Abstract
Metabolic syndrome (MetS) is a globally prevalent disorder and poses a significant threat to human health and social harmony. Consumption of anthocyanins has been proven to improve disrupted gut microbiota and obesity in mice and humans. However, the potential specific gut microbiota and metabolites that might mediate these beneficial effects on MetS remain unknown. Here, the MetS-mice model, induced by a high-fat diet (HFD), was employed to investigate the specific effects of ACNs. Additionally, 16S rRNA sequencing and targeted metabolomics analysis of short-chain fatty acids (SCFAs) were utilized to evaluate the influence on gut microbiota composition and SCFAs levels. More importantly, we also utilized antibiotics to construct a pseudo-germ-free mouse model for fecal microbiota transplantation (FMT) to further confirm the regulation of gut microbiota by ACNs and demonstrating that the related effects on MetS could be transferable through FMT. Our data demonstrated that the amelioration of MetS by ACNs might be achieved through modulation of the gut microbiota, which was validated through FMT, and the related benefits could be transferable by FMT. Furthermore, acetic acid and Prevotella histicola might be key microbial metabolites and bacteria, respectively, in this process. These findings highlight the diet-gut-metabolites-diseases system crosstalk and provide new research perspectives for plant-derived ingredients with poor bioavailability.

Similar content being viewed by others
Introduction
Metabolic syndrome (MetS) is a multiplex risk factor for cardiovascular disease (CVDs), type 2 diabetes mellitus (T2DM), and other health outcomes, and is a major challenge to public health1. In 2016, 39% of the adult world population was overweight, and it is estimated that by 2035, 51% of the population will be overweight or obese2. The occurrence of MetS arises from an intricate interplay among genetic, microbial, environmental factors, and diet, with dysbiosis of the gut microbiota playing a central role in its pathogenesis3.
Host and gut microbiota, coevolving through diet, metabolism, pathogen defense mechanisms, and other factors, can be collectively viewed as a holobiont. The gut microbiota performs indispensable functions in nurturing the intestinal epithelial barrier, modulating the immune system, thwarting the colonization of pathogenic bacteria, and fulfilling other vital roles, all of which are essential for maintaining human health, and known as the “virtual organ”4. Notably, the composition of the gut microbiota varies considerably among individuals. In mice with MetS, gut microbiota dysbiosis is characterized by changed microbial composition, including increased abundance of harmful microbiota, such as Firmicutes, Allobaculum, and decreased abundance of beneficial microbiota, including Bacteroidetes, Akkermansia, and Lactobacillus5. Moreover, mice with obesity exhibited impaired intestinal structure, marked by damage to the morphology of small intestinal villi, reduced villus length, and a decrease in the number of goblet cells and the thickness of the mucus layer6. In addition, individuals with MetS exhibit altered metabolic patterns7. The metabolites secreted or modified by gut microbiota are crucial for maintaining the functions of the host, such as bile acids, branched-chain amino acids, and short-chain fatty acids (SCFAs). SCFAs, primarily generated by the gut microbiota through the fermentation of indigestible dietary components, play regulatory roles in glucose and lipid metabolism, inflammation, and immune modulation8. It is reported that Bifidobacterium are major producers of acetate, while the Lachnospiraceae and Ruminococcaceae families are considered primary producers of butyrate9. Therefore, modulating SCFAs may be an important target for gut microbiota to exert therapeutic effects. Recent years, the potential of probiotics in the treatment of obesity has drawn the attention of researchers10. Studies have demonstrated that certain specific bacterial species interventions can target and treat diseases. For instance, the administration of Akkermansia muciniphila by gavage to genetically and diet-induced obese mice has been shown to reduce their body weight, improve glucose tolerance, and lower insulin concentrations, even improving overall metabolic status11. Lactobacillus plantarum could alleviate inflammation, obesity, dyslipidemia, and gut microbiota dysbiosis induced by glycerol monolaurate12. Prevotella, a major constituent of the Bacteroidetes phylum, demonstrates substantial health benefits. Studies have shown that glucose metabolism improves in healthy subjects after consuming barley kernel bread for 3 days, which is attributed to an increase in the Prevotella-to-Bacteroidetes ratio, with Prevotella copri (P. copri) being the most significantly altered species13. Further feeding mice with P. copri for 7 days also observed improvements in glucose metabolism13. Administering a polyphenol-rich Mediterranean diet to abdominal obesity patients for 8 weeks resulted in weight reduction, enhanced lipid metabolism, and significant shifts in the fecal microbiota composition. These changes were driven by less prevalent “non-core” microbial taxa, especially the increase in Prevotella14. In addition, compared to typical westernized diets, populations that follow traditional diets have a higher proportion of Prevotella15,16. The above might support the benefits of the Prevotella genus. Prevotella histicola (P. histicola), as an emerging probiotic, has also received widespread attention. It has been reported that P. histicola could effectively inhibit multiple sclerosis by enhancing anti-inflammatory immune responses and inhibiting pro-inflammatory immune responses17. Furthermore, Fan et al.18 found that P. histicola could significantly inhibit the expression of inflammatory cytokines (such as interleukin-1β, interleukin-6, tumor necrosis factor-α) and also reduce the levels of ZO-1 and Occludin, thereby improving dextran sulfate sodium (DSS)-induced intestinal barrier damage.
Blueberries and blackberries are two fruits that we commonly consume in our daily lives, rich in various nutrients and demonstrating anti-inflammatory, antioxidant, anti-obesity, and anti-diabetic effects7,19,20. Although they belong to different botanical families, both contain a large amount of anthocyanins, which are closely related to their benefits21. A study involving 12,400 individuals found that higher intake of anthocyanins showed a strong correlation with less weight gain22. Tsuda et al.23 made the first report on the anti-obesity effects of anthocyanins. They found that supplementing HFD-mice with purple corn anthocyanins could significantly reduce adipose tissue accumulation and body weight gain. Subsequently, an increasing number of animal experiments and clinical studies have demonstrated that anthocyanins can improve metabolic disorders24,25,26. Our previous research also showed that both blueberry anthocyanins and blackberry anthocyanins exhibit beneficial effects on MetS by restoring disrupted gut microbiota induced by HFD. However, the potential specific gut bacteria and microbial metabolites through which the anthocyanins from blueberry and blackberry exert their effects on MetS remain unclear. In this study, we utilized the mouse model of MetS induced by HFD, combined with FMT, to explore the interaction mechanism between anthocyanins, the host, the gut microbiota, and microbial metabolites. We found that anthocyanins from blueberries and blackberries (ACNs) exhibited therapeutic effects on metabolic disorders, including reducing body weight, blood glucose levels, blood lipid levels, inflammation, and improving gut structure damage, while also improving the disturbed gut microbiota. We further demonstrated that acetic acid might be a key active metabolite and verified its role by supplementing mice with sodium acetate. Additionally, through FMT, we discovered that the therapeutic effect of ACNs on MetS was mediated through the gut microbiota, and the characteristic change in gut microbiota is a highly enriched of Prevotella. Furthermore, both in vitro and in vivo experiments demonstrated that P. histicola might be the key bacterium responsible for the effect. In summary, these results collectively revealed the intricate interactions among the total anthocyanins from blueberries and blackberries, gut microbiota, microbial metabolites, and host health.
Results
ACNs demonstrated robust metabolic protective effects in HFD-fed mice
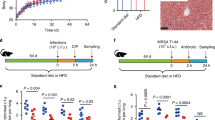
To investigate the anti-MetS effects of ACNs, we induced the MetS model by feeding mice a high-fat diet for 8 weeks. Subsequently, we administered ACNs at a dosage of 100 mg/kg to the mice via gavage once a day for the next 8 weeks (Fig. 1A). The results indicated that an 8-week HFD successfully induced a MetS model. Compared to the CON group, the HFD group exhibited significant increases in body weight and white adipose tissue mass, accompanied by a significant decrease in brown adipose tissue weight. As expected, intervention with ACNs significantly reversed these indicators, showing a trend towards recovery to the CON group levels. Similarly, the Met group also demonstrated comparable effects (Figs. 1B, C, E and S1A). What’s more, there was no significant difference in average food intake among groups (Fig. 1D). Additionally, the density maps of white fat weight-to-body weight and brown fat weight-to-body weight further highlighted the distinct ratio distributions among the different groups (Figs. 1H and S1B). Morphological observations of the liver revealed that the HFD group had enlarged liver and a yellowish color, whereas both the ACNs and Met groups exhibited normal, relatively reddish-hued appearances (Fig. 1F). Furthermore, we found that the amount of white fat in the ACNs group was significantly reduced (Fig. 1G).
