Abstract
This study investigates crossmodal fear generalization, testing whether conditioned fear spreads between different sensory modalities. Participants in the unimodal group were presented with visual stimuli—images of a sparrow (CS+) and a laptop (CS−)—while the crossmodal group received auditory stimuli—sparrow calls (CS+) and keyboard typing sounds (CS−). During the generalization phase, both groups were presented with conceptually similar visual stimuli (GSs) with varying similarity to the CS+ (e.g. high: Pigeon, moderate: Duck, low: Goat). Measures included US expectancy ratings, skin conductance responses (SCR), and functional near-infrared spectroscopy (fNIRS). Results showed successful fear acquisition in both groups, with significantly higher US expectancy ratings, SCR, and mPFC HbO activity for CS+ compared to CS−. Both groups exhibited a gradient effect during the generalization phase, with GSs that were more perceptually similar to the CS+ eliciting higher US expectancy ratings. These findings support crossmodal fear generalization and offer new insights into the overgeneralization of fear in anxiety disorders.
Similar content being viewed by others
Introduction
Fear learning is essential for human survival, as it enables individuals to adaptively respond to future threats based on prior experiences1. Through this mechanism, people can even avoid potentially dangerous situations they have not directly encountered—a phenomenon known as fear generalization2,3. Fear generalization refers to the extension of learned fear responses to stimuli or situations that are similar to the original threatening event3,4. Specifically, a conditioned fear response to a stimulus (CS+) that reliably predicts an unconditioned (aversive) stimulus (US) may generalize to stimuli (GSs) that share similarities with the CS+2,3,4. While fear generalization is a natural and adaptive process that aids in avoiding potential dangers, excessive or maladaptive fear generalization is thought to contribute to the development of anxiety-related disorders, including generalized anxiety disorder, post-traumatic stress disorder, and specific phobias5,6,7,8.
Current research on fear generalization predominantly focuses on single sensory modality9,10,11,12,13, such as the visual or auditory sensory, with limited evidence supporting crossmodal fear generalization. For instance, one study presented participants with 10 rings of gradually increasing size (including CS+, CS−, and 8 intermediate-sized GS) to examine fear acquisition and generalization14. The results revealed that participants exhibited stronger fear responses to the CS+ ring and to rings of similar size, compared to the CS−, confirming the presence of fear generalization. Additionally, a visual search task demonstrated that after pairing colors from the blue-green spectrum (489–500 nm) with an electric shock, participants displayed heightened attention to color stimuli similar to the CS+ during the task15. This attentional bias increased as the color more closely resembled the CS+ and diminished as it deviated, illustrating a fear generalization gradient. However, numerous studies suggest that crossmodal information processing enhances individuals’ ability to identify and avoid potential threats more effectively16,17,18. For example, a person bitten by a dog may develop a fear not only of other dogs but also of the sound of barking. This phenomenon, in which fear generalizes across sensory modalities, reflects the transfer of fear responses between visual and auditory stimuli.
A recent study investigated crossmodal fear generalization by using images of typewriters and telephones as the conditioned stimuli (vCS+) and the safety stimuli (vCS−), respectively, and their corresponding sounds (typewriter typing and telephone ringing) as generalization stimuli (aGS+ and aGS−)19; There were two groups in their study: a crossmodal group, which watched visual stimuli during the acquisition phase and heard auditory stimuli during the generalization phase, and a unimodal group, which watched visual stimuli in both phases. The crossmodal group showed significantly higher US expectancy ratings for aGS+ compared to aGS−. Notably, the unimodal group exhibited stronger responses to vCS+ than the crossmodal group did to aGS+. These findings provide the first direct evidence of crossmodal fear generalization, demonstrating that fear learned from visual stimuli can generalize to semantically consistent auditory stimuli. This study lays a foundation for further exploration into the mechanisms underlying crossmodal fear generalization and its implications for anxiety-related disorders. However, it should be noted that in the generalization phase of the study conducted by Gerdes et al., the experimental design included only two categories of stimuli (GS+, GS−). Additionally, the measurement of US expectancy rating was the sole assessment method employed during this phase. Thus, it remains unclear whether crossmodal generalization follows a stimulus gradient and what the neural correlates of crossmodal generalization are.
Fear generalization extends beyond perceptually similar stimuli to include conceptual similarity as a critical factor13,14,15,20,21,22. For instance, after experiencing a traumatic car accident, an individual may develop fear not only toward other cars but also toward seemingly unrelated vehicles, such as cruise ships or airplanes. This phenomenon illustrates that fear is influenced by deeper conceptual similarity, in addition to perceptual resemblance23. Although these stimuli differ substantially in their sensory features, they are categorized as “vehicles” within the individual’s conceptual framework, thereby eliciting a generalized fear response. This type of fear generalization engages not only perceptual-level mechanisms but also higher-order cognitive processes, such as conceptual representation and analogical reasoning24,25. Research suggests that individuals facing fear-inducing stimuli frequently rely on conceptual thinking rather than solely on direct sensory input25,26. This cognitive mechanism is particularly relevant to understanding the development of anxiety-related emotional disorders. Patients with anxiety disorders often exhibit exaggerated fear responses to stimuli that are unrelated to the original traumatic event, likely due to excessive analogy and generalization of “similar” concepts26,27. Understanding the role of conceptual similarity in fear generalization provides important insights into the pathological mechanisms of anxiety disorders. Furthermore, it highlights the potential for developing novel therapeutic strategies focused on cognitive interventions and emotion regulation to address excessive fear generalization.
