Abstract
Social dysfunction remains a core feature of schizophrenia (SCZ), particularly in individuals exhibiting prominent negative symptoms. The amygdala (AMYG), a key structure in emotional and social processing, may contribute to this dysfunction. This study investigated whether structural and functional alterations in the AMYG mediate the effects of negative symptoms on social functioning in SCZ. A total of 205 male participants were included: 53 with deficit schizophrenia (DS), 76 with non-deficit schizophrenia (NDS), and 76 matched healthy controls (HCs). Negative symptoms were assessed using the Scale for the Assessment of Negative Symptoms, and social functioning was evaluated with the Scale of Social Function in Psychosis Inpatients. Structural and resting-state functional MRI data were acquired. Amygdala volumes and region-of-interest-based functional connectivity (FC) were analyzed, and path analysis was used to test mediation effects. Patients with SCZ showed significantly reduced bilateral AMYG volumes compared to HCs. Within the SCZ group, the left amygdala (AMYG.L) was smaller than the right, with further reduction observed in DS compared to NDS. FC between the AMYG.L and the left superior temporal gyrus (STG.L) was also decreased in DS. Mediation analysis revealed that both AMYG.L volume and its FC with STG.L partially mediated the association between negative symptoms and poor social function. These findings suggest that AMYG.L abnormalities may involve social dysfunction in DS, offering potential targets for early intervention aimed at improving social outcomes in male patients with schizophrenia.
Similar content being viewed by others
Introduction
Schizophrenia (SCZ) is a severe psychiatric condition characterized by a combination of positive symptoms, negative symptoms, and cognitive impairment1. The negative symptoms in SCZ comprise affective flattening, alogia, avolition, asociality, stereotyped thinking, and anhedonia, and are reported to be associated with social dysfunction2,3. Long-term social dysfunction in SCZ patients adversely affects their prognosis and poses a burden on both their families and society.
In recent decades, magnetic resonance imaging (MRI) has enabled exploration of the neurological correlates of the symptoms of SCZ. The amygdala (AMYG), a subcortical component of the limbic system, plays a crucial role in decision-making and adaptation of instinctive and motivated behavior to environmental change, which are two functions commonly compromised in SCZ patients4,5,6. According to a meta-analysis, structural abnormalities of the left amygdala (AMYG.L) relate to enduring negative symptoms in SCZ7. Furthermore, structural asymmetries in the bilateral AMYG have been associated with affective and communicative aspects of negative symptoms in SCZ8. Rahm et al.9 found that reduced functional activity of the AMYG.L was linked to blunted emotional responses in SCZ patients, while structural alterations in the right amygdala (AMYG.R) were linked to stereotyped thinking. These results imply a potential involvement of AMYG dysfunction in the development of negative symptoms in SCZ. Prior studies have identified an inverse relationship between the severity of negative symptoms and functional connectivity (FC) strength between the bilateral AMYG and the middle temporal gyrus in SCZ10. Cumulatively, these findings suggested that abnormalities in structure and function of AMYG are associated with negative symptoms in SCZ.
Structural and functional alterations in AMYG have also been implicated in social dysfunction in SCZ. Studies of social function report that SCZ patients show a decline in daily function and difficulties interacting with others11,12,13,14. A prior study demonstrated that the responses of patients with SCZ to external expressions of anger are positively correlated with the activation of the bilateral AMYG, highlighting the association between abnormal functional activity in the amygdala and social functioning15. Other studies have demonstrated enhanced amygdala responsivity to social stimuli in SCZ16,17. Another study revealed that reduced bilateral AMYG volume is associated with social dysfunction in SCZ. Multiple meta-analyses support that abnormal neural AMYG activity is associated with severe deficits in emotional face perception in SCZ patients5,18,19. Taken together, these studies indicate that social dysfunction in SCZ is likely linked to alterations in the structural integrity and neural activity of the AMYG. However, whether structural and functional AMYG abnormalities modulate the relationship between negative symptoms and social dysfunction in SCZ remains unknown.
