Abstract
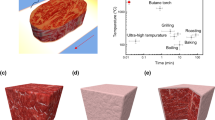
This study investigated the effects of combining sous vide (SV) and high-pressure (HP, 0.1–200 MPa) on the structural changes of meat proteins and the eating quality of pork loin in comparison to commercial cooking (CC). Differential scanning calorimetry (DSC) and Fourier transform infrared (FTIR) spectroscopy showed that SV under HP stabilized the secondary structure of sarcoplasmic proteins. SV under HP treatments significantly reduced cooking loss and improved water-retention ability. Texture profile analysis (TPA) showed that SV under HP reduced the hardness and chewiness while increasing springiness. Instrumental color analysis revealed that SV under 200 MPa resulted in higher L* and a* values with a lower b* value than SV-AT (p < 0.05). These findings demonstrated that SV cooking under HP was a promising cooking method for enhancing the eating quality of pork loin, potentially expanding the utilization of lean cuts, which are generally less favored by consumers.
Similar content being viewed by others
Introduction
Traditionally, meat has been valued as a rich source of dietary protein. However, in recent years, consumer preferences have increasingly shifted toward sensory attributes, healthfulness, practicality, and sustainability. Among these factors, tenderness plays a crucial role in consumer satisfaction and preference1. With rising global aging trends and growing interest in health-conscious diets, the importance of meat tenderization has gained increasing attention from nutritional, industrial, and social perspectives2,3. Pork is one of the most widely consumed meats worldwide, with various cuts used in diverse product development. Loin, known for its high protein and low fat content, holds strong nutritional appeal4. Nonetheless, the low intermuscular fat and high proportion (~70%) of type IIb fast-twitch glycolytic muscle fibers of the pork loin lead to greater cooking loss and a tougher texture than tenderloin, belly, and Boston butt5,6. To enhance the value and eating quality of this underutilized cut, novel thermal processing methods that improve tenderness and sensory attributes are warranted.
Sous vide (SV) is a cooking method where vacuum-sealed food is cooked at a precisely controlled temperature. SV conditions vary depending on the type of food, with meat and meat products typically processed at 50 °C to 85 °C for 2–48 h7. Low-temperature SV cooking (55–65 °C) offers several benefits for meat quality, including uniform internal heating, reduced cooking loss, oxidative stability, improved tenderness, and enhanced sensory attributes7,8,9. However, compared to commercial high-temperature short-time (HTST) pasteurization (70 °C for 2 min), low-temperature SV cooking has raised microbiological safety concerns. Nonetheless, previous studies have demonstrated that prolonged cooking times in SV cooking can achieve microbial lethality levels against Escherichia coli O157:H7, Listeria monocytogenes, and Salmonella spp., comparable to those achieved by the HTST method, effectively compensating for the lower temperatures used7,10.
Muscle fibers contract transversely between 40 °C and 60 °C, increasing the inter-fiber space and enhancing water retention. Moreover, the slow heating rate in SV cooking promotes partial protease activity and collagen solubilization initiation at approximately 55 °C, both of which contribute to the improved tenderness of the meat7,9,10. In contrast, longitudinal muscle fiber contraction and excessive collagen shrinkage under commercial HTST processing lead to reduced juiciness and tenderness10. Kim et al. reported that the juiciness and tenderness of SV-cooked meat depend on meat composition, with low-fat cuts being less tender than high-fat cuts11. Previous studies have shown that pork loins subjected to low-temperature SV cooking (50–65 °C for 3–24 h) exhibited around 15–25% cooking loss12,13. In particular, 55 °C for 24 h was found to minimize the cooking loss of pork loin among low-temperature SV conditions12. Although these findings indicate that SV-cooked pork loin generally has lower cooking loss and toughness than the control (HTST treatment), SV-induced moisture loss did not result in significant improvements in the sensorial acceptability of pork loins14.
