Abstract
In Parkinson’s disease (PD), several key factors influence patient selection for deep brain stimulation (DBS) as they directly affect long-term outcomes. A comprehensive interdisciplinary assessment is the first step to evaluating risks, benefits, and establishing appropriate goals. Patient-defined symptom priorities play a critical role in selecting the brain target for DBS. The entry of multiple manufacturers of hardware has spurred a rapid acceleration of technological progress. While innovations in programming such as sensing-based physiology-guided programming have introduced the concept of delivering an optimal or “Goldilocks dose”, a precise, personalized therapy to address specific PD symptoms, image-guided programming acts like a “GPS,” enabling faster determination of dose parameters. Emerging tools such as adaptive and automated programming offer clinicians the potential to provide optimal, energy-efficient stimulation. This review, integrating both old and well-established knowledge and new insights, provides a comprehensive summary of the multidimensional aspects of patient selection, target-specific benefits, advancements in hardware technology, and innovative strategies that are either currently available or on the horizon for DBS programming.
Similar content being viewed by others
Introduction
Deep brain stimulation (DBS) is FDA approved, established and a powerful therapy for treating Parkinson’s disease (PD)1,2. DBS is considered for individuals with PD who have uncontrollable tremors despite adequate medication trials, experience significant motor fluctuations on dopaminergic therapies and find dyskinesia induced by these therapies bothersome1,3. A Delphi expert consensus panel recently proposed specific criteria for advanced PD which are (1) individuals requiring ≥5-times oral levodopa doses/day, yet experiencing troublesome motor fluctuations with ≥2 h “off” symptoms/day and ≥1 h of troublesome dyskinesia/day (also referred to as 5-2-1 criteria) and (2) difficulty with activities of daily living4. Other factors also prompting a discussion in the clinic include significant intolerance to oral medications, complaints of medication-resistant dystonia causing discomfort, and deteriorating motor function hindering employment prospects. These circumstances should trigger conversations related to DBS and other device aided therapies5 such as levodopa-carbidopa infusion (intestinal and subcutaneous pumps) and apomorphine subcutaneous infusion pump. Patients and their care partners should be informed about all device-aided therapies available at their treatment center and within their geographic region with discussions on advantages and disadvantages6. When the clinical team and patient jointly decide to pursue DBS, particularly when there is severe dyskinesia or tremor (and at the same time there are no significant neuropsychiatric concerns), it is essential to establish appropriate goals and expectations from the outset. In this DBS-focused narrative review, we intend to summarize the multidimensional aspects of patient selection, the variety of brain targets and hardware technology available, and several novel approaches introduced for precise and personalized programming of electrical parameters. These considerations are critical for successful clinical outcomes. We believe this review will serve as a useful introduction to a broad range of DBS topics, particularly for those new to the field.
Patient Selection Process
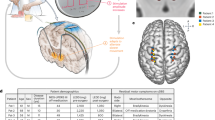
DBS has demonstrated consistent efficacy in managing many aspects of PD in at least eight high-quality randomized controlled DBS trials published between 2006 and 2020 (Table 1)7,8,9,10,11,12,13,14. In clinical practice, the first step deemed critical for a successful DBS outcome is a selection of appropriate patient candidates through an interdisciplinary evaluation process15. The interdisciplinary team comprising a neurologist, neurosurgeon, psychiatrist, neuropsychologist, rehab disciplines, and preferably also a social worker, aims to evaluate whether the advantages of the surgery significantly outweigh the associated risks through a risk-benefit analysis. The interdisciplinary team also plays a key role in guiding the selection of the optimal surgical target, such as the subthalamic nucleus (STN) vs. the globus pallidus internus (GPi), as well as determining the most suitable hardware components, including the choice between a rechargeable or non-rechargeable device. Equally important is evaluating the support system available for post-operative care. In addition, the team is responsible for providing thorough patient education about the procedure and ultimately confirming the patient’s candidacy for DBS (Fig. 1). Besides the disease related characteristics, the patient selection process should consider factors such as demographics (age, sex, race), response to levodopa medication, presence of axial symptoms, nonmotor symptom burden (particularly cognitive and psychiatric features), etiology of PD (genetic vs nongenetic), timing of surgery, comorbidities, and results from brain imaging for final decision making (Fig. 2).
Workflow for patient selection process for DBS: Potential consideration for DBS is first initiated in the clinic. The next step is an interdisciplinary evaluation by a team comprising a neurologist, neurosurgeon, psychiatrist, neuropsychologist, rehab disciplines, and a social worker to discuss risks and benefits in individual patients, provide education, and finalize candidacy. The final step for the selected candidate is scheduling surgery.
Age: biological and not chronological matters
Although clinical studies haven’t established a precise cut-off point, age remains a crucial factor to weigh when evaluating the balance between risks and benefits. Most DBS centers are comfortable when the patient candidates are under 70 or 75 years of age16. Some studies found age at surgery correlated negatively with the clinical outcomes17. However, trials involving older patients reported that these patients still benefit equally from surgery without apparent further risks8. A retrospective study analyzing a large market-based database found that older patients with PD ( > 75 years) showed a similar 90-day complication risk (including postoperative hemorrhage or infection) compared with younger counterparts18. As the elderly population continues to grow and life expectancy increases, along with the rising acceptance of DBS therapy, real-world data on its use among individuals in their late 80s or 90s is much needed. Such an analysis would highlight that biological factor—including comorbidities, cognitive decline, brain atrophy, and a high burden of levodopa-resistant symptoms—are more critical for patient selection than simply considering chronological age.
Sex, race, ethnicity and social structure: need for additional counselling and support
Although PD is more prevalent in men, women appear to be at higher risk for developing dyskinesias, potentially due to differences in levodopa metabolism and lower body weight19. Women with PD also tend to report greater psychological distress, higher self-perceived disability, and lower health-related quality of life (QoL) at their initial visits compared to men20. Despite this, multiple studies have shown that women are significantly underrepresented in referrals for DBS21,22,23, comprising less than 25% of those evaluated24,25. Data from a Medicare database further supports this disparity, showing that men, particularly younger men, are more likely to receive DBS26, they may have greater improvements in bradykinesia and levodopa reduction after STN DBS27. Women tend to decline DBS more often as during midlife they may face increased caregiving duties, occupational responsibilities, and other competing demands, which may influence their willingness or ability to pursue surgical options.
Racial and ethnic disparities are also evident in many studies28. For example, in a National Inpatient Sample database study, Black (Odds Ratio/OR 0.16), and Hispanic patients (OR 0.76) were significantly less likely than White patients to undergo DBS, while Asian/Pacific Islander patients showed no significant difference compared to White patients29.
While qualitative research will help understand the decision-making processes, and barriers faced by underrepresented groups, in clinical practice these patients would benefit from enhanced preoperative counseling, with more time devoted to discussing risks, benefits, and postoperative expectations, as well as providing comprehensive education on DBS technology. Adequate and reliable social support in such cases is essential. Care partners and families play a critical role in helping patients manage the anxiety and stress surrounding the procedure, ensuring timely attendance at appointments, recognizing and reporting potential DBS-related side effects, and supporting recovery. Integrating social worker support into the multidisciplinary care team can further strengthen care by connecting patients and their care partners to relevant resources30, facilitate seamless navigation through both preoperative and postoperative phases and foster more equitable access to and engagement with DBS treatment.
Levodopa responsiveness: pre-surgical levodopa challenge predicts short-term but not long-term outcomes
The motor responsiveness determined during the levodopa challenge test, involving the administration of a single supra-threshold dose of levodopa in the early morning following a 12-hour medication-free period, can serve as a predictor for the motor outcomes following DBS31. According to the CAPSIT-PD, the test should induce at least > 30% decrease in the Unified Parkinson’s Disease Rating Scale, part III, motor score32. A more significant response to levodopa, specifically in the range of a 50% improvement, has been regarded as indicating a more favorable outcome, particularly among patients undergoing STN DBS33. In keeping with this hypothesis, a meta-analysis of DBS studies found that UPDRS motor improvements after DBS correlated with the individual’s response to levodopa challenge before surgery34. While the rule of > 30% improvement with levodopa is helpful for patient selection, DBS is often used to treat PD tremor, particularly when it is poorly responsive to levodopa. In such cases, an incomplete levodopa response is a reason for, rather than a contraindication to DBS treatment. The notion of motor improvements with ~ 30% threshold may be seen as a simplified interpretation35, as it’s uncertain whether such improvements observed in the clinic extend to activities of daily living and QoL and whether the degree of response can consistently forecast improvements in axial symptoms such as gait slowness, gait freezing, postural instability, postural abnormalities, and speech disorders36. However, it has been demonstrated that the long-term outcomes with DBS do not consistently relate to levodopa responsiveness. Even when pooling data from multiple DBS centers there is a wide variability in individual responses to DBS, suggesting that the levodopa challenge test has a limited role in the long run37. Furthermore, recent data investigating long-term outcomes of DBS for 15 years after surgery show that levodopa does not predict rigidity, and that DBS seems to lose its effects on bradykinesia38. These results indicate that the current levodopa challenge might primarily serve to identify non-responders, highlighting the importance of finding better predictors.
Axial symptoms: technology may aid in quantitative preoperative assessment
Axial symptoms typically emerge in advanced stages of the disease and frequently exhibit resistance or a varied response to levodopa treatment, presenting a challenge in their management. Levodopa is considered a double-edged sword, improving gait speed and step length as well as turning and arm swing, but possibly worsening other walking related skills, such as gait initiation and postural sway39. While STN DBS can effectively address freezing of gait especially when levodopa responsive40,41,42, postural abnormalities, such as camptocormia and Pisa syndrome have reportedly improved even when there is poor or no improvement after a levodopa challenge43,44. Assessing the impact of DBS on freezing of gait presents unique challenges because of the episodic nature of this clinical phenomenon. Advanced wearable sensor technologies designed for home monitoring offer the potential to assess axial symptoms in real-life settings longitudinally. For example, in one study, kinematic assessment of gait preoperatively has revealed connections between preoperative levodopa response metrics—such as stride length and range of motion—and the freezing of gait outcomes postoperatively45. Unfortunately, the quantification of these parameters through kinematics remains largely confined to research tools and has not been widely adopted in routine clinical practice. It is important to recognize that long-term follow-up of PD patients undergoing STN DBS reveals a gradual loss of benefit, particularly concerning axial symptoms. In a large retrospective analysis of over 300 patients followed for 15 years, worsening of axial features was noted, likely reflecting the natural progression of the disease. The study identified preoperative axial scores, frontal dysfunction, and older age at PD onset as significant risk factors for post-surgical progression of axial impairment46.