To address this research gap, we recruited patients with deficit schizophrenia (DS), a subtype of SCZ characterized by primary, persistent negative symptoms. Patients with deficit schizophrenia exhibit more severe negative symptoms, greater impairment in social functioning, poorer insight into their illness and a less favorable functional prognosis compared to those with non-deficit schizophrenia20,21. Neuroimaging studies have revealed that patients with DS show altered functional connectivity within key brain networks, including the default mode network (DMN), salience network (SN), and nucleus accumbens (NAc) network22. These alterations are closely linked to the severity of negative symptoms and deficits in cognitive functioning. Focusing on DS increases patient homogeneity and may help minimize the confounding effects of other psychiatric symptoms on our results. Briefly, we compared structural and functional alterations in the bilateral AMYG among DS, non-DS (NDS), and healthy control (HC) groups. Path analysis was subsequently conducted to elucidate the relationships between negative symptoms, structural and functional AMYG alterations, and social function in DS patients23. It was hypothesized that AMYG volume would differ between the DS and NDS groups. We also explored group differences in region of interest (ROI)-wise functional (FC) connectivity based on the AMYG. Our findings of altered AMYG structure and function suggest that this brain region may mediate the influence of negative symptoms on social dysfunction in DS.
Methods
Study design
For this study, we enrolled 129 male individuals diagnosed with SCZ and 76 male HCs. Diagnoses were confirmed independently by three experienced psychiatrists in accordance with the criteria specified in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Additional inclusion criteria were: right-handed, aged 30-70 years. on a stable course of antipsychotic medication, and exhibited consistent psychotic symptoms for a minimum of 1 year. Exclusion criteria included a history of traumatic brain injury, substance abuse, intellectual disability, or prior electroconvulsive therapy. Patients were further classified into the DS based on scores from the Chinese version of the Syndrome Diagnostic Scale (SDS)24. This scale comprises of two parts. The first section evaluates the clinical manifestations and specific features of negative symptoms. Symptoms severity is rated on a 5-point scale ranging from 0 to 4, while the presence of primary and enduring symptoms is assessed using binary (“yes” or “no”) criteria. The second section provides a diagnostic summary based on the initial assessment, determining whether the patient meets the criteria for DS. To fulfill the diagnostic threshold for DS, individuals must exhibit at least two negative symptoms—such as diminished emotional expression, social withdrawal, restricted affect, poverty of speech, avolition, or lack of goal-directed behavior—that are rated at a moderate level or higher and persist for more than one year. These symptoms must be primary in nature and not attributable to secondary factors such as depression, paranoid ideation, or anxiety. For the HC group, participants were matched to SCZ patients on sex, age, handedness, and years of education. Exclusion criteria for controls included any diagnosis of neurological illness or significant head trauma. Individuals with a personal or first-degree family history of psychiatric disorders were also excluded. All participants or their legal guardians provided written informed consent prior to participation. The research protocol was reviewed and approved by the Institutional Ethics Committee of Zhongda Hospital, Southeast University.
Assessment of clinical and social function
In SCZ patients, negative symptoms were evaluated using the Scale for Assessment of Negative Symptoms (SANS) and social function was evaluated using the Scale of Social Function in Psychosis Inpatients (SSPI)25,26. To provide a realistic assessment of actual living conditions of psychiatric patients and the content of their rehabilitation, the SSPI contains 12 items divided into 3 categories based on item similarity. These categories were utilized to evaluate daily living skills, motility and social competence, and social activity skills, with each category comprising four items. Each item was rated on a 5-point scale, where scores ranged from 0 (indicating extreme functional impairment) to 4 (representing intact functioning), yielding a total score ranging from 0 to 48. A higher score indicates superior social functioning.
Data acquisition
All participants underwent neuroimaging using a 3.0 T MR scanner (GE HDx, Chicago, Illinois). Functional MRI scans were acquired using a gradient echo-planar imaging (EPI) sequence with the following parameters: repetition time (TR) = 2000 ms, echo time (TE) 25 ms, flip angle 90°, field of view (FOV) 240 mm × 240 mm2, matrix size 64 × 64, and 35 contiguous axial slices (thickness 4 mm, no inter-slice gap), resulting in isotropic voxels of 4 mm3. A total of 240 volumes were obtained over an 8-minute resting-state scan. High-resolution structural images were collected using a magnetization-prepared rapid gradient echo (MPRAGE) sequence with TR = 8.16 ms, TE = 3.18 ms, inversion time = 450 ms, FOV = 240 × 240 mm2, matrix = 256 × 256, slice thickness = 1.0 mm, and a flip angle of 15°, yielding whole-brain T1-weighted anatomical coverage. To reduce motion artifacts and acoustic noise, participants were fitted with foam padding and provided earplugs during scanning. They were instructed to lie still with their eyes closed, remain alert, and avoid any head movement throughout the scan session.
