Abstract
Alpha-synuclein (αSyn) aggregates are pathognomonic of Parkinson’s disease (PD) and play a critical role in its pathogenesis. However, existing diagnostic approaches rely on invasive cerebrospinal fluid (CSF) sampling or tissue biopsies, limiting their accessibility and scalability in clinical practice. Here, we present the Constant Shake-Induced Conversion (CSIC) assay, a novel plasma-based technique for the detection of αSyn aggregates. A total of 102 participants, comprising 42 PD patients and 60 healthy controls (HCs), were enrolled. Plasma samples were subjected to CSIC and validated via αSyn depletion, enzyme-linked immunosorbent assay (ELISA), and Western blotting. Diagnostic performance was assessed using receiver operating characteristic (ROC) analysis, and clinical associations were examined using Spearman’s correlation. The CSIC assay achieved an area under the curve (AUC) of 0.91, with 81% sensitivity and 85% specificity in distinguishing PD from HCs. Assay specificity was confirmed through αSyn depletion, and reproducibility assessments yielded intra- and inter-assay coefficients of variation below 10% and ~20%, respectively. Notably, plasma αSyn aggregate levels correlated with Hoehn and Yahr (H&Y) stage (r = 0.69), Unified Parkinson’s Disease Rating Scale (UPDRS) (r = 0.68), and Montreal Cognitive Assessment scores (r = −0.47). These findings establish CSIC as a robust, non-invasive diagnostic method with strong potential for clinical implementation in PD.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder characterized by the progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc)1, leading to hallmark motor symptoms such as bradykinesia, rigidity, resting tremor, and postural instability. Among the key molecular mechanisms implicated in PD2,3, the misfolding and aggregation of alpha-synuclein (αSyn) is central to disease pathogenesis and is shared across multiple synucleinopathies, including dementia with Lewy bodies (DLB)4,5,6,7.
Despite decades of research, the diagnosis of PD remains largely clinical, relying on tools such as the Hoehn and Yahr (H&Y)8,9 staging scale and the Unified Parkinson’s Disease Rating Scale (UPDRS)10. While these tools are well-validated, they are limited by subjectivity and inter-rater variability. Neuroimaging techniques, such as dopamine transporter (DAT) positron emission tomography (PET), provide objective biomarkers and are commonly used to support the diagnosis of Parkinsonian syndromes. However, DAT imaging lacks specificity for synucleinopathies, as similar striatal uptake reductions are observed in other atypical Parkinsonian disorders such as multiple system atrophy (MSA) and DLB. Therefore, accurate clinical diagnosis of idiopathic PD remains essential, especially in research and early-stage disease contexts11.
To address the pressing need for objective and accessible biomarkers, several fluid-based approaches have been explored. ELISA-based assays for αSyn detection in cerebrospinal fluid (CSF) or blood show promise12,13, yet they suffer from limited diagnostic specificity due to overlapping values between PD patients and controls. Skin biopsies assessing phosphorylated αSyn (pS129) offer another diagnostic avenue but are invasive and somewhat technically demanding, which can compromise reproducibility14,15,16,17.
Recent advances in seed amplification assays (SAAs), particularly real-time quaking-induced conversion (RT-QuIC), have substantially improved sensitivity for detecting misfolded αSyn in CSF18,19. Nevertheless, the requirement for lumbar puncture and the lack of assay standardization present barriers to widespread clinical adoption. Plasma-based detection methods offer an attractive alternative; however, the high abundance of plasma proteins such as albumin (>99% of total protein) poses significant analytical challenges for detecting trace αSyn aggregates20,21.
To overcome these obstacles, we have developed the Constant Shake-Induced Conversion (CSIC) assay—a non-invasive, plasma-based seed amplification method optimized for sensitive detection of αSyn aggregates. This study aims to evaluate the diagnostic performance, analytical reliability, and clinical relevance of the CSIC assay in differentiating PD patients from healthy individuals, establishing a potential new paradigm for blood-based biomarker development in neurodegenerative disease.
Results
Development and optimization of the CSIC assay
αSyn aggregates are present at higher levels in PD patients compared to healthy controls (HCs), yet their detection in plasma remains challenging. Despite advances in SAAs, no existing method reliably distinguishes PD from HC based on plasma-derived αSyn aggregates. To address this, we developed the CSIC assay—a novel SAA method tailored to amplify αSyn aggregates in plasma while overcoming the limitations of existing approaches.
