Abstract
Individuals with schizophrenia face significantly higher mortality rates than the general population, with a typical reduction in life expectancy of 15–20 years. This study investigated 10-year all-cause mortality and its clinical correlates in a Romanian cohort of patients with schizophrenia, using real-world clinical and hospital and forensic records. A total of 635 individuals hospitalized between 2010 and 2013 were followed for 10 years. Mortality rates, causes of death, and risk factors were assessed using Cox regression models and standardized mortality ratios (SMRs). During the follow-up, 123 patients (19.37%) died, corresponding to a mortality rate of 21.3 per 1000 person-years. The SMR was 1.58 compared to the Romanian general population. Non-violent causes predominated, with cardiovascular disease (27.64%) and infections (17.07%) being the most frequent. Violent deaths, including suicides and accidents, accounted for 17.07% of all mortality. The mean age at death was 58.97 years, reflecting a 17-year reduction in life expectancy. Age was the strongest independent predictor of mortality (HR = 1.07, p < 0.001). Use of second-generation antipsychotics (HR = 0.37, p < 0.001) and low frequency hospitalization (HR = 0.09, p < 0.001) were significantly associated with reduced all-cause and cause-specific mortality. Schizophrenia is associated with significantly increased premature mortality, primarily due to preventable physical illnesses and violent deaths. Early intervention, sustained treatment adherence, and integrated medical care are essential to improve survival outcomes.
Similar content being viewed by others
Introduction
Schizophrenia is a chronic psychiatric disorder affecting ~20 million people globally. It is characterized by hallucinations, delusions, and disorganized thinking, which severely impair daily functioning and quality of life1. Individuals with schizophrenia experience significantly reduced life expectancy—typically 15–20 years shorter than the general population2,3. This mortality gap has been observed across diverse populations; for example, recent data from Western China reported marked reductions in life expectancy and increased years of potential life lost among individuals with schizophrenia4. Moneta et al. found life expectancy reductions of 8–11 years among individuals with schizophrenia in Catalonia5, while a meta-analysis by Chan et al. reported an average of 15.37 years [95% CI 14.18–16.55] of potential life lost across studies of schizophrenia6. Although this disparity is well recognized, the underlying factors remain insufficiently understood. Modifiable factors include unhealthy lifestyle habits, limited access to healthcare, comorbid physical conditions, and inconsistent adherence to antipsychotic medication7. Younger individuals are particularly vulnerable, with increased mortality linked to both suicide and physical health complications8.
Both natural and unnatural causes contribute to the elevated mortality observed in this population, often worsened by systemic barriers, substance use disorders, and coexisting illnesses. Cardiovascular disease, respiratory illness, and diabetes are common natural causes of death, often exacerbated by medication side effects, physical inactivity, and poor diet9.
Approximately two-thirds of excess mortality is due to natural causes, particularly cardiovascular disease and cancer9,10. Cardiovascular disorders, especially acute myocardial infarction and coronary artery disease, are leading causes of sudden death in schizophrenia11. First-generation antipsychotics are associated with cardiovascular risks such as QT interval prolongation and arrhythmias. Second-generation antipsychotics are more often linked to metabolic complications, including diabetes and dyslipidemia, which lead to long-term cardiovascular risk12,13.
Recent work has drawn attention to the high burden of infectious diseases in schizophrenia, particularly pneumonia, which contributes significantly to premature mortality14. This vulnerability is thought to result not only from immune system alterations and barriers to timely medical care15 but also from the effects of antipsychotic medications, which can increase the risk of aspiration due to sedation and hypersalivation16. Among these, clozapine has been strongly associated with elevated pneumonia risk. De Leon and colleagues17 report that pneumonia accounts for nearly 30% of all clozapine-related deaths, surpassing even the risk of agranulocytosis. Additionally, institutionalized patients with schizophrenia—such as those in long-term psychiatric care—exhibit especially high pneumonia-related mortality18, highlighting the combined impact of clinical severity, limited mobility, and reduced access to somatic healthcare.
Suicide remains a leading cause of death, particularly during the early stages following diagnosis, underscoring the need for ongoing mental health care14. The suicide rate in individuals with schizophrenia is 8.5–14 times higher than in the general population, with an 80-fold increase in first attempts19. Older antipsychotics, such as thioxanthene, are associated with greater suicide and all-cause mortality, while atypical antipsychotics appear to offer some protective effect20. Co-occurring substance use disorders significantly increase suicide risk by contributing to instability, impulsivity, and exposure to violence21. The first year after diagnosis is particularly high risk, especially among young men. A longer duration of untreated psychosis (DUP) is also associated with poorer outcomes22.
This study aimed to examine mortality rates, causes of death, and associated risk factors over a 10-year period in patients with schizophrenia, providing valuable insights into long-term health outcomes in this population.
Methods
Study design and setting
This retrospective observational cohort study was conducted at the Clinical Hospital of Psychiatry and Neurology Brașov, Romania, an academic center with 100 beds for acute psychiatric patients and 315 beds for long-term admissions. The study assessed clinical characteristics, mortality, and causes of death in a cohort of 635 patients diagnosed with schizophrenia, hospitalized between 2010 and 2013, and followed for a period of 10 years.