Data preprocessing
T1-weighted structural MRI data were preprocessed using the DPABISurf_V2.0 toolbox (http://rfmri.org/DPABISurf), which automates surface-based processing workflows in MATLAB 2016b (http://www.mathworks.com/products/matlab/). Initially, image intensity non-uniformity was corrected using the N4BiasFieldCorrection algorithm provided in ANTs (v2.3.3)27,28. The bias-corrected T1 image was subsequently designated as the anatomical reference for the entire pipeline. Skull-stripping was conducted via a Nipype-integrated version of the antsBrainExtraction.sh script, employing the OASIS30ANTs template as the extraction target. Cortical reconstruction was performed using the recon-all function from FreeSurfer (version 7.2.0)29. To ensure accurate cortical delineation, segmentation outputs from ANTs and FreeSurfer were harmonized through a customized Mindboggle-based reconciliation procedure30. Subsequent volume-based spatial normalization to MNI152NLin2009cAsym space was achieved through nonlinear registration using antsRegistration, applied to skull-stripped T1 images and templates. The bilateral amygdala volumes were then extracted for downstream statistical analyses.
Functional MRI data were processed using methods similar to those described in our previous study31. The details of the spatial smoothing was performed using a Gaussian kernel with a full width at half maximum (FWHM) of 8 mm3. There were no statistically significant differences in head motion parameters among the DS, NDS, and HC groups (all p-values > 0.05).
Brain FC analysis
ROI-wise FC was conducted using DPARS. Following preprocessing, seed-based correlation analysis was performed to examine the connectivity patterns of the AMYG. The brain regions of the anatomical automatic labeling (AAL) template were chosen as seed points for the functional connectivity. Brain regions that exhibited significant volumetric differences between the SCZ subgroups were selected as seed ROIs for connectivity analysis. Group-level comparisons of amygdala-centered FC between different subgroups were assessed using two-sample t-tests, with age and education as the covariates. To account for multiple comparisons, results were corrected using Gaussian random field (GRF) (voxel significance: p < 0.01, cluster significance: p < 0.01).
Statistical analysis
All statistical procedures were conducted using SPSS software (version 24.0; IBM, Armonk, New York). The Kolmogorov-Smirnov test was employed to assess whether the continuous variables followed a normal distribution.Comparison of the bilateral AMYG volume among the HC, DS, and NDS groups was performed using analysis of variance (ANOVA). Differences in AMYG volume and social function between subgroups were compared using two-sample t-tests. The relationship between negative symptoms and social functioning was explored through path analysis conducted using AMOS software (version 24.0). The assessment of model fit included evaluations based on χ² statistics, the comparative fit index (CFI), and the root mean square error of approximation (RMSEA). A CFI greater than 0.80, coupled with an RMSEA < 0.08, generally suggests acceptable model fit.
Results
Comparison of demographics characteristics and social functioning between SCZ and HC groups
As shown in Table 1, there were no notable differences among the DS, NDS, and HC groups in terms of age (F (2, 202) = 0.016, p = 0.984), years of education (F (2, 202) = 1.404, p = 0.248), or body mass index (F (2, 202), p = 0.241). The data are normally distributed. All participants were male. In terms of social functioning, the DS group scored significantly lower than the NDS and HC groups in all evaluated areas (all p < 0.001).