Chow-diet fed mice were treated daily with sterile water (CON), or high-fat diet fed mice were orally administrated daily sterile water (HFD), the mixture of blueberry anthocyanins and blackberry anthocyanins (ACNs, 100 mg/kg), or Metformin (Met, 100 mg/kg). A Experiment design. B Body weight changes of the four groups during 16 weeks. C Weight gain. D Average daily food intake. E Organ weight/Body weight. Representative morphological observations of liver (F) and white fat (G). H White fat weight-to-body weight density map. n = 8 for each group, data are presented as mean ± sd, # significant difference between CON and HFD groups; * significant difference between HFD and ACNs or Met groups; # or *, P < 0.05, ## or **, P < 0.01; ### or ***, P < 0.001.
The histological examination of livers indicated that ACNs improved liver structure and lipid storage compared to the HFD group (Figs. S1C and 2A), which could be further demonstrated by the decreased serum levels of alanine transaminase (ALT) and aspartate transaminase (AST) (Figs. 2B and S1D). In addition, the histological analysis of white fat showed that, compared to the HFD group, the ACNs group exhibited a reduction in cell size but an increase in the total number of adipocytes (Fig. 2C, D). Besides, the levels of total cholesterol (TC), triglycerides (TG) and low-density lipoprotein cholesterol (LDL-C) in HFD groups significantly increased compared to the CON group, while the high-density lipoprotein cholesterol (HDL-C) levels significantly decreased. In contrast, the trends of the four lipid indicator levels in the ACNs group were completely opposite compared to those observed in the HFD group (Figs. 2E, F and S1E, F). For the glucose metabolism, the levels of fasting blood glucose (FBG), fasting insulin (FIN), homeostasis model assessment of insulin resistance (HOMA-IR), and glycosylated hemoglobin A1C (HbAc1) increased by HFD were decreased after administration of ACNs for 8 weeks (Figs. 2G, H and S1G, H). Meanwhile, glucose tolerance and insulin resistance were markedly ameliorated in the ACNs group, which even exhibited stronger effects than metformin (Fig. 2I, J). We further investigated the impacts of ACNs on oxidative stress, inflammation, and intestine structure. The results indicated that ACNs supplementation could significantly decrease the levels of tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), and malondialdehyde (MDA), and increase the level of superoxide dismutase (SOD) (Fig. S1I–L). ACNs possessing gut structure protection improved by a more compact and regular structure of the small intestine (Fig. S1M) and higher ratio of villus height-to-crypt length (Fig. S1N). Taken together, these data suggested that ACNs demonstrated robust metabolic protective effects in HFD-fed mice.
Chow-diet fed mice were treated daily with sterile water (CON), or high-fat diet fed mice were orally administrated daily sterile water (HFD), the mixture of blueberry anthocyanins and blackberry anthocyanins (ACNs, 100 mg/kg), or Metformin (Met, 100 mg/kg) A Representative oil red-stained liver of four groups. B Serum ALT levels. C Representative H&E-stained white adipose tissue. D Density map of white adipose cell size. E Serum level of TC. F Serum level of TG. G Fasting blood glucose. H Fasting insulin. I OGTT and AUC. J ITT and AUC. Scale bar, 100 μm, n = 8 for each group, data are presented as mean ± sd, # significant difference between CON and HFD groups; * significant difference between HFD and ACNs or Met groups; # or *, P < 0.05, ## or **, P < 0.01; ### or ***, P < 0.001.
ACNs alleviated HFD-induced gut microbiota dysbiosis
To investigate the mechanism of ACNs on treating MetS through modulating microbiome community structures, we examined the gut microbiota characters by performing a sequencing-based analysis of bacterial 16S rRNA in feces. The results showed that HFD could significantly decrease the α diversity, including the Chao1, Shannon, Simpson, and Pielou_e indexes. Conversely, ACNs supplementation could significantly increase the richness and evenness (Figs. 3A, B and S2A, B). The NMDS and PCoA based on Bray-Curtis distance metric showed distinct clustering of the gut microbiota composition of HFD group, and CON group, or ACNs group. However, no significant differences were observed between the HFD and Met groups, echoing similar trends observed in α-diversity indices. (Figs. 3C and S2C). The Venn diagram showed the unique ASVs in each group and common ASVs among groups. In total, 1265 ASVs were found to be shared among all four groups, and the unique ASVs counts within each group were 9984 (CON group), 4184 (HFD group), 8403 (ACNs group) and 5017 (Met group), respectively (Fig. 3D). Additionally, we analyzed the relative abundance of microbial species in each mouse across the four groups, focusing on the phylum and genus taxonomic levels. Our findings revealed that the three most abundant phyla were Bacteroidetes, Firmicutes, and Actinobacteria, while the three most prevalent genera identified were unidentified_S24-7, Allobaculum, and Lactobacillus (Fig. S2D and 3E). It is noteworthy that the unidentified_S24-7 was the genus with the highest abundance across all groups. Moreover, compared with the CON group (46.88%), the proportion of the unidentified_S24-7 in the HFD group was significantly decreased (33.76%). However, supplementation with ACNs and Met could restore the proportion of the unidentified_S24-7 (Fig. 3E). Moreover, compared to the HFD mice, both ACNs and metformin treatment displayed a decrease in relative abundance of Firmicutes and lower Firmicutes-to-Bacteroidetes ratio, although no significant difference was observed in the Met group. (Fig. 3F–H). Besides, both ACNs and Met supplementation resulted in a decrease in the abundance of the Actinobacteria phylum (Fig. 3I).
Gut microbiota compositions of CON group, HFD group, ACNs group, and Met group were analyzed by 16S rRNA sequencing. α diversity, evaluated by Chao1 (A), Shannon (B). C PCoA plot based on bray_curtis distance metric. D Venn diagram based on comparisons of the specific and shared amplicon sequence variants (ASVs). E Bacterial taxonomic profiling at the genus level of gut microbiota from different mouse. F Relative abundance of Firmicutes. G Relative abundance of Bacteroidetes. H The ratio of Firmicutes-to-Bacteroidetes. I Relative abundance of Actinobacteria. J Discriminative taxa determined by LEfSe between CON and HFD groups (LDA score log10 > 3). K Discriminative taxa determined by LEfSe between HFD and ACNs groups (LDA score log10 > 3). n = 8 for each group, data are presented as mean ± sd, # significant difference between CON and HFD groups; * significant difference between HFD and ACNs or Met groups; # or *, P < 0.05, ## or **, P < 0.01; ### or ***, P < 0.001.
Next, we performed linear discriminant analysis effect size (LEfSe) (LDA > 3) to identify differentially enriched bacterial taxa associated with ACNs treatment. The analysis revealed that 18 taxa were significantly enriched in the CON group compared to the HFD group, with a particular increase on the phylum Bacteroidetes. Meanwhile, in the ACNs group, 18 taxa were enriched, including the phylum Bacteroidetes and the genus Prevotella, and unclassified_Clostridiales, unidentified_Clostridiales, and unidentified_Lachnospiraceae (Fig. 3J, K). Furthermore, in order to assess whether certain bacterial phyla and genera altered by ACNs supplementation are involved in regulating metabolic parameters, we conducted a Spearman correlation coefficient analysis. The results indicated that the abundance of phylum Firmicutes and Actinobacteria, as well as the abundance of genus Allobaculum, exhibited positive correlations with parameters that promote obesity, inflammation, or oxidative stress, like FBG, FIN, TC, TG, LDL-C, ALT, AST, TNF-α, IL-1β, and MDA. However, the phylum Bacteroidetes, the genus Prevotella, and unidentified_Lachnospiraceae were negatively correlated with these parameters (Fig. S2E). These results provided evidence that ACNs intervention could alter the gut microbiota homeostasis of the host, and changes in specific bacterial taxa may mediate the anti-MetS effects of ACNs.
ACNs modulated MetS-associated acetic acid levels in HFD-fed mice
Metabolites secreted or modified by microbial play a crucial role in the interaction between the gut microbiota and the host27. To assess whether SCFAs contribute to the anti-MetS effects of ACNs, we measured the concentrations of the seven SCFAs in the feces and found that only acetic acid responded to the ACNs treatment. Specifically, ACNs supplementation significantly reversed the reduction in acetic acid levels induced by HFD. In contrast, there were no significant differences in the levels of propionic acid, butyric acid, caproic acid, isobutyric acid, and isovaleric acid among the four groups (Figs. 4A, B and S2F–K). To further illustrate the potential role of acetic acid in the interaction between the gut microbiota and the host, we conducted a Spearman’s correlation analysis between the dominant bacteria and the concentration of acetic acid. The results indicated that the phylum Bacteroidetes, as well as the genera Prevotella and Lactobacillus, were significantly positively correlated with acetic acid levels, while the phyla Firmicutes and Actinobacteria, as well as the genera Allobaculum and Bifidobacterium, were significantly negatively correlated (Fig. 4C). Next, we conducted a linear correlation analysis between the concentration of acetic acid and several MetS-related traits. The analysis results indicated a negative correlation between acetic acid and all indicators of metabolic abnormalities (Fig. 4D–K). Taken together, acetic acid exhibited a strong response to ACNs supplementation and demonstrated a significant correlation with MetS disorders. It suggested that acetic acid might be a key metabolite mediated by the gut microbiota in exerting anti-MetS effects.
