Abstract
Emerging evidence suggests that maternal nutrition plays a critical role in fetal development and pregnancy outcomes. This study explores the epigenetic link between maternal nutrition and preterm birth (PTB) by analyzing DNA methylation (DNAm) in placental and cord blood samples from PTB and full-term pregnancies among Karen and Burmese populations in Myanmar and Thailand. Mothers who experienced PTB exhibited significantly lower intake of several nutrients, especially protein. DNAm profiling revealed hypomethylation of the LIPF promoter in placenta and hypermethylation of the SSB promoter in cord blood, with corresponding downregulation of SSB gene expression. Gene ontology analysis highlighted PTB-specific enrichment in inflammatory, developmental, and metabolic pathways, with cord blood notably enriched in genes involved in “embryo development ending in birth.” Low protein intake correlated with SSB hypermethylation and differential methylation of IGKV1D-39. These findings provide novel insight into how protein deficiency may epigenetically predispose to PTB and suggest potential biomarkers for early detection and intervention. The clinical trial was registered on ClinicalTrials.gov registry, under NCT02797327, on 13-06-2016.
Similar content being viewed by others
Introduction
Preterm birth (PTB), also known as premature birth, is a significant global health concern that refers to the birth of a baby before 37 weeks of gestation has been completed1. Each year, PTB affects approximately 15 million newborns worldwide, making it a critical area of focus in maternal and neonatal health research2. The health implications associated with PTB are profound and far-reaching, with preterm infants, often referred to as “preemies”, facing higher risks of morbidity and mortality during both the neonatal period and infancy when compared to full-term infants3. PTB occurrence and etiology can significantly vary among various racial and ethnic populations and could be dependent on diverse geographic regions2.
Among the different factors influencing gestation length, maternal nutrition has surfaced as a critical area of research4. Maternal nutrition is important for fetal development and significantly influences pregnancy outcomes5,6,7. The Barker Hypothesis, also known as the Developmental Origins of Health and Disease (DOHaD) hypothesis, proposes that environmental factors, particularly nutrition during prenatal and early postnatal development, can have long-lasting effects on an individual’s health and susceptibility to diseases later in life8. A study has shown that maternal undernutrition, especially during early pregnancy, can disrupt placental development, potentially leading to adverse outcomes such as PTB4.
During pregnancy, DNA methylation (DNAm) patterns undergo profound, tissue-specific shifts to meet the evolving demands of fetal development, maternal metabolism, and immune regulation, ensuring a supportive environment for fetal growth9. Placental and cord blood methylation changes impact nutrient transport, hormonal signaling, and immune interactions between mother and fetus10.
The famine studies, particularly the one stemming from the Dutch Hunger Winter that took place near the end of World War II (1944–1945)11, were fundamental in establishing the connection between maternal undernutrition and adverse fetal developmental outcomes, introducing the idea that inadequate maternal nutrition during critical periods of fetal development could result in persistent changes to DNAm patterns and disease susceptibility across the lifespan12. Heijmans et al. provided compelling evidence of how early-life environmental conditions, specifically nutrition, can induce lasting epigenetic changes in humans13. Their study demonstrated that amino acid deprivation, such as low methionine intake during pregnancy, was associated with hypomethylation of IGF2 in whole blood, detectable even six decades later.
Macro- and micronutrient-driven changes in DNAm may impact critical biological functions, leading to increased PTB risk14. Key nutrients like folate, choline, methionine, vitamins B6 and B12, and other methyl donors are required to maintain the pool of S-adenosylmethionine (SAM), the universal methyl donor for methylation15,16. Nutritional deficiencies, particularly in folate, can cause global hypomethylation17, potentially affecting genes related to fetal development and placental functions18. Animal studies have demonstrated that a low-protein diet fed to rat dams was associated with hypermethylation of cytosine residues in the DNA of fetal liver and significantly lower fetal weight compared to controls19,20.
Despite the well-established importance of maternal nutrition, discrepancies persist among studies exploring the link between specific nutrient intake during pregnancy and PTB risk and their epigenetic effect at the fetal level. While some findings suggest that supplementation may lower PTB risk, others reveal no significant associations21. Moreover, previous research has faced several limitations in establishing the exact DNAm changes in both the placenta and cord blood associated with PTB. The latter was attributed to inconsistent methodologies, insufficient control of confounding factors, and a lack of comprehensive clinical data, all of which contribute to varying and sometimes contradictory findings. Thus, to identify the exact nutrient involved in DNAm alterations underlying PTB, our study aims to investigate whether maternal dietary intake, particularly specific nutrients, can explain DNAm changes in the placenta and cord blood in PTB-affected mothers compared to controls.
Results
Characteristics of study subjects and pregnancy outcomes
The study included 48 maternal-infant dyads: 18 PTB vs 30 controls, respectively. Maternal and neonatal demographic and clinical outcomes are described in Table 1. All deliveries in both groups were conducted vaginally, except for one participant in the control group who underwent a cesarean section. None of the recruited pregnant women reported a history of smoking during the prenatal period. All subjects were confirmed to have normal glucose-6-phosphate dehydrogenase (G6PD) levels and tested negative for HIV.
No significant differences were observed between the PTB and control groups in terms of maternal age, weight, height, BMI, pulse, and respiratory rates at conception, systolic and diastolic blood pressure, ethnicity, gravidity, or basic literacy skills (reading and writing). However, prenatal antibiotic use was significantly higher in PTB-affected mothers compared to controls, with the major difference at delivery. No statistically significant differences in antibiotic exposure were shown during the first, second, and third trimesters (chi-square, p > 0.05) (Table 1).
Concerning the neonates, statistically significant differences were observed, as expected, between the PTB and control groups in several parameters: gestational age (days), birth weight (kg), length at birth (cm), and head circumference (cm). No significant differences were detected in the gender distribution between the groups (Table 1). All subjects were supplemented with folic acid, as per the guidelines, with prophylactic folic acid (5 mg per week). If clinically required, vitamin C, B1, and B12 were prescribed with the same distribution between the two groups (Supplementary Fig. 1).
Profound nutritional deficits characterize mothers with PTB
We thoroughly investigated the differences in dietary intake components between PTB cases and controls. All observed differences demonstrated that mothers who experienced PTB had a notably lower kilocalories (KCal) intake (Fig. 1A) and multiple macro- and micronutrients. The main differences in macronutrients were in protein (g) (Fig. 1B), carbohydrate (g) (Fig. 1C), available carbohydrate (g) (Fig. 1D), lactose (g) (Fig. 1E), and total dietary fiber (g) (Fig. 1F). The main differences in micronutrients were in vitamins, minerals, phytonutrients, and fatty acids, detailed as follows: Vitamins: vitamin D (IU) (Fig. 2A), vitamin E (IU) (Fig. 2B), vitamin K (mcg) (Fig. 2C), vitamin A (IU) (Fig. 2D), pyridoxine (Vitamin B6) (mg) (Fig. 2E), riboflavin (vitamin B2) (mg) (Fig. 2F), thiamin (vitamin B1) (mg) (Fig. 2G), cobalamin (vitamin B12) (mcg) (Fig. 2H), total folate (vitamin B9) (mcg) (Fig. 2I).