In this context, theories of learning transfer and semantic consistency offer robust frameworks for understanding the role of conceptual stimuli in the crossmodal fear generalization15,28,29,30. Learning transfer is an essential evolutionary mechanism that allows organisms to apply acquired knowledge to novel situations, facilitating predictions of future events15,28. When stimuli across different sensory modalities share similarities in cognitive processing, they can enhance the transfer of learned associations29. For instance, Bratzke et al. demonstrated the effects of crossmodal transfer in a temporal discrimination task, showing that training with auditory stimuli improved temporal discrimination performance in the visual modality30. Similarly, in the study of fear generalization, it has been observed that fear responses can generalize not only to perceptually similar stimuli (e.g., those with similar shapes or sizes) but also to stimuli that are conceptually and semantically related28. These findings indicate that conceptually similar stimuli may facilitate crossmodal fear generalization between different sensory modalities, extending the understanding of how fear responses are transferred and generalized across sensory and conceptual domains.
Neuroscientific studies have demonstrated that the amygdala, hippocampus, and prefrontal cortex (PFC) play central roles in fear learning and generalization31,32,33,34,35,36,37,38. Among these, the PFC is crucial for regulating fear emotions and inhibiting amygdala activity, thereby modulating the expression of fear responses39,40,41,42,43,44. Specifically, the medial PFC has been implicated in top-down regulation of fear, influencing both the expression and suppression of fear responses triggered by cues36,37. For instance, research on patients with mPFC dysfunction has revealed that, compared to healthy controls, these individuals exhibit significantly enhanced amygdala activation when exposed to threatening stimuli38. Moreover, previous research has also indicated that when the GS more closely resembles the CS+, it elicits a more pronounced activation of the mPFC45. In summary, existing research underscores the significant relationship between mPFC activity and both fear learning and generalization.
Building on previous findings, we adopted a crossmodal conceptual fear generalization experimental design to investigate the presence of a crossmodal fear generalization gradient. First, we extended the visual generalization paradigm from the study by Gerdes et al. to include auditory generalization. Specifically, our crossmodal generalization design transitioned from auditory stimuli during the acquisition phase to visual stimuli during the generalization phase. In the unimodal group, visual stimuli were presented in both phases, whereas in the crossmodal group, auditory stimuli were used during the acquisition phase and visual stimuli during the generalization phase. Second, the GSs were designed to extend beyond modality to include conceptual generalization. The stimuli in the generalization phase were conceptually categorized as highly similar, moderately similar, or minimally similar to the CS+. This approach allowed us to explore generalization gradients both across modalities and conceptual dimensions. Finally, given the critical role of the mPFC in fear processing and regulation, we employed fNIRS to examine neural activities in this region. fNIRS is a non-invasive neuroimaging technique that measures changes in hemoglobin concentration in the brain, reflecting neural activity41. The advantage of the fNIRS is less sensitive to head motion and eliminates the potential auditory interference caused by scanner noise (e.g., fMRI) during fear acquisition42,43. In addition to employing fNIRS to assess frontal cortex activity, we measured US expectancy ratings and SCR as behavioral and physiological indicators of fear learning and generalization.
Our study proposed two hypotheses: (1) Both the unimodal and crossmodal groups will successfully acquire and generalize fear, demonstrating a generalization gradient effect. Specifically, fear responses will increase as the conceptual similarity between the GSs and the CS+ increases19. (2) The HbO activities of the mPFC will show a growing trend as the conceptual similarity between the GSs and the CS+ increases45.
Results
US expectancy ratings
The main effect of stimulus type on US expectancy ratings was significant in the acquisition phase (F1, 79 = 1312.167, p < 0.001, ηp2 = 0.943), with ratings for the CS+ being significantly higher than those for the CS−. The interaction between time block and stimulus type was also significant (F2, 158 = 45.389, p < 0.001, ηp2 = 0.365), indicating that the difference in ratings between the CS+ and CS− increased over time. This suggests that participants successfully learned to associate the CS+ with the electronic shock. No significant effects were observed for other main effects or interactions.
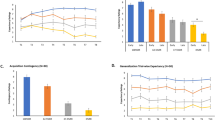
Similarly, in the generalization phase, the main effect of GS type on US expectancy ratings was significant (F2, 158 = 85.506, p < 0.001, ηp2 = 0.520). Ratings for GS1 were significantly higher than for GS2 and GS3 (both p < 0.001), with ratings for GS2 also being significantly higher than for GS3 (p < 0.001). These findings indicate a gradient of generalization, with US expectancy ratings decreasing as similarity to the CS+ decreased. The main effect of time block was significant (F2, 158 = 63.404, p < 0.001, ηp2 = 0.445), reflecting an overall decrease in US expectancy ratings across time. Additionally, the main effect of group was significant (F1, 79 = 10.586, p = 0.002, ηp2 = 0.118), with US expectancy ratings being higher in the unimodal group (3.689 ± 0.185) compared to the crossmodal group (3.072 ± 0.130). Significant interactions were observed between time block and stimulus type (F4, 316 = 3.171, p = 0.020, ηp2 = 0.039), time block and group (F2, 158 = 4.488, p = 0.014, ηp2 = 0.054), and stimulus type and group (F2, 158 = 3.448, p = 0.039, ηp2 = 0.042). Post-hoc t-tests revealed that in the unimodal group, US expectancy ratings followed a clear gradient in time block 1 (GS1 > GS2, p = 0. 010; GS2 > GS3, p = 0.001), whereas in the crossmodal group, no such gradient was evident (GS1 vs GS2, p = 0.057; GS2 vs GS3, p = 0.062). In time block 2, the unimodal group demonstrated a generalization gradient (GS1 > GS2, p < 0.050; GS2 > GS3, p = 0.029), while the crossmodal group showed a partial gradient (GS1 > GS2, p < 0. 001; GS2 vs GS3, p = 0.544). In time block 3, both groups showed a partial generalization gradient: for the unimodal group (GS1 > GS2, p = 0. 029; GS2 vs GS3, p = 0. 218) and for the crossmodal group (GS1 > GS2, p < 0.001; GS2 vs GS3, p = 0.371) (Fig. 1). These results suggest delayed fear generalization in the crossmodal group. The three-way interaction among time block, stimulus type, and group was not significant (F4, 316 = 1.602, p = 0.180, ηp2 = 0.020).
a The unimodal group. b The crossmodal group. Each dot represents the mean score across all participants in each group. The error bars indicate the standard error of the mean. A1, A2, and A3 are the time blocks of the acquisition phase, and G1, G2, and G3 are those of the generalization phase. *p < 0.05, **p < 0.01, and ***p < 0.001.
