Abstract
Highly pathogenic avian influenza (HPAI) H5 viruses from different clades have been circulating globally, threatening wild/domestic birds and mammals. Given frequent spillovers and high mortality among mammals, coupled with our inability to predict which clade of H5 virus has pandemic potential, cross-clade protective HPAI H5 vaccines are urgently needed. Here, we demonstrate the applicability of a lipid nanoparticle-based mRNA vaccine modality to induce cross-protective immunity against lethal HPAI virus infection.
Similar content being viewed by others
Highly pathogenic avian influenza (HPAI) H5 viruses first emerged in 1996 in Guangdong China. Since then, the viruses have diversified into different antigenic clades, threatening wild and poultry birds and causing sporadic infections in humans with high mortality1. In 2016, HPAI H5 viruses of the clade 2.3.4.4b were first detected in Europe. The virus actively reassorted with different neuraminidases (NA) such as N6, N8 and N1 NA and spread globally, causing mortality among multiple species of wild and farm mammals/birds as well as sporadic human cases1,2. Meanwhile, HPAI H5 viruses of clades 2.3.2.1a and 2.3.2.1c have been circulating in poultry in Southeast Asian countries, leading to human fatal cases3,4,5,6,7. Given the recent global outbreaks of HPAI H5 viruses in wild birds and mammals, the risk of more frequent spillover to humans has increased. The most effective countermeasure to reduce influenza disease severity is vaccination; accordingly, vaccines that can mount cross-protective immunity against different clades of H5 viruses are urgently needed.
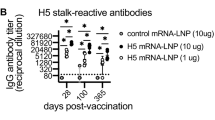
We generated a monovalent lipid nanoparticle-based mRNA (LNP-mRNA) vaccine candidate encoding hemagglutinin (HA) from the clade 2.3.4.4b A/chicken/Ghana/AVL-76321VIR7050-39/2021 (ch/Ghana) virus (DS8390). BALB/c mice (N = 5) were intramuscularly mock-immunized or immunized with 1 or 10 μg of DS8390 twice with a 2-wk interval (Fig.1a). At 2 wks after the second immunization, sera and spleens were collected. HA-binding IgG antibody titers were analyzed in an ELISA against the vaccine-homologous ch/Ghana HA and a heterologous HA from A/India/SARI-4571/2021 (India/S4571; H5N1 virus of clade 2.3.2.1a). India/S4571 HA has 43 amino acid substitutions compared to ch/Ghana HA (Supplementary Fig. 1). Binding IgG antibodies against the homologous ch/Ghana HA were significantly induced in a DS8390 dose-dependent manner (Fig. 1b), whereas the binding titers against the heterologous HA were lower compared to the homologous HA, as anticipated (Fig. 1c). Neutralizing antibody titers were also analyzed. Although the CPE-based neutralizing titer against the authentic virus was below the detection threshold in the assay, possibly due to the high replication efficiency of the virus, when we used pseudotyped virus bearing the homologous ch/Ghana HA, neutralizing antibodies were elicited in sera with a high dose (10 μg) of DS8390 (Table 1). We also examined antigen-specific splenic T-cell responses. Single cell suspensions (splenocytes) were prepared from spleens of individual animals, and were re-stimulated with the vaccine antigen-homologous ch/Ghana recombinant HA protein or heterologous India/S4571 HA in vitro. After a 3-day incubation, the frequency of IFN-γ-secreting cells were detected at equivalent levels with homologous or heterologous HA stimulation in an ELISpot assay (Fig. 1d, e), suggesting that T-cell responses were mounted at similar levels against homologous and heterologous HAs.
a Timeline of mouse immunization. Groups of BALB/c mice (7-wk-old females; N = 5/group) were intramuscularly (i.m.) mock-vaccinated or vaccinated with 1 or 10 μg of DS8390 by using a prime & boost regimen. Two weeks after the boost immunization, serum was collected and single cell suspensions (splenocytes) were prepared from spleens of individual animals. Binding antibody titers against the homologous ch/Ghana HA (b) or heterologous India/S4571 HA (c) in sera were analyzed in an ELISA. Area under the curve (AUC) values for individual animals were plotted. Bars show the median of the groups. Splenocytes (2 × 105 live cells/well) were stimulated with 3 μg of the homologous ch/Ghana HA (d) or heterologous India/S4571 HA protein (e) at 37 °C for 3 days. IFN-γ-secreting cells were detected by use of an ELISpot assay. Statistical analyses were performed by use of a one-way analysis of variance (ANOVA) and corrected for multi-group comparisons by using Dunnett’s test. (*P < 0.05; **P < 0.01; ***P < 0.001, ****P < 0.0001. n.s., not significant).