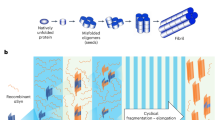
To ensure reagent quality, His-tagged αSyn was purified from Escherichia coli (E. coli), and its purity confirmed by liquid Chromatography with tandem mass spectrometry (LC-MS/MS) (Supplementary Fig. 1a, b), yielding highly pure monomeric αSyn. To optimize αSyn seed amplification conditions and determine the detection limit for the CSIC assay, we induced αSyn oligomerization using a thermomixer over 6 days. αSyn seed formation was verified using Coomassie Blue staining (Supplementary Fig. 2a) and Western blot analysis (Supplementary Fig. 2b). Our results indicated a significant increase in oligomeric αSyn from day 3 of incubation, with a notable reduction in monomeric αSyn by day 6, indicating successful oligomerization. To evaluate the seeding activity of synthetic αSyn seeds, we conducted an in vitro CSIC assay under buffer conditions. The results confirmed that the generated seeds possessed seeding activity, as shown in Supplementary Fig. 2c. Based on these findings, we established the CSIC assay for detecting and amplifying αSyn aggregates in plasma (Fig. 1a). Sensitivity testing revealed that the assay could detect as little as 1 fg of αSyn seed within 48 h in normal plasma (Supplementary Fig. 2d). Control reactions containing only monomeric αSyn failed to induce aggregation, confirming that signal amplification occurred specifically in the presence of preformed seeds.
a Schematic diagram illustrating the proposed CSIC assay. αSyn seeds present in blood are amplified through constant shaking. His-αSyn is used as the spiking material, and ThT fluorescence is measured to confirm the presence of amplified aggregates. b, c Dose-dependent αSyn aggregation kinetics measured by CSIC in normal plasma. Synthetic αSyn seeds at varying concentrations (1000 pg, 100 pg, 10 pg, 1 pg, 0.1 pg, and 0.01 pg) were spiked into normal plasma along with monomeric His-tagged αSyn. Each seed concentration was tested in duplicate (n = 2). The seed-free condition contained only monomeric His-αSyn. Colors indicate synuclein seed concentrations, ranging from 1000 pg (dark red) to 0.01 pg (gray). The black line represents the no-seed control. Aggregation kinetics were monitored in real time (b), with data shown as mean ± standard error of the mean (S.E.M.). T₅₀ was determined for each seed concentration (c).
To evaluate the quantitative capability of CSIC, we conducted a dose-response experiment using serial dilutions of synthetic αSyn seeds ranging from 1000 to 0.01 pg (Fig. 1b). The time to reach 50% of maximum Thioflavin T (ThT) fluorescence (T₅₀) showed a linear relationship with the logarithmic concentration of the seeds, demonstrating a clear dose-dependent response (R² = 0.9921) (Fig. 1c). These results indicate that the CSIC assay provides a robust and quantifiable signal across a dynamic range spanning at least three orders of magnitude.
Verification of CSIC specificity for αSyn aggregates
To ensure that the amplified signal observed when using the CSIC assay was due to αSyn present in plasma, we conducted αSyn depletion followed by αSyn amplification assays using the CSIC assay. αSyn was removed from plasma, and its depletion was confirmed using ELISA and Western blot analysis with immunoprecipitated samples (Fig. 2a). Both the HC and PD plasma samples exhibited lower αSyn levels after immunodepletion with ELISA (Fig. 2b), while Western blot analysis confirmed the precipitation of s αSyn in the immunoprecipitated samples using an anti-αSyn antibody (Fig. 2c). When CSIC was applied to plasma samples from which αSyn had been immunodepleted, no amplification was observed in the depleted PD samples (Fig. 2d). These results confirmed that the CSIC assay effectively amplified αSyn aggregates present in plasma, allowing for the distinction between PD and HC groups.
a Schematic representation of the experimental design. HC and PD plasma samples were subjected to αSyn depletion, followed by verification using b ELISA, c Western blotting, and d CSIC. b The αSyn-depleted supernatant was analyzed using sandwich ELISA to detect total αSyn. The red and blue bars represent PD and HC plasma, respectively. c αSyn was immunoprecipitated from HC and PD plasma samples using an anti-αSyn antibody (MJFR1). The presence of immunoprecipitated αSyn was verified by Western blotting using an anti-αSyn (211)-HRP antibody. The immunoprecipitated αSyn is indicated by an arrow. d CSIC was used to amplify αSyn aggregates in αSyn-depleted and untreated HC and PD plasma samples. The bar graph shows αSyn aggregates derived from CSIC. The red bar represents PD plasma, and the blue bar represents HC plasma. e CSIC was used to amplify αSyn aggregates in plasma samples from HCs (n = 3) and PD patients (n = 3). The red and blue lines represent amplified αSyn aggregates from PD and HC plasma, respectively. f Following CSIC, the final product was subjected to a sedimentation assay. The supernatant (sup) and pellet were separated, and equal volumes were analyzed using Western blotting. Immunoblotting was performed using an anti-αSyn (211)-HRP antibody. Arrows indicate the αSyn bands.
To further confirm that the ThT signal amplified by CSIC in plasma samples originated from αSyn aggregates, we performed sedimentation assays to separate high- and low-molecular-weight fractions, followed by immunoblotting with an anti-αSyn antibody22. CSIC-induced amplification was observed in PD plasma samples, while no signal was detected in HC plasma samples (Fig. 2e). Subsequent analysis of the pellet fraction confirmed αSyn oligomerization in PD plasma, whereas no oligomeric species were detected in HC plasma under the same experimental conditions (Fig. 2f). These results indicate that CSIC promotes αSyn oligomerization in PD plasma, leading to ThT signal amplification, and further support the specificity and diagnostic potential of this method for distinguishing PD patients from healthy individuals.