SCR results
In the acquisition phase, the main effect of stimulus type was significant (F1, 79 = 47.569, p < 0.001, ηp2 = 0.376), with SCR being significantly higher for the CS+ compared to the CS−. A significant main effect of time block was also observed (F2, 158 = 46.788, p < 0.001, ηp2 = 0.372), such that SCR fell over time. The main effect of group was also significant (F1,79 = 4.055, p = 0.047, ηp2 = 0.049), with higher SCR in the unimodal group (0.711 ± 0.026) than in the crossmodal group (0.651 ± 0.024). None of the interactions between factors were significant.
In the generalization phase, the main effect of time block was significant (F2, 156 = 11.280, p < 0.001, ηp2 = 0.126). The other main effects and all interactions were nonsignificant (Fig. 2).
a The unimodal group. b The crossmodal group. Each dot represents the mean score across all participants in each group. The error bars indicate the standard error of the mean. A1, A2, and A3 are the time blocks of the acquisition phase, and G1, G2, and G3 are those of the generalization phase. *p < 0.05, **p < 0.01, and ***p < 0.001.
fNIRS results
The repeated-measures ANOVA for the acquisition phase showed a main effect of stimulus type on brain activity in the mPFC (F1,38 = 20.191, p < 0.001, ηp2 = 0.347), but no significant main effect of group (F1,38 = 0.299, p = 0.588, ηp2 = 0.008) or stimulus type × group interaction (F1,38 = 0.123, p = 0.728, ηp2 = 0.003). The post-hoc test showed that mPFC activity was significantly higher in responses to the CS+ than to the CS− in both the unimodal group (p = 0.006) and the crossmodal group (p = 0.001).
The repeated-measures ANOVA for the generalization phase indicated no significant main effects of stimulus type or group on mPFC HbO concentration (stimulus type: F2, 76 = 1.191, p = 0.315, ηp2 = 0.061; group: F1, 38 = 0.099, p = 0.755, ηp2 = 0.003), and no significant interaction between the two (F2, 76 = 0.043, p = 0.958, ηp2 = 0.002). Further, responses to the GSs did not differ in either group (see Fig. 3). Given that pairwise comparisons among the GSs did not yield significant differences, we conducted an exploratory analysis to further examine the overall engagement of the mPFC in fear generalization. Specifically, we compared mPFC activity in response to the GSs with responses to the CS− during the acquisition phase, which served as a non-threat-related baseline. Exploratory paired-sample t-tests comparing mPFC responses to the CS− with those to GS1, GS2, and GS3 revealed that some GSs elicited significantly higher activity, while others showed trend-level differences than those to the CS− in the unimodal group (GS1: t19 = 1.871, p = 0.077; GS2: t19 = 1.632, p = 0.119; GS3: t19 = 2.876, p = 0.010). The results for the crossmodal group were similar (GS1: t19 = 3.761, p = 0.001; GS2: t19 = 1.990, p = 0.061; GS3: t19 = 2.667, p = 0.015). This pattern can be interpreted to mean that threat generalization on the behavioral level was successfully mirrored in the mPFC responses. However, we did not observe a generalization gradient or any group differences. Descriptive statistics summary of all results see Supplementary Table 1.
Different activation levels of mPFC in the two groups during the acquisition and generalization phases. *p < 0.05, **p < 0.01, and ***p < 0.001. Note: GS–CS− comparisons were conducted as an exploratory analysis and are not shown in the figure for consistency with other measures (i.e., US expectancy ratings and SCR).
Discussion
This study extended the traditional Pavlovian fear conditioning paradigm by developing and validating a crossmodal fear generalization paradigm based on conceptual stimulation. It further investigated the gradient effect of crossmodal fear generalization and its underlying neural correlates. We found that both experimental groups successfully acquired fear responses to CS+ during the acquisition phase, with no significant group differences. During the generalization phase, both groups including the crossmodal and unimodal generalization exhibited a gradient generalization effect in US expectancy ratings, such that the GS1 more similar to the CS+ elicited higher US expectancy ratings. Regarding neural correlates, both groups demonstrated significantly stronger HbO activites in the mPFC in response to the GS1 compared to the CS−.
The behavioral data of this study provided robust evidence supporting the gradient effect of fear generalization. Specifically, participants exhibited higher US expectancy ratings for stimuli that were more similar to the CS+. Specfically, the GS1 elicited significantly higher US expectancy ratings compared with the GS2 and the GS3 in both experimental groups. This finding suggests that the fear response followed a gradual gradient across different generalization stimuli, aligning with previous studies14,15. This gradient effect is consistent with the classical theory of fear generalization, which posits that after individuals associate a specific stimulus with threat, they exhibit an exaggerated fear response to stimuli resembling the original threat stimulus44,45. We extended the generalization gradient effect from the unimodal generalization to the crossmodal generalization. Interestingly, although the GS1 in the crossmodal group elicited significantly higher US expectancy ratings than GS2 or GS3, this effect did not emerge during the first block. In the unimodal group, the GS1 were significantly higher than the GS2 and the GS3. Thus, we propose that the delayed generalization gradient effect in crossmodal generalization may be attributed to the shift in stimulus modality as compared to unimodal generalization. Individuals undergoing crossmodal generalization require additional time to acclimate to the novel modality. Further research is needed to replicate this delayed generalization gradient effect within the context of crossmodal generalization.