Developing strategies to minimize moisture loss during SV cooking is essential for improving the juiciness and sensory qualities of lean cuts such as pork loin. High-pressure (HP) processing has been proposed as a potential method to reduce cooking loss in SV-cooked pork loins. Moderate pressurization (100–200 MPa) has been reported to improve protein hydration capacity, thereby enhancing water retention in cooked meat15,16. However, studies indicate that the combination of HP and SV may have no or even negative effects on cooking loss and meat tenderness. Kenesei et al. applied HP (300–600 MPa for 5 min) either before or after SV cooking (60 °C for 60 min) on pork loins and reported slight increases in cooking loss and hardness compared to the non-pressurized control, regardless of the processing sequence17. Similar results have been observed in studies on beef steaks and chicken breasts18,19, indicating that higher pressure levels tend to result in greater cooking loss and reduced tenderness. At extreme pressure levels (250–600 MPa), irreversible protein denaturation and aggregation likely occurred, diminishing any positive effects of SV cooking on juiciness and tenderness. Conversely, at relatively low pressures (<200 MPa), reversible protein dissociation may occur under HP20. When moderate pressure levels (100–200 MPa) are combined with simultaneous SV cooking, this condition could promote protein denaturation in a dissociated state, potentially enhancing meat tenderness20. However, few studies have explored the effects of SV cooking under HP on the physicochemical properties of meat. Although Ma and Ledward examined the textural properties of beef cooked under HP (200–800 MPa for 20 min at 20–70 °C)21, their studies primarily focused on protein structural changes induced by short-term HP combined with thermal treatment, offering limited insight into the effects of SV under HP processing. In comparison of fresh control (FC), commercial cooking (CC) and normal SV at atmospheric pressure (SV-AT), therefore, this study investigated the effects of SV cooking at 100 MPa (SV-100) and 200 MPa (SV-200) on the structural changes of meat proteins based on differential scanning calorimetry (DSC) and Fourier transform infrared (FTIR) spectroscopy and on quality characteristics of cooked pork loin including water-binding properties, texture profile analysis (TPA) and color.
Results and discussion
Thermal denaturation of meat proteins based on cooking methods
The maximum peak temperatures and enthalpies of cooked pork loins, estimated from DSC thermograms, are shown in Fig. 1. The FC exhibited three endothermic peaks corresponding to the denaturation of myosin (54.2 °C), sarcoplasmic and connective tissue proteins (62.9 °C), and actin (77.4 °C)6,22. These peaks were absent in the CC treatment, suggesting that the applied HTST conditions were sufficient to induce substantial thermal denaturation of the major meat proteins. In the SV-AT treatment, the first two peaks were not detected, while the actin peak remained at 78.0 °C. However, the enthalpy of the actin peak in SV-AT (0.12 J/g) was significantly lower than that of the actin peak in FC (0.35 J/g, P < 0.05). This suggests that prolonged low-temperature SV cooking influenced the partial unfolding of actin, a protein with relatively higher thermal stability than other meat proteins. Consistently, Hwang et al. demonstrated that the actin peak was detectable in pork loins cooked via SV below 55 °C but disappeared completely when the SV temperature reached 60 °C or higher12. Similarly, Dominguez-Hernandez et al. reported that prolonged holding times reduced the amount of native actin in beef and pork, even when the processing temperature remained below 60 °C23.
In contrast, several endothermic peaks absent in the SV-AT treatment were detected in SV cooking under HP treatment, although the peak temperatures varied depending on the applied pressure level. For SV-100 treatment, three peaks were observed at 56.3 °C, 68.7 °C, and 75.6 °C, whereas the SV-200 treatment exhibited only two major peaks at 61.3 °C and 66.3 °C. The results suggest that SV under HP can modify the thermal stability of meat proteins depending on the applied pressure level. Although pressure and temperature are key factors influencing protein structural changes, previous studies indicate that a phase diagram of protein supports the idea that moderate pressure (100–200 MPa) combined with mild heating (40–60 °C) can enhance protein thermal stability24. Pressure affects the tertiary and quaternary structures of proteins; nonetheless, recent studies suggest that pressure-induced volume changes may shift the folding equilibrium, potentially influencing the secondary structure25. The destabilization of tertiary and quaternary structures at HP of 100 MPa likely reduces the endothermic enthalpy of meat proteins compared to FC samples. Conversely, the stabilization of secondary structure may be associated with an increase in DSC peak temperature. The enthalpy observed in the SV-100 treatment may reflect the interaction between heat-induced disruption and pressure-mediated stabilization of hydrogen bonds. In contrast, the thermal stability of proteins under combined temperature and pressure conditions has been reported to be protein-specific. The thermal stabilities of globular proteins in the sarcoplasm and connective tissue proteins, stabilized by hydrogen bonds, were likely enhanced by SV cooking under 200 MPa26. Contrastingly, fibrous myofibrillar proteins underwent complete unfolding under the same conditions27. Therefore, the results suggest that SV and HP exert antagonistic effects on the thermal stability of sarcoplasmic proteins and collagen. This interaction may influence lysosomal protease activity or suppress the shrinkage of thermally denatured collagen11,26,28. Consequently, the typically undesirable eating quality of pork loin observed after CC may be improved by SV cooking combined with HP treatment.
