Abstract
Rapid and sensitive detection of mycotoxins in foodstuffs is of great significance. As a powerful detection tool, biosensing technologies and microfluidic devices have shown a great potential in rapid and on-site detection of mycotoxins. This review comprehensively summarized the latest advances on the construction of microfluidic biosensors and their promising applications in on-site detection of mycotoxins. Finally, future challenges and chances in this significant and promising field were proposed.

Similar content being viewed by others
Introduction
Mycotoxins are the secondary metabolites of the fungi, which are the most toxic and important contaminants in agricultural and food products1,2,3. At present, hundreds of mycotoxins have been found, including aflatoxin B1 (AFB1), aflatoxin M1 (AFM1), zearalenone (ZEN), ochratoxin A (OTA), fumonisin B1 (FB1), patuli, and T-2 toxin, etc.4,5. Among them, aflatoxins are the most toxic and widespread potential carcinogens, classified as group 1A carcinogens by the International Agency for Research on Cancer (IARC), while FB1 and OTA are classified as group 2B possible carcinogens6. Mycotoxins have shown extremely potent teratogenic, mutagenic, and immunosuppressive properties, which can cause multiple diseases (e.g., cancer, chronic tubulointerstitial nephropathy, neural tube defects, and cardiovascular diseases), posing a serious threat to humans worldwide7,8,9. Due to the high toxicity of mycotoxins, many countries have established the maximum permissible limits of mycotoxins in foods10. For example, the European Union has established a maximum residue level (MRL) for AFM1 of 0.050 µg/kg for adults and a lower level (0.025 µg/kg) for children and infants, respectively1. The US Food and Drug Administration has determined a MRL for aflatoxin in all foods of 20 μg/kg11, while the acceptability maximum limit for AFM1 in milk has been set as 0.5 μg/kg12. China has stipulated that the OTA content in cereals, beans and their products is less than 5.0 pg/kg. Moreover, the MRL of DON and ZEN in grain and its products have been limited to 1000 μg/kg and 60 μg/kg, respectively13. Consequently, to control mycotoxins within a reasonable concentration range, the development of sensitive detection methods for the detection of mycotoxins is of great importance to protect the public health.
So far, a variety of analytical methods have been developed for quantitative detection of mycotoxins, such as liquid chromatography tandem mass spectrometry (LC-MS/MS) and high performance liquid chromatography (HPLC), etc.14. However, these methods usually suffer from some limits (e.g., complex sample preparation procedures, time-consuming operation, expensive equipment, and professional operators) to a certain extent, which make it difficult to realize on-site and portable detection, especially in remote and poor areas15. To address these issues, many rapid detection methods have been widely developed, such as biosensing technologies16. Biosensing as an emerging technology has been widely applied for sensitive and rapid detection of mycotoxins in the last decades due to its simple operation, portable design, as well as high selectivity and sensitivity17,18. A biosensor is an analytical tool for identifying a target analyte by using a biometric element for converting the recognition event into a measurable signal19. A biosensor usually comprises a biometric element, a signal transduction element, and a display for recording data20. To realize on-site detection, biosensors are successfully integrated with some advanced portable devices (e.g., microfluidic devices) for improving their on-site detection capacities.
Microfluidic chips, also known as Micro Total Analysis System (μTAS), allow precise control of small volumes of fluid (10−6–10−15 mL) in channels of tens of micro21. Microfluidic systems possess many advantages, such as simultaneous detection of multiple parallel samples, high throughput, high sensitivity, short analysis times, as well as usage of only small amounts of samples and reagents22. Microfluidics is an emerging field involving interdisciplinary fields (such as micromechanics, nanotechnology, microelectronics, and bioengineering), and has been widely used in many fields, such as genomic and proteomic studies, medical diagnostics, analytical chemistry, biohazard detection, and environmental monitoring21,23,24. Notably, these advantages are crucial for adapting to the new era of rapid and on-site detection, where diverse biosensors have been successfully integrated with microfluidic devices to achieve portable, high-throughput, and on-site detection of mycotoxins25.
Currently, Microfluidic biosensors with high efficiency and outstanding detection performances have been widely applied in the mycotoxin detection20. However, the applications of microfluidic biosensors for on-site detection of mycotoxins have not been specifically summarized. In this review, we summarized the latest progresses on microfluidic biosensing technologies for on-site detection of mycotoxins in food samples. Firstly, the preparation and characteristics of commonly used microfluidic devices (i.e., silicon, glass, PDMS, PMMA, and paper-based microfluidic chips) for detection of mycotoxins were summarized, and their advantages and disadvantages were discussed and compared. Then, the roles of microfluidic devices in the mycotoxin detection process (i.e., sample preparation, separation, multiplex analysis, as well as the integration with the detection system) were summarized. After that, characteristics and functions of the recognition elements towards mycotoxins (e.g., antibodies, aptamers, and MIPs) were simply introduced and discussed. In addition, surface chemical methods for different recognition elements modified on the microfluidic chips were also discussed. In the end, applications of microfluidic biosensing technologies based on different sensing modes (i.e., colorimetric, fluorescence, SERS, electrochemical, photoelectrochemical, and dual-mode) for mycotoxin detection were summarized and compared. Impressively, future challenges and opportunities in this important and promising field were put forward (Scheme 1).
Preparation and roles of microfluidic devices
Microfluidic analytical platforms are characterized by low reagent and sample consumption, low fabrication costs, high throughput, high efficiency, high sensitivity, and high portability, which facilitate on-site detection26. Moreover, microfluidic devices can be used to simultaneously react, separate, and detect various compounds on a single chip27. Benefiting from their superior performances, these advanced microfluidic devices have been successfully applied in mycotoxin detection. In this section, we briefly described the types of microfluidic devices and the role they play in mycotoxin detection.
Preparation and characterization of microfluidic devices
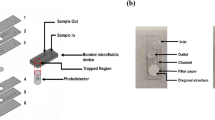
The microfluidic devices as one of the core elements play an important role in the development of microfluidic detection technologies. In this section, we focused on discussing the preparation and respective characteristics of different types of microfluidic devices (microfluidic chips and paper-based microfluidic devices). Moreover, the advantages and disadvantages of these microfluidic devices were also discussed and compared. According to their application and functional characteristics, microfluidic chips can be fabricated using different materials via different fabrication methods. The characteristics of fabrication materials can affect the functionality, absorbability, mobility, and biocompatibility of microfluidic devices28. Moreover, the diversity and quality of materials are also improving according to application requirements. Initially, microfluidic chips are usually prepared using silicon and glass. Silicon was the first material used in preparation of microfluidic chips due to its excellent chemical compatibility and thermal stability28. A simple silicon-based microfluidic chip consists of a silicon (Si) slot waveguide, a silicon dioxide (SiO2) substrate, and a covering cladding layer (Fig. 1A)29. However, its high price and fragile nature, as well as its opacity makes it incompatible with the optical detection in the visible and ultraviolet regions, which limits its wide applications to a certain extent30. Due to their excellent optical transparency and good compatibility with biological samples, glass-based microfluidic chips have been widely used in analytical applications (Fig. 1B)28,31. Nevertheless, glass-based microfluidic chips usually suffer from the complex glass manufacturing process and brittle characteristics, as well as require high temperatures during the bonding process, which limit their widespread applications. Due to the inherent shortcomings of glass and silicon, some emerging materials (e.g., polymer substrates, and paper) have been successfully used to fabricate cost-effective microfluidic devices. Compared with inorganic materials, polymers have many advantages (e.g., low cost, easy fabrication, and simple fabrication techniques), which greatly facilitate fabrication of novel microfluidic chips32. Currently, the most commonly used polymer materials (e.g., polydimethylsiloxane (PDMS) and polymethylmethacrylate (PMMA)) have played an important role in preparation of microfluidic devices. PMMA as a common thermoplastic material has been widely used in preparation of microfluidic chips due to its good insulating properties, compatibility, and surface gloss33. PMMA is usually pre-prepared in solid form and then manufactured by thermoforming, which allows for low-cost and fast production. PMMA-based microfluidic chips (Fig. 1C) have been widely used for optical sensing (e.g., colorimetric and fluorescence sensing) due to their excellent light transmission and optical properties34,35. However, this manufacturing method usually requires high temperatures and is time-consuming during processing36. PDMS, a strong, optically transparent, plastic, stable, and biocompatible polymer, is one of the most commonly used materials in the fabrication of microfluidic chips, which has attracted wide attention. Soft lithography is commonly applied to PDMS-based chip fabrication, which allows for the formation of high-resolution patterns on the PDMS layer. However, PDMS materials also suffer from some limitations, such as non-specific adsorption to proteins37,38. As shown in Fig. 1D, PDMS-based microfluidic chips usually consist of an array of crossed digital electrode transducers, a PDMS microfluidic structure, and a microarray for performing analysis39. Although microfluidic chips based on PMMA or PDMS materials have been applied in fields of separation and analytical science, they still suffer from some deficiencies (such as high cost and complex preparation processes). Therefore, the design and development of low-cost and easily prepared microfluidic chips are of great importance40. Compared with microfluidic chips based on PMMA or PDMS materials, paper-based microfluidic analytical devices (μPADs) have many advantages (e.g., low cost, simpler manufacturing process, and higher plasticity), which have been widely developed and obtained widespread applications. μPADs are considered a slightly different technology from polymers and inorganic microfluidics, and their porous nature allows them to be modified with a variety of chemical and biological reagents41. Besides, their capillary effect eliminates the need for an external power supply and simplifies device construction42. μPADs are typically constructed by forming surface patterning on a paper substrate by screen printing techniques, inkjet printing, or batik, forming various shapes of hydrophilic microchannels and reaction chambers by layer-by-layer printing, wherein the hydrophilic channels are separated by hydrophobic barriers43. In addition, many other techniques have been used in the manufacture of μPADs (e.g., flexographic printing, photolithography, hand-painting, laser processing, and plasma processing)44. For example, Fig. 1E showed the fabrication of microfluidic devices from omniphobic paper using embossing technique45. Mold a and Mold b are pressed together on the paper with pressure (about 0.2 kg/cm2). The “T” shaped channel is embossed. This three-dimensional microchannel system can be used as a phase separator and droplet generator. As shown in Fig. 1F, μPADs were prepared using an inkjet printing technique by shearing and pasting the hydrophilic regions printed on filter paper onto transparent adhesive tape to use as a hydrophobic barrier46. Moreover, in order to solve the problem of poor biocompatibility of PDMS, more biologically relevant hydrogel materials (e.g., gelatin, alginate, polyethylene glycol diacrylate, and gelatin methacrylate) have been developed for the construction of microfluidic chips47. Due to the high water content, hydrogels also show the excellent biocompatibility and biodegradability48. Benefiting from their outstanding perforces, hydrogel materials have also been widely used for the preparation of microfluidic devices49. However, the low mechanical strength and swelling properties of hydrogel may lead to serious deformation of chip structure, and thus it is necessary to strengthen its mechanical properties in future.
