Abstract
Alterations in brain volume and subcortical myelin content (MyC) between tremor-dominant (TD) and postural instability and gait difficulty (PIGD) subtypes of Parkinson’s (PD) disease remain unclear. This study investigated whole-brain volume and subcortical myelin content differences among PD motor subtypes using synthetic MRI. Ninety-two PD patients (39 TD and 53 PIGD) and 39 healthy controls were included. ANCOVA identified that PD patients exhibited reduced gray matter, white matter, and MyC, with no distinct atrophy patterns between TD and PIGD. Most subcortical nuclei showed decreased myelin, while PIGD presented more extensive alterations and increased bilateral caudate myelin; TD exhibited asymmetric thalamic myelin changes, with lateralized increases and decreases. Significant MyC differences in the left caudate and right GPi were observed between TD and PIGD. Synthetic MRI-derived parameters provide insights into whole-brain atrophy patterns and dynamic myelin changes in PD, contributing to a deeper understanding of PD subtypes.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disorder, following Alzheimer’s disease. More than 10 million people worldwide are living with PD, and the incidence of PD tends to increase with age1,2. The primary motor symptoms of PD include resting tremors, muscle rigidity, bradykinesia, and abnormalities in posture and gait. In addition, patients may experience various non-motor symptoms, including olfactory dysfunction, cognitive impairment, sleep disorders, and autonomic dysfunction. The clinical presentation of PD exhibits significant heterogeneity. To characterize this variability, neurologists categorize PD into three subtypes: tremor-dominant (TD), postural instability and gait difficulty (PIGD), and mixed type, each reflecting distinct symptom profiles3. The TD and PIGD subtypes of PD differ significantly in clinical manifestations, disease progression, treatment response, and prognosis. Compared to the TD subtype, PIGD is characterized by more rapid disease progression, impaired postural control, poorer prognosis, and a higher prevalence of non-motor symptoms. It is also more challenging treat due to its limited responsiveness to levodopa and the complexities of deep brain stimulation therapy4,5,6. Understanding the neuropathological changes across PD subtypes is essential for gaining deeper insights into disease pathogenesis and progression, ultimately facilitating the development of more personalized diagnostic and treatment strategies.
The pathological hallmark of PD is dopaminergic neuron loss in the substantia nigra, which progressively spreads to the subcortical nucleus and is closely associated with disease pathogenesis1,7. Considering the role of subcortical nuclei in PD pathogenesis, various quantitative MRI techniques have been used to characterize brain changes, with microstructural alterations in these regions widely acknowledged8,9. Charroud et al.10 assessed subcortical nuclei volumes at baseline and 48 months post-diagnosis, revealing widespread atrophy and confirming extensive subcortical damage in PD. Additionally, widespread iron deposition in subcortical nuclei is a key pathological feature of PD11. Furthermore, studies have investigated the structural and functional differences between PD subtypes, suggesting that distinct degradation patterns of subcortical structures may provide insight into these variations12,13. Diffusion tensor imaging (DTI) is a widely used MRI technique for investigating the microstructural integrity of white matter (WM). A large-scale multicenter study utilizing DTI revealed widespread WM abnormalities in PD, primarily characterized by reduced fractional anisotropy and increased mean diffusivity. The extent of these changes progressively increases with disease severity14. Additionally, studies have shown that PD patients with different subtypes exhibit distinct patterns of WM damage. Among them, the PIGD subtype shows more severe WM degradation, suggesting it is a more advanced or aggressive form of the disease15,16.
PD is associated with global brain atrophy and ventricular enlargement, particularly in individuals with cognitive impairment17,18. However, there is limited research on the comprehensive analysis of whole brain tissue, including gray matter (GM), WM, and cerebrospinal fluid (CSF), particularly across different PD subtypes. In addition, PD patients also exhibit atrophy of local gray matter nuclei, which may be related to the distribution of certain specific neurotransmitters, and this has certain significance in explaining the symptoms of the disease19,20.
Myelin sheath plays an important role in the transmission of neural information, and the loss of myelin is crucial in evaluating the progression of degenerative or demyelinating diseases. Synthetic magnetic resonance imaging (SyMRI) is a novel quantitative imaging technique based on relaxation values that enables rapid, automatic segmentation and volume measurement of GM, WM, CSF, and myelin content (MyC). The examination process is more time-efficient, completed in about 4 min, and post-processing is also simplified, enabling multi contrast images (such as T1, T2, PD mapping) and whole tissue segmentation to be achieved within 1 minute21. SyMRI shows strong consistency with Three-dimensional T1-weighted imaging (3D T1WI) in measuring brain volume and morphology22, and yields results similar to standard myelin imaging techniques, such as magnetic transfer imaging, for assessing myelin content, which correlates well with histological findings23,24. Although the value of SyMRI for assessing brain myelin development in premature infants25 and demyelinating diseases like multiple sclerosis26 is well-documented, studies in PD patients and its subtypes are lacking.
Subcortical nuclei play a crucial role in the pathogenesis of PD. However, while most previous studies have focused on gray matter atrophy and neuronal loss, the role of myelin—which is critical for efficient signal transmission—has been largely overlooked in these regions. Addressing this gap in research may offer valuable insights into the neurobiological mechanisms underlying different PD subtypes. Given previous studies19,27 demonstrating structural and functional brain differences across PD motor subtypes—particularly in subcortical nuclei—and growing evidence supporting myelin alterations in PD28, we hypothesized that patients with different motor subtypes may exhibit distinct patterns of myelin changes. In this study, we aimed to evaluate the utility of SyMRI to: (1) assess whole brain tissue content in PD patients with different motor subtypes; (2) measure myelin content in subcortical nuclei across these subtypes and explore potential associations between quantitative parameters and clinical symptoms; and (3) evaluate myelin damage in subcortical nuclei and investigate degenerative patterns to better understand the neuropathological mechanisms behind different PD subtypes. To our knowledge, this is the first application of SyMRI in investigating motor subtype PD patients, with a focus on deep gray matter nucleus damage.