Secondary structure of meat proteins based on cooking methods
The FC exhibited two peaks in the wavenumber range of 2800–3000 cm−1 and nine peaks in the range of 1000–1800 cm−1 (Fig. 2a). Among these, the peaks at 1638 cm−1 (amide I), 1530 cm−1 (amide II), and 1237 cm−1 (amide III) are associated with protein structures, while the remaining eight peaks correspond to lipids, fatty acids, cholesterol esters, phospholipids, and nucleic acids29. Changes in peak wavenumbers and peak intensities were observed across all cooked treatments, suggesting structural modifications in lipids and proteins of pork loin under cooking conditions. However, the structural changes in lipids induced by cooking methods are expected to have a minimal effect on the physicochemical properties of cooked loin owing to the low fat content of loin (<5%). Therefore, this study focused on a detailed analysis of FTIR spectra, with an emphasis on changes in protein structure.
The peak wavenumbers in the three amide spectra exhibited distinct changes depending on the cooking method. Compared to FC samples, the peak wavenumbers in the CC and SV-AT treatments shifted to lower values, whereas those in the SV cooking under HP treatment remained similar to the FC. This suggests that temperature and pressure exert different effects on the secondary structure of proteins. The relative proportions of secondary structural elements in meat proteins were analyzed using amide I spectra (Fig. 2b). In the FC, α-helix and β-sheet structures accounted for 74.5% of the secondary structural elements, while random coil structures comprised 9.5%. However, CC treatment led to a significant decrease in the proportion of α-helix, accompanied by an increase in β-sheet and random coil structures (P < 0.05). In addition, no change in β-turn structure between FC and CC was observed. Zhou et al. analyzed the Raman spectra of myofibrillar proteins heated from 70 °C to 120 °C and observed a similar pattern of protein secondary structure changes as found in the present study30. The authors suggested that the relative proportion of α-helix structures decreased owing to the unfolding of meat proteins, leading to the exposure of nonpolar residues. Moreover, the proportion of β-sheet structures gradually increased with temperature, likely due to thermal aggregation and restructuring of unfolded proteins. In contrast, Zhou et al. also reported that the proportion of random coils increased up to 100 °C but remained unchanged at higher temperatures30.
In the SV-AT treatment, the changes in protein secondary structure were similar to those observed in the CC treatment. Nonetheless, the proportion of β-sheet structures was lower in the SV-AT treatment than in the CC treatment, while the proportion of random coils was the highest in the SV-AT treatment among all treatments (P < 0.05). This suggests that the lower temperature used in the SV treatment had a limited impact on protein aggregation than the higher temperature applied in the CC treatment. The proportion of β-sheet structures in the SV cooking under HP treatment remained unchanged compared to that in the SV-AT treatment. However, the proportion of random coils decreased as the pressure increased, leading to a corresponding increase in the proportion of α-helix structures (P < 0.05). The proportion of β-turn structure in SV-cooked pork loins tended to increase with higher pressure; nevertheless, the differences in β-turn structure among treatments were relatively minor compared to the changes seen in other secondary structural elements. These findings are likely related to the DSC patterns observed in the SV cooking under HP treatment, suggesting that HP contributed to the stabilization of α-helix structures. Similarly, Okuno et al. observed that the β-sheet structure of albumin completely disappeared at 130 MPa of HP without cooking, likely due to the inhibition of protein aggregation31. Although this study did not perform a qualitative analysis of each protein fraction affected by HP, which warranted further exploration, it is likely that HP influenced the thermal properties of collagen and sarcoplasmic proteins, with hydrogen bonding playing a key role in their structural stabilization11,26,28. These results provide complementary evidence to the thermal properties observed in the DSC analysis, supporting the idea that SV cooking under HP induces distinct effects on protein structural stability and physicochemical characteristics compared to CC or SV-AT treatment.
Water-binding properties of cooked pork loin
The cooking loss of SV-AT treatment was 26.5%, which was significantly higher than that of CC (21.4%, P < 0.05, Fig. 3a). Hwang et al. applied similar cooking conditions to 10-mm pork loin steaks and reported that the cooking loss of SV-cooked pork loin (19.8%) was lower than that of CC (32.6%)12. Compared to the results of the present study, these findings suggest that the cooking loss of pork loin is influenced by sample size (thickness). Nevertheless, these findings indicate that SV cooking resulted in a cooking loss of pork loins exceeding 20%. In contrast, pork loins treated with SV under HP showed a significant decrease in cooking loss as the pressure increased (P < 0.05), reaching 6.58% at 200 MPa. These results suggest that SV under HP is an effective cooking method for minimizing cooking loss in pork loin.