Participants
Inclusion criteria were: patients aged 18 years or older at the time of hospitalization, with a confirmed diagnosis of schizophrenia based on DSM-5 criteria23, admitted between 2010 and 2013, and who provided written informed consent. All patients had a confirmed diagnosis of schizophrenia made prior to the hospitalization episode included in the study, by board-certified psychiatrists using structured assessments consistent with DSM-5 criteria23. Socio-economic status was assessed at the time of hospital admission using a standardized intake protocol that included educational level, employment status, and monthly income. This information was used to construct a basic socio-economic index for each patient and was available for the entire cohort. Exclusion criteria included diagnoses of schizoaffective disorder, acute or substance-induced psychosis, brief or delusional disorders, intellectual disability, and organic mental disorders. Gender was recorded as a binary variable (male/female) based on hospital records; gender identity was not assessed.
Data collection and ethics
Patients were identified through hospital records and matched in the National Health Insurance (CNAS) database using their Unique Personal Number (CNP) to confirm survival status. Deaths were verified through the Forensic Medicine Service Brașov. Ethical approval was obtained from the ‘’Ethics Committee of the Brașov Clinical Hospital of Psychiatry and Neurology ‘’ (decision no. 13, 13 November 2023). All patients had previously signed the hospital’s general informed-consent form, which permits the use of fully anonymized clinical data for ethically approved research. Because the data were retrospective and de-identified, the committee waived the need for additional written consent. Data were fully anonymized and no identifiable information was processed. All procedures conformed to the Declaration of Helsinki24 and EU GDPR.
Statistical analysis
Statistical analyses were conducted to investigate factors associated with all-cause and cause-specific mortality. Statistical analyses were performed using SPSS version 25.0 (IBM Corp.) and GraphPad Prism version 9.0 (GraphPad Software, LLC). Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals for variables including age, sex, duration of untreated psychosis, age of onset, hospital readmission frequency, and antipsychotic treatment type. Age of onset was categorized based on the age at first psychotic symptoms, as recorded in clinical files. Typical onset was defined as occurring between 18 and 40 years, while early onset was defined as <18 years and late onset as >40 years, in accordance with common epidemiological criteria for schizophrenia onset23. Standardized mortality ratios (SMRs) were calculated to compare observed mortality in the cohort with expected rates in the general population, adjusted for age and sex. Effect sizes are reported as hazard ratios with 95% confidence intervals, which are standard for time-to-event (survival) analyses. All statistical tests were two-sided, with significance set at p < 0.05. The proportional hazards assumption for Cox regression was assessed and found to be met. No corrections for multiple comparisons were applied, as analyses were exploratory and aimed at identifying potential associations rather than testing predefined hypotheses.
Outcomes
The primary outcome was death, categorized as violent (e.g., suicide, accidents) or non-violent (e.g., comorbid medical conditions). Demographic, clinical, and treatment-related factors—such as the use of clozapine and long-acting injectable antipsychotics—were analyzed as potential predictors of mortality.
Results
The sampling and inclusion process is summarized in Fig. 1, which presents the total number of psychiatric inpatients initially screened, the exclusion criteria applied, and the final sample included in the analysis.
The study included 635 patients, with a mean age at baseline of 45.26 years (SD = ± 11.52) and an average age of onset of 26.68 years (SD = ± 8.01). As shown in Table 1, which presents the demographic characteristics of the cohort, 57.96% were females and 42.04% were males. The average duration of illness was 18.57 years (SD = ± 11.50), with a mean duration of untreated psychosis (DUP) of 1.84 years (SD = ± 1.51). On average, patients experienced 18.09 relapses (SD = ± 9.39) that required hospitalization throughout the observation period. The majority of patients (92.28%) were classified as having low to medium socio-economic status, typically receiving disability pensions or sickness allowances. A small subset (3.94%) had no formal insurance but still accessed care, while 3.78% had moderate to high socio-economic status, maintaining employment and higher premorbid functioning.
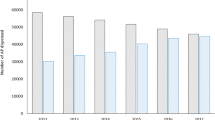
During the 10-year follow-up period, 123 patients (19.37%) died. The mean age at death in the study cohort was 58.97 years (SD = ± 11.58). Compared to the Romanian general population life expectancy (75.94), this corresponds to a mean of ~17 years of life lost per patient (YLL). Figure 2 presents individual-level age at death and YLL.
The mortality rate was calculated based on the number of observed deaths relative to the follow-up time contributed by all patients. The overall mortality rate was 21.3 deaths per 1000 person-years.
Mortality rates in the cohort were compared to those of the general Romanian population, which averaged 12.22 deaths per 1000 inhabitants annually between 2010 and 2023, according to the Romanian National Statistical Institute25. Over the ten-year period, 77.6 deaths were expected in the cohort of 635 patients, while 123 were observed, resulting in a standardized mortality ratio (SMR) of 1.58—indicating a 58% higher mortality risk. In males, the SMR was 1.50 (49 observed vs. 32.7 expected), and in females, 1.64 (74 observed vs. 45.0 expected). Table 2 presents these findings, including slightly higher mortality rates among females (22.08) than males (20.26 per 1000 person-years). Patients with a DUP exceeding three years had the highest mortality rate (26.27 per 1000 person-years; 23.81% mortality proportion), while those treated within one year had a lower rate (19.82 per 1000 person-years). However, this difference was not statistically significant (RR = 1.33, 95% CI: 0.76–1.89; χ² = 0.76, p = 0.38), indicating that additional factors may influence mortality.