The figure shows the significant differences in dietary intake of kilocalories (KCal) and various macronutrients with a consistently higher intake of all components in the control group compared to PTB (median ± IQR): A KCal, B protein (g), C carbohydrate (g), D available carbohydrate (g), E lactose (g), and F dietary fiber (total) (g). Error bars represent the IQR, with statistical significance determined by the Mann–Whitney U test, **p < 0.01; ***p < 0.001. PTB preterm birth, KCal kilocalories, g grams.
The figure shows the significant differences in dietary intake of vitamins with a consistently higher intake of all vitamins in the control group compared to PTB (median ± IQR): A vitamin D (IU), B vitamin E (IU), C vitamin K (mcg), D vitamin A (IU), E pyridoxine (vitamin B6) (mg), F riboflavin (vitamin B2) (mg), G thiamin (vitamin B1) (mg), H cobalamin (vitamin B12) (mcg), I total folate (vitamin B9) (mcg). Error bars represent the IQR, with statistical significance determined by the Mann–Whitney U test,*p < 0.05; **p < 0.01; ***p < 0.001. PTB preterm birth, IU international units, mg milligrams, mcg micrograms.
Different minerals, phytonutrients, and fatty acids include: calcium (mg) (Fig. 3A), potassium (mg) (Fig. 3B), iron (mg) (Fig. 3C), iodine (mcg) (Fig. 3D), beta-carotene (mcg) (Fig. 3E), phosphorus (mg) (Fig. 3F), magnesium (mg) (Fig. 3G), manganese (mg) (Fig. 3H), copper (mg) (Fig. 3I), and PFA 22:6, DHA (g) (Fig. 3J).
The control group showed higher intake in all the presented dietary components compared to PTB group (median ± IQR): A calcium intake (mg), B potassium (mg), C iron (mg), D iodine (mcg), E beta-carotene (mcg), F phosphorus (mg), G magnesium (mg), H manganese (mg), I copper (mg), J PFA 22:6, DHA (g). Error bars represent the IQR, with statistical significance determined by the Mann–Whitney U test,*p < 0.05; **p < 0.01; ***p < 0.001. PTB preterm birth, g grams, mg milligrams, mcg micrograms, PFA 22:6, DHA: polyunsaturated fatty acid 22: 6, docosahexaenoic acid.
Placenta and cord blood differential methylation profiles: Perturbations in PTB
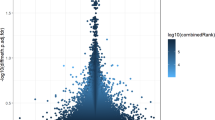
First, an exploratory analysis was conducted to examine the general trends in DNAm associated with PTB in placental samples. The scatterplot analysis to explore the DNAm patterns in the placenta revealed a potential hypomethylation trend in the PTB group versus the controls at genes (Fig. 4A) and promoters (Fig. 4B) levels assessed by the top 500 most differentially methylated regions; however, the trend was less obvious at the CpG sites level (top 10000 most differentially methylated CpG sites) (Fig. 4C). We then performed a deep analysis focusing on the 1000 most differentially methylated promoters (based on combined rank among the 1000 best-ranking regions) to confirm the differential methylation profile between the PTB group and controls. We observed 606 hypomethylated promoters in the PTB group versus 394 that were hypomethylated in the controls (Supplementary Data 1). We delved further into the analysis to explore the most differentially methylated promoter according to FDR-adjusted p-value, and we identified only one differentially methylated promoter corresponding to lipase F, gastric type (LIPF) (ID: ENSG00000182333, Chromosome 10, mean differential methylation value (Δβ) = −0.11776, adjusted p = 0.0005, number of CpG sites = 1) was retained (Fig. 5A). Interestingly, the LIPF promoter showed a significant hypomethylation demonstrated by the lowest combined rank (combined rank = 24) and the highest Δβ (β methylation difference of >11%), which was reflected in cg11231018 hypomethylation within this promoter (Δβ = −0.11776) (Fig. 5B, C).
Scatter plots for A the genes, B the promoters, and C the top 10000 most differentially methylated CpG sites and the top 500 most differentially methylated regions between PTB (x-axis) and controls (y-axis). The sparsest 1% of points are explicitly plotted, with a maximum of 10,000 points displayed, while colored points indicate differentially methylated sites or regions. mean.mean.diff (PTB vs. Controls), log2 of the mean quotient in means (mean.mean.quot.log2), and the combined p-value that is computed using Fisher’s method are ranked for all regions, to calculate the combinedRank.
A the most differentially methylated (hypomethylated) promoter of LIPF in placental samples of PTB and B significant lower β values in PTB group compared to controls, after performing the Mann–Whitney U test and Holm–Šídák method for multiple comparisons, *** p < 0.001. C the characteristics of the CpG site (cg11231018) in the LIPF promoter. id: promoter id; Chrm: chromosome; Start & End: start and end coordinates of the promoter; symbol: associated gene symbol to the given promoter; mean.mean.PTB/control: mean of mean methylation levels for PTB and control groups across all sites in a region; mean.mean.diff: Mean difference in means across all sites in a region; comb.p.adj.fdr: FDR-adjusted combined p-value; combinedRank: ranks data by combining mean.mean.diff, mean.mean.quot.log2 and comb.p.val; num.sites: number of sites associated with the region; cdid: cg site id; mean.PTB/control: mean methylation in each of the two groups; mean.diff: difference in methylation means between the two groups; diffmeth.p.val: p-value obtained from linear models employed in the limma package.
With respect to the cord blood samples, the scatterplot analysis for the top 10000 most differentially methylated CpG sites indicated a hypermethylation profile in the PTB group compared to the controls (Fig. 6A). Additionally, consistent hypermethylation profiles were observed at genes’ (Fig. 6B) and promoters’ (Fig. 6C) levels by assessing the top 500 most differentially methylated regions. For a more rigorous quantitative assessment, the focused analysis on the 1000 most differentially methylated promoters resulted in the identification of 652 differentially methylated promoters that were hypermethylated in the PTB group, whereas 348 differentially methylated promoters were hypermethylated in the control group (Supplementary Data 2). A similar approach of in-depth analysis was followed in the case of the cord blood compared to that of the placenta. After applying filtering criteria using an FDR-adjusted p-value < 0.05, only one differentially methylated promoter of small RNA binding exonuclease protection factor La (SSB) was identified: (ID: ENSG00000138385, Chromosome 2, Δβ = 0.19073, adjusted p = 0.0267, number of CpG sites = 1) (Fig. 7A and Fig. 7B). Notably, the SSB promoter exhibited the highest Δβ (β methylation difference of >19%) and held the lowest combined rank in our analysis (combinedRank = 12), highlighting its potential significance. We next explored the CpG site that is located in the SSB promoter, which corresponds to cg04956445, and as expected, it was hypermethylated in PTB compared to controls and reflecting the overall methylation levels in each group (Δβ = 0.19073) (Fig. 7C).
Scatter plots for A the top 10000 most differentially methylated CpG sites and the top 500 most differentially methylated regions, B genes, and C promoters between PTB (x-axis) and controls (y-axis). The sparsest 1% of points are explicitly plotted, with a maximum of 10,000 points displayed, while colored points indicate differentially methylated sites or regions. mean.mean.diff (PTB vs. Controls), log2 of the mean quotient in means (mean.mean.quot.log2), and the combined p-value that is computed using Fisher’s method are ranked for all regions, to calculate the combinedRank.
