Abstract
Food contamination by Listeria monocytogenes usually occurs in food processing environments, where bacteria coexist in multi-species biofilms. Using a stochastic modelling approach, this study addressed the cross-contamination dynamics of cold-smoked salmon by L. monocytogenes from multi-species biofilms (3) formed by bacterial consortia from food industry surfaces under conditions reproducing low L. monocytogenes loads. Single-species biofilms were also formed for comparison. Transfer rates (TR) were determined over successive contacts of each biofilm with 25 salmon slices. Modelling of TR probabilities revealed distinct contamination profiles. Differences were particularly marked between multi- and single-species biofilms, likely indicating the influence of the coexisting microbiota. Subsequently, the growth of L. monocytogenes in cold-smoked salmon after transfer from multi-species and single-species biofilms was examined. L. monocytogenes from multi-species biofilms showed higher growth than single-species biofilms, which would markedly increase food safety risk. These findings are critical for designing realistic challenge studies and improving contamination control of ready-to-eat foods.
Similar content being viewed by others
Introduction
Listeria monocytogenes is the causative agent of listeriosis, a foodborne illness with a fatality rate of up to 20–30%1,2,3, making it the subject of health concerns and surveillance and causing significant economic losses. Listeriosis can be particularly life-threatening in the population groups known as Young, Old, Pregnant, and Immunocompromised people4, and it is mainly caused by consuming contaminated ready-to-eat (RTE) food5,6. These products pose a significant food safety risk due to the absence of a final bactericidal treatment before consumption7, with high occurrences in meat and fish commodities, such as smoked salmon8. Despite intensified surveillance and control measures, the notification rate has increased consistently in the last few years3, indicating a recurrent presence of the pathogen in foods.
The presence of L. monocytogenes in foods is related to its ability to tolerate adverse environmental conditions, such as low pH9, low temperatures10, high salt concentrations11, desiccation12 and the presence of antimicrobials13, among others. Food contamination frequently occurs in food processing environments (FPE), where L. monocytogenes usually coexist with other bacteria in multi-species biofilms14. The biofilm provides a favourable environment to survive under adverse conditions, while facilitating nutrient acquisition and exchange of genetic material15. Thus, biofilms serve as reservoirs for L. monocytogenes, increasing the likelihood of cross-contamination of foods16,17,18. Also, biofilms may colonise areas on equipment or installations that are difficult to access due to poor design19, which often impedes the effective action of disinfectants. Accordingly, cross-contamination research has progressively moved from planktonic cells20,21,22 to biofilm systems23,24,25. However, studies have primarily focused on single-species and, to a much lesser extent, dual-species systems, which represent simplified models of the complex multi-species biofilms commonly present in real FPEs, leaving the influence of coexisting microbiota largely unexplored.
Therefore, this study aimed to examine the cross-contamination of cold-smoked salmon with L. monocytogenes from multi-species biofilms found in the food industry. For this purpose, typical conditions of FPEs were simulated, using low initial loads of L. monocytogenes and low nutrient availability. The study included: (i) a comparative analysis of transfer dynamics from three different multi-species biofilms systems; (ii) a comparison of transfer dynamics of L. monocytogenes from three different multi-species biofilms; (iii) a comparison of transfer dynamics between multi-species and single-species biofilms of L. monocytogenes to a the influence of coexisting microbiota on contamination; and (iv) an assessment of the growth potential of L. monocytogenes transferred from both types of biofilm during the shelf life of smoked salmon, complemented by a case study illustrating how this growth potential could impact the risk of listeriosis associated with the consumption of smoked salmon.
Results
Dynamics of cross-contamination from multi-species biofilms
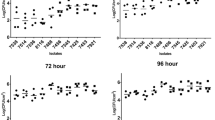
The numbers of L. monocytogenes cells transferred to smoked salmon from the multi-species biofilms under study, namely F96, F107 and F168, decreased progressively with each successive contact (Fig. 1A). Cell densities of L. monocytogenes varied significantly between biofilms, ranging from 104 CFU/cm2 in F96 and F107 to 105 CFU/cm2 in F168 (p < 0.05, Supplementary Table 1). Given those differences in the number of L. monocytogenes among the biofilms, transfer rates (TRs) were used to compare cross-contamination from the three biofilms.
A first approach based on pairwise comparisons revealed no significant differences in TR between biofilms in any of the 25 contacts (p > 0.05). Also, the dynamics of TR were analysed using three different models, namely, exponential decay20,26, logistic25 and Weibull27,28. Estimations and statistics from subsequent goodness-of-fit analyses of such models to the experimental values of TR showed that the Weibull distribution was the best fit for all three biofilms (Supplementary Table 2).
Based on the experimental data, a Monte Carlo method was used to generate data simulations, and their means were fitted to a Weibull distribution, followed by a noise correction. Fitting and noise correction were iterated 104 times to improve the accuracy of the model (Fig. 1B; for more information, see 'Methods'). A comparison of the model parameter values (shape and scale) obtained for each of the biofilms revealed no significant differences either (p > 0.05, Supplementary Table 3).
Given the results, a stochastic approach was attempted, grouping contacts into different clusters to increase the number of replicates. The contacts of each biofilm were grouped into three clusters based on the absence of significant differences in experimental TR values (p > 0.05). As a result, the contacts within each cluster were the same for all three biofilms, except the third and tenth contacts, respectively, included in clusters 2 and 3 of F96 (Fig. 2 and Table 1). These three clusters could be associated with different stages of the transfer dynamics, namely, cluster 1, initial detachment; cluster 2, unstructured biofilm detachment; and cluster 3, the transfer of cells tightly attached to the inner layers of biofilms.