Speech impairment is another common axial feature, which may not respond to standard dopaminergic treatments. Research has shown that DBS effects on speech are inconsistent, with both improvements and declines noted across various measures. Studies on STN DBS frequently report speech and language deterioration, including issues with naming and verbal fluency. However, in a long-term follow-up of 25 STN DBS patients, speech intelligibility stayed at the same level when compared with preoperative values47. In another GPi DBS study, while bilateral stimulation worsened several aspects of speech, overall intelligibility remained relatively stable48. In contrast, a recent meta-analysis of studies involving both STN and GPi targets highlighted that long-term DBS therapy can worsen phonation as well as articulation49. The implementation of acoustic analytical technology for appropriate patient selection could enable a more detailed and precise assessment of speech impairments, offering valuable insights for treatment and monitoring strategies50. Similar to sensor-based gait assessment, speech technology has not turned into a clinical reality for DBS practitioners.
Patients with significant axial disability should be thoroughly counseled on the lack of long-term benefits of DBS, with shared decision-making emphasized. It is conceivable that patients may achieve better outcomes when offered preoperative physical, occupational, and speech therapy, an approach of employing “prehab” or preventative rehabilitation.
Nonmotor symptoms: plan for setting expectations before surgery
DBS has garnered increasing attention over the past decade regarding its potential impact on non-motor symptoms (NMS) seen in PD including cognition, neuropsychiatric features (depression, anxiety, hallucinations), autonomic features, sleep, pain and fatigue. While the DBS lead is intended to target the motor subregions of the STN or GPi, the spread of electrical fields to the limbic and associative subregions could potentially affect NMS in both beneficial and adverse manner. A few uncontrolled and controlled studies observed that NMS improvements could contribute to QoL enhancement51,52,53. A systematic review further emphasized that control of NMS is essential for patient satisfaction with DBS therapy54.
Previous research found that STN DBS could worsen verbal fluency, cognition, and mood, with some of these effects attributed to the reduction of dopaminergic drug doses after DBS. However, certain NMS, such as impulse control disorders, primarily linked to long-term exposure to dopaminergic medications can improve when these drug doses are reduced following STN DBS55. Recent studies also noted improvements across various NMS—including sleep/fatigue, hallucinations, cardiovascular, gastrointestinal and urinary symptoms, three years following STN DBS52,56,57,58. Such large-scale data on the impact of GPi DBS on NMS and how it relates to patient satisfaction is not available.
Although there are no clear guidelines on how to precisely incorporate the burden of NMS into the patient selection process, caution needs to be exercised when NMS predominantly contributes to the disease burden. It may be helpful to ask patients to identify the three or four most troublesome symptoms they hope will improve with DBS; essentially, to “make a wish.” This can guide in setting goals, manage expectations, and help tailor postoperative programming. Patients with unrealistic expectations have been found to experience post-operative psychological distress and social maladjustments59,60. Future studies should focus on identifying the pathogenic networks that contribute to the NMS and are responsive to DBS.
Genetic etiology: prediction of outcomes is not definitive
Genetic testing is becoming accessible and affordable in clinical practice in many countries and may be used to inform DBS candidacy61. In one study, LRRK2-G2019S variant, the most prevalent variant associated with a phenotype resembling late-onset PD, characterized by frequent tremors and a positive response to dopaminergic medications, demonstrated an excellent response to STN DBS in over 85% of subjects62,63. In contrast, LRRK2-R1441G variant, characterized by more motor fluctuations, tremor, and issues of postural instability revealed higher rates of unsatisfactory outcome after STN DBS64.
Another important gene for DBS outcomes is the glucosylceramidase β1 (GBA1) mutation, which typically presents at an earlier age, follows a more aggressive disease course, and carries a higher burden of neuropsychological issues65,66. Notably, severe variants like p.L444P have been linked to an elevated risk of disease, an earlier onset age, and more pronounced cognitive dysfunction when compared to less severe mutations such as p.N370S67. A recent retrospective multicenter study involving 366 PD patients found that individuals with GBA1 mutations treated with STN DBS experienced a more pronounced or accelerated decline in cognitive scores when followed longitudinally after surgery68. Although cognitive decline post-surgery did not necessarily imply the onset of dementia, these findings were indeed concerning. The authors postulated that STN might not be an optimal target for GBA1-positive patients, and analysis of cerebrospinal fluid biomarkers, such as glucocerebrosidase activity levels, could potentially uncover the pathogenic mechanisms. In contrast, a recent multicenter study from Italy found that despite a selective decline in cognitive scores three years after DBS, most GBA-PD patients had not developed dementia by the five-year follow-up. In this cohort, 92% received bilateral STN DBS, while the remaining 8% were treated with GPi. The authors concluded that DBS continues to be a valid therapeutic strategy for patients with the GBA1 mutation66. While some suggest that GPi may offer a safer cognitive option in such situations, data from large cohorts is still pending.
A mutation of the Parkin gene, which is the most common genetic mutation associated with young-onset PD presenting before age 50 has been linked to an increased frequency and severity of impulse control behaviors69,70,71. In a cohort of 40 patients with Parkin mutation who underwent STN DBS, the majority experienced significant improvements, with only 15% reporting unsatisfactory outcomes at a shorter-term follow-up of 2 years. However, at longer follow-up ranging from 2 to 6 years, nearly a third of patients reported an unsatisfactory response62. Many other forms of genetic mutations have been linked with the pathogenesis of PD. In a recent systematic review, comprising 135 patients with diverse genetic mutations, most patients showed marked or satisfactory response in the short term with few adverse events reported62. Despite an increasing awareness for the role of genes, considering the body of evidence, definitive conclusions cannot be drawn.
Timing of DBS: consider early
For many years, DBS was offered to individuals with advanced PD, experiencing severe motor complications with an average disease duration of 12 to 15 years. However, findings of a pivotal EARLY-STIM trial demonstrated the clinical benefits of employing DBS earlier when motor complications were present for four years or less72. These findings prompted a paradigm shift in approach as an earlier intervention would conceptually prevent motor, social, and psychological disability and thereby provide a much better QoL. The FDA in the United States extended the indication for DBS to patients with “of at least 4 years duration and with recent onset motor complications, or motor complications of longer-standing duration that are not adequately controlled with medication.” Although the EARLY-STIM data is compelling there are several considerations that need attention. An important concern is the risk of subjecting patients with atypical parkinsonism to unnecessary DBS, as early in the course, differentiation of the two forms of parkinsonism can be challenging73. However, the longitudinal follow-up of the EARLY-STIM cohort found that the diagnosis of PD was switched to atypical parkinsonism in only a small percentage of patients74. Then, a shorter disease duration accompanied by a greater and faster burden of disability can sometimes be indicative of severe GBA1 gene variants, which may impact clinical outcomes. Finally, an early DBS intervention poses a risk of selecting patients with motor fluctuations that would have otherwise remained mild for a long time, given a slow trajectory of progression. These critical concerns highlight the need for a detailed interdisciplinary discussion and the importance of shared decision making with the patient when discussing the potential benefits of early DBS.
Target Selection
Although STN target is more frequently selected, during the era of lesioning procedures, GPi was widely targeted to address the motor symptoms. Historically, in 1993, Pollak and Benabid decision to pursue STN DBS was primarily driven by data from preclinical studies75,76. Although Siegfried and Lippitz identified the GPi as a potential DBS target as early as 199477, initial enthusiasm was limited; largely due to the perception that bilateral STN DBS offered more robust and sustained control of PD symptoms, with less attenuation of benefits over time, leading to greater preference for the STN target in clinical practice78. Now it is well accepted that each target indeed presents unique benefits (Fig. 3).
Subthalamic nucleus (STN) and globus pallidum internus (GPi) are two main DBS targets. The sweet spot that leads to optimal control of symptoms is located in dorsolateral STN and posteroventral GPi. Bradykinesia, rigidity, and tremor symptoms listed in blue occur to similar extents with DBS targeted to both STN and GPi. Target-specific clinical benefits are listed in orange.
Medication reduction: STN scores over GPi
The use of bilateral STN stimulation is accompanied by a significant reduction of medication doses. The majority of randomized STN DBS studies indicate about 50% or more reduction in dopaminergic medication dosage. For instance, in the NSTAPS study, medication decreased from a median levodopa equivalent daily dose (LEDD) of ~1200 mg at baseline to ~600 mg at the 3-year mark79. In another prospective 5-year study involving the initial 49 consecutive patients treated with bilateral STN DBS, Krack et al. reported a significant reduction in LEDD from ~1400 mg at baseline to ~500 mg at the 5-year follow-up80. This decrease in dopaminergic medications effectively addresses challenges like excessive levodopa-induced involuntary movements, as well as complications related to dopamine dysregulation syndrome and impulse control disorders, which are particularly common in younger patients. Unlike STN DBS, GPi DBS is generally not associated with a significant reduction in dopaminergic medication dosage.
Tremor control: should the target be STN, GPi or an alternative (Vim/Zona incerta)?
Tremor is a prevalent feature of PD, impacting nearly 80% of patients at some stage of the disease81. A severe rest tremor in PD can lead to significant social anxiety, and STN DBS is often perceived as offering superior symptom management. However, a meta-analysis of tremor outcomes revealed comparable benefits from both STN and GPi stimulation82. While action tremors affecting the arms likely have a greater deleterious impact on daily functioning from a practical standpoint. Most studies in literature evaluated DBS effects on rest and action tremors combined. A recent study specifically investigating the effects of DBS on action tremor found that both GPi and STN DBS resulted in similar tremor control at one-year follow-up, but the clinical benefits of GPi DBS tended to manifest more slowly83. Although PD tremors respond well to both STN and GPi DBS, residual tremors may persist despite multiple programming sessions. Ventral intermediate nucleus (Vim) has been found to improve PD tremor with benefits reportedly as high as 70% improvement84. Some DBS centers therefore consider simultaneous dual implantation in both the GPi and Vim as a preferred strategy for patients dealing with severe tremors85. Others have found dorsal STN or zona incerta to be a potent alternate target for controlling PD tremor86. In one study, albeit small, unilateral zona incerta DBS was found to improve PD tremor by nearly 80% at 18 months of follow-up87.
Bradykinesia and rigidity (STN same as GPi); dyskinesia (GPi scores over STN)
GPi target has a simpler approach for programming and has been linked to better maintenance of gait and cognitive function compared to STN DBS1. On the other hand, one study found that the extent of improvement in off-period bradykinesia with STN DBS seems to be somewhat better than GPi DBS79. Nevertheless, overall data from large-scale randomized studies involving more than 500 patients has conclusively shown that both targets offer similar long-term benefits in terms of bradykinesia and rigidity. There is a notable enhancement in motor fluctuations resulting in reduced off-time and increased on-time, (Table 1) and that the improvements are accompanied by an acceptable adverse effect profile. Studies including randomized trials listed in Table 1 have shown that GPi DBS has the capability to directly suppress dyskinesia (60% to 70% improvements), which is something not observed with STN88,89.