Results
Demographic and clinical characteristics in PIGD, TD and HC group
The classification of PD subtypes was based on the ratio of tremor to postural instability/gait difficulty scores from the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), with patients classified as TD if the ratio was ≥1.15, and as PIGD if ≤0.90. The demographic and clinical characteristics of all subjects are summarized in Table 1. No significant differences were found between the three groups in terms of age (P = 0.145), sex (P = 0.485), and education level (P = 0.345). There was a significant difference in symptom-dominant side between the TD and PIGD groups (P = 0.030). PIGD subtype patients exhibited significantly higher scores on the MDS-UPDRS-I (P = 0.018), MDS-UPDRS-II (P = 0.040), and MDS-UPDRS-III (P < 0.001), as well as on the modified Hoehn and Yahr scale (MHY) (P < 0.001), compared to those with the TD subtype.
Comparison of brain volume among three groups
Significant differences in volume values were observed between PD patients with different motor subtypes and HC in terms of WM, GM, CSF, MyC, Brain Parenchymal Fraction (BPF), Brain Parenchymal volume (BPV), and various ratio indicators, including WM/ICV (intracranial volume), GM/ICV, CSF/ICV, and MyC/ICV. However, no statistically significant differences were found between the TD and PIGD groups for these variables (Table 2).
Comparison of MyC in subcortical nuclei among three groups
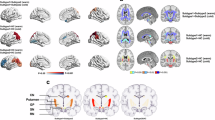
Figure 1 illustrates the entire processing workflow. Significant differences in the MyC of 16 subcortical nuclei were observed in the following regions: caudate_L, caudate_R, left and right external of globus pallidus (GPe_L, GPe_R), left and right internal of globus pallidus (GPi_L, GPi_R), and the thalamus_L. Compared to HC, the PIGD subtype has a wider range of regions with significant changes in MyC. Furthermore, when compared to the TD group, PIGD patients showed significantly higher MyC in the left caudate (P = 0.042; 7.937 ± 2.264 vs. 6.835 ± 2.074) and significantly lower MyC in the right GPi (P = 0.011; 14.698 ± 3.584 vs. 16.867 ± 3.380). No significant differences in MyC were observed between the two groups in other regions. For further details, refer to Table 3 and Fig. 2.
This figure illustrates two main processing streams: whole-brain volumetric analysis (left panel) and subcortical myelin content extraction (right panel). For volumetric analysis, raw SyMRI data were processed using Synthetic MR software to automatically segment brain tissues and compute structural volumes, including gray matter (GM), white matter (WM), cerebrospinal fluid (CSF), brain parenchymal volume (BPV), and intracranial volume (ICV), along with relevant volumetric ratios. For subcortical analysis, 3D T1-weighted anatomical images were rigidly registered to the T1 mapping and then normalized to Montreal Neurological Institute (MNI) space using Advanced Normalization Tools (ANTs). The PD25 subcortical atlas was subsequently transformed from MNI space back to each participant’s native space and aligned with the corresponding MyC maps. Manual inspection was performed to ensure accurate anatomical correspondence. Myelin content (MyC) values were then extracted from 16 subcortical nuclei, including the substantia nigra (SN), red nucleus (RN), caudate nucleus, putamen, internal and external globus pallidus (GPi and GPe), subthalamic nucleus (STN), and thalamus.
Statistical significance is indicated as follows: asterisk-based annotations (*, **, and ***) represent p-values of <0.05, <0.01, and <0.001, respectively; “ns” denotes no significant difference (p > 0.05). caudate_L left caudate, caudate_R right caudate, GPe_L left external of the globus pallidus, GPe_R right external of the globus pallidus, GPi_L left internal of the globus pallidus, GPi_R right internal of the globus pallidus, thalamus_L left thalamus.
Correlation analysis between whole-brain tissue volume, MyC of subcortical nuclei and clinical indicators
MyC and WM were significantly negatively correlated with MDS-UPDRS-I score (r = −0.276, P = 0.008; r = −0.270, P = 0.009) (Fig. 3A). BPF and WM showed a significant negative correlation with MDS-UPDRS-II score (r = −0.226, P = 0.030; r = −0.287, P = 0.006) (Fig. 3B). MyC and WM were also significantly negatively correlated with MDS-UPDRS-III score (r = −0.249, P = 0.017; r = −0.206, P = 0.049) (Fig. 3C). Furthermore, MyC of caudate_L, GPi_L, and thalamus_L were significantly positively correlated with disease duration (r = 0.357, P = 0.017; r = 0.240, P = 0.021; r = 0.440, P = 0.011) (Fig. 3D). MyC of GPi_R was significantly negatively correlated with MDS-UPDRS-II score (r = −0.224, P = 0.032) (Fig. 3E).
A Correlations between the MyC, WM and UPDRS-I scores in PD; B Correlations between the BPF, WM and UPDRS II scores in PD; C Correlations between the MyC, WM and UPDRS III scores in PD; D Correlations between the MyC of caudate_L, GPe_L, thalamus_L and duration; E Correlations between UPDRS II scores and MyC of GPi. All the volume and MyC of subcortical nuclei for the shown were residuals adjusted for age and gender and education level. MyC Myelin Content, WM White Matter, BPF Brain Parenchymal Fraction, caudate_L Left Caudate Nucleus, GPe_L left external segment of the globus pallidus, thalamus_L left thalamus, GPi_R right internal segment of the globus pallidus, MDS-UPDRS Movement Disorder Society-Unified Parkinson’s Disease Rating Scale.