Causes of death
Among the 123 deaths identified in the cohort, the cause of death was successfully determined in 104 cases (84.55%). These were classified as either external (violent) or resulting from medical conditions (non-violent). In 19 cases (15.45%), the cause of death remained unknown. The classification of these cases was limited by data accessibility. Non-violent deaths predominated (67.48%), with cardiovascular disease (27.64%), infectious diseases (17.07%), and cancer (12.19%) most common, as summarized in Table 3. Violent deaths accounted for 17.07% of total mortality, including 8 suicides (6.50%), by methods such as jumping from a height, intentional overdose, drowning or hanging. Most suicides (6 out of 8; 75%) occurred within the first three years of follow-up. Accidental deaths (13 cases; 10.57%) were primarily due to mechanical asphyxia from airway obstruction caused by aspiration of food during eating (7 cases; 5.69%), possibly linked to sedative side effects of antipsychotic medication. Of the accidental deaths, 6 (46.2%) occurred in the first six years, and 7 (53.8%) in the last 3 years. A summary of demographic and clinical characteristics for patients who died from violent causes (n = 21) is presented in Supplementary Table 1.
Infection-related deaths occurred in 21 patients (17.07%). Pneumonia was the most frequent infectious cause, responsible for 9 deaths (7.32%). An additional 5 patients (4.07%) died during confirmed COVID-19 infections in which pneumonia was present but not listed as the immediate cause of death; instead, complications such as pulmonary thromboembolism or multi-organ failure were recorded. The remaining 7 deaths (5.69%) were due to other infections, including sepsis (originating from urinary tract or post-surgical infections), influenza, and peritonitis secondary to intestinal tuberculosis. Infection-related deaths increased over time: 2 (9.5%) occurred during the first three years, 5 (23.8%) between years 4–6, and 14 (66.7%) in the final four years. Cardiovascular mortality remained relatively stable throughout the study period.
To highlight mortality, we used cumulative incidence curves (1 − S(t)), the complement of the Kaplan–Meier survival function, which emphasize the accumulating probability of death over time. As shown in Fig. 3, all-cause mortality increased progressively across the 10-year follow-up (Fig. 3a), largely driven by non-violent deaths (Fig. 3b), while violent deaths remained less frequent but occurred steadily over time (Fig. 3c).
Predictors of mortality
Univariable Cox regression analyses were conducted to examine predictors of all-cause mortality among patients with schizophrenia. The results are detailed in Supplementary Table 2. Age was the strongest predictor of mortality, with each additional year associated with a 7% increase in mortality risk (HR = 1.07, 95% CI: 1.06–1.09, p < 0.001). Compared to patients under 30 years, those aged 50–59 had nearly an eightfold increased risk (HR = 7.75, p = 0.005), while those aged 70–75 had a 34-fold increase (HR = 34.17, p < 0.001), reflecting the cumulative burden of ageing in this population. In contrast, sex (HR = 0.92, p = 0.63), age of onset (HR = 1.00, p = 0.905), and DUP (HR = 1.05, p = 0.276) were not significantly associated with mortality.
Regarding antipsychotic treatment, SGAs were associated with a 63% reduction in mortality risk compared to FGAs (HR = 0.37, p < 0.001), indicating a possible protective effect. Antipsychotic polypharmacy showed no statistically significant association (HR = 0.24, p = 0.155), likely due to the small sample size (n = 25, 3.94%). Clozapine, prescribed to 112 patients, also showed no significant effect on mortality (HR = 0.65, p = 0.123). LAI use overall was not significantly associated with mortality (HR = 0.84, p = 0.448); however, SGA LAIs significantly reduced mortality risk by 81% (HR = 0.19, p = 0.020), while FGA LAIs showed a non-significant increase (HR = 1.27, p = 0.334).
To approximate illness course and continuity of care, we calculated the average number of psychiatric admissions per patient and per follow-up year. Three categories were defined: low frequency (no more than one hospitalization every two years) moderate frequency (approximately one hospitalization per year) and high frequency (more than one hospitalization per year). Readmission frequency was strongly associated with all-cause mortality (χ² = 22.08, p < 0.001). High readmission frequency was associated with the greatest all-cause mortality. Relative to the high-frequency group, moderate frequency was linked to a 74% lower risk (HR = 0.26, p = 0.008) and low frequency to a 91% lower risk (HR = 0.09, p < 0.001).
In cause-specific analyses, age remained a significant predictor: each additional year increased the risk of non-violent deaths by 8% (HR = 1.08, p < 0.001) and violent deaths by 6% (HR = 1.06, p = 0.003). SGAs reduced the risk of non-violent death by 58% (HR = 0.42, p < 0.001) and violent death by 79% (HR = 0.21, p < 0.001). SGA LAIs were especially protective against non-violent deaths (HR = 0.13, p = 0.044), while FGA LAIs were associated with a threefold increased risk of violent death (HR = 3.20, p = 0.012).
Hospital readmission frequency was also associated with cause-specific mortality patterns. No violent deaths were recorded among patients with low readmission frequency (HR = 0; p = 0.949), while those with moderate readmission had an estimated 80% lower risk compared to the high-frequency group, though this was not statistically significant (HR = 0.20; p = 0.113). For non-violent deaths, patients with low readmission frequency had an 84% reduction in risk (HR = 0.16; p = 0.016), and those with moderate frequency showed a non-significant 66% reduction (HR = 0.34; p = 0.132).
DUP was not associated with non-violent deaths (HR = 0.99, p = 0.849). A DUP of 1 and 2 years showed a non-significant trend toward increased risk of violent death (HR = 2.60, p = 0.060). Sex was not a significant predictor in either model.
We performed a multivariable Cox regression analysis including all significant predictors (Table 4. Multivariate Cox regression analysis for overall and non-violent mortality and Supplementary Table 3. Multivariate Cox regression analysis for violent deaths). Age remained the strongest predictor of all-cause mortality. However, it was not a reliable predictor of violent deaths, as extreme hazard ratio estimates suggested high variability and statistical instability.