Finally, we examined the protective effects of DS8390 against homologous or heterologous HPAI H5 virus challenge. BALB/c mice were immunized by the same vaccination regimen as used previously. Pre-challenge sera analysis at 2 wks post-boost (Supplementary Fig. 2 and Supplementary Table 1) showed similar results as the immunogenicity assay (Fig. 1). At 3 wks post-boost, the mice were challenged with a lethal dose of homologous ch/Ghana virus or heterologous India/S4571 virus (Fig. 2a). The vaccinated animals were all protected from ch/Ghana virus, showing no body weight loss even with the low-dose (1 μg) vaccination, whereas the mock-vaccinated animals all died or were euthanized due to severe ( > 25%) weight loss or neurological symptoms by Day 10 post-challenge (Fig. 2b, c). Upon challenge with the lethal dose of heterologous India/S4571 virus, the vaccinated animals all survived regardless of whether they were vaccinated with the high (10 μg) or low dose (1 μg) of DS8390 whereas the mock-vaccinated animals all died by Day 10 post-challenge (Fig. 2d, e). These results demonstrate that the LNP-mRNA-H5HA vaccine potently induced cross-protective immunity in vivo.
a Timeline of the virus challenge study. Groups of BALB/c mice (7-wk-old females; N = 5/group) were intramuscularly (i.m.) mock-vaccinated or vaccinated with 1 or 10 μg of DS8390 by using a prime & boost regimen. Pre-challenge sera collected 2 wks post-boost were analyzed in an ELISA (Supplementary Fig. 2) and a neutralization assay (Supplementary Table 1). At 3 wks post-boost immunization, the animals were intranasally (i.n.) challenged with 10 MLD50 of homologous A/chicken/Ghana/AVL-76321VIR7050-39/2021 (ch/Ghana; 6.1 × 102pfu/animal) virus (b, c) or heterologous A/India/SARI-4571/2021 (India/S4571; 3.2 × 102pfu/animal) virus (d, e). Survival rate (b, d) and body weight change (c, e) were monitored for 14 days (% compared to Day 0; mean + SD).
Serum binding antibodies were observed at higher levels against vaccine-homologous clade HA than heterologous HA (Fig. 1b, c), whereas the elicited spleen T-cell responses were similar levels against homologous and heterologous HA (Fig. 1d, e). Neutralizing antibodies titers were detected with pseudotyped virus, but were below or around the detection threshold with authentic homologous virus (Table 1, Supplementary Table 1), possibly due to the high replication efficiency of the virus in cells. A previous study found that binding antibody responses were induced against diverse H5 virus strains in vitro8. Here, we demonstrated that animals vaccinated with DS8390 are protected from a lethal homologous or heterologous virus challenge (Fig. 2), which may indicate that the T-cell immunity elicited by DS8390 is involved in protective immunity in vivo. Recent studies by other groups also showed that T-cell immunity induced by mRNA-based vaccine contributes to protective effects against H5 virus infection8,9,10. Our data demonstrate that DS8390 can induce potent cross-clade anti-HPAI H5 protective immunity against lethal infection in mice, validating the applicability of the LNP-mRNA vaccine modality for preparedness against the increasing risk of spillover events of HPAI viruses to humans and potential transmissibility among humans.
Methods
Cells
Madin-Darby Canine Kidney (MDCK) cells were maintained in Eagle’s minimal essential medium (MEM) containing 5% newborn calf serum (NCS) and 1% Penicillin/streptomycin. Human embryonic kidney 293 T cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS). Lenti-X 293 T cells were maintained in DMEM supplemented with 10% FBS, and 1% Penicillin/streptomycin. MDCK-SIAT1 cells were maintained in DMEM supplemented with 1 mg/ml G418 Geneticin, 10% FBS, and 1% Penicillin/streptomycin .
Viruses
H5N1 viruses [A/chicken/Ghana/AVL-76321VIR7050-39/2021 (H5N1; clade 2.3.4.4b), and A/India/SARI-4571/2021 (H5N1; clade 2.3.2.1a)] were generated by reverse genetics11 with synthesized gene fragments cloned to pHH21 plasmids based on the sequences from GISAID, and were propagated in MDCK cells in MEM containing 0.3% bovine serum albumin (BSA) and 0.5 µg/mL N-p-Tosyl-L-phenylalanine chloromethyl ketone (TPCK)-treated trypsin. The experiments with highly pathogenic avian influenza viruses were performed in enhanced biosafety level 3 (BSL3) containment laboratories at the University of Tokyo, Japan, which are approved for such use by the Ministry of Agriculture, Forestry, and Fisheries, Japan.