The association between myelin asymmetry and symptom dominance in PD
Point-biserial correlation analysis revealed a significant negative correlation between the asymmetry index (AI) of myelin content in GPi and the symptom-dominant side in PD patients (r = −0.336, p = 0.023,95CI: [−0.421, −0.133]), as shown in Fig. 4. This association was also evident in the PIGD group (r = −0.290, p = 0.035, 95CI: [−0.392, −0.104]), but did not reach significance in the TD group (p > 0.05). In contrast, the correlation between caudate AI and symptom dominance was not statistically significant at the group level (p > 0.05) or within either the TD or PIGD subgroups.
The asymmetry index (AI) of myelin content was calculated as: AI = [(left − right)/(left + right)] × 100. The x-axis represents the AI of the GPi, and the y-axis represents the symptom-severe side. Point-biserial correlation analysis was performed to assess the association between AI and symptom laterality. The upper marginal density plot illustrates the distribution of GPi AI values in the TD and PIGD subgroups. The right panel displays the distribution of symptom-dominant sides (left vs. right) within each group.
Discussion
We investigated whole-brain tissue volumetric and MyC changes in subcortical structures across different PD subtypes. Our findings suggest that PD is characterized by reduced GM, WM, and MyC, along with increased CSF volume compared to HC. However, these indicators did not show significant differences between the PIGD and TD subtypes. Notably, we observed differences in MyC within the subcortical gray matter nuclei between these subtypes, specifically in the caudate_L and GPi_R. These findings may reflect subtype-specific pathophysiological alterations. Furthermore, correlations between brain tissue volume, MyC in subcortical structures, and clinical measures provide additional insights into the underlying mechanisms and heterogeneity of PD.
Misfolded alpha-synuclein can propagate across brain regions via the brain connectome, triggering neuronal death and subsequent atrophy in affected areas. At a macroscopic level, this process manifests as progressive whole-brain and region-specific tissue atrophy, with a more pronounced reduction in gray matter volume18,29. This study found reduced GM, WM, and MyC, along with increased CSF, in PD patients compared to HC, consistent with previous studies17,18. Similar trends were observed in the WM/ICV, GM/ICV, CSF/ICV, and MyC/ICV ratios. The TD group showed a significant reduction in gray matter volume compared to HC, whereas WM and the WM/ICV ratio did not differ significantly. This suggests that WM atrophy occurs in PD but may be less pronounced than gray matter atrophy. Similar findings have been reported in previous studies, further supporting the notion that PD-related atrophy predominantly affects gray matter29. However, some studies have failed to detected volumetric differences in PD brain tissue compared to healthy controls, possibly due to the relatively mild disease severity in the included patients, where neuronal loss-induced atrophy might not be sufficiently pronounced to be detected by MRI30,31. Additionally, the limited sample size in these studies may have contributed to these findings.
Moreover, WM and MyC were negatively correlated with MDS-UPDRS-I and MDS-UPDRS-III scores, respectively, while WM and BPF were negatively correlated with MDS-UPDRS-II scores in this study. These findings suggest that WM atrophy, reduced myelin content, and decreased overall brain parenchymal volume are associated with more severe motor and non-motor dysfunction31,32. It is noteworthy that, no significant associations were observed between MyC and MDS-UPDRS-II, or between BPF and MDS-UPDRS-III. This discrepancy may be attributable to the distinct constructs assessed by the two subscales. MDS-UPDRS-II evaluates activities of daily living and is subject to influence from non-motor factors such as affective symptoms, cognitive impairment, and social context. These broader factors may be more effectively reflected by global volumetric measures such as BPF, rather than by myelin-specific metrics. In contrast, MDS-UPDRS-III reflects clinician-rated motor performance and may be more directly linked to motor-specific pathology. Such alterations may be more sensitively captured by MyC, given the essential role of myelin in maintaining efficient neural conduction along motor pathways, while global atrophy markers such as BPF may lack the specificity needed to detect these changes. Beyond whole-brain volume loss, extensive regional atrophy has also been observed in the subcortical nuclei and limbic system. studies have demonstrated that the patterns of subcortical degeneration vary across different PD subtypes20,33.
The detection of myelin content enables precise in vivo assessment of myelin integrity, facilitating the identification of abnormalities at early stages of disease progression—potentially even before the onset of brain atrophy—thereby enabling timely intervention34.Our study also demonstrated a significant reduction in whole-brain MyC in PD patients. There was no significant difference in total MyC between the PIGD and TD subtypes. This may be due to overlapping pathological changes across subtypes or the limited sensitivity of global MyC measures in detecting subtle regional differences. Therefore, research focusing on regional myelin changes—particularly in subcortical nuclei—may provide deeper insights into subtype-specific neurodegeneration. Previous studies on myelin content in PD have primarily focused on WM structures35, while the myelin content of deep gray matter in PD has been largely overlooked. In contrast, this aspect has been more extensively discussed in demyelinating diseases such as multiple sclerosis36,37.
The maturation and myelin content of the basal ganglia are closely linked to motor and executive functions and are more susceptible to external influences than deep WM. The presence of extensive iron deposition in subcortical nuclei in PD is a widely recognized finding38,39. While ironis essential for myelin synthesis and maintenance, iron deficiency may delay myelin formation during brain development, whereas iron overload can contribute to neurodegeneration and demyelination. A study using SyMRI found reduced MyC in Alzheimer’s patients, but the small sample size limited the analysis, and no significant difference was observed40. Another study analyzing myelin content in PD found higher myelin water fraction in the frontal and temporal WM as well as the bilateral thalamus, though their analysis was restricted to WM structures28. Additionally, Tommy et al.41 demonstrated that approximately 80% of connections originating from the basal ganglia exhibited reduced myelin content based on myelin connectomics.