SCFAs levels of CON group, HFD group, ACNs group, and Met group were analyzed. A Acetic acid. B The total SCFAs levels in serum among groups. C Spearman correlation heatmap between 7 SCFAs levels in serum and top 10 phylum or genus in feces (Red represents a positive correlation, and blue represents a negative correlation. The darker the color, the stronger the correlation; the lighter the color, the weaker the correlation. *, **, *** indicate p < 0.05, p < 0.01, and p < 0.001, respectively.). The correlation between acetic acid levels in serum and body weight gain (D), TC (E), ALT (F), AST (G), FIN (H), FBG (I), TNF-α (J), and IL-1β (K). n = 8 for each group, data are presented as mean ± sd, # significant difference between CON and HFD groups; * significant difference between HFD and ACNs or Met groups; # or *, P < 0.05, ## or **, P < 0.01; ### or ***, P < 0.001.
The metabolic protective benefits of ACNs were transferable by FMT
FMT is currently regarded as a promising therapeutic approach, and its therapeutic effects may be achieved by reconstructing the disrupted gut microbiota28. To further evaluate whether the protective effect of ACNs on MetS is indeed mediated by the gut microbiota and whether the associated benefits could be transferable through gut microbiota, we performed FMT from ACNs-treated mice to HFD-induced MetS model mice (Fig. 5A, B). After 8 weeks of horizontal FMT, the HFD-fed mice that received feces from ACNs-treated mice exhibited significant improvement in body weight, as well as in indicators related to obesity, lipid metabolism, glucose metabolism, and inflammation (Figs. 5C–K and S3E–H, K–N). Furthermore, the histopathological analysis clearly demonstrated that FMT from mice treated with ACNs could restore disrupted small intestinal structure, including the villous morphology, villus length, and increase the number of goblet cells in the small intestine (Fig. 5L, M). For the colon, the HFD-HFD group exhibited inflammatory cell infiltration (indicated by black arrows) and thinning of the mucosal layer. However, the ACNs-HFD group could reverse these phenomena (Fig. S3B). We also evaluated the impact of FMT on liver and oxidative stress. In the ACNs-HFD group, we observed a compact liver structure with reduced fatty vacuole amount, indicating an improvement in HFD-induced liver injury (Fig. S3A). However, there were no significant differences in serum ALT and AST levels among the groups (Fig. S3C, D). Additionally, serum SOD and MDA levels suggested that FMT from mice treated with ACNs could significantly ameliorate oxidative stress (Fig. S3I, J). These findings indicated that the anti-MetS effects of ACNs were indeed mediated through modulation of the gut microbiota. Furthermore, the associated benefits, which were similar to those observed in the donors, could be transferred by FMT.
Chow-diet fed mice received fecal microbiota from the CON group mice daily (CON-CON), high-fat diet fed mice were received fecal microbiota from the HFD group mice daily (HFD-HFD), and high-fat diet fed mice were received fecal microbiota from the ACNs group mice (ACNs-HFD). A Animal experiment design. B FMT scheme. C Body weight changes of the four groups during 16 weeks. D Weight gain. E Average daily food intake. Serum levels of TC (F), TG (G), FBG (H), FIN (I), TNF-α (J), and IL-1β (K). Representative H&E-stained intestine of four groups (L) and Representative AB-PAS-stained intestine of four groups (M). Scale bar, 200 μm, n = 8 for each group, data are presented as mean ± sd, # significant difference between CON-CON and HFD-HFD groups; * significant difference between HFD-HFD and ACNs-HFD groups; # or *, P < 0.05, ## or **, P < 0.01; ### or ***, P < 0.001.
FMT improved the imbalanced gut microbiota induced by HFD
To further investigate the impact of FMT on gut microbiota in mice, we collected feces from FMT-treated mice and performed 16S rRNA sequencing. Based on the analysis results, we found that compared with the CON-CON group, the α-diversity of the gut microbiota in the HFD-HFD group mice was significantly decreased, while no significant difference was observed between the HFD-HFD and ACNs-HFD groups (Fig. S4A–D). The composition of the gut microbiota revealed that the most abundant phyla were Firmicutes, Bacteroidetes, and Proteobacteria, and the most abundant genus was Lactobacillus and unidentified_S24-7 (Figs. 6A and S4E). Notably, FMT from ACNs-treated mice could enhance the amount of ASVs compared to the HFD-HFD group (Fig. 6B). Additionally, it slightly reduced the Firmicutes-to-Bacteroidetes ratio, although this difference was not statistically significant (Fig. 6C). The Spearman correlation heatmap showed that Firmicutes and Lactobacillus were positively correlated with MetS-related indicators, while the phylum Bacteroidetes was negatively correlated with these indicators (Fig. 6D). Taken together, these results indicated that FMT from donor mice treated with ACNs could improve the gut microbiota of recipient mice; however, it couldn’t fully replicate the gut microbiota state of the donor.
Gut microbiota compositions of CON-CON group, HFD-HFD group, and ACNs-HFD group were analyzed by 16S rRNA sequencing. A Bacterial taxonomic profiling at the phylum level of gut microbiota from different mouse. B Venn diagram based on comparisons of the specific and shared amplicon sequence variants (ASVs). C The ratio of Firmicutes-to-Bacteroidetes. D Spearman correlation heatmap between the bacteria and MetS-associated parameters (Red represents a positive correlation, and blue represents a negative correlation. The darker the color, the stronger the correlation; the lighter the color, the weaker the correlation. *, **, *** indicate p < 0.05, p < 0.01, and p < 0.001, respectively.). n = 8 for each group, data are presented as mean ± sd, # significant difference between CON-CON and HFD-HFD groups; * significant difference between HFD-HFD and ACNs-HFD groups; # or *, P < 0.05, ## or **, P < 0.01; ### or ***, P < 0.001.
P. histicola might be a potential probiotic bacterium exerting anti-MetS effects
To uncover the key microbiota involved in the treatment of MetS with ACNs, we employed bioinformatics techniques utilizing the “Metagenomeseq” package to conduct differential abundance analysis of ASVs, comparing the ACNs, CON, and Met groups against the HFD group. Based on the expression levels of these ASVs in the ACNs group, we ranked them, and a heatmap illustrating the expression abundances of the top 50 ASVs is presented in Fig. S5A. Among these 50 ASVs, ASV_59643, belonging to the Allobaculum genus and ASV_78758, belonging to the Bifidobacterium genus, both exhibited a trend of lower expression in both the CON and ACNs groups. In contrast, ASV_51335, belonging to the genus Prevotella was significantly enriched in both the CON and ACNs groups (Fig. S5A). To further pinpoint the key bacteria responsible for the observed beneficial effects, we conducted a random forest analysis on the top 10 genera. The results indicated that Allobaculum and Prevotella remained the most significant bacterial taxa (Fig. 7A). RDA revealed that Prevotella, Allobaculum, and unclassified_Clostridiales were the most dominant factors driving the overall changes in the gut microbiota structure. Among them, Prevotella had the smallest angle with acetic acid and was in the same direction as acetic acid, SOD, and HDL-C. In contrast, it was in the opposite direction to other indicators. Conversely, Allobaculum demonstrated the completely opposite trends (Fig. 7B). In summary, these predictions and analyses suggested that Prevotella might be a key bacterium involved in ACNs’ anti-MetS effects in mice.
A Random Forest analysis of the four groups to identify key bacteria at genus level. B Redundancy analysis (RDA) of the correlation of the dominant genus and Mets-associated parameters. C The schematic diagram of in vivo and in vitro experiments related to P. histicola. D Body weight changes of the five groups during 16 weeks and average food intake per day. E OD600 of P. histicola cultures with/without different ACNs concentrations over 48 h (n = 3). Serum levels of FBG (F), TC (G), TG (H), LDL-C (I), HDL-C (J), ALT (K), and AST (L). n = 8 for each animal group, data are presented as mean ± sd, # significant difference between CON and HFD groups; * significant difference between HFD and P.h, P.h ko or P.h A groups; # or *, P < 0.05, ## or **, P < 0.01; ### or ***, P < 0.001.