The water-holding capacity of cooked meat is closely related to the physical state of myofibrils. According to the review of Tornberg22, transverse shrinkage of myofibrils occurs between 40 °C and 60 °C, causing the expansion of the gap spaces between muscle fibers and endomysium. The longitudinal shrinkage of muscle fibers begins at 40–50 °C but becomes dominant at 60–70 °C. The transverse shrinkage contributes to less moisture loss in SV-cooked meat, whereas excessive longitudinal shrinkage leads to greater moisture loss in CC treatment. A similar pattern has been observed in myofibrils exposed to HP (40–70 MPa), where sarcomere length decreases under pressure32. These microstructural changes in the sarcomere may not only facilitate physical entrapment of water within myofibrillar gaps but also be linked to the molecular stabilization of proteins under pressure. As observed in the DSC and FTIR analyses, HP contributed to the stabilization of meat proteins, likely allowing them to retain water more effectively during thermal processing, thereby enhancing the overall water retention ability. Kim et al. reported that the proportion of intra-myofibrillar water in HP-treated pork loins increased, with no changes observed in bound water or extra-myofibrillar water, based on transverse relaxation time (T2) analysis using nuclear magnetic resonance33. These myofibrillar changes likely explain the improved water-binding property of HP-treated meat15,16. However, the limited or negligible impact of HP on the cooking loss of SV-cooked pork loins reported in previous studies may be attributed to the short holding times (<20 min) and the application of HP as a separate pretreatment prior to SV cooking17,18,19.
Additionally, the expressible moisture of all cooked treatments ranged from 12.8% to 18.7%, which was significantly higher than that of FC (5.7%, P < 0.05, Fig. 3b). Expressible moisture is an indicator of sensorial juiciness and is associated with intramuscular lipid and moisture content34,35. From this perspective, cooked pork loins exhibited a tough texture after cooking, potentially leading to a negative eating quality. Among the cooked treatments, the expressible moisture of SV-AT did not differ significantly from that of CC. However, expressible moisture significantly increased with pressure, with the SV-200 treatment showing the highest expressible moisture (18.7%) among all cooked treatments (P < 0.05). This result aligns with the moisture content of cooked treatments, suggesting that SV under HP effectively minimized moisture loss, enhanced juiciness, and thereby advanced the eating quality of cooked meat. Therefore, this study suggests that SV cooking under HP is a promising cooking technique with the potential to increase consumer acceptance of lean meats such as pork loin.
Texture profiles of cooked pork loin
The texture profiles of pork loins subjected to different cooking conditions are shown in Fig. 4. The hardness and springiness of SV-AT treatment were significantly lower than those of CC treatment (P < 0.05), whereas cohesiveness showed no significant difference between SV-AT and CC treatments. These textural traits led to a 42.6% reduction in chewiness for pork loins subjected to the SV-AT (11.6 mJ) compared to CC treatment (20.2 mJ, P < 0.05). A similar pattern of change in TPA was reported in SV-cooked pork ham (61 °C for 90 min) when compared with pork ham subjected to the boiling method36, supporting the idea that SV cooking improves meat tenderness compared to the CC treatment12. The thermal shrinkage of connective tissue and thermal denaturation of actin likely contribute to the toughness observed in samples subjected to the CC treatment, explaining the textural differences between the CC and SV-AT treatments6,22. It should be noted that 70-mm thick loin steaks were used in this study. According to Baldwin10, heating a 65-mm steak (assumed to be in a slab) to the target SV temperature requires approximately 5.5 h, significantly longer than the 19 min needed for a 10-mm steak. This extended heating time may provide sufficient time for endogenous protease activity before thermal denaturation37, potentially serving as a key factor in the lower hardness and chewiness observed in samples subjected to the SV-AT treatment compared to those subjected to the CC treatment.
Alternatively, meat samples subjected to SV cooking under HP exhibited distinct TPA characteristics, including lower hardness but higher cohesiveness and springiness than samples subjected to the CC and SV-AT treatments (P < 0.05). These textural changes became more pronounced as pressure increased, with SV-200 showing a 50.4% reduction in chewiness (5.7 mJ) compared to that observed with the SV-AT treatment (P < 0.05). Kim et al. reported that HP treatment (200 MPa for 15 min at 4 °C) resulted in a tough texture in pork loin, and this pressure-induced toughness persisted during 2 weeks of chilled storage (4 °C)33. The authors suggested that HP-mediated toughness was likely due to changes in myofibril density. However, the effect of HP on meat texture may depend on the applied temperature, as HP-induced toughness has been observed primarily at low temperatures (below 4 °C)33. In contrast, at higher temperature ranges, HP is more likely to alter the protein structure of meat differently38. In this study, the improved tenderness observed in samples subjected to the SV cooking under HP may be attributed to the prolonged application of HP combined with SV, which likely enhanced the activity of endogenous muscle enzymes and consequently reduced actomyosin-related toughness compared to that in samples subjected to the SV-AT treatment. Although research suggests that moderate pressurization combined with mild thermal processing can increase the activity of lysosomal proteases26, the effects of prolonged HP treatment (12–24 h) on muscle proteases, such as calpain and cathepsins, remain largely unexplored. Moreover, recent studies report that HP incubation (100–300 MPa for 5–48 h at 37–50 °C) can enhance the activities of various hydrolytic enzymes, including trypsin, α-chymotrypsin, and cellulase39,40. Furthermore, Iwasaki et al. found that moderate pressure (~200 MPa) improved the solubility of chicken muscle proteins by dissociating the thick and thin filaments41. After cooking, the myofibrils in HP-treated chicken appeared highly dispersed, in contrast to the thin, filamentous structure observed in the unpressurized control. The authors reported that these structural characteristics contributed to the increased apparent elasticity of HP-treated samples. These findings demonstrate that SV cooking under HP can enhance the overall palatability of pork loin by simultaneously improving textural properties and water retention. These synergistic effects likely stem from HP-induced structural stabilization of muscle proteins and the preservation of endogenous enzymatic activity during the prolonged thermal process.