Our study found that the PIGD group exhibited MyC differences in more regions than the TD group when compared to HC, suggesting more extensive neuronal damage in this subgroup. In particular, the PIGD subtype showed myelin content differences in the left caudate nucleus and the right GPi relative to the TD subtype, indicating potential regional differences in neuronal damage across the motor subtypes of PD. This is consistent with previous studies on brain volume reduction7,13, which have reported inflammation and myelin loss in subcortical nuclei of PD patients, leading to corresponding decrease in myelin content. The globus pallidus is a key downstream structure in the substantia nigra-striatum pathway, playing a crucial role in neural circuit regulation. Neuronal loss and dendritic degeneration are common pathological features in PD42. Our findings demonstrate a reduction in MyC expression, supporting this pathophysiological model. Previous cross-sectional volumetric and DTI studies have reported greater gray matter loss in the globus pallidus and increased diffusivity in PIGD compared to TD27. Furthermore, structural changes in the globus pallidus progressively worsen in the PIGD subtype but remain relatively stable in TD13,43. Our study revealed varying degrees of myelin reduction in both the external and internal portions of the pallidum, with a more pronounced decrease in the PIGD subtype. Specifically, the MyC of GPi_R differed significantly between PIGD and TD, showing a negative correlation with motor scores. Additionally, the MyC of GPe_L exhibited a positive correlation with disease duration, suggesting that myelin alterations in the globus pallidus may be closely associated with disease progression and motor dysfunction. However, given the relatively modest correlations, these findings warrant further validation.
In the comparison between PIGD and TD subtypes, our study revealed a lateralized pattern of myelin alterations, which may be attributed to several factors: handedness44, which has been proposed as a factor contributing to hemispheric asymmetry; the asymmetrical onset and dominant side of symptom in PD patient45; and the asymmetrical dopaminergic degeneration in the striatum and globus pallidus in PD46,47. Collectively, these factors may contribute to the observed asymmetry in myelin alterations. In the current study, we found a significant negative correlation between GPi myelin asymmetry and symptom-dominant side, consistent with the known contralateral motor symptom pattern in PD. Lateralization was also pronounced in the PIGD subtype, likely due to a higher proportion of left-dominant patients, resulting in greater myelin loss in the right GPi. This pattern was not observed in the TD subtype, which featured more right-dominant patients. No significant correlation was observed between caudate asymmetry and symptom laterality, possibly due to limited sample size or disease heterogeneity. Given the limited research on myelin lateralization in PD, larger studies are needed to confirm these findings. In summary, evidence for lateralized myelin changes in PD remains sparse, warranting further investigation into their mechanisms and clinical relevance.
Surprisingly, myelin content in the bilateral caudate nucleus was significantly increased in the PIGD group, with no significant difference between the TD and HC groups. Of note, a trend-level difference in the left caudate was identified between the PIGD and TD groups(adjusted p = 0.042). As key components of the extrapyramidal system, the caudate nucleus and globus pallidus play crucial roles in muscle tone regulation and postural adjustments. Notably, the caudate nucleus is primarily responsible for balance and movement, which may explain the more severe motor impairments observed in PIGD patients. Most morphological studies on PD suggest that caudate nucleus atrophy is a common finding, reflecting neuronal inflammation and necrosis48,49. Additionally, caudate atrophy has been associated with the severity of gait instability50,51,52. However, some studies have reported increased caudate volume in PD, particularly in the PIGD subtype, possibly due to greater global brain atrophy relative to the caudate. Our findings revealed increased myelin content in the caudate nucleus, with a more pronounced trend in PIGD patients. Considering that increased myelin content reflects myelin repair and regeneration, this phenomenon may reflect a compensatory response for motor symptoms in PD, although further longitudinal and histological validation is needed53. Moreover, further research is warranted to validate and clarify the observed differences in MyC in the left caudate nucleus between the PIGD and TD subtypes. Besides, given that iron accumulation is a well-recognized feature of PD, differentiating compensatory myelination from iron-related artifacts in deep gray matter nuclei remains a challenge for SyMRI; therefore, this hypothesis warrants further validation. In contrast, gait deficits are a hallmark of the PIGD subtype, indicating more severe impairment of neural circuits involved in PIGD. This may be linked to disruptions in motor mechanisms, such as gait-related alterations, and suggests a potential compensatory response as the disease progresses, though further studies are needed to confirm this. Additionally, correlation analysis revealed a positive association between MyC in the left caudate nucleus and disease duration, indicating a progressive and compensatory increase in myelin content with disease advancement. Nonetheless, such compensatory mechanisms, though reported in some studies50, require further validation.
