Abstract
Protein subunit vaccines, lacking pathogen-associated molecular patterns that trigger immune responses, rely on adjuvants to induce robust immune responses against the target pathogen. Thus, selection of adjuvants plays a crucial role in the design of protein subunit vaccines. Recently, there has been growing interest in utilizing cGAS-STING agonists as vaccine adjuvants. In this study, we investigated the adjuvant effect of manganese (Mn), a cGAS-STING agonist, on the tuberculosis subunit vaccine Bfrb-GrpE (BG) in a mouse model. Initially, mice were administered with BG-Mn(J), and its immunogenicity and protective efficacy were assessed six weeks after the final immunization. The results showed that Mn(J) enhanced both the cellular and humoral immune responses to the BG vaccine and conferred effective protection against M. tuberculosis H37Ra infection in mice, leading to a significant reduction of 2.0 ± 0.17 Log10 CFU in spleens and 1.3 ± 0.17 Log10 CFU in lungs compared to the PBS control group. Additionally, we assessed the BG-Mn(J) vaccine in a surrogate model of tuberculosis in rabbit skin model. The vaccination with BG-Mn(J) also provided effective protection in the rabbit model, as indicated by a decreased bacterial load at the infection site, minimal pathological damage, and accelerated healing. These findings suggest that Mn(J) holds promise as an adjuvant for tuberculosis vaccines, underscoring its potential to enhance vaccine efficacy and offer protection against tuberculosis infection.
Similar content being viewed by others
Introduction
Tuberculosis is a persistent infectious disease caused by Mycobacterium tuberculosis (M. tuberculosis) and poses a substantial threat to human health. Recent data from the World Health Organization (WHO) estimates approximately 10.6 million cases of tuberculosis and 1.3 million deaths worldwide in 20221. Moreover, the increasing prevalence of multidrug-resistant tuberculosis (MDR-TB) presents greater challenges for tuberculosis control2. From an immunological perspective, the effective management of infectious diseases is primarily achieved through vaccination. Therefore, the development of effective tuberculosis vaccines is paramount for public health.
Currently, the Bacillus Calmette-Guerin (BCG) vaccine remains the sole tuberculosis vaccine in clinical use. Although it is effective in preventing severe tuberculosis in children, its efficacy in preventing adult tuberculosis is constrained3. Hence, there is a critical need to prioritize research on new TB vaccines. Several new TB vaccines, such as recombinant BCG vaccines, live attenuated vaccines, viral vector vaccines, recombinant protein subunit vaccines, and DNA vaccines, are currently under investigation in clinical or preclinical research4. Among these, protein subunit vaccines are particularly promising due to their well-defined composition, high safety profile, and capacity to elicit long-term immune protection with well-designed formulations5.
Adjuvants are critical components of protein subunit vaccines, as they are nonspecific substances that modulate the magnitude and the quality of immune responses to protein antigens6. An ideal vaccine adjuvant should not only improve the protective effect of antigens and reduce their required dose, but also be suitable for large-scale production, safe, and cost-effective. Currently, adjuvants such as ASO1E, IC31, GLA-SE, CpG ODN, and BC02 have been utilized for tuberculosis subunit vaccine preparations7. While these adjuvants have undergone evaluation in clinical trials, none currently exhibit all the essential qualities of an ideal vaccine adjuvant8. Therefore, the development of novel adjuvants is imperative for the advancement of tuberculosis vaccines.
The cyclic guanosine monophosphate (GMP)- adenosine monophosphate (AMP) synthase stimulator of interferon gene (cGAS-STING) DNA-sensing pathway plays a distinctive role in both innate and adaptive immunity. Research has demonstrated its involvement in regulating macrophage polarization9, activating NK cells10, and promoting the maturation and activation of dendritic cells (DCs)11,12, thus enhancing antigen presentation. Moreover, activation of the cGAS-STING pathway can subtly orchestrate the type, magnitude, and quality of many immune responses, including humoral immune responses13, CD4+ T cell differentiation14, and CD8+ T cell immunological responses15. Notably, cGAS- STING agonists have recently been found to contribute to memory T cell formation for durable immune responses16. Consequently, there has been growing interest in using agonists of this pathway as vaccine adjuvants17,18.
Manganese ions have been demonstrated to enhance antigen uptake and presentation through activation of cGAS-STING pathways, thereby bolstering immune responses19. Studies indicate that Mn2+ serves as a crucial sensitizer for bacterial or viral DNA, directly binding to cGAS in the cytoplasm and significantly augmenting cGAMP and dsDNA sensing. Furthermore, Mn2+ enhances the binding affinity of cGAMP and STING20. Additionally, research suggests that even in the absence of dsDNA, Mn2+ can bind to and activate cGAS21,22,23. The adjuvant potential of manganese has been verified in various vaccines, such as SARS-CoV-224, rabies and influenza25,26. It has been observed that manganese adjuvant, functioning both as an immune activator and a delivery system elicits humoral, cellular, and mucosal immune responses with minimal side effects27. However, the potential for manganese to be used in tuberculosis vaccines is not yet known.
In our previous study, we developed a novel fusion protein BG by combining M. tuberculosis ferritin BfrB and heat shock protein GrpE28. Furthermore, we demonstrated that combining BG with composite adjuvant DDA-poly I:C-cholesterol (DPC) stimulated strong cellular and humoral immune responses in mice28. DPC is a cationic liposome adjuvant composed of cationic liposome N, N’-dimethyl-N, N’-dioctadecylammonium bromide (DDA), Toll-like receptor agonist polyinosinic-polycytidylic acid (poly I:C) and cholesterol. Among them, DDA and Poly I:C are the effective components for antigen delivery, with cholesterol serving as a stabilizer for large-scale production29. DP is a simplified version of DPC adjuvant, composed of DDA and Poly I:C. Considering that DPC and DP induced almost similar immune responses and protective efficacy29, in this study, we selected the simpler DP adjuvant as a reference to evaluate the adjuvant effect of Mn(J) on tuberculosis subunit vaccine Bfrb-GrpE both in mice and rabbits.