However, the results above didn’t prove a direct relationship between Prevotella and metabolic disorders. Therefore, we selected Prevotella histicola for in vitro bacterial culture and in vivo validation in MetS-mice (Fig. 7C). P.histicola was co-incubated with different concentrations of ACNs and found that 25 μg/mL of ACNs could promote the growth of P.histicola (Fig. 7E). Subsequently, we orally administered P.histicola after been co-incubated with ACNs for 24 h (P.hA), live P. histicola (P.h), and pasteurized P.histicola (P.h ko) to MetS-mice. The animal results revealed that the administration of P.histicola could significantly inhibit body weight gain and improve abnormalities in glucose and lipid metabolism (Fig. 7B, K–J). Treatment with P.histicola also significantly improved the serum level of ALT, while no considerable improvement in AST was observed (Fig. 7K, L). Moreover, pasteurized P.histicola exhibited similar effects (Fig. 7B, F–L). Together, these findings indicated that ACNs could enrich the growth of P. histicola, which attenuated HFD-induced obesity and metabolic disorders may be associated with the ameliorative effects of ACNs on MetS.
Acetic acid is a key metabolite exerting anti-MetS effects
We observed that acetic acid strongly responded to ACNs treatment in MetS-mice and exhibited a significantly negative correlation with indicators associated with MetS. Moreover, the abundance of Prevotella was significantly positively correlated with acetic acid levels, hinting at the potential function of acetic acid. To further determine the role of acetic acid in MetS-mice, we orally administered sodium acetate daily to MetS mice fed a high-fat diet, continuing for 8 weeks. The animal results indicated that sodium acetate supplementation significantly increased the acetic acid content in mouse feces and improved HFD-induced lipid metabolism abnormalities and liver injury (Fig. 8A, C–H). However, there was no significant effect on FBG (Fig. 8B). These results suggest that sodium acetate might be a potential gut microbiota metabolite of ACNs in regulating MetS.
Discussion
MetS continues to rise globally, posing a significant challenge to clinical practice and public health1. While the underlying mechanisms remain largely unclear, gut microbiota dysbiosis has been identified as a core inducer of obesity, inflammation, MetS, and other metabolic diseases5,29,30. Increasing evidence indicates that plant-derived compounds such as anthocyanins, polymethoxyflavones, and resveratrol, owing to their remarkable safety and efficacy, hold great potential in the management of oxidant stress, obesity, and disorders in glycemic and lipidemic metabolism, thus bringing hope for medical advancement and human well-being5,20,31. Notably, the beneficial effects of these phytochemicals are closely related to their modulation of gut microbiota5,20,31. Our previous research has shown that interventions with blueberry anthocyanins and blackberry anthocyanins have positive effects on MetS32, findings that are highly consistent with those of other previous studies7,20. However, research on the specific gut bacteria and microbial metabolites that may be involved in the process by ACNs to improve MetS remains insufficient. Therefore, the current study aimed to explore the mechanism of ACNs on MetS and to identify the key bacteria and metabolites that might be responsible for ACNs’ anti-MetS effects. The results showed that ACNs supplementation significantly improved HFD-induced MetS, and this improvement was closely related to its modulation of gut microbiota. Additionally, we found that Prevotella was significantly enriched in the ACNs group, and further in vivo and in vitro experiments confirmed that P.histicola might be the key bacterium involved. Meanwhile, we also discovered significant positive correlations between acetic acid and both MetS and the abundance of Prevotella, Furthermore, combined with in vivo experiments in which mice were fed sodium acetate, these findings collectively indicated that acetic acid might play a role in the process of ACNs ameliorating MetS.
Feeding high-fat diet is one of the methods widely used to induce MetS model in mice33. In this study, supplemented MetS-mice with ACNs significantly ameliorated obesity, liver injury, and lipid accumulation. In addition, we also found that ACNs treatment significantly reduced the levels of FBG and FIN induced by HFD, improving insulin sensitivity and glucose tolerance, thereby effectively lowering the risk of diabetes and MetS in host. Inflammation and oxidative stress are two interrelated pathological features that act as both cause and effect, mutually promoting each other and exacerbating the risk of MetS in host34. Numerous studies have provided compelling evidence for the antioxidant and health-promoting effects of anthocyanins. In clinical studies, continuous supplementation of anthocyanins to patients with dyslipidemia for 6 weeks significantly increased the serum levels of SOD and decreased the levels of MDA in a dose-dependent manner, thereby exerting a lipid-lowering effect35. Animal experiments have also demonstrated similar results. For example, after diabetic mice were gavaged with blueberry anthocyanins for 5 weeks, their body weight and insulin resistance were improved. Meanwhile, the activities of SOD and glutathione peroxidase in the liver were significantly increased36. In our study, the HFD resulted in a significant decrease in serum SOD levels and a significant increase in MDA levels in mice. However, after 8 weeks of ACNs supplementation, these indicators were significantly improved. Furthermore, ACNs also reduced the levels of inflammatory factors induced by HFD, including TNF-α and IL-1β. More importantly, studies have demonstrated that long-term high-fat diets could disrupt the intestinal barrier structure, allowing pathogenic factors such as pro-inflammatory cytokines to enter the systemic circulation, thereby impairing host health37. Previous research has already shown that anthocyanins extracted from purple red rice have a repairing effect on intestinal structure damage, which is manifested by the restoration of intestinal structure and an increase in the ratio of villus length to crypt depth38. Taken together, the results above illustrated the potential role of ACNs in improving metabolic disorders.
The bioavailability of anthocyanins is relatively low. A substantial portion of anthocyanins reach the colon, where they are broken down or metabolized by gut microbiota into substances such as aldehydes or phenolic acids, thereby amplifying the benefits of anthocyanins39. In addition, anthocyanins can specifically alter the structure and composition of the gut microbiota. For example, raspberry anthocyanins supplementation enhanced the growth of Eubacterium rectale, Faecalibacterium prausnitzii, and Lactobacillus, and inhibited the growth of Desulfovibrio spp. and Enterococcus spp40. In our study, we found that HFD significantly reduced the α-diversity of the gut microbiota and demonstrated a clear separation between the CON group, which is consistent with the findings of previous studies41,42. ACNs treatment significantly restored the reduction in α-diversity induced by HFD, and in terms of β-diversity, the composition of the gut microbiota in the ACNs group was more similar to that of the CON group. Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria are the dominant phyla in the gut microbiota, accounting for more than 90% of the total bacterial content43. In a normal, healthy state, the gut microbiota of the host is characterized by higher abundance of Bacteroidetes and lower abundance of Firmicutes. Conversely, mice44 or human43 suffering from MetS or obesity display an elevated ratio of Firmicutes-to-Bacteroidetes ratio. In our study, both the ACNs and Met groups exhibited higher Bacteroidetes abundance and a lower Firmicutes-to-Bacteroidetes ratio, which is completely contrary to the findings of the HFD group. Moreover, both metformin and ACNs interventions significantly reduced the proportion of Actinobacteria, which is consistent with our previous research45. Notably, the genus unidentified_S24-7 had the highest abundance among all groups, but its abundance was the lowest in HFD group. Supplementation with ACNs could significantly restore the abundance of unidentified_S24-7. S24-7 is also known as Muribaculaceae. Recent studies have indicated that mice with a higher proportion of Muribaculaceae at baseline may be protected from diet-induced obesity46. Morissette et al. also found that FMT from mice treated with proanthocyanidins significantly increased the abundance of Muribaculaceae intestinale7. The results above seem to suggest that the MetS-improving effect of ACNs is mediated through modulation of the gut microbiota.
Currently, studies on the treatment of metabolic diseases by berry anthocyanins through improving the gut microbiota are not limited to blueberries and blackberries. Cyanidin-3-rutinoside derived from Mori Fructus reversed the reduction in the abundance of Muribaculaceae_ge, Lachnospiraceae_unclassified, Lachnospiraceae_NK4A136_group, and Lachnospiraceae_uncultured induced by HFD, thereby ameliorating obesity and dyslipidemia47. In addition, anthocyanins from Lycium ruthenicum Murray primarily ameliorated HFD-induced obesity by regulating Streptococcaceae and Helicobacteraceae48. In our study, to explore the relationship between metabolic disorders and ACNs changed bacteria, Spearman’s correlation analysis was conducted. Consistent with previous studies49,50, Allobaculum and Firmicutes were significantly positively correlated with obesity, glucose metabolism, lipid metabolism, liver injury, and inflammation-related indicators, whereas Bacteroidetes and Prevotella were negatively correlated with these indicators. The above results suggested that ACNs might exert potential protective effects on MetS by restoring gut microbiota diversity, increasing the proportion of beneficial bacteria, and decreasing the proportion of harmful bacteria.