CSIC Assay: plasma αSyn aggregate levels and clinical correlations in PD
A total of 102 participants were included in the study, comprising 60 HCs and 42 patients with PD. Demographic and clinical characteristics are summarized in Table 1. The PD group was significantly older than HCs (mean age: 73.2 vs. 65.3 years, p < 0.001) and had a higher proportion of males (50.0% vs. 28.3%, p = 0.043). The mean disease duration in PD patients was 2.1 years. Cognitive performance, assessed using the MoCA, was significantly lower in the PD group (median score: 21.0) compared to HCs (median score: 27.4, p < 0.001). The median H&Y stage was significantly higher in PD patients than in HCs (2.1 vs. 0, p < 0.001), and the median UPDRS Part III motor score was also elevated in PD patients compared to HCs (16.5 vs. 0.3, p < 0.001).
To evaluate the diagnostic potential of the CSIC assay, we assessed its performance in distinguishing between PD patients and HCs. As presented in Fig. 3a, b, the CSIC assay achieved an AUC of 0.91, with a sensitivity of 81% and specificity of 85%, demonstrating its efficacy in differentiating PD patients from HCs.
a Box and whisker plots showing the range and average distribution of αSyn aggregates detected using CSIC. Statistical analysis was conducted using Mann-Whitney U tests due to the non-parametric distribution, with significant differences between the HC and PD groups at p < 0.0001 (****) indicated. b ROC curve of αSyn aggregates in accordance with CSIC parameters for discriminating patients with PD from HC. c Repeatability and reproducibility tests. Repeatability assays were conducted on plasma samples from healthy individuals (n = 3) and patients with PD (n = 3). Each sample was tested three times by three different experimenters to assess the reproducibility of the assay.
To test its technical robustness, we examined batch-to-batch variability. Three independent preparations of His-tagged αSyn were produced under identical conditions. Protein quality was confirmed by Coomassie blue staining and Western blotting (Supplementary Fig. 3a, b). CSIC assays with each batch (Supplementary Fig. 3c) consistently distinguished PD from HCs. Although minor signal differences were observed in a subset of samples depending on the protein lot, overall performance remained consistent across batches. We further tested repeatability and reproducibility. Three experimenters performed assays on plasma samples from three HCs and three PD patients, with each sample tested three times. The results showed a repeatability coefficient of variation (CV) of less than 10%, and a reproducibility of around 20% over a 48-h incubation period based on RFUs (Fig. 3c). These findings support the reliability of the CSIC assay across operators and experimental runs.
We assessed the association between αSyn aggregates detected by the CSIC assay and clinical variables using Spearman’s correlation analysis (Fig. 4a). This analysis was conducted in the same cohort described in Table 1 and used for the CSIC results shown in Fig. 3a. Significant correlations were observed between αSyn aggregate levels and clinical measures of motor and cognitive function in the PD group. Specifically, αSyn aggregate levels correlated with the H&Y stage (r = 0.69; Fig. 4b), the UPDRS Part III score (r = 0.68; Fig. 4c), and negatively with the MoCA score (r = −0.47; Fig. 4d). To address potential confounding by age and sex, we performed additional correlation analyses in a subset of participants matched for these demographic variables. The results remained consistent, with the H&Y stage showing r = 0.71, the UPDRS score r = 0.63, and the MoCA score r = −0.41 (Supplementary Fig. 4, Supplementary Table 1). These results suggest that αSyn aggregate levels measured using CSIC were correlated with both motor impairment and cognitive decline in PD. Collectively, these results highlight the association of the CSIC results with both motor and cognitive markers of PD, underscoring its potential as an accurate tool for evaluating disease severity and progression.
a Heatmap representation of spearman correlation coefficients (r) between CSIC and clinical scales. The correlation coefficients are color-coded as shown in the vertical bar. 0 indicates no linear relationship, +1/−1 indicates a perfect linear positive/negative relationship. Correlations between CSIC parameters and clinical scores: b H&Y, c UPDRS, and d MoCA. All r values were calculated using raw data.
Discussion
This study introduces the CSIC assay, a novel and robust tool for amplifying αSyn aggregates in plasma samples. Our findings demonstrate its potential for PD diagnosis and monitoring, supported by high sensitivity, specificity, and strong correlations with established clinical metrics.
The timeline for diagnosing PD varies depending on symptom onset23, healthcare accessibility, and medical expertise. Typically, the time from initial symptom emergence to confirmation spans several weeks to months24,25. In this context, the ability of the CSIC to enable early detection and optimize patient management is noteworthy.
We first successfully produced high-purity monomeric αSyn from E. coli to ensure reliable experimental outcomes via the establishment of a robust in vitro seeding protocol (Supplementary Fig. 1). The CSIC assay employs continuous shaking instead of cyclic agitation, facilitating reliable amplification of αSyn seeds under plasma conditions.