The 25 contacts were clustered (1–3) based on the absence of significant differences in experimental values (p > 0.05); the contacts included in each cluster are listed in Table 2. Different letters in each cluster indicate significant differences between biofilms (p < 0.05).
The experimental TR values of each cluster were then represented in histograms for each biofilm (Fig. 3), and differences in frequency distributions between biofilms were evaluated within each cluster by a Kolmogorov–Smirnov (KS) test. The tests revealed significant differences between F96 and F168 in cluster 1 (p = 0.01, D = 0.27), as well as between F96 and F107 (p = 0.02, D = 0.23) and F96 and F168 (p = 0.01, D = 0.27) in cluster 3. In contrast, no differences were found between biofilms in cluster 2. Subsequently, the experimental values of each of the nine clusters were fitted to log-normal, gamma, and Weibull distributions. The goodness-of-fit analysis showed that the gamma distribution provided the best fit for clusters 1 and 2 of F96, whereas the log-normal distribution was the best fit for the others (Supplementary Table 4).
The probability density functions (PDF) with best goodness-of-fit were then determined using the adjusted parameter values for each case. PDFs allow quantifying the probabilities (P) of transfer events directly related to the risk of cross-contamination. Accordingly, within cluster 1, a TR > 20% would be more likely to occur for F168 (P = 0.12) than for F96 (P = 0.08) or F107 (P = 0.09), indicating a higher risk of initial cross-contamination from that biofilm. In contrast, in cluster 2, the probability of a TR > 5% would be 0 for F168 but not for F96 (P = 0.09) or F107 (P = 0.01), whereas F96 would show the highest probability of TR > 1% in cluster 3 (0.12, versus 0.03 for F107 and 0 for F168). Therefore, the distribution analysis revealed differences between biofilms. While F168 showed the highest cross-contamination potential in the first stages of the dynamic (first cluster), F96 maintained high cross-contamination likelihoods throughout the 25 contacts.
Transfer dynamics of single-species biofilms versus multi-species biofilms
A second set of transfer dynamics studies was conducted with single-species biofilms formed by the L. monocytogenes strains under study, namely L96, L107 and L168. A single assay was carried out to explore the ability of the three L. monocytogenes strains to form single-species biofilms under several low-nutrient conditions, specifically 1000-, 900-, 750- and 500-fold diluted TSB, and to find out which conditions would replicate the low loads of L. monocytogenes generally found in FPEs. The ability to form single-species biofilms under starvation varied significantly among the three strains, but a sharp decline in the number of biofilm cells of all strains was only found in 1000-fold diluted TSB, presenting a reduction of 2–5 orders of magnitude relative to 500-fold diluted TSB (Supplementary Fig. 1). Therefore, to replicate the low loads of L. monocytogenes generally found in FPEs29 and attempt to make them as similar as possible to those of multi-species biofilms, 1000-fold diluted TSB was used in single-species biofilm formation assays conducted subsequently. Subsequent trials with L107 and L168 did not detect attached cells; that is, the number of L. monocytogenes cells attached to the coupons was found to be below the detection limit of the method, i.e. less than 100 CFU per coupon (4 CFU/cm2). Therefore, the comparative analysis of transfer dynamics between single-species and multi-species biofilms was conducted only with strain L1.96.
As depicted in Fig. 5A, the transfer dynamics of L. monocytogenes were similar in both biofilm systems, displaying a decreasing pattern that reached very low values in the last contacts. Nonetheless, more cells were transferred from L96 than from F96, which was consistent with the initial load of L. monocytogenes in each biofilm (5.68 ± 0.27 log CFU/cm2 in L96 and 4.52 ± 0.68 log CFU/cm2 in F96). However, in terms of TR, significant differences were only observed between L96 and F96 first contact (p < 0.05), with higher values for F96, while all subsequent contacts showed similar values.
Among the three distributions tested, it was observed that the Weibull distribution also provided the best fit for L96 (Fig. 4B and Supplementary Table 2). Similarly, the values obtained for the parameters of the final model approach did not reveal significant differences with those of F96 (Supplementary Table 3). The values of TR obtained for L96 were also clustered into three groups of contacts based on the absence of significant differences (p > 0.05, Fig. 5). The Kruskal–Wallis test reported differences only in cluster 1, in which L96 showed significantly lower TR than F96. Similar results were obtained when comparing frequency distributions in each cluster, with F96 and L96 only showing significant differences in cluster 1 (KS, p < 0.05, D = 0.2).
The 25 contacts were clustered (1–3) based on the absence of significant differences in experimental values (p > 0.05); the contacts included in each cluster are listed in Table 2. Different letters in each cluster indicate significant differences between biofilms (p < 0.05).
Concerning the modelled PDFs, slight variations were found between L96 and F96 (Fig. 6). PDFs obtained for L96 consistently exhibited a narrower distribution with a lower degree of tailing in all clusters. This was particularly evident in cluster 1, with no TR higher than 20%. In terms of probabilities, differences were reported only for cluster 1, with P (TR > 20%) being 0 for L96 and 0.1 for F96. Thus, the cross-contamination potential of multi-species biofilm F96 was initially higher than the single-species counterpart L96.
Risk of growth of L. monocytogenes from single-species and multi-species biofilms in cold-smoked salmon
Figure 7 shows the evolution of L. monocytogenes loads in smoked salmon contaminated with either F96 or L96 biofilms at the first and sixth contact over a 15-day storage period. Significant differences (p < 0.05) were observed between the two biofilm systems throughout the storage period. By the end of the storage period, L. monocytogenes growth from multi-species biofilms significantly exceeded that from single-species biofilms. For the first contact, loads reached 679.27 ± 252.33 CFU/g and 8764 ± 289.93 CFU/g for L96 and F96, respectively. Similar trends were observed for the sixth contact, with final loads of 144.19 ± 11.32 CFU/g and 4556.54 ± 1197.81 CFU/g for L96 and F96, respectively, indicating that smoked salmon contaminated with F96 presents a significantly higher load of L. monocytogenes than those slices contaminated with L96.