SCFAs are the end products formed by the fermentation of non-digestible carbohydrates by the gut microbiota, representing the interaction between diet and gut microbiota51, play crucial roles in maintaining the integrity of the intestinal barrier and regulating neurological and immune processes9. Research has found that specific gut microbiota bacteria could regulate specific SCFAs; for example, the Lachnospiraceae and Ruminococcaceae families are major producers of butyrate52, while Akkermansia municiphilla has been identified as a propionate producer53. Recent research has indicated that anthocyanins extracted from purple red rice could significantly ameliorate the decrease in acetic acid levels induced by HFD, while having no significant impact on butyric acid and isovaleric acid levels38. Similarly, we also investigated the impact of ACNs on SCFAs and found that acetic acid exhibited a strong response to ACNs treatment, while the intervention of ACNs had no significant effect on other SCFAs. This suggested that acetic acid might be the key metabolite in the interaction between gut microbiota and ACNs. Spearman’s correlation analysis further confirmed the interaction among acetic acid, gut microbiota, and ACNs. We found that ACNs promoted the abundance of beneficial bacteria, including Prevotella, Lactobacillus, Bacteroidetes, and unidentified_S24-7, which showed a significant positive correlation with acetic acid levels. This suggested that ACNs might increase the production of acetic acid by promoting the growth and metabolism of these beneficial bacteria. According to previous reports, acetic acid treatment could upregulate the expression of genes involved in lipid metabolism in mouse livers, such as acetyl-CoA oxidase and uncoupling protein 2, thereby inhibiting HFD-induced weight gain and hepatic lipid accumulation54. Similarly, Ying et al.55 found that acetic acid dietary supplementation significantly inhibited weight gain in HFD mice and suppressed the expression of the liver fatty acid synthase protein, reducing the risk of non-alcoholic fatty liver disease in mice. In our study, to further determine the relationship between acetic acid and MetS, we conducted a linear correlation analysis between key metabolic indicators and acetic acid. We found that acetic acid levels were significantly negatively correlated with indicators related to lipids (TG), liver injury (ALT, AST), glucose metabolism (FIN, FBG), and inflammation (TNF-α, IL-1β).
However, Spearman’s correlation and linear analyses only inferred the correlations between the gut microbiota or physiological indicators and acetic acid content. They cannot directly demonstrate that the increase in acetate content in the ACNs group is a direct result of the increased abundance of Prevotella, Lactobacillus, Bacteroidetes, and unidentified_S24-7. Therefore, in subsequent experiments, we further validated the effects of acetic acid as a supplement on HFD-induced MetS-mice. The results showed that acetic acid supplementation significantly improved the lipid metabolism abnormalities and liver injury induced by HFD, which might provide evidence for the beneficial effects of acetic acid.
In human studies, FMT could reduce fat accumulation in the liver by improving gut microbiota dysregulation, thereby alleviating fatty liver disease56. Based on the great potential of FMT in treating metabolic diseases, we also explored the role of gut microbiota in MetS-mice using FMT. Fresh feces collected daily from donor mice (CON, HFD, ACNs groups) were processed and fed to MetS-mice. What’s more, prior to FMT, the mice were subjected to a one-week antibiotic treatment to establish a pseudo-germ-free mouse model57. Our research results found that after FMT, the traits of the donor mice were successfully transferred to the recipient mice. Depending on the characteristics of the donor mice, the recipient mice exhibited corresponding obese or lean states. Furthermore, glucose metabolism, lipid metabolism, inflammation, and even the status of the gut barrier were consistent with those of the donor mice. This demonstrated that the anti-MetS effects of ACNs are indeed achieved through the regulation of gut microbiota, and these beneficial effects could be transferable through FMT. This result is consistent with previous research5,58. To further explore the mechanism of action of FMT, we analyzed the gut microbiota of the recipient mice. The results showed that compared to the CON-CON group, the α-diversity of the gut microbiota in the HFD-HFD group was significantly reduced, whereas there was no significant increase in α-diversity in the ACNs-HFD group. Additionally, the Firmicutes-to-Bacteroidetes ratio in the ACNs-HFD group did not differ significantly from that in the HFD-HFD group. However, the Venn diagram revealed that the number of ASVs in the ACNs-HFD group was increased compared to the HFD-HFD group. It is worth noting that the results of this study indicated that the most abundant bacterial species in genus level is Lactobacillus, rather than the unidentified_S24-7, which is the same as that in the donor mice. However, our previous research also revealed that after FMT, the predominant bacterial genus in the gut microbiota was Lactobacillus58. Moreover, Morissette et al.7 found that in mice fed a high-fat, high-sucrose (HFHS) diet, FMT also partly replicated the metabolic characteristics of the donor mice. Specifically, germ-free mice that received FMT from blueberry anthocyanins-treated mice were leaner and exhibited improved glucose tolerance compared to those that received FMT from HFHS-fed mice. Further analysis of the gut microbiota in these mice revealed that FMT effectively colonized the gut microbiota in the recipient germ-free mice, driving them to display characteristics similar to those of the donor mice. These findings suggest that Lactobacillus may be the major colonizing bacterial genus in the gut following FMT. The results above suggested that while FMT could replicate the characteristics of the donor, it couldn’t fully replicate the donor’s gut microbiota pattern. The efficacy on MetS of FMT might be achieved through specific gut microbiota, and further investigation is needed to identify the key bacteria that play a role in the process of FMT.
To identify the key bacteria responsible for the anti-MetS effects of ACNs, we conducted a detailed analysis of the gut microbiota in mice and found that the Prevotella genus was highly enriched in the ACNs group and showed a correlation with metabolic indicators. In addition, random forest analysis and RDA indicated that Prevotella was the most dominant species driving the overall changes in the gut microbiota structure. Research indicated that Prevotella is more common in non-Western populations consuming plant-rich diets, and the association with such diets suggests that Prevotella is a beneficial bacteria59. Marietta et al.60 used two species, Prevotella histicola and Prevotella melanogenica, to explore their potential in arthritis-susceptible HLA-DQ8 mice. They found that Prevotella histicola possessed anti-inflammatory properties, protected the intestinal barrier, and modulated systemic immunity, ultimately exerting a therapeutic impact on arthritis. Furthermore, supplementing non-obese diabetic mice with Prevotella histicola couldsignificantly increase the α-diversity of the gut microbiota in mice, reduce the abundance of Lachnospiraceae Anaerostipes, and increase the abundance of Rikenellaceae and S24-761, leading to a significant increase in regulatory T cells in pancreatic lymph nodes, thereby delaying the onset of diabetes61. It is noteworthy that our previous studies have demonstrated that interventions with either blueberry anthocyanins or blackberry anthocyanins also promoted an increase in the abundance of Prevotella45. However, after analyzing the gut microbiota of the recipients, Lactobacillus might be the key microbial group. This discrepancy might be attributed to the differences in the composition of anthocyanins used in the two research58.
Next, we selected Prevotella histicola to explore its potential impact on MetS. A 16-week in vivo experiment found that Prevotella histicola significantly inhibited HFD-induced weight gain, improved abnormalities in glucose and lipid metabolism, and alleviated liver damage. Notably, pasteurized P.histicola exhibited similar beneficial effects. According to the definition by the International Scientific Association for Probiotics and Prebiotics (ISAPP), preparation of inanimate microorganisms that confers a health benefit on the host is referred to as ‘postbiotics'62. Research conducted by Liu et al.63 indicated that both live and pasteurized Akkermansia muciniphila could reduce the susceptibility of mice to Salmonella typhimurium infection. Furthermore, both oral fecal gavage and pasteurized fecal gavage can enrich Lachnospiraceae and butyrate production, and activate the AMPK-Nrf2 pathway and mitochondrial autophagy to improve acute liver injury64. These findings suggested that Prevotella histicola might have a potential association with the regulation of MetS by ACNs, but the specific effects and underlying mechanisms still require further investigation.
However, the limitations of this study need to be discussed. Regarding the identification of key microbiota, although we have identified the Prevotella genus and reviewed extensive literature on its association with diseases, selecting Prevotella histicola for identification based solely on previous studies is somewhat one-sided, despite the relatively limited research on species within this genus. Therefore, the efficacy of the Prevotella genus should be further investigated.