A Silicon29. This is an open access article under the CC BY-NC-ND license. B Glass31. Copyright 2018, The Royal Society of Chemistry. C PMMA34. Copyright 2021, American Chemical Society. D PDMS39. Copyright 2015, Elsevier B.V. E Fabrication of microfluidic devices for omniphobic paper using embossing technique45. This is an open access article under the CC BY-NC-ND license. F Paper-based microfluidic chip preparation using inkjet printing technology46. Copyright 2017, The Royal Society of Chemistry.
Roles of microfluidic devices in mycotoxin analysis
Encouraged by their advantages of portability, high throughput, and high sensitivity, microfluidic devices have been successfully applied in the construction of multi-functional and integrated detection platforms. In a microfluidic detection system of mycotoxins, the microfluidic devices mainly play two roles: (1) Sample separation and preparation; (2) Integration with the detection system. Therefore, in this section, we mainly focused on discussing the roles of microfluidic devices in mycotoxin detection.
Sample separation on microfluidic devices
Sample separation is the first step in the detection of mycotoxins in food and a prerequisite for the sample-to-result capability of the integrated microfluidic device. Appropriate sample preparation can increase detection accuracy and recovery rate. Routine sample preparation typically includes (1) an extraction method for extracting the substance from a test sample using a suitable solvent, and (2) a cleaning or purification step to reduce interference from the food matrix and to concentrate an analyte containing a low abundance of the substance for measurement50. Liquid-liquid extraction, solvent extraction, solid-phase extraction, and immunoaffinity solid-phase extraction have been applied for sample pretreatment before determination of mycotoxins by capture agents15. The capture agent is then used to capture the target substance, thereby facilitating complete separation of the target substance. However, these methods usually suffer from cumbersome and time-consuming operations, which limit their applications in on-site detection. Integrating sample separation into a microfluidic system can greatly simplify the detection process and improve detection efficiency51. For example, a microfluidic system integrating with the bacterial cell enrichment, cell lysis, DNA extraction, and loop mediated isothermal amplification (LAMP) for the pathogen detection has been developed52. Remarkably, Gowda et al. utilized the highly adsorbent polymer beads to retain the bacteria in a microfluidic chip through size repulsion and negative surface charge for realizing efficient separation. Target cells are enriched and then lysed by knockdown to extract DNA. Finally, the detection is achieved by specific LAMP. Microchannels as the basic geometry of microfluidic chips can form connected networks for rapid sample separation, sorting, and focusing, offering many competitive advantages (e.g., convenience, high throughput, flexible integration of interrogation units, low cost, as well as low reagent consumption)53. As shown in Fig. 2A, a microchannel was designed for realizing the extraction, concentration, and detection of OTA54. First, the salt-rich phase (SRP) and the PEG-rich phase (PRP) were present in part I. Then, molecular distribution was carried out along the 2.72 cm long separation channel, and OTA was completely distributed into PRP. A small part of the primary PRP was collected in part II. Then, the collected PRP was mixed with the solution containing the primary antibody in part III. After passing through the micro mixing area, 1/3 of the channel is diverged to the icFLISA channel (Part IV). Finally, as shown in part V, the OTA-BSA conjugate was previously adsorbed. For instance, Hervás et al. developed a competitive immunoassay-microfluidic device for separation and detection of ZEN55. The strategy creatively utilized a double t-shaped microchannel layout. The fluids in the different chambers were driven by an electric field procedure appropriately attached to the vessel. This resulted in the reactions between the different chambers, which sequentially performed immune interactions and enzymatic reactions, thus achieving the sample separation15.
Integration of microfluidic devices with detection systems
Microfluidic chips can provide controlled microenvironments for precise manipulation of mycotoxins on a microscopic scale. On this basis, various analytical techniques (e.g., optical and electrochemical) have been successfully integrated into microfluidic systems to establish a highly sensitive, reproducible, and portable detection platform53,56. Optical detection methods mainly include colorimetric assays, fluorescent assays, and SERS-based assays. The colorimetric detection method is a rapid and simple method, which mainly relies on the color change generated by a chemical reaction. Colored nanomaterials have been widely used in mycotoxin detection due to their excellent visibility. Colloidal AuNPs (e.g., irregular-shaped gold nanoflowers, gold nanorods, and spherical AuNPs) are the most commonly colored nanomaterials because of their bright colors and excellent chemical stability13. In addition, compared with colloidal AuNPs, carbon nanomaterials (e.g., carbon nanospheres, carbon nanotubes, and carbon nanofibers) have the higher sensitivity. Additionally, they have unique advantages in terms of mechanical strength, optical properties, surface and interface properties, and chemical stability57. Compared to spectroscopic or chromatographic methods, colorimetric methods are more intuitive, cheaper, simpler, and more visually appealing58. At present, colorimetric methods have been integrated into microfluidic devices for real-time, rapid, and on-site detection of mycotoxins in food samples59,60,61,62,63,64,65,66,67. Fluorescent detection is an important branch of optical detection methods based on the fluorescence enhancement, fluorescence extinction variations (i.e., “turn on” or “turn off”), or fluorescence resonance energy transfer (FRET) caused by the target analytes68. Fluorescence detection methods have shown many advantages (e.g., high sensitivity, simple operation, and good precision)69. Fluorescent nanoparticles, such as quantum dots (QDs), dye-doped nanoparticles, and upconversion nanoparticles (UCNPs), have also been considered as promising reporters in sensing analysis due to their strong luminescence and strong photostability70. Benefiting from their advantages, fluorescence detection methods have been successfully combined with microfluidic devices for on-site detection of mycotoxins24,71,72,73,74,75,76,77. SERS is a powerful optical technique based on the enhancement of the inherent weak Raman signals of molecules adsorbed on or near the surface of precious metal nanostructures (such as silver (Ag) and gold (Au)) via the mechanisms of chemical enhancement (CM) and electromagnetic enhancement (EM)78. SERS has exhibited many advantages (e.g., high sensitivity, rapidity, narrow spectral bands, and unique fingerprints of target molecules)79. In recent years, polystyrene (PS) ball lithography and three-dimensional (3D) printing techniques have been used to fabricate an integrated platform of PDMS-based microfluidic devices and SERS analysis for mycotoxin detection80,81. This strategy can produce uniform nanoparticle arrays with high regeneration rates, which facilitate the production of uniform and reproducible plasma enhancement51. Electrochemical transduction methods, such as electrochemical (ECL) assays and photoelectrochemical (PEC) assays. ECL detection methods can be classified as amperometric and conductivity methods, which can be used for quantitative analysis of targets using the processable and quantifiable electrical signals produced from a variety of electrochemical reactions82. ECL detection of targets through electrical signals (e.g., as capacitance, current, potential, impedance, and conductivity) has shown many advantages (e.g., low cost, fast response time, controllability, and high sensitivity)83. It’s worth mentioning that ECL detection methods have been integrated with microfluidic systems for the detection of mycotoxins84,85,86,87,88,89. PEC detection is established based on a photoexcitation and electrochemical detection process90. PEC detection has exhibited many advantages, such as simple operation, high sensitivity, and fast response91,92. Currently, PEC biosensors are replacing electrochemical sensors in a wide range of applications due to their low background signal caused by the independent excitation source and detection signal93. Microfluidics and PEC biosensors have been integrated to construct a multifunctional PEC biosensing platform, which improves the versatility of PEC biosensors94,95,96. By now, plenty of detection systems have been integrated with microfluidics for mycotoxin detection, but the integration and intellectualization of these detection platforms remain to be improved.
Multiplex analysis in microfluidic devices
Because the reaction space on the microfluidic device is relatively independent, the sensitivity and specificity of each measurement can be ensured in multiple analysis97. Therefore, the multiple analysis technologies based on microfluidic devices show great potential in the mycotoxin detection, which can reduce the sample size, shorten the detection time, achieve high throughput, and reduce the experimental error, without affecting the detection sensitivity98. For example, physical or chemical modification technologies (e.g., wax, silk screen or inkjet printing, plasma processing, photolithography, and even cutting) can be used to reasonably design the channels of μPADs, so as to guide several microliters of liquid to a specific detection area for multiple analysis99. Moreover, a microfluidic biosensing device using 3D printing die and micro valve to realize multiple analysis was developed100. As shown in the Fig. 2B, the device was composed of five main channels (A, B, C, D, and E), which could be coated or filled with different samples. Channel X is designed for filling reaction solution, which can pass through all 5 corresponding channels. The application of positive and negative pressure to these channels can control the valve opening and closing mode, and then cause each channel to be filled on demand. The device provides the ability to perform sequential and parallel biochemical analysis on demand. Although microfluidic platforms with multiple analysis show a great potential in the field of analysis science, the development of more efficient microfluidic platforms remain to be further explored.
Recognition elements towards mycotoxins
Recognition elements
Currently, common recognition elements for mycotoxin detection mainly include antibodies, aptamers, molecularly imprinted polymers, etc. According to their types, we mainly discussed and summarized the characteristics and preparation methods of these recognition elements, as well as compared their advantages and disadvantages in the detection of mycotoxins in this section.
Antibodies
Classical antibodies are considered to be the most popular bioreceptor components that can be integrated into immunoassays through antibody/antigen interactions for highly specific target recognition101. And the integration of different antibodies into a multichannel microfluidic device allows the quantitative analysis of mycotoxins in a high-throughput manner15. Based on different production methods, antibodies can be classified into three different types: monoclonal, polyclonal, and recombinant. Among these antibodies, monoclonal antibodies are the most commonly used in the detection of mycotoxins102. However, traditional antibodies usually suffer from many shortcomings (e.g., high production costs, low yields, large batch-to-batch variations, and poor stability), which limit the development of immunoassays to a certain extent. In recent years, nanoantibodies (Nbs) have been rapidly developed due to their advantages (e.g., small molecular mass (about 15–17 kDa), good thermal stability, high solubility, low immunogenicity, and easy production)103,104,105. Pure heavy chain antibodies (HcAb) are naturally free of light chains and lack the CH1 structural domain, which were first identified in camel (Camelus dromedarius) serum by Hamers et al. Since then, camel antibodies have received increasing attention due to their convenience for biotechnological applications106. As shown in Fig. 3A, the basic structure of a classical antibody is a Y-shaped tetrapeptide chain, which consists of two identical heavy chains and two identical light chains. Camelid HcAb consists only of heavy chains, in which the antigen-binding site is formed only by variable domain of heavy chain of heavy-chain antibody (VHH). These VHHs can be expressed recombinantly and are referred to as VHH antibodies or single domain antibodies (sdAb)107. VHH antibodies are also referred to as nanobodies due to their nanoscale size. As shown in Fig. 3B, recombinant antibody technology has been used to create phage-displayed Nb libraries108. To benefit from the exquisite in vivo antibody affinity maturation process, camelids are usually immunized before isolation of antibodies. Following weekly booster immunization, total Ribonucleic Acid (RNA) was extracted from peripheral blood lymphocytes, which are isolated from whole blood. And their cDNA was synthesized using a reverse transcriptase technology. Nb coding fragments from Immunoglobulin G (IgG) 2 and IgG3 (a subset of IgG antibodies that lack the light chain) were amplified and cloned into M13 phage vectors to construct phage-displayed Nb libraries109. For mycotoxins, competitive elution is generally used to elute phage binding to immobilized targets, resulting in the isolation of high-affinity Nbs. Nbs have been found to be easily expressed in eukaryotic or prokaryotic expression systems, which are more robust under extreme conditions. It’s worth noting that Nbs are readily used as substitutes for artificial antigens. Numerous studies have shown that Nb technology is a powerful and promising technology for completing advanced applications in mycotoxin detection108. Although antibodies and Nb have been widely applied in mycotoxin detection, they still suffer from high costs and complex preparation to a certain extent. Thus, it is urgent to design and develop novel low-cost alternatives to antibodies and Nb for mycotoxin detection110.