Results
The subunit vaccines BG-DP and BG-Mn(J) induced a high level of antigen-specific IFN- γ in mice
The fusion protein BfrB-GrpE (BG), without any tags, was successfully purified by a two-step process involving ammonium sulfate precipitation followed by hydrophobic interaction chromatography (Fig. 1A). To determine the endotoxin content in the purified BG protein, we conducted the LAL test and the result revealed an endotoxin level of 0.091 EU/mL, which falls below the limit for animal use. The purified BG was then combined with adjuvants DP and Mn(J) separately to produce subunit vaccines BG-DP and BG-Mn(J). To evaluate the adjuvant effect of these two different adjuvants on BG, the C57BL/6 mice were subcutaneously vaccinated with BG-DP and BG-Mn(J) three times at weeks 0, 2, and 4 (Fig. 1B). Six weeks post the final immunization, antigen-specific IFN-γ secretion in splenocytes were detected by ELISA.
A The purified BG fusion protein. Lane 1, protein marker; Lane 2, E. coli lysate supernatant; Lane 3, BG protein precipitated with 35% saturated ammonium sulfate; Lane 4, the purified BG fusion protein by ammonium sulfate precipitation and chromatography. B Vaccination procedure for mice. The BCG-immunized group had a single inoculation at week 0, while the other groups had three inoculations at weeks 0, 2, and 4. Antigen-specific immune responses were assessed 6 weeks post the final immunization. All mice received subcutaneous injections. C Vaccination procedure for rabbits. The BCG-immunized group had a single inoculation at week 0, while the other groups had three inoculations at weeks 0, 2, and 4. Antigen-specific immune responses were assessed 6 weeks post-final immunization. All rabbits received subcutaneous injections. The figure was generated by ourself.
It was observed that both the BG-DP and BG-Mn(J) vaccines induced significantly higher levels of BfrB and GrpE specific cytokine IFN-γ compared to the PBS and BCG groups (p < 0.05) (Fig. 2). However, the subunit vaccine BG-DP elicited notably higher levels of antigen-specific IFN- γ than BG-Mn(J) (p < 0.001) (Fig. 2). These findings suggest that both the adjuvants DP and Mn(J) enhance the vaccine’s capacity to stimulate a stronger cellular immune response compared to BCG.
Six weeks post the final immunization, fresh splenocytes were isolated and stimulated with BfrB or GrpE (5 μg/mL) for 72 h. Subsequently, the culture supernatants were collected, and the IFN-γ ELISA kit was used to quantify cytokine levels. A BfrB-specific IFN-γ levels. B GrpE-specific IFN-γ levels. *p < 0.05, **p < 0.01, and ***p < 0.001. Data are presented as means ± standard deviations.
The subunit vaccines BG-DP and BG-Mn(J) induced high levels of antigen-specific antibodies in mice and rabbits
Six weeks after the last immunization, mouse serum was collected to measure the levels of antigen-specific antibodies IgG1, and IgG2c, and also total IgG against BfrB and GrpE. We found that BCG vaccination failed to stimulate mice to produce high levels of antibodies, with levels comparable to those in the PBS group (Fig. 3A–C, E–G). In contrast, mice receiving BG-DP and BG-Mn(J) vaccinations exhibited higher levels of antigen-specific IgG and IgG1 antibodies against BfrB and GrpE, compared to the BCG and PBS groups (Fig. 3A–D). However, it is important to note that while the levels of IgG1 in mice vaccinated with BG-Mn(J) were similar to those in BG-DP vaccinated mice, the levels of IgG2c were lower (Fig. 3C, G). Consequently, the ratio of IgG2c/IgG1 in the BG-DP group was higher than that in the BG-Mn(J) group (p < 0.05) (Fig. 3D, H), indicating a stronger Th1-biased immune response in the BG-DP vaccinated mice.
Serum samples were collected 6 weeks post the final injection, and the levels of anti-BfrB and anti-GrpE IgG1, and IgG2c, and also total IgG were assessed. A BfrB-specific IgG. B BfrB-specific IgG1. C BfrB-specific IgG2c. D GrpE-specific IgG. E GrpE-specific IgG1. F GrpE-specific IgG2c. G The ratio of BrfB-specific IgG2c and IgG1. H The ratio of GrpE-specific IgG2c and IgG1. Data are presented as means ± standard deviations.
Furthermore, we evaluated the production of antibodies induced by immunization with these two vaccines in rabbits. Following the same immunization protocol as in mice, rabbits were subjected to three immunizations with a two-week interval between each dose (Fig. 1C). Six weeks after the final immunization, serum samples were collected to measure the production of antibodies. Due to the availability of only rabbit serum IgG antibodies, we focused our testing on IgG level. The results were consistent with those observed in mice, as both BG-DP and BG-Mn(J) vaccinations induced significantly higher levels of BfrB and GrpE-specific IgG antibodies compared to the BCG and PBS groups in rabbits as well (Fig. 4). However, the difference observed after vaccination in the two animal models is that BCG vaccination could stimulate antibody production in rabbits, whereas it did not stimulate antibody production in mice (Fig. 4).
BG-Mn(J) vaccination reduces the bacterial burden in the spleens and lungs following challenge in mice
To evaluate the protective effects of the vaccine BG-Mn(J), mice were vaccinated and subsequently infected with M. tuberculosis H37Ra via tail vein injection. Three weeks post-infection, the bacterial load in the lung and spleen tissues was quantified by CFU counting. Notably, the PBS group exhibited the highest bacterial load in both spleen and lung. In contrast, vaccination with BCG and BG-Mn(J) significantly reduced the bacterial load (Fig. 5). Compared to the spleen bacterial load in the PBS-vaccinated mice (6.5 ± 0.19 Log10 CFU), BG-Mn(J) vaccination reduced the bacterial load by 2.0 ± 0.17 Log10 CFU, while BCG vaccination reduced it by 1.95 ± 0.18 Log10 CFU. Similarly, compared to the lung bacterial load in the PBS-vaccinated mice (7.28 ± 0.08 Log10 CFU), BG-Mn(J) vaccination reduced the bacterial load by 1.3 ± 0.17 Log10 CFU, while BCG vaccination reduced it by 2.62 ± 0.15 Log10 CFU. These observations indicate that BG-Mn(J) provides a level of protection to mice that is comparable to BCG vaccination. Surprisingly, we found that BG-DP did not provide significant protection to mice (Fig. 5).
Six weeks after the final vaccination, the vaccinated mice were infected with M. tuberculosis H37Ra (5 × 106 CFU/mouse) by tail-vein injection. Three weeks post-challenge, bacterial loads in lung and spleen tissues were quantified using CFU counting. A Bacterial loads in spleens. B Bacterial loads in lungs. Data are presented as means ± standard deviations. *p < 0.05, and **p < 0.01.