While CSF-based αSyn SAAs such as RT-QuIC have demonstrated diagnostic potential, their widespread use is limited by the invasive nature of CSF collection and associated costs18,19. The CSIC assay addresses these limitations by enabling sensitive aggregate detection in plasma—a more accessible biofluid.
The CSIC assay effectively distinguished αSyn aggregates between HC and PD patients, demonstrating its ability to detect as little as 1 fg of αSyn seeds in plasma within 48 h (Supplementary Fig. 2). High sensitivity and specificity were confirmed, with no amplification observed in monomeric αSyn samples. αSyn depletion experiments, validated through ELISA and Western blot analysis, further demonstrated that amplified signals in CSIC assays arose exclusively from αSyn aggregates present in plasma.
The CSIC assay demonstrated high sensitivity and specificity in distinguished αSyn aggregates between HC and PD patients, achieving a sensitivity of 81%, specificity of 85%, and an AUC of 0.91. The performance metrics of the CSIC assay surpass those of existing tools like ELISA and immunostaining, which struggle with limited sensitivity and specificity. Furthermore, repeatability and reproducibility tests demonstrated the robustness of the assay, with CV below 10% for repeatability and ~20% for reproducibility over a 48-h period.
We observed strong correlations between CSIC assay results and clinical markers of disease severity, highlighting its potential utility in distinguishing early PD from other Parkinsonian disorders and monitoring disease progression. αSyn aggregate levels positively correlated with the H&Y scale and UPDRS scores, and inversely with MoCA scores, consistent with previous findings linking αSyn pathology to both motor and cognitive impairment in PD18,19. These findings suggest that CSIC-derived αSyn levels may reflect disease progression and cognitive decline over time.
Among the optimization parameters, the quality of the spiking αSyn protein emerged as a critical determinant for reproducibility. This observation aligns with previous studies highlighting the role of protein quality in such assays. During the optimization of the αSyn seed amplification assay, we found that RPM, buffer composition, and αSyn lot quality were closely interconnected, with adjustments in one factor often requiring compensatory changes in others. Notably, buffer composition and shaking conditions had a significant impact on the amplification process, while the quality of αSyn spiking materials remained essential for consistent and accurate results.
These findings underscore the potential of CSIC as a non-invasive diagnostic tool, warranting further exploration of plasma αSyn aggregates for advancing diagnosis and therapeutic monitoring in PD and related disorders. The high sensitivity and specificity of the CSIC method suggest that it could be integrated into clinical practice, offering a more accessible and reliable diagnostic option for PD. Additionally, investigating the applicability of the CSIC method in other neurodegenerative diseases with αSyn pathology could expand its utility and impact in the field of neurology.
While this study demonstrates the diagnostic potential of the CSIC assay for detecting αSyn aggregates, several limitations constrain the interpretation and generalizability of the findings. The cohort analyzed was relatively small and demographically narrow, and, critically, did not include other major α-synucleinopathies such as DLB and MSA. Both DLB and MSA are characterized by pathological αSyn aggregation but differ from PD in clinical presentation, disease progression, and the anatomical distribution of aggregates. The absence of these disease groups makes it impossible to directly assess the disease specificity of the assay and limits conclusions regarding its discriminative power across the synucleinopathy spectrum. Although previous studies have reported that abundant blood components (e.g., albumin, lipoproteins) may interfere with αSyn aggregation dynamics—for instance, albumin’s chaperone-like inhibitory effects and lipoprotein-mediated modulation of fibrillization—their effects on the CSIC assay remain unexplored21,26,27. Investigating how these factors influence CSIC’s seeding detection could further refine assay robustness and diagnostic accuracy.
To improve the clinical applicability of the CSIC assay, several refinements are currently underway. These include efforts to reduce the overall assay duration, enhance the batch-to-batch consistency of His-tagged αSyn, and expand the sample size to support the establishment of a clinically meaningful diagnostic threshold. Although all tested protein batches consistently enabled differentiation between PD and HC samples, we observed minor variations in absolute ThT fluorescence signals. These subtle differences did not affect the overall diagnostic classification but may introduce slight variability in signal intensities for individual samples. To minimize such inter-batch variation and enhance assay reproducibility, we are currently standardizing protein production protocols and evaluating the inclusion of internal reference controls. These efforts are expected to improve signal consistency and contribute to the development of a more robust and reliable diagnostic platform.
Additionally, investigating the applicability of the CSIC assay in synucleinopathies beyond PD, such as DLB, and in Alzheimer’s disease (AD) cases with coexisting Lewy body pathology, represents an important direction for future studies28,29. Validating the assay with plasma samples from DLB and AD patients could help confirm its utility in detecting Lewy body pathology and provide insights into the broader impact of αSyn aggregates on cognitive decline and disease progression. Such advancements may enable early detection and preemptive interventions, ultimately improving disease management and patient quality of life.