Case study
Based on the results presented, a hypothetical scenario was simulated (Fig. 8). The simulation specifically involved a single smoked salmon slice (~5 g) within an 80 g package. Utilising the contamination data reported on single- and multi-species biofilms (Fig. 7), the microbial load transferred to a 5 g slice was calculated, and the final contamination level (CFU/g) was reported for the entire 80 g package.
Estimates of L. monocytogenes load in cold-smoked salmon during storage at 5 ºC. In this specific case study, contamination is modelled to occur within a 5 g portion of a single slice in each 80 g package. L. monocytogenes was transferred at 1st or 6th contact from both single- (L96) and multi-species (F96) biofilms. Estimates were obtained from the experimental results of this study. The red dashed line marks the 100 CFU/g limit set in legislation. This image was created with BioRender.com.
According to the scenario results, the legal limit of 100 CFU/g of L. monocytogenes load would not be exceeded after 7 days of storage in both biofilm systems. However, by day 15, the limit would be significantly surpassed on multi-species biofilm, reaching 547.76 CFU/g and 284.78 CFU/g for the first and sixth contacts, respectively. In contrast, L. monocytogenes from single-species L96 remained below the detection limit in both contacts throughout the shelf life. Therefore, in this scenario, L. monocytogenes from multi-species biofilms poses a significantly higher risk than those from single-species biofilms.
Discussion
Multi-species biofilms are highly complex and variable systems that are challenging to study. Numerous factors modulate the variability of the system, affecting biofilm structure and cell transfer dynamics. Many of these factors are difficult or impossible to control and replicate consistently in laboratory settings. Despite these challenges, investigating multi-species biofilms provides a more realistic representation of how bacteria, such as L. monocytogenes, interact and behave within the context of FPE, offering valuable insights into the dynamics of contamination events.
The similarity in the TR profiles of the three multi-species biofilms under study could be explained by the high variability, i.e. stochasticity, which makes it difficult to appreciate possible differences, unless they were large. In fact, differences among biofilms arose when the contacts were grouped into three clusters (thus increasing the number of TR data), as shown by the PDFs. To the best of the author’s knowledge, cross-contamination with L. monocytogenes from multi-species biofilms had not been addressed previously, which prevents any comparison with other works. The vast majority of studies on the issue have focused on single-species biofilms24,25 or at most, dual-species biofilms23. In dual-species biofilms, Pang and Yuk23 reported a TR of 10% at a single contact of smoked salmon with dual-species biofilms of L. monocytogenes and Pseudomonas fluorescens.
These studies represent simplified models of those complex multi-species biofilms commonly present in FPEs, leaving the influence of coexisting microbiota largely unexplored. Specifically, Pang & Yuk23 combined initial concentrations of 106 CFU/mL of each L. monocytogenes and P. fluorescens to form dual-species biofilms. As a result, L. monocytogenes densities of 107 UFC/cm2 were found in 48 h old biofilms. These loads are substantially higher than those generally found in FPE. Additionally, they relied on a small number of replicates (n = 6), which reduces the statistical power and the ability to detect meaningful effects. In contrast, we present a stochastic modelling approach based on using a high number of replicates (n > 60, after grouping contacts). By modelling contamination as a probabilistic process, this approach enables us to capture the inherent variability of contamination, enhance our ability to detect differences, and provide a more robust understanding of transfer dynamics.
The differences in the contamination profiles observed among the biofilms are probably due to the different microbiota present in each of them, rather than to the L. monocytogenes strains. Thus, the co-existing microbiota would probably determine the biofilm structure and cross-contamination potential. In this sense, Kim and Oh30 suggested that differences in cross-contamination of chicken meat and skin from dual-species formed either by Salmonella enterica and Campylobacter jejuni or S. enterica and Clostridium perfringens and multi-species biofilms formed by all three bacteria could be due to the different bacterial composition of each biofilm, which affects the attachment and production of extracellular polymeric substances (EPS). Similarly, a recent study has shown that the microscopic 3D structure of mixed-species biofilms is determined by the coexisting microbiota rather than the specific strain of L. monocytogenes31. In addition, it is well established that L. monocytogenes survives but does not outcompete other bacteria within biofilms32. Previous studies on multi-species biofilms harbouring L. monocytogenes showed that coexisting bacterial species outnumber the pathogen29,33,34. Similarly, surface sampling in FPEs revealed a low abundance of L. monocytogenes at contamination hotspots, in which many other bacterial species predominated in the microbial communities35. Accordingly, in our experimental systems, the inoculum of L. monocytogenes (102 CFU/mL) was set three orders of magnitude below those of each coexisting bacterium (105 CFU/mL) to reflect conditions found in industrial settings. However, little is known about the interactions of these bacteria with L. monocytogenes in biofilms, apart from enhancing its resistance against disinfectants31,36 and increasing L. monocytogenes counts in dual-species biofilms with Acinetobacter or Pseudomonas37. Therefore, further studies are needed to elucidate these complex interactions fully.