Patients treated with SGAs consistently demonstrated improved survival outcomes compared to those receiving FGAs. Use of SGAs was associated with a 58% reduction in all-cause mortality (HR = 0.42, p < 0.001), a 57% lower risk of non-violent deaths (HR = 0.43, p = 0.001), and a 69% reduced risk of violent deaths (HR = 0.31, p = 0.046). SGA LAIs demonstrated a non-significant protective effect for non-violent deaths (HR = 0.24, p = 0.169), while FGA LAIs were also not significantly associated (HR = 0.47, p = 0.144).
Hospital readmission frequency was strongly associated with mortality outcomes across models. Compared to the high-frequency group, patients with moderate readmission frequency had an 86% lower risk of all-cause mortality (HR = 0.14, p = 0.001), and those with low frequency had a 95% lower risk (HR = 0.05, p < 0.001). For non-violent deaths, low readmission frequency was linked to an 88% risk reduction (HR = 0.12, p = 0.015), while moderate frequency showed a non-significant trend toward lower risk (HR = 0.23, p = 0.069). In terms of violent mortality, moderate readmission frequency was significantly protective (HR = 0.04, p = 0.029), and no violent deaths were recorded in the low-frequency group, though confidence intervals were wide. Duration of untreated psychosis (DUP), sex, and age of onset were not independently associated with mortality in any model.
Discussion
This study offers valuable insight into long-term mortality in schizophrenia, based on a clinically well-defined cohort with confirmed diagnoses, using real-world data and verified mortality information collected over a 10-year period through hospital and forensic records. To the best of our knowledge, it is the first study from Romania to examine mortality outcomes in this population over a 10-year period.
The sex distribution in our sample, with a higher proportion of female participants (57.96%), contrasts with the typically reported higher incidence of schizophrenia in males. This discrepancy may reflect differences in symptom presentation and patterns of service use rather than true prevalence variation. Evidence suggests that women with schizophrenia are more likely to exhibit affective and positive symptoms, which are associated with earlier detection and increased hospitalization rates, while men are more often characterized by predominant negative symptoms and poorer social functioning, which may delay help-seeking and reduce contact with services26,27. These differences may contribute to the overrepresentation of women in hospital-based or longitudinal follow-up cohorts. The majority of patients (92.28%) had low to medium socio-economic status, yet all had access to inpatient psychiatric care under Romania’s universal coverage system. While this likely reduced disparities in acute care access, differences in outpatient follow-up and preventive care may still exist and could influence long-term outcomes.
As a reference for interpreting survival outcomes, we reviewed national demographic data for Brașov County between 2010 and 202325. During this period, the average life expectancy ranged from 69.9 to 73.9 years for men and from 77.6 to 81.0 years for women, with an overall average of 75.94 years, which served as a reference point for evaluating survival in our cohort.
At baseline, five patients had already reached the average life expectancy, and four of them died during the follow-up period. An additional 18 individuals (2.83%) had the potential to exceed this threshold if they had survived the entire 10-year follow-up; however, only five ultimately reached the age of 76. Although patients over 65 years of age would exceed the general life expectancy within the 10-year follow-up period, they were included to ensure a more comprehensive assessment of survival outcomes in schizophrenia. Excluding them would have introduced selection bias and limited the capacity to capture later-life schizophrenia-related outcomes. Older patients often remain engaged in care and face distinct health challenges, such as chronic physical conditions and age-related vulnerabilities, that influence mortality. Moreover, life expectancy estimates are population-based averages and do not reflect individual variability.
The average age at death in the cohort was 58.97 years (SD ± 11.58), nearly 17 years below the general population life expectancy of 75.94 years. This difference is consistent with international findings, which report a 10- to 25-year reduction in YLL among individuals with schizophrenia compared to the general population28,29.
The observed all-cause mortality rate in the cohort was 21.3 per 1000 person-years, markedly higher than Romania’s general population rate of 12.2225. The resulting standardized mortality ratio (SMR) of 1.58 indicates a 58% excess mortality risk. This elevated mortality reflects well-documented associations between schizophrenia and increased vulnerability to physical illnesses, unhealthy lifestyle factors, and barriers to accessing appropriate healthcare28,30,31,32.
Patients with a DUP greater than three years exhibited the highest mortality rates. While DUP was not a statistically significant predictor, the observed trend toward higher mortality is consistent with evidence linking prolonged DUP to symptom persistence, cognitive deterioration, and reduced functional outcomes—all of which may contribute cumulatively to increased mortality risk33,34,35.
Non-violent deaths accounted for 67.48% of all mortality. Cardiovascular disease was the most frequently recorded cause, with a stable incidence throughout the study period. This persistent burden is likely influenced by cardiovascular risk factors, antipsychotic-related metabolic effects, and limitations in preventive health access36,37.
A rise in infection-related mortality was observed during the latter part of the follow-up period, particularly between 2020 and 2023, likely reflecting the impact of the COVID-19 pandemic. Pneumonia was the most frequently documented infectious cause of death, either as the immediate cause or part of the clinical presentation. This pattern aligns with international studies reporting increased susceptibility to infections—especially respiratory ones—among individuals with schizophrenia, attributed to immune dysregulation, physical comorbidities, and barriers to preventive care. Prior research has emphasized the need to prioritize this population for preventive strategies such as influenza and pneumococcal vaccination15. Schizophrenia has also been linked to worse COVID-19 outcomes, potentially due to biological and systemic vulnerabilities38. However, some studies have found no COVID-19-related deaths in schizophrenia cohorts, despite high comorbidity rates, and have speculated on protective effects from antipsychotic treatment, including lower levels of inflammatory markers such as CRP and fibrinogen39.