In conclusion, the CSIC assay represents a significant advancement in non-invasive biomarker technology for PD. Its high sensitivity, specificity, and strong clinical correlations position it as a promising tool for early diagnosis and disease monitoring. By enabling plasma-based detection of αSyn aggregates, CSIC analysis provides a reliable, accessible, and scalable diagnostic alternative and requires further validation for broad clinical adoption.
Methods
Study participants
This study included 102 participants, consisting of 60 HCs and 42 PD patients. Participants were prospectively recruited between December 2022 and May 2024 at our medical center. The HCs were recruited through an IRB-approved announcement targeting volunteers from the community and caregivers of patients visiting the hospital. The inclusion criteria for the HCs were an age of 45 years or older, the absence of cognitive impairment, no motor or non-motor symptoms indicative of PD, and no history of degenerative brain diseases or significant neurological conditions. Of the 60 HCs, six individuals with mild hand tremors that were diagnosed with essential tremor and who had no 18F-FP-CIT PET abnormalities were included. Exclusion criteria included brain lesions causing motor or cognitive impairments observed on brain MRI, history of stroke, thyroid dysfunction, vitamin deficiencies, untreated syphilis, and severe physical conditions preventing clinical trial participation.
PD patients were diagnosed according to the UK Parkinson’s Disease Society Brain Bank Clinical Diagnostic Criteria1 and were aged 45 years or older. Exclusion criteria included suspected atypical Parkinsonism, vascular Parkinsonism, secondary Parkinsonism due to hydrocephalus on brain MRI, other neurological or psychiatric conditions, clinically significant abnormalities on brain MRI, severe white matter changes (FAZEKAS grade 3 or higher), or multiple lacunar infarcts (5 or more). Patients with unstable physical conditions preventing clinical trial participation, a history of severe mental illness, drug addiction, or alcohol abuse (3 or more drinks per day) within the past 10 years, or drug-induced Parkinsonism were also excluded.
Individuals with conditions making it difficult to obtain consent or for them to participate, such as severe cognitive impairment, implanted metallic devices or cochlear implants, or those undergoing treatment for malignant tumors, were excluded from both groups. This study was approved by the Institutional Review Board of Eunpyeong St. Mary’s Hospital (IRB PC22TISI0182). Written informed consent was obtained from all participants prior to enrollment, in accordance with the Declaration of Helsinki.
Imaging acquisition
Prior to the PET/ computed tomography (CT) scan, medications affecting DAT binding, including benzatropine, D-amphetamine, and methylphenidate, were discontinued. PET scans were conducted using a Biograph Vision 600 PET/CT scanner (Siemens Healthineers, Hoffman Estates, IL). Participants underwent a 10-min scan 3 h after an intravenous injection of 185 MBq (5.0 mCi) 18F-FP-CIT. CT was conducted for attenuation correction, followed immediately by static PET imaging. PET images were reconstructed using ultraHD technology (point spread function and time-of-flight) with eight iterations, five subsets, a matrix size of 440 × 440, and a voxel size of 0.82 × 0.82 × 1.64 mm. No post-filtering was employed.
Image analysis
PET images were visually assessed by two nuclear medicine physicians with 15 and 7 years of experience who were blind to the clinical information. Discrepancies were resolved through discussion. Visual interpretation was based on the pattern of DAT loss in the anterior and posterior putamen and caudate nucleus. For quantitative analysis, syngo.via MI Neurology software (Siemens Healthineers) was used. Spatial normalization was performed using an automated anatomical labeling atlas and a volume of interest (VOI) set. The distribution volume ratio was calculated for each VOI as the mean of the 75% brightest voxels within the target VOI divided by the mean uptake in the occipital lobe.
Materials
Mouse anti-αSyn (211)-horseradish peroxidase (HRP) antibody (Santa Cruz Biotechnology, Cat. No. sc-12767, Dallas, TX, USA) was used. Rabbit anti-αSyn MJFR1 (Abcam, Cat. No. ab138501, Cambridge, UK) and anti-αSyn 4B12 (BioLegend, Cat. No. 807803, San Diego, CA, USA) antibodies were obtained. An HRP conjugation kit (Lightning-Link®, Abcam, Cat. No. ab102890, Cambridge, UK) was purchased from Abcam. Recombinant full-length human His-αSyn (residues 1–140) was purified in-house. Standard chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise noted.
Immunoprecipitation and Western blot analysis
For the immunodepletion of αSyn, diluted plasma in CSIC buffer was mixed with αSyn antibody MJFR1 on a rocking platform for 3 h at 4 °C. Pre-washed protein A/G-Sepharose beads (Thermo Fisher, Cat. No. 20422) were added to the plasma and antibody pre-mixture and incubated overnight at 4 °C on a rocking platform. After incubation, the mixture was centrifuged at 1000 × g for 3 min at 4 °C. The supernatant was transferred to a new microcentrifuge tube for ELISA to confirm αSyn depletion. The beads were extensively washed three times with Immunoprecipitation washing buffer to obtain protein-antibody complexes. For Western blot analysis, samples were boiled in 1× sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (150 mM Tris-HCl, pH 8.45, 0.4% SDS, 11% glycerol, 1% β-mercaptoethanol, and 0.03% Coomassie blue G), followed by SDS-PAGE and Western blot analysis to determine the presence of αSyn.