A (a) Schematic representation of a conventional antibody (IgG) and (b) a heavy chain camel antibody108. This is an open access article under the CC BY-NC-ND license. B Schematic diagram of Nb library construction by recombinant antibody technology108. This is an open access article under the CC BY-NC-ND license. C Schematic illustration of the cell-based aptamers selection114. This is an open access article under the CC BY-NC-ND license. D Schematic illustration for preparation of MIPs126. Copyright 2014, The Royal Society of Chemistry.
Aptamers
Aptamers are single-stranded oligonucleotides that can fold into unique secondary or tertiary structures to recognize and bind to target molecules111,112. The affinity between the aptamer and target molecules can reach the level of mM-pM in comparison to that of the antibody level113. Compared with antibodies, aptamers have many superior advantages (i.e., short screening cycles, low cost, tunable selectivity and affinity, inherent stability to denaturation and re-denaturation, as well as structural analogs or cross-reacting substances). Encouraged by their advantages, aptamers have been widely applied in the construction of biosensors for the detection of mycotoxins (e.g., AFB1, AFM1, OTA, and FB1)13. The development of aptamers is closely linked to the advances in screening techniques. Nucleic acid aptamer screening techniques are basically performed by systematic evolution of ligands by exponential enrichment (SELEX), which mainly involves the binding, isolation, elution, amplification, and conditioning. Nucleic acid aptamers are generally obtained by SELEX screening, which usually begins with a library of random oligonucleotides. During this process, the complexity and diversity of the screen depend on the number of random nucleotides. In addition, a newer process called cell-SELEX has been used to produce the aptamers that specifically bind to a certain cell line, specifically cell surface molecules, based on unique extracellular properties (Fig. 3C)114. Briefly, pools of single-stranded DNA (ssDNA) were incubated with target cells. After washing, the bound DNA is eluted and then incubated with negative cells for reverse selection. After centrifugation, the supernatant is collected and the selected DNA is amplified by polymerase chain reaction (PCR). Subsequently, the PCR products are isolated as the ssDNA for the next round of selection, or cloned and sequenced for aptamer identification in the final round of selection. Although aptamers have been widely used in mycotoxin detection, they still suffer from the relatively high cost and complex preparation process to some extent. Moreover, the binding affinity of aptamers may also suffer from low efficiency in mycotoxin recognition. Therefore, there is an urgent need to design and develop novel and low-cost alternatives to aptamers and antibodies for mycotoxin detection115.
Molecularly imprinted polymers (MIPs)
MIPs are polymers that contain specific cavities complementary to the spatial and functional groups of the template molecule, which can be used for analyte recognition or identification of polymers with similar structures. MIPs are prepared and synthesized using the molecular imprinting technologies116,117. Molecular imprinting technology can be imaginatively described as a method of creating molecular locks to match molecular keys118. Compared with natural antibodies, MIPs have many advantages (such as high mechanical strength, low synthetic cost, no requirements for special storage environment, long storage time, and a wide range of applicable temperatures), which enable MIPs to be an emerging alternative to antibodies in mycotoxin detection119. Benefiting from their numerous merits, MIPs have been widely used in many fields, such as solid-phase extraction, nano-enzymes, food safety analysis, chromatographic separation, and biosensors120,121,122,123,124,125. Currently, it has been combined with microfluidic chips for the detection of ZEN in food77. As shown in Fig. 3D, MIPs can be synthesized in the following three main steps. Firstly, the template molecule interacts with the functional monomer in the solution system to form a reversible template molecule-functionalized monomer pre-polymer. Secondly, under the synthetic conditions of heating or light, the initiator triggers the cross-linking agent to cause the polymerization of the functional monomer around the template, forming the polymer with a certain degree of mechanical strength. At last, after the polymerization, the template molecules are eluted through the disruption of the force between the template and the binding sites126. After that, 3D cavities fully complementary to the template in shape, size, and position of functional groups are produced. These three-dimensional cavities can match the template molecules, which allows the MIPs to efficiently recognize the molecules. Although MIPs have many advantages (e.g., high mechanical strength, low cost, and reusability), their types are relatively few compared with those of antibodies. Therefore, developing novel types of MIPs for mycotoxin recognition to realize the mycotoxin detection is of great significance.
Surface chemical modification of recognition elements
Surface chemistry is one of the important factors to improve the analytical performance of microfluidic biosensing technology, which is used for modifying the recognition elements on the microfluidic chip127. The immobilization methods of recognition elements play an important role in maintaining their binding activity. Physical adsorption is a common fixation method for surface chemical modification of recognition elements. However, the orientation of sensor elements fixed by physical adsorption is small and the conformation is difficult to control. Moreover, physical adsorption has certain limitations on the interaction strength128. Therefore, surface chemical reactions that produce three-dimensional structures are usually preferred because they have higher loading capacity and preserve the molecular structure, and maintain unaltered molecular activity129. For example, polymer coating is a common method to immobilize molecules on sensing surfaces130. First, the bare surface is activated to expose reactive groups. Then, the activated surface reacts with the polymer to form a uniform and stable coating. Finally, the biomolecules deposited on the surface react with the functional groups of the polymer to achieve immobilization. In addition, to improve the immobilization efficiency, the existing thin-layer materials can also be molded into nanostructures. It has higher load capacity and better spot morphology, and can control the wettability of the microfluidic surface131. To further improve the detection performance of microfluidic biosensing technologies, much more efficient surface chemical modification methods remain to be further explored.
Applications of microfluidic biosensors for detection of mycotoxins
To date, a large number of microfluidic biosensors have been successfully developed for mycotoxin detection in food samples. In this section, we mainly systematically discussed and summarized the applications of microfluidic biosensors in mycotoxin detection (Table 1). According to different sensing modes, these microfluidic biosensors for mycotoxin detection were mainly classified into the following types, including colorimetric, fluorescent, SERS, electrochemical, photoelectrochemical, and dual-mode detection, etc.
Colorimetric detection
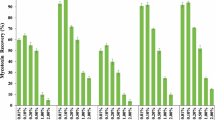
Due to their advantages (e.g., simple operation, low cost, and visual readout), colorimetric microfluidic biosensors have been used for real-time, rapid, and on-site detection of mycotoxins in food samples58,59. For example, Gandhi’s group constructed a colorimetric μPADs for the detection of OTA using 36-aggregate aptamers (Fig. 4A). In this microfluidic system, μPADs consisted of three zones (i.e., a control zone, a detection zone, and a sample zone), which were connected to each other through channels. The aptamer could be physically bound to the surface of the gold nanoparticles (AuNPs) and was displaced by the existence of OTA. The detection zone and control zone contain the AuNPs coated with OTA aptamers. OTA was added to the sample zone and allowed to pass through the microfluidic channel. Therefore, in the presence of OTA, the AuNPs/OTA aptamer detection zone showed a significant aggregation (color change to dark gray). However, no aggregation was observed in the absence of OTA (color is red). To perform quantitative analysis, OTA at different concentrations were added into the test tube with fixed aptamer concentration, and NaCl was added into the OTA aptamer adsorbed on the surface of AuNPs, and finally the color changes were observed. The resulting solution was transferred into a 96 well plate, and absorbance ratios of A630 and A520 corresponding to the wavelengths of aggregated (gray) and dispersed (red) AuNPs were measured by UV-vis spectroscopy63. The developed colorimetric μPADs possessed low limit of detection (LOD) of 242.00 ng/mL (water), 545.45 ng/mL (maize), and 95.69 ng/mL (peanut), respectively. By combining colorimetric detection with the image recognition technology and smartphones, the rapid and portable detection could be realized. In this method, the smartphone was usually used for data processing and output, providing better visual effects132. For example, Jessica et al. established a PDMS microchannel-based, colorimetric, autonomous capillary microarray for enabling a multiplexed and semi-quantitative immunoassay analysis. Results were obtained using a standard smartphone camera and analyzed using a simple greyscale quantification procedure60. The device completed the multiple detection of OTA, AFB1, and DON in approximately 10 min, with LODs of <40 ng/mL for OTA, 0.1–0.2 ng/mL for AFB1, and <10 ng/mL for DON, respectively. Subsequently, Tang et al. constructed a μPAD using a smartphone for the rapid detection of AFB1. The colorimetric mechanism of μPADs was on the basis of the common starch-iodine reaction, which was enhanced by the addition of gelatin/chitosan. Competition analysis occurred during detection of AFB1, in which AFB1 monoclonal antibodies were used for the recognition of free AFB1 and the functionalized antigens. Glucose oxidase-labeled IgG was used as secondary antibody. Hydrogen peroxide, an oxidation product of glucose, led to the color change in μPADs65. The colorimetric change was proportional to the change in AFB1 concentration. Under the optimized conditions, the LOD of AFB1 in the buffer was 9.45 ng/mL. This strategy allowed the quantification of AFB1 content by color change with the naked eye or a smartphone, which was more sensitive and easier to observe than before. Hydrogel, as an emerging material, has also been applied to microfluidic devices with satisfactory properties, such as fast molding speed, and good hydrophilicity47. For example, Ma et al. applied hydrogels to a microfluidic volumetric bar chart chip (v-chip) for highly sensitive and portable AFB1 detection (Fig. 4B). The hydrogels were preloaded with AuNPs for visual AFB1 detection. Upon introduction of AFB1, the aptamer bound to AFB1 led to disruption of the hydrogel to release AuNPs. After that, the color of the supernatant changed from colorless to red66. The method for detection of AFB1 in beer showed a linear range from 0.25 μM to 40 μM with a LOD of 1.77 nM (0.55 ppb). Although plenty of colorimetric microfluidic biosensors have been widely established for real-time, rapid, and on-site detection of mycotoxins, they usually suffer from potential background interferences (e.g., complex sample matrices), which may affect the detection results. In addition, the sensitivity of colorimetric microfluidic biosensors remain to be further improved56.
A Schematic diagram of the paper-based microfluidic device for colorimetric detection using aptamer recognition of OTA63. This is an open access article under the CC BY-NC-ND license. B Schematic diagram of hydrogel microfluidic v-chip colorimetric detection of AFB166. Copyright 2016, The Royal Society of Chemistry. C Schematic diagram of gravity-driven fluorescent microfluidic chip73. Copyright 2021, Elsevier B.V. D Schematic diagram of a fluorescent microfluidic biosensor based on CRISPR/Cas12a71. Copyright 2023, American Chemical Society.