The basal ganglia-thalamocortical circuitry plays a crucial role in the development of PD tremors54,55 and the thalamus is also a key target for deep brain stimulation (DBS). A longitudinal study of PD patients also reported a significant correlation between thalamic volume and tremor scores in a subgroup of tremor-dominant PD patients49. Chen et al.9 calculated the volumes of the thalamus and its subregions, finding that the left posterior parietal thalamus was significantly smaller in TD patients than in PIGD patients. They hypothesized that the thalamus plays a crucial role in the mechanism underlying tremor symptoms in TD-PD patients. Lin et al.56 employed the VBM method to investigate volumetric alterations in brain regions associated with the basal ganglia-thalamocortical and cerebellar-thalamocortical circuits, identifying both increases and decreases. Notably, the PD group exhibited increased thalamic volume, suggesting a compensatory effect. In our study, thalamic nuclei—structures critically involved in PD and tremor mechanisms—were also examined. We observed a significant reduction in MyC values in the left thalamus in both TD and PIGD groups compared to HC, although no statistically significant difference was found between the two PD subtypes. Conversely, a slight increase in MyC values was noted in the right thalamus in PD patients, particularly in the TD group, but with non-significant trend. The thalamus contributes to the pathophysiology of different PD subtypes through distinct pathways. Current evidence indicates that non-TD patients exhibit disruptions in striato-thalamo-cortical and other thalamocortical projections associated with cognitive and sensorimotor functions, whereas TD patients show greater involvement of cerebello-thalamo-cortical circuitry33,57. The asymmetric changes observed in the bilateral thalamus in our study may reflect differential engagement of thalamic circuits across PD subtypes or more pronounced tremor symptoms in TD, warranting further investigation. Correlation analysis revealed a positive association between MyC in the left thalamus and disease duration, suggesting a potential compensatory mechanism in the later stages of PD. These findings may imply that myelin alterations in PD are not static but could involve dynamic compensatory processes, rather than exhibiting a simple linear increase or decrease over time. However, given the relatively modest correlation strength, this interpretation should be viewed with caution and requires further investigation.
However, in this study, the SN and STN, which are closely associated with PD, did not exhibit significant MyC alterations. This may be attributed to the heterogeneity of PD and differences in pathological stages among patients. Additionally, numerous studies have demonstrated widespread iron accumulation in the subcortical nuclei of PD patients, which may affect the quantification of myelin content11,12. Notably, research has found a negative correlation between iron and myelin content across multiple brain regions in PD. Increased iron deposition and reduced myelin content in motor symptom-dominant hemispheres have been associated with greater motor dysfunction58. Although iron content was not directly quantified in this study, we acknowledge that its presence in these regions may have influenced the estimation of MyC. Future studies incorporating iron quantification techniques, such as Quantitative Susceptibility Mapping (QSM), may help clarify the mechanisms underlying myelin alterations and better isolate the effects of iron. It is also worth noting that some postmortem studies have shown that variations in myelin content within the SN can influence the relationship between R2* values and QSM measurements, suggesting a bidirectional interaction between iron deposition and myelin content in subcortical nuclei59. Incorporating additional imaging modalities may help elucidate this complex relationship.
Also, this study have several limitations. First, given the widespread iron deposition in subcortical nuclei in PD and its potential impact on myelin content, iron quantification techniques like QSM may help elucidate the mechanisms behind myelin alterations, warranting further research. Second, as gray matter components more accurately reflect neuronal degeneration and the loss of dopaminergic terminals, some scholars propose that subdividing deep gray matter nuclei into gray and WM components could improve tissue classification. This approach may better capture subtle histological changes and represents a promising direction for future research50. Despite these limitations, this study assessed volumetric differences among PD motor subtypes using SyMRI. In addition, myelin content in subcortical regions was automatically quantified using PD—specific subcortical nuclei templates, enabling comparisons across subtypes and analyses of correlations with clinical features—parameters that have been largely overlooked to date. The observed similarities and differences in brain tissue volume and myelin content between the TD and PIGD subtypes may reflect distinct underlying pathophysiological mechanisms, offering valuable insights for more accurate clinical diagnosis and improved disease management.
Methods
Participants
Sample size estimation was performed using G*Power based on a one-way analysis of covariance (ANCOVA). An effect size of 0.25 was assumed (with covariates explaining 20% of the variance), with α = 0.05, power = 0.80, and three groups. The analysis indicated that 127 participants were required, corresponding to approximately 42 participants per group. Actually, a total of 92 patients diagnosed with PD based on the UK PD Society Brain Bank Criteria were recruited from the First People’s Hospital of Foshan between September 2022 and July 2024. In addition, 39 healthy individuals, matched for age, gender, and educational background, were recruited from the local community as controls. The PD participants underwent a series of evaluations, including the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) and modified Hoehn and Yahr scale (MHY). The classification of PD subtypes was based on the ratios of tremor and postural instability/gait difficulty scores from the MDS-UPDRS: patients with a ratio ≥1.15 were categorized as TD those with a ratio ≤0.90 as PIGD, and those with ratios ranging from 0.90 to 1.15 as Mixed type3. MHY > 2.5 is considered advanced stage. Ethical approval for this study was obtained from the Medical Ethics Committee of the First People’s Hospital of Foshan, and informed consent was obtained from all participants.
Inclusion Criteria for Participants: (1) Participants were diagnosed with PD based on the diagnostic criteria established by the International Movement Disorder Society in 2015. (2) Participants had no prior history of other neurological disorders. (3) PD patients were classified into two subtypes, PIGD or TD, based on their MDS-UPDRS scores. Exclusion Criteria: (1) Individuals with secondary PD or Parkinson-plus syndromes were not included. (2) A history of encephalitis, brain tumors, surgery, chronic alcohol consumption, or dependence on drugs or substances led to exclusion. (3) Patients with significant psychiatric conditions or those with poor adherence to the study protocol were excluded from the study.