ELISA
Each well was loaded with 100 μL for ELISA analysis. ELISA plates (Maxisorp; Nunc, Roskilde, Denmark) were coated overnight at 4 °C with a capture antibody (anti-αSyn MJFR1, 100 ng/well) in carbonate/bicarbonate buffer. The plates were washed three times with phosphate-buffered saline (PBS) using a Bio-Rad plate washer. Blocking was performed with 1% Block Ace in DW for 2 h at room temperature. Standards were prepared by diluting recombinant αSyn in PBS. The plates were washed again and incubated with standards or samples for 1 h at room temperature. After washing, the plates were incubated with a detection antibody (anti-αSyn 4B12, 50 ng/well) in 0.4% Block Ace in TBST for 1 h at room temperature. Following a final wash, relative luminescence units (RLUs) were developed using enhanced chemiluminescence (ECL) (Rockland, FEMTOMAX-110). The plates were read with a CLARIOstar Microplate Reader (BMG Labtech, Ortenberg, Germany).
In-Gel digestion with trypsin and extraction of peptides
Protein bands from SDS-PAGE gels were excised and in-gel digested with trypsin according to established procedures30. Protein bands were excised from the stained gels and cut into pieces. The gel pieces were washed for 1 h at room temperature in 25 mM ammonium bicarbonate buffer (pH 7.8), containing 50% (v/v) acetonitrile (ACN). Following dehydration in a centrifugal vacuum concentrator (SpeedVacTM SPD1030, Thermo Scientific) for 10 min, gel pieces were rehydrated in 20 ng of sequencing-grade trypsin solution (Promega, Madison, WI, USA). After incubation in 25 mM ammonium bicarbonate buffer, pH 7.8, at 37 °C overnight, tryptic peptides were extracted with 100 μL of 1% formic acid (FA) containing 50% (v/v) ACN for 20 min with mild sonication. The extracted solution was concentrated using a centrifugal vacuum concentrator. Prior to mass spectrometric analysis, the peptide solution was desalted using a reversed-phase column31. The peptide solution was loaded on the column, washed with 10 μL of 5% (v/v) FA, and eluted with 8 μL of 70% ACN with 5% (v/v) FA.
Identification of proteins using LC-MS/MS
LC–MS/MS analysis was performed using a nanoACQUITY UPLC and an LTQ-Orbitrap mass spectrometer (Thermo Electron, San Jose, CA). A BEH C18 1.7 μm, 100 μm × 100 mm column (Waters, Milford, MA, USA) was used for this analysis. The mobile phase A for LC separation was 0.1% FA in deionized water, and mobile phase B was 0.1% FA in ACN. The chromatography gradient was established to ensure a linear increase from 10% B to 40% B for 16 min, from 40% B to 95% B for 8 min, and from 90% B to 10% B for 11 min. The flow rate was 0.5 μL/min. Mass spectra were acquired using data-dependent acquisition with a full mass scan (300–2000 m/z) followed by MS/MS scans. Each MS/MS scan was an average of one microscan on the LTQ mass spectrometer. The ion transfer tube temperature was held at 275 °C, and the spray voltage was set to 2.3 kV. The normalized collision energy was set at 35% for MS/MS. The individual spectra from MS/MS were processed using SEQUEST software (Thermo Quest, San Jose, CA), and peak lists were used to query an in-house database using the MASCOT program (Matrix Science Ltd., London, UK). Carbamidomethyl (C), deamidated (NQ), and oxidation (M) modifications were employed for MS analysis with a peptide mass tolerance of 10 ppm. The MS/MS ion mass tolerance was 0.8 Da, with an allowance for two missed cleavages and charge states +2 and +3 considered for data analysis. Significant hits were defined according to MASCOT probability analysis.
E. coli expression of recombinant αSyn
The pET19b plasmid containing human αSyn cDNA (c.408 C to T mutant), introduced to prevent mistranslation of cysteine in E. coli, was transformed into E. coli BL21(DE3) (Thermo Fisher). The bacterial cultures were grown in lysogeny broth (LB) supplemented with ampicillin (100 µg/mL) at 37 °C with shaking. Expression of αSyn was induced by adding 0.5 mM isopropyl-β-D-thiogalactopyranoside (IPTG) when the culture reached an OD600 of 0.6, followed by incubation at 150 rpm for 4 h. Cells were then harvested by centrifugation at 4000 rpm for 20 min. The bacterial pellet was resuspended in lysis buffer (20 mM Tris, pH 7.6, 150 mM NaCl) supplemented with a protease inhibitor (cOmplete, Roche) and lysed on ice. Sonication was performed on ice for 5 min (5-s on/off pulses, 30% power), and the lysate was subsequently centrifuged at 14,000 rpm for 30 min. The supernatant containing His-tagged αSyn was collected and loaded onto a Ni-NTA agarose column (Qiagen, Cat. No. 30230). The column was washed, and αSyn was eluted using 250 mM imidazole. The eluted protein underwent overnight dialysis, followed by filtration through a 100 kDa molecular weight cutoff (MWCO) filter to remove high-molecular-weight aggregates, ensuring predominantly monomeric αSyn. The concentration of αSyn was determined using bicinchoninic acid (BCA) assays, and small aliquots of purified protein were stored at −80 °C. Protein purity was confirmed via SDS-PAGE and LC-MS/MS.