A recent retrospective study from our center, conducted between 2018 and 2024, found relatively low pneumonia incidence among hospitalized schizophrenia patients—1.02% pre-pandemic and 1.63% post-pandemic—but identified advanced age, underweight status, high-dose atypical antipsychotics, polypharmacy, and physical restraint as risk factors40. These findings support broader concerns about infection vulnerability in this population.
In the Romanian context, however, patients with schizophrenia may benefit from relatively easy access to medical services. Psychiatric admissions are permitted without restriction through emergency departments, and patients are promptly transferred to general hospitals when required. Multidisciplinary teams—including internists and infectious disease specialists—are routinely involved in inpatient psychiatric care, supporting early identification and treatment of somatic conditions. This integrated approach may contribute to better infection management and fewer complications. As such, preventive efforts—such as vaccination, timely recognition of respiratory symptoms, and routine somatic screening—should be considered essential components of comprehensive care for individuals with schizophrenia.
Violent causes accounted for 17.07% of all deaths, with suicides comprising 6.5% (n = 8) of the total. Most suicide cases (75%) occurred during the first three years of follow-up. While literature often describes an elevated risk of suicide early in the course of schizophrenia41,42, the present cohort had a mean age of 45 years and consisted of individuals with established diagnoses rather than first-episode cases. Suicide risk in schizophrenia is known to be associated with younger age, male sex, depressive symptoms, previous suicide attempts, active psychosis, insight into illness, and substance use43, whereas consistent treatment engagement remains one of the most reliable protective factors44. Given the small number of suicide cases in our sample, these findings are descriptive and should be interpreted with caution.
Accidental deaths accounted for 10.57% of all mortality in our cohort, with 5.69% resulting specifically from mechanical asphyxia due to food aspiration. These cases are clinically significant and highlight a preventable cause of death linked to antipsychotic-induced sedation, extrapyramidal side effects, and impaired swallowing function45,46. In our sample, three aspiration-related deaths occurred during psychiatric hospitalization, raising important concerns about monitoring and risk management. Contributing factors included not only pharmacological effects but also patient-related vulnerabilities such as edentulism, advanced age (mean = 61 years, SD = ± 12.33), and nonadherence to ward safety rules (e.g., eating in bed or consuming inappropriate foods). In response, institutional safety protocols were revised to restrict food brought by visitors and require meals to be taken only in supervised dining areas. These findings underscore the importance of routine swallowing assessments, age-adjusted medication selection, and stricter inpatient monitoring—particularly for patients receiving sedating agents or those with pre-existing risk factors for aspiration.
Although a general gradual cumulative increase in all-cause and non-violent mortality was observed over time, violent deaths appeared sporadically, without a consistent pattern. This highlights the importance of continuous suicide risk assessment and the need for sustained, long-term support strategies—particularly during high-risk periods such as the early years following diagnosis or after hospital discharge47,48.
All-cause mortality
Age was the strongest predictor of all-cause mortality, with each additional year associated with a 7% increase in risk (HR = 1.07, 95% CI: 1.06–1.09, p < 0.001), consistent with existing evidence regarding ageing and declining health in schizophrenia49. Treatment with SGAs was associated with significantly lower mortality (HR = 0.37, p < 0.001), aligning with data from meta-analyses showing reduced all-cause and cardiovascular mortality with SGAs and LAIs13,50,51. However, the protective effect of SGA LAIs lost significance in multivariable models, possibly due to younger and healthier patients being more likely to receive these treatments.
In our study, patients with fewer psychiatric readmissions over the 10-year follow-up had significantly lower risks of both all-cause and cause-specific mortality. In the Romanian healthcare context, over 95% of patients with schizophrenia are insured through the national health system, often receiving disability benefits or early retirement, which ensures free access to all antipsychotic medications—including first- and second-generation drugs, clozapine, and long-acting injectables. Structural barriers to care and medication cost are thus minimal. As a result, when relapse and hospitalization do occur, they are more likely to reflect treatment non-adherence or clinical instability rather than financial inaccessibility. According to Kane et al.52, ~30–40% of patients with schizophrenia are non-adherent to medication, and those who remain free of hospitalizations for extended periods (e.g., two or more years) are more likely to be adherent in clinical practice. In our cohort, hospital readmission frequency can be viewed as a composite indicator of several clinically relevant factors, including illness severity, longitudinal stability and—implicitly—treatment adherence. Individuals with infrequent admissions often exhibit more stable disease courses and steadier follow-up, whereas those with repeated hospitalizations may experience greater symptom burden, psychosocial stress, or non-compliance. These findings reflect the well-described “revolving door” pattern in psychiatric care, wherein recurrent hospitalizations signal more complex clinical trajectories and reduced continuity of community-based care53. Substance use, especially cannabis, is another known cause of relapse. However, its prevalence remains relatively low in Romania. Among young adults cannabis use was reported at 6% in 2019, compared to 15% across the EU and over 30% in the United States54,55. These data further support our interpretation that treatment non-adherence is the main factor behind repeated hospitalizations in our cohort.
This finding is consistent with previous large-scale research showing that stable engagement with antipsychotic treatment is associated with improved survival outcomes in schizophrenia. For example, Taipale et al.56 and Tiihonen et al.57 found that consistent antipsychotic use reduced the risk of both suicide and natural death in long-term follow-up cohorts. Supporting patient stability through better outpatient monitoring, early intervention for relapse, and sustained community engagement may help reduce premature mortality in this population.