To gain further insight into the role of the coexisting microbiota in cross-contamination, single-species biofilms of L. monocytogenes were formed under the same conditions. Based on a screening experiment, a 1000-fold dilution of TSB in PBS was selected as the low-nutrient medium that better replicated the low microbial load of L. monocytogenes found in FPEs. Unexpectedly, strains L1.107 and L1.168 did not form single-species biofilms in 1000-fold diluted TSB, unlike previous screening results. The lack of experimental reproducibility observed for those two strains could be due to severe starvation and low inoculum. Conditions close to the growth/no-growth limit and low inoculum (102 CFU/mL) have been reported to lead to experimental variations that are very difficult or even impossible to control38. Stochastic variations in growth kinetics are known to become apparent at low inoculum due to individual cell heterogeneity, which affects small populations much more than large ones39. Similarly, growth limits for planktonic L. monocytogenes cells were found to be distributed over a range of values and were affected by inoculum size40,41.
These findings contrast with the results obtained for multi-species biofilms, in which attached L. monocytogenes cells were found for the three strains. In fact, L. monocytogenes did not seem to experience growth limitations when coexisting with other bacteria. It has been noted that the presence of diverse metabolic pathways within multi-species biofilms could lead to metabolic cross-feeding42, as certain bacterial metabolites can serve as an energy source for other biofilm members. Recently, Ch’ng et al.43 have shown that Enterococcus faecalis benefits from the heme group produced by Staphylococcus aureus to use in aerobic respiration within dual-species biofilms. However, to the author’s knowledge, the specific metabolic interactions between L. monocytogenes and coexisting bacteria have not been addressed. Therefore, that possibility should be substantiated through future analyses evaluating microbial interactions within biofilms.
Since no cell counts were detected in single-species biofilms of L107 and L168, the comparison between single-species and multi-species biofilms was limited to those formed by the strain L1.96. Both the initial L. monocytogenes load in biofilms and the number of cells transferred were higher for single-species (L96) than for multi-species biofilms (F96). These results are consistent with those reported by Pang and Yuk23, who found higher L. monocytogenes cell densities in single-species biofilms compared to dual-species biofilms formed with P. fluorescens. These authors attributed their findings to a competitive advantage of P. fluorescens over L. monocytogenes. Similarly, it could be that the co-existing microbiota competes with L. monocytogenes in F96, leading to a lower load than L96.
However, the TRs of F96 were higher than those of L96, suggesting the involvement of the coexisting microbiota in the potential for cross-contamination by L. monocytogenes. TR does not solely depend on the initial microbial load but also on the properties of the biofilm surface, which are highly conditioned by the content and composition of EPS, such as carbohydrates, extracellular DNA and lipids, which affect the viscoelasticity of biofilms44. As an example, a comparative study of EPS-producing and non-EPS-producing strains of Staphylococcus epidermidis showed that the number of cells transferred to nanopillared silicon surfaces was higher in the case of EPS-producing biofilms45. L. monocytogenes produces biofilms with a low EPS content, consisting mainly of proteins46. Unpublished results from our laboratory on single-species biofilms formed by various strains of L. monocytogenes confirm these findings. Thus, it would be expected that L96 would barely present EPS. On the contrary, bacteria that coexist in F96, such as A. johnsonii and Rhodococcus sp, are known to produce EPS and have a high biofilm-forming ability47,48. Although EPS were not analysed in the multi-species biofilms, the preponderance of EPS-producing strains would be expected to provide F96 with a viscoelasticity that would lead L. monocytogenes to detach from biofilms, enhancing the cross-contamination potential.
Few studies have conducted a comparative analysis of the contamination risk posed by L. monocytogenes from single-species versus multi-species biofilms, which limits direct comparisons with our present findings. One notable exception is the work of Pang and Yuk23, who investigated mixed-species biofilms of L. monocytogenes and P. fluorescens. The authors observed significantly higher TRs and numbers of transferred cells for single-species biofilms of L. monocytogenes than for mixed-species biofilms. However, their approach differed significantly from ours. We used a more complex biofilm system consisting of multiple bacterial species and employed experimental conditions—low inoculum and starvation—that mirrored the reality of FPEs.
Another issue of concern is the lack of knowledge regarding the behaviour of L. monocytogenes following cross-contamination of food from multi-species biofilms. L. monocytogenes can grow under refrigerated conditions; thus, challenge studies are essential to ensure the safety of RTE products. In the European Union, Regulation (EC) 2073/2005 mandates that RTE foods susceptible to L. monocytogenes growth must maintain a microbial load below 100 CFU/g throughout their shelf-life. In such products, the risk of listeriosis is highly dependent on the ability of L. monocytogenes to grow. Consequently, we conducted a study to investigate if there was an increased risk of L. monocytogenes growth during storage of cold-smoked salmon, whether it came from single- or multi-species biofilms.
It was thus found that L. monocytogenes cells from multi-species biofilms were more likely to thrive in vacuum-packaged cold-smoked salmon than those from single-species biofilms. It is considered that having previously been part of multi-species biofilms seems to confer a pre-adaptative advantage, enhancing L. monocytogenes growth. Whether this is also the case in other foods configured as different ecological niches is a question to be examined in the future. Consequently, when encountering the microbiota-rich environment of smoked salmon, it is better prepared to proliferate significantly. This environment is primarily dominated by lactic acid bacteria49, known producers of bacteriocins and other inhibitory metabolites50,51. L. monocytogenes cells from multi-species biofilms are likely better adapted to coexist with other bacteria, such as those in the salmon microbiota. Therefore, it is hypothesised that the co-existence of various bacterial species within multi-species biofilms may induce modifications in L. monocytogenes, conferring a competitive advantage that accelerates its growth relative to single-species biofilm-derived cells.
The growth differences observed between multi-species and single-species biofilm cells are particularly relevant within the context of European legislation. For this reason, we conducted a case study simulating a scenario where a single smoked salmon slice from an 80 g package was contaminated with L. monocytogenes from F96 or L96 biofilms. After 15 days of refrigerated storage, the L. monocytogenes load in samples contaminated from F96 exceeded the regulatory limit of 100 CFU/g. This underscores a significantly greater food safety risk and highlights the importance of considering multi-species biofilm contamination as real case scenarios that would then be incorporated into the design of more realistic and protective challenge studies, ultimately leading to more effective control of L. monocytogenes.