Pseudotyped viruses
A pseudotyped virus bearing the HA and NA proteins from the A/chicken/Ghana/AVL-76321VIR7050-39/2021 strain was generated in Lenti-X 293 T cells by co-transfection with four plasmids: pCAG-H5HA and pCAG-N1NA containing the coding sequences of the H5-HA and N1-NA proteins, respectively; the lentiviral backbone plasmid pCI-neo synHIVgp-RRE, carrying the gag and env genes of HIV; and the pGreenFire Transcriptional Reporter Lentivector, which expresses green fluorescent protein (GFP) and the firefly luciferase reporter. Supernatants containing the pseudotyped H5N1 virus were collected 48 h after transfection and filtered. The TCID50 value of the pseudotyped virus was determined in MDCK-SIAT1 cells by measuring luciferase reporter activity.
Preparation of DS8390
T7 RNA polymerase-mediated transcription in vitro was used to synthesize the mRNA from a linearized DNA template, in which the open-reading frame of the human codon-optimized HA gene from A/chicken/Ghana/AVL-76321VIR7050-39/2021(H5N1; clade 2.3.4.4b) was flanked by 5′ and 3′ untranslated regions and a poly-A tail. Messenger RNA for the HA was purified and then encapsulated into lipid nanoparticles (LNPs) composed of ionizable lipid, phospholipid, cholesterol, and PEG-lipid.
Animal experiments
The experiments with mice were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee at the Animal Experiment Committee of the Institute of Medical Science, the University of Tokyo (approval number: PA20-06). Virus inoculations were performed under anesthesia with isoflurane, and animals were humanely euthanized by cervical dislocation under deep anesthesia with isoflurane to minimize any suffering after virus infection.
Mouse immunization
BALB/c mice (7-wk-old females; obtained from Japan SLC, Inc.) were anesthetized with isoflurane and intramuscularly mock-immunized with vehicle buffer [10 mM histidine, 300 mM sucrose (pH 7.0)] or immunized with 1 or 10 μg of DS8390 in 20 μL twice with a 2-wk interval between immunizations for immunogenicity analysis and for virus challenge studies.
Immunogenicity analysis in immunized mice
For the humoral immunity and T-cell response analysis, the immunized mice were anesthetized with isoflurane 2 weeks after their second immunization with DS8390, and blood was collected by cardiac puncture under deep anesthesia. After euthanasia by cervical dislocation, spleens were collected to prepare single cell suspensions.
Micro-neutralization assay
Virus neutralizing antibody titers against H5 influenza viruses were evaluated in serum samples. Serum samples were treated with receptor-destroying enzyme (RDE; Denka seiken) at 37 °C for 20 h, inactivated at 56 °C for 1 h, and diluted 1:10 in phosphate-buffered saline (PBS). Two-fold serial dilutions of sera were prepared in MEM, and each dilution was incubated with the same volume of virus diluent (100 TCID50/50 µL) in MEM containing 1 µg/mL of TPCK-trypsin at room temperature for 1 h. The serum/virus mixture was added to 100% confluent MDCK cells that were plated a day prior in 96-well plates. The cells were incubated for 3 days at 37 °C and then cytopathic effect (CPE) was microscopically assessed by eye. Virus neutralization titers were determined as the reciprocal of the highest serum dilution that completely prevented CPE. Each sample was analyzed in duplicate for geometric mean titers.
Pseudotyped virus neutralization assay
Serum neutralizing activity against the pseudotyped virus bearing HA and NA protein from H5 influenza viruses was evaluated. Sera were treated with receptor-destroying enzyme (RDE; Denka seiken) at 37 °C for 18–20 h, inactivated at 56 °C for 30 min, and diluted 1:10 in OPTI/MEM. Two-fold serial dilutions of sera were prepared in OPTI/MEM, and each dilution was incubated with the same volume of virus diluent (100 TCID50/50 µL) in OPTI/MEM at 37 °C for 30 min. The serum/virus mixture was added to 100% confluent MDCK-SIAT1 cells that were plated a day prior in 96-well plates. The cells were incubated for 2 days at 37 °C then luciferase activity was measured by using Bright-Glo Luciferase detection system (Promega). The luminescence signal was measured using a plate reader (BMG Labtech). Cut-off value to determine that the virus is not infected based was set on the value of the negative control well, and virus neutralization titers were determined as the reciprocal of the highest serum dilution that completely prevented. Each sample was analyzed in duplicate for geometric mean titers.