αSyn fibrillization
Monomeric αSyn (2 mg/mL) was prepared in 50 mM MES and 150 mM NaCl (pH 6.5). The tubes were cap-locked and sealed with parafilm. All steps were performed aseptically in a particle-free environment under a microbiological safety laminar flow hood. For comparative fibrillization, all samples were loaded into a thermomixer with a 1.5 mL Eppendorf tube holder and a heating lid. The temperature was set to 37 °C, and shaking was conducted at 1200 rpm for 6 days to allow fibril formation.
αSyn seed amplification assay
We established a novel SAA method, termed CSIC (Constant Shaking and Induced Conversion), by optimizing buffer conditions and replacing the conventional shaking–resting cycles with constant shaking. The aggregation of αSyn was monitored using a CLARIOstar fluorescence microplate reader (BMG Labtech, Ortenberg, Germany) at 37 °C in black, clear-bottomed 96-well plates (Thermo Fisher Scientific), sealed with Axygen sealing tape. Each reaction was carried out in 200 μL aliquots containing 200 μg/mL of αSyn mutant and 20 μM ThT in an optimized BES buffer (pH 7.2 ± 0.2). Plasma was added at 5 μL per reaction. The assay buffer was systematically optimized with a focus on ionic strength, using NaCl concentrations ranging from 0 to 150 mM to determine the optimal condition for αSyn aggregation. Fluorescence signals were recorded using an excitation filter of 448 ± 10 nm, an emission filter of 482 ± 10 nm, and a dichroic cutoff of 465 nm. Throughout the experiment, the plate was constantly shaken in double orbital mode. Fluorescence measurements were taken every hour for a total of 48 h.
Sedimentation assays
HC and PD plasma samples were subjected to CSIC to amplify the αSyn aggregates. The amplified αSyn aggregates in plasma samples were centrifuged at 14,000 rpm for 60 min at 4 °C to separate the protein fractions by size. After centrifugation, the supernatant was carefully transferred to a new microcentrifuge tube. The pellet was washed once with PBS to remove residual supernatant. The washed pellet was resuspended in 1× sample loading buffer (150 mM Tris-HCl, pH 8.45, 0.4% SDS, 11% glycerol, and 0.03% Coomassie blue G). Equal volumes of the supernatant and resuspended pellet from each sample were loaded onto SDS-PAGE gel.
Statistical analysis
Data analysis was conducted using GraphPad Prism (version 8.3) software. Statistical methods included calculating SAA kinetic parameters (fluorescent intensity at the final point), generating ROC curves to evaluate diagnostic accuracy, and using Spearman’s correlation to explore associations between αSyn aggregate levels and PD clinical data. A significance level of p < 0.05 was considered statistically significant.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Levivier, M., Przedborski, S., Bencsics, C. & Kang, U. J. Intrastriatal implantation of fibroblasts genetically engineered to produce brain-derived neurotrophic factor prevents degeneration of dopaminergic neurons in a rat model of Parkinson’s disease. J. Neurosci. 156, 7810–7820 (1995).
Giasson, B. I. et al. Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science 290, 985–989 (2000).
Dauer, W. & Przedborski, S. Parkinson’s disease: mechanisms and models. Neuron 39, 889–909 (2003).
Spillantini, M. G. et al. α-Synuclein in Lewy bodies. Nature 388, 839–840 (1997).
Tokuda, T. et al. Levels of cerebrospinal fluid α-synuclein oligomers are increased in Parkinson’s disease with dementia and dementia with Lewy bodies compared to Alzheimer’s disease. Acta Neuropathol. 120, 131–136 (2010).
Simuni, T. et al. A biological definition of neuronal α-synuclein disease: towards an integrated staging system for research. Lancet Neurol. 23, 178–190 (2024).
Goedert, M. Alpha-synuclein and neurodegenerative diseases. Nat. Rev. Neurosci. 2, 492–501 (2001).
Hughes, A. J., Daniel, S. E., Kilford, L. & Lees, A. J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 55, 181–184 (1992).
Hoehn, M. M. & Yahr, M. D. Parkinsonism: onset, progression and mortality. Neurology 17, 427–442 (1967).
Goetz, C. G. et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23, 2129–2170 (2008).
Kerstens, V. S. et al. Longitudinal DAT changes measured with [18F]FE-PE2I PET in patients with Parkinson’s disease; a validation study. Neuroimage Clin. 37, 103347 (2023).