BG-Mn(J) vaccination demonstrates effective protection against M. bovis BCG challenge in the rabbit skin model
The characteristic of tuberculosis infection in the lungs in humans is characterized by immunopathological damage like liquefaction, which can be produced in the rabbit skin model27,28, while the protective immune response established by vaccination can reduce the immunopathological damage and promote lesion repair. In order to further evaluate the protective effect of BG-DP and BG- Mn(J), we utilized the rabbit skin model. As stated earlier, six weeks after the final vaccination, rabbits were intradermally injected at two sites on each flank with M. bovis BCG. Subsequently, we conducted daily observations of the immunopathological changes at the injection sites and recorded the skin lesions. To quantitatively assess the skin lesions following infection, we measured their width, length and height, and calculated their volumes by multiplying these three dimensions.
After M. bovis BCG infection in the rabbit skin model, we observed that the rabbits in the vaccine-immunized groups and those in the PBS control group developed significant immunopathological lesions typical of tuberculosis, such as tuberculosis granulomas, caseous necrosis and liquefaction, ulceration, and healing process (Fig. 6) (Table 1). Interestingly, the rabbits that received BG-Mn(J) vaccination exhibited the largest size of tuberculous granuloma, measuring 1174.62 ± 599.05 mm3 before liquefaction and 7252.61 ± 2946 mm3 when the liquefaction aera was at its maximum (Fig. 6), followed by BG-DP, BCG and PBS (Table 1). Furthermore, we determined the bacterial load in the liquefied caseum necrotic material at the peak of liquefaction by CFU counting. We found that the PBS group had the highest mycobacterial load (5.4 × 106 CFU/g), while the BG-Mn(J) vaccine group had the lowest bacterial load (1.33 × 106 CFU/g), followed by BG-DP (2.4 × 106 CFU/g) and BCG (3.49 × 106 CFU/g) (Fig. 7A). To confirm that the bacteria causing the liquefaction of lesions were mycobacteria rather than contamination by other bacteria, the liquefied necrotic material before and after culture underwent acid-fast staining. All bacteria in every test sample exhibited acid-fast traits, and no other bacteria were observed under the microscope (Fig. 7). These results indicated that the bacterial load in the liquefied caseum necrosis is consistent with the size of the granuloma.
A Volumes of the skin lesions in different vaccine groups. B Photographic representation of various pathological stages of tubercles. Six weeks after the last vaccine immunization, rabbits received intradermal injections of 5 × 106 CFU BCG in the skin on each flank. The volume of the skin lesions was measured daily by assessing width, length, and thickness. The figure was generated by ourself.
Additionally, based on the criteria for tuberculous granulomas, caseous necrosis and liquefaction, ulceration, and healing, the onset of these typical pathological manifestations was also recorded. After M. bovis BCG challenge, the rabbits in the vaccinated groups showed an earlier immunopathological response and healing than that in the PBS control group (Table 1). Among the vaccinated groups, the rabbits that received BG-DP exhibited the earliest onset of liquefaction and ulceration, occurring on days 7 and 9 post-challenge, respectively. But their lesions healed the last, with the healing process commencing on day 24 and completing on day 29 (Table 1). For the rabbits that received BG- Mn(J) or BCG, the onsets of liquefaction and ulceration were the same, occurring on days 8 and 10 post-challenge, respectively. However, it is worth noting that, compared to the BCG group, the rabbits in the BG-Mn(J) group had a shorter healing time by 4 days, despite both groups started the healing process on the 18th day (Table 1).
At the peak of liquefaction and during the complete healing stage, the skin lesions were subjected to tissue pathological examination. Hematoxylin and eosin (HE) staining revealed that the pathological changes in skin lesions in each group were consistent with mycobacterial infection, including infiltration of inflammatory cells, lymphocytes and the presence of liquefied necrotic tissues and Langhan’s giant cells (Fig. 8). These results further demonstrate the success of the surrogate rabbit model for pulmonary tuberculosis. At the peak of liquefaction, there were no significant differences in pathological changes between the different groups. However, during the complete healing stage, the skin lesions in rabbits immunized with BG-Mn(J) exhibited typical fibrotic tubercles and displayed a reduction in the number of newly formed blood vessels which signifies the resolution of inflammation and healing of the lesion (Fig. 8), indicating a favorable prognosis. In addition, Masson’s trichrome staining indicated that the area of the region expressing collagen was significantly higher in the vaccine group compared to the PBS group, with the highest value observed in the BG-Mn group.
A Tissue sections stained with HE staining. At the peak of caseous necrosis and during the complete healing period, rabbits were sacrificed, and the lesion site and surrounding skin tissues were removed and subjected to HE staining. The original scan (1×) and local magnification (200×) of the skin lesions are displayed. The various cells and necrosis are indicated on the enlarged pathological sections using different colored arrows (Red arrow, eosinophils; purple arrow, lymphocytes; black arrow, Langhan’s giant cells; yellow arrow, necrotic area or abscess; blue arrow, fibroplasia; green arrow, blood vessel or congestion). B Tissue sections stained with Masson’s trichrome staining.
Cytokine profiles in the caseum necrosis lesions at the peak of liquefaction
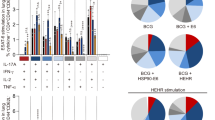
To investigate the impact of vaccine immunizations on the immune micro-environment at the lesion sites, we conducted further analysis of cytokine profiles during the peak of liquefaction in the rabbit skin model. A quantitative rabbit cytokine antibody array was utilized to detect 10 cytokines, including IL-1α, IL-1β, IL-8, IL-17A, IL-21, leptin, MMP-9, NCAM-1, MIP-1β, and TNF-α (Fig. 9A). The findings revealed that, compared to rabbits in the PBS control group, IL-1α, leptin, and TNF-α levels were higher in the BG-Mn(J) group, while IL-1β, IL-8, IL-21 and MMP-9 levels were lower. In comparison to rabbits in the PBS group, IL-1α, leptin, IL-17A, and MIP-1β levels were higher in the BG-DP group, while IL-1β, IL-8, and IL-21 levels were lower (Fig. 9A). This suggests that rabbits immunized with subunit vaccines BG-Mn(J) and BG-DP exhibited a comparable immune micro-environment at the infection sites compared to those immunized with PBS, with elevated IL-1α and leptin levels and decreased IL-1β, IL-8, and IL-21 levels. Notably, the BG-DP group showed significant differences in MMP9, leptin, and IL-17A levels compared to the BG-Mn(J) group in the post-infection immune microenvironment (Fig. 9L). These variations in cytokine levels may underlie the differing protective effects between the BG-Mn(J) and BG-DP vaccines. Furthermore, the BCG immunized group displayed higher IL-21 and NCAM-1 levels and lower IL-1α and leptin levels compared to rabbits immunized with subunit vaccines BG-Mn(J) or BG-DP (Fig. 9L). These distinct cytokine profiles may contribute to the varying protective effects observed between subunit vaccines and the live attenuated BCG vaccine.