MRI data acquisition
MRI data were acquired using a GE 3.0 Tesla scanner (SIGNA Architect, GE Healthcare, United States) with a 48-channel head coil. T1-weighted images were acquired with a fast spoiled gradient echo (FSPGR) sequence: sagittal orientation, TR = 6.6 ms, TE = 2.6 ms, FOV = 256 × 256 mm, voxel size = 1 × 1 × 1 mm³ (isotropic), matrix = 256 × 256, 152 slices. SyMRI was performed using the MAGnetic resonance image Compilation (MAGiC) sequence, with scans conducted in the transverse plane, aligned with the anterior-posterior commissures. Parameters as follows: FOV = 256 × 256 mm, matrix = 128 × 128, slice thickness/inter-slice gap = 2 mm/0 mm, voxel size = 2 × 2 × 2 mm³, 68 slices, TR = 10205 ms, TE = 13.1/91.9 ms, ETL = 16, scan time = 6:08 min.
MRI data processing
The raw data obtained from SyMRI were further processed using the Synthetic MR software (version 11.2.2; Synthetic MR AB, Sweden) to generate Myelin Content (MyC), proton density (PrD) mapping, T1 mapping, and T2 mapping. This processing also involved calculating various structural parameters, including GM, WM, and CSF volumes. The CSF volume mainly included fluid in the lateral, third, and fourth ventricles and the subarachnoid space. This processing also involved calculating various structural parameters, including GM, WM, and CSF volumes. The CSF volume mainly included fluid in the lateral, third, and fourth ventricles and the subarachnoid space. Additionally, we computed the brain parenchymal volume (BPV), intracranial volume (ICV), and several ratios: GM/BPV, WM/BPV, MyC/BPV, GM/ICV, WM/ICV, CSF/ICV, MyC/ICV, and the brain parenchymal fraction (BPF, defined as BPV/ICV). To obtain MyC values for the subcortical nuclei, the 3D T1-weighted images were first rigidly aligned with the T1 mapping. These registered T1 images were then transformed into MNI space using the Advanced Normalization Tools (ANTs) for further analysis. The PD25 atlas60 was subsequently mapped from MNI space back to the individual subject’s space and aligned with the corresponding MyC mapping for each participant. To ensure precise alignment between the PD25 subcortical atlas and the MyC maps in the individual native space, we conducted a thorough manual inspection of the overlay between the transformed atlas and the individual anatomical structures. Specifically, we focused on the accuracy of alignment within subcortical regions. In cases where misalignment was observed—such as spatial mismatch or anatomical boundary deviation—subjects were excluded from further analysis. This step was critical for maintaining anatomical accuracy and minimizing measurement bias. Finally, the transformed PD atlas was applied to each subject’s post-processed SyMRI images, allowing the extraction of MyC values for 16 subcortical structures, including the substantia nigra (SN), red nucleus (RN), caudate nucleus, putamen, both internal and external globus pallidus (GPi and GPe), subthalamic nucleus (STN), and thalamus.
Statistical analyses
Statistical analyses were conducted using SPSS 26.0 (IBM Corp., Armonk, NY) and R software. Continuous variables are presented as mean ± standard deviation, while categorical variables are expressed as frequencies. Demographic characteristics, MDS- UPDRS scores, and H-Y grades were compared using analysis of variance (ANOVA), Student’s t-test, or the chi-square test, as appropriate. ANCOVA was conducted to compare brain volume parameters and myelin content across groups, with adjustments for sex, age, and years of education as covariates. Post hoc comparisons were performed using the Tukey HSD method, and P-values were adjusted for multiple comparisons using the false discovery rate (FDR) method. Partial η² was reported as a measure of effect size for the main group effect. Additionally, partial correlation analysis, adjusted for sex, age, and education level, was used to investigate the relationship between significantly altered regional quantitative parameters and clinical measures within the PD group. To assess the relationship between hemispheric asymmetry in myelin content and clinical symptom dominance, asymmetry indices (AI) were calculated for subcortical nuclei using the formula: AI = [(left − right)/ (left + right)] × 100, where “left” and “right” represent the MyC in the respective nuclei. The symptom-dominant side was determined based on clinical assessment and coded as a binary variable (0 = left-dominant, 1 = right-dominant). Point-biserial correlation analyses were conducted to assess the relationship between AI values (continuous variables) and symptom dominance (binary variable), both in the overall cohort and separately within the TD and PIGD subgroups.
Data availability
The data are not publicly available due to privacy concerns. The datasets used in this study are available from the corresponding author upon reasonable request and subject to institutional review board approval.
References
Kalia, L. V. & Lang, A. E. Parkinson’s disease. Lancet 386, 896–912 (2015).
Yang, J., Burciu, R. G. & Vaillancourt, D. E. Longitudinal progression markers of parkinson’s disease: current view on structural imaging. Curr. Neurol. Neurosci. Rep. 18, 83 (2018).
Stebbins, G. T. et al. How to identify tremor dominant and postural instability/gait difficulty groups with the Movement Disorder Society Unified Parkinson’s disease rating scale: Comparison with the unified Parkinson’s disease rating scale. Mov. Disord. 28, 668–670 (2013).
Wickremaratchi, M. M. et al. The motor phenotype of Parkinson’s disease in relation to age at onset. Mov. Disord. 26, 457–463 (2011).
Wu, Y. et al. Non-motor symptoms and quality of life in tremor dominant vs postural instability gait disorder Parkinson’s disease patients. Acta Neurol. Scand. 133, 330–337 (2016).
Boonstra, J. T., Mcgurran, H., Temel, Y. & Jahanshahi, A. Nigral neuropathology of Parkinson’s motor subtypes coincide with circuitopathies: a scoping review. Brain Struct. Funct. 227, 2231–2242 (2022).
Laansma, M. A. et al. A worldwide study of subcortical shape as a marker for clinical staging in Parkinson’s disease. NPJ Parkinsons Dis. 10, 223 (2024).