Alternative targets for treating axial symptoms and cognitive impairment
Axial burden in PD is known to worsen with disease progression. Several strategies have been adopted to address axial symptoms but with inconsistent outcomes90. One example is DBS targeted to the pedunculopontine nucleus (PPN) area, a rich cholinergic source that initially garnered significant attention as a promising approach to address gait and balance disturbances91. Initial studies indicated that both unilateral and bilateral PPN could improve gait freezing and some were observed to improve other axial features including falls91. However, further evaluations found that the extent of improvement varies, that benefits are often not sustained, and that it is unclear whether such partial benefits on gait and balance are clinically meaningful as assessment of QoL was rarely reported. It is well known that defining the boundaries of PPN, a part of the mesencephalic locomotor region can be challenging92, and the precise neuronal population requiring targeting is still unknown90. Therefore, despite more than 20 years of history of the use of PPN DBS in patients with PD, the cohort of implanted patients worldwide remains limited. In addition, only a few centers have generated the bulk of the cases and published literature93,94. Some studies that pursued simultaneous or separate stimulation of the STN and the substantia nigra pars reticulata (SNr) located in the midbrain (known to control locomotion) found improvement in freezing of gait but not in balance95. One study found that SNr stimulation alone could not improve freezing of gait when compared to STN stimulation alone or STN combined with SNr stimulation96. The inconsistency in results could be attributed to the limited precision of currently available imaging sequences combined with the small size of the targeted nuclei. Alongside the PPN, the nucleus basalis of Meynert (NBM) in the basal forebrain has been studied as a DBS target to enhance cholinergic tone and improve cognition. While a few randomized controlled trials97,98 have found NBM-DBS to be well tolerated, they have not demonstrated cognitive improvement even after follow-up for one year99 and three years100.
Role of imaging in target selection
There is no doubt that simply selecting an appropriate target may not be adequate for achieving a successful outcome with DBS. In recent years, neuroimaging advances have led to substantial enhancement of the precision and clarity of target visualization. For instance, the STN target, often challenging to visualize on routine T2-weighted MRI sequences (used for surgical planning), especially its ventral border towards the substantia nigra, can now be better delineated through the application of quantitative susceptibility mapping on gradient-echo sequences. This technique, highly sensitive to iron, notably improves the visualization of the STN101. Additionally, the internal medullary lamina, which separates the GPi and globus pallidus externus and is frequently not visible on routine T1-weighted sequences, can be more effectively visualized using a fast-grey matter acquisition T1 inversion recovery sequence102.
Besides these developments relating to MRI sequences, the availability of ultra-high field 7T MRI at many DBS centers shows promise in further improving target selection as it can visualize intrathalamic nuclei and other smaller basal ganglia subregions that are not possible with the current 3 T technology103. There is also growing enthusiasm and experience in employing diffusion tensor imaging and tractography to target specific white matter tracts involved in pathogenic networks104. However, these dense white matter bundles have crossing fibers specifically at the STN’s periphery, making it challenging to use diffusion MRI for direct STN targeting. Nevertheless, using optimized MRI sequences (30 diffusion gradients or higher, applying multiple b-values, and a voxel size 2 mm), in combination with tractography can visualize the white matter tracts in the STN region (and its surrounding fibers system)105. In one study, a greater improvement in PD tremor was observed when the distance of the DBS electrode from the crossing fibers of the dentato-rubro-thalamic tract was smaller106. Similarly, another tractography-based study suggested that the therapeutic effects of STN DBS may, in part, be due to the stimulation of nearby white matter tracts, such as the dentato-rubro-thalamic tract, rather than just the targeted structure itself107.
Microelectrode recording for target mapping
DBS centers can utilize microelectrode recordings (MER) on 2 or 3 parallel tracks simultaneously, starting approximately 5–10 mm above the calculated target depth, to capture multiunit activity, which reflects the summed neuronal activity within a 50–350 µm radius of the microelectrode tip108. After outlining the anterior and lateral borders of the STN or the posterior and medial borders of the GPi through these recordings, macroelectrode stimulation can be applied along the track with the longest section or strongest signal of target activity, while assessing symptom reduction and adverse effects of stimulation. Interpretation of electrophysiological patterns during MER often depends on the expertise of a neurophysiologist, introducing the risk of subjective bias. Additionally, both single- and multi-unit recordings are vulnerable to ambient noise, and signal distortion from fluid in the electrode track109. Many emerging solutions integrate MER data with oscillatory activity in specific local field potential (LFP) frequency bands (LFPs discussed later in the sensing technology section). For example, advanced algorithms like HaGuide, integrated into the Neuro Omega and NeuroSmart MER systems are now available for clinical use. The system quantitatively displays background noise changes and plots dominant LFP frequencies along the MER trajectory thus enabling real-time detection of subregion boundaries within the STN, specifically distinguishing the motor dorsolateral oscillatory region from the nonmotor ventromedial non-oscillatory region. Based on LFP analysis, the algorithm can also recommend the optimal location for maximal therapeutic benefit110. While this novel approach to MER is certainly beneficial, further validation and real-world implementation of these advanced systems remain necessary.
While traditionally, DBS is performed with the patient awake under local anesthesia to allow for physiologic mapping of the target nucleus, intraoperative test stimulation to assess clinical benefit, and identification of motor or sensory “off-target” effects, some patients may poorly tolerate awake surgery due to anxiety, being off medication, or fatigue from prolonged testing. With advancements in preoperative imaging and the availability of intraoperative MRI there has been growing use of asleep DBS performed under general anesthesia111. Meta-analyses of DBS outcomes have found no significant differences in clinical efficacy between asleep and awake surgical techniques111,112. An important consideration is that LFPs can be affected by propofol anesthesia; dexmedetomidine may be a more suitable agent for general anesthesia, as it better preserves neurophysiological signals113.
Hardware Selection
DBS technology has evolved primarily from cardiac pacemaker technology. After the inception of the ‘modern’ DBS era in the late 1980s, DBS technology experienced relatively little advancement for nearly two decades. Though quadripolar electrodes were accessible at certain centers in the 1970s, the electrodes first used in Grenoble had just one contact at the tip, and chronic stimulation was achieved through radiofrequency coupled coils114. Initially, hardware technology primarily addressed concerns related to implantable pulse generator (IPG) or battery like bulky battery size, short battery life, and frequent battery replacements115. Nevertheless, in recent years, there has been a notable change, with multiple manufacturers of DBS technology entering the global market for clinical use (Medtronic, Boston Scientific, Abbott, PINS, SceneRay). This development has spurred international competition, leading to a rapid acceleration in technological progress within the field116. Each hardware system has both overlapping (common) and distinctive unique features (technical specifications presented in Table 2). The choice of a DBS hardware system for an individual patient is ultimately influenced by patient-specific factors, the planned anatomical target, accessibility to full-body MRI, and the preferences of the treating neurologists and neurosurgeons117.
Considerations for DBS lead specifications
DBS leads have electrodes consisting of platinum–iridium wires that have minimal toxicity and excellent conduction properties. DBS leads are available in various designs such as traditional quadripolar electrode, directional electrode, and multi-electrode configurations. The number of contacts, electrode length, and interelectrode spacing are important factors that allow precise targeting of different anatomical structures during stimulation therapy. Leads with 0.5 mm inter-electrode spacing may be used for smaller targets such as the STN which measures approximately 6–8 mm in the anterior-posterior direction, 9–11 mm in the mediolateral direction, and 3–5 mm in the dorsoventral direction. Whereas in GPi target that has approximate dimensions of about 10–12 mm in the anterior-posterior direction,15-18 mm in the mediolateral direction, and 4–6 mm in the dorsoventral direction, leads with inter-electrode spacing of 1.5 mm may be preferred. The new directional leads are like traditional omnidirectional leads, featuring four vertically spaced cylindrical contacts. However, the two center contacts are divided into three segments, each spanning roughly 120° radially. These segments can either be activated together for omnidirectional spherical or ring stimulation or individually to steer stimulation in a cone-like pattern. This design theoretically enhances DBS programming by directing stimulation toward therapeutic targets especially in smaller or more complex target areas, improving the effectiveness of symptom relief in PD and avoiding areas that may cause side effects, thereby widening the therapeutic window (discussed later in Programming section). When placing directional leads, it may be required to insert the lead deeper than usual to ensure that the second and third levels of contacts, which include the directional contacts, are positioned within the target region116. Using the linear, 8-contact lead instead of the traditional quadripolar lead offers the opportunity for a multi-target approach, which may enable addressing multiple PD symptoms. For e.g., in one study, the 8-contact linear lead was employed to stimulate the zona incerta dorsal to STN to alleviate dyskinesia and tremor and stimulate the substantia nigra pars reticulata ventral to STN to enhance gait and balance. The study found a slightly more favorable patient global impression of change when comparing 8-contact linear lead with 4-contact standard lead118. Early this year, the FDA approved Boston Scientific’s 16-contact directional leads; Cartesia X (6 levels, 11.5 mm) and HX (8 levels, 15.5 mm). These leads offer multi-level directionality (more directional span), enabling full coverage of the STN, GPi, and Vim, as well as the white matter tracts beneath the Vim. It is worth noting that while new lead designs offer greater flexibility and precision in programming, the expanded parameter space and options with newer electrode models may also impose a significant time burden. The higher number of electrodes in directional systems has led to a 1680-fold increase in electrode permutations, increasing programming complexity119,120.
Choosing rechargeable vs. non-rechargeable battery
Battery longevity refers to the duration for which a single IPG can effectively deliver the required current before needing surgical replacement. Non-rechargeable (primary cell) batteries typically last 3–5 years, depending on usage. However, high continuous stimulation settings, such as those often required for PD patients with significant dystonia (e.g., high pulse widths), can drain the batteries more quickly. Implantation of a rechargeable IPG extends battery longevity, reducing the need for surgical replacement and thereby alleviating both economic and psychosocial burdens. Rechargeable IPG devices, which were first introduced in 2008 and have a predicted lifespan of 15 or more years are now offered by most manufacturers (Table 2). Rechargeable devices have the potential to reduce the need for frequent battery changes, consequently lowering costs associated with multiple surgical procedures. In one study conducted at a single center, the utilization of a rechargeable IPG (specifically, the Activa RC device) over a 9-year period, aligning with the initially approved lifespan of the device, led to cost savings totaling $60,900 per patient121. Additionally, rechargeable IPGs potentially decrease the likelihood of hardware infections associated with multiple surgeries122,123. The latest rechargeable IPGs available for commercial use are lighter, smaller, and designed with smoother contours and some can offer a lifespan of about 25 years (For eg Boston Scientific Vercise GenusTM system). The reduced profile of rechargeable IPGs, compared to non-rechargeable ones, could further improve wound healing and cosmetic results. Although the newer rechargeable IPGs require relatively superficial implantation (1 to 1.5 cm beneath the skin surface) to facilitate easy recharging121, this may increase the risk of skin erosions and cosmetic complications, particularly in thinner patients, potentially negating the benefits of the smaller device size. In the multicenter, open-label trial assessing IPGs (Multi Recharge Trial), failed recharges occurred in 8.7% of patients, only 3.6% experienced a therapy interruption; surgical revision and infection rates were 4.1% and 2.1%, respectively124.