Our fNIRS data revealed that the stimulus-induced HbO activities of the mPFC during the acquisition phase were significantly higher for the CS+ compared to the CS− in both groups, indicating that the mPFC is more responsive to the CS+ and plays a role in encoding threat-related stimuli. This finding is consistent with previous research that the mPFC might show an important role in threat discrimination during the fear acquisition stage38,45,46. However, although the slope of activites in mPFC might show a generalization gradient, the mPFC activities elicited by the GSs did not differ from each other. Further exploratory analyses revealed that during the generalization phase, the mPFC in both groups showed differential responses to the GSs compared to the CS− during the acquisition phase. Specifically, some GSs elicited significantly higher mPFC responses than the CS−, while others showed only trend-level differences. This pattern suggests that the mPFC is more engaged in processing stimuli conceptually related to the CS+ than in processing the non-threat CS−, though the strength of this engagement varied across stimuli.
Our study builds on the work of Gerdes et al., which provided direct evidence for crossmodal generalization of conditioned fear. Consistent with their findings, our results confirm the bidirectional transmission of fear information between auditory and visual modalities. While Gerdes et al. demonstrated that learned fear associated with visual stimuli can generalize to semantically consistent auditory stimuli19, our study extends this understanding by showing that conditioned fear can also generalize from auditory stimuli to semantically consistent visual stimuli. Additionally, our experiment introduced GSs with varying degrees of conceptual similarity to the CS+—high, medium, and low similarity. The need to judge complex conceptual similarity likely engages higher-order cognitive processes, suggesting that fear generalization spans multiple levels of processing47,48,49. This includes not only simple perceptual similarity but also higher-order conceptual evaluations50,51,52, highlighting the intricate mechanisms underlying fear generalization.
From a theoretical perspective, our findings validated the crossmodal generalization gradient of conditioned fear, aligning with the hierarchical network model53,54,55. This model posits that concepts (e.g., sparrows) are stored as nodes within a network, with connections to other related concepts (e.g., orioles, fish) varying by similarity and relevance53,54. During concept processing, a distance effect emerges: the greater the conceptual distance, the greater the difficulty in categorization and generalization55,56. In the context of fear generalization, stimuli with higher conceptual similarity are more likely to be perceived as analogous threat sources, eliciting stronger fear responses. Conversely, stimuli with lower similarity are harder to associate with the conditioned fear stimulus, resulting in weaker responses14,15,37. Our findings support this hierarchical network theory, demonstrating that fear generalization is influenced not only by perceptual similarity but also by complex conceptual hierarchies. This provides a theoretical framework for understanding the cognitive mechanisms underlying crossmodal fear generalization.
Overgeneralization is widely regarded as a key mechanism in the development of anxiety-related disorders. Patients with such disorders exhibit a steeper generalization gradient compared to healthy individuals5,57,58, reflecting impaired discrimination of fear-related stimuli and excessive fear generalization. Exposure therapy, the most common treatment for anxiety disorders, aims to reduce fear responses through repeated exposure to conditioned stimuli that are not paired with the unconditioned stimulus59,60,61. However, real-world environments are rich in multisensory information. Future advancements in exposure therapy could explore crossmodal approaches, leveraging the interplay between sensory modalities to enhance treatment efficacy.
Our study did not observe crossmodal fear generalization in SCR. Both groups successfully acquired fear during the acquisition phase, but no significant differences in SCR were found during the generalization phase. This discrepancy between SCR and US expectancy ratings may stem from their distinct measures of fear response. SCR reflects physiological arousal controlled by the sympathetic nervous system62,63, whereas US expectancy ratings capture subjective cognitive evaluations. One possible reason for the flat trend in SCR during the generalization phase is the highly habituative nature of SCR, which can become less sensitive over time, especially in the absence of reinforcement. This aligns with findings that SCR is more responsive to direct threat signals40, but less sensitive to conceptual or crossmodal fear generalization. Our results are consistent with similar findings in related studies19,40,45. The divergence between SCR and US expectancy ratings highlights that physiological and cognitive indicators may represent different mechanisms underlying fear generalization. Future research should investigate how these mechanisms interact to influence the fear generalization process.
This study has several limitations. First, the sample may lack representativeness, and the findings should be replicated in larger and more diverse populations to improve statistical power and generalizability. Accordingly, the present study should be considered exploratory. Second, the imbalance between the CSs could have influenced the results. For instance, individual preferences for specific stimuli may have impacted the conditioning process and generalization outcomes. Third, to maintain consistency in stimulus modality, the original CS was not presented during the generalization phase. Since the two groups had already experienced different modalities during acquisition, reintroducing the original CS could have introduced confounding effects. This methodological choice may help explain the rapid decline in participants’ fear responses to the CS+, potentially attenuating group differences in generalization. Finally, while a 0.05 Hz high-pass filter is not the most commonly used setting, it has been applied in prior studies40. Future work may explore how different preprocessing choices, including filter parameters, impact SCR measures in fear conditioning paradigms. And the functions of the prelimbic and infralimbic cortex in the mPFC can not be clearly distinguished with the fNIRS technique, future studies could explore the different functions of these mPFC areas in crossmodal generalization.
Additionally, in this study, the GSs systematically varied in conceptual similarity to the CS+ but did not include stimuli similar to the CS−. This approach focused on examining conceptual fear generalization gradients while avoiding the additional complexity of incorporating stimuli from a different category, such as tools. However, this design limits direct comparisons with traditional paradigms that include stimuli similar to both the CS+ and CS−. Future research could address this by including GSs similar to both the CS+ and CS−, allowing for a more comprehensive analysis of the interaction between conceptual similarity and categorical differences in fear generalization. And this study focused solely on the audio-visual modality, and future research could consider investigating the impact of other sensory modalities64,65, such as olfaction, taste, and touch, on fear learning and generalization.