A the most differentially methylated (hypermethylated) promoter of SSB in cord blood samples of PTB and B significant higher β values in the PTB group compared to controls, after performing the Mann–Whitney U test and Holm–Šídák method for multiple comparisons, *** p < 0.001. C the characteristics of the CpG site (cg04956445) in the SSB promoter. id: promoter id; Chrm: chromosome; Start & End: start and end coordinates of the promoter; symbol: associated gene symbol to the given promoter; mean.mean.PTB/control: mean of mean methylation levels for PTB and control groups across all sites in a region; mean.mean.diff: Mean difference in means across all sites in a region; comb.p.adj.fdr: FDR-adjusted combined p-value; combinedRank: ranks data by combining mean.mean.diff, mean.mean.quot.log2 and comb.p.val; num.sites: number of sites associated with the region; cdid: cg site id; mean.PTB/control: mean methylation in each of the two groups; mean.diff: difference in methylation means between the two groups; diffmeth.p.val: p-value obtained from linear models employed in the limma package.
The gene expression analysis demonstrated a decreased expression of the SSB gene in both placenta and cord blood samples from PTB subjects compared to controls (p = 0.0033 and p = 0.0093, respectively). This finding substantiates the hypothesis that gene function is impaired on both maternal and fetal sides, potentially due to epigenetic modulation (Fig. 8).
Enriched biological processes in PTB compared to controls
To interpret the resulting list of differentially methylated promoters in the context of biological processes and molecular functions, we performed GO enrichment analysis on the hypomethylated promoters in placental samples from PTB cases compared to controls using the GOstats package. Different processes were enriched, and the top ones were related to interleukin (IL)-33-mediated signaling pathway (GO:0038172, OR = 210.59, p = 0.0095), galactosylceramide catabolic process (GO:0006683, OR = 210.59, p = 0.0095), beta-glucoside catabolic process (GO:1901805, OR = 210.59, p = 0.0095), beta-alanine transport (GO:0001762, OR = 210.59, p = 0.0095), humoral immune response (GO:0006959, OR = 38.02, p = 0.0), maltose catabolic process (GO:0000025, OR = 31.05, p = 0.0048), positive regulation of peptidyl-serine phosphorylation of STAT protein (GO:0033141, OR = 25.05, p = 0.0037), complement activation (GO:0006956, OR = 11.95, p = 0.0026), etc. (Fig. 9A).
Next, we tested the enrichment of GO sets on the hypermethylated promoters in cord blood samples. The results indicated that dopamine catabolic process was the most enriched pathway (GO:0042420, OR = 152.72, p = 0.0005), followed by vitamin K metabolic process (GO:0042373, OR = 21.80, p = 0.0057), cell proliferation involved in metanephros development (GO:0072203, OR = 19.08, p = 0.0071), metanephric collecting duct development (GO:0072205, OR = 16.96, p = 0.0086), positive regulation of acute inflammatory response (GO:0002675, OR = 9.57, p = 0.005), digestive tract morphogenesis (GO:0048546, OR = 6.98, p = 0.0035), positive regulation of IL-8 production (GO:0032757, OR = 5.38, p = 0.0082), digestive system development (GO:0055123, OR = 5.21, p = 0.0001), etc. Of relevance in the contest of PTB, the embryo development ending in birth pathway was significantly enriched (GO:0009792, OR = 4.69, p = 0.0056), etc. (Fig. 9B).
Protein consumption can explain the differential methylation profiles associated with PTB
The observed differences in nutrient intake between the PTB and control groups may help explain the detected distinct methylation changes, suggesting that specific dietary components could influence methylation patterns associated with PTB. The National Academy of Medicine (NAM) recommends a daily intake of 175 × g of carbohydrates for pregnant women regardless of the presence of diabetes22,23. This recommendation is intended to ensure adequate glucose supply for both the fetus and the brain, meeting the increased metabolic demands during pregnancy. Five PTB-affected mothers were considered low-carbohydrate consumers compared to nine who were consuming normal amounts. However, the analysis revealed that carbohydrate consumption was not able to explain the differential methylation profiles in the PTB group (data not shown). Eight out of fourteen mothers were classified as low-protein consumers, according to the Food and Nutrition Board within the Health and Medicine Division at the National Academies24,25, which advises women to consume a minimum of 60 grams of protein per day to support fetal growth, maternal tissue development, and overall pregnancy health24.
Therefore, we re-analyzed the methylation alterations in both cord blood and placenta, according to maternal protein intake. We also attempted to investigate whether any other dietary components could explain the differential methylation profiles observed in the PTB group. However, the analysis was limited by the insufficient sample size when dividing the subjects based on nutrient consumption levels. Consequently, these factors hindered the ability to draw definitive conclusions regarding the influence of different dietary component intakes on methylation patterns in this group, except for protein consumption.
Starting with the placenta, low protein consumption was associated with hypomethylation profiles at CpG sites (Fig. 10A), genes’ (Fig. 10B), and promoters’ levels (Fig. 10C) as shown by volcano plots, with lower density of points on the right side of the x-axis (where mean.diff and mean.mean.diff > 0). To confirm this general observation, we focused on the 1000 most differentially methylated promoters, which showed 760 hypomethylated and 240 hypermethylated promoters in low-protein consumers in comparison to normal-protein consumers among the PTB subjects (Supplementary Data 3). Interestingly, immunoglobulin kappa variable 1D-39 (IGKV1D-39) promoter was the only filtered promoter according to significant FDR-adjusted p-value (ID: ENSG00000251546, Chromosome 2, Δβ = −0.27016, adjusted p = 0.0313, number of CpG sites = 1) (Fig. 10D). Moreover, low protein consumption was not shown to be associated with LIPF promoter hypomethylation (adjusted p = 0.2630).
Volcano plots for differential methylation quantified by combined p values for (A, B) CpG sites, (B, F) genes, and (C, G) promoters in (A–D) placenta and (E–H) cord blood samples. The x-axis represents the mean difference in methylation (mean.diff) (low protein consumers vs. normal protein consumers), while the y-axis shows the statistical significance as the −log10 of the differential p-value. The color gradient indicates the log10 of the combined rank, with darker colors representing CpG sites or regions with higher combinedRank values. Tables (D and H) report the most differentially methylated (hypomethylated and hypermethylated) promoters in placental and cord blood samples of low protein consumers in the PTB group. id: promoter id; Chrm: chromosome; Start & End: start and end coordinates of the promoter; symbol: associated gene symbol to the given promoter; mean.mean.Low/Normal: mean of mean methylation levels for each group across all sites in a region; mean.mean.diff: Mean difference in means across all sites in a region; comb.p.adj.fdr: FDR-adjusted combined p-value; combinedRank: ranks data by combining mean.mean.diff, mean.mean.quot.log2 and comb.p.val; num.sites: number of sites associated with the region.
With respect to the cord blood, the volcano plots revealed a higher density of points on the right side of the x-axis (where mean.diff and mean.mean.diff > 0) at CpG sites (Fig. 10E), genes’ (Fig. 10F) and promoters’ (Fig. 10G) levels, suggesting a hypermethylation trend in low-protein consumers compared to normal-protein consumers in the PTB cohort. After checking the 1000 most differentially methylated promoters, we identified 553 hypermethylated and 447 hypomethylated promoters in low-protein consumers versus the normal-protein consumers (Supplementary Data 4). Three differentially methylated promoters were identified using FDR-adjusted p-value: (i) IGKV1D-39: (ID: ENSG00000251546, Chromosome 2, Δβ = 0.236664, adjusted p = 0.0003, number of CpG sites = 1), (ii) microRNA 181a-2 (MIR181A2): (ID: ENSG00000207595, Chromosome 9, Δβ = 0.195762, adjusted p = 0.0009, number of CpG sites = 16), (iii) SSB: (ID: ENSG00000138385, Chromosome 2, Δβ = 0.200546, adjusted p = 0.0030, number of CpG sites = 1) (Fig. 10H).