The rabbits were sacrificed at the peak of caseous necrosis of the nodules, and the nodules were ground and quantitative rabbit cytokine antibody arrays were used to evaluate the levels of cytokines in the nodules of each group. A The results of images were captured using an Imager system, and the different cytokines are marked with various colors. B Cytokine IL-1α levels. C Cytokine IL-1β levels. D Cytokine IL-8 levels. E Cytokine IL-17A levels. F Cytokine IL-21 levels. G Cytokine leptin levels. H Cytokine MIP-1β levels. I Cytokine MMP-9 levels. J Cytokine NCAM-1 levels. K Cytokine TNF-α levels. L Heatmap of cytokine. Data are presented as mean ± standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
In this study, we evaluated the effect of Mn(J) as an adjuvant for a tuberculosis subunit vaccine Bfrb-GrpE (BG) in mice and rabbits. Our findings showed that Mn(J) enhanced the cellular and humoral immune responses to the BG vaccine and provided protection to the vaccinated mice and rabbits by reducing bacterial load and promoting the healing of immunopathological injury. Therefore, Mn(J) exhibits significant potential as an adjuvant candidate for tuberculosis vaccines.
The identification of preclinical evaluation models and indicators of protective immunity with predictive value for vaccine efficacy in humans has always been a challenge in the development of tuberculosis vaccines30,31. Mouse models are widely used in the evaluation of tuberculosis vaccines, because they offer the advantage of producing strong cell mediated immunity, as well as their ease of handling, cost-effectiveness, and the availability of reagents. In this study, to evaluate the adjuvant effect of manganese on the tuberculosis subunit vaccine Bfrb-GrpE, mice were initially administered BG-Mn(J) and BG-DP vaccines three times at weeks 2, 4, and 6. The protective efficacy was assessed 6 weeks after the final immunization. The results showed that BG-Mn(J) provided effective protection against M. tuberculosis H37Ra infection in mice, even better than BG-DP. Specifically, BG-Mn(J) vaccination resulted in a 2.0 ± 0.17 Log10 CFU reduction in spleens and a 1.3 ± 0.17 Log10 CFU reduction in lungs compared to the PBS control.
Caseous necrosis and liquefaction in tuberculous granulomas is a crucial process that leads to cavity formation, facilitating the spread of the disease. One criticism of the mouse tuberculosis model is its failure to faithfully replicate the pathological features observed in humans, particularly the formation of liquefaction in granuloma31. Therefore, it is believed that mouse models may not entirely reflect the true protective efficacy in humans. In contrast, rabbits infected with tubercle bacilli develop cell-mediated immunity and tissue-damaging delayed-type hypersensitivity (DTH) similar to humans32,33, resulting in the successive development of granulomas, caseous necrosis and liquefaction, and cavities. Consequently, we further assessed the BG-Mn(J) vaccine in a surrogate model for pulmonary tuberculosis in rabbit skin. In this model, BG-Mn(J) vaccination exhibited effective protection as well (Figs. 6–8), as evidenced by a lower bacterial load at the infection site, minimal pathological damage, and rapid healing compared to BCG and BG-DP.
Based on the comparison of the adjuvant effects of DP and Mn(J) on the fusion protein BG in mice and rabbits, the findings suggest that manganese provides a stronger effect in enhancing the vaccine’s protective effect. The control adjuvant DP, which consists of the Toll-like receptor 9 agonist poly I:C and cationic liposome DDA, was previously developed by our laboratory and has been utilized in the assessment of various tuberculosis subunit vaccines34,35. However, the component DDA needs to be dissolved in a water bath at 80 °C, and after mixing with the water-soluble poly I:C and protein, flocculent precipitates appear, which can lead to erratic effectiveness. These adverse factors affect the large-scale application of the DP adjuvant in subunit vaccines. In contrast, manganese salt, like the traditional aluminum adjuvant, offers the advantages of simplicity and robustness. In terms of protection effects, preparation and stability, we believe that Mn(J) has the potential to be employed in tuberculosis protein subunit vaccines.
Given the pivotal role of IFN-γ in tuberculosis immunity, it has been proposed that T cell production of IFN-γ could serve as immunological correlates of protection. Therefore, the Th1-type cytokine IFN-γ has been commonly utilized to assess the immunogenicity of tuberculosis vaccines in clinical and pre-clinical studies36,37. In this study, our findings in mice showed that both BG-Mn(J) and BG-DP vaccinations elicited higher levels of IFN-γ in splenocytes compared to BCG and PBS vaccinations. However, when comparing BG-Mn(J) and BG-DP, BG-DP elicited higher levels of IFN-γ. The comparisons of the protective effects and IFN-γ production between BG-Mn(J) and BG-DP suggest that the vaccine-induced Th1-type immune responses may not directly correlate with the protective efficacy in mice. It is important to note that this inconsistency in the immune response induced by tuberculosis vaccines and their protective efficacy in mice was also observed with the BCG vaccine in mice. In these cases, BCG-inoculated mice produced very low levels of IFN-γ and antibodies, yet still offered significant protection28,34,35. These results suggest that relying solely on the detection of vaccine-induced IFN-γ may not accurately reflect the true level of protection provided by the vaccine. This hypothesis has also been supported by other studies31,38,39. In addition, take MVA85A vaccine as an example, it induced potent Th1 responses and imparted better protection than BCG in preclinical animal models but failed to deliver superior protection compared to BCG in Phase IIb clinical trial40,41. It should be pointed out that vaccine specific cellular immune responses in rabbits in this study has not been detected since there are currently no suitable reagents.