Furthermore, our results demonstrate the importance of using an experimental distribution-based stochastic approach, followed by robust statistical analysis, in studying the transfer dynamics of L. monocytogenes to food. It allows researchers to reproduce variable contamination scenarios that better reflect actual conditions in FPEs and thus capture key differences between biofilms that conventional methods may overlook.
This approach could also enable us to develop more accurate Quantitative Microbial Risk Assessments of L. monocytogenes in RTE food, particularly those that support its growth during their shelf-life (e.g. cold-smoked salmon). Challenge studies could thus move beyond deterministic inoculation schemes and instead model real-world cross-contamination risks based on observed stochastic dynamics. For food manufacturers, this would support more accurate microbial risk assessments and the development of tailored strategies to control L. monocytogenes that improve current food safety management systems. For example, manufacturers can prioritise sanitation efforts and monitoring in areas where multi-species biofilms are likely to form and enhance L. monocytogenes transfer. In addition, integrating these probabilistic contamination models into HACCP plans or predictive modelling tools can help set more protective critical limits and better manage residual risk in RTE products.
Methods
Bacterial strains and culture conditions
Bacterial strains under this study are listed in Table 2. The list comprises three L. monocytogenes strains, namely L1.96 (serogroup IIc, CC9), L1.107 (serogroup IIc, CC9) and L1.168 (serogroup IVb, CC217) and several representative strains of the most abundant genera coexisting with each of those strains of L. monocytogenes. All strains were isolated from three locations in FPEs where L. monocytogenes had been found, and were thus considered potential contamination sites52.
All strains were part of the Instituto de Investigaciones Marinas—Consejo Superior de Investigaciones Científicas (IIM-CSIC) culture collection, where stock cultures of each were maintained at −80 °C in sterile Brain-Heart Infusion Broth (BHI; Scharlab, Barcelona, Spain) with 50% (v v-1) glycerol. Short-term working cultures of each strain were stored at −20 °C under identical conditions. A maximum of 15 freeze-thaw cycles were permitted per cryovial. Strains were reactivated by adding 100 µL of working culture to 5 mL of Tryptone Soy Broth (TSB, Scharlab, Barcelona, Spain), followed by overnight incubation without shaking at 37 °C for L. monocytogenes strains, or 25 °C for the coexisting strains.
Biofilm formation
All biofilms were formed under starvation conditions using TSB diluted at a 1:1000 ratio in Phosphate-Buffered Saline (PBS, Thermo Fisher Scientific, Massachusetts, USA). Multi-species biofilms F96, F107 and F168 were created by combining L. monocytogenes with the corresponding coexisting microbiota listed in Table 2. Single-species biofilms, L96, L107 and L168, were formed using each of the three L. monocytogenes strains separately.
The inoculum was individually standardised by adjusting reactivated cultures of each strain to an optical density (OD) at 700 nm of 0.100 ± 0.01, corresponding to ~108 CFU/mL. Once adjusted, ten-fold serial dilutions were performed to obtain the desired microbial loads, which were verified in all cases by plating on Tryptone Soy Agar (TSA, Scharlab, Barcelona, Spain).
Biofilms were formed on sterile AISI 316 stainless steel coupons (1.0 mm thickness, 50 × 50 mm). Each coupon was placed into a plastic Petri dish (90 × 16.2 mm) with 25 mL of bacterial culture. Bacterial cultures contained ~102 CFU/mL of L. monocytogenes and, in the case of multi-species biofilms, 105 CFU/mL of each of the other bacteria in the consortium. Biofilms were cultured under static conditions for 72 h at 25 °C.
The concentration of L. monocytogenes in biofilms was determined using three biological replicates (coupons) in each trial. Once formed, biofilms were washed twice with 25 mL of PBS to remove non-attached cells. Subsequently, biofilms were sonicated for 2 min at 40 kHz and then scraped for 1 min to release the cells. Detached cells were serially diluted in peptone water (Scharlab, Barcelona, Spain), and appropriate serial dilutions were plated on Listeria Octavi Agostini Agar (ALOA, Scharlab, Barcelona, Spain) and cultured at 37 °C for 48 h.
Transfer trials to smoked salmon fillets
Four independent transfer trials, unless otherwise stated, were conducted for each biofilm, using a minimum of 60 units of 80 g packs of sliced cold-smoked salmon in each trial. All packs were purchased from a local retailer and, where possible, were from the same production batch within each trial, with an expiry date of 9–15 days from purchase. Upon arrival at the laboratory, the packages were aseptically opened, and individual slices were trimmed into 4.5 cm diameter portions, each weighing ~5 g. In addition, slices were homogenised with peptone buffer at a 1:4 ratio in a stomacher blender, and aliquots of the homogenates were then plated onto ALOA agar to ensure that the load of L. monocytogenes was below the detection limit of 20 CFU/g.
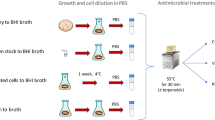
Before transferring L. monocytogenes cells, the biofilms (coupons) were washed twice with PBS to remove non-attached cells. Subsequently, 25 portions of smoked salmon slices (5 g each) were sequentially contacted for 30 s with the biofilm surface, making 25 contacts per biofilm. Contacts were made by placing each portion on the biofilm without exerting pressure. Unless otherwise stated, four transfer trials were performed on different days for each biofilm, with five biological replicates (coupons) in each trial. Consequently, 20 distinct coupons were used to transfer biofilm cells to 25 portions of smoked salmon slices each, yielding 500 contact events per system. Figure 9 summarises the experimental process.