We tested the gene expression of both SSB and IGKV1D-39 genes in placenta and cord blood according to protein intake, and unfortunately, the number of samples producing reliable results do not allow statistical analysis. We expect a very low expression of both genes in PTB samples, which are probably not detectable (data not shown).
Enriched biological processes in PTB based on maternal protein intake
GO enrichment analysis on the hypomethylated promoters in placental samples from maternal low protein consumers compared to normal protein consumers within the PTB cohort revealed several enriched processes including neural crest cell fate specification (GO:0014036, OR = 289.72, p = 0.0036), diencephalon morphogenesis (GO:0048852, OR = 284.84, p = 0.0054), regulation of meiotic chromosome separation (GO:1905132, OR = 184.86, p = 0.0074), and dendritic transport of messenger ribonucleoprotein complex (GO:0098963, OR = 189.88, p = 0.0072) (Fig. 11A). Next, we tested the enrichment of GO sets on the hypermethylated promoters in cord blood samples from maternal low protein consumers compared to normal protein consumers within the PTB cohort. The results indicated that positive regulation of IL-8 production (GO:0032757, OR = 260.89, p = 0.0022) was among the top enriched pathways, along with embryonic heart tube anterior/posterior pattern specification (GO:0035054, OR = 260.89, p = 0.0059) and positive regulation of microtubule nucleation (GO:0090063, OR = 215.40, p = 0.0001) (Fig. 11B).
Discussion
The saying “you are what you eat” holds true in every way. Our dietary choices directly influence and shape the composition, function, and structure of our cells. The impact of diet becomes even more significant during pregnancy, a time when “eating for two” takes on a deeper meaning. What a mother consumes not only nourishes her own body but also shapes the development of the growing fetus. Essential nutrients act as the building blocks for a healthy pregnancy, and its lack thereof can disrupt this delicate balance, potentially leading to complications, including PTB. In its consensus study report, the Institute of Medicine (US) Committee on Nutritional Status During Pregnancy focused on compiling scientific evidence and offering recommendations for nutrient intake26. This initiative sparked scientists’ curiosity to discover the relationship between dietary intake and maternal and infant health and disease conditions26.
In our study, significant disparities in nutrient intake were observed between PTB-affected mothers and controls. The PTB group demonstrated a substantially reduced intake of kilocalories and essential macronutrients, including protein, carbohydrates, and dietary fiber, as well as key micronutrients such as vitamins D, E, B6, and B12, and minerals like calcium, potassium, and iron. Previous studies have thoroughly reviewed the impact of maternal nutrition on PTB risk by highlighting the role of different macro- and micro-nutrients, especially during pre-pregnancy period, and the consequences of its deficiency on decreasing the gestation length4. Wu, Guoyao et al. reviewed the positive association between the reduced PTB risk and calcium and magnesium supplementation as well as the increased PTB risk with iron deficiency27. In line with our findings, the study by Zhang, Yan et al. explored maternal dietary intake between mothers with PTB and term deliveries among Chinese pregnant women in the last month before delivery by taking into consideration five macronutrients and seventeen micronutrients28. Specifically, they observed that mothers in the PTB group had a lower intake of vitamin E and total fat compared to those who delivered at term. However, a limitation of their study was that it only represented dietary intake differences at a single time point, close to delivery, rather than during the entire course of pregnancy28.
Hashmi, A. H. et al.29 showed that pregnant women in a marginalized population along the Thailand-Myanmar border have limited knowledge and awareness about healthy and proper dietary practices. Here, we further demonstrated that, within the same population, and particularly among PTB-affected mothers, undernutrition emerged as a concerning profile during pregnancy, likely contributing to the increased risk and pathophysiology of PTB.
In our analysis of placental DNA methylation, LIPF emerged as the most differentially methylated promoter between PTB and controls, showing significant hypomethylation in PTB group. Gastric lipase is an enzyme primarily responsible for digesting lipids, converting dietary fats into fatty acids and monoglycerides30. The placenta plays a vital role in nutrient transfer, including fats, to the fetus, which is crucial for fetal development; thus, hypomethylation in the promoter region of LIPF in the placenta could alter its expression and activity and consequently affect lipid metabolism. Hamosh, M et al. have found that preterm infants born before 26 weeks of gestation have very low gastric lipase activity compared to those born after 26 weeks and its activity reaches peak level after week 3031. By studying the biological processes associated with the placental hypomethylated promoters in the PTB group, we have detected processes related to inflammation and immune responses. Given its role as a pro-inflammatory cytokine, IL-33 is known as an “alarmin” because it is released in response to cellular injury or stress32. A study by Lei, Wen-Jia et al. has shown that the increased levels of secreted IL-33 in human amnion fibroblasts, accompanied with an elevated level of IL-33 receptor, suppression of tumorigenicity 2 (ST2), could induce a cascade of inflammatory reactions that can result in parturition, and consequently PTB33. They have also demonstrated that the intraperitoneal injection of IL-33 in pregnant mice induced PTB; thus, IL-33 or its receptor, ST2, could act as a target of interest to manage inflammation-associated PTB33. Evidence from previous studies has consistently shown that complement activation and humoral immunity were linked to PTB onset and pathogenesis34,35,36. Enriched metabolic processes, such as starch and maltose catabolic processes, supported the hypothesis of activation of adaptive compensatory mechanism in case of maternal undernutrition to meet the high energy demands of the fetus37. The remaining enriched processes (e.g., galactosylceramide and beta-glucoside catabolic processes) were identified for the first time in relation to PTB, highlighting the need for further investigation.
A clear hypermethylation pattern was detected in cord blood DNA samples of the PTB group at different DNA levels, including CpG sites and functional genomic regions. Remarkably, the most differentially methylated promoters in cord blood in the PTB group were hypermethylated. The promoter of SSB, also known as “La protein”, was identified as the most differentially methylated between PTB and controls in cord blood samples and the consequent reduced gene activity was confirmed by the gene expression experiment. The protein encoded by SSB is an RNA-binding protein38, and it is also referred to as Sjogren’s syndrome antigen B because it functions as an autoantigen in autoimmune diseases such as systemic lupus erythematosus (SLE), which are characterized by aberrant immune responses resulting in the production of autoantibodies targeting SSB. During pregnancy, the presence of a condition such as SLE is suggested to increase the risk of PTB from 5% to 33%39. Similarly, the published report from the EWAS atlas revealed that the most related trait to differentially methylated SSB (hypermethylated gene body) is SLE40, with the highest number of associations (AssociationCount = 2). To date, no studies have directly reported methylation alterations in SSB specifically associated with PTB. Considering its important functions, hypermethylation of SSB promoter could impact fetal development by influencing RNA processing and immune regulation. The impact on immune regulation was more evident after conducting GO enrichment analysis of the hypermethylated promoters identified in cord blood samples from PTB cases. The most attractive enriched biological process was “embryo development ending in birth”, suggesting a disruption in the normal regulatory mechanisms of genes critical for fetal development and successful birth outcomes, thus interfering with full-term embryonic development, potentially leading to premature termination of gestation and contributing to the pathophysiology of PTB. Our results also showed that the most enriched biological process was the dopamine catabolic process. A study by Moloney, Roisin A. et al. revealed that PTB was associated with alteration in dopamine pathways in both the frontal cortex and hippocampus of preterm neonates of guinea pigs after 24 h from birth41. Furthermore, the negative regulation of cholesterol efflux could suggest an accumulation of cholesterol, impacting lipid metabolism and potentially contributing to PTB and cardiovascular stress in infants42,43. From a developmental perspective, the pathways associated with regulation of stem cell proliferation, digestive system development, and digestive tract morphogenesis were found to be enriched in the PTB group. The digestive system of preterm neonates is often underdeveloped and characterized by immature gastrointestinal (GI) motility44. Preterm infants are subjected to a high risk of developing CKD, primarily due to a reduced number of nephrons at birth45. Our findings indicated that processes related to kidney development, including cell proliferation involved in metanephros development, metanephric collecting duct development, and cell differentiation involved in kidney development, were enriched.