In summary, this study developed a crossmodal fear generalization paradigm using conceptual stimulation to investigate its gradient effect and underlying neural mechanisms. The results demonstrated that individuals successfully learned fear in both unimodal and crossmodal conditions, exhibiting a gradient generalization effect based on similarity to CS+. In terms of neural mechanisms, the both groups displayed significant mPFC activation. This suggests that crossmodal generalization may involve more complex cognitive processing mechanisms. The study also validated the use of conceptual stimulation, highlighted the potential role of crossmodal fear generalization in the excessive generalization observed in anxiety disorders, and provided new insights for future emotion regulation-based intervention strategies.
Methods
Participants
Fifty-one healthy participants (30 female) were recruited from Sichuan Normal University through campus advertisements. Participants were randomly assigned to either the unimodal group (n = 26) or the crossmodal group (n = 25). All participants were right-handed, had normal hearing and normal or corrected-to-normal vision, no history of psychiatric or neurological disorders, and no prior participation in similar studies. They received 40 RMB (~6 USD) as compensation. Participation was voluntary, and written informed consent was obtained from all participants before the start of the experiment. The study was approved by the Ethics Committee of Sichuan Normal University (reference number: SICNU-230211), in accordance with the Declaration of Helsinki54. Due to poor SCR data quality or excessive noise, six participants from the unimodal group and five from the crossmodal group were excluded. As a result, data from 40 participants (20 per group, 12 females per group; age range: 18–22 years, M ± SD = 19.87 ± 1.14) were included in all subsequent analyses. A post hoc power analysis was conducted using G*Power (ANOVA: repeated measures, within-between interaction) to evaluate whether the collected sample size was sufficient. With an effect size of Cohen’s f = 0.25 (medium), α = 0.05, and 1 – β = 0.80, the analysis indicated that a total of 22 participants (11 per group) would be required to achieve the desired power. The actual power achieved with our sample was 0.836.
Stimuli and apparatus
The visual stimuli included photographs of a sparrow (vCS+), a laptop (vCS−). And the GSs were selected based on their conceptual similarity to the CS+, systematically varying across high similarity (GS1), medium similarity (GS2), and low similarity (GS3) levels (see Table 1). These stimuli were primarily associated with animal and environmental concepts related to the CS+ and did not include stimuli from the tool category. This design aimed to focus on examining gradient effects driven by conceptual similarity without introducing confounding elements associated with stimuli similar to the CS−. The auditory stimuli comprised a sparrow call (aCS+) and the sound of keyboard typing (aCS−) (see Supplementary Note 1).
The US was a mild electric shock (50 ms) delivered to the left wrist. It was produced by a multichannel electrical stimulator (type: SXC-4A, Sanxia Technique Inc., China) and delivered through a pair of Ag/AgCl surface electrodes. Participant pain thresholds were assessed and the current level was adjusted individually to a level that the participants described as “highly uncomfortable but not painful”40,45. The average current intensity was 6.094 ± 2.133 μA.
SCR was measured using the BIOPAC MP160 system at 2000 Hz (Pushengda Science & Trade, Beijing, China), with Ag/AgCl gel sensors adhered to the hypothenar surface of the left hand.
Brain activity was monitored using a Nirscan-2430 fNIRS system (Danyang Huichuang, Jiangsu, China). The system operated at two wavelengths, 760 nm and 830 nm, with an average inter-channel distance of 3 cm and a sampling rate of 11 Hz. The acquisition cap was equipped with 19 light sources (transmitters) and 20 detectors (receivers), resulting in a total of 56 channels. The probe in the middle of the cap’s lower edge was positioned at Frontal midpoint (Fpz). The sources and detectors were strategically positioned on the scalp according to the 10–20 system37 (Fig. 4). The mPFC was designated as the region of interest (ROI) based on extensive prior literature elucidating its involvement in fear13,38,45.
Procedure and design
Before the experiment, several potential confounding variables were assessed. In particular, depression and anxiety levels were assessed using the Patient-Health Questionnaire-9 (see Supplementary Note 2), Center for Epidemiologic Studies Depression Scale66, and the Generalized Anxiety Disorder 7-item Scale67 (Table 2). Next, the participants were seated in front of a monitor in a quiet room and the pain threshold for the electric shock was assessed.
The experiment consisted of two phases: the acquisition phase and the generalization phase, and was implemented using E-Prime 2.0 software (Fig. 5). In the acquisition phase, the unimodal group was presented with visual stimuli, while the crossmodal group was presented with auditory stimuli. The CS+ was either an image of a sparrow or the sound of a sparrow call, paired with an electric shock (unconditioned stimulus, US) on 9 out of 12 trials (reinforcement rate: 75%). Conversely, the CS− was either an image of a laptop or the sound of typing/clicking, presented in all 12 trials but never paired with the electric shock. The presentation order of the CS+ and CS− was pseudo-randomized, with no more than two consecutive presentations of the same stimulus type.
The generalization phase introduced three types of stimuli—GS1, GS2, and GS3—that varied in their conceptual and physical similarity to the CS+. GS1 was the most similar to the CS+, while GS3 was the least similar. Notably, no electric shocks were delivered during this phase. Each stimulus was presented once, resulting in a total of 36 trials (Table 1).
Across both phases, each stimulus was presented for 8 s. After the stimulus disappeared, participants rated the perceived risk of receiving an electric shock on a 9-point Likert scale, where 1 indicated “no risk”, 5 indicated “moderate risk”, and 9 indicated “high risk”. The rating period was self-paced, and participants advanced to the next trial by pressing a key to submit their response. If a shock was associated with the stimulus, it was administered immediately following the submission of the rating. The intertrial interval (ITI), represented by a blank black screen, varied randomly between 9 and 12 s to minimize predictability and occurred after the rating was submitted.