Comparison of bilateral AMYG volume between SCZ and HC groups
The structural MRI data indicated significant differences in both the AMYG.L (F (2, 202) = 25.779, p < 0.001) and AMYG.R (F (2, 202) = 24.283, p < 0.001) volumes between the SCZ patient and HC groups, with the SCZ patient group having a smaller volume. Furthermore, individuals in the DS group exhibited a significantly reduced volume in AMYG.L compared to those in the NDS group (t = −2.27, p = 0.025). In contrast, no statistically significant differences were found between groups regarding the volume of AMYG.R. All results are displayed in Table 1. Furthermore, the AMYG.L volume was found to be smaller than the AMYG.R volume in both the DS (t = −9.020, p < 0.001) and NDS (t = −9.077, p < 0.001) groups.
Group differences in amygdala FC
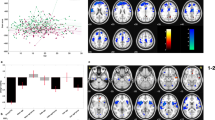
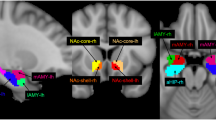
FC analyses encompassing the entire brain were performed independently for the DS, NDS, and HC groups. As depicted in Fig. 1 and detailed in Table 2, individuals with DS demonstrated significantly lower FC strength between the AMYG.L and several regions, including the right insula, right superior frontal gyrus, left superior temporal gyrus, medial orbital gyrus (ORBsupmed.R), and left supplementary motor area (SMA.L), when compared to healthy controls. Within the DS group, there was notable enhancement of FC between the AMYG.L and regions such as the right calcarine cortex, right superior occipital gyrus, and right superior temporal gyrus (Fig. 1A). In the NDS group, relative to HCs, enhanced FC was evident between the AMYG.L and both the right middle temporal gyrus and the left lingual gyrus. In contrast, decreased FC was detected between the AMYG.L and bilateral insula, as well as the SMA.L (Fig. 1C). Additionally, when directly comparing DS and NDS groups, the DS group showed significantly diminished FC between the AMYG.L and the left superior temporal gyrus (Fig. 1B).
FC functional connectivity, DS deficit schizophrenia, NDS non-deficit schizophrenia, HC healthy controls. Z represents the location of the transect. ORBsupmed.R right superior frontal gyrus, medial orbital, INS.R right insula, CAL.R right calcarine fissure and surrounding cortex, SOG.R right superior occipital gyrus, STG.L left superior temporal gyrus, STG.R right superior temporal gyrus, SMA.L left supplementary motor area, MTG.R right middle temporal gyrus, INS.L left insula, LING.L left lingual gyrus. A Significant differences in FC between the DS and HC groups. Red and blue, respectively, indicate regions where the DS group had higher or lower FC than the HC group. B Significant differences in FC between the DS and NDS groups. Red and blue, respectively, represent regions where the DS group had higher and lower FC compared to the NDS group. C Significant differences in FC between the NDS and HC groups. Red and blue, respectively, represent regions where the NDS group had higher and lower FC compared to the HC group.
Path analysis results
The fit indices from the path analysis, including χ2/df =1.413, CFI = 0.920, and RMSEA = 0.06, suggest a good model fit. For the DS group, the analysis demonstrated that negative symptoms exerted indirect effects on motivation and socialization. Specifically, AMYG.L volume and FC between the AMYG.L and STG.L were identified as mediators of the negative indirect effect of negative symptoms on social functioning in DS. The calculated indirect effect of negative symptoms on social function was −0.058 (−0.35 × −0.40 × −0.42; Table 3). Notably, negative symptoms did not have direct effects on motivation or socialization of patients within the DS group (Fig. 2).
Squares represent measured variables (scale or scale dimension scores). Arrows connecting circles and rectangles in one direction show a hypothesized direct relationship between two variables. The numbers next to the paths are standardized regression coefficients. The bold black line represents that the effect between the variables is statistically significant (p < 0.05). The letter “e” represents the associated error. SANS Scale for Assessment of Negative Symptoms, AMYG.L left amygdala, STG.L left superior temporal gyrus, GMV volume of gray matter, FC functional connectivity.
Discussion
Our findings indicated that individuals with DS experience significantly greater impairments in social functioning compared to those with NDS. Additionally, SCZ patients demonstrated bilateral reduction in AMYG volume, with the AMYG.L being smaller than the AMYG.R. Notably, the DS group displayed a significantly smaller AMYG.L volume relative to the NDS group, while no substantial difference was observed for AMYG.R volume between the two subgroups. Furthermore, AMYG.L volume and its FC with the STG.L were found to mediate the indirect effects of negative symptoms on social functioning in the DS group. To the best of our knowledge, this is the first study to simultaneously examine how negative symptoms are associated with both structural and functional amygdala alterations and their joint impact on social dysfunction in DS.