Twenty replicates of single-species (L96, L107 and L168) and multi-species (F96, F107 and F168) biofilms were cultured and subsequently used for cell transfer to cold-smoked salmon. For each biofilm, 25 consecutive contacts were made to salmon fillets. L. monocytogenes loads on the salmon were quantified and reported as CFU/g. This image was created with BioRender.com.
The number of L. monocytogenes cells transferred to the portions (CFU/g) corresponding to 1st, 2nd, 3rd, 4th, 6th, 8th, 10th, 15th, 20th and 25th contacts was determined by homogenising the portions in peptone buffer at a 1:4 ratio and plating homogenates in ALOA. Next, TRs were calculated for each of those contacts as follows:
The number of cells of L. monocytogenes transferred to 5 g of smoked salmon is expressed as CFU/g, whereas the number of biofilm cells of L. monocytogenes is expressed as total CFU per coupon (Supplementary Table 1). The latter was determined by multiplying the number of L. monocytogenes cells per cm2 of coupon surface area (CFU/cm2) by the surface area of the coupon in contact with the salmon slice (i.e. 15.90 cm2), as not all the coupon surface area (25 cm2) was in contact with the slice.
Storage in vacuum package trials
L. monocytogenes L1.96 cells were transferred from 72 h biofilms, both single (L96) and multi-species (F96), to smoked salmon slices as previously described. Portions contaminated during the first and sixth contacts were vacuum-packed in plastic bags and stored at 5 °C for 15 days. The L. monocytogenes load in the salmon slices (CFU/g) was reported at the initial time point (day 0) and after 7 and 15 days of storage by plating on ALOA. This experiment was performed in triplicate.
Data analysis and modelling
Data analysis was conducted using R software version 4.3.053 and RStudio version 4.1.354. All graphs were generated using ggplot255. The Shapiro-Wilk test was used to check data normality, and Levene’s test was used to determine the homogeneity of variances. Significant differences were determined via one-way ANOVA, followed by Tukey’s HSD test post hoc in normally distributed data. The Kruskal–Wallis rank sum test was used non-normally, followed by Dunn’s post hoc test.
Experimental TR data for each biofilm were first fitted to the exponential, logistic and Weibull distributions using the fitdistrplus package56. This initial step aimed to identify how well each standard distribution could describe the variability observed in raw, ungrouped TR data from successive transfer events. Then, a Monte Carlo method was applied to generate 10,000 synthetic row datasets to improve the estimates of the best fit distribution (Weibull) and mitigate the impact of possible outliers and unstable data points. Each synthetic row (yi) was based on experimental data (xi) by adding white noise sampled from a normal distribution with a mean of zero and a standard deviation proportional to the original data. Column-wise (contact) means were calculated, obtaining a new representative row vector (y), a robust approximation of the original data distribution. However, the added noise was substantial due to large standard deviations. A Weibull distribution was fitted to (y) to reduce noise, resulting in a new vector (w). The error (e) between \(w\) and y was calculated by the root of the sum of squared differences and then subtracted from each row vector of the expanded database, yielding an adjusted data matrix, where each row is denoted as \({z}_{i}=\left|{y}_{i}-e\right|\). Subsequently, column-wise means were recalculated, and a new Weibull distribution was fitted to the resulting adjusted row vector (\(z\)). Once the bias correction was applied, it was confirmed to be sufficient through empirical simulations, as established in statistical references57.
Next, TR values from different contacts that showed no significant statistical differences (p > 0.05, Table 2) were grouped into clusters via Hierarchical Clustering, under the rationale that these sets could represent a more homogeneous contamination dynamic. For these clustered datasets, we shifted the fitting to gamma, log-normal, and Weibull distributions (fitdistrplus package). The KS test was used to assess the statistical differences between the frequency distributions obtained for each cluster. The optimal parameters of each fitted distribution were then used to calculate probabilities via the empirical cumulative distribution function from the stats package53.
Data availability
The authors declare that all data supporting the findings of this study are available in the paper, and the statistical analysis code will be made available upon reasonable request to the corresponding author.
References
Tack, D. M. et al. Preliminary incidence and trends of infections with pathogens transmitted commonly through food—foodborne diseases active surveillance network, 10 U.S. Sites, 2016–2019. Morb. Mortal. Wkly. Rep. 69, 509–514 (2020).
Fan, Z., Xie, J., Li, Y. & Wang, H. Listeriosis in mainland China: a systematic review. Int. J. Infect. Dis. 81, 17–24 (2019).
EFSA & ECDC. The European Union One Health 2023 Zoonoses Report. EFSA J. 22, 62-73 (2024).
Charlier, C. et al. Clinical features and prognostic factors of listeriosis: the MONALISA national prospective cohort study. Lancet Infect. Dis. 17, 510–519 (2017).
Pinner, R. W. Role of foods in sporadic listeriosis. JAMA 267, 2046 (1992).
Ricci, A. et al. Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 16, e05134 (2018).
FAO & WHO. Risk Assessment of Listeria Monocytogenes in Ready-to-Eat Foods. http://www.fao.org/es/esn (2004).
ECDC & EFSA. Prolonged Multi-Country Cluster of Listeria Monocytogenes ST155 Infections Linked to Ready-to-Eat Fish Products. https://efsa.onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2023.EN-8538 (2023).
Lundén, J., Tolvanen, R. & Korkeala, H. Acid and heat tolerance of persistent and nonpersistent Listeria monocytogenes food plant strains. Lett. Appl. Microbiol. 46, 276–280 (2007).