The differential DNAm patterns, hypomethylation in placental tissue and hypermethylation in cord blood, may reflect the distinct biological roles and environmental exposures of these tissues during pregnancy and support the notion that each tissue possesses a distinct methylation landscape46. Which reflects tissue-specific responses to various maternal and fetal risk factors associated with PTB.
Dietary habits can significantly impact the epigenetic patterns in different tissues47. In our study, we highlighted the differential methylation profiles in the placenta and cord blood from pregnancies affected by PTB, which could be explained by the low intake of protein during pregnancy. We were not able to detect the impact of the other differentially consumed nutrients in our study due to the insufficient sample size when dividing the subjects based on nutrient consumption levels within the PTB group.
Given the importance of dietary protein intake as a main source of essential amino acids like arginine (Arg) and tryptophan (Trp), which play critical roles in placental function, immune modulation, and fetal growth, a deficiency in these AAs can disrupt these processes, contributing to PTB risk27. A study has suggested that inadequate levels of Arg may contribute to the onset of PTB by increasing uterine contractility, a consequence of reduced nitric oxide (NO) bioavailability48. However, a deeper understanding of how low dietary protein levels may influence PTB through epigenetic mechanisms can add more evidence to the importance of monitoring protein intake through nutritional guidelines and interventions that could alter these pathological mechanisms, potentially reducing PTB rates. Our findings demonstrated that low protein consumption in the PTB group could act as a factor that can explain the dominant hypo/hypermethylation profiles in the placenta and cord blood, respectively. Low protein intake was linked to the hypomethylation of the IGKV1D-39 promoter in the placenta, but it could not explain the hypomethylation in the LIPF promoter. Of interest is that IGKV1D-39 emerged as one of the most differentially methylated promoters in cord blood, showing hypermethylation in the low protein consumers group in the PTB cohort. Additionally, low protein intake was associated with SSB promoter hypermethylation in the cord blood, further underscoring its potential role in PTB risk and pathogenesis.
The IGKV1D-39 gene encodes a segment of the immunoglobulin kappa light chain and is important for humoral immune responses, where B-cells produce antibodies for targeted immune responses against specific antigens. Recognizing IGKV1-39 as a key paralog of IGKV1D-39, a study by Huang, Jianxia et al. reported downregulation of IGKV1-39 protein in the serum of PTB-affected mothers in China compared to those who gave birth at term49. Further studies are necessary to explore the relationship between differential methylation of IGKV1D-39 in response to dietary protein intake and its implications for immune modulation in PTB.
Of note, the positive regulation of IL-8 production emerged as a commonly enriched pathway linked to hypermethylated promoters in cord blood samples from both PTB compared to controls and maternal low protein consumption compared to normal protein consumption within the PTB cohort. IL-8 is a pro-inflammatory cytokine implicated in various pregnancy-related processes50. This suggests a profound impact of maternal low protein consumption and a shared inflammatory response mechanism that may contribute to PTB. Elevated prenatal cervicovaginal fluid and serum IL-8 levels at 24–34 weeks of gestation were suggested to serve as a predictor for PTB51,52. A study demonstrated that elevated IL-8 levels in umbilical cord blood were strongly associated with histologic chorioamnionitis, an inflammatory condition in the uterus that is a major contributor to preterm birth53.
This study has certain limitations that should be noted. First, the relatively small sample size (18 PTB cases vs. 30 healthy subjects) may limit the generalizability of the results. Additionally, the reliance on 24hDR, a method that is subjective, may introduce inaccuracies in dietary reporting. While the study provides valuable insights, the findings may be specific to the population studied. Broader population-based studies across diverse geographical and ethnic groups are necessary to validate the generalizability of these findings. Although protein intake was highlighted, other macronutrients (e.g., dietary fiber) could not be assessed as extensively in the PTB cohort.
We provided foundational insights to pave the way for developing dietary intervention strategies aimed at mitigating the detrimental outcomes associated with PTB. Our findings open new avenues for understanding how nutritional deficiencies can alter the epigenome in a way that predisposes them to adverse pregnancy outcomes, specifically PTB. Thus, analyzing the complete macronutrient profile may provide further insights into dietary factors associated with PTB. Moreover, nutritional interventions in a high-risk PTB pregnancy could be promising, such as focusing on increasing protein intake to evaluate their impact on modulating epigenetic profiles and potentially reducing PTB risk.
Our study revealed an association between maternal undernutrition and DNAm alterations in the context of PTB. We highlighted distinct nutritional deficiencies in essential macro- and micronutrients among mothers who experienced PTB, as well as significant epigenetic alterations in the placenta and cord blood, with marked hypo- and hypermethylation patterns, respectively. We also studied the possibility of common differentially methylated promoters or genes. Altered methylation in the placenta may reflect changes in the intrauterine environment triggered by PTB, which could have lasting effects on the child’s health. Methylation changes in cord blood can provide insights into how PTB may impact the fetus directly. This is crucial for understanding potential transgenerational effects. Our study also identified LIPF promoter hypomethylation and SSB promoter hypermethylation in placental and cord blood samples, respectively. The latter are promoters of genes related to immune function and metabolic pathways, which are fundamental to maternal-fetal health. Hypermethylation of SSB promoter was associated with low protein intake in PTB, emphasizing the important role of adequate protein intake during pregnancy. This data suggests novel epigenetic biomarkers for PTB, and it implies that protein consumption may reverse or modify the DNAm status of these biomarkers associated with PTB.
Overall, this study emphasizes the importance of comprehensive nutritional interventions during pregnancy to promote optimal epigenetic profiles, ultimately aiming to reduce PTB incidence and improve long-term health outcomes for both mothers and infants. Future research should build upon these findings with broader, population-based studies and explore other influencing factors such as lifestyle, socioeconomic status, and environmental exposures to develop globally relevant recommendations and therapeutic approaches focused on dietary optimization to mitigate the adverse effects associated with PTB.