We found that SCZ patients exhibited significant deficits in various domains of social functioning compared with HCs. These deficits included living skills, motility and social competence, and social activity skills. These findings are consistent with previous reports32. In terms of social functioning, the DS group performed worse than the NDS group in terms of activity autonomy, social initiative, and social skills such as learning, labor, and organizational skills. This disparity may be attributed to the fact that patients with DS, which is characterized by reduced expression and a lack of motivation, experience significant difficulties processing social information33. Consequently, impaired social cognition negatively impacts social functioning14,33,34. Take together, these symptoms support the notion that DS may constitute a distinct SCZ subtype, characterized by persistent and primary negative symptoms, which contribute to significant impairments in social functioning.
Moreover, we found that the AMYG.L volume was significantly smaller than the AMYG.R volume in each SCZ subgroup. This finding aligns with previous studies reporting structural asymmetry in the brains of patients with SCZ, specifically that the AMYG.L is smaller than the AMYG.R35,36. Previous studies by Guo et al. have demonstrated a negative correlation between the volume of the left amygdala and the severity of schizophrenia symptoms37. The authors proposed that this relationship might be attributed to compensatory atrophy resulting from prolonged abnormal functional activity of the left amygdala during the acute phase of the disorder. Similarly, Reynolds38 observed increased dopamine content in the left amygdala of schizophrenia patients, suggesting that the abnormal volume of this region may be linked to dysregulation of the dopamine system. SCZ patients who exhibit persistent negative symptoms showed reduced volume of the AMYG.L relative to the right8. We also observed that AMYG.L volume in the DS group was significantly smaller than that in the NDS group. Notably, Toll et al.39 reported that reduced AMYG.L volume predicted the severity of negative symptoms in SCZ patients. These findings suggest that the AMYG.L may be involved in the pathogenesis of negative symptoms in SCZ. However, Buchanan et al. did not observe volume differences between the AMYG.R and AMYG.L in DS or NDS patients. This discrepancy may relate to limitations of the sample size. Future research should aim to increase sample sizes to further investigate potential differences in AMYG volume across various SCZ subgroups. Identifying structural asymmetry in the AMYG among SCZ patients could enhance prediction of social functioning and inform early interventions aimed at improving long-term prognoses.
Abnormalities in the FC of the AMYG.L with multiple brain regions, including the bilateral insula, bilateral temporal lobes, left supplementary motor area, and left lingual gyrus, were also observed in patients with SCZ. Previous studies have reported similar results, with reduced FC between the AMYG and insula suggesting impaired functioning of social brain networks40. Notably, the insula serves as a key hub of the salience network, while the bilateral temporal cortices are integral components of the default mode network (DMN). The involvement of these areas supports the hypothesis that social impairment in schizophrenia may stem from disturbed inter-network communication, particularly across the salience, DMN, and social cognitive networks41. In addition, our results revealed a specific reduction in FC between the AMYG.L and the left superior temporal gyrus in the DS subgroup relative to NDS patients. This observation aligned with findings from Abram et al., who reported that decreased connectivity between these regions was associated with the severity of negative symptoms, especially diminished emotional and verbal expression10. In light of the aberrant brain regions identified within relevant neural networks, non-invasive brain stimulation (NIBS) techniques may offer a promising approach to modulate neuroplasticity, strengthen functional connectivity, and ultimately enhance social functioning in patients42. By selectively targeting these network nodes with NIBS, it is possible to modulate intra-network connectivity as well as inter-network interactions, thereby alleviating negative symptoms associated with schizophrenia43,44. Our findings strengthen the evidence linking AMYG.L dysconnectivity to the pathophysiology of negative symptoms and suggest potential targets for early identification and intervention strategies.