Liu, M. et al. Cold-tolerance mechanisms in foodborne pathogens: Escherichia coli and Listeria monocytogenes as examples. Crit. Rev. Food Sci. Nutr. 1–15 https://doi.org/10.1080/10408398.2024.2322141 (2024).
Bae, D. et al. Global gene expression of Listeria monocytogenes to salt stress. J. Food Prot. 75, 906–912 (2012).
Vogel, B. F., Hansen, L. T., Mordhorst, H. & Gram, L. The survival of Listeria monocytogenes during long term desiccation is facilitated by sodium chloride and organic material. Int. J. Food Microbiol. 140, 192–200 (2010).
Møretrø, T. et al. Tolerance to quaternary ammonium compound disinfectants may enhance growth of Listeria monocytogenes in the food industry. Int. J. Food Microbiol. 241, 215–224 (2017).
Puga, C. H., Dahdouh, E., SanJose, C. & Orgaz, B. Listeria monocytogenes colonizes Pseudomonas fluorescens biofilms and induces matrix over-production. Front. Microbiol. 9, 1706 (2018).
Flemming, H. C. et al. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 14, 563–575 (2016).
Abebe, G. M. The role of bacterial biofilm in antibiotic resistance and food contamination. Int. J. Microbiol. 2020, 1–10 (2020).
Carrascosa, C., Raheem, D., Ramos, F., Saraiva, A. & Raposo, A. Microbial biofilms in the food industry—a comprehensive review. Int. J. Environ. Res. Public Health 18, 2014 (2021).
Bolocan, A. S. et al. Putative cross-contamination routes of Listeria monocytogenes in a meat processing facility in Romania. J. Food Prot. 78, 1664–1674 (2015).
Carpentier, B. & Cerf, O. Review-persistence of Listeria monocytogenes in food industry equipment and premises. Int. J. Food Microbiol. 145, 1–8 (2011).
Aarnisalo, K., Sheen, S., Raaska, L. & Tamplin, M. Modelling transfer of Listeria monocytogenes during slicing of ‘gravad’ salmon. Int. J. Food Microbiol. 118, 69–78 (2007).
Lin, C.-M. et al. Cross-contamination between processing equipment and deli meats by Listeria monocytogenes. J. Food Prot. 69, 71–79 (2006).
Vorst, K. L., Todd, E. C. D. & Ryser, E. T. Transfer of Listeria monocytogenes during mechanical slicing of turkey breast, bologna, and salami. J. Food Prot. 69, 619–626 (2006).
Pang, X. & Yuk, H. G. Effects of the colonization sequence of Listeria monocytogenes and Pseudomonas fluorescens on survival of biofilm cells under food-related stresses and transfer to salmon. Food Microbiol. 82, 142–150 (2019).
Truelstrup Hansen, L. & Vogel, B. F. Desiccation of adhering and biofilm Listeria monocytogenes on stainless steel: survival and transfer to salmon products. Int. J. Food Microbiol. 146, 88–93 (2011).
Wang, Z. et al. Characterization of Listeria monocytogenes biofilm formation kinetics and biofilm transfer to cantaloupe surfaces. Food Res. Int. 161, 111839 (2022).
Scollon, A. M., Wang, H. & Ryser, E. T. Transfer of Listeria monocytogenes during mechanical slicing of onions. Food Control 65, 160–167 (2016).
Possas, A. M. M., Posada-Izquierdo, G. D., Pérez-Rodríguez, F. & García-Gimeno, R. M. Modeling the transfer of Salmonella Enteritidis during slicing of ready-to-eat turkey products treated with thyme essential oil. J. Food Sci. 81, M2770–M2775 (2016).
Xiao, X. et al. Modeling transfer of Vibrio Parahaemolyticus during peeling of raw shrimp. J. Food Sci. 83, 756–762 (2018).
Rodríguez-López, P., Saá-Ibusquiza, P., Mosquera-Fernández, M. & López-Cabo, M. Listeria monocytogenes-carrying consortia in food industry. Composition, subtyping and numerical characterisation of mono-species biofilm dynamics on stainless steel. Int. J. Food Microbiol. 206, 84–95 (2015).
Kim, U. & Oh, S. W. Characterizing the formation and cross-contamination potential of multi-species biofilms in poultry processing environments: S. Enteritidis, C. jejuni and C. perfringens. Food Control 163, 110481 (2024).
Rodríguez-López, P., Rodríguez-Herrera, J. J. & López Cabo, M. Architectural features and resistance to food-grade disinfectants in Listeria monocytogenes-Pseudomonas spp. Dual-species biofilms. Front. Microbiol. 13, 917964 (2022).
Heir, E., Møretrø, T., Simensen, A. & Langsrud, S. Listeria monocytogenes strains show large variations in competitive growth in mixed culture biofilms and suspensions with bacteria from food processing environments. Int. J. Food Microbiol. 275, 46–55 (2018).
Fagerlund, A., Langsrud, S. & Møretrø, T. Microbial diversity and ecology of biofilms in food industry environments associated with Listeria monocytogenes persistence. Curr. Opin. Food Sci. 37, 171–178 (2021).
Langsrud, S., Moen, B., Møretrø, T., Løype, M. & Heir, E. Microbial dynamics in mixed culture biofilms of bacteria surviving sanitation of conveyor belts in salmon-processing plants. J. Appl. Microbiol. 120, 366–378 (2016).
Rodríguez-López, P., Bernárdez, M., Rodríguez-Herrera, J. J., Comesaña, ÁS. & Cabo, M. L. Identification and metagenetic characterisation of Listeria monocytogenes-harbouring communities present in food-related industrial environments. Food Control 95, 6–17 (2019).