In conclusion, our study elucidates that ACNs could ameliorate obesity, abnormalities in glucose and lipid metabolism, inflammation, and gut structure damage in HFD-induced MetS-mice. Through gut microbiota analysis and FMT, we found that the anti-MetS effects of ACNs were achieved by modulating the gut microbiota, and the corresponding therapeutic effects could partly be replicated through FMT. Additionally, we identified acetic acid as the key microbial metabolite. Furthermore, P. histicola might be a pivotal bacterium for the anti-MetS effects of ACNs (Fig. 9). Our research here opens a new window for understanding the therapeutic effects of functional substances derived from plants from a microbiome-targeted perspective.
Methods
Preparation of ACNs
The extraction and purification methods for blueberry anthocyanins and blackberry anthocyanins are as described in our previous work45,65. In brief, fresh blueberries and blackberries were juiced separately using 50% and 75% (v/v) ethanol containing 0.1% formic acid. The mixtures were then subjected to ultrasonic extraction at 30 °C for 40 min, with a liquid-to-solid ratio of 1:4 (w/v), and this process was repeated three times. Subsequently, the filtrates were collected, centrifuged, and concentrated before being purified using macroporous resin. Finally, the eluates were collected, concentrated, and freeze-dried to obtain freeze-dried powders of total anthocyanins from blueberry and blackberry. A mixture of these powders, prepared at a 1:1 ratio and at a concentration of 100 mg/Kg, was designated as ACNs.
MetS-mice and drug administration
All animal experiments had been approved by the Committee for Animal Research of China Pharmaceutical University, and all procedures were conformed to the Guide for the Care and Use of Laboratory Animals. Male C57BL/6 J mice (6 weeks old) were purchased from Huachuang Xinnuo Pharmaceutical Technology Co., Ltd (Jiangsu, China). All mice were kept under specific pathogen-free (SPF) conditions with a temperature of 22 ± 2°C, with the relative humidity ranging from 45 to 75%, and with a 12 h light−dark cycle. The mice were free to food and water. The first week was for acclimation, after that, mice were randomly divided to chow diet (CON) group (8 mice) and HFD group (24 mice). After 8 weeks feeding, the mice in HFD groups were further arranged to three groups, namely HFD group (gavaged with water), ACNs group (gavaged with 100 mg/kg mixture of blueberry anthocyanins and blackberry anthocyanins), and Met group (gavaged with 100 mg/kg metformin). After the second round of grouping, the mice were continuedly fed for 8 weeks. During the trail, the body weight of each mouse and food intake were recorded every week. All animal food used was purchased from Junke Bioengineering Co., Ltd (Nanjing, China).
Antibiotic treatment and FMT
The mice in the CON, HFD, and ACNs served as donor mice to provide transplant materials for FMT. In this study, the FMT materials were prepared according to previous research with slight modifications66. Specifically, fresh feces from the donor mice were collected daily during the 8th week. The feces were then dissolved (100 mg feces per 1 ml of PBS), suspended, filtered, and centrifuged. The resulting supernatant was stored at 4 °C as the transplant material. Twenty four male C57BL/6 J mice were purchased from the same company as before as recipient mice. The entire experiment procedures were kept the same as that of donor mice. After 1 week acclimation, the mice were arranged to 2 groups, namely chow group (8 mice, fed with chow diet) and HFD group (16 mice, fed with HFD diet), after 7 weeks feeding, all mice with treated with antibiotics by drinking for 7 days, four antibiotics were added into the water according to the following concentration: ampicillin 1 g/L, neomycin sulfate 1 g/L, metronidazole 1 g/L, and vancomycin 500 mg/L. Then, all recipient mice received FMT daily from donor mice, with each mouse receiving 200 μL of bacterial suspension. The mice in the chow group received bacterial materials from CON group mice and were named the “CON-CON” group. Likewise, mice in the HFD groups were treated similarly and named the “HFD-HFD” group and the “ACNs-HFD” group, respectively. FMT were conducted for 8 weeks as described before5. Briefly, fresh feces were collected from donor mice daily and mixed with sterile phosphate-buffered saline (PBS) at a ratio of 100 mg feces per 1 mL of PBS. The mixture was then vortexed for 2 min and centrifuged at 500 g/min for 3 min at 4 °C.
P. histicola cultivation and treatment
Prevotella histicola was purchased from BeNa Culture Collection Ltd and culture under anaerobic conditions in BHI broth supplemented with 0.4% mucin (Sigma-Aldrich Co.). For the vivo experiment, 25 μg/mL, 50 μg/mL, and 100 μg/mL concentrations of ACNs were added to the broth in order to assess the growth status of P. histicola based on the OD600 value. For the vitro experiment, the 200 μL alive P. histicola, pasteurized P. histicola, or P. histicola treated with ACNs were gavaged to the HFD mice daily for 8 weeks, the CON and HFD groups were given an equivalent volume of sterile saline.
Sodium acetate treatment in MetS-mice
Mice were feed with HFD for 8 weeks to establish the MetS model, then the HFD mice were assigned to HFD group (gavaged with sterile saline) and AA group (gavaged with 0.1 mol/L sodium acetate) for another 8 weeks.
Oral glucose tolerance test
At the 16th week, all mice were fasted for 12 h with free access to water. After that, they were gavaged with a dose of 2 g/kg glucose based on their body weight, and then the blood samples were obtained from the inner orbit using a capillary tube, and this time point was recorded as 0 min. Blood samples were then collected at 30, 60, 90, and 120 min thereafter, making a total of 5 time points. After obtaining the blood samples, they were promptly centrifuged, and the serum was collected. Commercial kits from Jiancheng Bioengineering Institute (Nanjing, China) were used to evaluate the glucose levels of all samples.
Insulin tolerance test
Three days after OGTT, the mice were fasted for 12 h with free access to water. All mice received an intraperitoneal injection of insulin at a concentration of 0.75 U/kg based on their body weight. Subsequently, blood was collected from the inner canthus of each mouse using a capillary tube, and this time point was recorded as 0 min. Blood samples were then collected at 30, 60, 90, and 120 min thereafter, making a total of 5 time points. After obtaining the blood samples, they were promptly centrifuged, and the serum was collected. Commercial kits from Jiancheng Bioengineering Institute (Nanjing, China) were used to evaluate the glucose levels of all samples. Moreover, the area under the curve (AUC) of OGTT/ITT with every blood glucose level multiplied by time.
HOMA-IR
The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as: \({\rm{HOMA}}-{\rm{IR}}={\rm{FBG}}* {\rm{FIN}}/22.5\)
Analysis of biochemical parameters
Alanine transaminase (ALT), aspartate transaminase (AST), fasting blood glucose (FBG), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were assessed using kits from Jiancheng Bioengineering Institute (Nanjing, China) based on the protocols. Malondialdehyde (MDA) and superoxide dismutase (SOD) were assessed using kits from Beyotime Bioengineering Institute (Nanjing, China) based on the protocols.
Elisa assays
The levels of tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), fasting insulin (FIN), and glycosylated hemoglobin A1C (HbAc1) were measured by ELISA kits.
Hematoxylin-eosin staining
Freshly collected adipose tissue, small intestine, and colon tissues were promptly placed in 4% paraformaldehyde solution for fixation for 24 h. Subsequently, the tissues were taken out and sequentially dehydrated in ethanol solutions of different concentrations. After dehydration, the tissue samples underwent processes of clearing, wax immersion, and embedding, and were then sectioned into 5 μm thick slices. After that, the slices were subjected to dewaxing and hydration, followed by staining, which involved hematoxylin staining and eosin staining in sequence. Finally, the slices were dehydrated and mounted for observation.
Alcian blue and periodic acid-schiff (AB-PAS) staining
Place the embedded small intestine sections through dewaxing and hydration procedures. Next, add alcian blue staining solution and allow it to act on the sections for 10–20 min. Then, wash the sections three times with distilled water. After that, place the sections in an oxidizing agent for 5 min, rinse them with tap water, and then immerse them in distilled water twice. Subsequently, immerse the sections in schiff staining solution for 10–20 min. Pour out the schiff staining solution and rinse the sections with running water for 10 min. Stain the cell nuclei with hematoxylin staining solution for 1–2 min and then wash the sections with water. Differentiate the sections using acidic differentiation solution for 2 s and wash with water again. Perform bluing by treating the sections with Scott’s bluing solution for 3 min and then washing them with water for 3 min. Finally, mount the sections for observation.