Methods
Study population and setting
A case-control study was conducted based on data and samples collected from a non-interventional and observational prospective cohort study of pregnant women. Data were collected from September 2016 to February 2019 involving women who were enrolled during the first trimester of their pregnancies. The study was carried out in strict accordance with the Declaration of Helsinki and adherence to the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines. The MSP study has obtained ethical approval from multiple institutions: the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand (Reference: TMEC 15-062), the Oxford Tropical Research Ethics Committee (OxTREC reference33-15:), and the Institutional Review Board (IRB) committee of Sidra Medicine (IRB protocol #1866255). Informed consent to participate in this study was obtained from all participants. The clinical trial was registered on ClinicalTrials.gov registry, under NCT02797327, on 13-06-201654.
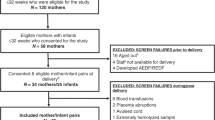
Briefly, pregnant women from either Myanmar or Thailand of either Karen or Burman ethnicity were recruited and followed every two weeks throughout their pregnancy (TM1, TM2, TM3) and at delivery at Shoklo Malaria Research Unit (SMRU). Participants were invited for the study when they met the inclusion criteria: a viable singleton pregnancy in the first trimester, age between 18 and 49 years, and no critical medical or obstetric history, including cesarean section. The exclusion criteria of the original pregnancy cohort included emergency care or medical or obstetric complications. In total, 381 pregnant women were enrolled in the original cohort study. In this study, we included only women who experienced PTB and matched with a control group. PTB was defined as birth occurring before completing 37 weeks of gestation. Eighteen women delivered at ≤37 weeks of gestational age and were defined as PTB subjects. A group of 30 healthy women from the same cohort who delivered at term or ≥ 37 weeks and matched by age, BMI, ethnicity, parity, and gravida were selected as the control group. Clinical and demographic measurements of both mother and baby, as well as pregnancy outcomes, were collected and analyzed in detail55. These include maternal age, weight, height, body mass index (BMI), pulse rate, respiratory rate, systolic and diastolic blood pressure at enrollment, ethnic background (Burmese or Karen), gravida status (1, 2, or ≥3), basic literacy skills (yes or no), and prenatal antibiotic exposure across different trimesters and at delivery. Additionally, information was gathered on gestational age, birth weight, baby length, head circumference, and gender (male or female). Patients’ recruitment was carried out at SMRU, placenta and cord blood samples and clinical data were collected at SMRU and shipped to Sidra Medicine for processing and data analysis.
Dietary assessment
Maternal dietary data was collected using 24-h dietary recalls at 4 different time points during pregnancy (Trimesters 1, 2, and 3 and at delivery) to correlate and investigate its association with DNAm changes. Participants were asked to provide a detailed recall of all foods and beverages consumed in the past 24 h. In total, 14 PTB-affected mothers and 30 healthy participants provided dietary recalls. Supplements intake was also recorded.
To ensure accurate reporting, portion sizes were estimated using standard household measurements, food models, and visual aids such as pictures. Then, the nutrient breakdown of the diet was calculated using Nutritionist Pro™ software (Axxya Inc)56. The local dishes not found initially in the NPro, were added after a careful search of the standardized recipes. The unit of grams was used for all food items during dietary data entry in NPro. The nutrient composition was then downloaded from NPro as .xsls files. For each participant, the average nutrient intake was calculated across the four time points and used in the statistical analyses.
The National Academy of Medicine (NAM) and the Food and Nutrition Board within the Health and Medicine Division at the National Academies24,25 were used as reference for the adequate nutrients intake for pregnant women22,23. As an approach to testing the association between single nutrient consumption and altered DNAm profiles in placenta and cord blood, we conducted a downstream analysis using RnBeads on the PTB group specifically by classifying its subjects according to food intake.
Placental and cord blood sample collection and Genomic DNA extraction
Following standard operating procedures, samples were collected at delivery in sterile conditions and immediately stored at −80 °C in an RNA-preservative solution. Specifically, 14 placenta samples (fetal side) and 13 cord blood samples were collected from mothers affected by PTB. In the control group, 25 placenta samples and 21 cord blood samples were collected following the same protocol. Details on the total number of subjects, available data and samples are presented in the Supplementary Fig. 2.
Genomic DNA was extracted from placental tissue (20–25 mg) and cord blood (250 µL) using the QIAGEN DNeasy Blood & Tissue Kit (QIAGEN, Hilden, Germany) following the manufacturer’s protocol. Samples were lysed with ATL buffer and proteinase K, incubated at 56 °C, and then processed with AL buffer and ethanol for DNA binding. Purification involved AW1/AW2 washes, and DNA was eluted with AE buffer.
Bisulfite conversion was performed using the EZ-96 DNA Methylation Kit (Zymo Research Corp., Irvine, CA, USA). DNA ( ~ 500 ng) was denatured with CT Conversion Reagent, incubated at 98 °C for 8 min and 64 °C for 3.5 h. After desulfonation and purification with M-Wash and Desulfonation Buffers, bisulfite-converted DNA was eluted in M-Elution Buffer for EPIC array methylation analysis. DNA quality and quantity were assessed using NanoDrop One (ThermoFisher Scientific, Waltham, MA, USA).
Genome-wide DNA methylation profiling
The bisulfite-treated DNA was submitted to Sidra Medicine Omics Core for processing. Briefly, the DNA was subjected to whole-genome amplification (WGA) to increase the quantity of DNA, ensuring there was sufficient material for further processing. This step was followed by DNA fragmentation, which created smaller DNA fragments optimal for hybridization to the array. The fragmented DNA was then precipitated and resuspended in a suitable buffer, incubated at 48 °C overnight to allow proper and efficient hybridization to the Infinium MethylationEPIC BeadChip (Illumina Inc., San Diego, CA, USA). This BeadChip allows for the assessment of over 850,000 CpG sites across the human genome, providing comprehensive methylation coverage. During hybridization, the DNA fragments annealed to specific probes on the chip, targeting the CpG sites. The array was then washed, stained using Illumina reagents to detect methylated and unmethylated cytosines, and scanned using iScan System (Illumina), which generates methylation intensity data to measure the methylation levels at each CpG site.
Gene expression analysis
Total RNA was first extracted using the AllPrep DNA/RNA/miRNA Universal Kit (Qiagen, Hilden, Germany), following the manufacturer’s protocol. Reverse transcription was performed using the iScript™ Reverse Transcription Supermix for RT-qPCR (Bio-Rad, CA, USA). Quantitative real-time PCR (qPCR) was performed using the QuantStudio™ Real-Time PCR System (Applied Biosystems, Thermo Fisher Scientific) with SYBR Green SYBR™ Green Universal Master Mix. Each 20 µL reaction mixture contained 10 µL of 2× SYBR Green Master Mix, 0.5 µL each of forward and reverse primers (final concentration 200 nM) targeting SBB, LIPF, and IGKVID-39 genes, 1 µL of cDNA template, and 8 µL of nuclease-free water. Thermal cycling conditions were initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 60 s. A melt curve analysis was performed to confirm amplification specificity. Gene expression data were analyzed using QuantStudio Design and Analysis Software. Relative expression levels were calculated using the 2−ΔΔCt method, with β-actin used as the internal control gene. All reactions were run in duplicates, with at least two biological replicates per group.