Fluorescent detection
Compared with colorimetric microfluidic biosensors, fluorescent microfluidic biosensors possess higher sensitivity and show many other advantages (such as simple implementation, good precision, and multi-detection capability). Encouraged by their superior performances, fluorescent microfluidic biosensors have been widely applied in the on-site detection of mycotoxins24. For example, by using a PDMS gravity-driven cycling microfluidic chip, Xiang et al. developed a two-signal microfluidic biosensor to achieve rapid and sensitive detection of AFB1 (Fig. 4C). Based on the PS microspheres coupled with a competitive antigen (AFB1), a system was designed to control the reaction process by adjusting the tilt angle and a 3D reaction interface. The quantum dot (QD)-coupled streptavidin as a reference signal enabled the system to achieve a stable operation. Utilizing the designed microfluidic chip, a fluorescent competitive immunometric assay based on the “turn on” mode was constructed, and the obtained fluorescence measurement results were proportional to the AFB1 concentration73. This microfluidic device enabled ultrasensitive quantitative detection of AFB1 within several minutes, with a LOD of 6 × 10−5 μg/mL in the linear range of 2 × 10−4–0.5 μg/mL, which had many advantages (e.g., high specificity, good precision, and good reusability). Soon after, based on the disruption of FRET between UCNPs and Cu-TCPP nanosheets, Lin et al. designed a portable fluorescent microfluidic biosensor for the simultaneous determination of OTA and ZEN in maize flour. Blue and green bicolor UCNPs were functionalized by OTA aptamer and ZEN aptamer, respectively. Moreover, Cu-TCPP nanosheets were used as bursting agents. After that, OTA aptamer-functionalized blue color UCNPs and ZEN aptamer-functionalized green color UCNPs were respectively mixed with Cu-TCPP, which were separately loaded into two different regions of the microfluidic device (i.e., region 1 and region 2), leading to the recovery of green and blue fluorescence, respectively. The blue-green fluorescence recovery was observed by visual observation or by taking pictures using a portable smartphone. On this basis, OTA and ZEN could be simultaneously and sensitively detected72. The developed fluorescent paper-based microfluidic aptasensor showed a linear range from 0.5 ng/mL to 100 ng/mL with a LOD of 0.44 ng/mL for ZEN and a linear range from 0.1 ng/mL to 50 ng/mL with a LOD of 0.098 ng/mL for OTA, respectively. The established platform had many advantages (such as simple operation, wide adaptability, and low sample consumption), which could realize the on-site detection of various hazards in food. Recently, clustered regularly interspaced short palindromic repeats (CRISPR) technology has shown multiple advantages (e.g., simple instrumentation, low cost, and ease of operation), which have attracted a lot of attention in the field of biosensing133. Based on a signal transduction CRISPR/Cas12a strategy, Xiang et al. developed a new functional DNA-guided transition-state CRISPR/Cas12a microfluidic biosensor (namely FTMB) to realize the highly sensitive and high-throughput determination of mycotoxins (Fig. 4D). In this FTMB, the signal transduction CRISPR/Cas12a strategy was established by using the specific recognition function of DNA sequences and activators for the formation of trigger switches. To realize the high responses towards low target mycotoxin concentrations, the transition-state CRISPR/Cas12a system could be established by adjusting the composition ratios between the activator and CRISPR RNA (crRNA). To realize the signal enhancement, the CdSe/ZnS QDs with highly matched emission bands to the photonic crystals (PCs) were designed to enhance the fluorescence signal by 45.6 times, which enabled the FTMB to have the ultra-sensitive determination capability for various mycotoxins. The microfluidic chip contained a three-layer PDMS film with eight external driver-regulated reaction chambers. Among the eight reaction chambers, two reaction chambers were used for the positive and negative control, the other six reaction chambers realized the simultaneously ultrasensitive and quantitative detection of six mycotoxins71. Overall, the FTMB had a wide detection range (10−5–101 ng/mL) with LODs of 2.3 fg/mL for AFB1, 3.9 fg/mL for OTA, 2.6 fg/mL for ZEN, 1.4 fg/mL for FB1, 1.7 fg/mL for T-2, and 1.5 fg/mL for DON, respectively. In addition, a short cycle time (~40 min), a high detection specificity, a good precision, and a satisfactory analytical capability for real samples were obtained. Despite its high sensitivity and selectivity, fluorescent detection still faces some challenges. Due to the inherent differences between absorbing materials, it is important to select the appropriate excitation wavelength for fluorescent detection. In addition, spontaneous background signals generated by complex food matrix components may produce an effect on the detection accuracy133.
Surface-enhanced Raman scattering (SERS) detection
As a promising new analytical device, SERS-based microfluidic biosensors have many advantages (e.g., high sensitivity, rich molecular information, less optical drift, rapidity, narrow spectral bands, and portability)79,134. Moreover, SERS-based microfluidic biosensors can realize the on-site detection and complete the detection using a small amount of solution in a confined space, which is beneficial for the detection of mycotoxins in food135,136,137. By using electron beam lithography, Galarreta et al. prepared a nanostructured Au 2D SERS microfluidic platform for the detection of OTA. Briefly, metal nanostructures were prepared by electron-beam lithography on the surface of glass coverslips, which were further embedded in a microfluidic channel made of PDMS. The localized surface plasmon resonance (LSPR) of designed Au structure matched the excitation wavelength for Raman measurements. The OTA aptamer was immobilized on the metal nanostructures, and the conformational changes upon binding with OTA were analyzed via the acquired SERS data80. However, any impurities during the production of SERS substrates using soft lithography might interfere with the target signal. In addition, stresses during the transfer process might lead to subtle changes in the internal gap of the nanocomponents, which resulted in a reduction of the “hot spot” enhancement. To further improve the analytical sensitivity of the SERS biosensor, Wu et al. subsequently developed a SERS-active microfluidic chip for detection of OTA using a lift-up lithography strategy. Briefly, the SERS substrate of this biosensor was prepared by electrostatically assembling AuNPs, and the designed pattern was formed by subsequent lift-up soft lithography. The SERS micropatterns could be easily integrated within the microfluidic channel. The resulting microfluidic SERS chip allowed ultrasensitive in situ SERS monitoring. By functionalizing AuNPs with double-stranded DNA (OTA aptamer), the detection of OTA could be accomplished81. However, when the concentration of OTA was 500 nM or lower, the change in signal ratio did not show a concentration-dependent variation, implying that the sensitivity of the developed device was not able to meet food safety requirements (~5 nM). Moreover, it is still difficult to obtain highly stable and reproducible SERS signals in practical food detection due to the interference of complex food matrix and the diversity of non-target molecules, thus affecting the accuracy of food mycotoxin detection to a certain extent138.
ECL detection
Microfluidic ECL biosensors are miniaturized platforms with integrated microelectrodes and liquid actuation, which have shown many advantages (such as low cost, fast response time, controllability, and high sensitivity). Benefiting from their superior performances, microfluidic ECL biosensors have been widely applied to the detection of mycotoxins84,85,86,139. For example, Lu et al. fabricated a microfluidic immunosensor for the single detection of FB1 and DON by using capillary-driven PDMS microfluidic channels for sample processing. The dual-channel three-electrode ECL sensor pattern was photolithographed on the transparent ITO coated glass. Polished AuNPs were electrochemically deposited on the working electrodes, and the working electrodes were further functionalized with anti-FB1 and anti-DON antibodies. The formation of mycotoxin-antibody immunocomplexes on the working electrode surface could produce the ECL signal responses84. The prepared microfluidic ECL biosensors had a liner range from 0.3 ppb to 140 ppb with a LOD of 97 pg/mL for FB1 and a liner range from 0.2 ppb to 60 ppb with a LOD of 35 pg/mL for DON, respectively. The developed simultaneous assay system was used for the ultrasensitive, fast, convenient, and sensor-stable detection of ground corn extract. Later, utilizing Ag-CeO2 as nanocomposite/ITO electrodes, Solanki’s group developed a PDMS-based microfluidic ECL system for FB1 detection85. The developed microfluidic ECL system showed a linear detection range from 10 pg/mL to 100 ng/mL with a LOD of 1.5 × 10−6 μg/mL, enabling rapid, label-free, and highly sensitive detection of the antigen FB1. Based on the similar mechanism, Solanki’s group developed a new PDMS-based microfluidic immunosensor by using Ab-AFB1 immobilized Mn3O4/ITO (BSA/Ab-AFB1/Mn3O4/ITO) for detection of AFB1. In this system, tri-manganese tetroxide nanoparticles (Mn3O4 nps) were synthesized via the co-precipitation route at room temperature. A three-electrode chip and microfluidic channels for the construction of microfluidic ECL biosensing system were prepared using maskless ultraviolet (UV) lithography. On this basis, microfluidic immunosensors were successfully prepared for detection of AFB186. The developed ECL biosensor showed a liner range from 1 pg/mL to 300 ng/mL with an ultra-low LOD of 0.295 pg/mL. Recently, by using the Pd@PCN-222 CRISPR nanozyme to amplify signals, Wu et. al developed a CRISPR/Cas ECL microfluidic system for on-site detection of OTA. The excellent catalytic properties of the nanozymes were synergized with the trans-cleavage ability of the CRISPR enzyme, which significantly enhanced ECL signals to improve detection sensitivity87. The established method showed a detection range from 0.005 ng/mL to 50 ng/mL with a LOD of 1.21 pg/mL. However, ECL detection still suffers from some challenges, such as the easily contaminated electrodes and the potential interference from complex sample matrices140.
PEC detection
PEC microfluidic biosensors have exhibited many advantages, such as high sensitivity, good selectivity, good stability, fast response, and wide detection range91,92. Compared with the ordinary PEC biosensors, PEC microfluidic biosensors combine the advantages of PEC biosensors and characteristics of microfluidic devices, and have been applied in the portable and on-site detection of mycotoxins95. For example, Lin and his co-workers, designed and developed a PEC immunosensor for the rapid and sensitive detection of AFB1 in food samples. Glucose oxidase (GOx)-labeled AFB1-bovine serum albumin (AFB1-BSA) coupling was used as a marker. By using a competitive immunoassay format, the immunoreaction was carried out on anti-AFB1 antibody modified magnetic beads. Moreover, a competitive immunoassay format was combined with visual and PEC evaluation for detection of AFB1 by using carbon quantum dots (CQDs)-functionalized MnO2 nanosheets. Furthermore, the system was aggregated into a high-throughput microfluidic device to construct a semi-automated detection unit96. Under optimal conditions, the photocurrent increased with increasing target AFB1, and this PEC biosensor had a linear range from 0.01 to 2.0 ng/mL with a LOD of 2.1 pg/mL. Later, by using a novel nano-array bismuth sulfide/bismuth oxyiodide/zinc oxide (Bi2S3/BiOI/ZnO) with excellent photocatalytic activity, Wei’s group developed a prode DNA (p DNA)@Ag2S signal-regulated competitive PEC microfluidic immunosensor for the sensitive determination of OTA in wheat flour (Fig. 5A). A strong photocurrent intensity was achieved by modifying Bi2S3/BiOI/ZnO onto ITO electrodes because its good matching cascade band-edge levels could improve efficient separation of photo-generated e−/h+ pairs. The captured DNA (c DNA) was first immobilized on the sensing platform by glutaraldehyde and chitosan. After that, the p DNA sulfydryl-modified aptamers could directly react with AgNO3 by combination of Ag-S bond to form the p DNA@Ag2S. The formed p DNA@Ag2S was exploited as the acceptor to hybridize with the c DNA for formation of DNA duplexes. The photocurrent intensity was significantly reduced because the photogenerated electrons were partially converted to Ag2S instead of Bi2S3. In the presence of target OTA, the photocurrent could be enhanced after the target-induced removal of p DNA@Ag2S because the photo-generated electrons were transferred to the Bi2S3/BiOI/ZnO electrode95. The sensor realized the highly selective and sensitive analysis of target OTAs with a LOD of 3.5 × 10−2 pg/mL in a wide linear response range of 1 × 10−5–200 ng/mL. Although PEC detection possesses the merits of high sensitivity and simple operation, it still suffers from some challenges, such as the limited detection range, high cost, and the potential interference from ambient light93.