As antibody-dependent mechanisms for controlling M. tuberculosis infection are observed42,43, there is a renewed interest in vaccine-elicited antibodies to M. tuberculosis. For instance, it had found that the administration of an anti-LAM IgG antibody to BALB/c mice reduced the bacterial burden in the infected animals and prolonged their survival42. Similarly, pre-incubation of M. tuberculosis with antibody specific to a M. tuberculosis reduced their pathogenicity in mice42,43. These findings suggest that antibodies can be protective against tuberculosis. Data from BCG vaccination in pre-clinical model with rhesus macaques or in clinical trials have all revealed an association between the antibody titers specific to BCG and a decreased tuberculosis infection44,45. Additionally, vaccine-induced antibody titers have been shown to correlate with the reduction in M. tuberculosis burden post infection, in the cases of the DNA vaccine using the M. bovis Ag85B antigen46,47 and subunit vaccine Mtb10.4-HspX39. In our study, we observed that, compared to BCG and PBS vaccinations, both BG-Mn(J) and BG-DP vaccinations elicited higher levels of IgG and IgG1 in mice, and IgG in rabbits (Figs. 3 and 4), which correlated with their protective efficacy. It is worth mentioning that the BCG vaccine did not induce the production of IgG in mice, but it did induce the production of IgG in rabbits. The results of BCG-induced IgG antibody production in rabbits are consistent with its protective effect and the generation of specific antibodies, mostly IgG, following BCG vaccination in humans. From this, we speculate that the rabbit model may be superior to the mouse model for predicting value for tuberculosis vaccines in humans.
In the rabbit skin model, our results showed that all BCG or subunit vaccine vaccinations accelerated the liquefaction and healing processes in the skin lesions, and reduced the bacterial load in the liquefied caseum, which are consistent with our previous findings32. The reason might be that, as described in Koch phenomenon, the immune response established by the vaccine immunization prompts a strong immune response at the site of infection. In order to examine the influence of the vaccine-induced immunity on the immune microenvironment at the lesion sites, we conducted additional analysis of cytokine profiles during the peak of liquefaction. We found that rabbits immunized with subunit vaccines BG-Mn(J) and BG-DP exhibited a comparable immune microenvironment at the infection site compared to those immunized with PBS, with elevated IL-1α and leptin levels and decreased IL-1β, IL-8, and IL-21 levels. In contrast, the BG-Mn(J) group exhibited significantly lower levels of MMP9, leptin, and IL-17A compared to the BG-DP group in the post-infection immune microenvironment. In addition, the BCG immunized group displayed higher IL-21 and NCAM-1 levels and lower IL-1α and leptin levels compared to rabbits immunized with subunit vaccines BG-Mn(J) or BG-DP (Fig. 9L). These findings revealed that, at the time of infection, rabbits immunized with different types of vaccines and subunit vaccines composed of different adjuvants produced different cytokine profiles in the immune microenvironment of infections. In addition, these cytokines are not higher the better, as evidenced by lower levels of cytokines MMP9, leptin, and IL-17A in the Mn(J) adjuvant group, which showed better protective effects compared to the DP group. However, further research is needed to understand why rabbits in the Mn(J) adjuvant group did not produce high levels of MMP9, leptin, and IL-17 in response to infection.
In summary, we employed manganese adjuvant Mn(J), primarily composed of Mn2+, in the tuberculosis subunit vaccine BG and observed that BG-Mn(J) vaccine induced robust cellular and humoral immune responses in mice and reduced bacterial loads in the lungs and spleens. Additionally, in rabbits, BG-Mn(J) immunization not only induced high levels of antibodies and decreased bacterial loads at the infection site but also shortened the immunopathological process and promoted healing of liquefied necrotic tissues. Therefore, despite the need for further research into the mechanisms by which Mn(J) adjuvant-assisted vaccines regulate the body’s anti-infection responses, we believe that Mn(J) has the potential to be used in tuberculosis subunit vaccines for improved vaccine efficacy. Further studies are needed to validate our findings with other vaccines and in different models.
Materials and methods
Animals
Six-week-old female C57BL/6 mice were obtained from Beijing Weitong Lihua Company and were housed in specific pathogen-free facilities at Lanzhou University. For M. tuberculosis H37Ra infection, the mice were kept in biosafety level 2 (BSL2) laboratory. At the end of the experiment, the mice were euthanized by intravenous injection of sodium pentobarbital (35 mg/kg) followed by cervical dislocation. The animal protocols followed the guidelines set by the Institutional Animal Care and Use Committee of the Lanzhou University.
Female New Zealand white rabbits, weighing 2.0 to 3.0 kg, were provided by Yuhang Kelian Rabbit Industry. Each rabbit was housed individually in a standard animal room with unrestricted access to food and water. For M. bovis BCG infection, the mice were kept in biosafety level 2 (BSL2) laboratory. At the end of the experiment, the rabbits were euthanized by intravenous injection of sodium pentobarbital (30 mg/kg) followed by intravenous injection of air. All experimental procedures were approved by the Experimental Animal Committee of Zhejiang Chinese Medical University.
Over-expression and purification of the fusion protein BG, as well as the single protein antigens BfrB and GrpE
To optimize the concentration of IPTG for the induction of the recombinant vector, we have tested a range of IPTG concentrations for BG expression, including 0.2 mM, 0.5 mM, and 1 mM. The results indicated no significant differences in protein expression levels among the different IPTG concentrations28. Considering factors such as protein expression levels, cell toxicity, and industrial cost for vaccine production, we have decided to select an intermediate concentration of IPTG, 0.5 mM, for the subsequent protein induction. This concentration strikes a balance between achieving optimal protein expression and minimizing potential cell toxicity.
For the over-expression and purification of the fusion protein BG, the E. coli BL21(DE3 pLySs) harboring the recombinant plasmid pET30a (+)-BfrB-GrpE, was cultured in LB medium supplemented with kanamycin (50 µg/mL) until reaching an optical density (OD) of 0.5 at 600 nm. Subsequently, the cells were induced with 0.5 mM IPTG (Solarbio, Beijing, China) and incubated at 37 °C for an additional 5 h. Following incubation, the cells were harvested, sonicated on ice using the SCIENTZ-IID ultrasonic cell disruptor (Scientz Biotechnology Co., Ltd, Ningbo, China). The sonication conditions involved three cycles with a power of 600–800w for 3 s and stopped for 3 s for 99 cycles. Subsequently, the resulting homogenate was centrifuged at 10,000 × g for 10 min at 4 °C. The supernatant, enriched with the BfrB-GrpE fusion protein, was subjected to purification.