SCR data pre-processing
The SCR data were pre-processed using AcqKnowledge 5.0 software. A high-pass filter with a cutoff frequency of 0.05 Hz was applied to remove low-frequency noise and artifacts from the SCR recordings. Trials in which no SCR peak was detected (i.e., no increase in SCR during the 1–6 s window following stimulus onset) were considered as zero-response trials. Additionally, trials were classified as zero-response if the difference between the maximum and minimum SCR amplitudes was less than 0.02 μs45. The response value for subsequent analyses was calculated as the square root of the difference between the maximum and minimum skin conductance values measured 6 s after stimulus onset62.
fNIRS data pre-processing
For data preprocessing of the fNIRS signals, we used the Homer2 in Matlab r2019b. The main calculation steps are described as follows: 1) Based on the modified Beer–Lambert law, the light density information was calculated and converted into the HbO concentration change values. 2) the light intensity information was converted into light density information. 3) the artifacts were corrected; 4) the signal was bandpass filtered (0.01 to 0.2 Hz). then, 5) the signal was normalized as Z-scores because the absolute concentration values significantly differed among participants37,45. We obtained the Z-scores as z = (μ1 – μ2)/σ, where μ2 is the mean of the baseline, μ1 is the mean concentration value, and σ is the SD during the baseline period. To obtain the mean values in the calculation, we extracted a time series of concentrations from 0 s before stimuli onset to 8 s after stimuli offset. The baseline period was from −2 s to 0 s and the concentration period was from 0 to 8 s. Finally, we averaged the values from 1 s to 8 s for the final analysis. A repeated-measures ANOVA was then conducted on the HbO Z-scores obtained from s the brain areas that showed significant activation during the acquisition and generalization phases. The alpha threshold for statistical significance was set at 0.05 after FDR correction.
Data analysis
All statistical analyses were conducted using IBM SPSS 23 (IBM Corporation, Armonk, NY, USA) and Matlab software (Version 2019, Natick, Mass., USA). Behaviorally, outcome measures included the self-reported US expectancy ratings for the stimuli. Physiologically, the study included SCR data and HbO concentration levels in the brain areas that showed significant activation. As the HbO signal is more sensitive to changes in cerebral blood flow than the deoxyHb signal68,69, only HbO time series were analyzed in this study. In the acquisition phase, the 12 trials for each stimulus type were divided into three blocks of four trials based on their presentation order within the task. A 3 (time block: A1, A2, A3) × 2 (stimulus type: CS+, CS−) × 2 (group: unimodal, crossmodal) repeated measures ANOVA was conducted for both the US expectancy ratings and the SCR data. For the generalization phase, the 12 trials for each GS type were also divided into three blocks of four trials based on their presentation order. A 3 (time block: G1, G2, G3) × 3 (stimulus type: GS1, GS2, GS3) × 2 (group: unimodal, crossmodal) repeated measures ANOVA was conducted for the US expectancy ratings and SCR data. For analysis of the fNIRS data, we did not divide the data into time blocks, as a certain number of trials per stimulus type is required for obtaining clear results. In the acquisition phase, a 2 (stimulus type: CS+, CS−) × 2 (group: unimodal, crossmodal) repeated measures ANOVA was conducted. In the generalization phase, a 3 (stimulus type: GS1, GS2, GS3) × 2 (group: unimodal, crossmodal) repeated measures ANOVA was employed. In instances where the sphericity assumption was violated, the Greenhouse-Geisser correction was applied for repeated-measures ANOVA to adjust the degrees of freedom and ensure valid statistical tests70. To control for the potential inflation of Type I error due to multiple comparisons, Bonferroni correction was applied during follow-up analyses. The alpha threshold for statistical significance was set at 0.05.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
The codes that support the findings of this study are available from the corresponding author upon reasonable request.
References
Beckers, T. et al. Understanding clinical fear and anxiety through the lens of human fear conditioning. Nat. Rev. Psychol. 2, 233–245 (2023).
Fraunfelter, L., Gerdes, A. B. M. & Alpers, G. W. Fear one, fear them all: a systematic review and meta-analysis of fear generalization in pathological anxiety. Neurosci. Biobehav. Rev. 139, 104707 (2022).
Webler, R. D. et al. The neurobiology of human fear generalization: meta-analysis and working neural model. Neurosci. Biobehav. Rev. 128, 421–436 (2021).
Pavlov, P. I. Conditioned Reflexes: an Investigation of the Physiological Activity of the Cerebral Cortex (Oxford University Press, 1927).
Cooper, S. E. et al. A meta-analysis of conditioned fear generalization in anxiety-related disorders. Neuropsychopharmacology 47, 1652–1661 (2022).
Kaczkurkin, A. N. et al. Neural substrates of overgeneralized conditioned fear in PTSD. Am. J. Psychiatry 174, 125–134 (2017).
Lissek, S. et al. Neural substrates of classically conditioned fear-generalization in humans: a parametric fMRI study. Soc. Cogn. Affect. Neurosci. 9, 1134–1142 (2014).
Onat, S. & Büchel, C. The neuronal basis of fear generalization in humans. Nat. Neurosci. 18, 1811–1818 (2015).
Dunsmoor, J. E., Martin, A. & LaBar, K. S. Role of conceptual knowledge in learning and retention of conditioned fear. Biol. Psychol. 89, 300–305 (2012).
Dunsmoor, J. E., Kroes, M. C. W., Braren, S. H. & Phelps, E. A. Threat intensity widens fear generalization gradients. Behav. Neurosci. 131, 168–175 (2017).
Ghosh, S. & Chattarji, S. Neuronal encoding of the switch from specific to generalized fear. Nat. Neurosci. 18, 112–120 (2015).
Resnik, J. & Paz, R. Fear generalization in the primate amygdala. Nat. Neurosci. 18, 188–190 (2015).
Wang, Y. et al. Exaggerated sensitivity to threat and reduced medial prefrontal engagement during threat generalization in reactive aggressive adolescents. NeuroImage 294, 120645 (2024).
Lissek, S. et al. Generalization of conditioned fear-potentiated startle in humans: experimental validation and clinical relevance. Behav. Res. Ther. 46, 678–687 (2008).
Dowd, E. W., Mitroff, S. R. & Labar, K. S. Fear generalization gradients in visuospatial attention. Emotion 16, 1011–1018 (2016).