A Schematic diagram of a competitive PEC microfluidic sensor95. Copyright 2020, Elsevier B.V. B Schematic diagram of the constructed magnetotropic microfluidic fluorescence/colorimetric sensor145. Copyright 2023, Elsevier B.V. C Schematic diagram of the constructed paper-based microfluidic colorimetric/electrochemical sensor146. Copyright 2024, Elsevier B.V.
Dual-mode detection
Although single-mode microfluidic biosensors have been widely developed for the detection of mycotoxins, they are still susceptible to a variety of factors (such as unstable experimental environments, different personnel handling, and differences in the amount of sample and substrate morphology between batches), all of which make it difficult to meet the requirements of reliable and accurate analysis141. Therefore, dual-mode microfluidic sensors have been developed to allow simultaneous introduction of dual-channel detection into a microfluidic identification system, which can output multiple response signals under the same or different test conditions. In this case, each signal is independent and can be used to mutually verify the experimental results to further improve the accuracy. Benefiting from their superior performances, dual-mode microfluidic detection has been widely applied in detection of mycotoxins142. For example, based on manganese dioxide nanoflowers (MnO2NFs), Li et al. developed a colorimetric and fluorescent dual-mode aptamer sensor for the accurate and sensitive detection of AFB1. In this sensor, MnO2NFs could catalyze the oxidation of 3,3’,5,5’-tetramethylbenzidine (TMB) to a blue oxidation product (TMBox) in the presence of H2O2. In addition, MnO2NFs could also act as a signal amplifier, which could be reduced by ascorbic acid to generate a large amount of Mn2+, thus bursting the fluorescence of calreticulin143. The established dual-mode detection method showed high sensitivity and accuracy for the detection of AFB1. Based on AuAg NCs-SPCN nanocomposites, Ke et al. constructed a competitive fluorescent and colorimetric dual-mode immunosensor for OTA detection. S and P co-doping decreased the band gap width of carbon nitride and prolonged the absorption of visible light. Moreover, the addition of silver significantly improved the fluorescence properties of the nanoclusters. In the presence of OTA, the aggregation of nanocomposites was induced, which led to a greatly enhanced fluorescence signal due to the principle of aggregated luminescence effect (AIE). A new emission peak appeared at 440 nm to readout the fluorescence signal. On this basis, fluorescent detection mode was established. Meanwhile, the synthesized AuAg NCs-SPCN could decompose H2O2 to produce ·OH groups, which could oxidize TMB to blue oxTMB. In the presence of OTA, by binding AuAg NCs-SPCN to the OTA antibody, the AuAg NCs-SPCN-AbOTA was prepared for colorimetric quantitative detection of OTA144. The developed method was used for portable and sensitive OTA detection, showing a wide linear response range of 0.001–10 μg/L with a LOD of 0.155 ng/L (fluorescence) and 0.213 ng/L (colorimetric), respectively. To realize on-site detection, a portable fluorescence and colorimetric microfluidic detection platform could be constructed by combining a dual-mode detection method with a microfluidic chip. For example, based on G-quadruplex DNAzyme, Lin et al. constructed a portable microfluidic fluorescence/colorimetric sensor for detection of OTA in wheat (Fig. 5B). The OTA aptamer coupled with magnetic beads (mb) could self-assemble with two segments of DNA and hemin to form a g-quadruplex DNAzyme structure. G-quadruplex DNAzyme could catalyze the oxidation of 10-Acetyl-3,7-dihydroxyphenoxazine (Amplex Red) with H2O2, resulting in the production of a red color and strong fluorescence in solution. However, in the presence of OTA, the structure of the G-quadruplex DNAzyme was disrupted, making it less catalytically active. On this basis, a magnet-controlled chip integrating the reaction, detection, and washing was designed for on-site detection. Meanwhile, the mb-DNAzyme probe was shuttled to the magnetron chip, which greatly reduced the background signal and improved the detection efficiency and sensitivity145. The established method showed a LOD of 1.1 × 10−5 μg/mL (fluorescence) and 8 × 10−6 μg/mL (colorimetric). Later, by using flow channels to assemble platinum nanoparticle-treated colorimetric regions with patterned paper electrodes, Huang et al. designed and fabricated a dual-mode paper-based microfluidic chip (Fig. 5C). The synthesized porous UiO-66-NH2MOFs acted as functional carriers for loading TMB indicator molecules, which were then controlled by DNA switches consisting of CdS quantum dot-modified aptamer. In the presence of AFB1, it would bound to the aptamer and then targeted to induce TMB release, triggering both colorimetric and ECL signals146. The established method achieved ultra-high sensitive detection with a LOD of 7.8 fg/mL. To improve the detection portability and accuracy, dual-mode microfluidic sensors remain to be further explored for on-site detection of mycotoxins.
Challenges and perspectives
Microfluidic biosensors have shown many advantages (such as low sample consumption, miniaturization, integration, fast analysis, and automation) and have been widely applied in the detection of mycotoxins in food samples. However, there are some remaining challenges and chances in this significant field. In this section, we proposed future challenges and chances in the following aspects, including the development of new recognition elements towards mycotoxins, exploration of new sensing modes, design and development of new microfluidic devices, as well as intellectualization of microfluidic devices.
-
(1)
Development of new recognition elements towards mycotoxins: although the antibodies of mycotoxins have obtained widespread applications, they still suffer from some shortcomings (such as high cost, complex preparation, and poor stability). To solve this problem, emerging nanoantibodies with small molecular mass (about 15–17 kDa), good thermal stability, high solubility, low immunogenicity, and easy preparation have been successively developed147. However, the cost of Nb is high, and Nb-based forms of immunodetection are still limited to a certain extent. Further improvements and exploration of new recognition elements remained to be explored. In addition, aptamers with high specificity and stability can be further designed and developed to improve the detectability and stability. Compared with antibodies, aptamers have shown many advantages (e.g., relatively low cost and relatively high stability), but they still need complex preparation process. In addition, MIPs as a new recognition element have shown many advantages (e.g., high mechanical strength, low synthetic cost, long storage time, and a wide range of applicable temperatures), but they are at an early stage. New MIPs for mycotoxins remain to be further developed. In future studies, machine learning may be combined into MIPs technology for the design and development of new MIPs.
-
(2)
Exploration of new sensing modes for microfluidic detection of mycotoxins: existing sensors (e.g., colorimetric, fluorescent, and electrochemical) have been integrated with microfluidic devices for the detection of mycotoxins, but their sensitivity and stability need to be further improved. Therefore, it is important to develop new sensing modes. Surface plasmon resonance (SPR) as a novel analytical technique based on optical principles has many advantages (i.e., no labeling of analytes, real-time dynamic monitoring, high throughput, small sample size for detection, and fast response time), which shows a great potential in the detection of mycotoxins in food148. Moreover, surface-enhanced Raman scattering spectroscopy as a powerful tool may be combined with microfluidic devices to offer a great opportunity for the detection of various mycotoxins at trace levels. Thus, microfluidic SERS sensors remain to be further explored for detection of mycotoxins. Dual-mode microfluidic sensors have been developed for the detection of mycotoxins, but they are still in their infancy. Thus, novel and highly stable dual-mode microfluidic sensors remain to be further studied. Furthermore, novel sensing modes (e.g., chemiluminescence and ECL) can also be integrated with microfluidic devices to further improve their detection performances. In addition, to further improve the analytical performances of microfluidic devices, a number of emerging nanomaterials (e.g., nano-enzymes, metal organic frameworks, and porous materials) may be used to construct microfluidic biosensors for the highly sensitive detection of mycotoxins.
-
(3)
Design and development of new microfluidic devices: the manufacturing process of microfluidic chips is relatively complex and the cost is high. To reduce the cost, the cost-effective paper-based microfluidic chips were developed for widespread detection of mycotoxins. However, paper-based microfluidic chips still have some shortcomings, such as short lifespan and high permeability. To solve this problem, paper-based microfluidic chips with better performances remain to be further explored. Currently, most microfluidic devices are designed for detection of single targets. However, multiple mycotoxins may be simultaneously present in one food sample. Thus, new microfluidic devices for multi-detection of mycotoxins remain to be further designed and developed. In addition, microfluidic devices may suffer from the microchannel clogging, which leads to the reduced detection accuracy and efficiency. Moreover, due to complex food matrices, the detection of mycotoxins may suffer from potential interference with the food matrix. Consequently, the microfluidic device with highly efficient sample pretreatment functions remains to be further designed to improve the detection reliability of mycotoxins.
-
(4)
Intellectualization of microfluidic sensors: common microfluidic devices require bulky instruments for signal output. To improve the portability of microfluidic devices, smartphones can be combined with microfluidic sensors to perform the signal acquisition and data analysis, which possesses great promise for the on-site detection of food mycotoxins. Meanwhile, to improve their intelligence, microfluidic sensors combined with artificial intelligence may be a novel idea. The combination of microfluidic devices and advanced artificial intelligence (e.g., machine learning and deep learning) is of great promise for simplifying operations, facilitating signal acquisition and data analysis, enabling automation, and realizing detection system intellectualization.