When deciding between a rechargeable and non-rechargeable IPG, factors such as the reliability in regularly recharging as the process may not be easy and convenient for all patients particularly those with advanced age and cognitive problems125. Then some patients find pairing the charging pad and IPG difficult. They feel “tethered” during charging as the current rechargeable IPGs require a minimal distance between the charging pad and the IPG during the charging session126,127. However, according to patient experience reports, the vast majority of patients including individuals with dystonia and PD found the process easy, and the incidence of adverse events and temporary interruptions in therapy was low128. In the Multi Recharge Trial, 93.8% of users reported feeling confident recharging their devices, with overall convenience rated as “easy” with average weekly recharge time of about 2 h124.
MRI safety: an important factor from long-term perspective
The primary dangers for patients with DBS devices undergoing MRI include heating at the electrode tips, induced currents, IPG dysfunction, and mechanical forces, all of which arise from exposure to magnetic fields and magnetic forces during the scan. It has been found that more than 70% of patients will need an MRI within 10 years of DBS implantation due to either comorbidities or device complications129. Many of the newly introduced Medtronic IPGs that have been tested in 3.0-T MRI environments are found to be MRI-conditional when eligibility criteria are fulfilled. Choosing specific MRI parameters, such as gradients, coils, radiofrequency pulses, and scan time, along with turning off the DBS device, are some examples of the eligibility criteria to mitigate risks. Medtronic, Boston Scientific and Abbott provide DBS leads and IPGs that are deemed as MRI safe when certain conditions are met (conditional approval). Patients must be informed about the potential risks before subjecting them to the scanning procedure. Medtronic devices also offer the unique ability to conduct MRI even with DBS turned on. Thus, patients with significant tremors or dyskinesia with DBS turned on can stay still in the scanner.
Accessibility and affordability
Finally, the preferences and familiarity of the surgical and neurology teams with specific DBS systems and accessibility to the chosen DBS system, including the availability of devices and support services, may also vary based on geographic location and healthcare infrastructure can influence the choice of devices. For example, the PINS and SceneRay DBS systems, both manufactured in China, are primarily available in select Asian countries.
Overall, the choice of hardware should be tailored to the specific needs and preferences of each patient, considering factors such as anatomical considerations, patient characteristics, and the expertise of the treating medical team. Collaborative decision-making involving the patient, neurologist, and surgeon is essential to ensure optimal outcomes and patient satisfaction with DBS therapy.
Programming Selection
DBS programming begins once lead placement is confirmed, and hardware integrity is ensured through impedance check (resistance encountered by electrical current). Because of the potential confounding effects of the microlesion effects such as the acute impact of local brain tissue manipulation with the electrode, along with the occurrence of micro-hemorrhages or edema that can lead to transient motor improvement—programming usually begins 3 to 4 weeks after lead implantation. The traditional method of identifying optimal contact with suitable stimulation parameters has been found to be time-consuming, involving numerous trial-and-error iterations and requiring expertise, experience, and patience130,131. The conventional approach lacks precision and may sometimes result in the delivery of excessive stimulation doses, leading to unwanted side effects such as speech dysarthria, freezing or stimulation-induced dyskinesia. While brief programming sessions can capture acute side effects, they may fail to identify the full range of side effects that could emerge with chronic stimulation. Recent advancements in programming techniques have enabled the idea of delivering the “Goldilocks dose” of precise and personalized DBS therapy. The new systems have wider flexibility in parameter selection to enable efficient programming. For example, the expansion of pulse width ranges and the ability to use various frequencies (both low and high) within a single lead has substantially increased flexibility of addressing both appendicular and axial symptoms while mitigating side effects. The electrical field can be steered horizontally perpendicular to lead shaft or vertically along the length of the lead (multiple independent current control or MICC, Coactivation, Optistim) allowing various configurations and patterns of stimulation. In one STN DBS study, the use of short pulse widths was found to improve speech intelligibility132. In another study, the use of short pulse widths, directional steering, or both resulted in significant and lasting improvements in dysarthria, dyskinesia, and pyramidal side effects at six months, while maintaining effective motor symptom control133. Another study found the use of interleaving stimulation of dorsal STN lead to improvement of dyskinesia134.
Other advances include image-guided programming to reduce the time required to achieve treatment goals and tele-programming to potentially reduce the patient or caregiver burden. Sensing technology or physiology-guided programming and adaptive DBS are now possible in the clinic for optimizing treatment outcomes and delivering the right amount of energy efficient stimulation. Finally advances in machine learning, artificial intelligence and automated programming are valuable tools in the pipeline. The section below discusses the various exciting technologies now available in the DBS clinic. It is also important to note that while evidence regarding efficiency and the best practice approach for programming is beginning to build, concrete data is still lacking, as most new programming studies are small, have short follow-up periods, and offer limited proof of superiority over conventional programming, aside from initial time savings during the first session.
Steering of current with directional programming and fractionation of current
The eight-contact directional leads feature segmented contacts at the middle levels, arranged in a circular formation, allowing programmable axial steering of current for more precise control and improved therapeutic outcomes135. Directional programming is used when the threshold for stimulation-related side effects is low or the therapeutic window (the range of currents that produce beneficial effects with minimal or no adverse effects) in the typical target area is less than 2 mA. In these cases, programmers often prefer segment mode (where electrical current is delivered directionally through individual segment contacts) over ring mode (where current is emitted omnidirectionally through all three segments of the contact). Programmers also opt for directional leads when programming parameters established during surgery cannot be directly applied postoperatively in the clinic to aspects such as gait, cognition, or behavioral disturbances, as these clinical features cannot be assessed intraoperatively. Moreover, if a DBS lead is implanted under general anesthesia, clinical features like dysarthria cannot be evaluated intraoperatively117. Caution should be exercised when transitioning from ring mode to segment mode. Smaller electrodes are linked to higher charge density. Therefore, intensity adjustments will be necessary with smaller step-size increments (ranging from 0.1 to 0.3 mA compared to the traditional 0.5 mA), and plans should ensure that no more than ~ 3.4 mA per contact is delivered. Additionally, activating multiple adjacent segmented electrodes can reduce directionality, as the electrical current tends to flow out through the electrode edges, creating a broader electrical field than the electrode surface itself117,136. The utilization of directional systems has been observed to decrease the current needed for experiencing clinical benefits while simultaneously raising the threshold for side effects137. Thus far, studies have shown that the therapeutic current requirements are reduced by around 40% or more and the therapeutic window is increased by approximately 40% or more138,139,140,141. While there is no clear evidence supporting increased efficacy with the use of directional system, one study conducted in patients with essential tremor suggested that power consumption could be reduced when comparing directional against omnidirectional stimulation119.
The MICC feature in the Boston Scientific system allows each contact to operate with a separate current source, facilitating the simultaneous fractionalization of current across multiple electrodes. Fractionation of current can be applied when addressing multiple clinical features that usually respond to distinct stimulation settings and require current delivery to specific subregions—such as low-frequency stimulation of the ventral STN for gait and high-frequency stimulation of the dorsal STN for tremor. The coactivation feature in the Abbott system permits the stimulation of multiple contacts together, effectively treating them as a single electrode. Meanwhile, Medtronic’s OptiStim feature allows for unique amplitude values to be assigned to each electrode. These features enable a better spatial precision of current delivery.
Image-guided programming: navigation aid for faster programming
Visualization tools have been developed to facilitate an “image-guided” programming approach, utilizing patient-specific anatomy reconstructed from MRI and co-registered with post-operative CT images displaying implanted leads. Examples of such tools include Medtronic SureTuneTM and Boston Scientific STIMVIEWTM-XT (or their latest Illumina 3D AI programming software). Imaging tools create a 3D model presenting the leads in relation to the predicted brain structure from preoperative processing. This functionality provides surgeons with feedback on the final lead location compared to the surgical plan and informs programming neurologists about lead location and contact orientation relative to anatomical and physiological targets, simplifying programming complexity142. Image-guided navigation during programming143,144 can be compared to the convenience of using a “GPS system” while driving a car. Just as a GPS guides the driver along the best route to their destination, image-guided navigation aids clinicians in accurate programming by offering clear guidance based on patient-specific anatomy. Additionally, the visualization tools allow modeling of the volume of tissue activated generated by various stimulation settings to identify the contact locations with the highest probability of success (maximal benefits and minimal side effects). Indeed, these tools have led to more efficient programming sessions by reducing programming time, minimizing patient discomfort, and alleviating burdens for clinical staff. Recent research indicates that the time required for programming decreased by almost half (reduced to almost 20 min from 45 min)145 when employing these semi-automated or automated methods for registering MRI and CT data, segmenting the STN, detecting leads, and generating volume of tissue activated146. In a recent study, a machine learning-based model utilizing imaging metrics was developed to automate the selection of stimulation parameters. This approach aims to achieve optimal motor improvement while minimizing the risk of stimulation-induced side effects147. However, there are potential limitations that one must take into consideration. These methods may not fully integrate individual electrode impedances, axon orientation, and brain tissue inhomogeneity. Additionally, factors like axial rotation or vertical migration of the DBS lead post-image acquisition could impact the accuracy of these tools.
Tele-programming: convenience with caution
Tele-programming offers DBS care to patients in remote areas, those facing mobility challenges, and those lacking caregiver support. Through telemedicine, clinicians can remotely adjust stimulation parameters based on clinical symptoms, troubleshoot issues, and all clinically approved DBS systems allow for remote checking of impedance and neurostimulator battery status148. Clinicians can unlock a specified range of stimulation parameters that patients can adjust using the patient programmer at home, with or without direct clinician advice. Alternatively, patients can select between multiple programs or groups with different parameters, empowering them to make minor adjustments within preset ranges or switch to another program or group with different preset programming parameters using a patient programmer. The Neurosphere™ Virtual Clinic platform offered by Abbott Inc. through their Infinity™ DBS system is a further step ahead as clinicians can remotely program new stimulation settings as if the patients were in the clinic. The platform ensures patient privacy with multiple layers of protection and encryption. Patients must approve clinician access at the start of each session and retain control to pause or end the session and in case of internet disruptions, the system reverts to a secure “recovery program”149.