Appearance and color of cooked pork loin
The visual appearance and instrumental color parameters of all treatments are shown in Fig. 5. Compared to the FC, the pork loins subjected to CC treatment exhibited a typical well-done appearance, characterized by a relatively dry, brownish surface. In contrast, SV-cooked pork loins appeared brighter and retained a moist surface, resulting in a distinct appearance suggestive of being undercooked. This rare-like color has been consistently reported in pork subjected to low-temperature cooking23. Among the SV treatments, pork subjected to SV cooking under HP exhibited a more pronounced pink color than that subjected to the SV-AT treatment. Unlike beef, which can be served at various levels of doneness, pork is traditionally cooked to a well-done state, reflecting concerns over microbiological safety and consumer anxiety regarding parasitic infections. However, applying HP (>200 MPa for 10 min) can completely inactivate Trichinella spiralis, a parasite commonly found in pork42, thereby making it possible to offer pork at different degrees of doneness when combined with low-temperature SV cooking. The rare-like appearance of pork subjected to SV treatments may positively influence consumer sensory preference. Honegger et al. reported that pork chops cooked using SV at 63 °C were preferred by consumers over those cooked at 71 °C, attributing this preference to the perceived greater tenderness and juiciness43.
Based on color analysis, meat samples from all cooked treatments exhibited higher L* values than the FC, with SV-cooked samples showing significantly higher L* values than those subjected to the CC treatment (P < 0.05). The lightness of cooked meat is influenced by factors such as protein denaturation and water-holding capacity. The highest L* value observed in the SV under HP treatment appears to be associated with the higher moisture content and greater expressible moisture of the pork loins. In contrast, thermal processing caused a steep decrease in a* values of pork loin; the b* values of pork loin increased (P < 0.05), a change commonly attributed to the denaturation of pigment proteins, particularly myoglobin denaturation. In this study, the a* values of samples subjected to the SV-AT and SV-100 treatments were slightly lower than those of samples subjected to the CC and SV-200 treatments (P < 0.05). The b* value was highest in CC, followed by SV-AT treatment, and it gradually decreased with increasing pressure in SV-treated samples (P < 0.05). These results suggest that the color attributes of cooked pork loin are influenced by the structural stabilization of sarcoplasmic proteins induced by HP. Furthermore, the pinking effect, commonly observed in meat applied to moderate HP, may occur during low-temperature SV cooking. Overall, these findings highlight the potential of SV cooking under HP as a promising thermal processing method capable of enhancing both the eating quality and visual appearance of pork loin, thereby increasing consumer acceptability.
In conclusion, the findings of this study demonstrate that SV cooking under HP not only reduces the excessive cooking loss typically observed in atmospheric SV of pork loin but also enhances tenderness and imparts a rare-like appearance characteristic of low-temperature thermal processing, which would positively influence consumer preference. These combined effects suggest that SV cooking under HP has the potential to significantly enhance the value of lean pork loin compared to conventional thermal processing methods. Although further research is needed to develop pilot-scale systems for the industrial application of the SV cooking under the HP technique proposed in this study, recent global advancements in HP technology support its potential as an upcycling technology for various underutilized pork cuts beyond pork loin.
Methods
Materials
Three pork loins (M. longissimus thoracis et lumborum), from 10-month-old crossbred hogs (Landrace × Yorkshire × Duroc), were randomly purchased per batch at 48 h post-mortem from a local market (Seoul, Korea). External fat and connective tissue were manually removed, and each loin was sliced into five thick pieces (7 cm in thickness). The slices were divided into five groups, with three slices per group. After weighing, each slice was vacuum-packed in a poly-nylon pouch and subjected to thermal processing. To ensure experimental replication, each batch was purchased and cooked on three separate days.