Conclusion
The persistent problem of mycotoxin residues in food urgently requires the development of rapid and practical detection technologies. Traditional detection methods are difficult to meet the requirements of rapid and on-site detection due to cumbersome operations and complex instruments. As an emerging technology, biosensors show great potential for rapid, accurate, and portable mycotoxin detection. Microfluidic chips have the advantages of small size, precise control, and rich functions. To realize the on-site detection, microfluidic chips have been successfully integrated with biosensors for the detection of mycotoxins. This review summarized the recent progress on microfluidic biosensors for the detection of mycotoxins. Firstly, the characteristics and preparation of microfluidic devices based on different materials (e.g., glass, silicon, PDMS, PMMA, and paper) were summarized and discussed. Next, we discussed the roles of microfluidic devices in mycotoxin analysis (i.e., sample preparation, separation, and analysis). Then, the characteristics and preparation of various recognition elements (e.g., antibodies, aptamers, and MIPs) for mycotoxin detection were discussed. In addition, microfluidic biosensors based on different sensing modes (colorimetric, fluorescence, SERS, electrochemical, and photoelectrochemical) for the on-site detection of mycotoxins in food were summarized and compared. Impressively, future opportunities and challenges in this promising field were proposed.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- 3D:
-
Three-dimensional
- 2D:
-
Two-dimensional
- AFB1:
-
Aflatoxin B1
- AFB1-BSA:
-
AFB1-bovine serum albumin
- AFM1:
-
Aflatoxin M1
- Ag:
-
Silver
- AIE:
-
Aggregated luminescence effect
- Amplex red:
-
10-Acetyl-3,7-dihydroxyphenoxazine
- Au:
-
Gold
- AuNPs:
-
Gold nanoparticles
- Bi2S3/BiOI/ZnO:
-
Bismuth sulfide/bismuth oxyiodide/zinc oxide
- cDNA:
-
Complementary DNA
- CM:
-
Chemical enhancement
- CQDs:
-
Carbon quantum dots
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- crRNA:
-
CRISPR RNA
- ECL:
-
Electrochemiluminescence
- EM:
-
Electromagnetic enhancement
- FB1:
-
Fumonisin B1
- FRET:
-
Fluorescence resonance energy transfer
- FTMB:
-
Functional DNA-guided transition-state CRISPR/Cas12a microfluidic biosensor
- GOx:
-
Glucose oxidase
- HcAb:
-
Heavy chain antibodies
- HPLC:
-
High-performance liquid chromatography
- IgG:
-
Immunoglobulin G
- ITO:
-
Indium tin oxide
- LAMP:
-
Loop mediated isothermal amplification
- LC-MS/MS:
-
Liquid chromatography tandem mass spectrometry
- LOD:
-
Limit of detection
- LSPR:
-
Localized surface plasmon resonance
- mb:
-
Magnetic beads
- MIPs:
-
Molecularly imprinted polymers
- MnO2NFs:
-
Manganese dioxide nanoflowers
- Mn3O4 nps:
-
Tri-manganese tetroxide nanoparticles
- MRL:
-
Maximum residue level
- Nbs:
-
Nanoantibodies
- OTA:
-
Ochratoxin A
- PCR:
-
Polymerase chain reaction
- PCs:
-
Photonic crystals
- PDMS:
-
Polydimethylsiloxane
- p DNA@Ag2S:
-
Ag2S-modified aptamers probe DNA
- PEC:
-
Photoelectrochemical
- PMMA:
-
Polymethyl methacrylate
- PS:
-
Polystyrene
- QD:
-
Quantum dot
- RNA:
-
Ribonucleic Acid
- SdAb:
-
Single domain antibodies
- SELEX:
-
Systematic evolution of ligands by exponential enrichment
- SERS:
-
Surface-enhanced Raman scattering
- Si:
-
Silicon
- SiO2 :
-
Silicon dioxide
- SPR:
-
Surface plasmon resonance
- ssDNA:
-
Single-stranded DNA
- TCPP:
-
Tetrakis(4-carboxyphenyl) porphyrin
- TMB:
-
3,3′,5,5′-Tetramethylbenzidine
- UCNPs:
-
Upconversion nanoparticles
- UV:
-
Ultraviolet
- V-chip:
-
Volumetric bar chart chip
- VHH:
-
Variable domain of heavy chain of heavy-chain antibody
- ZEN:
-
Zearalenone
- μPADs:
-
Microfluidic paper-based analytical devices
- μTAS:
-
Micro total analysis system
References
Guo, X. D. et al. Aptamer-based biosensor for detection of mycotoxins. Front. Chem. 8, 195 (2020).
Atar, N., Eren, T. & Yola, M. L. A molecular imprinted SPR biosensor for sensitive determination of citrinin in red yeast rice. Food Chem. 184, 7–11 (2015).
Liu, J. et al. A portable automated microfluidic platform for point-of-care testing for multiple mycotoxins in wine. Foods 13, 2066 (2024).
Ebrahim, S., Labeb, M., Abdel-Fattah, T. & Soliman, M. CdTe quantum dots capped with different stabilizing agents for sensing of ochratoxin A. J. Lumin. 182, 154–159 (2017).
Deng, Y. J. et al. Fungal diversity and mycotoxin contamination in dried fish products in Zhanjiang market, China. Food Control 121, https://doi.org/10.1016/j.foodcont.2020.107614 (2021).
Chen, Y. et al. Rapid detection of four mycotoxins in corn using a microfluidics and microarray-based immunoassay system. Talanta 186, 299–305 (2018).
Li, P. W. et al. Current development of microfluidic immunosensing approaches for mycotoxin detection via capillary electromigration and lateral flow technology. Electrophoresis 33, 2253–2265 (2012).
Zhai, S. S. et al. Ochratoxin A: its impact on poultry gut health and microbiota, an overview. Poult. Sci. 100, 101037 (2021).
Yan, X. et al. Recent trends in fluorescent aptasensors for mycotoxin detection in food: principles, constituted elements, types, and applications. ACS Sens. 3, 428–452 (2022).
Kebede, H., Liu, X. M., Jin, J. & Xing, F. G. Current status of major mycotoxins contamination in food and feed in Africa. Food Control 110, 106975 (2020).
Akgönüllü, S., Yavuz, H. & Denizli, A. SPR nanosensor based on molecularly imprinted polymer film with gold nanoparticles for sensitive detection of aflatoxin B1. Talanta 219, 121219 (2020).
Akgönüllü, S., Yavuz, H. & Denizli, A. Development of gold nanoparticles decorated molecularly imprinted–based plasmonic sensor for the detection of aflatoxin M1 in milk samples. Chemosensors 9, 363 (2021).
Xing, K.-Y., Shan, S., Liu, D.-F. & Lai, W.-H. Recent advances of lateral flow immunoassay for mycotoxins detection. Trends Anal. Chem. 133, 116087 (2020).
Chen, D. et al. Determination of ochratoxin A and ochratoxin alpha in wine by ultra-performance liquid chromatography tandem mass spectrometry. Wei Sheng Yan Jiu = J. Hyg. Res. 48, 646–650 (2019).
Guo, L. et al. Application of microfluidic “lab-on-a-chip” for the detection of mycotoxins in foods. Trends Food Sci. Technol. 46, 252–263 (2015).
Puiu, M. & Bala, C. Microfluidics-integrated biosensing platforms as emergency tools for on-site field detection of foodborne pathogens. Trends Anal. Chem. 125, 115831 (2020).
Ji, C., Zhou, Y., Leblanc, R. M. & Peng, Z. Recent developments of carbon dots in biosensing: a review. ACS Sens. 5, 2724–2741 (2020).
Zhu, D. et al. Recent progresses on emerging biosensing technologies and portable analytical devices for detection of food allergens. Trends Food Sci. Technol. 148, 104485 (2024).
Lee, W. et al. Ultrarapid detection of pathogenic bacteria using a 3D immunomagnetic flow assay. Anal. Chem. 86, 6683–6688 (2014).
Fattahi, Z. & Hasanzadeh, M. Nanotechnology-assisted microfluidic systems for chemical sensing, biosensing, and bioanalysis. Trends Anal. Chem. 152, 116637 (2022).
Whitesides, G. M. The origins and the future of microfluidics. Nature 442, 368–373 (2006).
Zhang, X. et al. Nanozyme-enabled microfluidic biosensors: a promising tool for on-site food safety analysis. Trends Food Sci. Technol. 148, 104486 (2024).
Nunes, J. & Stone, H. Introduction: microfluidics. Chem. Rev. 122, 6919–6920 (2022).
Xue, L. et al. Lab-on-chip separation and biosensing of pathogens in agri-food. Trends Food Sci. Technol. 137, 92–103 (2023).
Zirath, H. et al. Bridging the academic–industrial gap: application of an oxygen and pH sensor-integrated lab-on-a-chip in nanotoxicology. Lab Chip 21, 4237–4248 (2021).
Atalay, Y. T. et al. Microfluidic analytical systems for food analysis. Trends Food Sci. Technol. 22, 386–404 (2011).
Zhu, L. et al. Research progress on pesticide residue detection based on microfluidic technology. Electrophoresis 44, 1377–1404 (2023).
Nielsen, J. B. et al. Microfluidics: innovations in materials and their fabrication and functionalization. Anal. Chem. 92, 150–168 (2019).
Liu, Y., Zhang, W., He, L. & Zhang, X. All-optical separation of chiral nanoparticles on silicon-based microfluidic chips with vector exceptional points. APL Photonics 8, 036112 (2023).
Martins, J. P., Torrieri, G. & Santos, H. The importance of microfluidics for the preparation of nanoparticles as advanced drug delivery systems. Expert Opin. Drug Deliv. 15, 469–479 (2018).
Zhao, S. et al. A laser-induced fluorescent detector for pesticide residue detection based on the spectral recognition method. Anal. Methods 10, 5507–5515 (2018).
Ren, K., Zhou, J. & Wu, H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 46, 2396–2406 (2013).
Huang, L. et al. Chip-based multi-molecularly imprinted monolithic capillary array columns coated Fe3O4/GO for selective extraction and simultaneous determination of tetracycline, chlortetracycline and deoxytetracycline in eggs. Microchem. J. 150, 104097 (2019).
Uka, B., Kieninger, J., Urban, G. A. & Weltin, A. J. Electrochemical microsensor for microfluidic glyphosate monitoring in water using MIP-based concentrators. ACS Sens. 6, 2738–2746 (2021).
Choi, Y.-J. et al. Label-free attomolar protein detection using a MEMS optical interferometric surface-stress immunosensor with a freestanding PMMA/parylene-C nanosheet. Biosens. Bioelectron. 172, 112778 (2021).
Kotz, F. et al. Fused deposition modeling of microfluidic chips in polymethylmethacrylate. Micromachines 11, 873 (2020).
Chen, S. et al. Present status of microfluidic PCR chip in nucleic acid detection and future perspective. Trends Analyt Chem. 157, 116737 (2022).
Chałupniak, A. & Merkoçi, A. Toward integrated detection and graphene-based removal of contaminants in a lab-on-a-chip platform. Nano Res. 10, 2296–2310 (2017).
Díaz-González, M. et al. A microfluidic device for the automated electrical readout of low-density glass-slide microarrays. Biosens. Bioelectron. 74, 698–704 (2015).
Gao, H. W., Yan, C. L., Wu, W. & Li, J. Application of microfluidic chip technology in food safety sensing. Sensors 20, 1792 (2020).
Mumtaz, Z. et al. Prospects of microfluidic technology in nucleic acid detection approaches. Biosensors 13, 584 (2023).
Li, W., Ma, X., Yong, Y.-C., Liu, G. & Yang, Z. Review of paper-based microfluidic analytical devices for in-field testing of pathogens. Anal. Chim. Acta 1278, 341614 (2023).
Carrilho, E., Martinez, A. W. & Whitesides, G. Understanding wax printing: a simple micropatterning process for paper-based microfluidics. Anal. Chem. 81, 7091–7095 (2009).
Alahmad, W., Varanusupakul, P. & Varanusupakul, P. Recent developments and applications of microfluidic paper-based analytical devices for the detection of biological and chemical hazards in foods: a critical review. Crit. Rev. Anal. Chem. 53, 233–252 (2023).
Thuo, M. M. et al. Fabrication of low-cost paper-based microfluidic devices by embossing or cut-and-stack methods. Chem. Mater. 26, 4230–4237 (2014).