While remote adjustment of DBS parameters can reduce the need for clinic visits and the burden and cost of traveling, one study found that self-adjustment was unexpectedly associated with a longer time needed to optimize programming and a higher rate of stimulation-induced side effects, as patients persistently changed parameters in attempts to improve their clinical condition150. Therefore, it is crucial to provide education on using the patient controller and telemedicine platform. Additionally, during a tele-programming session, ensuring a high-speed broadband internet connection, providing an open examination area, ensuring privacy and safety, and encouraging patients to compile a list of medications and DBS-related symptoms or concerns to discuss are essential steps.
Sensing technology: potential application for identifying optimal contact for stimulation and for adaptive DBS
LFPs are generally considered to be caused by the sum of excitatory and inhibitory postsynaptic currents within a specific tissue volume surrounding the DBS electrode, with minimal contribution from the recorded discharges or action potentials of the activated neurons. Various factors, including neuronal and synaptic sources, recording volume, electrode surface, and electrode-tissue interface impedance, can influence LFPs. With recent advances in sensing technology, it is now possible to conduct in-clinic as well as at-home monitoring of LFPs and deliver precise stimulation doses of DBS to effectively regulate these signals. Newer DBS systems for clinical use (Percept PCTM and Percept RCTM, Medtronic, USA) have ‘sensing’ (use of the stimulation electrodes as recording systems to capture LFPs) capabilities for real-time monitoring of neural activity and providing valuable insights into the brain’s electrical activity. Another DBS system currently used in research is the Newronika AlphaDBS, which features sensing technology capable of recording noise-free LFPs even during active electrical stimulation151.
Numerous studies have demonstrated that peaks in the beta band (13–30 Hz) often correlate strongly with bradykinesia and rigidity. Reductions in beta power in response to oral medications or DBS is used for dose titration and programming120,152. The ability to chronically and objectively sense physiological signals offers numerous benefits, such as gaining a deeper understanding of the disease state and traits, monitoring the effects of medication wash-in and wash-out periods (ON-OFF cycling), insights into how circadian rhythm variations could impact ON-OFF cycling, and identifying potential issues with medication compliance117. Peaks in the theta (4–8 Hz) and alpha (8–12 Hz) bands correlate with tremor152. Monitoring theta or alpha peaks can help troubleshoot breakthrough tremor but sometimes these low-frequency bands can also be seen in patients with levodopa-induced dyskinesias153. The higher frequency band or the gamma band (30–90 Hz) has been found to correlate with levodopa-induced dyskinesia. A recently published study from 2025 examined finely tuned gamma (FTG) activity that has two distinct spectral patterns: a levodopa-induced signal (70–90 Hz) and a stimulation-entrained signal at half the DBS frequency (e.g., 62.5 Hz with 125 Hz stimulation). In this study, FTG recorded over several weeks from the implanted leads in the STN using the Percept™ PC system demonstrated a reliable correlation with dyskinesia, as measured by wearable sensors and patient-reported diaries154. Another study found that cross-frequency interactions between FTG oscillations and the stimulation frequency may facilitate dynamic neural processing that contributes to motor symptom relief within the targeted network155. The use of sensing technology to capture various LFPs may help guide optimal electrode contact selection. While sensing technology marks a significant advance, it comes with limitations. LFP peaks may lack disease and symptom specificity; beta peaks can appear in both PD and essential tremor, while theta activity may relate to tremor or tics. There may be challenges related to the low sampling rate for the device, and the difficulties in interpretation because of stimulation, movement, or EKG artifacts156. Finally, long-term LFP monitoring may drain battery life, however the use of rechargeable devices could mitigate this challenge.
Adaptive DBS (aDBS), which adjusts therapy in real time based on physiological signals, offers more efficient symptom control with fewer side effects by aligning stimulation with the patient’s clinical state and avoiding unnecessary stimulation. While steering technology enhances spatial precision, aDBS provides temporal precision. These physiological signals can be recorded not only from DBS electrodes but also through electrocorticography from cortical regions157,158, as well as peripherally using wearable sensors (especially tremor)159. Most studies to date have involved LFPs recorded from the STN. Although visualization of the LFP peaks may not be possible in all individuals, in the ADAPT-PD trial (n = 68) that enrolled both STN and GPi DBS receiving Medtronic Percept™ PC system usable LFP signals (8–30 Hz, ≥ 1.2 μVp) were identified in 84.8% of patients on medication (65% bilateral) and 92% off medication (78% bilateral)160. Fast adaptation may better manage bradykinesia and speech effects, while slow adaptation may be more effective for dyskinesias161,162. Though still evolving, growing clinical evidence supports aDBS as a promising strategy to improve outcomes, reduce adverse effects, and conserve device energy. PD aDBS studies that used beta band as the feedback signal and published their final results are illustrated in Table 3. While no major side effects were reported, these studies have involved small samples of STN DBS with limited follow-up158,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176. It should be noted that these studies mostly used a broader beta band as the feedback signal for aDBS. Some consider that low beta band (13–20 Hz) may reveal a better correlation with PD motor features and some experts advocate using the individual beta peak or beta-related movement-modulation frequency as a critical feedback signal, as even slight deviations from the selected target frequency can significantly alter the timing of stimulation onset in aDBS177.
Another important point for aDBS is that the rapid shift in voltage or frequency driven by the feedback signal could be perceived to be uncomfortable or intolerable for patients receiving chronic stimulation. In the ADAPT-PD trial, two beta-driven aDBS modes driven by fast and slow changes in beta power were tested: single threshold, which adjusted stimulation rapidly (within 250 ms), and dual threshold, which changed stimulation more gradually (ramping up over 2.5 min, ramping down over 5 min). The study found that even though at least one mode was reported to be acceptable (88% of patients) as they received aDBS over a period of 60 days, more patients showed a preference for the slower dual threshold mode178.
With the FDA approval of the BrainSense™ aDBS system in early 2025 (some countries like Japan had aDBS available earlier), clinicians can now implement automatic stimulation adjustments based on real-time beta signal feedback for managing PD symptoms. The new system can trigger DBS even when brain rhythm feedback arises from the contralateral STN or GPi, offering greater flexibility in sensing and stimulation without the need to restrict stimulation to middle-level contacts of the lead. The FDA also approved the ‘Electrode Identifier’ tool, which identifies optimal electrodes using a monopolar survey of beta signals, an upgrade from the bipolar survey in the Percept system. This advancement is expected to significantly reduce DBS programming time.
A recent Delphi consensus panel concluded that while aDBS shows promise, it still requires skilled clinicians and further algorithm refinement to address complex PD symptoms179. The panel agreed on its safety and potential to provide faster, more stable symptom control than conventional DBS, particularly for patients with tremor-dominant PD, motor fluctuations, and dyskinesias. Echoing the evolution of cardiac pacing from fixed-rate to on-demand in the 1980s, the panel concluded that aDBS is on a similar path. However, questions remain about whether aDBS can induce neuroplastic changes or mitigate habituation (gradual loss of benefits over time). The panel opined that widespread adoption into routine clinical use will likely need more time as evidence continues to accumulate and the clinical infrastructure and training needed to support aDBS are developed.
Summary
Selecting PD patients for DBS requires a thorough assessment by a multidisciplinary team to evaluate risks, benefits, and establish appropriate goals. Biological age deserves more consideration than chronological age, especially with the growing elderly population, longer lifespans, and the increasing acceptance of DBS therapy. Women and minorities will need greater support structure and counselling. Genetics and nonmotor symptoms should be given due consideration when selecting patients. With regards to target selection, STN and GPi each offer unique advantages. The emergence of multiple DBS manufacturers has accelerated technological advancements. However, the final selection of hardware is based on patient-specific considerations, clinician preferences, MRI compatibility, accessibility and affordability. Traditional DBS programming methods are time-consuming and imprecise, sometimes leading to excessive stimulation and unwanted side effects. New systems provide greater flexibility in parameter selection and stimulation configurations. Physiology-guided programming enhances precision, while image-guided programming facilitates targeted stimulation delivery in less time. Advances in machine learning and artificial intelligence have introduced adaptive and automated programming tools, optimizing treatment outcomes by delivering energy-efficient stimulation.
References
Fasano, A., Daniele, A. & Albanese, A. Treatment of motor and non-motor features of Parkinson’s disease with deep brain stimulation. Lancet Neurol. 11, 429–442 (2012).
Wagle Shukla, A. & Okun, M. S. Surgical treatment of Parkinson’s disease: patients, targets, devices, and approaches. Neurotherapeutics 11, 47–59 (2014).
Deuschl, G. et al. European Academy of Neurology/Movement Disorder Society—European Section guideline on the treatment of Parkinson’s disease: I. Invasive therapies. Eur. J. Neurol. 29, 2580–2595 (2022).
Antonini, A. et al. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: a multi-country Delphi-panel approach. Curr. Med. Res. Opin. 34, 2063–2073 (2018).
Siddiqui, J. et al. Rationale and patient selection for interventional therapies in Parkinson’s disease. Expert Rev. Neurother. 18, 811–823 (2018).
Auffret, M., Weiss, D., Stocchi, F., Vérin, M. & Jost, W. H. Access to device-aided therapies in advanced Parkinson’s disease: navigating clinician biases, patient preference, and prognostic uncertainty. J. Neural Transm. (Vienna) 130, 1411–1432 (2023).
Deuschl, G. et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med. 355, 896–908 (2006).
Weaver, F. M. et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 301, 63–73 (2009).
Follett, K. A. et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N. Engl. J. Med. 362, 2077–2091 (2010).
Williams, A. et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol. 9, 581–591 (2010).
Okun, M. S. et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson’s disease: an open-label randomised controlled trial. Lancet Neurol. 11, 140–149 (2012).
Odekerken, V. J. et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurol. 12, 37–44 (2013).
Schuepbach, W. M. et al. Neurostimulation for Parkinson’s disease with early motor complications. N. Engl. J. Med. 368, 610–622 (2013).
Vitek, J. L. et al. Subthalamic nucleus deep brain stimulation with a multiple independent constant current-controlled device in Parkinson’s disease (INTREPID): a multicentre, double-blind, randomised, sham-controlled study. Lancet Neurol. 19, 491–501 (2020).
Wagle Shukla, A. & Okun, M. S. State of the art for deep brain stimulation therapy in movement disorders: a clinical and technological perspective. IEEE Rev. Biomed. Eng. 9, 219–233 (2016).
Ory-Magne, F. et al. Does ageing influence deep brain stimulation outcomes in Parkinson’s disease?. Mov. Disord. 22, 1457–1463 (2007).
Du, T. et al. The effect of age and disease duration on the efficacy of subthalamic nuclei deep brain stimulation in Parkinson’s disease patients. CNS Neurosci. Ther. 28, 2163–2171 (2022).
DeLong, M. R. et al. Effect of advancing age on outcomes of deep brain stimulation for Parkinson disease. JAMA Neurol. 71, 1290–1295 (2014).
Abraham, D. S. et al. Sex differences in Parkinson’s disease presentation and progression. Parkinsonism Relat. Disord. 69, 48–54 (2019).