Non-violent deaths
Age remained a strong predictor of non-violent deaths, increasing risk by 8% per year (HR = 1.08, p < 0.001). Cognitive decline and reduced health awareness may delay medical help-seeking and contribute to poor outcomes58,59,60,61. SGAs reduced the risk of non-violent mortality by 58% (HR = 0.42, p < 0.001), and SGA LAIs showed a non-significant trend toward benefit.
Violent deaths
Age was not a reliable predictor of violent deaths, reflecting substantial variability and small event counts. SGAs were associated with a 69% reduction in violent death risk (HR = 0.31, p = 0.045). FGA LAIs were linked to increased risk in univariate models (HR = 3.20, p = 0.012), but not in multivariate analysis. Clozapine has consistently been shown to reduce suicide risk in schizophrenia, and is often recommended for treatment-resistant cases or patients at high risk of self-harm62,63. In our sample, 112 patients (17.6%) were treated with clozapine, with 14 recorded deaths during the follow-up period. However, no statistically significant association was observed between clozapine use and reduced mortality —either overall or cause-specific.
In line with our findings, several practical measures could support improved outcomes and reduce premature mortality in schizophrenia. Patients with frequent psychiatric readmissions—often reflecting more complex clinical courses—may benefit from structured follow-up and strengthened community-based care. Closer integration of psychiatric and general medical services is needed to ensure early detection and management of somatic comorbidities, particularly cardiovascular and metabolic conditions. Preventive efforts should also prioritize infection-related risks through routine vaccination (e.g., pneumococcal, influenza, and COVID-19) and prompt intervention for respiratory symptoms.
Additionally, individuals with schizophrenia should have facilitated access to medical specialists such as internists, cardiologists, and gynecologists, alongside routine preventive screenings. These may include mammography, Pap smears, FIT or colonoscopy, PSA testing, and low-dose chest CT scans for high-risk smokers. Embedding these services within psychiatric care pathways could help address modifiable risk factors and reduce the persistent mortality gap observed in this population.
Limitations
This study provides valuable insight into long-term mortality patterns in schizophrenia based on a 10-year follow-up. Nonetheless, certain limitations should be acknowledged. The cohort was drawn from a single psychiatric center serving a catchment area of ~550,000 inhabitants in central Romania. While the population is socio-demographically diverse—including Romanian, Hungarian, German, and other ethnic minorities and while socioeconomic data were available and indicated a relatively uniform distribution in our cohort, we lacked detailed information on ethnicity, comorbidity burden, and outpatient care engagement, which limits generalizability and subgroup analysis. The cohort included only patients who had signed the hospital’s general informed consent form, which may introduce selection bias. Individuals who consent to the use of their data are likely to differ from those who are more severely ill, disengaged from services, or socially marginalized.
In 84.55% of cases, the cause of death was successfully identified—a considerable proportion for a retrospective study. However, in 15.45% of cases, the cause of death could not be determined due to limited access to complete forensic and medical records. Many individuals were from other Romanian counties, and our access was restricted to the forensic database in Brașov. Some deaths occurred at home, with death certificates issued by general practitioners that were not part of centralized records. Attempts to obtain information from family members were often inconclusive.
Hospital readmission frequency was used in this study as an indicator of clinical stability and treatment continuity, but it does not directly measure medication adherence. Although antipsychotic treatment is widely accessible in Romania, with free coverage for all patients regardless of income or insurance status, admission frequency may still be influenced by factors such as illness severity, treatment resistance, social support, and engagement with outpatient services.
While our findings suggest a substantial gap between the observed age at death and general population life expectancy, this estimate should not be interpreted as a direct measure of life expectancy in schizophrenia, but rather as an indicator of premature mortality within the followed cohort.
Treatment regimens were also adjusted over time, with changes in doses and medications not systematically examined. Additionally, the absence of data on other prescribed psychotropic medications, such as benzodiazepines or mood stabilizers, may have influenced clinical outcomes. The study did not explore gender identity or gender-related factors, which may limit the generalizability of the findings.
Conclusion
Over a 10-year follow-up, our study highlights a significant burden of premature mortality among individuals with schizophrenia, with an average age at death nearly 17 years below that of the general population. Cardiovascular disease, infections, and violent causes such as suicide were the most common contributors. Use of second-generation antipsychotics and fewer psychiatric readmissions were associated with lower mortality risk, suggesting that clinical stability and treatment quality remain important factors in long-term outcomes.
These findings underscore the importance of integrated physical and mental healthcare, particularly in monitoring somatic diseases. Preventive strategies—including vaccination, routine cancer screening, early detection of somatic illness, and improved coordination with medical specialists—should be standard practice. These interventions, if widely implemented, may help reduce the gap in life expectancy and improve overall survival in this high-risk population. Continued research is warranted to better understand subgroup differences and optimize long-term care strategies in real-world settings.
Data availability
The data used in this study were obtained from patients’ paper and electronic medical records. The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
McCutcheon, R. A., Reis Marques, T. & Howes, O. D. Schizophrenia-an overview. JAMA Psychiatry 77, 201–210 (2020).
Correll, C. U. et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 16, 163–180 (2017).
Vermeulen, J. et al. Antipsychotic medication and long-term mortality risk in patients with schizophrenia; a systematic review and meta-analysis. Psychol. Med. 47, 2217–2228 (2017).
Liu, X. et al. Life expectancy and potential years of life lost for schizophrenia in western China. Psychiatry Res. 308, 114330 (2022).
Moneta, M. V., Haro, J. M., Plana-Ripoll, O. & Olaya, B. Life expectancy associated with specific mental disorders and the contribution of causes of death: a population-based study in the region of Catalonia. Psychiatry Res. 348, 116480 (2025).