Chen, Y.-C. & Spence, C. When hearing the bark helps to identify the dog: semantically-congruent sounds modulate the identification of masked pictures. Cognition 114, 389–404 (2010).
Molholm, S. et al. Multisensory auditory-visual interactions during early sensory processing in humans: A high-density electrical mapping study. Cogn. Brain Res. 14, 115–128 (2002).
Sun, X. W., Sun, Y. & Qiu-Fang, F. U. Cross-modal learning and its cognitive and neural mechanisms. Prog. Biochem. Biophys. 46, 565–577 (2019).
Gerdes, A. B. M., Fraunfelter, L. & Alpers, G. W. Hear it, fear it: fear generalizes from conditioned pictures to semantically related sounds. J. Anxiety Disord. 69, 102174 (2020).
Bennett, M., Vervoort, E., Boddez, Y., Hermans, D. & Baeyens, F. Perceptual and conceptual similarities facilitate the generalization of instructed fear. J. Behav. Ther. Exp. Psychiatry 48, 149–155 (2015).
Dunsmoor, J. E., White, A. J. & LaBar, K. S. Conceptual similarity promotes generalization of higher order fear learning. Learn. Mem. 18, 156–160 (2011).
Morey, R. A., Haswell, C. C., Stjepanović, D., Mid-Atlantic MIRECC Workgroup, Dunsmoor, J. E. & LaBar, K. S. Neural correlates of conceptual-level fear generalization in posttraumatic stress disorder. Neuropsychopharmacology45, 1380–1389 (2020).
Boyle, S., Roche, B., Dymond, S. & Hermans, D. Generalisation of fear and avoidance along a semantic continuum. Cognit. Emot. 30, 340–352 (2016).
Lei, Y., Wang, J., Zhu, Y., Chen, Q. & Li, H. P300 and positive slow waves reveal the plausibility in inductive reasoning. Psychophysiology 56, e13337 (2019).
Mei, Y., Dai, Y. & Lei, Y. The influence of hierarchical masks on masked repetition priming: Evidence from event-related potential investigation. Front. Hum. Neurosci. 13, 70 (2019).
Lei, Y., Mei, Y., Dai, Y. & Peng, W. Taxonomic relations evoke more fear than thematic relations after fear conditioning: an EEG study. Neurobiol. Learn. Mem. 167, 107099 (2020).
Wang, J. et al. Influence of perceptual and conceptual information on fear generalization: a behavioral and event-related potential study. Cogn., Affect. Behav. Neurosci. 21, 1054–1065 (2021).
Foa, E. B. & Kozak, M. J. Emotional processing of fear: exposure to corrective information. Psychol. Bull. 99, 20–35 (1986).
Kouvaris, K., Clune, J., Kounios, L., Brede, M. & Watson, R. A. How evolution learns to generalise: using the principles of learning theory to understand the evolution of developmental organisation. PLoS Comput. Biol. 13, e1005358 (2017).
Bratzke, D., Seifried, T. & Ulrich, R. Perceptual learning in temporal discrimination: asymmetric cross-modal transfer from audition to vision. Exp. Brain Res. 221, 205–210 (2012).
Dunsmoor, J. E., Bandettini, P. A. & Knight, D. C. Impact of continuous versus intermittent CS−UCS pairing on human brain activation during Pavlovian fear conditioning. Behav. Neurosci. 121, 635–642 (2007).
Liu, X. et al. Medial prefrontal and occipito-temporal activity at encoding determines enhanced recognition of threatening faces after 1.5 years. Brain Struct. Funct. 227, 1655–1672 (2022).
Lopresto, D., Schipper, P. & Homberg, J. R. Neural circuits and mechanisms involved in fear generalization: implications for the pathophysiology and treatment of posttraumatic stress disorder. Neurosci. Biobehav. Rev. 60, 31–42 (2016).
Xin, F. et al. Oxytocin differentially modulates amygdala responses during top-down and bottom-up aversive anticipation. Adv. Sci. 7, 2001077 (2020).
Zhou, F. et al. A distributed fMRI-based signature for the subjective experience of fear. Nat. Commun. 12, 6643 (2021).
Zhou, F. et al. Human extinction learning is accelerated by an angiotensin antagonist via ventromedial prefrontal cortex and its connections with basolateral amygdala. Biol. Psychiatry 86, 910–920 (2019).
Zhou, X. et al. Intolerance of uncertainty enhances adolescent fear generalization in both perceptual-based and category-based tasks: fNIRS studies. Behav. Res. Ther. 183, 104650 (2024).
Motzkin, J. C., Philippi, C. L., Wolf, R. C., Baskaya, M. K. & Koenigs, M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol. Psychiatry 77, 276–284 (2015).
Kroes, M. C. W. et al. Patients with dorsolateral prefrontal cortex lesions are capable of discriminatory threat learning but appear impaired in cognitive regulation of subjective fear. Soc. Cogn. Affect. Neurosci. 14, 601–612 (2019).
Zhang, J. et al. Sleep deprivation increases the generalization of perceptual and concept-based fear: an fNIRS study. J. Anxiety Disord. 105, 102892 (2024).
Quaresima, V. & Ferrari, M. Functional near-infrared spectroscopy (fNIRS) for assessing cerebral cortex function during human behavior in natural/social situations: a concise review. Organ. Res. Methods 22, 46–68 (2019).
Li, K. et al. Functional near-infrared spectroscopy-informed neurofeedback: regional-specific modulation of lateral orbitofrontal activation and cognitive flexibility. Neurophotonics 6, 025011 (2019).
Yang, X. et al. Secondary rewards acquire enhanced incentive motivation via increasing anticipatory activity of the lateral orbitofrontal cortex. Brain Struct. Funct. 226, 2339–2355 (2021).