Subramanian, I. et al. Unmet needs of women living with parkinson’s disease: gaps and controversies. Mov. Disord. 37, 444–455 (2022).
Dalrymple, W. A. et al. Comparison of Parkinson’s disease patients’ characteristics by indication for deep brain stimulation: men are more likely to have DBS for tremor. Tremor Other Hyperkinet Mov (N Y) 9 (2019).
Maccarrone, G. et al. Gender disparity in access to advanced therapies for patients with Parkinson’s disease: a retrospective real-word study. Front. Neurol. 15, 1429251 (2024).
Jost, S. T. et al. Gender gap in deep brain stimulation for Parkinson’s disease. NPJ Parkinsons Dis. 8, 47 (2022).
Willis, A. W. et al. Disparities in deep brain stimulation surgery among insured elders with Parkinson disease. Neurology 82, 163–171 (2014).
Shpiner, D. S. et al. Gender disparities in deep brain stimulation for Parkinson’s disease. Neuromodulation 22, 484–488 (2019).
Jimenez-Shahed, J. et al. Association of patient characteristics, social drivers of health, and geographic location on access to device-aided therapies among medicare beneficiaries with advanced Parkinson’s disease. Parkinsonism Relat. Disord. 133, 107322 (2025).
Hendriks, M., Vinke, R. S. & Georgiev, D. Gender discrepancies and differences in motor and non-motor symptoms, cognition, and psychological outcomes in the treatment of Parkinson’s disease with subthalamic deep brain stimulation. Front. Neurol. 14, 1257781 (2023).
Memon, A. A. et al. A systematic review of health disparities research in deep brain stimulation surgery for Parkinson’s disease. Front. Hum. Neurosci. 17, 1269401 (2023).
Venkatraman, V. et al. Disparities in the treatment of movement disorders using deep brain stimulation. J. Neurosurg. 141, 241–251 (2024).
Currie, A. D. et al. The role of a social worker in the deep brain stimulation preoperative evaluation: the DBS-FACTS screening tool. Mov. Disord. Clin. Pract. https://doi.org/10.1002/mdc3.70026 (2025).
Defer, G. L., Widner, H., Marié, R. M., Rémy, P. & Levivier, M. Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD). Mov. Disord. 14, 572–584 (1999).
Merello, M., Nouzeilles, M. I., Arce, G. P. & Leiguarda, R. Accuracy of acute levodopa challenge for clinical prediction of sustained long-term levodopa response as a major criterion for idiopathic Parkinson’s disease diagnosis. Mov. Disord. 17, 795–798 (2002).
Deuschl, G. et al. Comparing two randomized deep brain stimulation trials for Parkinson’s disease. J. Neurosurg. 132, 1376–1384 (2019).
Kleiner-Fisman, G. et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov. Disord. 21, S290–S304 (2006).
Artusi, C. A., Lopiano, L. & Morgante, F. Deep brain stimulation selection criteria for parkinson’s disease: time to go beyond CAPSIT-PD. J. Clin. Med. 9 (2020).
Fasano, A., Aquino, C. C., Krauss, J. K., Honey, C. R. & Bloem, B. R. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat. Rev. Neurol. 11, 98–110 (2015).
Wolke, R. et al. The role of Levodopa challenge in predicting the outcome of subthalamic deep brain stimulation. Mov. Disord. Clin. Pr. 10, 1181–1191 (2023).
Zampogna, A. et al. Disentangling Bradykinesia and rigidity in Parkinson’s disease: evidence from short- and long-term subthalamic nucleus deep brain stimulation. Ann. Neurol. 96, 234–246 (2024).
Bryant, M. S. et al. Gait variability in Parkinson’s disease: influence of walking speed and dopaminergic treatment. Neurol. Res. 33, 959–964 (2011).
Kim, R. et al. Long-term effect of subthalamic nucleus deep brain stimulation on freezing of gait in Parkinson’s disease. J. Neurosurg. 131, 1797–1804 (2019).
Barbe, M. T. et al. Deep Brain Stimulation for Freezing of Gait in Parkinson’s Disease With Early Motor Complications. Mov. Disord. 35, 82–90 (2020).
Di Rauso, G. et al. Freezing of gait in Parkinson’s disease patients treated with bilateral subthalamic nucleus deep brain stimulation: a long-term overview. Biomedicines 10 (2022).
Artusi, C. A. et al. Subthalamic deep brain stimulation and trunk posture in Parkinson’s disease. Acta Neurol. Scand. 137, 481–487 (2018).
Roediger, J. et al. Effect of subthalamic deep brain stimulation on posture in Parkinson’s disease: A blind computerized analysis. Parkinsonism Relat. Disord. 62, 122–127 (2019).
Silva de Lima, A. L. et al. Freezing of gait and fall detection in Parkinson’s disease using wearable sensors: a systematic review. J. Neurol. 264, 1642–1654 (2017).
Zampogna, A. et al. Axial impairment and falls in Parkinson’s disease: 15 years of subthalamic deep brain stimulation. NPJ Parkinsons Dis. 8, 121 (2022).
Gessani, A. et al. Long-term effects of subthalamic nucleus deep brain stimulation on speech in Parkinson’s disease. Sci. Rep. 13, 11462 (2023).
Chiu, S. Y. et al. Dysarthria and speech intelligibility following parkinson’s disease globus pallidus internus deep brain stimulation. J. Parkinsons Dis. 10, 1493–1502 (2020).
Tabari, F. et al. Speech, voice, and language outcomes following deep brain stimulation: a systematic review. PLoS ONE 19, e0302739 (2024).
van Brenk, F. et al. No differential effects of subthalamic nucleus vs. globus pallidus deep brain stimulation in Parkinson’s disease: Speech acoustic and perceptual findings. IBRO Neurosci. Rep. 16, 361–367 (2024).
Diaz, A. P. et al. Variables associated with physical health-related quality of life in Parkinson’s disease patients presenting for deep brain stimulation. Neurol. Sci. 37, 1831–1837 (2016).
Dafsari, H. S. et al. Nonmotor symptoms evolution during 24 months of bilateral subthalamic stimulation in Parkinson’s disease. Mov. Disord. 33, 421–430 (2018).
Gronostay, A. et al. Stratifying quality of life outcome in subthalamic stimulation for Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry (2023).
Geraedts, V. J., Feleus, S., Marinus, J., van Hilten, J. J. & Contarino, M. F. What predicts quality of life after subthalamic deep brain stimulation in Parkinson’s disease? A systematic review. Eur. J. Neurol. 27, 419–428 (2020).
Paschen, S. & Deuschl, G. Patient evaluation and selection for movement disorders surgery: the changing spectrum of indications. Prog. Neurol. Surg. 33, 80–93 (2018).
Lhommee, E. et al. Behavioural outcomes of subthalamic stimulation and medical therapy versus medical therapy alone for Parkinson’s disease with early motor complications (EARLYSTIM trial): secondary analysis of an open-label randomised trial. Lancet Neurol. 17, 223–231 (2018).
Ricciardi, L., Sorbera, C., Barbuto, M. & Morgante, F. Sleep disturbances are mainly improved by deep brain stimulation of the subthalamic nucleus. Mov. Disord. 34, 154–155 (2019).
Jost, S. T. et al. A prospective, controlled study of non-motor effects of subthalamic stimulation in Parkinson’s disease: results at the 36-month follow-up. J. Neurol. Neurosurg. Psychiatry 91, 687–694 (2020).
Geraedts, V. J. et al. Selecting candidates for Deep Brain Stimulation in Parkinson’s disease: the role of patients’ expectations. Parkinsonism Relat. Disord. 66, 207–211 (2019).
Mameli, F. et al. Role of expectations in clinical outcomes after deep brain stimulation in patients with Parkinson’s disease: a systematic review. J. Neurol. 270, 5274–5287 (2023).
Artusi, C. A. et al. Association of subthalamic deep brain stimulation with motor, functional, and pharmacologic outcomes in patients with monogenic parkinson disease: a systematic review and meta-analysis. JAMA Netw. Open 2, e187800 (2019).
De Oliveira, L. M. et al. Deep brain stimulation in patients with mutations in Parkinson’s disease-related genes: a systematic review. Mov. Disord. Clin. Pr. 6, 359–368 (2019).
Kuusimäki, T. et al. Deep brain stimulation for monogenic Parkinson’s disease: a systematic review. J. Neurol. 267, 883–897 (2020).
Gómez-Esteban, J. C. et al. Outcome of bilateral deep brain subthalamic stimulation in patients carrying the R1441G mutation in the LRRK2 dardarin gene. Neurosurgery 62, 857–862 (2008).
Mangone, G. et al. Early cognitive decline after bilateral subthalamic deep brain stimulation in Parkinson’s disease patients with GBA mutations. Parkinsonism Relat. Disord. 76, 56–62 (2020).
Avenali, M. et al. Are patients with GBA-Parkinson disease good candidates for deep brain stimulation? A longitudinal multicentric study on a large Italian cohort. J. Neurol. Neurosurg. Psychiatry 95, 309–315 (2024).
Cilia, R. et al. Survival and dementia in GBA-associated Parkinson’s disease: the mutation matters. Ann. Neurol. 80, 662–673 (2016).
Pal, G. et al. Parkinson disease and subthalamic nucleus deep brain stimulation: cognitive effects in GBA mutation carriers. Ann. Neurol. 91, 424–435 (2022).
Lohmann, E. et al. Are Parkinson's patients particularly suited for deep-brain stimulation?. Mov. Disord. 23, 740–743 (2008).
Kim, H. J. et al. Parkin mutation and deep brain stimulation outcome. J. Clin. Neurosci. 21, 107–110 (2014).
Prendes Fernández, P. et al. Analysis of deep brain stimulation of the subthalamic nucleus (STN-DBS) in patients with monogenic PRKN and LRRK2 forms of Parkinson’s disease. Parkinsonism Relat. Disord. 107, 105282 (2023).
Cabrera, L. Y., Goudreau, J. & Sidiropoulos, C. Critical appraisal of the recent US FDA approval for earlier DBS intervention. Neurology 91, 133–136 (2018).
Mestre, T. A. et al. Subthalamic nucleus-deep brain stimulation for early motor complications in Parkinson’s disease-the EARLYSTIM trial: early is not always better. Mov. Disord. 29, 1751–1756 (2014).
Schupbach, W. M. et al. Myths and facts about the EARLYSTIM study. Mov. Disord. 29, 1742–1750 (2014).
Pollak, P. et al. [Effects of the stimulation of the subthalamic nucleus in Parkinson disease]. Rev. Neurol. (Paris) 149, 175–176 (1993).
Blomstedt, P. & Hariz, M. I. Deep brain stimulation for movement disorders before DBS for movement disorders. Parkinsonism Relat. Disord. 16, 429–433 (2010).