The non-tagged BfrB-GrpE fusion protein was purified from the supernatant through two sequential steps: ammonium sulfate precipitation followed by chromatography. Initially, a 35% volume ratio of saturated ammonium sulfate was added to the supernatant and incubated overnight at 4 °C to induce protein precipitation. Subsequently, the precipitated protein was collected by centrifugation, resuspended in PBS, and refolded to its native conformation through gradual dilution. The refolded protein was then subjected to purification through hydrophobic interaction chromatography (HIC) using a butyl-Sepharose high-performance column, employing buffers A (ddH2O) and B (20 mM phosphate buffer, 2 M sodium chloride, pH 8.0). Chromatography was conducted utilizing the AKTA Purifier 100 system (GE, NJ, USA). Recombinant single protein antigens BfrB and GrpE, tagged with His-tags, were overexpressed in E. coli BL21 cells and subsequently purified from the supernatant through affinity chromatography using Ni-NTA His-Bind Resin (GE, NJ, USA).
Finally, the purified proteins were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) for analysis, and the concentration of the purified proteins after ultrafiltration was determined utilizing the Bradford protein assay (Yuanye Bio., Shanghai, China).
Vaccine preparation and immunization procedure
The purified BG fusion protein was suspended in PBS at a concentration of 0.4 mg/mL for vaccine preparation. For the BG-DP vaccine, BG was initially mixed with 1 mg/mL poly (I:C) (Sigma-Aldrich, Poole, UK). Subsequently, a suspension of 5 mg/mL DDA (Source Leaf Biology, Shanghai, China) was gradually added to the mixture of BG and poly (I:C) to achieve a uniform emulsion. In the case of the BG-Mn(J) vaccine candidate, BG were directly combined with the 5 mg/mL Mn(J) suspension (MnStarter Biotechnology, Jiangsu, China). The BCG was cultured on Lowenstein-Jensen medium for a period of 3–4 weeks. Subsequently, the BCG vaccine was harvested, washed, and its concentration was adjusted to 5 × 107 CFU/mL (colony-forming units of bacteria).
In both mouse and rabbit experiments, the animals were divided into four groups separately. The first group received PBS inoculation, the second group received BCG inoculation, the third group received vaccine BG-DP, and the fourth group received vaccine BG-Mn(J). The vaccination dose for the BG-DP vaccine for mice consisted of 20 μg protein, 250 μg DDA and 50 μg poly (I: C) in 200 μL. For rabbits, each vaccination dose of the BG-DP vaccine included 50 μg protein, 625 μg DDA, and 125 μg poly (I:C) in 1000 μL. The vaccination dose for the BG-Mn(J) vaccine for mice contained 20 μg protein and 100 μg Mn(J) in 200 μL, while for rabbits, each vaccination dose included 50 μg protein and 250 μg Mn(J) in 1000 μL. The vaccination dose for the BCG vaccine for mice consisted of 5 × 106 CFU in 200 μL, and for rabbits, each vaccination dose includes 1 × 107 CFU in 400 μL. All the animals received a subcutaneous injection of the vaccine. The PBS and submit vaccine groups were administered three injections at zero, second, and fourth week, while the BCG immunization group received a single injection at week zero. The temperature and weight of the vaccinated animals were measured before and after each immunization.
Enzyme-linked immunosorbent assay (ELISA) for the determination of IFN-γ levels in spleen lymphocytes
Following immunization of vaccines, ELISA was performed 6-weeks after the final immunization to determine the level of IFN-γ secreted from splenic lymphocytes of mice (n = 4 per group). Firstly, the EZ-SepTM Mouse Lymphocyte Isolation Kit (Dakewe, Beijing, China) was used to isolate splenic lymphocytes. The freshly separated splenocytes were then resuspended at a concentration of 1 × 107 cells/mL. After individual stimulation with BfrB or GrpE (5 μg/mL), lymphocytes (2 × 106 cells/well) were placed in 24-well plates and incubated for 72 h at 37 °C with 5% CO2. The culture supernatants were harvested, and the cytokine levels released by spleen lymphocytes were measured using mouse IFN-γ ELISA kits (U-CyTech, Utrecht, Netherlands) according to the manufacturer’s instructions. Absorbance at 450 nm was measured using a microplate reader (Thermo Fisher, Waltham, USA). The data was evaluated using CurveExpert software (Abbkine, Georgia, USA).
ELISA for the quantification of antigen-specific antibodies in the serum of vaccinated mice and rabbits
Six weeks post the last immunization, serum samples from mice (n = 4 per group) or rabbits (n = 3 per group) were collected to measure the levels of BfrB and GrpE antigen-specific IgG1, and IgG2c, and also total IgG antibodies using ELISA. To initiate the experiment, 96-well plates were coated with either BfrB or GrpE (5 μg/mL) and incubated overnight at 4 °C. The plates were then washed multiple times with PBST (PBS containing 0.05% Tween 20) and blocked for one hour at 37 °C using 5% BSA. Serum samples were diluted 1:100 with PBS and added to the plates in two-fold successive dilutions up to 1:25,600 or 1:509,600. After incubation and washing, anti-IgG, IgG1 and IgG2c antibodies were applied separately at dilutions of 1:15,000, 1:10,000, or 1:5,000 with a volume of 200 μL per well. Subsequently, the plates were washed with PBST and the TMB substrate was added. Following a fifteen-minute incubation at room temperature, the optical density (OD) was recorded at 450 nm upon the addition of 50 μL of a 1 M sulfuric acid solution, which terminated the reaction.
Evaluation of vaccine efficacy against M. tuberculosis H37Ra infection in mice
Following the final vaccination, six weeks later, mice (n = 3–4 per group) that received PBS, BCG, BG-DP, or BG-Mn(J) vaccines were subjected to a challenge with 5 × 106 CFU of M. tuberculosis H37Ra via tail vein injection. Three weeks after the challenge, the mice were euthanized, and their lungs and spleens were homogenized. Tissue homogenates were serially diluted ten-fold in sterile PBS, and the number of CFUs was determined by plating in triplicate on 7H10 agar plates supplemented with 10% OADC.
Assessment of vaccine efficacy in an attenuated M. bovis BCG challenge rabbit skin model
The rabbit skin model was carried out as we described previously32,33. Six weeks after the final vaccination, rabbits (n = 3 per group) were intradermally injected at two sites on each flank with 100 µl BCG containing 5 × 106 CFU, with the two sites spaced 2–3 cm apart. Each rabbit received a total of four injections. Subsequently, we conducted daily observations of the pathological changes at the injection sites and recorded the skin lesions. To assess the skin lesions following BCG infection, we measured their width, length and height, and calculated their volumes by multiplying these three dimensions. The timeline of typical pathological manifestations, such as the formation of tuberculous granulomas, caseous necrosis and liquefaction, ulceration, and healing processes, was also recorded.