Zhu, J. et al. Highly sensitive and label-free determination of thiram residue using surface-enhanced Raman spectroscopy (SERS) coupled with paper-based microfluidics. Anal. Methods 9, 6186–6193 (2017).
Zhao, P. et al. Fabrication of a novel hydrogel-based microfluidic chip and its application in pathogen analysis. Anal. Methods 13, 5240–5246 (2021).
Nie, J., Fu, J. & He, Y. Hydrogels: the next generation body materials for microfluidic chips? Small 16, 2003797 (2020).
Shen, C., Li, Y., Wang, Y. & Meng, Q. Non-swelling hydrogel-based microfluidic chips. Lab Chip 19, 3962–3973 (2019).
Adunphatcharaphon, S. et al. The evolution of multiplex detection of mycotoxins using immunoassay platform technologies. J. Hazard Mater. 432, 128706 (2022).
Liao, X. Y. et al. Advancing point-of-care microbial pathogens detection by material-functionalized microfluidic systems. Trends Food Sci. Technol. 135, 115–130 (2023).
Gowda, H. N. et al. Development of a proof-of-concept microfluidic portable pathogen analysis system for water quality monitoring. Sci. Total Environ. 813, 152556 (2022).
Shi, H. et al. Recent advances of integrated microfluidic systems for fungal and bacterial analysis. Trends Anal. Chem. 158, 116850 (2023).
Soares, R. et al. On-chip sample preparation and analyte quantification using a microfluidic aqueous two-phase extraction coupled with an immunoassay. Lab Chip 14, 4284–4294 (2014).
Hervás, M., López, M. A. & Escarpa, A. J. A. Integrated electrokinetic magnetic bead-based electrochemical immunoassay on microfluidic chips for reliable control of permitted levels of zearalenone in infant foods. Analyst 136, 2131–2138 (2011).
Gao, D., Ma, Z. & Jiang, Y. Recent advances in microfluidic devices for foodborne pathogens detection. Trends Anal. Chem. 157, 116788 (2022).
Goud, K. Y., Reddy, K. K., Satyanarayana, M., Kummari, S. & Gobi, K. V. A review on recent developments in optical and electrochemical aptamer-based assays for mycotoxins using advanced nanomaterials. Microchim. Acta 187, 29 (2019).
Murugesan, P., Raj, G. & Moses, J. Microfluidic devices for the detection of pesticide residues. Rev. Environ. Sci. Bio/Technol. 22, 625–652 (2023).
Yang, F.-Q. & Ge, L. J. Colorimetric Sensors: Methods and Applications. Sensors 23, 9887 (2023).
Machado, J. M., Soares, R. R., Chu, V. & Conde, J. P. Multiplexed capillary microfluidic immunoassay with smartphone data acquisition for parallel mycotoxin detection. Biosens. Bioelectron. 99, 40–46 (2018).
Jiang, Q. et al. Paper-based microfluidic device (DON-Chip) for rapid and low-cost deoxynivalenol quantification in food, feed, and feed ingredients. ACS Sens 4, 3072–3079 (2019).
Kasoju, A. et al. Fabrication of microfluidic device for Aflatoxin M1 detection in milk samples with specific aptamers. Sci. Rep. 10, 4627 (2020).
Shahdeo, D. et al. Molecular diagnostic of ochratoxin A with specific aptamers in corn and groundnut via fabrication of a microfluidic device. Front. Nutr. 9, 851787 (2022).
Kasoju, A. et al. Microfluidic paper device for rapid detection of aflatoxin B1 using an aptamer based colorimetric assay. RSC Adv. 10, 11843–11850 (2020).
Tang, X. et al. Colorimetric detection of Aflatoxin B1 by using smartphone-assisted microfluidic paper-based analytical devices. Food Control 132, 108497 (2022).
Ma, Y. et al. Portable visual quantitative detection of aflatoxin B 1 using a target-responsive hydrogel and a distance-readout microfluidic chip. Lab Chip 16, 3097–3104 (2016).
Feng, S., Hua, M. Z., Roopesh, M. S. & Lu, X. Rapid detection of three mycotoxins in animal feed materials using competitive ELISA-based origami microfluidic paper analytical device (μPAD). Anal. Bioanal. Chem. 415, 1943–1951 (2023).
Qiu, M. Y. et al. Recent advances on emerging biosensing technologies and on-site analytical devices for detection of drug-resistant foodborne pathogens. Trends Anal. Chem. 167, 117258 (2023).
Pang, L. et al. Optical nanosensors based on noble metal nanoclusters for detecting food contaminants: a review. Compr. Rev. Food Sci. Food Saf. 23, 1–34 (2024).
Jin, B. et al. Lateral flow aptamer assay integrated smartphone-based portable device for simultaneous detection of multiple targets using upconversion nanoparticles. Sens Actuators B Chem. 276, 48–56 (2018).
Xiang, X. R. et al. Microfluidic biosensor integrated with signal transduction and enhancement mechanism for ultrasensitive noncompetitive assay of multiple mycotoxins. Anal. Chem. 95, 7993–8001 (2023).
Lin, X. et al. A portable paper-based aptasensor for simultaneous visual detection of two mycotoxins in corn flour using dual-color upconversion nanoparticles and Cu-TCPP nanosheets. Food Chem. 404, 134750 (2023).
Xiang, X. et al. Quantitative detection of aflatoxin B1 using quantum dots-based immunoassay in a recyclable gravity-driven microfluidic chip. Biosens. Bioelectron. 190, 113394 (2021).
Epifania, R., Soares, R. R. G., Pinto, I. F., Chu, V. & Conde, J. P. Capillary-driven microfluidic device with integrated nanoporous microbeads for ultrarapid biosensing assays. Sens Actuators B Chem. 265, 452–458 (2018).
Nakamura, A. et al. Determination of deoxynivalenol in wheat, barley, corn meal, and wheat-based products by simultaneous multisample fluorescence polarization immunoassay using a portable analyzer. ACS Food Sci. Technol. 1, 1623–1628 (2021).
Wang, X., Lu, D., Huang, Q. & Yang, J. Microfluidics-based time-resolved fluorescence immunoassay for the on-site detection of aflatoxins B1 zearalenone and deoxynivalenol in cereals. Foods 11, 1319 (2022).
Hua, M. Z., Li, S., Roopesh, M. S. & Lu, X. Development of a microfluidic device to enrich and detect zearalenone in food using quantum dot-embedded molecularly imprinted polymers. Lab Chip 24, 2700–2711 (2024).
Hassan, M. M. et al. Au@ Ag nanostructure based SERS substrate for simultaneous determination of pesticides residue in tea via solid phase extraction coupled multivariate calibration. LWT Food Sci. Technol. 105, 290–297 (2019).
Yang, Y. et al. Low background interference SERS aptasensor for highly sensitive multiplex mycotoxin detection based on polystyrene microspheres-mediated controlled release of Raman reporters. Anal. Chim. Acta 1218, 340000 (2022).
Galarreta, B. C. et al. Microfluidic channel with embedded SERS 2D platform for the aptamer detection of ochratoxin A. Anal. Bioanal. Chem. 405, 1613–1621 (2013).
Wu, Y. et al. Facile fabrication of microfluidic surface-enhanced Raman scattering devices via lift-up lithography. R. Soc. Open Sci. 5, 172034 (2018).
Golubchik, A., Lopes, L. C., Singh, V. & Kuss, S. J. Pharma-molecule transport across bacterial membranes: detection and quantification approaches by electrochemistry and bioanalytical methods. Angew. Chem. 133, 22284–22296 (2021).
Campuzano, S., Yáñez-Sedeño, P. & Pingarrón, J. M. Molecular biosensors for electrochemical detection of infectious pathogens in liquid biopsies: current trends and challenges. Sensors 17, 2533 (2017).
Lu, L. & Gunasekaran, S. J. T. Dual-channel ITO-microfluidic electrochemical immunosensor for simultaneous detection of two mycotoxins. Talanta 194, 709–716 (2019).
Dhiman, T. K. et al. Rapid and label-free electrochemical detection of fumonisin-B1 using microfluidic biosensing platform based on Ag-CeO2 nanocomposite. J. Electrochem. Soc. 168, 077510 (2021).
Singh, A. K. et al. Rapid and label-free detection of Aflatoxin-B1 via microfluidic electrochemical biosensor based on manganese (III) oxide (Mn3O4) synthesized by co-precipitation route at room temperature. Nanotechnology 33, 285501 (2022).
Wu, C. et al. CRISPR-powered microfluidic biosensor for preamplification-free detection of ochratoxin A. Talanta 269, 125414 (2024).
Mondal, K., Ali, M. A., Srivastava, S., Malhotra, B. D. & Sharma, A. Electrospun functional micro/nanochannels embedded in porous carbon electrodes for microfluidic biosensing. Sens. Actuators B Chem. 229, 82–91 (2016).
Laza, A. et al. Origami paper-based electrochemical immunosensor with carbon nanohorns-decorated nanoporous gold for zearalenone detection. Chemosensors 12, 10 (2024).
Qiu, Z., Shu, J., Liu, J. & Tang, D. J. A. C. Dual-channel photoelectrochemical ratiometric aptasensor with up-converting nanocrystals using spatial-resolved technique on homemade 3D printed device. Anal. Chem. 91, 1260–1268 (2018).
Zhao, W.-W., Xu, J.-J. & Chen, H.-Y. Photoelectrochemical bioanalysis: the state of the art. Chem. Soc. Rev. 44, 729–741 (2015).
Tu, W., Wang, Z. & Dai, Z. Selective photoelectrochemical architectures for biosensing: design, mechanism and responsibility. Trends Anal. Chem. 105, 470–483 (2018).
Li, J. et al. A novel photoelectrochemical microfluidic chip for multi-index determination of diabetes and its complications. Biosens. Bioelectron. 217, 114719 (2022).
Zhuo, Y., Xu, W., Chen, Y. & Long, F. Rapid and sensitive point-of-need aflatoxin B1 testing in feedstuffs using a smartphone-powered mobile microfluidic lab-on-fiber device. J. Hazard Mater. 460, 132406 (2023).
Feng, J. et al. A signal amplification of p DNA@ Ag2S based photoelectrochemical competitive sensor for the sensitive detection of OTA in microfluidic devices. Biosens. Bioelectron. 168, 112503 (2020).
Lin, Y., Zhou, Q., Tang, D., Niessner, R. & Knopp, D. Signal-on photoelectrochemical immunoassay for aflatoxin B1 based on enzymatic product-etching MnO2 nanosheets for dissociation of carbon dots. Anal. Chem. 89, 5637–5645 (2017).
Somvanshi, S. B. et al. Microfluidic paper-based aptasensor devices for multiplexed detection of pathogenic bacteria. Biosens. Bioelectron. 207, 114214 (2022).
Chen, F. et al. Multiplex detection of infectious diseases on microfluidic platforms. Biosensors 13, 410 (2023).
Brito-Pereira, R., Ribeiro, C., Lanceros-Méndez, S. & Fernandes Cardoso, V. Biodegradable polymer-based microfluidic membranes for sustainable point-of-care devices. Chem. Eng. J. 448, 137639 (2022).