Li, M. G. et al. Structural and functional thalamic changes in parkinson’s disease with mild cognitive impairment. J. Magn. Reson. Imaging 52, 1207–1215 (2020).
Chen, Y. et al. Structural and functional differences of the thalamus between drug-naive Parkinson’s disease motor subtypes. Front Neurol. 14, 1102927 (2023).
Fearnley, J. M. & Lees, A. J. Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain 114, 2283–2301 (1991).
Jin, J., Su, D., Zhang, J., Lam, JST., Zhou, J. & Feng, T. Iron deposition in subcortical nuclei of Parkinson's disease: A meta- analysis of quantitative iron-sensitive magnetic resonance imaging studies. Chin Med J (Engl). 138, 678–692 (2025).
Zhang, X. et al. Distribution pattern of iron deposition in the basal ganglia of different motor subtypes of Parkinson’s disease. Neurosci. Lett. 807, 137249 (2023).
Rosenberg-Katz, K. et al. Subcortical volumes differ in parkinson’s disease motor subtypes: New insights into the pathophysiology of disparate symptoms. Front. Hum. Neurosci. 10, 356 (2016).
Owens-Walton, C. et al. A worldwide study of white matter microstructural alterations in people living with Parkinson’s disease. NPJ Parkinsons Dis. 10, 151 (2024).
Luo, C. et al. White matter microstructure damage in tremor-dominant Parkinson’s disease patients. Neuroradiology 59, 691–698 (2017).
Wen, M. C. et al. Differential white matter regional alterations in motor subtypes of early Drug-Naive parkinson’s disease patients. Neurorehabil Neural Repair 32, 129–141 (2018).
Mak, E. et al. Longitudinal whole-brain atrophy and ventricular enlargement in nondemented Parkinson’s disease. Neurobiol. Aging 55, 78–90 (2017).
Pan, G. et al. Identification of Parkinson’s disease subtypes with distinct brain atrophy progression and its association with clinical progression. Psychoradiology 4, e2 (2024).
Pang, H. et al. Disentangling gray matter atrophy and its neurotransmitter architecture in drug-naive Parkinson’s disease: an atlas-based correlation analysis. Cereb. Cortex 34, bhae420 (2024).
Rosenberg-Katz, K. et al. Gray matter atrophy distinguishes between Parkinson disease motor subtypes. Neurology 80, 1476–1484 (2013).
Hagiwara, A. et al. SyMRI of the brain: rapid quantification of relaxation rates and proton density, with synthetic MRI, automatic brain segmentation, and myelin measurement. Invest. Radiol. 52, 647–657 (2017).
Liu, S. et al. Brain volumetric and fractal analysis of synthetic MRI: a comparative study with conventional 3D T1-weighted images. Eur. J. Radiol. 141, 109782 (2021).
Akifumi, H. et al. Myelin measurement: comparison between simultaneous tissue relaxometry, magnetization transfer saturation index, and T1w/T2w ratio methods. Sci. Rep. 8, 10554 (2018).
Warntjes, J., Persson, A., Berge, J. & Zech, W. Myelin detection using rapid quantitative MR imaging correlated to macroscopically registered luxol fast Blue-Stained brain specimens. AJNR Am. J. Neuroradiol. 38, 1096–1102 (2017).
Schmidbauer, V. et al. SyMRI detects delayed myelination in preterm neonates. Eur. Radiol. 29, 7063–7072 (2019).
Saccenti, L. et al. Brain tissue and myelin volumetric analysis in multiple sclerosis at 3T MRI with various in-plane resolutions using synthetic MRI. Neuroradiology 61, 1219–1227 (2019).
Nagae, L. M. et al. Microstructural changes within the basal ganglia differ between parkinson disease subtypes. Front. Neuroanat. 10, 17 (2016).
Dean, D. R. et al. Alterations of myelin content in parkinson’s disease: a Cross-Sectional neuroimaging study. PLoS One 11, e163774 (2016).
He, H. et al. Progressive brain changes in Parkinson’s disease: a meta-analysis of structural magnetic resonance imaging studies. Brain Res. 1740, 146847 (2020).
Menke, R. A. et al. Comprehensive morphometry of subcortical grey matter structures in early-stage Parkinson’s disease. Hum. Brain Mapp. 35, 1681–1690 (2014).
Tessa, C. et al. A whole-brain analysis in de novo Parkinson disease. AJNR Am. J. Neuroradiol. 29, 674–680 (2008).
Guevara, C. et al. Whole-Brain atrophy differences between progressive supranuclear palsy and idiopathic parkinson’s disease. Front. Aging Neurosci. 8, 218 (2016).
Boonstra, J. T., Michielse, S., Temel, Y., Hoogland, G. & Jahanshahi, A. Neuroimaging detectable differences between parkinson’s disease motor subtypes: a systematic review. Mov. Disord. Clin. Pract. 8, 175–192 (2021).
Mchinda, S. et al. Whole brain inhomogeneous magnetization transfer (ihMT) imaging: sensitivity enhancement within a steady-state gradient echo sequence. Magn. Reson. Med. 79, 2607–2619 (2018).
Cai, J. et al. A multi-sequence MRI study in parkinson’s disease: association between rigidity and myelin. J. Magn. Reson. Imaging 55, 451–462 (2022).
Xie, Y. et al. Atrophy-Independent and dependent iron and myelin changes in deep gray matter of multiple sclerosis: a longitudinal study using chi-Separation imaging. Acad. Radiol. 32, 988–999 (2025).
Pontillo, G. et al. Unraveling deep gray matter atrophy and iron and myelin changes in multiple sclerosis. AJNR Am. J. Neuroradiol. 42, 1223–1230 (2021).