Enrichment analysis of differentially methylated promoters
We tested the enriched pathways associated with the hypo/hypermethylated promoters in PTB using the GOStats package57. As recommended by the RnBeads team58, GOStats enables the calculation of gene ontology (GO) enrichment from differential methylation results, providing both odds ratios and p-values. In this study, we examined the GO enrichment of the top 500 hypo/hypermethylated promoters identified in PTB-exposed placenta and cord blood for biological process (BP) and molecular function (MF) ontology terms. For visualization and plotting of GO data, we used SRplot (Science and Research Plot), which is an online and freely accessible tool designed to facilitate the graphical representation of data by generating a wide range of plots and visualizations59.
Statistical analysis
Descriptive statistics for dietary variables, including medians, and ranges, were computed using GraphPad Prism (version: 10.1.2) (https://www.graphpad.com/) to characterize the data distribution within each group. Comparison of dietary, clinical and demographic characteristics between both groups were performed based on the Mann–Whitney U test for continuous data, given the non-normal distribution of the data, and the Chi-square test for categorical data. P values were corrected for multiple testing using the Holm–Šídák method, with a p < 0.05 was considered significant.
Raw methylation data (IDAT files) were analyzed in R software (version 4.3.2, https://www.r-project.org/) using the RnBeads package. RnBeads provided quality control, including sample quality assessment, outlier detection, and array performance evaluation. Normalization was performed using the Dasen method, and preprocessing involved the greedycut algorithm for background correction, removal of low-quality probes, and exclusion of probes on sex chromosomes, focusing on autosomal methylation. Differential methylation analysis was conducted at CpG sites, promoters, and gene levels. The combinedRank metric was used to prioritize CpG sites or regions based on mean.diff (average β value difference), mean.quot.log2 (log2 ratio of methylation levels), and diffmeth.p.val (statistical significance from limma package). Data were adjusted for maternal age, gestational age, gender, and BMI. Significant methylation differences were identified using a false discovery rate (FDR) with Benjamini–Hochberg correction applied to p-values from Welch’s t-tests. The preprocessing of the placental methylation dataset involved filtering out unreliable and confounding data, removing 17,371 SNP-enriched sites to avoid confounding effects from genetic variations and 79,627 low-quality probes using the Greedycut algorithm. Additionally, 16,390 probes from sex chromosomes were excluded to avoid gender-related methylation biases. After normalization using the “Dasen” method, 39 samples (PTB: n = 14; controls: n = 25) and 751,312 high-quality probes remained for analysis, and annotations were made for genes (n = 34,472) and promoters (n = 43,657) based on Ensembl Genes version 75. Similarly, for the cord blood methylation dataset, the preprocessing of the involved the removal of 17,371 SNP-enriched sites, 68,663 low-quality probes, and 16,754 probes from sex chromosomes. After “Dasen” normalization, 34 samples (PTB: n = 13; controls: n = 21) and 761,857 high-quality probes remained for analysis, and annotations were made for genes (n = 34,578) and promoters (n = 43,935) based on Ensembl Genes version 75.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Khandre, V., Potdar, J. & Keerti, A. Preterm birth: an overview. Cureus 14, e33006 (2022).
Purisch, S. E. & Gyamfi-Bannerman, C. Epidemiology of preterm birth. Semin. Perinatol. 41, 387–391 (2017).
Institute of Medicine Committee on Understanding Premature, B. & Assuring Healthy, O. In Preterm Birth: Causes, Consequences, and Prevention (eds Behrman, R. E. & Butler, A. S.) (National Academies Press (US) Copyright © 2007, National Academy of Sciences, 2007).
Bloomfield, F. H. How is maternal nutrition related to preterm birth?. Annu. Rev. Nutr. 31, 235–261 (2011).
Abu-Saad, K. & Fraser, D. Maternal nutrition and birth outcomes. Epidemiol. Rev. 32, 5–25 (2010).
Qin, Y. & Xie, L. Nutrition and supplements during pregnancy: a vital component in building the health and well-being of both the mother and the developing baby. Nutrients 15, https://doi.org/10.3390/nu15153395 (2023).
de Seymour, J. V., Beck, K. L. & Conlon, C. A. Nutrition in pregnancy. Obstet. Gynaecol. Reprod. Med. 32, 253–258 (2022).
Barker, D. J. The fetal and infant origins of adult disease. Br. Med. J. 301, 1111–1111 (1990).
Lin, M. W. et al. Comparison of DNA methylation changes between the gestation period and the after-delivery state: a pilot study of 10 women. Front. Nutr. 9, 829915 (2022).
Sainty, R., Silver, M. J., Prentice, A. M. & Monk, D. The influence of early environment and micronutrient availability on developmental epigenetic programming: lessons from the placenta. Front. Cell Dev. Biol. 11, https://doi.org/10.3389/fcell.2023.1212199 (2023).
Stein, Z., Susser, M., Saenger, G. & Marolla, F. Famine and Human Development: The Dutch Hunger Winter of 1944-1945 (Oxford University Press, 1975).
Lumey, L. H., Stein, A. D. & Susser, E. Prenatal famine and adult health. Annu. Rev. Public Health 32, 237–262 (2011).
Heijmans, B. T. et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA 105, 17046–17049 (2008).
Fradin, D. et al. DNA methylation dynamics during pregnancy. Front. Cell Dev. Biol. 11, https://doi.org/10.3389/fcell.2023.1185311 (2023).
Niculescu, M. D. & Zeisel, S. H. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J. Nutr. 132, 2333s–2335s (2002).
Kadayifci, F. Z., Zheng, S. & Pan, Y. X. Molecular Mechanisms underlying the link between diet and DNA methylation. Int. J. Mol. Sci. 19, https://doi.org/10.3390/ijms19124055 (2018).
Duthie, S. J., Narayanan, S., Brand, G. M., Pirie, L. & Grant, G. Impact of folate deficiency on DNA stability. J. Nutr. 132, 2444S–2449S (2002).
Cao, R., Xie, J. & Zhang, L. Abnormal methylation caused by folic acid deficiency in neural tube defects. Open Life Sci. 17, 1679–1688 (2022).
Rees, W. D., Hay, S. M., Brown, D. S., Antipatis, C. & Palmer, R. M. Maternal protein deficiency causes hypermethylation of DNA in the livers of rat fetuses. J. Nutr. 130, 1821–1826 (2000).
Lillycrop, K. A., Phillips, E. S., Jackson, A. A., Hanson, M. A. & Burdge, G. C. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring1. J. Nutr. 135, 1382–1386 (2005).
Catov, J. M., Bodnar, L. M., Olsen, J., Olsen, S. & Nohr, E. A. Periconceptional multivitamin use and risk of preterm or small-for-gestational-age births in the Danish National Birth Cohort. Am. J. Clin. Nutr. 94, 906–912 (2011).
Roskjær, A. B. et al. Dietary intake of carbohydrates in pregnant women with type 1 diabetes—A narrative review. Food Sci. Nutr. 9, 17–24 (2021).
14. Management of Diabetes in Pregnancy Standards of medical care in diabetes-2020. Diabetes Care 43, S183–s192 (2020).
Kominiarek, M. A. & Rajan, P. Nutrition recommendations in pregnancy and lactation. Med. Clin. North Am. 100, 1199–1215 (2016).
Trumbo, P., Schlicker, S., Yates, A. A. & Poos, M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Dietetic Assoc. 102, 1621–1630 (2002).
Marshall, N. E. et al. The importance of nutrition in pregnancy and lactation: lifelong consequences. Am. J. Obstet. Gynecol. 226, 607–632 (2022).