Siegfried, J. & Lippitz, B. Bilateral chronic electrostimulation of ventroposterolateral pallidum: a new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery 35, 1126–1129.
Limousin, P. et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N. Engl. J. Med. 339, 1105–1111 (1998).
Odekerken, V. J. et al. GPi vs STN deep brain stimulation for Parkinson disease: Three-year follow-up. Neurology 86, 755–761 (2016).
Krack, P., Fraix, V., Mendes, A., Benabid, A. L. & Pollak, P. Postoperative management of subthalamic nucleus stimulation for Parkinson’s disease. Mov. Disord. 17, S188–S197 (2002).
Pirker, W., Katzenschlager, R., Hallett, M. & Poewe, W. Pharmacological treatment of tremor in Parkinson’s disease revisited. J. Parkinsons Dis. 13, 127–144 (2023).
Wong, J. K. et al. STN vs. GPi deep brain stimulation for tremor suppression in Parkinson disease: a systematic review and meta-analysis. Parkinsonism Relat. Disord. 58, 56–62 (2019).
Wong, J. K. et al. STN versus GPi deep brain stimulation for action and rest tremor in Parkinson’s disease. Front. Hum. Neurosci. 14, 578615 (2020).
Cury, R. G. et al. Thalamic deep brain stimulation for tremor in Parkinson disease, essential tremor, and dystonia. Neurology 89, 1416–1423 (2017).
Azghadi, A., Rajagopal, M. M., Atkinson, K. A. & Holloway, K. L. Utility of GPI+VIM dual-lead deep brain stimulation for Parkinson’s disease patients with significant residual tremor on medication. J. Neurosurg. 136, 1364–1370 (2022).
Martinez-Ramirez, D., Hu, W., Bona, A. R., Okun, M. S. & Wagle Shukla, A. Update on deep brain stimulation in Parkinson’s disease. Transl. Neurodegener. 4, 12 (2015).
Blomstedt, P. et al. Unilateral caudal zona incerta deep brain stimulation for Parkinsonian tremor. Parkinsonism Relat. Disord. 18, 1062–1066 (2012).
Honey, C. R. et al. Deep brain stimulation target selection for Parkinson’s disease. Can. J. Neurol. Sci. 44, 3–8 (2017).
Mainardi, M. et al. Deep brain stimulation of globus pallidus internus and subthalamic nucleus in Parkinson’s disease: a multicenter, retrospective study of efficacy and safety. Neurol. Sci. 45, 177–185 (2024).
Cury, R. G., Pavese, N., Aziz, T. Z., Krauss, J. K. & Moro, E. Gaps and roadmap of novel neuromodulation targets for treatment of gait in Parkinson’s disease. NPJ Parkinsons Dis. 8, 8 (2022).
Thevathasan, W. et al. Pedunculopontine nucleus deep brain stimulation in Parkinson’s disease: a clinical review. Mov. Disord. 33, 10–20 (2018).
Zrinzo, L. & Zrinzo, L. V. Surgical anatomy of the pedunculopontine and peripeduncular nuclei. Br. J. Neurosurg. 22, S19–S24 (2008).
Thevathasan, W. et al. Pedunculopontine nucleus stimulation improves gait freezing in Parkinson disease. Neurosurgery 69, 1248–1253 (2011).
Welter, M. L. et al. PPNa-DBS for gait and balance disorders in Parkinson’s disease: a double-blind, randomised study. J. Neurol. 262, 1515–1525 (2015).
Weiss, D. et al. Nigral stimulation for resistant axial motor impairment in Parkinson’s disease? A randomized controlled trial. Brain 136, 2098–2108 (2013).
Valldeoriola, F. et al. Simultaneous low-frequency deep brain stimulation of the substantia nigra pars reticulata and high-frequency stimulation of the subthalamic nucleus to treat levodopa unresponsive freezing of gait in Parkinson’s disease: A pilot study. Parkinsonism Relat. Disord. 60, 153–157 (2019).
Gratwicke, J. et al. Bilateral deep brain stimulation of the nucleus basalis of Meynert for Parkinson Disease Dementia: A Randomized Clinical Trial. JAMA Neurol. 75, 169–178 (2018).
Bogdan I. D. et al. Serendipitous stimulation of nucleus basalis of Meynert—the effect of unintentional, long-term high-frequency stimulation on cognition in Parkinson’s disease. J Clin Med. 11. 2022
Sasikumar, S. et al. Single-trajectory multiple-target deep brain stimulation for parkinsonian mobility and cognition. Mov. Disord. 37, 635–640 (2022).
Cappon, D. et al. Deep brain stimulation of the nucleus basalis of meynert for Parkinson’s disease dementia: a 36 months follow up study. Mov. Disord. Clin. Pr. 9, 765–774 (2022).
Liu, Y. et al. Predicting motor outcome of subthalamic nucleus deep brain stimulation for Parkinson’s disease using quantitative susceptibility mapping and radiomics: a pilot study. Front. Neurosci. 15, 731109 (2021).
Rijks, N. et al. Combining 7T T2 and 3T FGATIR: from physiological to anatomical identification of the subthalamic nucleus borders. J. Neurol. Neurosurg. Psychiatry 93, 1019–1020 (2022).
Patriat, R. et al. Individualized tractography-based parcellation of the globus pallidus pars interna using 7T MRI in movement disorder patients prior to DBS surgery. Neuroimage 178, 198–209 (2018).
Shih, Y. C., Tseng, W. I. & Montaser-Kouhsari, L. Recent advances in using diffusion tensor imaging to study white matter alterations in Parkinson’s disease: A mini review. Front Aging Neurosci. 14, 1018017 (2022).
Li, Y. et al. Mapping motor pathways in Parkinson’s disease patients with subthalamic deep brain stimulator: a diffusion MRI tractography study. Neurol. Ther. 11, 659–677 (2022).
Deuter, D. et al. Amelioration of Parkinsonian tremor evoked by DBS: which role play cerebello-(sub)thalamic fiber tracts?. J. Neurol. 271, 1451–1461 (2024).
Abdulbaki, A., Kaufmann, J., Galazky, I., Buentjen, L. & Voges, J. Neuromodulation of the subthalamic nucleus in Parkinson’s disease: the effect of fiber tract stimulation on tremor control. Acta Neurochir. (Wien.) 163, 185–195 (2021).
Jones, J. D. et al. Cognitive outcomes for essential tremor patients selected for thalamic deep brain stimulation surgery through interdisciplinary evaluations. Front Hum. Neurosci. 14, 578348 (2020).
Thompson, J. A., Lanctin, D., Ince, N. F. & Abosch, A. Clinical implications of local field potentials for understanding and treating movement disorders. Stereotact. Funct. Neurosurg. 92, 251–263 (2014).
Thompson, J. A. et al. Semi-automated application for estimating subthalamic nucleus boundaries and optimal target selection for deep brain stimulation implantation surgery. J. Neurosurg. 130, 1224–1233 (2018).
Maroufi, S. F. et al. Awake versus asleep deep brain stimulation for Parkinson’s disease: a comprehensive systematic review and meta-analysis. J. Neurosurg. 142, 324–338 (2025).
Li, L., Rae, A. I. & Burchiel, K. J. A meta-analysis of medication reduction and motor outcomes after awake versus asleep deep brain stimulation for Parkinson disease. Neurosurgery 96, 481–493 (2025).
Huang, P. H., Pan, Y. S., Chen, S. Y. & Lin, S. H. Anesthetic effect on the subthalamic nucleus in microelectrode recording and local field potential of Parkinson’s disease. Neuromodulation 28, 414–424 (2025).
Krauss, J. K. et al. Technology of deep brain stimulation: current status and future directions. Nat. Rev. Neurol. 17, 75–87 (2021).
Sarica, C. et al. Implantable pulse generators for deep brain stimulation: challenges, complications, and strategies for practicality and longevity. Front Hum. Neurosci. 15, 708481 (2021).
Paff, M., Loh, A., Sarica, C., Lozano, A. M. & Fasano, A. Update on current technologies for deep brain stimulation in Parkinson’s disease. J. Mov. Disord. 13, 185–198 (2020).
Merola, A. et al. New frontiers for deep brain stimulation: directionality, sensing technologies, remote programming, robotic stereotactic assistance, asleep procedures, and connectomics. Front Neurol. 12, 694747 (2021).
Soh, D. et al. Flexible vs. standard subthalamic stimulation in Parkinson disease: a double-blind proof-of-concept cross-over trial. Parkinsonism Relat. Disord. 89, 93–97 (2021).
Rebelo, P. et al. Thalamic directional deep brain stimulation for tremor: spend less, get more. Brain Stimul. 11, 600–606 (2018).
Neumann, W. J., Köhler, R. M. & Kühn, A. A. A practical guide to invasive neurophysiology in patients with deep brain stimulation. Clin. Neurophysiol. 140, 171–180 (2022).
Hitti, F. L., Vaughan, K. A., Ramayya, A. G., McShane, B. J. & Baltuch, G. H. Reduced long-term cost and increased patient satisfaction with rechargeable implantable pulse generators for deep brain stimulation. J. Neurosurg. 131, 799–806 (2018).
Thrane, J. F., Sunde, N. A., Bergholt, B. & Rosendal, F. Increasing infection rate in multiple implanted pulse generator changes in movement disorder patients treated with deep brain stimulation. Stereotact. Funct. Neurosurg. 92, 360–364 (2014).
Fytagoridis, A. et al. Surgical replacement of implantable pulse generators in deep brain stimulation: adverse events and risk factors in a multicenter cohort. Stereotact. Funct. Neurosurg. 94, 235–239 (2016).
Jakobs, M. et al. A multicenter, open-label, controlled trial on acceptance, convenience, and complications of rechargeable internal pulse generators for deep brain stimulation: the Multi Recharge Trial. J. Neurosurg. 133, 821–829 (2020).
Jakobs, M. et al. A multicenter, open-label, controlled trial on acceptance, convenience, and complications of rechargeable internal pulse generators for deep brain stimulation: the Multi Recharge Trial. J. Neurosurg. 133, 821–829 (2019).
Qiu, X. et al. Fixed-life or rechargeable battery for deep brain stimulation: preference and satisfaction in Chinese patients with Parkinson’s disease. Front Neurol. 12, 668322 (2021).
Wolf, M. E. et al. Implementation of new technology in patients with chronic deep brain stimulation: switching from non-rechargeable constant voltage to rechargeable constant current stimulation. Stereotact. Funct. Neurosurg. 97, 362–368 (2019).
Jakobs, M., Kloß, M., Unterberg, A. & Kiening, K. Rechargeable internal pulse generators as initial neurostimulators for deep brain stimulation in patients with movement disorders. Neuromodulation 21, 604–610 (2018).
Falowski, S., Safriel, Y., Ryan, M. P. & Hargens, L. The rate of magnetic resonance imaging in patients with deep brain stimulation. Stereotact. Funct. Neurosurg. 94, 147–153 (2016).