Oil red O staining
Oil Red O (ORO) staining was performed following the description of the previous study67. Liver tissues were immersed in a 4% formalin solution and fixed for a duration of 24 h; after that, tissues were processed by embedding in paraffin for sectioning. Five μm slices were fixed in 4% paraformaldehyde for 15 min. Following fixation, the slices were rinsed thoroughly using pure water and 60% isopropyl alcohol. Afterward, the frozen sections were exposed to a freshly prepared ORO working solution for 10 min at a temperature of 37 °C, allowing for effective staining. The samples were submerged in a solution of 60% isopropanol and subsequently subjected to staining with hematoxylin to facilitate the visualization of nuclei. After washing with PBS, the sections were visualized and captured using a light microscope (Olympus, Tokyo, Japan).
16S rRNA amplicon sequencing and data analysis
Mice feces were collected and immediately stored at −80 °C for further analysis. The microbial genomic DNA of each mice fecal sample was extracted using the OMEGA Soil DNA Kit (M5635-02) (Omega Bio-Tek, Norcross, GA, USA) based on the manufacturer’s instructions. Then the DNA quantity and quality were checked by NanoDrop NC2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and agarose gel electrophoresis, respectively. Then the paired-end 2 × 250 bp sequencing was performed at Suzhou PANOMIX Biomedical Tech Co., LTD (Suzhou, China) using the Illlumina TruSeq Nano DNA LT Library Prep.
Then the raw data was analyzed using QIIME2 2019.4 with slight modifications following the official tutorials. In brief, the raw reads were filtered, denoised, merged, and chimeric sequences were removed using the DATA2 plugin, followed by annotation of each amplicon sequence variant (ASV). The raw data were for the assessment of α and β diversity with QIIME2. The α diversity was indicated by Chao1, Shannon, Simpson, and Pielou_e indexes, and the β diversity was indicated by non-metric multidimensional scaling (NMDS) and principal coordinate analysis (PCoA) basing the Bray-Curtis distance. Additionally, the linear discriminant analysis effect size (LEfSe) was used to identify the microbial biomarkers among groups using the OmicStudio tools at https://www.omicstudio.cn/tool. Furthermore, the random forest analysis and Spearman correlation heatmaps were also evaluated and visualized using OmicStudio tools. Additionally, the linear correlation analysis between acetic acid concentration and metabolic indexes was calculated and visualized using the “ggplot”, “ggscatter”, and “ggprism” packages in R software (version 4.3.0) (https://www.r-project.org/).
Targeted SCFAs quantification
Mice feces were collected and immediately stored at −80 °C for further SCFAs analysis. Samples were extracted in 50 μL of 15% phosphoric acid with 100 μL of 125 μg/mL 4-methylvaleric acid solution as IS and 400 μL ether. Subsequently, the samples were centrifuged at 4 °C for 10 min at 12000 rpm after vortexing for 1 min, and the supernatant was transferred into the vial prior to GC-MS analysis. Then, the GC analysis was performed on a trace 1310 gas chromatograph (Thermo Fisher Scientific, USA), and it was fitted with an Agilent HPINNOWAX capillary column (30 m × 0.25 mm ID × 0.25 μm); helium gas was employed as the carrier gas at a flow rate of 1 mL/min. The analysis was conducted using an lSQ LT mass spectrometer (Thermo Fisher Scientific) with electron impact ionization mode. The column temperature was ramped up from 90 to 250 °C at specific rates, and metabolite detection was performed in single ion monitoring mode with an electron energy of 70 eV.
Statistical analysis
The statistical analysis was conducted using Graphpad Prism 9.0 software (Graphpad Software Inc., CA) and presented as the mean ± sd. Statistical significance among more than two groups was assessed using an analysis of variance (ANOVA) with Dunnett’s test, except that the analysis of concentrations of SCFAs in Fig. 4 was evaluated using ANOVA followed by Fisher’s least significant difference (LSD) test. Statistical significance of two groups was evaluated with the unpaired two-tailed Student’s t-test. In all data analysis, P < 0.05 was considered to be statistically significant (Detailed data statistical analysis can be found in the Supplementary Information).
Data availability
The data presented in the study are submitted in the NCBI Sequence Read Archive (SRA) repository, accession number PRJNA1207214.
References
Neeland, I. J. et al. Metabolic syndrome. Nat. Rev. Dis. Prim. 10, 77 (2024).
Busch, C. B. E., Bergman, J., Nieuwdorp, M. & van Baar, A. C. G. Role of the intestine and its gut microbiota in metabolic syndrome and obesity. Am. J. Gastroenterol. 119, 1038–1046 (2024).
Park, S., Sadanala, K. C. & Kim, E. K. A metabolomic approach to understanding the metabolic link between obesity and diabetes. Mol. Cells 38, 587–596 (2015).
Schuijt, T. J. et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 65, 575–583 (2016).
Zeng, S. L. et al. Citrus polymethoxyflavones attenuate metabolic syndrome by regulating gut microbiome and amino acid metabolism. Sci. Adv. 6, eaax6208 (2020).
Zhang, X. Y. et al. Phlorizin ameliorates obesity-associated endotoxemia and insulin resistance in high-fat diet-fed mice by targeting the gut microbiota and intestinal barrier integrity. Gut Microbes 12, 1–18 (2020).
Morissette, A. et al. Blueberry proanthocyanidins and anthocyanins improve metabolic health through a gut microbiota-dependent mechanism in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 318, E965–e980 (2020).
den Besten, G. et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340 (2013).
Fusco, W. et al. Short-chain fatty-acid-producing bacteria: key components of the human gut microbiota. Nutrients 15, 2211 (2023).
Tang, C., Kong, L., Shan, M., Lu, Z. & Lu, Y. Protective and ameliorating effects of probiotics against diet-induced obesity: a review. Food Res. Int. 147, 110490 (2021).
Plovier, H. et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 23, 107–113 (2017).
Li, Y. et al. Lactobacillus plantarum helps to suppress body weight gain, improve serum lipid profile and ameliorate low-grade inflammation in mice administered with glycerol monolaurate. J. Funct. Foods 53, 54–61 (2019).
Kovatcheva-Datchary, P. et al. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 22, 971–982 (2015).
Rinott, E. et al. The effects of the Green-Mediterranean diet on cardiometabolic health are linked to gut microbiome modifications: a randomized controlled trial. Genome Med. 14, 29 (2022).
Hansen, M. E. B. et al. Population structure of human gut bacteria in a diverse cohort from rural Tanzania and Botswana. Genome Biol. 20, 16 (2019).
De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 107, 14691–14696 (2010).
Shahi, S. K. et al. Prevotella histicola, a human gut commensal, is as potent as COPAXONE® in an animal model of multiple sclerosis. Front. Immunol. 10, 462 (2019).
Fan, X. et al. Prevotella histicola ameliorates DSS-induced colitis by inhibiting IRE1α-JNK pathway of ER stress and NF-κB signaling. Int. Immunopharmacol. 135, 112285 (2024).
Johnson, M. H., Wallig, M., Luna Vital, D. A. & de Mejia, E. G. Alcohol-free fermented blueberry-blackberry beverage phenolic extract attenuates diet-induced obesity and blood glucose in C57BL/6J mice. J. Nutritional Biochem. 31, 45–59 (2016).
Wu, T., Gao, Y., Guo, X., Zhang, M. & Gong, L. Blackberry and blueberry anthocyanin supplementation counteract high-fat-diet-induced obesity by alleviating oxidative stress and inflammation and accelerating energy expenditure. Oxid. Med. Cell. Longev. 2018, 4051232 (2018).
Wu, X. et al. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 54, 4069–4075 (2006).
Bertoia, M. L. et al. Dietary flavonoid intake and weight maintenance: three prospective cohorts of 124,086 US men and women followed for up to 24 years. BMJ Clin. Res. 352, i17 (2016).
Tsuda, T., Horio, F., Uchida, K., Aoki, H. & Osawa, T. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. 133, 2125–2130 (2003).
Prior, R. L. et al. Purified blueberry anthocyanins and blueberry juice alter development of obesity in mice fed an obesogenic high-fat diet. J. Agric. Food Chem. 58, 3970–3976 (2010).
Meireles, M. et al. Effect of chronic consumption of blackberry extract on high-fat induced obesity in rats and its correlation with metabolic and brain outcomes. Food Funct. 7, 127–139 (2016).
Yang, L. et al. Anthocyanins regulate serum adipsin and visfatin in patients with prediabetes or newly diagnosed diabetes: a randomized controlled trial. Eur. J. Nutr. 60, 1935–1944 (2021).
Wu, T. et al. Cyclocarya paliurus polysaccharide improves metabolic function of gut microbiota by regulating short-chain fatty acids and gut microbiota composition. Food Res. Int. 141, 110119 (2021).