Recombinant protein expression and purification
To construct expression plasmids for soluble-form recombinant HAs (rHA) of A/chicken/Ghana/AVL-76321VIR7050-39/2021 or A/India/SARI-4571/2021, the HA signal peptide and ectodomain (amino acid residues HA1-1–HA2-176) with stabilizing mutations to form disulfide bonds (HA1-M30C and HA2-K51C) and a detoxified cleavage site (KRRKR/G was substituted with A/G; the slash indicates the cleavage site), followed by a T4 foldon trimerization domain and a hexa-histidine tag at the C-terminus, were cloned into pCAGGS plasmids. Proteins were expressed in Expi293F cells (Thermo Fisher Scientific) and purified by using TALON metal affinity resin (TaKaRa Clontech).
Enzyme-linked immunosorbent assay (ELISA)
The ELISA was performed using rHAs for mouse sera. The ELISA plates were coated overnight at 4 °C with 50 µl of the antigen protein at a concentration of 2 µg/ml in PBS. After blocking with PBS containing 1% BSA, the plates with incubated in triplicate with heat-inactivated (56 °C for 30 minutes) serum that was 5-fold serially diluted in PBS containing 0.5% BSA and 0.05% Tween 20 (PBS-BT). After a 1-h incubation at room temperature, the plates were washed with PBS containing 0.1% Tween 20 (PBS-T) four times and then incubated with anti-mouse IgG (H + L) secondary antibody conjugated with horseradish peroxidase (1:20,000 dilution in PBS-BT) at room temperature for 1 h. Then, the plates were washed four times with PBS-T, and developed with 1-Step Ultra TMB-ELISA Substrate Solution (Thermo Scientific). After a 10-minute incubation, the reaction was stopped with the addition of 1 N sulfuric acid. The absorbance was measured immediately at a wavelength of 450 nm.
Enzyme-linked immunospot (ELISpot) assay
Multiscreen 96-well plates with PVDF membrane (Millipore) were coated with anti-mouse IFN-γ capture antibody at 4 °C for overnight, and blocked in RPMI 1640 media containing 10% FCS for 2 h. To prepare single cell suspensions from spleen, the spleen of each mouse was individually cut into 2–3 mm pieces, incubated with collagenase D in Hanks’ Balanced Salt Solution (HBSS) at 37 °C for 30 mins, and gently mushed on a 70-µm cell strainer (BD Falcon). The dissociated cells were centrifuged, treated in 1X RBC lysis buffer (eBioscience) for 2 mins, and washed in HBSS. The cells were then re-suspended in RPMI 1640 containing 10% FCS, 1X MEM non-essential amino acid solution (Gibco), 1 mM Sodium Pyruvate (Gibco), and 0.05 mM 2-mercaptoethanol. Live cell numbers were determined by using trypan blue staining. Then, 2 × 105 live cells/well from each animal were plated in the pre-coated assay plates with 3 µg of the homologous ch/Ghana rHA or heterologous India/S4571 rHA protein in 120 µL of RPMI media and cultured at 37 °C for 3 days. The plates were then washed in water and processed to stain spots by using the mouse IFN-gamma ELISpot kit (BD) according to the manufacturer’s instructions. Stained spots were counted by use of an ImmunoSpot analyzer (CTL).
Virus challenge of immunized mice
For virus challenge studies, blood was collected from the immunized animals via the submandibular vein under anesthesia with isoflurane at 2 weeks after the second immunization. At 3 weeks after the second immunization, the mice were intranasally infected with 10 mouse median lethal dose (MLD50) of A/chicken/Ghana/AVL-76321VIR7050-39/2021 (H5N1; clade 2.3.4.4b), or A/India/SARI-4571/2021 (H5N1; clade 2.3.2.1a). Body weights and survival rates were monitored daily for 14 days. Mice with more than 25% body weight loss or with neurological symptoms were euthanized by cervical dislocation under deep anesthesia with isoflurane.
Data availability
All data generated or analyzed during this study are included in this manuscript. All relevant data are available upon reasonable request from the corresponding author.
References
Verhagen, J. H., Fouchier, R. A. M. & Lewis, N. Highly Pathogenic Avian Influenza Viruses at the Wild-Domestic Bird Interface in Europe: Future Directions for Research and Surveillance. Viruses 13 https://doi.org/10.3390/v13020212 (2021).