Furthermore, our path analysis revealed the impact of negative symptoms on social functioning in the DS group, highlighting the mediating roles of altered volume and FC of the AMYG.L. These findings indicate that negative symptoms adversely affect patients’ motivation and socialization, consistent with previous research reporting that these symptoms detrimentally influence social well-being in SCZ patients45,46. In our analysis, both the volume of the AMYG.L and altered FC between the AMYG.L and STG.L mediated these effects. Previous studies have also reported a significant correlation between AMYG.L volume and negative symptom severity in SCZ47,48. Similarly, reduced AMYG volume has been found to predict the severity of negative symptoms in SCZ patients39. Thus, abnormalities in the volume or FC of the AMYG.L may potentially contribute to the neurobiological signature of SCZ. Our model further indicated that structural and functional AMYG.L abnormalities mediate the influence of negative symptoms on specific aspects of social functioning (motivation and socialization) in patients with DS. Nevertheless, future research with larger sample sizes and more comprehensive metrics is essential to thoroughly explore the effects of negative symptoms on social functioning in SCZ.
Despite the potential significance of this study, several limitations should be noted. First, the cross-sectional design precludes exploration of dynamic structural and functional changes in the amygdala over time. Second, to ensure sample homogeneity, this study was limited to a small cohort of male patients; future research should involve larger sample sizes that include both male and female participants and use more comprehensive assessment tools. Third, all patients in this study were chronic SCZ sufferers receiving long-term antipsychotic treatment, resulting in stable psychotic symptoms. Notably, prior studies have demonstrated that patients on antipsychotic medication experience notable alterations in both brain structure and function49,50. Specifically, following treatment with clozapine or olanzapine, patients exhibited reduced activation of the left amygdala, along with decreased functional connectivity between the left amygdala and the right ventral striatum51. Future investigations that include first-episode SCZ patients and assess their brain structure and function longitudinally could provide valuable insights.
In conclusion, our preliminary findings underscore the presence of coupled structural and functional abnormalities of the AMYG.L in DS, which mediate the impact of negative symptoms on social function. These results provide important insights into the treatment of social dysfunction in male SCZ patients.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to them containing information that could compromise research participant consent.
References
Owen, M. J., Sawa, A. & Mortensen, P. B. Schizophrenia. Lancet (Lond., Engl.) 388, 86–97 (2016).
Kirkpatrick, B., Fenton, W. S., Carpenter, W. T. Jr. & Marder, S. R. The NIMH-MATRICS consensus statement on negative symptoms. Schizophrenia Bull. 32, 214–219 (2006).
Ventura, J. et al. Negative symptoms and functioning during the first year after a recent onset of schizophrenia and 8 years later. Schizophrenia Res. 161, 407–413 (2015).
Gallagher, M. & Chiba, A. A. The amygdala and emotion. Curr. Opin. Neurobiol. 6, 221–227 (1996).
Kohler, C. G., Walker, J. B., Martin, E. A., Healey, K. M. & Moberg, P. J. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophrenia Bull. 36, 1009–1019 (2010).
Šimić, G. et al. Understanding Emotions: Origins and Roles of the Amygdala. Biomolecules 11, 823 (2021).
Zhu, T. et al. Meta-analysis of structural and functional brain abnormalities in schizophrenia with persistent negative symptoms using activation likelihood estimation. Front. Psychiatry 13, 957685 (2022).
Huang, Z. et al. Negative symptoms correlate with altered brain structural asymmetry in amygdala and superior temporal region in schizophrenia patients. Front. Psychiatry 13, 1000560 (2022).
Rahm, C. et al. Negative symptoms in schizophrenia show association with amygdala volumes and neural activation during affective processing. Acta Neuropsychiatrica 27, 213–220 (2015).
Abram, S. V. et al. Oxytocin Enhances an Amygdala Circuit Associated With Negative Symptoms in Schizophrenia: A Single-Dose, Placebo-Controlled, Crossover, Randomized Control Trial. Schizophrenia Bull. 46, 661–669 (2020).
Favrod, J. et al. Improving Pleasure and Motivation in Schizophrenia: A Randomized Controlled Clinical Trial. Psychother. Psychosom. 88, 84–95 (2019).
Velthorst, E. et al. The 20-Year Longitudinal Trajectories of Social Functioning in Individuals With Psychotic Disorders. Am. J. Psychiatry 174, 1075–1085 (2017).