Saá Ibusquiza, P., Herrera, J. J. R., Vázquez-Sánchez, D. & Cabo, M. L. Adherence kinetics, resistance to benzalkonium chloride and microscopic analysis of mixed biofilms formed by Listeria monocytogenes and Pseudomonas putida. Food Control 25, 202–210 (2012).
Lake, F. B., van Overbeek, L. S., Baars, J. J. P., Abee, T. & den Besten, H. M. W. Growth performance of Listeria monocytogenes and background microbiota from mushroom processing environments. Int. J. Food Microbiol. 395, 110183 (2023).
Koutsoumanis, K. A study on the variability in the growth limits of individual cells and its effect on the behavior of microbial populations. Int. J. Food Microbiol. 128, 116–121 (2008).
Koyama, K., Hiura, S., Abe, H. & Koseki, S. Application of growth rate from kinetic model to calculate stochastic growth of a bacteria population at low contamination level. J. Theor. Biol. 525, 110758 (2021).
Koutsoumanis, K. P. & Sofos, J. N. Effect of inoculum size on the combined temperature, pH and a w limits for growth of Listeria monocytogenes. Int. J. Food Microbiol. 104, 83–91 (2005).
Koutsoumanis, K. P., Kendall, P. A. & Sofos, J. N. A comparative study on growth limits of Listeria monocytogenes as affected by temperature, pH and aw when grown in suspension or on a solid surface. Food Microbiol. 21, 415–422 (2004).
Smith, N. W., Shorten, P. R., Altermann, E., Roy, N. C. & McNabb, W. C. The classification and evolution of bacterial cross-feeding. Front. Ecol. Evol. 7, 153 (2019).
Ch’ng, J.-H. et al. Heme cross-feeding can augment Staphylococcus aureus and Enterococcus faecalis dual species biofilms. ISME J. 16, 2015–2026 (2022).
Gusnaniar, N. et al. Physico-chemistry of bacterial transmission versus adhesion. Adv. Colloid Interface Sci. 250, 15–24 (2017).
Gusnaniar et al. Transmission of monospecies and dualspecies biofilms from smooth to nanopillared surfaces. Appl. Environ. Microbiol. 84, 1–11 (2018).
Colagiorgi, A., Di Ciccio, P., Zanardi, E., Ghidini, S. & Ianieri, A. A look inside the Listeria monocytogenes biofilms extracellular matrix. Microorganisms 4, 22 (2016).
Andrews, J. S., Rolfe, S. A., Huang, W. E., Scholes, J. D. & Banwart, S. A. Biofilm formation in environmental bacteria is influenced by different macromolecules depending on genus and species. Environ. Microbiol. 12, 2496–2507 (2010).
Jia, J., Xue, X., Guan, Y., Fan, X. & Wang, Z. Biofilm characteristics and transcriptomic profiling of Acinetobacter johnsonii defines signatures for planktonic and biofilm cells. Environ. Res. 213, 113714 (2022).
Maillet, A. et al. Characterization of bacterial communities of cold-smoked salmon during storage. Foods 10, 362 (2021).
Golmoradi Zadeh, R., Asgharzadeh, S., Darbandi, A., Aliramezani, A. & Masjedian Jazi, F. Characterization of bacteriocins produced by Lactobacillus species against adhesion and invasion of Listeria monocytogenes isolated from different samples. Microb. Pathog. 162, 105307 (2022).
Duffes, F., Corre, C., Leroi, F., Dousset, X. & Boyaval, P. Inhibition of Listeria monocytogenes by in situ produced and semipurified bacteriocins of carnobacterium spp. on Vacuum-packed, refrigerated cold-smoked salmon. J. Food Prot. 62, 1394–1403 (1999).
Rodríguez-López, P., Rodríguez-Herrera, J. J. & Cabo, M. L. Tracking bacteriome variation over time in Listeria monocytogenes-positive foci in food industry. Int. J. Food Microbiol. 315, 108439 (2020).
R Core Team. R: a language and environment for statistical computing. https://www.R-project.org/ (2024).
Posit team. RStudio: Integrated Development Environment for R. http://www.posit.co/ (2024).
Wickham, H. Ggplot2. (Springer International Publishing, Cham, 2016). https://doi.org/10.1007/978-3-319-24277-4.
Delignette-Muller, M. L. & Dutang, C. fitdistrplus: An R Package for Fitting Distributions. J. Stat Softw. 64, 1–34 (2015).
Beran, R. Prepivoting test statistics: a bootstrap view of asymptotic refinements. J. Am. Stat. Assoc. 83, 687–697 (1988).
Acknowledgements
This study was funded by the Spanish Ministry of Science and Innovation (ASEQURA, PID2019-10 8420RB-C31, ASEQURA2, PID2023-152192OB-C21) and the Spanish Research Council (PIE 202170E074). The funders played no role in study design, data collection, analysis and interpretation of data, or the writing of this manuscript. The authors thank S. Rodríguez Carrera and T. Blanco for their helpful support in all the experimental work.
Author information
Authors and Affiliations
Contributions
R.A.N. was responsible for the investigation, formal analysis, and writing of the original draft. J.J.R.H. provided conceptualisation and supervision, contributed to the writing of the original draft and was involved in funding acquisition. J.L.L.C. contributed to the methodology and performed the formal analysis. A.V. contributed to the methodology, performed the formal analysis, and was involved in funding acquisition. M.L.C. provided conceptualisation and supervision, contributed to the writing of the original draft and was involved in funding acquisition. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nogueira, R.A., Rodríguez-Herrera, J.J., López-Carmona, J.L. et al. A formal analysis of Listeria monocytogenes cross-contamination dynamics in multi-species biofilms. npj Sci Food 9, 168 (2025). https://doi.org/10.1038/s41538-025-00535-3
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41538-025-00535-3