SV cooking under HP
For SV-AT, one group was randomly selected and cooked in a water bath at 55 °C for 24 h. Two additional groups were randomly selected for SV-100 and SV-200 treatments and subjected to SV under 100 MPa and 200 MPa, respectively. A laboratory-installed HP device, as described by Kim et al., was used for SV under HP conditions33. A thermostatic jacket was externally mounted on the pressure vessel (2 L working volume), with hot water circulation maintaining the internal temperature at 55 °C (Fig. 6). Each group was placed in the vessel and pressurized to either 100 MPa or 200 MPa at a rate of 2.4 MPa/s. Once the target pressure was reached, SV cooking was performed for 24 h under constant pressure. After cooking, the vessel was depressurized to atmospheric pressure at a rate of 24 MPa/s, and the samples were collected for analysis. Another group was randomly immersed in a water bath set at 75 °C for 30 min and served as CC. The final group remained uncooked as the FC.
Water-binding properties
All sample slices were tempered at ambient temperature (~20 °C) for 30 min before being removed from their packaging. Surface exudates were gently blotted using tissue paper, and the samples were weighed. Cooking loss for each treatment was calculated based on the percentage of weight change before and after cooking, using three slices per treatment (n = 9; 3 batches × 3 slices). Expressible moisture was measured according to the method described by Kim et al.33. A 1 g sample, taken in duplicate from each slice, was placed in a centrifuge tube lined with gauze to absorb released moisture. After centrifugation at 3000×g for 15 min at ambient temperature, the pellet was carefully removed, and the tube with gauze was weighed. The tubes were then dried at 105 °C for 24 h and weighed again. Expressible moisture was calculated as the percentage of moisture loss relative to the initial sample weight (n = 18; 3 batches × 3 slices × 2 replications).
Instrumental color
All slices were placed at ambient temperature for 10 min, and the CIE color of pork loins was measured using a color reader (CR-400, Konica-Minolta Sensing Inc., Tokyo, Japan) equipped with a D65 illuminant, a CIE 2° standard observer, and an 8 mm aperture. The device was calibrated using a standard white tile. CIE L* (lightness), a* (redness), and b* (yellowness) values were recorded at three randomly selected surface positions on each slice (n = 27; 3 batches × 3 slices × 3 replications).
Differential scanning calorimetry
The thermal behavior of meat proteins was analyzed using a differential scanning calorimeter (200F3, Netzsch GmbH, Selb, Germany) at the Biopolymer Research Center for Advanced Materials (NFEC-2023-07-289071; Seoul, Korea) following the method of Kim et al.24 Approximately 25 mg of each sample was randomly selected, hermetically sealed in an aluminum pan, and subjected to thermal scanning from 20 °C to 95 °C at a rate of 5 °C/min, with an empty pan used as the reference. Thermal transitions were analyzed using Proteus software (Netzsch GmbH) to determine endothermic peak temperatures and enthalpy changes in each treatment (n = 9; 3 batches × 3 slices).
FTIR spectroscopy
The FTIR spectrum of each treatment was obtained using an FTIR spectrometer (Nicolet iS20, Thermo Fisher Scientific Inc., Waltham, MA, USA) following the method of Kim et al.11. Approximately 1 mm cube was sampled in duplicate from a random position on each slice and placed on an attenuated total reflectance crystal (Smart iTX, Thermo Fisher Scientific Inc.), equilibrated at 25 °C. FTIR spectra were recorded with 32 accumulated scans across a wavenumber range of 400–4000 cm−1, using a resolution setting of 4 cm−1. Heat-induced structural changes in proteins were analyzed by second derivative analysis of the amide I region (1580–1710 cm−1) using PeakFit software (ver. 4.12, Systat Software Inc., Palo Alto, CA, USA) based on the method of Candoğan et al. (n = 18; 3 batches × 3 slices × 2 replications)29.
Texture profile analysis
From each slice, six cubes (1 cm × 1 cm × 1 cm) were sampled, aligned parallel to the muscle fiber direction, and subjected to TPA using a texture analyzer (CT3, Brookfield Engineering Laboratories Inc., Middleboro, MA, USA) equipped with a cylindrical probe (38.1 mm in diameter). Each cube underwent double compression, perpendicular to the fiber direction, with a trigger load of 5 gf, compression to 70% of its original height, and a compression speed of 1 mm/s. The texture parameters, including hardness, cohesiveness, springiness, and chewiness, were assessed (n = 54; 3 batches × 3 slices × 6 replications).
Statistical analysis
A randomized complete block design was used to evaluate the main effect of cooking conditions. Data from three independent experimental replicates were analyzed by one-way analysis of variance (ANOVA) using SPSS software (ver. 24, IBM, Armonk, NY, USA). Results were expressed as mean ± standard deviation (SD), and significant differences among means were determined using Duncan’s multiple range test (P < 0.05).