Bellack, A. S. et al. Assessment of community functioning in people with schizophrenia and other severe mental illnesses: a white paper based on an NIMH-sponsored workshop. Schizophrenia Bull. 33, 805–822 (2007).
Green, M. F., Horan, W. P. & Lee, J. Social cognition in schizophrenia. Nat. Rev. Neurosci. 16, 620–631 (2015).
Pinkham, A. E. et al. Abnormal modulation of amygdala activity in schizophrenia in response to direct- and averted-gaze threat-related facial expressions. Am. J. Psychiatry 168, 293–301 (2011).
Adolphs, R. The social brain: neural basis of social knowledge. Annu. Rev. Psychol. 60, 693–716 (2009).
Brunet-Gouet, E. & Decety, J. Social brain dysfunctions in schizophrenia: a review of neuroimaging studies. Psychiatry Res. 148, 75–92 (2006).
Delvecchio, G., Sugranyes, G. & Frangou, S. Evidence of diagnostic specificity in the neural correlates of facial affect processing in bipolar disorder and schizophrenia: a meta-analysis of functional imaging studies. Psychological Med. 43, 553–569 (2013).
Taylor, S. F. et al. Meta-analysis of functional neuroimaging studies of emotion perception and experience in schizophrenia. Biol. Psychiatry 71(Jan), 136–145 (2012).
Galderisi, S. et al. Categorical and dimensional approaches to negative symptoms of schizophrenia: focus on long-term stability and functional outcome. Schizophr. Res 147, 157–162 (2013).
Kirkpatrick, B., Buchanan, R. W., Ross, D. E. & Carpenter, W. T. Jr. A separate disease within the syndrome of schizophrenia. Arch. Gen. Psychiatry 58, 165–171 (2001).
Zhou, C. et al. Convergent and divergent genes expression profiles associated with brain-wide functional connectome dysfunction in deficit and non-deficit schizophrenia. Transl. Psychiatry 14, 124 (2024).
Bowie, C. R., Reichenberg, A., Patterson, T. L., Heaton, R. K. & Harvey, P. D. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am. J. Psychiatry 163, 418–425 (2006).
Wang, X., Yao, S., Kirkpatrick, B., Shi, C. & Yi, J. Psychopathology and neuropsychological impairments in deficit and nondeficit schizophrenia of Chinese origin. Psychiatry Res. 158, 195–205 (2008).
Bhola, P. et al. Development of a Social Skills Assessment Screening Scale for Psychiatric Rehabilitation Settings: A Pilot Study. Indian J. Psychological Med. 38, 395–403 (2016).
Yu, W. et al. Analysis of Medication Adherence and Its Influencing Factors in Patients with Schizophrenia in the Chinese Institutional Environment. Int. J. Environ. Res. Public Health 18, 4746 (2021).
Tustison, N. J. et al. N4ITK: improved N3 bias correction. IEEE Trans. Med. Imaging 29, 1310–1320 (2010).
Avants, B. B., Epstein, C. L., Grossman, M. & Gee, J. C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 12, 26–41 (2008).
Dale, A. M., Fischl, B. & Sereno, M. I. Cortical surface-based analysis. I. Segmentation Surf. reconstruction. NeuroImage 9, 179–194 (1999).
Klein, A. et al. Mindboggling morphometry of human brains. PLoS Computational Biol. 13, e1005350 (2017).
Fang, J. et al. Aberrant brain functional connectivity mediates the effects of negative symptoms on cognitive function in schizophrenia: A structural equation model. J. Psychiatr. Res. 177, 109–117 (2024).
van Os, J. & Kapur, S. Schizophrenia. Lancet (Lond., Engl.) 374, 635–645 (2009).
Blanchard, J. J. & Cohen, A. S. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophrenia Bull. 32, 238–245 (2006).
Green, M. F., Hellemann, G., Horan, W. P., Lee, J. & Wynn, J. K. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch. Gen. Psychiatry 69, 1216–1224 (2012).
Okada, N. et al. Abnormal asymmetries in subcortical brain volume in schizophrenia. Mol. Psychiatry 21, 1460–1466 (2016).