Jasnow, A. M., Lynch, J. F., Gilman, T. L. & Riccio, D. C. Perspectives on fear generalization and its implications for emotional disorders. J. Neurosci. Res. 95, 821–835 (2017).
Dou, H., Lei, Y., Cheng, X., Wang, J. & Leppänen, P. Social exclusion influences conditioned fear acquisition and generalization: a mediating effect from the medial prefrontal cortex. Neuroimage 218, 116735 (2020).
Roesmann, K. et al. Transcranial direct current stimulation of the ventromedial prefrontal cortex modulates perceptual and neural patterns of fear generalization. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 7, 210–220 (2022).
Spalding, K. N. The role of the medial prefrontal cortex in the generalization of conditioned fear. Neuropsychology 32, 1–17 (2018).
Roesmann, K. et al. Behavioral and magnetoencephalographic correlates of fear generalization are associated with responses to later virtual reality exposure therapy in spider phobia. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 7, 221–230 (2022).
Morrison, N. M., Burnham, D. & Morrison, B. W. Cognitive load in cross-modal dual-task processing. Appl. Cogn. Psychol. 29, 436–444 (2015).
Sandhu, R. & Dyson, B. J. Cross-modal perceptual load: the impact of modality and individual differences. Exp. Brain Res. 234, 1279–1291 (2016).
Likhtik, E. & Paz, R. Amygdala-prefrontal interactions in (mal)adaptive learning. Trends Neurosci. 38, 158–166 (2015).
Likhtik, E., Stujenske, J. M., Topiwala, M. A., Harris, A. Z. & Gordon, J. A. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat. Neurosci. 17, 106–113 (2014).
Lei, Y., Liang, X. & Lin, C. How do the hierarchical levels of premises affect category-based induction: diverging effects from the P300 and N400. Sci. Rep. 7, 11758 (2017).
Liang, X., Chen, Q., Lei, Y. & Li, H. How types of premises modulate the typicality effect in category-based induction: diverging evidence from the P2, P3, and LPC effects. Sci. Rep. 6, 37890 (2016).
Collins, A. M. & Loftus, E. F. A spreading-activation theory of semantic processing. Psychol. Rev. 82, 407 (1975).
Springer, U. S., Murphy, G. L., Hampton, J. A. & Milovanovic, G. S. Semantic memory redux: an experimental test of hierarchical category representation. J. Mem. Lang. 67, 521–539 (2012).
Dymond, S., Dunsmoor, J. E., Vervliet, B., Roche, B. & Hermans, D. Fear generalization in humans: systematic review and implications for anxiety disorder research. Behav. Ther. 46, 561–582 (2015).
Reinhard, J. et al. Fear conditioning and fear generalization in children and adolescents with anxiety disorders. Eur. Child Adolesc. Psychiatry 33, 2163–2172 (2024).
Raeder, F., Merz, C. J., Margraf, J. & Zlomuzica, A. The association between fear extinction, the ability to accomplish exposure and exposure therapy outcome in specific phobia. Sci. Rep. 10, 4288 (2020).
Telch, M. J., York, J., Lancaster, C. L. & Monfils, M. H. Use of a brief fear memory reactivation procedure for enhancing exposure therapy. Clin. Psychol. Sci. 5, 367–378 (2017).
Hofmann, S. G. Cognitive processes during fear acquisition and extinction in animals and humans: implications for exposure therapy of anxiety disorders. Clin. Psychol. Rev. 28, 199–210 (2008).
Bach, D. R., Friston, K. J. & Dolan, R. J. An improved algorithm for model-based analysis of evoked skin conductance responses. Biol. Psychol. 94, 490–497 (2013).
Baumann, C. et al. Effects of an anxiety-specific psychometric factor on fear conditioning and fear generalization. Z. Psychol. 225, 200–213 (2017).
Janssens, T., Martens, F., Storms, N. & Van den Bergh, O. Generalization of respiratory symptom triggers. Behav. Ther. 46, 689–698 (2015).
Wesson, D. W. & Wilson, D. A. Smelling sounds: olfactory-auditory sensory convergence in the olfactory tubercle. J. Neurosci.30, 3013–3021 (2010).
Radloff, L. S. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1, 385–401 (1977).
Spitzer, R. L., Kroenke, K., Williams, J. B. W. & Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 166, 1092–1097 (2006).
Hoshi, Y. Functional near-infrared optical imaging: Utility and limitations in human brain mapping. Psychophysiology 40, 511–520 (2003).
Lindenberger, U., Li, S.-C., Gruber, W. & Müller, V. Brains swinging in concert: cortical phase synchronization while playing guitar. BMC Neurosci. 10, 22 (2009).
Greenhouse, S. W. & Geisser, S. On methods in the analysis of profile data. Psychometrika 24, 95–112 (1959).
Acknowledgements
The research was supported by the following grants: STI 2030-Major Projects 2022ZD0210900, National Natural Science Foundation of China [NSFC32300928 and NSFC32271142], The Ministry of Education of Humanities and Social Science project [23YJC190003], Natural Science Foundation of Sichuan Province [2025ZNSFSC1023], Guangdong Key Project in “Development of new tools for diagnosis and treatment of Autism” [2018B030335001], Ministry of Education Key Projects of Philosophy and Social Sciences Research [21JZD063], and the Shenzhen Science and Technology Research Funding Program [JCYJ20200109144801736].
Author information
Authors and Affiliations
Contributions
X.L., B.B., H.D. and Y.L. conceived and designed the project. Y.L. supervised the project. X.L. created the experiment and collected the data. X.L., B.B., H.D. and Y.W. analyzed the data. X.L. wrote the initial draft of the paper. All authors contributed to the writing and editing of the manuscript, and all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, X., Becker, B., Wang, Y.J. et al. A visual generalization gradient of conceptual stimuli based on fear acquisition in visual and auditory modalities. npj Sci. Learn. 10, 37 (2025). https://doi.org/10.1038/s41539-025-00318-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41539-025-00318-1