Guan, X. et al. Influence of regional iron on the motor impairments of Parkinson’s disease: a quantitative susceptibility mapping study. J. Magn. Reson. Imaging 45, 1335–1342 (2017).
Li, J., Zhang, Q., Che, Y., Zhang, N. & Guo, L. Iron deposition characteristics of deep gray matter in elderly individuals in the community revealed by quantitative susceptibility mapping and multiple factor analysis. Front. Aging Neurosci. 13, 611891 (2021).
Lou, B. et al. Quantitative analysis of synthetic magnetic resonance imaging in alzheimer’s disease. Front. Aging Neurosci. 13, 638731 (2021).
Boshkovski, T. et al. The Myelin-Weighted connectome in parkinson’s disease. Mov. Disord. 37, 724–733 (2022).
Zaja-Milatovic, S. et al. Dendritic degeneration in neostriatal medium spiny neurons in Parkinson disease. Neurology 64, 545–547 (2005).
Bower, A. E., Crisomia, S. J., Chung, J. W., Martello, J. P. & Burciu, R. G. Free water imaging unravels unique patterns of longitudinal structural brain changes in Parkinson’s disease subtypes. Front Neurol. 14, 1278065 (2023).
Melamed, E. & Poewe, W. Taking sides: is handedness involved in motor asymmetry of Parkinson’s disease?. Mov. Disord. 27, 171–173 (2012).
Barrett, M. J., Wylie, S. A., Harrison, M. B. & Wooten, G. F. Handedness and motor symptom asymmetry in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 82, 1122–1124 (2011).
Zhong, Z. et al. High-Spatial-Resolution diffusion MRI in parkinson disease: Lateral asymmetry of the substantia nigra. Radiology 291, 149–157 (2019).
Madelung, C. F. et al. High-resolution mapping of substantia nigra in Parkinson’s disease using 7 Tesla magnetic resonance imaging. NPJ Parkinsons Dis. 11, 113 (2025).
Lee, S. H. et al. Regional volume analysis of the Parkinson disease brain in early disease stage: gray matter, white matter, striatum, and thalamus. AJNR Am. J. Neuroradiol. 32, 682–687 (2011).
Jia, X. et al. Longitudinal study of gray matter changes in parkinson disease. AJNR Am. J. Neuroradiol. 36, 2219–2226 (2015).
Fang, E. et al. Differentiating Parkinson’s disease motor subtypes using automated volume-based morphometry incorporating white matter and deep gray nuclear lesion load. J. Magn. Reson. Imaging 51, 748–756 (2020).
Baumeister, T. R., Kim, J. L., Zhu, M. & Mckeown, M. J. White matter myelin profiles linked to clinical subtypes of Parkinson’s disease. J. Magn. Reson. Imaging 50, 164–174 (2019).
Charroud, C. & Turella, L. Subcortical grey matter changes associated with motor symptoms evaluated by the Unified Parkinson’s disease Rating Scale (part III): a longitudinal study in Parkinson’s disease. Neuroimage Clin. 31, 102745 (2021).
Jeong, S. H. et al. Local striatal volume and motor reserve in drug-naive Parkinson’s disease. NPJ Parkinsons Dis. 8, 168 (2022).
Zhong, Y. et al. A review on pathology, mechanism, and therapy for cerebellum and tremor in Parkinson’s disease. NPJ Parkinsons Dis. 8, 82 (2022).
Milosevic, L. et al. Physiological mechanisms of thalamic ventral intermediate nucleus stimulation for tremor suppression. Brain 141, 2142–2155 (2018).
Lin, C. H. et al. VBM reveals brain volume differences between parkinson’s disease and essential tremor patients. Front. Hum. Neurosci. 7, 247 (2013).
Barbagallo, G. et al. Structural connectivity differences in motor network between tremor-dominant and nontremor Parkinson’s disease. Hum. Brain Mapp. 38, 4716–4729 (2017).
Li, X. et al. Association of Myelin Disruption and Iron Accumulation on MRI With Parkinson's Disease Severity. J Magn Reson Imaging. https://doi.org/10.1002/jmri.29832 (2025).
Lee, H. et al. Differential effect of iron and myelin on susceptibility MRI in the substantia nigra. Radiology 301, 682–691 (2021).
Xiao, Y. et al. An accurate registration of the BigBrain dataset with the MNI PD25 and ICBM152 atlases. Sci. Data 6, 210 (2019).
Acknowledgements
This work was supported by the National Natural Scientific Foundation of China (No. 82172012) and the Foshan 14th Five-Year Plan Key Discipline Foundation (No. FSGSP145036).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study as follows: Conception and Design: Dongliang Cheng, Ge Wen; Data Curation and Methodology: Dongliang Cheng and Junyan Wen, Yulin Liu, Yunjun Yang; Patient Diagnosis and Collection of Clinical Evaluations: Nan Ding, Zhenpeng Duan and Biqing Lin. Magnetic Resonance Data Acquisition: Yaozhong Wu, Hang Wang, and Jincheng Ma; Analysis and Interpretation of Data: Dongliang Cheng, Junyan Wen, and Yulin Liu, JiaLu Zhang; Writing of the Manuscript: Dongliang Cheng; Guide the Researcher: Zhifeng Xu, Hai Zhao, and Ge Wen; Review of the Manuscript and Study Supervision: Ge Wen.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cheng, D., Wen, J., Ding, N. et al. Synthetic MRI study of brain volume and subcortical myelin in various Parkinson’s disease motor subtypes. npj Parkinsons Dis. 11, 255 (2025). https://doi.org/10.1038/s41531-025-01120-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-025-01120-x