Picillo, M., Lozano, A. M., Kou, N., Puppi Munhoz, R. & Fasano, A. Programming deep brain stimulation for Parkinson’s disease: the Toronto Western Hospital Algorithms. Brain Stimul. 9, 425–437 (2016).
Wagle Shukla, A., Zeilman, P., Fernandez, H., Bajwa, J. A. & Mehanna, R. DBS Programming: an evolving approach for patients with Parkinson’s disease. Parkinsons Dis. 2017, 8492619 (2017).
Fabbri, M. et al. Deep brain stimulation fine-tuning in Parkinson’s disease: Short pulse width effect on speech. Parkinsonism Relat. Disord. 87, 130–134 (2021).
Dayal, V. et al. Novel programming features help alleviate subthalamic nucleus stimulation-induced side effects. Mov. Disord. 35, 2261–2269 (2020).
Aquino, C. C. et al. Interleaved deep brain stimulation for dyskinesia management in Parkinson’s disease. Mov. Disord. 34, 1722–1727 (2019).
Patel, B. et al. Deep brain stimulation programming strategies: segmented leads, independent current sources, and future technology. Expert Rev. Med Devices 18, 875–891 (2021).
Munhoz, R. P. & Albuainain, G. Deep brain stimulation: new programming algorithms and teleprogramming. Expert Rev. Neurother. 23, 467–478 (2023).
Dembek, T. A. et al. Directional DBS increases side-effect thresholds—A prospective, double-blind trial. Mov. Disord. 32, 1380–1388 (2017).
Contarino, M. F. et al. Directional steering: a novel approach to deep brain stimulation. Neurology 83, 1163–1169 (2014).
Pollo, C. et al. Directional deep brain stimulation: an intraoperative double-blind pilot study. Brain 137, 2015–2026 (2014).
Steigerwald, F., Müller, L., Johannes, S., Matthies, C. & Volkmann, J. Directional deep brain stimulation of the subthalamic nucleus: a pilot study using a novel neurostimulation device. Mov. Disord. 31, 1240–1243 (2016).
Schnitzler, A. et al. Directional deep brain stimulation for parkinson’s disease: results of an international crossover study with randomized, double-blind primary endpoint. Neuromodulation 25, 817–828 (2022).
Constanthin, P. E., Zemzemi, N., Cuny, E. & Engelhardt, J. Comparison of two segmentation software tools for deep brain stimulation of the subthalamic and ventro-intermedius nuclei. Acta Neurochir. (Wien.) 165, 3397–3402 (2023).
Pourfar, M. H. et al. Model-based deep brain stimulation programming for parkinson’s disease: the GUIDE Pilot study. Stereotact. Funct. Neurosurg. 93, 231–239 (2015).
Pavese, N., Tai, Y. F., Yousif, N., Nandi, D. & Bain, P. G. Traditional trial and error versus neuroanatomic 3-dimensional image software-assisted deep brain stimulation programming in patients with Parkinson disease. World Neurosurg. 134, e98–e102 (2020).
Lange, F. et al. Reduced programming time and strong symptom control even in chronic course through imaging-based DBS programming. Front Neurol. 12, 785529 (2021).
Waldthaler, J. et al. Imaging-based programming of subthalamic nucleus deep brain stimulation in Parkinson’s disease. Brain Stimul. 14, 1109–1117 (2021).
Roediger, J. et al. StimFit-A data-driven algorithm for automated deep brain stimulation programming. Mov. Disord. 37, 574–584 (2022).
Meng, F. et al. Utilization, surgical populations, centers, coverages, regional balance, and their influential factors of deep brain stimulation for Parkinson’s disease: a large-scale multicenter cross-sectional study from 1997 to 2021. Int J. Surg. 109, 3322–3336 (2023).
Sharma, V. D. et al. Telemedicine and deep brain stimulation—current practices and recommendations. Parkinsonism Relat. Disord. 89, 199–205 (2021).
Jitkritsadakul, O., Rajalingam, R., Toenjes, C., Munhoz, R. P. & Fasano, A. Tele-health for patients with deep brain stimulation: The experience of the Ontario Telemedicine Network. Mov. Disord. 33, 491–492 (2018).
Marceglia, S. et al. Double-blind cross-over pilot trial protocol to evaluate the safety and preliminary efficacy of long-term adaptive deep brain stimulation in patients with Parkinson’s disease. BMJ Open 12, e049955 (2022).
Yin, Z. et al. Local field potentials in Parkinson’s disease: a frequency-based review. Neurobiol. Dis. 155, 105372 (2021).
Alonso-Frech, F. et al. Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson’s disease. Brain 129, 1748–1757 (2006).
Colombo A. et al. Finely tuned γ tracks medication cycles in Parkinson’s disease: an ambulatory brain-sense study. Mov. Disord. (2025).
Muthuraman, M. et al. Cross-frequency coupling between gamma oscillations and deep brain stimulation frequency in Parkinson’s disease. Brain 143, 3393–3407 (2020).
Wilkins, K. B., Melbourne, J. A., Akella, P. & Bronte-Stewart, H. M. Unraveling the complexities of programming neural adaptive deep brain stimulation in Parkinson’s disease. Front. Hum. Neurosci. 17, 1310393 (2023).
Cernera, S. et al. Sustained clinical benefit of adaptive deep brain stimulation in Parkinson’s disease using gamma oscillations: a case report. Mov. Disord. 40, 345–350 (2025).
Oehrn, C. R. et al. Chronic adaptive deep brain stimulation versus conventional stimulation in Parkinson’s disease: a blinded randomized feasibility trial. Nat. Med. 30, 3345–3356 (2024).
Ferleger, B. I. et al. Fully implanted adaptive deep brain stimulation in freely moving essential tremor patients. J. Neural Eng. 17, 056026 (2020).
Stanslaski, S. et al. Sensing data and methodology from the Adaptive DBS Algorithm for Personalized Therapy in Parkinson’s Disease (ADAPT-PD) clinical trial. NPJ Parkinsons Dis. 10, 174 (2024).
Little, S. et al. Adaptive deep brain stimulation for Parkinson’s disease demonstrates reduced speech side effects compared to conventional stimulation in the acute setting. J. Neurol. Neurosurg. Psychiatry 87, 1388–1389 (2016).
Rosa, M. et al. Adaptive deep brain stimulation controls levodopa-induced side effects in Parkinsonian patients. Mov. Disord. 32, 628–629 (2017).
Little, S. et al. Adaptive deep brain stimulation in advanced Parkinson disease. Ann. Neurol. 74, 449–457 (2013).
Rosa, M. et al. Adaptive deep brain stimulation in a freely moving Parkinsonian patient. Mov. Disord. 30, 1003–1005 (2015).
Little, S. et al. Bilateral adaptive deep brain stimulation is effective in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 87, 717–721 (2016).
Piña-Fuentes, D. et al. Adaptive DBS in a Parkinson’s patient with chronically implanted DBS: a proof of principle. Mov. Disord. 32, 1253–1254 (2017).
Arlotti, M. et al. Monitoring subthalamic oscillations for 24 h in a freely moving Parkinson’s disease patient. Mov. Disord. 34, 757–759 (2019).
Arlotti, M. et al. Eight-hours adaptive deep brain stimulation in patients with Parkinson disease. Neurology 90, e971–e976 (2018).
Swann, N. C. et al. Adaptive deep brain stimulation for Parkinson’s disease using motor cortex sensing. J. Neural Eng. 15, 046006 (2018).
Velisar, A. et al. Dual threshold neural closed loop deep brain stimulation in Parkinson disease patients. Brain Stimul. 12, 868–876 (2019).
Petrucci, M. N. et al. Neural closed-loop deep brain stimulation for freezing of gait. Brain Stimul. 13, 1320–1322 (2020).
Piña-Fuentes, D. et al. Acute effects of adaptive deep brain stimulation in Parkinson’s disease. Brain Stimul. 13, 1507–1516 (2020).
Bocci, T. et al. Eight-hours conventional versus adaptive deep brain stimulation of the subthalamic nucleus in Parkinson’s disease. NPJ Parkinsons Dis. 7, 88 (2021).
Gilron, R. et al. Long-term wireless streaming of neural recordings for circuit discovery and adaptive stimulation in individuals with Parkinson’s disease. Nat. Biotechnol. 39, 1078–1085 (2021).
He, S. et al. Beta-triggered adaptive deep brain stimulation during reaching movement in Parkinson’s disease. Brain 146, 5015–5030 (2023).
Busch, J. L. et al. Single threshold adaptive deep brain stimulation in Parkinson’s disease depends on parameter selection, movement state and controllability of subthalamic beta activity. Brain Stimul. 17, 125–133 (2024).
Alva, L. et al. Clinical neurophysiological interrogation of motor slowing: A critical step towards tuning adaptive deep brain stimulation. Clin. Neurophysiol. 152, 43–56 (2023).
Herrington, T. M. B. et al., H. Bronte-Stewart. Programming adaptive deep brain stimulation in the clinic: lessons from the ADAPT-PD Trial. Movement Disorders Society 2024.
Guidetti M. et al. Adaptive deep brain stimulation in Parkinson’s disease: a Delphi consensus study. medRxiv 2024.
Acknowledgements
There was no funding allocated for the review article
Author information
Authors and Affiliations
Contributions
A.W.S.: study concept and design, interpretation of data, critical revision of manuscript for intellectual content. M.B.: critical revision of manuscript for intellectual content. M.M.: critical revision of manuscript for intellectual content.
Corresponding author
Ethics declarations
Competing interests
A.W.S. reports grant support from the NIH R01NS122943 as PI and Ro1 NS121120-01 as a Co-I. She reports past funding from Benign Essential Blepharospasm Research foundation, Dystonia coalition, Dystonia Medical Research foundation, National Organization for Rare Disorders. AWS has received consultant fees from Merz, Jazz and Acadia. She is the current Vice President for the Tremor Research Group and recent advisor for Supernus, Biogen-Sage and Encora. AWS and MM are currently serving as the associate editors for Nature Parkinson’ disease journal.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wagle Shukla, A., Bange, M. & Muthuraman, M. Patient, target, device, and program selection for DBS in Parkinson’s disease: advancing toward precision care. npj Parkinsons Dis. 11, 195 (2025). https://doi.org/10.1038/s41531-025-01015-x
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41531-025-01015-x
This article is cited by
-
Real-world multicenter assessment of sustained clinical outcomes after digital deep brain stimulation
npj Digital Medicine (2026)
-
Decoding the impact of visual states on adaptive deep brain stimulation feedback signals in movement disorders
npj Parkinson's Disease (2026)
-
Motor and non-motor fluctuations in Parkinson’s disease: the knowns and unknowns of current therapeutic approaches
Journal of Neural Transmission (2026)