Graziosi, G., Lupini, C., Catelli, E. & Carnaccini, S. Highly Pathogenic Avian Influenza (HPAI) H5 Clade 2.3.4.4b Virus Infection in Birds and Mammals. Animals (Basel) 14 https://doi.org/10.3390/ani14091372 (2024).
Potdar, V. et al. Identification of Human Case of Avian Influenza A(H5N1) Infection, India. Emerg. Infect. Dis. 28, 1269–1273 (2022).
Tare, D. S., Keng, S. S., Walimbe, A. M. & Pawar, S. D. Phylogeography and gene pool analysis of highly pathogenic avian influenza H5N1 viruses reported in India from 2006 to 2021. Arch. Virol. 169, 111 (2024).
Guan, L. et al. Highly Pathogenic H5 Influenza Viruses Isolated between 2016 and 2017 in Vietnamese Live Bird Markets. Viruses 15 https://doi.org/10.3390/v15051093 (2023).
Zhong, G. et al. Isolation of Highly Pathogenic H5N1 Influenza Viruses in 2009-2013 in Vietnam. Front Microbiol 10, 1411 (2019).
Chang, P. et al. Characterization of the haemagglutinin properties of the H5N1 avian influenza virus that caused human infections in Cambodia. Emerg. Microbes Infect. 12, 2244091 (2023).
Furey, C. et al. Development of a nucleoside-modified mRNA vaccine against clade 2.3.4.4b H5 highly pathogenic avian influenza virus. Nat. Commun. 15, 4350 (2024).
Cui, X. et al. Immunogenicity and biodistribution of lipid nanoparticle formulated self-amplifying mRNA vaccines against H5 avian influenza. NPJ Vaccines 9, 138 (2024).
Li, Y. et al. Protective efficacy of a universal influenza mRNA vaccine against the challenge of H1 and H5 influenza A viruses in mice. mLife 2, 308–316 (2023).
Neumann, G. et al. Generation of influenza A viruses entirely from cloned cDNAs. Proc. Natl Acad. Sci. USA 96, 9345–9350 (1999).
Acknowledgements
We thank Susan Watson for scientific editing, and Kyoko Yokota, Rie Onoue and Nao Yamasaki for technical assistance. This study was supported by grants from the Japan Program for Infectious Diseases Research and Infrastructure (JP24wm0125002), the Japan Initiative for World-leading Vaccine Research and Development Centers (JP243fa627001), and the Program on R&D of new generation vaccine including new modality application (JP243fa827010) from Japan Agency for Medical Research and Development (AMED).
Author information
Authors and Affiliations
Contributions
S.C. developed the initial concept and designed the study, conducted experiments, analysed the data, and wrote a draft of the manuscript; M.K., conducted experiments; Sh.Y., developed the initial concept and designed the study, conducted experiments, analysed the data, and wrote a draft of the manuscript; K.S, developed the initial concept and design of the study; Y.O., conducted experiments; A.Y., conducted experiments; S.M., conducted experiments; R.U., designed the study and conducted experiments; K.I-H., conducted experiments; Se.Y., designed the study; F.T., developed the initial concept and designed the study, and acquired funding; Y.K. developed the initial concept and design of the study, analysed the data, wrote a draft of the manuscript, and acquired funding. All the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.Y., K.S., Y.O., A.Y., T.M., and F.T. are employees of Daiichi Sankyo Co., Ltd. Y.K. has received unrelated funding support from FUJIFILM Toyama Chemical Co., Ltd.; TAUNS Laboratories, Inc.; Shionogi & Co., Ltd.; Otsuka Pharmaceutical Co., Ltd.; KM Biologics Co., Ltd.; Kyoritsu Seiyaku Corporation; Shinwa Corporation; and Fujirebio Diagnostics. Y.K. is also a co-founder of FluGen, Inc. Y.K. is supported by grants from the Japan Program for Infectious Diseases Research and Infrastructure (JP24wm0125002) and the Japan Initiative for World-leading Vaccine Research and Development Centers (JP243fa627001) from Japan Agency for Medical Research and Development (AMED). Y.K. and Daiichi Sankyo Co., Ltd. are also supported by the Program on R&D of new generation vaccine including new modality application (JP243fa827010) from AMED. All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chiba, S., Kiso, M., Yamada, S. et al. An mRNA vaccine candidate encoding H5HA clade 2.3.4.4b protects mice from clade 2.3.2.1a virus infection. npj Vaccines 9, 189 (2024). https://doi.org/10.1038/s41541-024-00988-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-024-00988-9