Chan, J. K. N. et al. Life expectancy and years of potential life lost in people with mental disorders: a systematic review and meta-analysis. eClinicalMedicine 65, 102294 (2023).
Bitter, I. et al. Mortality and the relationship of somatic comorbidities to mortality in schizophrenia. A nationwide matched-cohort study. Eur. Psychiatry 45, 97–103 (2017).
Saha, S., Chant, D. & McGrath, J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time?. Arch. Gen. Psychiatry 64, 1123–1131 (2007).
Newman, S. C. & Bland, R. C. Mortality in a cohort of patients with schizophrenia: a record linkage study. Can. J. Psychiatry 36, 239–245 (1991).
Westman, J. et al. Increased cardiovascular mortality in people with schizophrenia: a 24-year national register study. Epidemiol. Psychiatr. Sci. 27, 519–527 (2018).
Ifteni, P., Correll, C. U., Burtea, V., Kane, J. M. & Manu, P. Sudden unexpected death in schizophrenia: autopsy findings in psychiatric inpatients. Schizophr. Res. 155, 72–76 (2014).
Goff, D. C. et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr. Res. 80, 45–53 (2005).
Tang, C.-H. et al. A nationwide study of the risk of all-cause, sudden death, and cardiovascular mortality among antipsychotic-treated patients with schizophrenia in Taiwan. Schizophr. Res. 237, 9–19 (2021).
Correll, C. U. et al. Mortality in people with schizophrenia: a systematic review and meta-analysis of relative risk and aggravating or attenuating factors. World Psychiatry 21, 248–271 (2022).
Ronaldson, A. et al. Severe mental illness and infectious disease mortality: a systematic review and meta-analysis. EClinicalMedicine 77, 102867 (2024).
Kuo, C.-J. et al. Second-generation antipsychotic medications and risk of pneumonia in schizophrenia. Schizophr. Bull. 39, 648–657 (2013).
de Leon, J. et al. Investigating in VigiBase over 6000 cases of pneumonia in clozapine-treated patients in the context of the literature: focus on high lethality and the association with aspiration pneumonia. Expert Opin. Drug Metab. Toxicol. 20, 857–871 (2024).
Yang, Q., Qiu, J., Chen, X., Hu, Y. & Shen, H. Risk factors for lower respiratory tract infections in a psychiatric hospital: a retrospective study. J. Infect. Dev. Ctries. 17, 560–566 (2023).
Kasckow, J., Felmet, K. & Zisook, S. Managing suicide risk in patients with schizophrenia. CNS Drugs 25, 129–143 (2011).
Auquier, P., Lançon, C., Rouillon, F., Lader, M. & Holmes, C. Mortality in schizophrenia. Pharmacoepidemiol. Drug Saf. 15, 873–879 (2006).
Pompili, M. et al. Suicide risk in schizophrenia: learning from the past to change the future. Ann. Gen. Psychiatry 6, 10 (2007).
Whiting, D., Gulati, G., Geddes, J. R. & Fazel, S. Association of schizophrenia spectrum disorders and violence perpetration in adults and adolescents from 15 countries: a systematic review and meta-analysis. JAMA psychiatry 79, 120–132 (2022).
Diagnostic and statistical manual of mental disorders: DSM-5TM (American Psychiatric Publishing, a division of American Psychiatric Association, 2013).
Ebihara, A. World medical association declaration of Helsinki. Jpn. Pharmacol. Ther. 28, 983–986 (2000).
National Institute of Statistics. Life expectancy at birth, by sex and area of residence, by counties and regions – POP217A (National Institute of Statistics, 2025).
Riecher-Rössler, A. & Häfner, H. Gender aspects in schizophrenia: bridging the border between social and biological psychiatry. Acta Psychiatr. Scand. 102, 58–62 (2000).
Abel, K. M., Drake, R. & Goldstein, J. M. Sex differences in schizophrenia. Int. Rev. Psychiatry 22, 417–428 (2010).
Laursen, T. M., Nordentoft, M. & Mortensen, P. B. Excess early mortality in schizophrenia. Annu. Rev. Clin. Psychol. 10, 425–448 (2014).
Hjorthøj, C., Stürup, A. E., McGrath, J. J. & Nordentoft, M. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. lancet Psychiatry 4, 295–301 (2017).
Correll, C. U. et al. Factors and their weight in reducing life expectancy in schizophrenia. Schizophr. Res. 250, 67–75 (2022).
Crump, C., Winkleby, M. A., Sundquist, K. & Sundquist, J. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. Am. J. Psychiatry 170, 324–333 (2013).
Yuen, K. et al. Long-term follow-up of all-cause and unnatural death in young people with first-episode psychosis. Schizophr. Res. 159, 70–75 (2014).
Barnes, T. R. E. et al. Duration of untreated psychosis and social function: 1-year follow-up study of first-episode schizophrenia. Br. J. Psychiatry 193, 203–209 (2008).
Yu, M. et al. Correlation between duration of untreated psychosis and long-term prognosis in chronic schizophrenia. Front. Psychiatry 14, 1112657 (2023).
Harris, M. G. et al. The relationship between duration of untreated psychosis and outcome: an eight-year prospective study. Schizophr. Res. 79, 85–93 (2005).
Hennekens, C. H., Hennekens, A. R., Hollar, D. & Casey, D. E. Schizophrenia and increased risks of cardiovascular disease. Am. Heart J. 150, 1115–1121 (2005).
Laursen, T. M., Munk-Olsen, T. & Vestergaard, M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr. Opin. Psychiatry 25, 83–88 (2012).