Zhao, Z. et al. Fecal microbiota transplantation protects rotenone-induced Parkinson’s disease mice via suppressing inflammation mediated by the lipopolysaccharide-TLR4 signaling pathway through the microbiota-gut-brain axis. Microbiome 9, 226 (2021).
Jian, T. et al. Total Sesquiterpenoids of Loquat Leaves Alleviated High-Fat Diet-Induced Obesity by Targeting Fecal Metabolic Profiling and Gut Microbiota Composition. J. Agric. Food Chem. 70, 13279–13288 (2022).
Tian, Y. et al. Phenolic acids from Chicory roots ameliorate dextran sulfate sodium-induced colitis in mice by targeting TRP signaling pathways and the gut microbiota. Phytomed. Int. J. Phytother. Phytopharmacol. 128, 155378 (2024).
Wang, P. et al. Resveratrol reduces obesity in high-fat diet-fed mice via modulating the composition and metabolic function of the gut microbiota. Free Radic. Biol. Med. 156, 83–98 (2020).
Du, L. et al. Blueberry and blackberry anthocyanins ameliorate metabolic syndrome by modulating gut microbiota and short-chain fatty acids metabolism in high-fat diet-Fed C57BL/6J Mice. J. Agric. Food Chem. 71, 14649–14665 (2023).
Panchal, S. K. & Brown, L. Rodent models for metabolic syndrome research. J. Biomed. Biotechnol. 2011, 351982 (2011).
Biswas, S. K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox?. Oxid. Med. Cell Longev. 2016, 5698931 (2016).
Zhang, H. et al. Anthocyanin supplementation improves anti-oxidative and anti-inflammatory capacity in a dose-response manner in subjects with dyslipidemia. Redox Biol. 32, 101474 (2020).
Herrera-Balandrano, D. D. et al. Hypoglycemic and hypolipidemic effects of blueberry anthocyanins by AMPK activation: in vitro and in vivo studies. Redox Biol. 46, 102100 (2021).
Rohr, M. W., Narasimhulu, C. A., Rudeski-Rohr, T. A. & Parthasarathy, S. Negative effects of a high-fat diet on intestinal permeability: a review. Adv. Nutr.11, 77–91 (2020).
Chen, T. et al. Purple red rice anthocyanins alleviate intestinal damage in cyclophosphamide-induced mice associated with modulation of intestinal barrier function and gut microbiota. Food Chem. 397, 133768 (2022).
Tian, L. et al. Metabolism of anthocyanins and consequent effects on the gut microbiota. Crit. Rev. Food Sci. Nutr. 59, 982–991 (2019).
Chen, L. et al. Chemoprevention of colorectal cancer by black raspberry anthocyanins involved the modulation of gut microbiota and SFRP2 demethylation. Carcinogenesis 39, 471–481 (2018).
Kong, C. Y. et al. An energy-restricted diet including yogurt, fruit, and vegetables alleviates high-fat diet-induced metabolic syndrome in mice by modulating the gut microbiota. J. Nutr. 152, 2429–2440 (2022).
Le Roy, T. et al. Dysosmobacter welbionis is a newly isolated human commensal bacterium preventing diet-induced obesity and metabolic disorders in mice. Gut 71, 534–543 (2022).
Ley, R. E., Turnbaugh, P. J., Klein, S. & Gordon, J. I. Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023 (2006).
Turnbaugh, P. J., Bäckhed, F., Fulton, L. & Gordon, J. I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3, 213–223 (2008).
Du, L. et al. Blueberry and blackberry anthocyanins ameliorate metabolic syndrome by modulating gut microbiota and short-chain fatty acids metabolism in high-fat diet-fed C57BL/6J mice. J. Agric. Food Chem. 71, 14649–14665 (2023).
Cao, W. et al. The role of gut microbiota in the resistance to obesity in mice fed a high fat diet. Int. J. Food Sci. Nutr. 71, 453–463 (2020).
Zhong, S. et al. Cyanidin-3-rutinoside from Mori Fructus ameliorates dyslipidemia via modulating gut microbiota and lipid metabolism pathway. J. Nutr. Biochem. 137, 109834 (2025).
Liu, P. et al. The main anthocyanin monomer from Lycium ruthenicum Murray fruit mediates obesity via modulating the gut microbiota and improving the intestinal barrier. Foods 11, 98 (2021).
Wang, H. et al. Effects on diabetic mice of consuming lipid extracted from foxtail millet (Setaria italica): gut microbiota analysis and serum metabolomics. J. Agric. Food Chem. 71, 10075–10086 (2023).
Zhu, L. et al. Cyanidin-3-O-glucoside alleviates alcoholic liver injury via modulating gut microbiota and metabolites in mice. Nutrients 16, 694 (2024).
Morrison, D. J. & Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7, 189–200 (2016).
Singh, V. et al. Butyrate producers, “The Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 13, 1103836 (2022).
Derrien, M., Vaughan, E. E., Plugge, C. M. & de Vos, W. M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evolut. Microbiol. 54, 1469–1476 (2004).
Kondo, T., Kishi, M., Fushimi, T. & Kaga, T. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. J. Agric. Food Chem. 57, 5982–5986 (2009).
Hong, Y. et al. Desulfovibrio vulgaris, a potent acetic acid-producing bacterium, attenuates nonalcoholic fatty liver disease in mice. Gut Microbes 13, 1–20 (2021).
Xue, L., Deng, Z., Luo, W., He, X. & Chen, Y. Effect of fecal microbiota transplantation on non-alcoholic fatty liver disease: a randomized clinical trial. Front. Cell. Infect. Microbiol. 12, 759306 (2022).
Chen, S. et al. High temperature and humidity in the environment disrupt bile acid metabolism, the gut microbiome, and GLP-1 secretion in mice. Commun. Biol. 7, 465 (2024).
Du, L. et al. Fecal microbiota transplantation from blueberry and blackberry anthocyanins-supplemented mice ameliorated metabolic syndrome by regulating gut microbiota in a high-fat diet model. Food Sci. Hum. Wellness https://doi.org/10.26599/FSHW.2024.9250238 (2024).
Ley, R. E. Gut microbiota in 2015: prevotella in the gut: choose carefully. Nat. Rev. Gastroenterol. Hepatol. 13, 69–70 (2016).
Marietta, E. V. et al. Suppression of inflammatory arthritis by human gut-derived prevotella histicola in humanized mice. Arth. Rheumatol.68, 2878–2888 (2016).
Marietta, E. et al. Administration of human-derived upper gut commensal Prevotella histicola delays the onset of type 1 diabetes in NOD mice. BMC Microbiol. 22, 8 (2022).
Salminen, S. et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 18, 649–667 (2021).
Liu, J. et al. Live and pasteurized Akkermansia muciniphila decrease susceptibility to Salmonella Typhimurium infection in mice. J. Adv. Res. 52, 89–102 (2023).
Yang, C. J. et al. Oral fecal transplantation enriches Lachnospiraceae and butyrate to mitigate acute liver injury. Cell Rep. 43, 113591 (2024).
Zhu, C. W. et al. Five blueberry anthocyanins and their antioxidant, hypoglycemic, and hypolipidemic effects in vitro. Front. Nutr. 10, 1172982 (2023).
Wang, L. et al. Prebiotic properties of the polysaccharide from Rosa roxburghii Tratt fruit and its protective effects in high-fat diet-induced intestinal barrier dysfunction: a fecal microbiota transplantation study. Food Res. Int.164, 112400 (2023).
Omori, K. et al. Effects of dapagliflozin and/or insulin glargine on beta cell mass and hepatic steatosis in db/db mice. Metabolism 98, 27–36 (2019).
Acknowledgements
This research was financially supported by grants from the National Natural Science Foundation of China (32170377), the Primary Research & Development Plan of Jiangsu Province (No. BE2022371) and the Postgraduate Research and Practice Innovation Program of Jiangsu Province (KYCX24-1240). Support was also received from Jiangsu Scientific and Technological Innovations Platform (Jiangsu Provincial Service Center for Antidiabetic Drug Screening).
Author information
Authors and Affiliations
Contributions
Authors’ contributions: L.D., X.D. and J.C. conceived and designed the study, L.D., X.D. W.Z. and L.H. performed the experiments, H.L., T.J., and J.L. performed the data analysis, X.M. and G.N. advised the experiment, H. L. J.C. and W.L. revised the manuscript and offered the funding support.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Du, L., Ding, X., Zhang, W. et al. Anthocyanins from blueberry and blackberry ameliorate metabolic syndrome by Prevotella histicola and acetic acid. npj Sci Food 9, 158 (2025). https://doi.org/10.1038/s41538-025-00526-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41538-025-00526-4