Dang, B. V. et al. Microfluidic actuation via 3D-printed molds toward multiplex biosensing of cell apoptosis. ACS Sens 4, 2181–2189 (2019).
Zhang, X. L. et al. A bifunctional core-shell gold@Prussian blue nanozyme enabling dual-readout microfluidic immunoassay of food allergic protein. Food Chem. 434, 137455 (2024).
Wang, L. et al. Recent progress in visual methods for aflatoxin detection. Crit. Rev. Food Sci. Nutr. 62, 7849–7865 (2022).
Del Rio, B. et al. Lactic acid bacteria as a live delivery system for the in situ production of nanobodies in the human gastrointestinal tract. Front. Microbiol. 9, 432854 (2019).
Zhang, X., Li, G., Wu, D., Liu, J. & Wu, Y. Recent advances on emerging nanomaterials for controlling the mycotoxin contamination: from detection to elimination. Food Front. 1, 360–381 (2020).
Wang, Y. et al. Nanobody-based food allergen surveillance: current status and prospects. Food Qual. Saf. 8, fyae018 (2024).
Robinson, G., Hamers, C., Bajyana Songa, E., Bendahman, N. & Hamers, R. Naturally occuring antibodies devoid of light chains. Nature 363, 3 (1993).
Narciso, J. E. T. et al. Analysis of the antibody structure based on high-resolution crystallographic studies. N. Biotechnol. 28, 435–447 (2011).
He, T. et al. Nanobody technology for mycotoxin detection in the field of food safety: current status and prospects. Toxins 10, 180 (2018).
Desmyter, A., Spinelli, S., Roussel, A. & Cambillau, C. Camelid nanobodies: killing two birds with one stone. Curr. Opin. Struct. Biol. 32, 1–8 (2015).
Zhang, X. L., Li, G. L., Liu, J. H. & Su, Z. Q. Bio-inspired nanoenzyme synthesis and its application in a portable immunoassay for food allergy proteins. J. Agric. Food Chem. 69, 14751–14760 (2021).
Lou, X. et al. Micromagnetic selection of aptamers in microfluidic channels. Proc. Natl Acad. Sci. USA 106, 2989–2994 (2009).
Pang, L. et al. Colorimetric biosensor based on aptamer recognition-induced multi-DNA release and peroxidase-mimicking three-way junction DNA-Ag/PtNCs for the detection of Salmonella typhimurium. Talanta 274, 125930 (2024).
Chang, A. L., McKeague, M., Liang, J. C. & Smolke, C. D. Kinetic and equilibrium binding characterization of aptamers to small molecules using a label-free, sensitive, and scalable platform. Anal. Chem. 86, 3273–3278 (2014).
Shangguan, D. et al. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc. Natl Acad. Sci. USA 103, 11838–11843 (2006).
Guo, W. F. et al. Advances in aptamer screening and aptasensors’ detection of heavy metal ions. J. Nanobiotechnol. 19, 166 (2021).
Wang, Q., Yang, Q. & Wu, W. Progress on structured biosensors for monitoring aflatoxin B1 from biofilms: a review. Front. Microbiol. 11, 408 (2020).
Delaunay, N., Combès, A. & Pichon, V. Immunoaffinity extraction and alternative approaches for the analysis of toxins in environmental, food or biological matrices. Toxins 12, 795 (2020).
Ma, X. H., Li, J. P., Wang, C. & Xu, G. B. Review on bio-macromolecular imprinted sensors and their applications. Chin. J. Anal. Chem. 44, 152–159 (2016).
Mamipour, Z., Nematollahzadeh, A. & Kompany-Zareh, M. Molecularly imprinted polymer grafted on paper and flat sheet for selective sensing and diagnosis: a review. Microchim. Acta 188, 279 (2021).
Alhamoud, Y. et al. Advances in biosensors for the detection of ochratoxin A: bio-receptors, nanomaterials, and their applications. Biosens. Bioelectron. 141, 111418 (2019).
Pacheco, J. G. et al. Molecularly imprinted electrochemical sensor for ochratoxin A detection in food samples. Sens. Actuators B Chem. 215, 107–112 (2015).
Huang, W. H. et al. Selective enrichment-release of trace dibutyl phthalate via molecular-imprinting based photo-controlled switching followed by high-performance liquid chromatography analysis. J. Sep Sci. 44, 513–520 (2021).
Xue, M. et al. Extraction of shikimic acid from Chinese star anise using flash column chromatography on a molecularly-imprinted polymer column. J. Liq. Chromatogr. Relat. Technol. 36, 2677–2686 (2013).
Wan, L. B., Liu, H., Huang, C. X. & Shen, X. T. Enzyme-like MOFs: synthetic molecular receptors with high binding capacity and their application in selective photocatalysis. J. Mater. Chem. A Mater. 8, 25931–25940 (2020).
Sawetwong, P., Chairam, S., Jarujamrus, P. & Amatatongchai, M. Enhanced selectivity and sensitivity for colorimetric determination of glyphosate using Mn-ZnS quantum dot embedded molecularly imprinted polymers combined with a 3D-microfluidic paper-based analytical device. Talanta 225, 122077 (2021).
Lofgreen, J. E. & Ozin, G. A. Controlling morphology and porosity to improve performance of molecularly imprinted sol-gel silica. Chem. Soc. Rev. 43, 911–933 (2014).
Ohashi, T., Mawatari, K. & Kitamori, T. On-chip antibody immobilization for on-demand and rapid immunoassay on a microfluidic chip. Biomicrofluidics 4, 032207 (2010).
Schasfoort, R. B. (ed.). Handbook of Surface Plasmon Resonance (Royal Society of Chemistry, 2017).
Chiodi, E., Marn, A. M., Geib, M. T. & Ünlü, M. S. The role of surface chemistry in the efficacy of protein and DNA microarrays for label-free detection: an overview. Polymers 13, 1026 (2021).
Nimse, S. B., Song, K., Sonawane, M. D., Sayyed, D. R. & Kim, T. J. Immobilization techniques for microarray: challenges and applications. Sensors 14, 22208–22229 (2014).
Tsougeni, K. et al. Three-dimensional (3D) plasma micro-nanotextured slides for high performance biomolecule microarrays: comparison with epoxy-silane coated glass slides. Colloids Surf. B Biointerfaces 165, 270–277 (2018).
Man, Y. et al. A microfluidic colorimetric biosensor for in-field detection of Salmonella in fresh-cut vegetables using thiolated polystyrene microspheres, hose-based microvalve and smartphone imaging APP. Food Chem. 354, 129578 (2021).
Qiu, M. et al. Recent advances on CRISPR/Cas system-enabled portable detection devices for on-site agri-food safety assay. Trends Food Sci. Technol. 129, 364–387 (2022).
Xia, L. & Li, G. Recent progress of microfluidics in surface‐enhanced Raman spectroscopic analysis. J. Sep. Sci. 44, 1752–1768 (2021).
Yang, M., Liu, G., Mehedi, H. M., Ouyang, Q. & Chen, Q. J. A universal SERS aptasensor based on DTNB labeled GNTs/Ag core-shell nanotriangle and CS-Fe3O4 magnetic-bead trace detection of Aflatoxin B1. Anal. Chim. Acta 986, 122–130 (2017).
Hassan, M. M., Zareef, M., Xu, Y., Li, H. & Chen, Q. J. SERS based sensor for mycotoxins detection: challenges and improvements. Food Chem. 344, 128652 (2021).
Zhai, W., You, T., Ouyang, X. & Wang, M. J. Recent progress in mycotoxins detection based on surface-enhanced Raman spectroscopy. Compr. Rev. Food Sci. Food Saf. 20, 1887–1909 (2021).
Zhang, X. L. et al. Biomineralization-inspired artificial clickase for portable click SERS immunoassay of Salmonella enterica serovar Paratyphi B in foods. Food Chem. 413, 135553 (2023).
Mohan, J. M., Amreen, K., Javed, A., Dubey, S. K. & Goel, S. J. Emerging trends in miniaturized and microfluidic electrochemical sensing platforms. Curr. Opin. Electrochem. 33, 100930 (2022).
Rezazadeh, M., Seidi, S., Lid, M., Pedersen-Bjergaard, S. & Yamini, Y. The modern role of smartphones in analytical chemistry. Trends Anal. Chem. 118, 548–555 (2019).
Chen, M. T. et al. Advances in immunoassay-based strategies for mycotoxin detection in food: from single-mode immunosensors to dual-mode immunosensors. Compr. Rev. Food Sci. Food Saf. 22, 1285–1311 (2023).
Liu, Z. et al. A smartphone-based dual detection mode device integrated with two lateral flow immunoassays for multiplex mycotoxins in cereals. Biosens. Bioelectron. 158, 112178 (2020).
Li, S. F. et al. MnO2 nanoflowers based colorimetric and fluorescent dual-mode aptasensor for sensitive detection of aflatoxin B1 in milk. Anal. Chim. Acta 1279, 341844 (2023).
Ke, C. X. et al. A novel competitive fluorescence colorimetric dual-mode immunosensor for detecting ochratoxin A based on the synergistically enhanced peroxidase-like activity of AuAg NCs-SPCN nanocomposite. Food Chem. 437, 137930 (2024).
Lin, X. Q. et al. Magnetically actuated microfluidic chip combined with a G-quadruplex DNAzyme-based fluorescent/colorimetric sensor for the dual-mode detection of ochratoxin A in wheat. Talanta 267, 125273 (2024).
Huang, S. et al. Portable dual-mode paper chips for highly sensitive and rapid determination of aflatoxin B1 via an aptamer-gated MOFs. Food Chem. 457, 140182 (2024).
Zhang, X. L. et al. Recent progress in the construction of nanozyme-based biosensors and their applications to food safety assay. Trends Anal. Chem. 121, 115668 (2019).
Chang, C. C. Recent advancements in aptamer-based surface plasmon resonance biosensing strategies. Biosensors 11, 233 (2021).
Acknowledgements
This research was supported by the Joint Funds of the National Natural Science Foundation of China (No. U21A20272) and Scientific Research Start-up Funds of Northeast Agricultural University (No. 54960612).
Author information
Authors and Affiliations
Contributions
J.W.Z.: conceptualization, writing—original draft preparation, writing—review and editing. X.R.Z.: writing—review and editing. Y.Z., X.Y.Y., and L.G.: supervision, writing—review and editing. C.X.M.: software and formal analysis. Y.J.J.: funding acquisition, software and formal analysis. W.Z.: supervision, writing—review and editing. X.L.Z.: conceptualization, supervision, writing—review and editing, and funding acquisition. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, J., Zhang, X., Zhang, Y. et al. Emerging biosensors integrated with microfluidic devices: a promising analytical tool for on-site detection of mycotoxins. npj Sci Food 9, 84 (2025). https://doi.org/10.1038/s41538-025-00444-5
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41538-025-00444-5
This article is cited by
-
Next-Generation Joint-on-a-Chip: Toward Precision Mechanical Control in Multi-Tissue Systems
Nano-Micro Letters (2026)
-
Biosensors at the crossroads of food safety and antimicrobial resistance control in Africa
Discover Electrochemistry (2025)