, H., Greenbaum, U., Djaldetti, R. et al. Lower prevalence of metabolic syndrome and its components in the prediagnostic phase of Parkinson’s disease. npj Parkinsons Dis. 11, 147 (2025). https://doi.org/10.1038/s41531-025-01003-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41531-025-01003-1