Magalhães, P. & Lashuel, H. A. Opportunities and challenges of alpha-synuclein as a potential biomarker for Parkinson’s disease and other synucleinopathies. NPJ Parkinsons Dis. 8, 93 (2022).
Tokuda, T. et al. Detection of elevated levels of α-synuclein oligomers in CSF from patients with Parkinson disease. Neurology 75, 1766–1770 (2010).
Waqar, S., Khan, H., Zulfiqar, S. K. & Ahmad, A. Skin biopsy as a diagnostic tool for synucleinopathies. Cureus 15, e47179 (2023).
Donadio, V. et al. A new potential biomarker for dementia with Lewy bodies: skin nerve α-synuclein deposits. Neurology 89, 318–326 (2017).
Gibbons, C. H. et al. Skin biopsy detection of phosphorylated α-synuclein in patients with synucleinopathies. JAMA 331, 1298–1306 (2024).
Al-Qassabi, A., Tsao, T. S. & Racolta, A. Immunohistochemical detection of synuclein pathology in skin in idiopathic rapid eye movement sleep behavior disorder and Parkinsonism. Mov. Disord. 36, 895–904 (2020).
Palmqvist, S. et al. Cognitive effects of Lewy body pathology in clinically unimpaired individuals. Nat. Med. 29, 1971–1978 (2023).
Quadalti, C. et al. Clinical effects of Lewy body pathology in cognitively impaired individuals. Nat. Med. 29, 1964–1970 (2023).
Pietrowska, M. et al. MS-based proteomic analysis of serum and plasma: problem of high abundant components and lights and shadows of albumin removal. Emerg. Sample Treat. Proteom 1073, 57–76 (2019).
Bellomo, G. et al. Dissecting the interactions between human serum albumin and α-synuclein: new insights on the factors influencing α-synuclein aggregation in biological fluids. J. Phys. Chem. B 123, 4380–4386 (2019).
Meng, L. et al. The yeast prion protein Sup35 initiates α-synuclein pathology in mouse models of Parkinson’s disease. Sci. Adv. 9, eadi3297 (2023).
Schrag, A. et al. Prediagnostic presentations of Parkinson’s disease in primary care: a case-control study. Lancet Neurol. 14, 57–64 (2015).
Tolosa, E., Garrido, A., Scholz, S. W. & Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 20, 385–397 (2021).
Goedert, M., Spillantini, M. G., Del Tredici, K. & Braak, H. 100 years of Lewy pathology. Nat. Rev. Neurol. 9, 13–24 (2013).
Galvagnion, C. et al. Chemical properties of lipids strongly affect the kinetics of the membrane-induced aggregation of α-synuclein. Proc. Natl. Acad. Sci. USA 113, 7065–7070 (2016).
Michaels, T. C. T. et al. Molecular mechanism of α-synuclein aggregation on lipid membranes revealed. Chem. Sci. 15, 3579–3592 (2024).
Twohig, D. & Nielsen, H. M. α-Synuclein in the pathophysiology of Alzheimer’s disease. Mol. Neurodegener. 14, 23 (2019).
Clinton, L. K., Blurton-Jones, M., Myczek, K. & LaFerla, F. M. Synergistic interactions between Aβ, tau, and α-synuclein: acceleration of neuropathology and cognitive decline. J. Neurosci. 30, 7281–7289 (2010).
Shevchenko, A., Wilm, M., Vorm, O. & Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1, 2856–2860 (2006).
Gobom, J., Nordhoff, E., Mirgorodskaya, E., Ekman, R. & Roepstorff, P. Sample purification and preparation technique based on nano-scale reversed-phase columns for the sensitive analysis of complex peptide mixtures by matrix-assisted laser desorption/ionization mass spectrometry. J. Mass Spectrom. 34, 105–116 (1999).
Acknowledgements
We would like to express our sincere gratitude to the following individuals and organizations for their invaluable contributions to this study: Kyeong Yeon Kim and Yu Jin Kim from Protia Inc., for performing the protein LC-MS/MS analysis; Chayon Laboratories, Inc., and HyungMin Im for his assistance with the operation of the Clariostar instrument. These contributions were instrumental in the successful completion of this study.
Author information
Authors and Affiliations
Contributions
H.R. conceived and designed the study, conducted the literature search, curated, acquired, analyzed, and interpreted the data, created figures, and drafted the manuscript. D.W., H.M., and T.H. performed the experiments. H.J., S.M., S.Y., and J.S. conceived and designed the study and accessed and verified the raw data. J.S. and N.Y. contributed to the study design, analyzed the data, and revised the manuscript. H.L. and S.J. participated in data analysis and manuscript revision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
Authors affiliated with PeopleBio declare that, while no product has been developed yet, the findings of this manuscript may contribute to the future development of a Research Use Only (RUO) kit. The remaining authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ko, H.R., Lee, D., Park, H. et al. A novel approach to detecting plasma synuclein aggregates for Parkinson’s disease diagnosis. npj Parkinsons Dis. 11, 219 (2025). https://doi.org/10.1038/s41531-025-01083-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-025-01083-z