Wu, G., Imhoff-Kunsch, B. & Girard, A. W. Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr. Perinat. Epidemiol. 26, 4–26 (2012).
Zhang, Y., Zhou, H., Perkins, A., Wang, Y. & Sun, J. Maternal dietary nutrient intake and its association with preterm birth: a case-control study in Beijing, China. Nutrients 9, https://doi.org/10.3390/nu9030221 (2017).
Hashmi, A. H. et al. Because the baby asks for it’: a mixed-methods study on local perceptions toward nutrition during pregnancy among marginalised migrant women along the Myanmar–Thailand border. Glob. Health Action 11, 1473104 (2018).
Prozialeck, J. D. & Wershil, B. K. In Fetal and Neonatal Physiology 5th edn (eds Polin, R. A. et al.) 876–881.e871 (Elsevier, 2017).
Hamosh, M. et al. Fat digestion in the newborn. Characterization of lipase in gastric aspirates of premature and term infants. J. Clin. Invest. 67, 838–846 (1981).
Keelan, J. A. et al. Cytokines, prostaglandins and parturition-a review. Placenta 24 Suppl A, S33–S46 (2003).
Lei, W. J. et al. IL-33/ST2 axis of human amnion fibroblasts participates in inflammatory reactions at parturition. Mol. Med. 29, 88 (2023).
Romero, R. et al. The role of inflammation and infection in preterm birth. Semin Reprod. Med 25, 21–39 (2007).
Girardi, G., Lingo, J. J., Fleming, S. D. & Regal, J. F. Essential role of complement in pregnancy: from implantation to parturition and beyond. Front. Immunol. 11, https://doi.org/10.3389/fimmu.2020.01681 (2020).
Busse, M. et al. Regulatory B cells are decreased and impaired in their function in peripheral maternal blood in pre-term birth. Front. Immunol. 11, 386 (2020).
Bell, A. W. & Ehrhardt, R. A. Regulation of placental nutrient transport and implications for fetal growth. Nutr. Res. Rev. 15, 211–230 (2002).
Maraia, R. J. & Intine, R. V. La protein and its associated small nuclear and nucleolar precursor RNAs. Gene Expr. 10, 41–57 (2002).
Skorpen, C. G. et al. Influence of disease activity and medications on offspring birth weight, pre-eclampsia and preterm birth in systemic lupus erythematosus: a population-based study. Ann. Rheum. Dis. 77, 264 (2018).
Xiong, Z. et al. EWAS Open Platform: integrated data, knowledge and toolkit for epigenome-wide association study. Nucleic Acids Res. 50, D1004–d1009 (2022).
Moloney, R. A. et al. Ongoing effects of preterm birth on the dopaminergic and noradrenergic pathways in the frontal cortex and hippocampus of guinea pigs. Dev. Neurobiol. 84, 93–110 (2024).
Baardman, M. E. et al. The role of maternal-fetal cholesterol transport in early fetal life: current insights1. Biol. Reprod. 88, https://doi.org/10.1095/biolreprod.112.102442 (2013).
Chen, J., Hua, L., Luo, F. & Chen, J. Maternal hypercholesterolemia may involve in preterm birth. Front. Cardiovasc. Med. 9, 818202 (2022).
Henderickx, J. G. E. et al. Maturation of the preterm gastrointestinal tract can be defined by host and microbial markers for digestion and barrier defense. Sci. Rep. 11, 12808 (2021).
Starr, M. C. & Hingorani, S. R. Prematurity and future kidney health: the growing risk of chronic kidney disease. Curr. Opin. Pediatr. 30, 228–235 (2018).
Wang, X. M. et al. Comparison of DNA methylation profiles associated with spontaneous preterm birth in placenta and cord blood. BMC Med. Genomics 12, 1 (2019).
Lee, H. S. Impact of maternal diet on the epigenome during in utero life and the developmental programming of diseases in childhood and adulthood. Nutrients 7, 9492–9507 (2015).
Herring, C. M., Bazer, F. W., Johnson, G. A. & Wu, G. Impacts of maternal dietary protein intake on fetal survival, growth, and development. Exp. Biol. Med. 243, 525–533 (2018).
Huang, J., Yang, Y. & He, P. Serum apolipoprotein A-II and alpha-2-antiplasmin levels in midtrimester can be used as predictors of preterm delivery. J. Int. Med. Res. 48, 300060520952280 (2020).
Vilotić, A. et al. IL-6 and IL-8: an overview of their roles in healthy and pathological pregnancies. Int. J. Mol. Sci. 23, https://doi.org/10.3390/ijms232314574 (2022).
Ashford, K. et al. Comparison of serum and cervical cytokine levels throughout pregnancy between preterm and term births. AJP Rep. 8, e113–e120 (2018).
Herrera-Muñoz, A., Fernández-Alonso, A. M., Fischer-Suárez, N., Chedraui, P. & Pérez-López, F. R. Maternal serum cytokine levels in pregnancies complicated with threatened preterm labour. Gynecol. Endocrinol. 33, 408–412 (2017).
Sullivan, G. et al. Interleukin-8 dysregulation is implicated in brain dysmaturation following preterm birth. Brain Behav. Immun. 90, 311–318 (2020).
Brummaier, T. et al. A prospective cohort for the investigation of alteration in temporal transcriptional and microbiome trajectories preceding preterm birth: a study protocol. BMJ Open 9, bmjopen–2018 (2019).
Prust, J. T. et al. Risk factor-based screening compared to universal screening for gestational diabetes mellitus in marginalized Burman and Karen populations on the Thailand-Myanmar border: an observational cohort. Wellcome Open Res. 7, 132 (2022).
Bank, F. D. Nutritionist Pro (San Bruno, CA, 2005).
Falcon, S. & Gentleman, R. Using GOstats to test gene lists for GO term association. Bioinformatics 23, 257–258 (2007).
Müller, F. et al. RnBeads 2.0: comprehensive analysis of DNA methylation data. Genome Biol. 20, 55 (2019).
Tang, D. et al. SRplot: a free online platform for data visualization and graphing. PLoS ONE 18, e0294236 (2023).
Acknowledgements
We acknowledge Sidra Medicine for funding this research to Dr. Terranegra (Sidra Medicine Internal Funds SDR400132). We acknowledge the Omics Core Facility at Sidra Medicine for providing DNA methylation service using the Illumina Infinium MethylationEPIC BeadChip array. We gratefully acknowledge the team at Shoklo Malaria Research Unit (SMRU), Mae Sot, Thailand, and the study participants. We thank Dr Mohamed Nadhir Djekidel for helping improve the resolution of Fig. 10.
Author information
Authors and Affiliations
Contributions
A.T. conceived and designed this study. A.T., S.K., D.C., F.N., R.M., T.B., T.K., A.M., and B.K. designed the cohort. T.B., R.M., and F.N. recruited and consented the study participants. F.A. performed experiments and data analysis. S.A. and S.H.A. performed the dietary data analysis. A.P.L., A.A., and S.A. helped with sample processing and data analysis. F.A. wrote the first manuscript. A.T. reviewed the manuscript with input from all co-authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ahmad, F., Lakshmanan, A.P., Alabduljabbar, S. et al. Placental and cord blood DNA methylation in preterm birth: exploring the epigenetic role of maternal dietary protein. npj Sci Food 9, 206 (2025). https://doi.org/10.1038/s41538-025-00566-w
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41538-025-00566-w