Data availability
All data generated or analyzed during this study are included in this published article.
References
Warner, R. D. et al. Meat tenderness: advances in biology, biochemistry, molecular mechanisms and new technologies. Meat Sci. 185, 108657 (2022).
Padeiro, M., Santana, P. & Grant, M. Global aging and health determinants in a changing world (ed Oliveira, P. J. & Malva, J. O.) pp 3–30 (Academic Press, 2023).
Yoon, D. Y., Lee, K. Y. & Lee, H. G. Effect of ultrasound-assisted treatment on meat tenderization for elderly individuals. Food Sci. Biotechnol. 33, 3029–3036 (2024).
Vicente, F. & Pereira, P. C. Pork meat composition and health: a review of the evidence. Foods 13, 1905 (2024).
Lee, Y. E. et al. Analysis on difference of consumer’s evaluation on visual features of pork cuts. J. Anim. Sci. Technol. 63, 614–625 (2021).
LeMaster, M. N. et al. Impact of cooking temperature on pork longissimus, and muscle fibre type, on quality traits and protein denaturation of four pork muscles. Meat Sci. 209, 109395 (2024).
Latoch, A., Gluchowski, A. & Czarniecka-Skubina, E. Sous-vide as an alternative method of cooking to improve the quality of meat: a review. Foods 12, 3110 (2023).
Pang, Z., Lee, J. W., Lee, Y. & Moon, B. K. Changes in quality characteristics and biogenic amine contents in beef by cooking methods. Food Sci. Biotechnol. 33, 2313–2321 (2024).
Thathsarani, A. P. K., Alahakoon, A. U. & Liyanage, R. Current status and future trends of sous vide processing in meat industry; a review. Trend Food Sci. Technol. 129, 353–363 (2022).
Baldwin, D. E. Sous vide cooking: a review. Int. J. Gastron. Food Sci. 1, 15–30 (2012).
Kim, M. et al. Impact of sous-vide cooking on quality attributes of high-fat and low-fat cuts of beef, pork, and chicken. Food Sci. Anim. Resour. https://doi.org/10.5851/kosfa.2025.e21 (2025).
Hwang, S. I., Lee, E. J. & Hong, G. P. Effects of temperature and time on the cookery properties of sous-vide processed pork loin. Food Sci. Anim. Resour. 39, 65–72 (2019).
Kurp, L. & Danowska-Oziewicz, M. Quality of pork loin subjected to different temperature-time combinations of sous vide cooking. Appl. Sci. 14, 9562 (2024).
Kurp, L., Bielecka, M. & Danowska-Oziewicz, M. Quality of sous vide-cooked pork loin stored in refrigerated conditions. Appl. Sci. 15, 850 (2025).
Ji, L. et al. Advances and applications in water retention technology for meat and meat products: status and future research directions. Int. J. Food Sci. Technol. 59, 2823–2836 (2024).
Yoon, Y., Lee, M. Y., Lee, S. Y. & Hong, G. P. Effects of high-pressure, sous-vide cooking and commercial freezing on the physicochemical properties of moisture-enhanced restructured pork. Food Sci. Anim. Resour. https://doi.org/10.5851/kosfa.2024.e136 (2025).
Kenesei, G., Kiskó, G. & Dalmadi, I. Combined sous-vide and high hydrostatic pressure treatment of pork: is the order of application decisive when using minimal processing technologies?. Appl. Sci. 14, 3583 (2024).
Sun, S., Rasmussen, F. D., Cavender, G. A. & Sullivan, G. A. Texture, color and sensory evaluation of sous-vide cooked beef steaks processed using high pressure processing as method of microbial control. LWT Food Sci. Technol. 103, 169–177 (2019).
Chen, Y. A., Sheen, S. & Hsu, H. Y. Combined effects of high pressure processing and sous-vide cooking on the tenderization of proteolytic enzyme-injected chicken breast. LWT Food Sci. Technol. 202, 116213 (2024).
Baldelli, A. et al. Effect of high-pressure on protein structure, refolding, and crystallization. Food Chem. Adv. 5, 100741 (2024).
Ma, H. J. & Ledward, D. A. High pressure/thermal treatment effects on the texture of beef muscle. Meat Sci. 68, 347–355 (2004).
Tornberg, E. Effects of heat on meat proteins—īmplications on structure and quality of meat products. Meat Sci. 70, 493–508 (2005).
Dominguez-Hernandez, E., Salaseviciene, A. & Ertbjerg, P. Low-temperature long-time cooking of meat: eating quality and underlying mechanisms. Meat Sci. 143, 104–113 (2018).