The criteria for identifying these typical pathological manifestations are as follows: (1) Solid granuloma: the nodule is obviously red and swollen, with a hard texture. (2) Caseous necrosis and liquefaction: the texture of the nodule begins to soften, and the presence of liquefied caseous necrosis in the center can be observed. Detection involves gently inserting a sterile syringe needle into the nodule’s center for examination. The onset of liquefaction is indicated by the presence of liquefied material on the needle upon withdrawal or the observation of liquefaction at the insertion site after gentle pressure on the tuberculosis nodule, resulting in material flow. (3) Ulceration: Ulceration occurs when the center of the nodule ruptures, leading to the discharge of a small amount of liquefied caseous necrosis material from the rupture site. The peak of liquefaction is reached when the nodule volume reaches its maximum, resulting in the discharge of a significant amount of liquefied caseous necrotic material. (4) Healing: initiated by a decrease in nodule volume, shrinking by 60% of the maximum volume, and the formation of a scab at the ulcer site. Complete healing is indicated by a 75% reduction in nodule volume, equivalent to 25% of the maximum volume, with visible epithelial formation at the site of nodule ulceration.
Quantification of bacterial loads in rabbit skin lesions
At the peak of caseous necrosis, as much liquefied caseum as possible was collected from the lesions of rabbits (n = 3 per group). The collected caseum was aseptically weighed and homogenized in one milliliter of sterile saline (0.9% NaCl). For CFU quantification, the homogenates were serially diluted and cultured for three weeks on Lowenstein-Jensen medium. Ziehl-Neelsen acid-fast staining was employed to confirm the presence of tubercle bacilli in the bacterial colonies.
Measurement of cytokine profiles in tuberculous granulomas during the peak of caseous necrosis
During the peak of caseous necrosis, the rabbit (n = 3 per group) skin tuberculous granulomas were excised using a scalpel blade and subsequently fully ground in 1 mL of PBS. To assess cytokine profiles in tuberculous granulomas, the Quantitative Rabbit Cytokine Antibody Array (Cat. #ab197459, Abcam, Boston, MA, USA) was utilized. Initially, the slides are incubated in blocking solution for 30 min to reduce nonspecific antibody binding. Subsequently, the samples being tested were incubated with primary coated antibodies in the slides for 2 h to allow for binding of target biomarkers. Following this, series of seven washes were performed to remove unbound antibodies and other impurities. Next, the slides were incubated with a cocktail of biotinylated detector antibodies for 2 h to enhance signal specificity. Another round of seven washes ensures removal of excess detector antibodies. Subsequent incubation with fluorophore-labeled streptavidin for 1 h was performed to enable visualization of bound detector antibodies. Finally, seven additional washes were conducted to eliminate unbound streptavidin. Plate images were captured with a LuxScan Imager system (CapitalBio, Beijing, China). Quantification of individual cytokines in the images was performed using ImageJ software (RRID: SCR_003070).
Pathological examination
During the peak of caseous necrosis and healing phase, tuberculous nodules in rabbits, both the lesions and normal skin tissues nearby were collected. The dissected nodular tissues were then fixed by immersion in 10% neutral buffered formalin solution for 24–48 h. After fixation, the tissues underwent dehydration using ascending grades of ethanol, followed by clearing with xylene, and infiltration, embedding, and blocking with paraffin. The resulting blocks were sectioned using an Ultra-Thin Semiautomatic Rotary microtome at a thickness of 4–5 μm and mounted on slides. All prepared slides were stained with the hematoxylin and eosin, and subsequently scanned using a pathological section scanner (Hamamatsu, Japan).
Statistical analyses
Data analysis was performed using GraphPad Prism 5.0 (GraphPad Software Inc., CA, USA). For data comparison, either a one-way or two-way analysis of variance (ANOVA) was conducted, followed by Bonferroni’s multiple comparison test. Statistical significance was considered at p < 0.05.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Bagcchi, S. WHO’s Global Tuberculosis Report 2022. Lancet Microbe 4, E20–E20 (2023).
Zhu, B. et al. Tuberculosis vaccines: opportunities and challenges. Respirology 23, 359–368 (2018).
Nguipdop-Djomo, P. et al. Duration of BCG protection against tuberculosis and change in effectiveness with time since vaccination in Norway: a retrospective population-based cohort study. Lancet Infect. Dis. 16, 219–226 (2016).
Ahsan, M. J. Recent advances in the development of vaccines for tuberculosis. Therapeutic Adv. Vaccines 3, 66–75 (2015).
Gong, W. P., Liang, Y. & Wu, X. Q. The current status, challenges, and future developments of new tuberculosis vaccines. Hum. Vaccines Immunotherapeutics 14, 1697–1716 (2018).
Bastola, R. et al. Vaccine adjuvants: smart components to boost the immune system. Arch. Pharmacal Res. 40, 1238–1248 (2017).
Schrager, L. K. et al. The status of tuberculosis vaccine development. Lancet Infect. Dis. 20, E28–E37 (2020).
Wan, X. H. et al. Polysaccharides derived from Chinese medicinal herbs: A promising choice of vaccine adjuvants. Carbohydrate Polymers 276 (2022).
Mosser, D. M. & Edwards, J. P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008).
Nicolai, C. J. et al. NK cells mediate clearance of CD8(+) T cell-resistant tumors in response to STING agonists. Sci. Immunol. 5 (2020).
Diamond, M. S. et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 208, 1989–2003 (2011).
Woo, S. R. et al. STING-Dependent Cytosolic DNA Sensing Mediates Innate Immune Recognition of Immunogenic Tumors (vol 41, pg 830, 2014). Immunity 42, 199–199 (2015).
Li, X. D. et al. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 341, 1390–1394 (2013).
Ishikawa, H. & Barber, G. N. The STING pathway and regulation of innate immune signaling in response to DNA pathogens. Cell. Mol. Life Sci. 68, 1157–1165 (2011).
Chattopadhyay, S. & Hu, C. M. J. Nanomedicinal delivery of stimulator of interferon genes agonists: recent advances in virus vaccination. Nanomedicine 15, 2883–2894 (2020).