Mohan, M., Perry, B. I., Saravanan, P. & Singh, S. P. COVID-19 in people with schizophrenia: potential mechanisms linking schizophrenia to poor prognosis. Front. Psychiatry 12, 666067 (2021).
Moga, S., Teodorescu, A., Ifteni, P., Gavris, C. & Petric, P.-S. Inflammatory response in SARS-CoV-2 infection of patients with schizophrenia and long-term antipsychotic treatment. Neuropsychiatr. Dis. Treat. 17, 3053–3060 (2021).
Miron, A.-A. et al. Pre- and post- COVID-19 pandemic pneumonia rates in hospitalized schizophrenia patients. Medicina 61, 1251 (2025).
Nordentoft, M., Madsen, T. & Fedyszyn, I. Suicidal behavior and mortality in first-episode psychosis. J. Nerv. Ment. Dis. 203, 387–392 (2015).
Zaheer, J. et al. Predictors of suicide at time of diagnosis in schizophrenia spectrum disorder: a 20-year total population study in Ontario, Canada. Schizophr. Res. 222, 382–388 (2020).
Sher, L. & Kahn, R. S. Suicide in schizophrenia: an educational overview. Medicina 55, 361 (2019).
Hor, K. & Taylor, M. Suicide and schizophrenia: a systematic review of rates and risk factors. J. Psychopharmacol. 24, 81–90 (2010).
Warner, J. Risk of choking in mental illness. Lancet 363, 674 (2004).
Cicala, G., Barbieri, M. A., Spina, E. & de Leon, J. A comprehensive review of swallowing difficulties and dysphagia associated with antipsychotics in adults. Expert Rev. Clin. Pharmacol. 12, 219–234 (2019).
Ventriglio, A. et al. Suicide in the early stage of schizophrenia. Front. Psychiatry 7, 116 (2016).
Krause, T. J. et al. Suicide risk after psychiatric discharge: study protocol of a naturalistic, long-term, prospective observational study. Pilot Feasibility Stud. 6, 145 (2020).
Jia, N. et al. Long-term effects of antipsychotics on mortality in patients with schizophrenia: a systematic review and meta-analysis. Rev. Bras. Psiquiatr. 44, 664–673 (2022).
Kiviniemi, M. et al. Antipsychotics and mortality in first-onset schizophrenia: prospective Finnish register study with 5-year follow-up. Schizophr. Res. 150, 274–280 (2013).
Aymerich, C. et al. All-cause mortality risk in long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis. Mol. Psychiatry 30, 263–271 (2025).
Kane, J. M., Kishimoto, T. & Correll, C. U. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry 12, 216–226 (2013).
Fonseca Barbosa, J. & Gama Marques, J. The revolving door phenomenon in severe psychiatric disorders: a systematic review. Int. J. Soc. Psychiatry 69, 1075–1089 (2023).
European Monitoring Centre for Drugs and Drug Addiction. European drug report 2021: trends and developments (Publications Office of the European Union, 2021).
Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2019 National Survey on Drug Use and Health (Center for Behavioral Health Statistics and Quality, 2020).
Taipale, H. et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr. Res. 197, 274–280 (2018).
Tiihonen, J. et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29823 patients with schizophrenia. JAMA Psychiatry 74, 686–693 (2017).
Starzer, M. S. K. et al. Predictors of mortality following a schizophrenia spectrum diagnosis: evidence from the 20-year follow-up of the OPUS randomized controlled trial. Schizophr. Bull. 49, 1256–1268 (2023).
Dickerson, F. et al. Risk factors for natural cause mortality in schizophrenia. JAMA Netw. Open 7, e2432401–e2432401 (2024).
Irwin, K. E., Henderson, D. C., Knight, H. P. & Pirl, W. F. Cancer care for individuals with schizophrenia. Cancer 120, 323–334 (2014).
Zhuo, C., Tao, R., Jiang, R., Lin, X. & Shao, M. Cancer mortality in patients with schizophrenia: systematic review and meta-analysis. Br. J. Psychiatry 211, 7–13 (2017).
Meltzer, H. Y. et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch. Gen. Psychiatry 60, 82–91 (2003).
Frogley, C., Taylor, D., Dickens, G. & Picchioni, M. A systematic review of the evidence of clozapine’s anti-aggressive effects. Int. J. Neuropsychopharmacol. 15, 1351–1371 (2012).
Acknowledgements
We are grateful to our co-authors for their contributions to the study’s conception, design, data collection, and analysis. We also acknowledge the support of our colleagues from the Clinical Hospital of Psychiatry and Neurology of Brașov, Romania. Our sincere thanks go to the medical staff for their continuous care of patients with schizophrenia and to the patients themselves for their participation.
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to the work reported, including aspects of study conception, design, data collection, analysis, and interpretation. They were involved in drafting, revising, or critically reviewing the manuscript, approved the final version for publication, agreed on the target journal, and accept responsibility for the integrity and accuracy of the work in its entirety. Conceptualization, PAV, and IP; Data curation, PAV, ȚD, PPS and TA; Formal analysis, PAV, and IP; Methodology, PAV, IP, and TA; Supervision, PAV, ȚD, TA and IP; Visualization, PAV, and IP; Writing – original draft, PAV, IP, and TA; Writing – review & editing, PAV, PPS, TA, ȚD and IP.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Popa, AV., Ifteni, P.I., Țâbian, D. et al. What is behind the 17-year life expectancy gap between individuals with schizophrenia and the general population?. Schizophr 11, 117 (2025). https://doi.org/10.1038/s41537-025-00667-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41537-025-00667-1