Dave, K. & Gruebele, M. Fast-folding proteins under stress. Cell. Mol. Life Sci. 72, 4273–4285 (2015).
Makarov, A., LoBrutto, R. & Karpinski, P. Effect of pressure on secondary structure of proteins under ultra high pressure liquid chromatographic conditions. J. Chromatogr. A 1318, 112–121 (2013).
Buckow, R., Sikes, A. & Tume, R. Effect of high pressure on physicochemical properties of meat. Crit. Rev. Food Sci. Nutr. 53, 7709–7786 (2013).
Chen, X. et al. Structural modification by high-pressure homogenization for improved functional properties of freeze-dried myofibrillar protein powder. Food Res. Int. 100, 193–200 (2017).
Potekhin, S. A., Senin, A. A., Abdurakhmanov, N. N. & Tiktopulo, E. I. High pressure stabilization of collagen structure. Biochim. Biophys. Acta 1794, 1151–1158 (2009).
Candoğan, K., Altuntas, E. G. & İğci, N. Authentication and quality assessment of meat products by Fourier-transform infrared (FTIR) spectroscopy. Food Eng. Rev. 13, 66–91 (2021).
Zhou, C. Y. et al. Evaluation of the secondary structure and digestibility of myofibrillar proteins in cooked ham. CyTA J. Food 17, 78–86 (2019).
Okuno, A., Kato, M. & Taniguchi, Y. Pressure effects on the heat-induced aggregation of equine serum albumin by FT-IR spectroscopic study: secondary structure, kinetic and thermodynamic properties. Biochim. Biophys. Acta 1774, 652–660 (2007).
Shintani, S. A. Effects of high-pressure treatment on the structure: and function of myofibrils. Biophys. Physicobiol. 18, 85–95 (2021).
Kim, H. et al. Effect of high pressure pretreatment on the inhibition of ice nucleation and biochemical changes in pork loins during supercooling preservation. Meat Sci. 208, 109393 (2024).
Schönfeldt, H. C. et al. Cooking- and juiciness-related quality characteristics of goat and sheep meat. Meat Sci. 34, 381–394 (1993).
Xu, S. & Falsafi, S. R. Juiciness of meat, meat products, and meat analogues: Definition, evaluation methods, and influencing factors. Food Rev. Int. 40, 2344–2377 (2024).
Jeong, K., O, H., Shin, S. Y. & Kim, Y. S. Effects of sous-vide method at different temperatures, times and vacuum degrees on the quality, structural, and microbial properties of pork ham. Meat Sci. 143, 1–7 (2018).
Vaskoska, R. et al. Myosin sensitivity to thermal denaturation explains differences in water loss and shrinkage during cooking in muscles of distinct fibre types. Meat Sci. 179, 108521 (2021).
Sun, X. & Holley, R. A. High hydrostatic pressure effects on the texture of meat and meat products. J. Food Sci. 75, R17–R23 (2010).
Kim, N., Maeng, J. S. & Kim, C. T. Effects of medium high pressure treatments on protease activity. Food Sci. Biotechnol. 22, 289–294 (2013).
Son, C. G., Lee, J. W., Lee, M. Y. & Hong, G. P. Conversion of rice husks into antioxidative and prebiotic biomaterials using subcritical water pretreatment and cellulase incubation under high-pressure. Food Sci. Biotechnol. 34, 1581–1588 (2025).
Iwasaki, T. et al. Studies of the effect of hydrostatic pressure pretreatment on thermal gelation of chicken myofibrils and pork meat patty. Food Chem. 95, 474–483 (2006).
Porto-Fett, A. C. S. et al. Evaluation of fermentation, drying, and/or high pressure processing on viability of Listeria monocytogenes, Escherichia coli O157:H7, Salmonella spp., and Trichinella spiralis in raw pork and Genoa salami. Int. J. Food Microbiol. 140, 61–75 (2010).
Honegger, L. T. et al. The effect of cooking method and cooked color on consumer acceptability of boneless pork chops. Foods 11, 106 (2022).
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant No. RS-2022-NR070874) and by the Ministry of Science and ICT (grant No. RS-2024-00341861).
Author information
Authors and Affiliations
Contributions
Y.Y. and S.Y.L. conducted formal analyses, study design, and data interpretation and drafted and edited the paper. S.J. and S.L. analyzed and interpreted the FTIR and NMR data and drafted and edited the paper. G.P.H. aided in the conceptualization of the work, supervised, edited the paper, and acquired funding for this work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yoon, Y., Lee, S., Jeong, S. et al. Effect of low-temperature sous vide under high-pressure on the moisture retention, tenderness, and color of pork loins. npj Sci Food 9, 189 (2025). https://doi.org/10.1038/s41538-025-00560-2
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41538-025-00560-2