Zheng, F. et al. Study on the sub-regions volume of hippocampus and amygdala in schizophrenia. Quant. Imaging Med. Surg. 9, 1025–1036 (2019).
Guo, H. et al. Amygdala signal abnormality and cognitive impairment in drug-naïve schizophrenia. BMC Psychiatry 23, 231 (2023).
Reynolds, G. P. Increased concentrations and lateral asymmetry of amygdala dopamine in schizophrenia. Nature 305, 527–529 (1983).
Toll, A. et al. Multidimensional predictors of negative symptoms in antipsychotic-naive first-episode psychosis. J. Psychiatry Neurosci. : JPN 47, E21–e31 (2022).
Mukherjee, P. et al. Altered amygdala connectivity within the social brain in schizophrenia. Schizophrenia Bull. 40, 152–160 (2014).
Seeley, W. W. et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. : Off. J. Soc. Neurosci. 27, 2349–2356 (2007).
Gainsford, K., Fitzgibbon, B., Fitzgerald, P. B. & Hoy, K. E. Transforming treatments for schizophrenia: Virtual reality, brain stimulation and social cognition. Psychiatry Res 288, 112974 (2020).
Lefaucheur, J. P. et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 125, 2150–2206 (2014).
Palm, U. et al. Prefrontal Transcranial Direct Current Stimulation for Treatment of Schizophrenia With Predominant Negative Symptoms: A Double-Blind, Sham-Controlled Proof-of-Concept Study. Schizophr. Bull. 42, 1253–1261 (2016).
Cai, R. et al. The motivation and pleasure deficits but not expressivity affects social functioning through cognitive function in male patients with schizophrenia: A structural equation model. Asian J. Psychiatry 85, 103616 (2023).
Kaneko, K. Negative Symptoms and Cognitive Impairments in Schizophrenia: Two Key Symptoms Negatively Influencing Social Functioning. Yonago Acta Med. 61, 91–102 (2018).
Prestia, A. et al. Hippocampal and amygdalar local structural differences in elderly patients with schizophrenia. Am. J. Geriatr. Psychiatry : Off. J. Am. Assoc. Geriatr. Psychiatry 23, 47–58 (2015).
Welch, K. A. et al. Amygdala volume in a population with special educational needs at high risk of schizophrenia. Psychological Med. 40, 945–954 (2010).
Kraguljac, N. V. et al. Aberrant Hippocampal Connectivity in Unmedicated Patients With Schizophrenia and Effects of Antipsychotic Medication: A Longitudinal Resting State Functional MRI Study. Schizophrenia Bull. 42, 1046–1055 (2016).
Zhang, Z. et al. Dynamic functional connectivity and its anatomical substrate reveal treatment outcome in first-episode drug-naïve schizophrenia. Transl. Psychiatry 11, 282 (2021).
Mier, D. et al. Reduced activity and connectivity of left amygdala in patients with schizophrenia treated with clozapine or olanzapine. Eur. Arch. Psychiatr. y. Clin. Neurosci. 269, 931–940 (2019).
Acknowledgements
This study was conducted in according to the local and international ethical principles including those in the Declaration of Helsinki. This study was approved by the Human Ethics Committee of ZhongDa Hospital Affiliated to Southeast University (approval number: 2013ZDSYLL52.0). Written informed consent was obtained from individual or guardian participants. This study was supported by the National Natural Science Foundation of China (82371510 and 82101572), Social Development Foundation of Jiangsu Province, China (No. BE2023668), and Nanjing Major Science and Technology Project (Life and Health, No 202305035).
Author information
Authors and Affiliations
Contributions
All authors contributed to the preparation of the manuscript. X.R.Z .and C.Z. designed and organized the research. X.W.T. provided the S.D.S. Y.D.L., C.R.W., and X.Y.F. collected the imaging and cognitive data. J.F., Y.S.H. and Y.L. analyzed the data and wrote the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fang, J., Hu, Y., Li, Y. et al. Left amygdala alterations mediate the effects of negative symptoms on social dysfunction in schizophrenia. Schizophr 11, 107 (2025). https://doi.org/10.1038/s41537-025-00655-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-025-00655-5