Wang, J. et al. Pulmonary surfactant–biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science 367 (2020).
Van Herck, S., Feng, B. & Tang, L. Delivery of STING agonists for adjuvanting subunit vaccines. Adv. Drug Delivery Rev. 179 (2021).
Tian, X. Y. et al. cGAS-STING pathway agonists are promising vaccine adjuvants. Med. Res. Rev. (2024).
Wang, C. G. et al. Manganese Increases the Sensitivity of the cGAS-STING Pathway for Double-Stranded DNA and Is Required for the Host Defense against DNA Viruses. Immunity 48, 675 (2018). -+.
Lv, M. Z. et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 30, 966–979 (2020).
Hooy, R. M. et al. Allosteric coupling between Mn2+ and dsDNA controls the catalytic efficiency and fidelity of cGAS. Nucleic Acids Res. 48, 4435–4447 (2020).
Zhao, Z. et al. Mn(2+) Directly Activates cGAS and Structural Analysis Suggests Mn(2+) Induces a Noncanonical Catalytic Synthesis of 2'3’-cGAMP. Cell Rep. 32, 108053 (2020).
Wu, S., Gabelli, S. B. & Sohn, J. The structural basis for 2’-5’/3’-5’-cGAMP synthesis by cGAS. Nat. Commun. 15, 4012 (2024).
Guo, M. Y. et al. Durable and enhanced immunity against SARS-CoV-2 elicited by manganese nanoadjuvant formulated subunit vaccine (vol 9, 22, 2024). Signal Transduction and Targeted Therapy, 9 (2024).
Wang, Z. M. et al. Colloidal manganese salt improves the efficacy of rabies vaccines in mice, cats, and dogs. J. Virol. 95 (2021).
Sheng, Y. et al. In situ bio-mineralized Mn nanoadjuvant enhances anti-influenza immunity of recombinant virus-like particle vaccines. J. Control Release 368, 275–289 (2024).
Zhang, R. et al. Manganese salts function as potent adjuvants. Cell. Mol. Immunol. 18, 1222–1234 (2021).
Niu, H. et al. Construction and evaluation of a novel multi-antigenic Mycobacterium tuberculosis subunit vaccine candidate BfrB-GrpE/DPC. Int Immunopharmacol. 124, 111060 (2023).
Liu, X. et al. A novel liposome adjuvant DPC mediates Mycobacterium tuberculosis subunit vaccine well to induce cell-mediated immunity and high protective efficacy in mice. Vaccine 34, 1370–1378 (2016).
Chugh, S. et al. Antigen identification strategies and preclinical evaluation models for advancing tuberculosis vaccine development. Npj Vaccines 9 (2024).
Lai, R. C. Y. et al. Key advances in vaccine development for tuberculosis-success and challenges. Npj Vaccines 8 (2023).
Chen, H. et al. A new rabbit-skin model to evaluate protective efficacy of Tuberculosis vaccines. Front Microbiol. 8, 842 (2017).
Dannenberg, A. M. Jr. Liquefaction and cavity formation in pulmonary TB: a simple method in rabbit skin to test inhibitors. Tuberculosis (Edinb 89, 243–247 (2009).
Liu, X. et al. A multistage mycobacterium tuberculosis subunit vaccine LT70 including latency antigen Rv2626c induces long-term protection against tuberculosis. Hum. Vaccines Immunotherapeutics 12, 1670–1677 (2016).
Niu, H. et al. Multi-stage tuberculosis subunit vaccine candidate LT69 provides high protection against Mycobacterium tuberculosis infection in mice. PLoS ONE 10, e0130641 (2015).
Li, J. L. et al. Tuberculosis vaccine development: from classic to clinical candidates. Eur. J. Clin. Microbiol. Infect. Dis. 39, 1405–1425 (2020).
Lewinsohn, D. A., Lewinsohn, D. M. & Scriba, T. J. Polyfunctional CD4(+) T Cells As Targets for Tuberculosis Vaccination. Front Immunol. 8, 1262 (2017).
Lu, L. L. et al. IFN-γ-independent immune markers of Mycobacterium tuberculosis exposure. Nat. Med. 25, 977–987 (2019).
Niu, H. et al. Construction and evaluation of a multistage Mycobacterium tuberculosis subunit vaccine candidate Mtb10.4-HspX. Vaccine 29, 9451–9458 (2011).
Tameris, M. D. et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381, 1021–1028 (2013).
Tameris, M. et al. The candidate TB vaccine, MVA85A, induces highly durable Th1 responses. PLoS ONE 9, e87340 (2014).
Hamasur, B. et al. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab′)2 fragment prolong survival of mice infected withMycobacterium tuberculosis. Clin. Exp. Immunol. 138, 30–38 (2004).
Teitelbaum, R. et al. A mAb recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc. Natl. Acad. Sci. USA 95, 15688–15693 (1998).
Fletcher, H. A. et al. T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat. Commun. 7, 11290 (2016).
Irvine, E. B. et al. Robust IgM responses following intravenous vaccination with Bacille Calmette-Guerin associate with prevention of Mycobacterium tuberculosis infection in macaques. Nat. Immunol. 22, 1515–1523 (2021).
Teixeira, F. M. et al. DNA Vaccine UsingMycobacterium bovisAg85B Antigen Induces Partial Protection against Experimental Infection in BALB/c Mice. Clin. Vaccin. Immunol. 13, 930–935 (2006).
Huygen, K. et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nature Medicine, 1996).
Acknowledgements
This study was supported by the Zhejiang Provincial Natural Science Foundation of China (LY24H290004), National Natural Science Foundation of China Youth Foundation (81701969) and the Fundamental Research Funds for the Central Universities (712213A12627).
Author information
Authors and Affiliations
Contributions
H.N., Y.Z., and A.Z. conceived the work, designed the study, analyzed the data and wrote the manuscript; S.Z. and Q.C. mainly performed the experiments, analyzed the data and wrote the manuscript; Z.Z., Y.D., Y.H., X.Z., Z.X.,Y.Z., and B.Z. participated in the evaluation of vaccines and immunological protection in animal models. All authors agree to be accountable for the content of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, S., Cao, Q., Zhang, Z. et al. The adjuvant effect of manganese on tuberculosis subunit vaccine Bfrb-GrpE. npj Vaccines 9, 248 (2024). https://doi.org/10.1038/s41541-024-01049-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-024-01049-x